Many bacterial protein toxins are highly potent and extremely toxic cytocidal agents. With the discovery of toxin targets and the elucidation of molecular mechanisms of toxin action, the question arose of how to use these powerful agents for therapy, especially for tumor therapy. Many toxins can kill target cells at very low concentrations. For example, one molecule of diphtheria toxin may be sufficient to kill a target cell (1). However, an obvious major problem of using toxins as antitumor drugs is their high toxicity for normal, noncancer cells. Now, Duru et al. (2) describe in an excellent preclinical study how the specificity of anthrax toxin can be greatly increased to enable the toxin to be targeted to ovarian carcinoma.
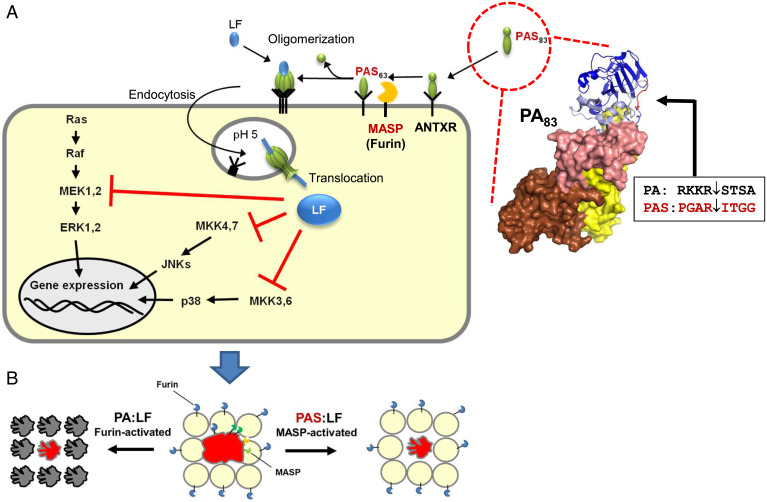
Anthrax toxin is the prototype of a family of binary toxins (3). Each binary toxin consists of a receptor-binding component and a separate enzyme component. For anthrax toxin, two enzyme domains are known: lethal factor (LF), a zinc protease that mainly cleaves MAP kinases (mitogen activated protein kinases) (4), and edema factor, a calmodulin-dependent adenylylcyclase. The binding, uptake, and action of anthrax toxin have been studied in great detail (3). The monomeric receptor-binding component of anthrax toxin, PA (protective antigen; 83 kDa), consists of four domains (Fig. 1 A, Right). Domain 1 contains the activation site (blue in Fig. 1), domain 2 (pink in Fig. 1) is mainly involved in pore formation, domain 3 (yellow in Fig. 1) is important for oligomerization, and domain 4 (brown in Fig. 1) is responsible for receptor binding. PA can bind to either of two ubiquitously expressed receptors, anthrax receptor 1 (ANTXR1; tumor endothelial marker-8) and anthrax receptor 2 (ANTXR2; capillary morphogenesis protein 2). Bound full-length PA (PA83) is cleaved by furin, thereby releasing a 20-kDa fragment and allowing oligomerization of PA63 to form heptamers, which bind up to three molecules of LF and/or EF. After endocytosis of the toxin–receptor complex, PA inserts into endosomal membranes, forms a β-barrel pore, and delivers the enzyme components into the cytosol of target cells (3).
Fig. 1.
The action of the zymogen-activated anthrax toxin PAS:LF. (A, Right) The monomeric 83-kDa binding component of anthrax toxin (PA83) is activated by cleavage after the sequence RKKR by furin (the box and arrow indicate the cleavage site). In PAS, eight residues of PA in the cleavage site were changed, resulting in activation by MASPs, which are overexpressed in ovarian cancer cells. (A, Left) Monomeric PAS83 binds to anthrax receptors (ANTXR). MASPs-induced cleavage causes heptamerization of PAS63 and binding of the enzyme component, LF. At low pH of endosomes, PAS inserts into the membrane and forms a pore for translocation of LF into the cytosol. In the cytosol, LF cleaves and inactivates the kinases MEK (MAP/ERK kinase)1,2 and MKK (mitogen-activated kinase kinase) 3,4,6,7. These kinases are involved in MAP kinase pathways, which act as signal hubs to the nucleus, modulating gene expression. MAP kinase pathways are frequently activated in cancer cells. (Ras, proto-oncogene product with GTPase activity; Raf, serine/threonine-protein kinase; ERK, extracellular signal-regulated kinase; p38, p38 mitogen-activated protein kinase; JNK, c-Jun N-terminal kinase) (B) Wild-type anthrax toxin PA:LF is activated by furin, which is ubiquitously expressed. Therefore, the toxin effects are not specific. Ovarian cancer cells (red) overexpress several types of MASPs, which can activate each other. PAS:LF is mainly selectively activated by MASPs from cancer cells. Therefore, the toxic effect of PAS:LF is specific for the cancer cell.
Attempts to make toxins more specific for cancer cells are often based on modifying receptor specificity. Early examples are immunotoxins, which are protein toxins or toxin fragments conjugated with specific antibodies directed against tumor cell proteins. Later, receptor ligands were conjugated with toxins (ligand toxins) or more often, with the active components of toxins (5, 6). In the present study, a different approach was used, namely the change of the specificity of the activation step. The action of anthrax toxin depends on the cleavage of domain 1 of PA83 by furin or furin-like proteases (Fig. 1). Furin is a transmembrane, calcium-dependent serine protease (7) from the family of proprotein convertases. The similarity to bacterial subtilisin and yeast kexin generated the abbreviation PCSK for this family, with furin as PCSK3. Furin is an essential protein (deletion is embryonic lethal) and mainly involved in the processing of numerous precursors, secreted by the constitutive secretion pathway. Originating in the ER, furin reaches the cell membrane, where it activates bacterial toxins (e.g., PA of anthrax toxin) and also, numerous viral proteins (e.g., SARS-CoV-2 spike proteins). Importantly, furin is proteolytically self-activated during its travel to the cell membrane (7, 8). The proteolytic activation of PA occurs within a surface loop at the sequence RKKR (residues 164 to 167). Because furin is ubiquitously expressed, the toxic effects of anthrax toxin can occur wherever toxin receptors are present. Here lies the Gordon knot of the toxin’s action.
How to solve this problem? Progressed and metastasized tumors are very often characterized by dysregulated protease activity. This appears to be especially true for advanced-stage ovarian tumors (9). The highly expressed membrane-anchored serine proteases (MASPs) are involved in invasion and metastasis. MASPs are synthesized and expressed on the cell surface as inactive precursors (zymogens). They require activation by other serine proteases present in the microenvironment (10). Moreover, active MASPs can activate inactive tumor MASPs.
Here, the authors describe the development of a zymogen activation prodrug toxin (ZMT). They changed eight amino acids (residues 164 to 171 in Fig. 1 A, Right) from the furin cleavage site of PA with sequences appropriate for cleavage by typical MASPs. They found that the cleavage site of prostasin, introduced into PA (named PAS), was the most appropriate. Prostasin is a GPI-anchored nonself-activated serine protease, which is a known substrate of MASPs. PAS was fully activated by the MASPs matriptase, testisin, and hepsin. By contrast, furin, matrix metallopeptidases MMP-2/-9, and urokinase-type plasminogen activator were not able to activate PAS, indicating specificity for MASPs. They tested the toxicity of the zymogen-activated prodrug toxin in cultured cells (HEK293T) that overexpressed different types of MASPs. Overexpression of MASPs greatly increased toxicity (up to 20- to 50-fold). Various cell lines derived from ovarian tumors exhibited high sensitivity as compared with nontumorigenic ovarian cells. ZMT toxicity appears to depend fully on serine protease activity because the serine protease inhibitor AEBSF (4-(2-aminoethyl)benzenesulfonyl fluoride) blocked the toxin effects.
Initial studies were performed with an LF anthrax toxin fusion protein that contained a toxin fragment (FP59) of Pseudomonas aeruginosa Exotoxin A, which causes inhibition of protein synthesis by ADP ribosylation of elongation factor 2. However, this conjugate was too toxic, even toward nontumorigenic ovarian cells. Therefore, subsequent studies were performed with PAS and unmodified LF, which is ∼50-fold less toxic than FP59 and targets the MAP kinase pathway by proteolytic cleavage of mitogen-activated protein kinase kinases (MEK1,2 and MKK3,4,6,7) (4) (Fig. 1). Notably, the MAP kinase pathway is critical for human cancer cell survival, is involved in metastasis and invasion, and is activated in ∼50% of all tumors (11). The MAP kinase pathway is a signaling hub with multiple inputs from numerous stimuli, including growth factors, cell matrix, and cell–cell interactions but also, metabolic stress or DNA damage pathways (11). PAS:LF was highly effective in killing tumor cells and significantly reduced the formation and growth of multicellular ovarian tumor spheroids. The adriamycin-resistant ovarian tumor cell line (NCI/ADR-Res ovarian tumor cells), which is in fact multidrug resistant, was initially also resistant toward PAS:LF. However, a repetitive sequential treatment regime demonstrated its susceptibility. Notably, nontumorigenic IOSE397 cells were not affected by PAS:LF treatment. Furthermore, PAS:LF was highly effective in a xenograft model that seems to mimic the key events of late-stage ovarian cancers. While in the controls of the xenograft model, multiple tumor foci were visible, the PAS:LF-treated mice exhibited no ascites accumulation or tumor foci throughout the peritoneal cavity, and the mean survival time of mice was prolonged. Importantly, the mice tolerated PAS:LF treatment without weight loss or obvious organ damage. When instead of PAS, a PA mutant not cleaved by any known protease was used, the positive treatment effects were completely blocked, indicating that selective protease activation is required. In another study, the authors used patient-derived ovarian tumor cells, obtained from patient ascites, that consistently overexpressed MASPs, like hepsin, matriptase, and testisin. Again, PAS:LF largely inhibited the development of organotypic multicellular spheroids from patient tumor cells. Final support for the efficiency and potential of the PAS:LF toxin design came from patient-derived xenograft (PDX) studies. When PDX tumors were treated intraperitonially with LF alone, the tumors’ volume increased approximately fourfold; however, no significant increase was observed in PAS:LF-treated tumors. Importantly, no off-target organ damage was observed with PAS:LF.
Ovarian cancer is the leading mortality cause of gynecologic malignancies. The classical treatment for advanced ovarian cancer recommended by several international guidelines is the combination of carboplatin and paclitaxel (12). Recently, bevacizumab and PARP (poly(ADP-ribose)polymerase) inhibitors were added as further options (13). Clearly, the therapeutic armamentarium against ovarian cancer is limited and at advanced stages, often without success. The 5-y survival of advanced ovarian cancer is still below 50% (13). Therefore, new innovative alternatives are urgently needed. Quite early, anthrax toxin was discussed as an anticancer agent (14, 15). The proteolytic cleavage and inhibition of MAP kinases by LF are especially interesting because the MAP kinase pathway is critical for the survival of cancer cells (11). Various subtypes of ovarian cancers have been described, which differ in their histomorphological and genetic profiles. Several oncogenic driver mutations were described, which often cause ERK1/2 activation (12). Many of them funnel at least partially to the Ras-Raf-MEK-ERK1/2 signaling pathway. Thus, LF appears to be a most effective tool to block this signal pathways. Moreover, the approach to increase the specificity of anthrax toxin as described may be of relevance for other tumor types. Future studies may show whether the specificity toward tumors could be further increased by combining modulation of the activation specificity with changes in receptor specificity. However, whether an increase in specificity alone is sufficient to cope with expected side effects (e.g., from the immune system) remains open.
Footnotes
The author declares no competing interest.
See companion article, “Selective targeting of metastatic ovarian cancer using an engineered anthrax prodrug activated by membrane-anchored serine proteases,” 10.1073/pnas.2201423119.
References
- 1.Yamaizumi M., Mekada E., Uchida T., Okada Y., One molecule of diphtheria toxin fragment A introduced into a cell can kill the cell. Cell 15, 245–250 (1978). [DOI] [PubMed] [Google Scholar]
- 2.Duru N., et al. , Selective targeting of metastatic ovarian cancer using an engineered anthrax prodrug activated by membrane-anchored serine proteases. Proc. Natl. Acad. Sci. U.S.A., 10.1073/pnas.2201423119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young J. A., Collier R. J., Anthrax toxin: Receptor binding, internalization, pore formation, and translocation. Annu. Rev. Biochem. 76, 243–265 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Duesbery N. S., et al. , Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science 280, 734–737 (1998). [DOI] [PubMed] [Google Scholar]
- 5.Kreitman R. J., Pastan I., Immunotoxins: From design to clinical application. Biomolecules 11, 1696 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antignani A., et al. , Targeting receptors on cancer cells with protein toxins. Biomolecules 10, E1331 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seidah N. G., Prat A., The biology and therapeutic targeting of the proprotein convertases. Nat. Rev. Drug Discov. 11, 367–383 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Leduc R., Molloy S. S., Thorne B. A., Thomas G., Activation of human furin precursor processing endoprotease occurs by an intramolecular autoproteolytic cleavage. J. Biol. Chem. 267, 14304–14308 (1992). [PubMed] [Google Scholar]
- 9.Pawar N. R., Buzza M. S., Antalis T. M., Membrane-anchored serine proteases and protease-activated receptor-2-mediated signaling: Co-conspirators in cancer progression. Cancer Res. 79, 301–310 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antalis T. M., Buzza M. S., Hodge K. M., Hooper J. D., Netzel-Arnett S., The cutting edge: Membrane-anchored serine protease activities in the pericellular microenvironment. Biochem. J. 428, 325–346 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burotto M., Chiou V. L., Lee J. M., Kohn E. C., The MAPK pathway across different malignancies: A new perspective. Cancer 120, 3446–3456 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matulonis U. A., et al. , Ovarian cancer. Nat. Rev. Dis. Primers 2, 16061 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lheureux S., Gourley C., Vergote I., Oza A. M., Epithelial ovarian cancer. Lancet 393, 1240–1253 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Bodart J. F., Chopra A., Liang X., Duesbery N., Anthrax, MEK and cancer. Cell Cycle 1, 10–15 (2002). [PubMed] [Google Scholar]
- 15.Bachran C., Leppla S. H., Tumor targeting and drug delivery by anthrax toxin. Toxins (Basel) 8, E197 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]