Significance
Disparities between Black and White Americans persist in medical treatment and health outcomes. One reason is that physicians sometimes implicitly favor White (over Black) patients. This paper presents results suggesting an approach for promoting more equitable interpersonal treatment during doctor–patient interactions. In our study, Black and White actors (“standardized patients” [SPs]) were trained to behave in an engaged or typical manner during appointments with unsuspecting doctors. When SPs were typical, doctors’ implicit racial bias was associated with racially biased interpersonal treatment. When SPs were engaged, however, doctors’ implicit bias had no effect on interpersonal treatment. These results suggest that patients who ask questions, advocate for themselves, and voice concerns or opinions can receive more fair-minded interpersonal treatment.
Keywords: intervention, implicit bias, health disparities, cancer
Abstract
Disparities between Black and White Americans persist in medical treatment and health outcomes. One reason is that physicians sometimes hold implicit racial biases that favor White (over Black) patients. Thus, disrupting the effects of physicians' implicit bias is one route to promoting equitable health outcomes. In the present research, we tested a potential mechanism to short-circuit the effects of doctors’ implicit bias: patient activation, i.e., having patients ask questions and advocate for themselves. Specifically, we trained Black and White standardized patients (SPs) to be “activated” or “typical” during appointments with unsuspecting oncologists and primary care physicians in which SPs claimed to have stage IV lung cancer. Supporting the idea that patient activation can promote equitable doctor–patient interactions, results showed that physicians’ implicit racial bias (as measured by an implicit association test) predicted racially biased interpersonal treatment among typical SPs (but not among activated SPs) across SP ratings of interaction quality and ratings from independent coders who read the interaction transcripts. This research supports prior work showing that implicit attitudes can undermine interpersonal treatment in medical settings and provides a strategy for ensuring equitable doctor–patient interactions.
Racial health disparities between Black and White patients persist in medical treatment and health outcomes (1, 2), including among patients with cancer (3–6). One factor responsible for these disparities, among others, is physicians’ implicit racial bias, an automatic and typically nonconscious preference or positive association for some groups over others (7, 8).
Prior research shows that most non-Black health professionals, including oncologists, have some level of implicit bias favoring White (over Black) patients (9, 10). More important, physicians’ implicit racial bias favoring White (over Black) patients is associated with worse doctor–patient interactions for Black patients (9, 11, 12). For instance, within oncology settings, one study found that oncologists’ implicit pro-White/anti-Black bias was associated with less time spent with patients and less patient-centered communication (10). In addition, doctors’ implicit racial bias is linked with racially biased decision-making (13, 14), although these effects are less consistent.
Little research has identified effective interventions or strategies to reduce implicit racial bias and its influence in the medical context. Some research has identified factors that increase or decrease doctors’ implicit racial bias (15–18), but this research has not connected these reductions in implicit bias to more equitable doctor–patient interactions. In addition, little work has focused on interventions or strategies that patients can use to mitigate the effects of physicians’ implicit bias on equitable doctor–patient interactions.
One promising strategy that addresses these gaps is patient activation, i.e., increasing levels of patients’ engagement with their physicians. Compared to typical patients, activated patients are more likely to ask questions, advocate for themselves, and voice concerns or opinions (19). Patient activation is associated with better doctor–patient interaction outcomes, such as better physician communication, increased shared decision-making, and higher patient satisfaction (20, 21). Relevant to the present research, prior work has shown that Black patients are generally less activated than White patients, which may be due, at least in part, to systematic oppression (e.g., lower-quality physician-patient encounters and inequitable access to resources) (22). Thus, we decided to test whether patient activation could reduce the effects of doctors’ implicit bias on quality of care.
We tested this question in a randomized field experiment with trained standardized patients (SPs). Our methods had three major advantages. First, by having Black and White SPs who were trained to behave similarly except for being either activated (vs. typical), we were able to test whether patient activation and race have causal roles in moderating the effect of doctors’ implicit bias. Second, unlike prior research on doctors’ implicit racial bias that has relied on vignette studies (13, 14), we examined doctor–patient interactions in high-stakes, realistic settings with actors who were extensively trained and monitored for role fidelity. Third, we tested our central hypothesis across two independently obtained measures of doctor–patient interaction quality (SP self-report and third-party coders), which bolsters the robustness of our findings.
Results
Fourteen SPs completed 181 visits with 96 physicians. Eighty-five physicians completed two visits, while 11 only completed one visit. Eighty-one (84%) physicians completed an implicit association test (IAT), where they matched positive and negative words with Black and White facial images with both pained and non-pained expressions. Three physicians did not provide their own age or race. Of the 181 visits, 162 (90%) had SP ratings of interaction quality (the SPs occasionally forgot to complete forms) and 175 (97%) had coder ratings of interaction quality (technological failures prevented some visits from being recorded). Analyses of doctor-level dependent variables included all doctors present in the visit–level analyses (n = 78). Analyses of visit level–dependent variables included all visits where all data were present for analyses of both SP and coder ratings of interaction quality (n = 133), although results did not change if analyses included visits for which the nonrelevant dependent variable was missing.
Physician demographics.
Physicians were mostly middle-aged (M = 52.3, SD = 12.5) and male (62%); most were White (63%), followed by another race/mixed race (18%), Asian (17%), and Black (3%); 42% were oncologists and 58% were primary care physicians (PCPs). Fifteen percent of SP visits were detected by physicians. Results did not differ significantly between physicians who detected SP visits and those who did not; thus, all physicians are included in our analyses.
Physician implicit bias.
Physicians overall indicated significant pro-White/anti-Black bias (M = 0.89, SD = 0.57, t(77) = 13.9, P < 0.001, d = 1.58). IAT scores did not differ significantly by physician specialty (t(76) = 0.8, P = 0.45, d = 0.17), physician sex (t(76) = 1.3, P = 0.20, d = 0.30), or age (r(76) = 0.11, P = 0.34). IAT scores did not differ by physician race (monoracial White vs. monoracial Asian vs. another race/mixed race) (F(2, 75) = 1.28, P = 0.28, partial η2 = 0.03).
Ratings of interaction quality.
We used mixed-model linear regressions to estimate the effects of SP race and activation on SP and coder ratings of interaction quality. Analyses accounted for the nesting of two visits within each physician. We tested for interactions between SP race, SP activation, and physician IAT. We used the same control variables as prior research using this dataset (23): physician specialty (oncologist or PCP), physician race, physician sex, physician age, and research site (i.e., sites in Michigan vs. sites in Indiana or New York). We standardized continuous predictors and outcome measures and coded the binary categorical predictors using simple coding (e.g., −0.5 vs. 0.5 for binary variables). Variance components were estimated using restricted maximum likelihood.
SP ratings of doctor–patient interaction.
We tested the hypothesis that physician implicit bias favoring White (over Black) SPs is associated with better SP ratings of interaction quality for White SPs and worse SP ratings of interaction quality ratings for Black SPs, and that this effect is moderated by patient activation (Tables 1 and 2).
Table 1.
Patient ratings of interaction quality by SP race, activation, IAT, and physician characteristics (mixed-model linear regression)
| Main Effects | Activation Moderation | |||||||
|---|---|---|---|---|---|---|---|---|
| b | 95% CI | P | b | 95% CI | P | |||
| Black SP | 0.82 | 0.53 | 1.12 | < 0.001 | 0.83 | 0.54 | 1.11 | < 0.001 |
| Activated SP | 0.01 | −0.26 | 0.27 | 0.967 | −0.05 | −0.32 | 0.22 | 0.712 |
| Physician IAT | 0.00 | −0.15 | 0.15 | 0.972 | 0.00 | −0.15 | 0.14 | 0.956 |
| Black SP * Activated SP | −0.21 | −0.75 | 0.33 | 0.456 | ||||
| Black SP * Physician IAT | −0.34 | −0.65 | −0.04 | 0.027 | ||||
| Activated SP * Physician IAT | −0.09 | −0.36 | 0.19 | 0.534 | ||||
| Black SP * Activated SP * Physician IAT | 0.59 | 0.04 | 1.14 | 0.037 | ||||
| Site | −0.47 | −0.76 | −0.19 | 0.002 | −0.36 | −0.65 | −0.06 | 0.019 |
| Oncologist (vs. PCP) | 0.22 | −0.08 | 0.53 | 0.151 | 0.25 | −0.04 | 0.54 | 0.099 |
| Physician female | 0.57 | 0.27 | 0.88 | < 0.001 | 0.61 | 0.32 | 0.91 | < 0.001 |
| Physician age (y) | −0.23 | −0.38 | −0.09 | 0.003 | −0.19 | −0.33 | −0.05 | 0.011 |
| Physician Asian | −0.12 | −0.51 | 0.26 | 0.532 | −0.14 | −0.51 | 0.23 | 0.462 |
| Physician other race | −0.13 | −0.52 | 0.26 | 0.506 | −0.09 | −0.47 | 0.30 | 0.652 |
| Fixed intercept | 0.08 | −0.13 | 0.25 | 0.401 | 0.11 | −0.06 | 0.29 | 0.211 |
| Variance | Variance | |||||||
| Random intercept | 0.04 | 0.03 | ||||||
Main effects columns include results from the model without any interactions. Activation Moderation columns include results with all interactions for SP race, SP activation, and physician IAT.
Table 2.
Effect of IAT on patient ratings of interaction quality at different levels of SP race and activation (mixed-model linear regression)
| Activated SP | Black SP | b | 95% CI | P | |
|---|---|---|---|---|---|
| Control | White | 0.36* | 0.07 | 0.65 | 0.014 |
| Black | −0.28 | −0.57 | 0.01 | 0.060 | |
| Activated | White | −0.02 | −0.28 | 0.23 | 0.856 |
| Black | −0.07 | −0.39 | 0.25 | 0.659 | |
CI, confidence interval. *P < 0.05.
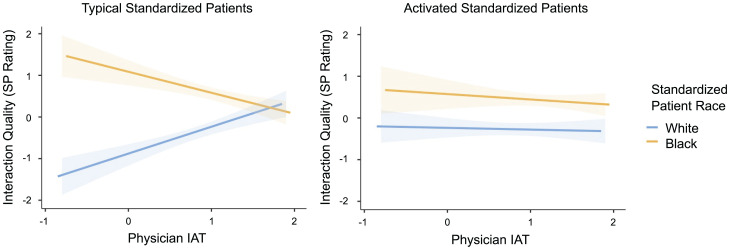
Consistent with predictions, patient activation reduced the effect of implicit racial bias on SP ratings of interaction quality (3-way interaction: b = 0.59, SE = 0.28, P = 0.04). We unpacked this interaction by examining the slopes capturing the IAT–interaction quality relationship for SPs of different races and activation levels. For typical SPs, implicit bias favoring White (over Black) SPs was associated with significantly better SP ratings of interaction quality for White SPs (b = 0.36, SE = 0.15, P = 0.01) and marginally (but not significantly) worse SP ratings of interaction quality for Black SPs (b = −0.28, SE = 0.15, P = 0.06). For activated SPs, implicit bias favoring White (over Black) SPs was not associated with SP ratings of interaction quality for White SPs (b = −0.02, SE = 0.12, P = 0.86) or Black SPs (b = −0.07, SE = 0.16, P = 0.66) (Fig. 1). SP ratings of interaction quality were also higher for Black (vs. White) SPs, female (vs. male) physicians, younger (vs. older) physicians, and at research site 3 (vs. other sites).
Fig. 1.
Interaction of SP race, SP activation level, and physician IAT on SP ratings of interaction quality. Among typical SPs (Left) but not activated SPs (Right), physicians’ implicit bias predicted bias-consistent effects in interaction quality among Black and White SPs. Shaded regions represent ± 1 SE.
Coder ratings of doctor–patient interaction.
We tested the hypothesis that implicit bias favoring White (over Black) SPs is associated with better coder ratings of interaction quality for White SPs and worse coder ratings of interaction quality ratings for Black SPs. We used the same model as the one using SP ratings of interaction quality but replaced the dependent variable with coder ratings of interaction quality (Tables 3 and 4).
Table 3.
Coder ratings of interaction quality by SP race, activation, IAT, and physician characteristics (mixed-model linear regression)
| Main Effects | Activation Moderation | |||||||
|---|---|---|---|---|---|---|---|---|
| b | 95% CI | P | b | 95% CI | P | |||
| Black SP | −0.22 | −0.57 | 0.14 | 0.243 | −0.23 | −0.58 | 0.12 | 0.211 |
| Activated SP | 0.29 | 0.04 | 0.53 | 0.026 | 0.24 | −0.01 | 0.49 | 0.069 |
| Physician IAT | −0.04 | −0.22 | 0.14 | 0.675 | −0.05 | −0.23 | 0.14 | 0.621 |
| Black SP * Activated SP | 0.08 | −0.43 | 0.58 | 0.767 | ||||
| Black SP * Physician IAT | −0.35 | −0.71 | 0.02 | 0.066 | ||||
| Activated SP * Physician IAT | −0.07 | −0.33 | 0.18 | 0.583 | ||||
| Black SP * Activated SP * Physician IAT | 0.57 | 0.05 | 1.08 | 0.034 | ||||
| Site | 0.66 | 0.31 | 1.01 | < 0.001 | 0.76 | 0.41 | 1.12 | < 0.001 |
| Oncologist (vs. PCP) | 0.32 | −0.05 | 0.69 | 0.094 | 0.33 | −0.03 | 0.69 | 0.074 |
| Physician female | 0.53 | 0.15 | 0.90 | 0.007 | 0.55 | 0.19 | 0.92 | 0.004 |
| Physician age (y) | −0.16 | −0.34 | 0.02 | 0.078 | −0.13 | −0.30 | 0.05 | 0.168 |
| Physician Asian | −0.49 | −0.97 | −0.02 | 0.046 | −0.51 | −0.97 | −0.05 | 0.035 |
| Physician other race | −0.02 | −0.49 | 0.46 | 0.937 | 0.02 | −0.45 | 0.49 | 0.937 |
| Fixed intercept | −0.03 | −0.25 | 0.18 | 0.761 | 0.00 | −0.22 | 0.22 | 1.000 |
| Variance | Variance | |||||||
| Random intercept | 0.30 | 0.27 | ||||||
Main Effects columns include results from the model without any interactions. Activation Moderation columns include results with all interactions for SP race, SP activation, and physician IAT. CI, confidence interval.
Table 4.
Effect of IAT on coder ratings of interaction quality at different levels of SP race and activation (mixed-model linear regression)
| Activated SP | Black SP | b | 95% CI | P | |
|---|---|---|---|---|---|
| Control | White | 0.31 | −0.01 | 0.62 | 0.056 |
| Black | −0.33* | −0.65 | −0.00 | 0.050 | |
| Activated | White | −0.05 | −0.32 | 0.23 | 0.719 |
| Black | −0.11 | −0.46 | 0.24 | 0.527 | |
CI, confidence interval. *P < 0.05.
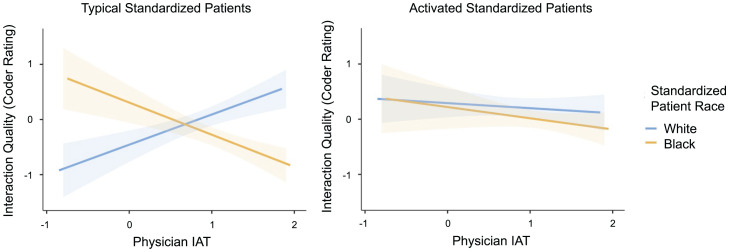
Consistent with the findings for SP ratings of interaction quality, patient activation reduced the effect of implicit racial bias on coders’ ratings of interaction quality (3-way interaction: b = 0.57, SE = 0.26, P = 0.03). For typical SPs, implicit bias favoring White (over Black) SPs was associated with marginally (but not significantly) better coder ratings of interaction quality for White SPs (b = 0.31, SE = 0.16, P = 0.06) and significantly worse coder ratings of interaction quality for Black SPs (b = −0.33, SE = 0.16, P = 0.05). For activated SPs, implicit bias favoring White (over Black) SPs was not associated with coder ratings of interaction quality for White SPs (−b = 0.05, SE = 0.14, P = 0.72) or Black SPs (−b = 0.11, SE = 0.18, P = 0.53) (Fig. 2). Coder ratings of interaction quality were also higher for activated (vs. typical) SPs, Black (vs. non-Black) physicians, female (vs. male) physicians, and at research site 3 (vs. other sites).
Fig. 2.
Interaction of SP race, SP activation level, and physician IAT on coder ratings of interaction quality. Among typical SPs (Left) but not activated SPs (Right), physicians’ implicit bias predicted bias-consistent effects in interaction quality among White SPs and marginally among Black SPs. Shaded regions represent ± 1 SE.
Discussion
The present research, based on a randomized field trial, demonstrates that patient activation can minimize the effect of physicians’ implicit racial bias on interaction quality as rated by SPs and third-party coders. In doing so, the present research makes five broad contributions to the existing research on implicit racial bias and Black–White disparities in quality of care.
First, several of our important findings accord with prior research on implicit bias in health care. For instance, in our data, physicians—regardless of race, sex, and specialty—had implicit bias measured by the IAT favoring White (over Black) standardized patients. Prior IAT estimates from larger, more diverse physician samples showed slightly less pro-White/anti-Black bias, and those are likely better estimates of population-level IAT scores among physicians (24). The mean of our full IAT was comparable to the nonpained portion(Mfull = 0.89, Mnopain = 0.86, Mpain = 0.96), suggesting that our higher IAT scores are not attributable to the presence of pained and nonpained expressions. Instead, the higher IAT scores in our sample, and the fact that we found no differences by physician race, may be attributable to our lack of Black physicians, who on average have unbiased IAT scores (24).
In addition, consistent with prior research, implicit racial bias favoring White (over Black) SPs was associated with better perceived treatment for White SPs and worse perceived treatment for Black SPs (among typical SPs). One caveat is that on the whole, Black (vs. White) SPs reported having better-quality doctor–patient interactions, although this finding did not generalize to the coder ratings of interaction quality. Although disparities between White and Black patients in quality of care are prevalent and well-established (1, 2), such disparities have not been shown in all studies (25–27) and could potentially reflect differences in reference standards between White and Black individuals based on different prior health care experiences.
Second, the present study demonstrates a mechanism that reduces the effects of implicit bias as we originally hypothesized. To date, prior research has predominantly focused on reducing implicit racial bias (i.e., IAT score), rather than its pernicious effects. Focusing on overcoming the negative effects of implicit bias—rather than the bias itself—is important because prior work shows that it is difficult to change people’s levels of implicit bias. The present research cannot explain why patient activation reduced the effects of implicit bias. One possibility, however, is that patient activation—which involved SPs being vocal and engaged—helped physicians learn specific information about the SPs, in turn leading them to perceive SPs as individuals rather than solely as representatives of their race (i.e., individuation) (28).
Third, the present research demonstrates a tool that can be used by patients to reduce the effects of physician bias. Prior research has typically focused on interventions aimed directly at doctors. Practically speaking, interventions to reduce biased treatment directed toward patients with minoritized identities must occur across multiple ecological systems for optimal results. However, it is important to note that ethically, the onus of receiving equitable care should not be on patients. Rather, it is the responsibility of physicians and medical institutions to provide unbiased and high-quality care to all patients. Patients from minoritized groups are disproportionately burdened to handle discrimination within the health care system, and asking them to actively engage to reduce biases can exacerbate this burden. Thus, for both practical and ethical reasons, scholars and clinicians must also focus on developing and implementing health equity interventions that focus on changing clinician attitudes and behavior, as well as policies in health care systems. At the same time, the capacity for patient activation to improve care more broadly while also disrupting bias is empowering for patients and encouraging for population health. That patients can short-circuit the effects of physicians’ implicit bias is even more important when considering research showing that people’s implicit biases are often resistant to change.
Fourth, these findings have important implications for activation training for patients. Previous studies suggest that activation can yield improved outcomes (20, 21). Our findings suggest that such training offers potential for reducing the effects of physician implicit bias and improves care for Black patients.
Fifth, the present research contributes to prior research on implicit bias in doctor–patient interactions through the use of high-quality methods and data quality. Most notably, we used a randomized field study with SPs, which had the advantages of having high ecological validity because of the real-world doctor–patient interactions, as well as demonstrating the causal role of race and patient activation. Adding to the robustness of the present research, our most important findings—the role of implicit bias affecting interaction quality and the power of patient activation to reduce this bias—held for both SP and coder ratings of interaction quality. Future research should focus on the mechanism through which patient activation reduces the effects of bias; for instance, it could lead to greater individuation of patients, attention toward important medical considerations, or increased perspective-taking.
Limitations.
The present research had several limitations. First, our study used actors (i.e., SPs) rather than real patients, making the interactions less organic than a study with real patients (29). On the other hand, we replicated findings with third-party coders, and there is some evidence that SPs can have greater expertise in evaluating interaction quality than typical patients due to the frequency of their encounters (30). Second, the costs of training SPs meant that we had few individuals in each role, meaning that findings could theoretically be attributed to individual characteristics independent of their role. We aimed to overcome this by high-fidelity training. Third, our sample contained few non-White and non-Asian doctors. Future research could include a more diverse physician sample, a more diverse set of SPs, and a larger overall sample to obtain the statistical power needed to test how our findings generalize to physicians and SPs across different racial groups.
Materials and Methods
Overview.
The methods for the present research are presented in detail elsewhere (31). We conducted a randomized field experiment in small metropolitan and rural areas of Indiana, Michigan, and New York. Each study site received institutional review board approval (1009009643 for the Purdue University Institutional Review Board; RSRB00033086 for the University of Rochester Research Subjects Review Board; HUM00067842 for the University of Michigan Human Research Protection Program; 2014–00098 for the McLaren Health Care Corporation Human Research Protections Program). By design, we collected data at sites 1 and 2 between July 2012 and October 2014 and from site 3 from March 2014 to November 2016. We trained Black and White men to portray a 62-year-old with stage IV lung cancer with bone metastases and uncontrolled pain. We constructed four roles that differed by race and activation; otherwise, the roles were identical. Each physician saw one activated and one typical SP of the same race. SP assignment to physicians was stratified by specialty. To reduce SP detection risk, we scheduled visit 1 and visit 2 at least 4 mo apart. Office visits were covertly audiorecorded, transcribed, and analyzed for content and process. Physicians completed questionnaires at baseline asking about demographics, as well as attitudinal scales used for secondary analyses not reported here. Approximately 2 mo after the physician saw the second SP, we sent the physician an email or fax asking whether they suspected that they had seen an SP, and they completed an online IAT designed specifically for the study.
Physicians.
Study investigators approached oncologists and PCPs individually and at meetings after obtaining approval from their practice medical directors. All study participants provided written, informed consent. In the informed consent form, physicians were told that study’s purpose was “to improve patient-physician communication and clinical decisions by examining social and personal characteristics that can affect clinical care and outcomes. More specifically, the study examines overall variations in communication patterns based on patient characteristics such as behavior, ethnicity, race, sex, literacy, socioeconomic status, and education.”
SPs.
SPs portrayed a divorced male who completed 1.5 y of college and worked as a carpenter/contractor before taking a job at a home improvement store 5 y earlier. The SPs presented a several-month history of stage IV lung cancer with painful bone metastases treated with radiation therapy and opioids. Before the visit, we mailed a realistic medical record detailing the medical history, medications, and contact information. The SPs were seeking care from a physician, having recently moved from another state to live closer to an adult child.
Using criteria derived from prior research (19, 21, 32, 33), we trained activated SPs to politely ask direct questions, to request information, to ask for clarification, and to redirect when their concerns were not addressed. They brought a list of questions and interrupted the physician at least once to ask for clarification. In contrast, we trained typical SPs to ask questions about following through with treatment, to express relatively few concerns, to appear satisfied with the information offered, and to say that they understood even when physician explanations were lacking.
Each SP only played the role that they were trained to portray. We trained activated and typical SPs separately at each site. SPs were blinded to the study hypotheses. Although SPs may have observed that they were of different races from one another during training, they were not informed that race was a primary factor examined in the study. Each site had its own trainer who reviewed audiorecordings with the SPs to assess role fidelity and provide feedback. Trainers listened to audiorecordings within two business days of each visit for the first 15 visits, after every third visit thereafter, and more frequently if needed. The standardized role–fidelity scale included items that distinguished between activated and typical roles; fidelity met our criterion of 90% or higher.
Data collection.
Immediately following the visit, the study coordinator at each site debriefed SPs about fidelity, logistics, and any difficulties encountered. Approximately 2 mo after the final SP visit, we asked physicians to complete a form asking whether they suspected that they saw an SP and asked for identifying data to confirm their suspicions. After receiving physicians’ responses, the study team requested a copy of the SP’s record and study physicians completed an online IAT, where they matched positive and negative words with Black and White facial images with both pained and nonpained expressions (34). The IAT showed good internal reliability when examined as a whole or when scores for pained and nonpained expressions were separated (αfull = 0.74, αnopain = 0.72, αpain = 0.72). Analyses presented here use the full IAT.
Outcome measures.
SP ratings of interaction quality.
After completing each visit, SPs rated their satisfaction with overall care, quality of pain discussion, and quality of prognosis discussion on a 6-point Likert scale (Cronbach’s α = 0.86).
SPs then answered questions from the Jefferson Scale of Patient Perceptions of Physician Empathy (35), a 5-item scale measuring patient perceptions of physician empathy (1 = strongly disagree, 7 = strongly agree; Cronbach’s α = 0.90).
SPs reported nonverbal communication using a measure developed for the study consisting of seven questions (e.g., “the physician maintained appropriate eye contact with me”) scored on a 5-point Likert scale (1 = poor, 5 = excellent) (Cronbach’s α = 0.95).
SPs assessed physicians’ communication skills using the Rochester Communication Rating Scale (RCRS; [36]), a 19-item scale with four subscales developed to assess the patient-centered communication skills of physicians (all items are on a 6-point Likert scale; 1 = strongly disagree, 6 = strongly agree).
The four RCRS subscales, patient satisfaction scale, empathy scale, and nonverbal scale were highly intercorrelated (Cronbach’s α = 0.97). In addition, an exploratory factor analysis using oblimin rotation, minimum residuals extraction, and factor determination based on parallel analysis indicated that these scales all loaded onto a single factor. Thus, we thus created a composite by taking the mean of each scale, converting that mean into a z-score, and computing a weighted average for the z-scored scales based on the number of items in the scale (37).
Coder ratings of interaction quality.
The recordings of the SP visits were professionally transcribed, stripped of any racial identifiers, and coded by the research team. For all items within a scale, coders used a 0 to 4 scale, with higher numbers representing better-quality discussion.
Patient-centered pain assessment was coded using the Measure of Physician Pain Assessment (38), consisting of nine items measuring the frequency and depth of physician responses to patients’ expressions of concerns about their pain (coders’ intraclass correlation coefficient (ICC) = 0.73; Cronbach’s α = 0.75).
We also coded for routine pain assessment (e.g., standard questions regarding pain location, intensity, etc.), using 9 items that captured the quality of pain assessment (coders’ ICC = 0.85; Cronbach’s α = 0.54).
Finally, coders assessed prognosis and treatment choice communication using the Prognosis and Treatment Choice Communication scale (PTCC) (39), which consisted of 11 items that measured physicians’ communication of diagnostic information and treatment options for patients with advanced cancer (coders’ ICC = 0.73, Cronbach’s α = 0.92).
The three scales combined had poor reliability (Cronbach’s α = 0.61). An exploratory factor analysis using oblimin rotation, minimum residuals extraction, and factor determination based on parallel analysis indicated that the PTCC had low factor loading (0.37). Thus, we removed the prognosis scale from the coder ratings of interaction quality, which improved the scale’s reliability (Cronbach’s α = 0.66). As with the SP ratings of interaction quality, we created a composite by taking the mean of each scale, converting that mean into a z-score, and computing a weighted average for the z-scored scales based on the number of items in the scale. Because the composite measure had only fair reliability, we present separate analyses for the two components that made up the composite as well as the third scale that was not included in the composite (SI Appendix, Tables S1–S6).
Supplementary Material
Acknowledgments
The National Cancer Institute of the NIH funded this study. The funder had no role in the study’s design, conduct, or reporting.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2203915119/-/DCSupplemental.
Data Availability
Anonymized (Jamovi file) data and results for the main text (https://osf.io/djxu3) and SI Appendix (https://osf.io/gc7md) have been deposited in the Open Science Framework (OSF), a publicly accessible database (40).
References
- 1.Orsi J. M., Margellos-Anast H., Whitman S., Black-White health disparities in the United States and Chicago: A 15-year progress analysis. Am. J. Public Health 100, 349–356 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Penner L. A., Albrecht T. L., Orom H., Coleman D. K., Underwood W., “Health and health care disparities” in The Sage Handbook of Prejudice, Stereotyping and Discrimination, Dovidio J. F., Hewstone M., Glick P., Esses V. M., Eds. (Sage, 2010), pp. 472–490. [Google Scholar]
- 3.Penner L. A., et al. , Life-threatening disparities: The treatment of Black and White cancer patients. J. Soc. Issues 68, 328–357 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jemal A., et al. , Factors that contributed to Black-White disparities in survival among nonelderly women with breast cancer between 2004 and 2013. J. Clin. Oncol. 36, 14–24 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Wheeler S. B., Reeder-Hayes K. E., Carey L. A., Disparities in breast cancer treatment and outcomes: Biological, social, and health system determinants and opportunities for research. Oncologist 18, 986–993 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Keefe E. B., Meltzer J. P., Bethea T. N., Health disparities and cancer: Racial disparities in cancer mortality in the United States, 2000–2010. Front. Public Health 3, 51 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenwald A. G., McGhee D. E., Schwartz J. L. K., Measuring individual differences in implicit cognition: The implicit association test. J. Pers. Soc. Psychol. 74, 1464–1480 (1998). [DOI] [PubMed] [Google Scholar]
- 8.FitzGerald C., Hurst S., Implicit bias in healthcare professionals: A systematic review. BMC Med. Ethics 18, 19 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maina I. W., Belton T. D., Ginzberg S., Singh A., Johnson T. J., A decade of studying implicit racial/ethnic bias in healthcare providers using the implicit association test. Soc. Sci. Med. 199, 219–229 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Penner L. A., et al. , The effects of oncologist implicit racial bias in racially discordant oncology interactions. J. Clin. Oncol. 34, 2874–2880 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper L. A., et al. , The associations of clinicians’ implicit attitudes about race with medical visit communication and patient ratings of interpersonal care. Am. J. Public Health 102, 979–987 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall W. J., et al. , Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: A systematic review. Am. J. Public Health 105, e60–e76 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabin J. A., Greenwald A. G., The influence of implicit bias on treatment recommendations for 4 common pediatric conditions: Pain, urinary tract infection, attention deficit hyperactivity disorder, and asthma. Am. J. Public Health 102, 988–995 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green A. R., et al. , Implicit bias among physicians and its prediction of thrombolysis decisions for black and white patients. J. Gen. Intern. Med. 22, 1231–1238 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castillo L. G., Brossart D. F., Reyes C. J., Conoley C. W., Phoummarath M. J., The influence of multicultural training on perceived multicultural counseling competencies and implicit racial prejudice. J. Multicult. Couns. Devel. 35, 243–255 (2007). [Google Scholar]
- 16.van Ryn M., et al. , Medical school experiences associated with change in implicit racial bias among 3547 students: A medical student CHANGES study report. J. Gen. Intern. Med. 30, 1748–1756 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson T. J., et al. , The impact of cognitive stressors in the emergency department on physician implicit racial bias. Acad. Emerg. Med. 23, 297–305 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dyrbye L., et al. , Association of racial bias with burnout among resident physicians. JAMA Netw. Open 2, e197457 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon H. S., Street R. L. Jr., Sharf B. F., Souchek J., Racial differences in doctors’ information-giving and patients’ participation. Cancer 107, 1313–1320 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Mishel M. H., et al. , Managing uncertainty about treatment decision making in early stage prostate cancer: A randomized clinical trial. Patient Educ. Couns. 77, 349–359 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Hibbard J. H., Greene J., What the evidence shows about patient activation: Better health outcomes and care experiences; fewer data on costs. Health Aff. (Millwood) 32, 207–214 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Adebayo C. T., et al. , Race and Blackness: A thematic review of communication challenges confronting the black community within the U.S. health care system. J. Transcult. Nurs. 31, 397–405 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Shields C. G., et al. , The influence of patient race and activation on pain management in advanced lung cancer: A randomized field experiment. J. Gen. Intern. Med. 34, 435–442 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sabin J., Nosek B. A., Greenwald A., Rivara F. P., Physicians’ implicit and explicit attitudes about race by MD race, ethnicity, and gender. J. Health Care Poor Underserved 20, 896–913 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blair I. V., et al. , Clinicians’ implicit ethnic/racial bias and perceptions of care among Black and Latino patients. Ann. Fam. Med. 11, 43–52 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirsh A. T., Hollingshead N. A., Ashburn-Nardo L., Kroenke K., The interaction of patient race, provider bias, and clinical ambiguity on pain management decisions. J. Pain 16, 558–568 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaa K. L., Roter D. L., Biesecker B. B., Cooper L. A., Erby L. H., Genetic counselors’ implicit racial attitudes and their relationship to communication. Health Psychol. 34, 111–119 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fiske S. T., Neuberg S. L., “A continuum of impression formation, from category-based to individuating processes: Influences of information and motivation on attention and interpretation” in Advances in Experimental Social Psychology, Zanna M. P., Ed. (Academic Press, 1990), pp. 1–74. [Google Scholar]
- 29.Hagiwara N., Dent R., Patient-physician communication during racially discordant medical interactions: Limitations with the current coding systems. TPM Test. Psychom. Methodol. Appl. Psychol. 23, 511–529 (2016). [Google Scholar]
- 30.Srinivasan M., et al. , Connoisseurs of care? Unannounced standardized patients’ ratings of physicians. Med. Care 44, 1092–1098 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Elias C. M., et al. , The social and behavioral influences (SBI) study: Study design and rationale for studying the effects of race and activation on cancer pain management. BMC Cancer 17, 575 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hibbard J. H., Stockard J., Mahoney E. R., Tusler M., Development of the Patient Activation Measure (PAM): Conceptualizing and measuring activation in patients and consumers. Health Serv. Res. 39, 1005–1026 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaplan S. H., Greenfield S., Gandek B., Rogers W. H., Ware J. E. Jr., Characteristics of physicians with participatory decision-making styles. Ann. Intern. Med. 124, 497–504 (1996). [DOI] [PubMed] [Google Scholar]
- 34.Fiscella K., Epstein R. M., Griggs J. J., Marshall M. M., Shields C. G., Is physician implicit bias associated with differences in care by patient race for metastatic cancer-related pain? PLoS One 16, e0257794 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hojat M., et al. , The Jefferson Scale of Physician Empathy: Development and preliminary psychometric data. Educ. Psychol. Meas. 61, 349–365 (2001). [Google Scholar]
- 36.Epstein R. M., et al. , Comprehensive assessment of professional competence: The Rochester experiment. Teach. Learn. Med. 16, 186–196 (2004). [DOI] [PubMed] [Google Scholar]
- 37.Epstein R. M., et al. , Effect of a patient-centered communication intervention on oncologist-patient communication, quality of life, and health care utilization in advanced cancer: The VOICE randomized clinical trial. JAMA Oncol. 3, 92–100 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shields C. G., et al. , Pain assessment: The roles of physician certainty and curiosity. Health Commun. 28, 740–746 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shields C. G., et al. , Patient-centered communication and prognosis discussions with cancer patients. Patient Educ. Couns. 77, 437–442 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gainsburg I., Data for "Patient Activation Reduces Effects of Implicit Bias on Doctor-Patient Interactions." OSF. https://osf.io/5ukxa/. Deposited 7 March 2022. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized (Jamovi file) data and results for the main text (https://osf.io/djxu3) and SI Appendix (https://osf.io/gc7md) have been deposited in the Open Science Framework (OSF), a publicly accessible database (40).