Significance
Adenosine-to-inosine RNA editing generates various levels of editing in a developmental and spatiotemporal manner. This posttranscriptional process can diversify protein functions or levels through recoding codons or through affecting alternative splicing or noncoding RNA binding. In mammals, including humans, the editome contains millions of editing sites. This paper focuses on the functional and neurophysiological significance of L-type voltage-gated calcium channel CaV1.3 RNA editing. It is not only evolutionarily conserved across species but also tunable in response to training in learning and memory. The unexpected role that the CaV1.3 channel plays in hippocampal learning and memory was also revealed as a result of a combination of gain of function in biophysical properties arising from the loss of CaV1.3 RNA editing .
Keywords: RNA editing, CaV1.3 calcium channel, hippocampal plasticity, spatial learning and memory
Abstract
L-type CaV1.3 calcium channels are expressed on the dendrites and soma of neurons, and there is a paucity of information about its role in hippocampal plasticity. Here, by genetic targeting to ablate CaV1.3 RNA editing, we demonstrate that unedited CaV1.3ΔECS mice exhibited improved learning and enhanced long-term memory, supporting a functional role of RNA editing in behavior. Significantly, the editing paradox that functional recoding of CaV1.3 RNA editing sites slows Ca2+-dependent inactivation to increase Ca2+ influx but reduces channel open probability to decrease Ca2+ influx was resolved. Mechanistically, using hippocampal slice recordings, we provide evidence that unedited CaV1.3 channels permitted larger Ca2+ influx into the hippocampal pyramidal neurons to bolster neuronal excitability, synaptic transmission, late long-term potentiation, and increased dendritic arborization. Of note, RNA editing of the CaV1.3 IQ-domain was found to be evolutionarily conserved in mammals, which lends support to the importance of the functional recoding of the CaV1.3 channel in brain function.
Hard-wired genomic mutations can be passed down the generations, but flexible RNA editing, which varies between 0 and 100%, can generate various levels of editing in a developmental and spatiotemporal manner. This posttranscriptional process can correspondingly alter protein function or level through recoding codons or through either affecting alternative splicing or noncoding RNA binding. Adenosine-to-inosine (A-to-I) RNA editing is catalyzed by adenosine deaminases acting on RNA (ADAR) enzymes. In humans, it has been reported that the editome contains millions of editing sites, but they are mostly found in noncoding sites (1, 2). However, there are also multiple substrates in human transcripts coding for ion channels or transporters that are important for brain function (3). Of interest, ∼60,000 recoding A-to-I RNA editing sites have been uncovered in the Drosophila and in cephalopods, such as squid and octopus (4), but there is a paucity of information on the functional and behavioral importance of RNA editing, especially in mammals. In cephalopods, voltage-gated potassium channel KV1.1 RNA editing is associated with cold adaptation, while causality has not been demonstrated (5).
It has been postulated that most mammalian nonsynonymous recoding RNA editing sites are nonadaptive, but it is still unclear whether the functional editing recoding—that is, the recoding of the genomic codon in the transcript that results in an amino acid and subsequent functional change—has critical physiological significance. However, a counterargument is that conserved high-level recoding RNA editing sites in mammals could be functionally advantageous. The ablation of RNA editing of glutamate ionotropic receptor AMPA type subunit 2 (GluA2) resulted in epilepsy and death in a few weeks (6), while the generation of knock-in VGV serotonin 2C receptors (5-HT2C) edited mice showed a loss of fat mass and characteristics of anxiety and depression (7, 8). The dearth of investigations into altered behavior and the functional effects of mammalian RNA editing in cognitive functions led us to investigate whether mice genetically targeted to harbor only unedited CaV1.3 channels would experience effects on hippocampal learning and memory because the editing level of the first site of the CaV1.3 IQ-domain IQDY (ATA→ATG; I→M) residue in the mouse hippocampus can reach 50% (9). In addition, such mice allowed us to resolve the CaV1.3 RNA editing paradox because RNA editing of the CaV1.3 IQ-domain slows Ca2+-dependent inactivation to allow larger entry of Ca2+ ions (9), while in contrast, editing attenuates apo-calmodulin interaction with the IQ domain to decrease channel open probability to reduce Ca2+ influx (10, 11).
Here, we show that CaV1.3 RNA editing is evolutionarily conserved and the editing level is down-regulated during learning. Loss of CaV1.3 IQ-domain RNA editing is a gain of function to admit larger Ca2+ influx, and thus the result obtained in neurons resolves the editing paradox. The neurophysiological consequence of a larger Ca2+ influx is to increase neuronal excitability, enhance hippocampal plasticity that leads to strengthened late long-term potentiation (LTP), and, behaviorally, improved spatial learning and memory.
Results
CaV1.3 RNA Editing Is Evolutionarily Conserved from Zebrafish to Humans.
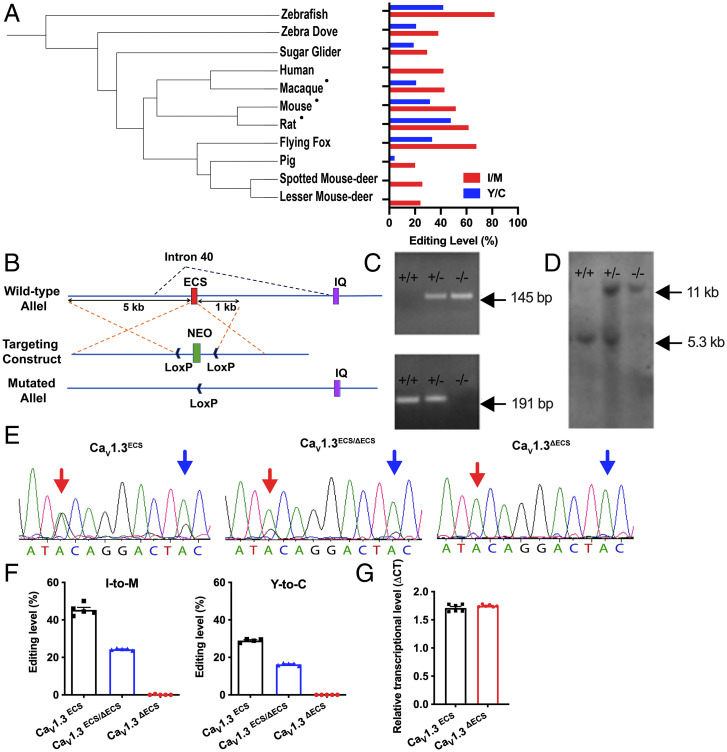
There are millions of editing sites identified among different species. Conservation of RNA editing across species, such as isoleucine/valine (I/V) editing in the KV1.1 channel and isoleucine/methionine (I/M) editing in the γ-aminobutyric acid type A receptor α3 receptor (GABRA3) (12, 13), points to their functional importance. We therefore examined brain CaV1.3 RNA editing across several mammalian species and discovered high conservation from zebrafish to humans (Fig. 1A), indicating that RNA editing of the CaV1.3 channel is evolutionarily conserved. The data suggest the neurophysiological importance of CaV1.3 RNA editing.
Fig. 1.
Generation of CaV1.3ΔECS IQ-domain RNA editing knockout mice. (A) Polygenetic tree of animals tested for RNA editing events in the IQ-domain of CaV1.3 channels and quantification of editing levels in all tested species using brain tissues or the cDNA library (for humans). •Hippocampal tissues were used. (B) Schematic illustrating the knockout strategy with homologous recombination used to knock out intronic ECS in Cacna1d. (C and D) Genotyping by PCR and Southern blot analysis. PCR: 145 bp and 191 bp bands indicated the presence of ECS and ΔECS alleles, respectively; Southern blot analysis: 5.3 kb and 11 kb bands indicated the presence of ECS and ΔECS alleles, respectively. (E) Direct DNA sequencing of RT-PCR products from whole brain of CaV1.3ECS, CaV1.3ECS/ΔECS, and CaV1.3ΔECS mice. Red arrow: I/M editing site; blue arrow: tyrosine/cysteine (Y/C) editing site. (F) Percentage of editing calculated by measuring electropherogram heights for adenosine versus guanosine. No editing in CaV1.3ΔECS mice (n = 5), while CaV1.3ECS/ΔECS mice (n = 5) exhibited half the editing level of CaV1.3ECS mice (n = 5). Left: I/M editing site; Right: Y/C editing site. (G) Transcript level of Cacna1d quantified in CaV1.3ΔECS mice (n = 6) by RT-PCR was not different from that in CaV1.3ECS mice (n = 6). Data represent means ± SEM. Scatters in bars indicate the number of analyzed animals. The two-tailed unpaired Student’s t test was used for data in (G).
Genetic Deletion of the Editing-Site Complementary Sequence Completely Abolished RNA Editing of the CaV1.3 Channel.
To address the twin questions of the physiological importance of CaV1.3 functional recoding of RNA editing and whether the loss of CaV1.3 editing results in greater or lower Ca2+ influx, we genetically targeted the mouse genome to remove the intronic editing-site complementary sequence (ECS) by homologous recombination (Fig. 1B). We have reported on the identity of the ECS (14), which forms the imperfect double-stranded stem structure that is essential for ADAR2 binding to initiate RNA editing. Replacing ECS with the loxP site by cre-recombinase disrupted the double-stranded stem structure that is critical for RNA editing. The success in generating the heterozygous CaV1.3ECS/ΔECS or homozygous CaV1.3ΔECS/ΔECS (CaV1.3ΔECS) mice (Fig. 1 C and D) is clearly demonstrated by the retention of half the editing level in CaV1.3ECS/ΔECS and the total abolition of CaV1.3 RNA editing in CaV1.3ΔECS detected from whole brain tissue as compared to wild-type (WT) CaV1.3ECS/ECS (CaV1.3ECS) mice (I/M: CaV1.3ECS/ECS 45.3% ± 1.352, CaV1.3ECS/ΔECS 24.32% ± 0.22, CaV1.3ΔECS/ΔECS 0.096% ± 0.096; Y/C: CaV1.3ECS/ECS 28.98% ± 0.4626, CaV1.3ECS/ΔECS 16.29% ± 0.2817, CaV1.3ΔECS/ΔECS 0.0% ± 0.0) (Fig. 1 E and F). Deletion of the intronic ECS sequence did not affect the production of CaV1.3 mRNA in the brain (Fig. 1G). We also did not detect any alterations in the C-terminal alternative splicing events of the CaV1.3 transcript (SI Appendix, Fig. S1 A and B). The gross phenotypes of CaV1.3ΔECS mice including hearing and vision were characterized to be normal, although the body weights of 3-month-old male mice were slightly heavier (SI Appendix, Fig. S2 A–C).
Lower RNA Editing Level in CaV1.3 IQ-Domain Detected in WT Mice after Water-Maze Training.
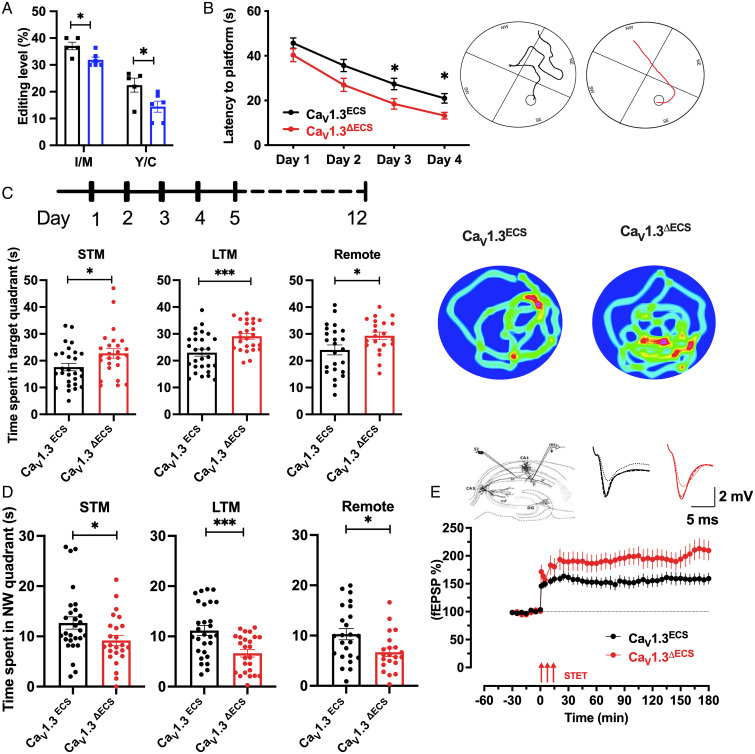
Per our previous report (9), editing at the first site (I/M) of the CaV1.3 IQ-domain could be as high as 50% in the hippocampus. It has also been shown that I to M editing has more significant effects on open probability (Po) and calcium-dependent inactivation (CDI) of the CaV1.3 channel, while the functional effect of Y/C editing regarding Po and CDI is not obvious (9, 10). To study the functional importance of CaV1.3 RNA editing, we surmised that IQ-domain RNA editing in the CaV1.3 channel could be dynamically regulated during hippocampus-dependent learning and memory. Therefore, when we characterized the behavioral phenotypes of CaV1.3ΔECS mice using a Morris water maze, we first examined the editing level of the cornu ammonis 1 (CA1) region from untrained and trained WT CaV1.3ECS mice. Intriguingly, the CaV1.3 IQ-domain RNA editing levels in the CA1 region as well as the whole hippocampus were altered and were reduced, by 10%, after short-term water-maze training (Fig. 2A and SI Appendix, Fig. S3). This result indicates that the RNA editing of CaV1.3 channels is regulated during water-maze training and made us wonder how the loss of CaV1.3 RNA editing would affect hippocampus-dependent learning and memory and whether the net influx of Ca2+ ions would increase or decrease.
Fig. 2.
Enhanced spatial learning and memory of CaV1.3ΔECS mice in Morris water maze. (A) Quantification of IQ-domain RNA editing levels at I-to-M and Y-to-C sites of CaV1.3 channels in hippocampal CA1 region from CaV1.3ECS mice with (n = 6) and without (n = 5) water maze training. (B) Learning curve of CaV1.3ECS (n = 28) and CaV1.3ΔECS mice (n = 25) during the Morris water maze training. Average latency to platform of training trials is presented. Inset: Representative swimming traces of CaV1.3ECS and CaV1.3ΔECS mice exhibited the training trails on Day 4. (C) Time spent in target quadrant during probe tests. Left: STM probe test; Middle: LTM probe; Right: Remote probe test. Inset: Representative heat map of CaV1.3ECS and CaV1.3ΔECS mice exhibits the time spent in each quadrant in LTM probe test. (D) Time spent in northwestern quadrant (opposite of the targeted southeastern quadrant) during STM (Left), LTM (Middle), and remote (Right) probe tests. (E) Late LTP in CaV1.3ECS and CaV1.3 ΔECS mice with STET delivered in the stratum radiatum to stimulate Schaffer collateral fibers (Left Inset). STET in CaV1.3 ΔECS mice also resulted in a long-lasting LTP with CaV1.3 ΔECS mice showing a significantly higher percentage of potentiation than CaV1.3ECS mice from 90 min onward until 180 min (Scale bar, 5 ms, 2 mV). Data represent means ± SEM. Scatters in bars indicate the number of analyzed animals. Two-way ANOVA with Sidak’s multiple comparisons test was used for data in (B). Difference between genotypes, F (1, 50) = 10.77, P = 0.0019), *P < 0.05. The two-tailed unpaired Student’s t test was used for data in (A, C, D); *P < 0.05, ***P < 0.001. The Mann–Whitney U test was used for data in (E) (90 min, P = 0.02; 120 min, P = 0.02; 180 min, P = 0.02).
CaV1.3ΔECS Mice Exhibited Enhanced Spatial Learning and Memory in Morris Water Maze.
Because CaV1.3 RNA editing was reduced after short-term water-maze training, we therefore hypothesized that hippocampal learning and memory could be affected in CaV1.3ΔECS mice. To test this hypothesis, we employed the Morris water maze behavioral tests in which the mice were guided by distal spatial cues to find a platform submerged in opaque water in a specified quadrant to evaluate learning ability. Probe tests, which involved removing the submerged platform, were performed on days 1, 5, and 12 to evaluate short-term, long-term, and remote memory, respectively. During the training trials on day 1, CaV1.3ΔECS and CaV1.3ECS mice had similar temporal latency in finding the platform. With further trainings from day 2 to day 4, both CaV1.3ECS and CaV1.3ΔECS mice showed clear improvements in their performances (Fig. 2B). However, CaV1.3ΔECS mice found the platform in a significantly shorter time latency as compared to WT mice, and the traces obtained on day 4 suggest that CaV1.3ΔECS mice had better performance in searching for the platform (Fig. 2B, Inset). One plausible explanation for the differences in water maze results could be differences in locomotor speed, but we found that the average swimming velocities of the CaV1.3ΔECS and CaV1.3ECS mice were similar (SI Appendix, Fig. S3). These results are indicative of CaV1.3ΔECS mice having better spatial learning ability. In addition, the CaV1.3ΔECS mice showed enhanced spatial memory because they spent significantly more time swimming in the target quadrant (the southeastern quadrant, where the platform was located during the training trials) in the probe tests. Of note, enhanced spatial memory of CaV1.3ΔECS mice was not only observed in the short-term memory (STM) probe test conducted 30 min after the last training trial on day 1 but also in the long-term memory (LTM) probe test performed on day 5, and significantly, it was maintained until the remote probe test on day 12 (Fg. 2C). To further corroborate the results of the memory probe tests, the CaV1.3ΔECS mice spent less time swimming in the diametrically opposite northwestern quadrant (Fig. 2D).
Enhanced Hippocampal Late LTP Underpinned Improved Spatial Learning and Memory in CaV1.3ΔECS Mice.
The persistence of enhanced spatial learning and memory observed in the Cav1.3ΔECS mice suggests that RNA editing strongly influences the contribution of CaV1.3 channels in hippocampus-dependent learning and memory. The logical next step was to interrogate how LTP, a major cellular mechanism that underlies learning and memory, might be altered in CaV1.3 ΔECS mice, especially in the CA1 area of the hippocampus. We examined persistent changes in the plasticity of the hippocampal CA3–CA1 synapses. Induced CA3–CA1 late LTP with a conventional high-frequency stimulation protocol using strong tetanus (STET) was recorded from both CaV1.3ΔECS and CaV1.3ECS mice with synaptic input S1 located at the Schaffer collateral projections. Single-pathway experiments were used for this study (Fig. 2E, Inset). A stable baseline was first recorded for a minimum of 30 min and STET was applied, which resulted in a long-lasting LTP until 180 min in CaV1.3ECS hippocampal slices. Next, we studied late LTP in CaV1.3 ΔECS mice using the same paradigm, which resulted in an enhanced and more pronounced potentiation. In particular, the potentiation in CaV1.3 ΔECS mice was significantly higher than that in CaV1.3ECS mice from 90 min onward, until 180 min (Fig. 2E). This showed that the CaV1.3ΔECS hippocampus after STET revealed enhanced late LTP, which is considered a cellular correlate of learning and memory.
Next, to determine whether a weaker form of LTP is affected in CaV1.3 ΔECS mice, we invoked early LTP using weak tetanus (WTET). When a WTET was delivered to hippocampal slices of CaV1.3ECS and CaV1.3ΔECS mice, it resulted in early LTP in both cases. When the profiles of early LTP were compared between CaV1.3ECS and CaV1.3 ΔECS hippocampal slices, they did not show any significant difference (SI Appendix, Fig. S4A). In addition, depotentiation and baseline recordings were also unaltered in CaV1.3ΔECS mice (SI Appendix, Fig. S4 B and C).
Loss of CaV1.3 RNA Editing Increased Ca2+ Influx into Hippocampal CA1 Pyramidal Neurons.
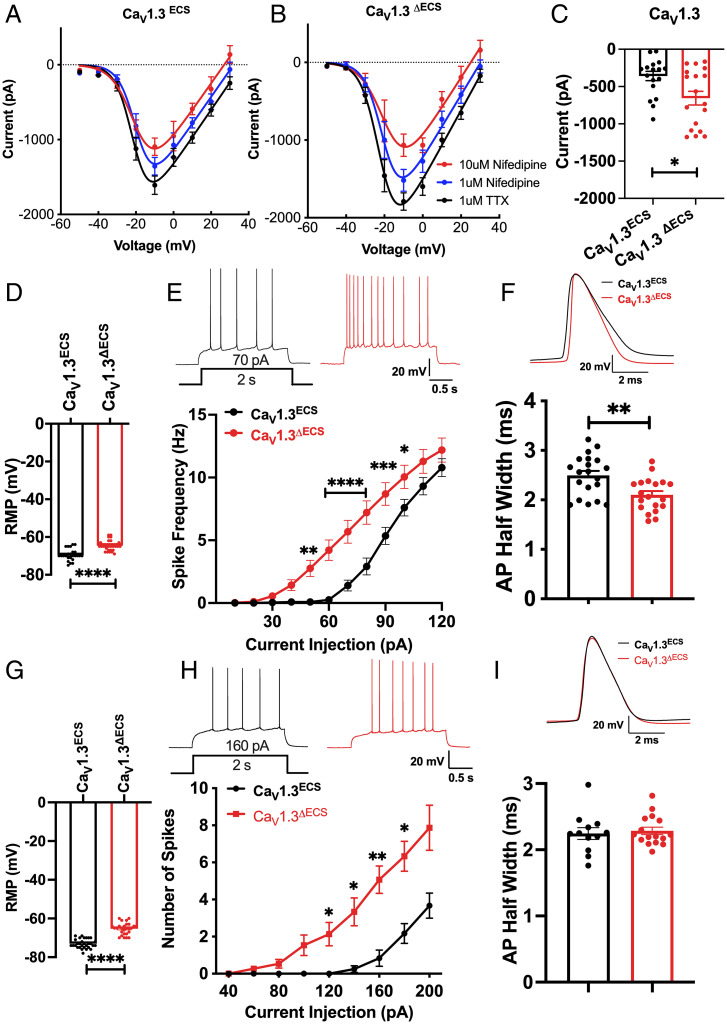
While functional recoding via RNA editing of CaV1.3 enhanced learning and memory, to further understand the physiological and behavioral changes in CaV1.3ΔECS mice, we needed to first resolve the CaV1.3 IQ-domain editing paradox. The critical question was about whether the next Ca2+ influx into neurons was increased or decreased arising from the opposing effects of editing, on the one hand, to slow CaV1.3 Ca2+-dependent inactivation to increase Ca2+ influx, and on the other, to reduce channel open probability to decrease Ca2+ influx. Total Ca2+ currents were therefore recorded directly from CA1 pyramidal neurons on the hippocampal slices, and the amount of low dihydropyridine (DHP)-sensitive current was isolated by first using low-dose nifedipine (1 µM) to substantively block primarily the CaV1.2 channels and then high-dose nifedipine (10 µM) to block the less-sensitive CaV1.3-mediated currents. The relative CaV1.3-mediated currents were determined by subtracting the peak Ca2+ currents recorded in 1 µM nifedipine from those recorded in 10 µM nifedipine at −10 mV. Therefore, as the CaV1.3 channel property has been modified in CaV1.3ΔECS mice, there is a strong likelihood that the difference in the observed low DHP-sensitive currents from WT would be contributed by CaV1.3 channels. We detected an approximately doubled CaV1.3 ICa in CaV1.3ΔECS CA1 neurons (Fig. 3C; CaV1.3ECS −359.0 ± 62.61, CaV1.3ΔECS −655.2 ± 91.62; P = 0.0116). Therefore, we concluded that the loss of CaV1.3 RNA editing generated increased Ca2+ influx, suggesting that in the in vivo milieu, an increased open probability arising from the higher affinity of binding of calmodulin to the unedited CaV1.3 channel had a larger effect to increase Ca2+ conduction (10, 11). This result resolved the CaV1.3 editing paradox because RNA editing of the IQ-domain affects Ca2+ influx with opposing effects of CDI and channel open probability.
Fig. 3.
Deletion of IQ-domain RNA editing increases CaV1.3 channel current and leads to enhanced neuronal excitability. (A and B) I-V curve of calcium current recorded from CA1 pyramidal neurons in hippocampal slices from CaV1.3ECS and CaV1.3ΔECS mice. The total Ca2+ currents were first recorded with 1uM TTX, and 1uM nifedipine was then applied to the bath solution to mostly block the high-affinity CaV1.2 channels. To isolate the low-affinity CaV1.3 currents, 10 μM nifedipine was subsequently added. (C) Increased amount of CaV1.3 currents calculated by the difference in peak Ca2+ currents recorded in 1uM versus 10 μM nifedipine at −10 mV. (D) Depolarized RMP of CA1 pyramidal neurons in CaV1.3ΔECS mice. (E) Representative traces of spike frequency (with 70 pA current injection for 2 s) and frequency–current curve showing spike frequency with current injection in CA1 pyramidal neurons. n = 24 and n = 19 for CaV1.3ECS and CaV1.3ΔECS mice, respectively. (F) Representative traces and quantification of CA1 pyramidal neurons AP half-width measured from average of the first AP in 5 trains with 10 s interval induced with 200 pA current injection. (G) Depolarized RMP of CA3 pyramidal neurons in CaV1.3ΔECS mice. (H) Representative traces of spike frequency (with 160 pA current injection for 2 s) and frequency–current curve showing spike frequency with current injection in CA3 pyramidal neurons of CaV1.3ECS (n = 12) and CaV1.3ΔECS mice (n = 15). (I) Representative traces and quantification of CA3 pyramidal neurons AP half-width measured from the average of the first AP in 5 trains with 10 s interval induced with 250 pA current injection did not show any difference between CaV1.3ECS and CaV1.3ΔECS mice. Data represent means ± SEM. Scatters in bars indicate the number of analyzed neurons. Two-way ANOVA with Bonferroni’s multiple comparisons test was used for data in (E and H); the two-tailed unpaired Student’s t test was used for analysis of data in all other panels. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Enhanced Excitability of CA1 and CA3 Pyramidal Neurons in CaV1.3ΔECS Mice.
The increased Ca2+ influx via unedited CaV1.3 channels provided the framework to uncover the underlying biophysical and molecular mechanisms for enhanced synaptic activity in the CA3–CA1 connections. DHP-sensitive voltage-gated calcium channels have been shown to contribute to low-voltage activated calcium current in hippocampal CA1 and CA3 pyramidal neurons (15, 16). Because CaV1.3 channels are activated close to the resting membrane potential (17) and unedited channels are also activated at a more hyperpolarized potential (9), our hypothesis was that CaV1.3ΔECS neurons would have more CaV1.3 channels available to depolarize the neurons at rest. Indeed, we recorded from CaV1.3ΔECS proximal CA1 pyramidal neurons and detected that the resting membrane potential (RMP) was depolarized by 5.70 ± 0.8209 mV (Fig. 3D; P < 0.0001), and the same difference was observed in distal CA3 pyramidal neurons (Fig. 3G; P < 0.0001). There was no difference in input resistance (SI Appendix, Fig. S5 A and D). To test whether the depolarized RMP could contribute to elevated membrane excitability, we determined the rheobase as an indicator of neuronal excitability and found consistently that CaV1.3ΔECS CA1 or CA3 neurons required lower injection of current to initiate action potential firings (Fig. 3E, CA1: CaV1.3ECS vs. CaV1.3ΔECS, 70 pA vs. 40 pA, F(1,32) = 15.39, P = 0.0004; Fig. 3H, CA3: CaV1.3ECS vs. CaV1.3ΔECS, 140 pA vs. 80 pA, F(1,14) = 6.355, P = 0.0245). To further characterize the increased excitability of CA1 and CA3 pyramidal neurons, the action potential duration (APD), action potential (AP) threshold, and spike frequency adaptation (SFA) ratio were recorded and analyzed. In CaV1.3ΔECS CA1 neurons, the APD was significantly narrower when compared to those recorded from CaV1.3ECS CA1 neurons (Fig. 3F; P < 0.01), and the threshold potential was significantly hyperpolarized (SI Appendix, Fig. S5C; P < 0.05). In CA3 neurons, both the APD and AP threshold were, however, comparable between CaV1.3ΔECS and CaV1.3ECS mice (Fig. 3I and SI Appendix, Fig. S5F). The SFA ratio detected from both CaV1.3ΔECS CA1 and CA3 neurons was lower, suggesting that there would be less adaptation during firing (SI Appendix, Fig. S5 B and E).
Increased Large-Conductance Ca2+-activated (BK) Potassium Channel Activation Contributes to Enhanced Neuronal Excitability by Close Coupling with CaV1.3 Channel in CA1 Pyramidal Neurons.
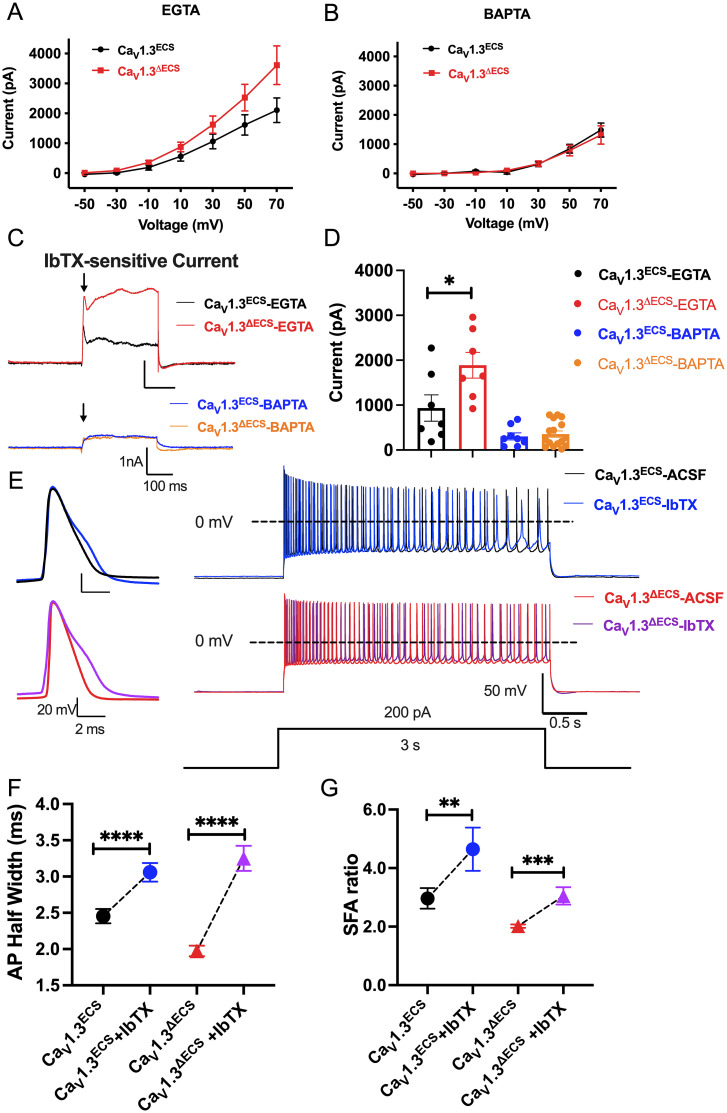
While the biophysical property of the unedited CaV1.3 channels provides an explanation for membrane excitability, we also observed reduced AP width and firing adaptation. Therefore, we next queried whether the increased influx of Ca2+ observed in CaV1.3ΔECS CA1 neurons may functionally couple with Ca2+-activated potassium channels to increase action potential firings. A candidate to test this hypothesis is the BK channel, as it has been shown that BK channels underlie repolarization and fast afterhyperpolarization to support high-frequency AP firing in hippocampal neurons (18). Furthermore, the reported proximal clustering of CaV1.3 and BK channels promotes the activation of the BK channel at low voltage and therefore may serve to regulate neuronal excitability in the rat hippocampus (19). Therefore, we performed whole-cell patch-clamp electrophysiological recordings with 1μM tetrodotoxin (TTX) and 100 nM iberiotoxin (IbTX, BK channel antagonist) to isolate the outward (IBK) K+ currents. With the presence of 5 mM ethylene glycol tetraacetic acid (EGTA) in the internal solution, we observed larger outward K+ currents (CaV1.3ECS: 2104.6 ± 410.7 pA; CaV1.3ΔECS: 3608.5 ± 645.7 pA) (Fig. 4A and SI Appendix, Fig. S6 A and B) that displayed a transient peak sensitive to IbTX-blocking in the CaV1.3ΔECS CA1 neurons. The IbTX-sensitive currents at 70 mV were ∼twofold larger as recorded from the CA1 neurons of the CaV1.3ΔECS mice (CaV1.3ECS: 935.2 ± 293.2 pA; CaV1.3ΔECS: 1888 ± 287.5 pA; P = 0.0043) (Fig. 4C). Therefore, we surmised that this transient peak represented the fast activation of the BK channel, triggered due to a close functional coupling with the CaV1.3 channels. To address this possibility, we used an internal solution with 10 mM 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid) (BAPTA), a fast Ca2+ chelator, to disrupt the close proximity coupling between CaV1.3 and the BK channel. Indeed, the IbTX-sensitive currents were mostly abolished in both mice (Fig. 4 B and C and SI Appendix, Fig. S6 A and B). To corroborate this finding, we investigated whether blocking the BK channels would affect the APD and the SFA and found that in the presence of IbTX, the APD became significantly wider and that the SFA ratio was increased for both CaV1.3ΔECS and CaV1.3ECS neurons (Fig. 4 D and E and SI Appendix, Fig. S6 C–F).
Fig. 4.
Increased BK current in CaV1.3ΔECS mice due to close coupling with CaV1.3 channel affects APD and SFA in CA1 neurons. (A) I-V curve of IK currents recorded with EGTA in the pipette solution from CA1 pyramidal neurons in hippocampal slices from CaV1.3ECS (n = 7) and CaV1.3ΔECS (n = 8) mice. (B) I-V curve of IK currents recorded with BAPTA in the pipette solution from CA1 pyramidal neurons in hippocampal slices from CaV1.3ECS (n = 13) and CaV1.3ΔECS (n = 22) mice. (C) Representative trace quantification of subtracted IbTX-sensitive IBK in CA1 pyramidal neurons with the presence of EGTA or BAPTA in the internal solution. (D) Quantification of IbTX-sensitive IBK in CA1 pyramidal neurons with the presence of EGTA or BAPTA in the internal solution. (E) Representative traces of the first AP and trains of APs induced by 200 pA current injection for 3 s before and after IbTX treatment in CA1 pyramidal neurons. (F) Quantification of AP half-width before and after 100 nM IbTX treatment from CaV1.3ECS (n = 20) and CaV1.3ΔECS mice (n = 14). (G) Quantification of SFA ratio before and after IbTX treatment in CaV1.3ECS (n =15) and CaV1.3ΔECS mice (n = 21). Data represent means ± SEM. Scatters in bars indicate the number of analyzed neurons. The two-tailed unpaired Student’s t test was used for analysis of data in (D); the two-tailed paired Student’s t test was used for analysis of data in (F and G). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Enhanced Synaptic Transmission at CA3–CA1 Synapses in CaV1.3ΔECS Mice.
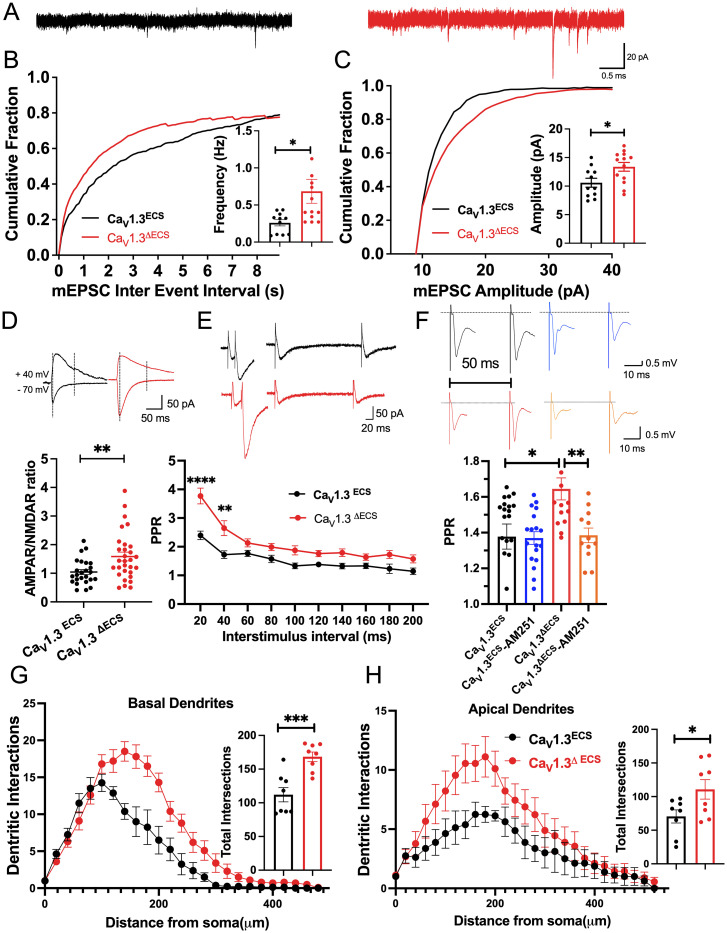
After assessing the enhanced excitability of CaV1.3ΔECS CA1 and CA3 pyramidal neurons due to a gain of function as a result of the loss of editing of the CaV1.3 channels, we probed how synaptic transmission is affected at the CA3–CA1 synapses. To investigate whether presynaptic activity or postsynaptic responses might be involved, miniature excitatory postsynaptic currents (mEPSCs) of CA1 pyramidal neurons were recorded at −70 mV in acute adult hippocampal slices prepared from both CaV1.3ΔECS and CaV1.3ECS mice (Fig. 5A). Because CaV1.3 channels are mostly expressed on the dendrites and soma of neurons, we were surprised by the significant increase in mEPSC frequency, as this result suggests the enhanced presynaptic release of neurotransmitters (Fig. 5B). Because little is known about how CaV1.3 channels contribute to hippocampal LTP (20–22), the increase in mEPSC amplitude, which suggests enhanced postsynaptic reception, was also intriguing (Fig. 5C). To further characterize the underlying postsynaptic mechanism, we determined the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR)/N-methyl-D-aspartate receptor (NMDAR) ratio, because this result showed the relative expression of AMPA and NMDA receptors at the postsynapse. With stimulation electrode located at the Schaffer collaterals, the AMPAR/NMDAR ratio recorded from CA1 pyramidal neurons was higher in the CaV1.3ΔECS mice, suggesting that there were relatively more AMPA receptors present in the postsynaptic membranes (Fig. 5D). The increased Ca2+ current through the CaV1.3 channel and the enhanced activity of CA1 neurons are likely to promote the trafficking and insertion of the AMPA receptor to the postsynaptic terminals, as previously reported (23).
Fig. 5.
Increased synaptic transmission and dendritic intersections of CA1 pyramidal neurons in CaV1.3ΔECS mice. (A) Representative trace of mEPSCs recorded in CA1 pyramidal neurons from CaV1.3ECS (black) and CaV1.3ΔECS mice (red) (Scale bar, 0.5 s and 20 pA). (B and C) Histogram and cumulative fraction of the interevent interval and amplitude of mEPSCs, n = 11 and n = 13 for CaV1.3ECS and CaV1.3ΔECS mice. (D) Representative traces and quantification of AMPAR/NMDAR ratio calculated by estimating the respective AMPAR and NMDAR component at +40 mV based on their different time courses (Scale bar, 50 ms and 50 pA). (E) Representative traces and quantification of PPR recorded in CA1 pyramidal neurons with stimulus located in the stratum radiatum at various interpulse intervals from 20 to 200 ms. n = 30 and n = 24 for CaV1.3ECS and CaV1.3ΔECS mice, respectively (Scale bar, 20 ms and 50 pA). (F) Quantification of PPR measured from the slope of fEPSPs with stimulus located in the stratum radiatum at a 50 ms interpulse interval before and after applying CB1R antagonist AM251 (10 µM). n = 20 and n = 15 for CaV1.3ECS and CaV1.3ΔECS mice, respectively, before AM251 treatment. n = 18 and n = 12 for CaV1.3ECS and CaV1.3ΔECS mice, respectively, after applying AM251. (G and H) Quantification of basal and apical dendritic intersections of CA1 pyramidal neurons. Total intersections were counted. n = 8 and n = 8 for CaV1.3ECS and CaV1.3ΔECS mice, respectively. Data represent means ± SEM. Scatters in bars indicate the number of analyzed neurons or slices. Two-way ANOVA with Bonferroni’s multiple comparisons test was used for data in (E); the two-tailed unpaired Student’s t test was used for analysis of data in all other panels. *P < 0.05; **P < 0.01; ***P < 0.001.
Up-Regulated Activation of Inhibitory Cannabinoid Type 1 Receptor (CB1R) for Homeostatic Feedback.
Apart from increased postsynaptic responses in the CA3–CA1 synapse, we also found that paired-pulse ratios (PPRs) were significantly higher at short interstimulus intervals in CaV1.3ΔECS hippocampus (Fig. 5E). Increased PPR could be associated with inhibited presynaptic neurotransmitters release upon the first pulse, while the local accumulation of Ca2+ leads to an enhanced release when the second pulse arrives. To uncover the explanation for increased PPR, we evaluated the inhibitory retrograde endocannabinoid signaling pathway (24). With a 50 ms interpulse interval, the PPR in the CaV1.3ΔECS hippocampus was significantly reduced to a level similar to that of CaV1.3ECS by 10 μM CB1 receptor antagonist AM251 (Fig. 5F). This finding suggests that there was enhanced basal activation of CB1R in the CaV0.3ΔECS CA1 presynapse. Therefore, the increased PPR in the CaV1.3ΔECS CA3–CA1 connection could be due to the disinhibition of presynaptic release after the first pulse so that the second pulse generates a larger response. It is possible that at the basal level, increased activation of CB1R in CaV1.3ΔECS mice leads to an increase in tonic Gβγ inhibition of CaV2 channels to suppress the neurotransmitter release (25–30). Upon the first stimulating pulse, due to depolarization of the membrane potentials, the inhibition of CaV2 by Gβγ was relieved and was further relieved in the CaV1.3ΔECS mice. The increased amount of presynaptic calcium influx then accumulated at the presynaptic terminal to trigger an increase in the synaptic release of the neurotransmitter upon the second pulse.
Increased Dendritic Arborization and Spine Density of CA1 Pyramidal Neurons Support Their Enhanced Synaptic Activity.
Since the Ca2+ ion as a signaling molecule is important for changes in dendritic structure and arborization, we scrutinized alterations in the dendritic arborization and spine number of CA1 pyramidal neurons. To visualize a CA1 pyramidal neuron in a hippocampal slice, a single neuron was dialyzed with Alexa fluorescent 488 dye via an attached patch pipette. The labeled neurons were subject to Sholl analysis for morphological characterizations. In congruence with enhanced synaptic activity, we observed increased dendritic intersections in the basal dendrites of CA1 pyramidal neurons of CaV1.3ΔECS mice (Fig. 5G; P = 0.0006). In addition, the dendritic intersections of the apical dendrites were also significantly higher in CaV1.3ΔECS CA1 pyramidal neurons (Fig. 5H; P = 0.0395). Enumeration of the spine density revealed that CaV1.3ΔECS CA1 pyramidal neurons had a higher density of spines in the basal dendrites (SI Appendix, Fig. S7).
Changes in Ca2+ Signaling Pathways in CaV1.3ΔECS and CaV1.3ECS CA1 Neurons after Spatial Learning.
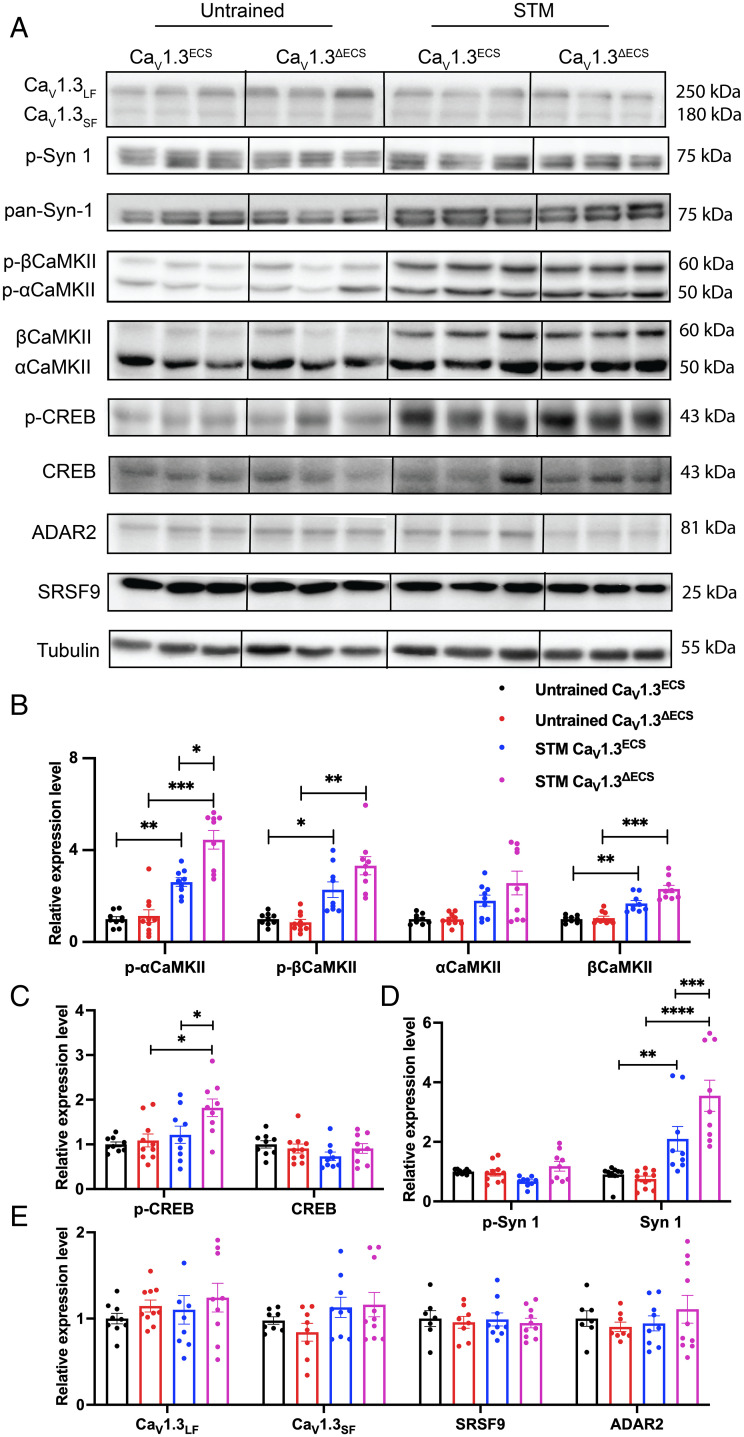
Our follow-up question was about how a larger Ca2+ influx observed in CaV1.3ΔECS mice may signal to contribute to enhanced hippocampal synaptic plasticity. Ca2+ influx through L-type calcium channels is known to drive local Ca2+/calmodulin-dependent protein kinase II (CaMKII) aggregation and trigger nuclear signaling for cAMP-response element binding protein CREB-dependent gene expression (31, 32). Here, we predicted that the increased Ca2+ current density would elevate the accumulation of CaMKII and downstream CREB activity in CaV1.3ΔECS mice during water-maze training. To substantiate this possibility, we measured the expression and phosphorylation level of CaMKII and CREB in CA1 regions from both untrained mice and the Morris water maze–trained CaV1.3ΔECS and CaV1.3ECS mice after the STM probe test (Fig. 6A). First, we detected increased levels of phosphorylated-αCaMKII and βCaMKII (threonine286) as well as nonphosphorylated βCaMKII in the trained CaV1.3ECS and CaV1.3ΔECS mice. Of note, the level of p-αCaMKII was significantly higher in trained Cav1.3ΔECS mice as compared to that in trained CaV1.3ECS mice (Fig. 6B). The increased phosphorylation of αCaMKII would support enhanced synaptic transmission in a kinase-dependent manner, while the increased βCaMKII would contribute toward an activity-induced kinase-independent role in the maintenance of dendritic spine architecture (33). Second, for CREB activity, we detected a significant increase of the p-CREB level in the trained CaV1.3ΔECS mice as compared to the untrained CaV1.3ΔECS mice and the trained CaV1.3ECS mice (Fig. 6C). Considering that hippocampal tissues were harvested 30 min after the short-term probe test, it may have still been too early to capture changes of CREB activity in CaV1.3ECS mice. Therefore, the elevated level of p-CREB indicated that the Ca2+-triggered signaling to the nucleus is promoted in CaV1.3ΔECS mice. Third, we also found that in both CaV1.3ECS and CaV1.3ΔECS mice, the Morris water-maze training elevated the expression of nonphosphorylated synapsin 1 (Syn-1) protein (Fig. 6D). Syn-1 has been reported to be associated with activity-induced vesicle release at presynaptic terminals (34). Syn-1 regulates the number of synaptic vesicles at the active zone in a bidirectional manner. The level of Syn-1 is positively associated with the number of vesicles at the presynaptic terminals, while the phosphorylation of Syn-1 removes the vesicles from the active zone (34). Parallel to what we observed for CaMKII, the level of Syn-1 was approximately 1.75-fold higher in trained CaV1.3ΔECS mice as compared to trained CaV1.3ECS mice, supporting the enhanced synaptic activity during learning in CaV1.3ΔECS mice. As shown earlier that the editing level of CaV1.3 was reduced after the STM probe test in the CaV1.3ECS mice, this reduction of editing would allow more Ca2+ influx and therefore trigger downstream signaling of CaMKII and CREB proteins. Although the editing level of CaV1.3 channels was down-regulated after training, the expression levels of CaV1.3 channels in the CA1 region and the editing regulator of ADAR2 such as Ser/Arg (SR)-rich splicing factor 9 (SRSF9) was unaltered (Fig. 6E).
Fig. 6.
Regulation of Ca2+-dependent signaling pathway after Morris water maze and the dynamics and conservation of RNA editing in CaV1.3 channels. (A–E) Immunoblotting analysis of plasticity-related proteins in hippocampal CA1 from untrained CaV1.3ECS and CaV1.3ΔECS mice as well as those trained for four trials and tested with STM probe test. Quantification of relative expression level of p-CaMKII and CaMKII (B); p-CREB and CREB (C); p-Syn-1 and Syn-1 (D); CaV1.3LF (long-form splicing variant CaV1.342 with predicted size of 250 kDa), CaV1.3SF (short-form splicing variants CaV1.342a, CaV1.343S and CaV1.343S2 with predicted size around 180 kDa), SRSF9, and ADAR2 (E) with and without water-maze training in CaV1.3ECS and CaV1.3ΔECS mice. (B–E) Data represent means ± SEM. Scatters in bars indicate the number of analyzed animals. Two-way ANOVA with Bonferroni’s multiple comparisons test was used. Mixed-effects analysis (type III) of genotype and training: p-CaMKII and CaMKII (F (1.767, 15.90) = 51.9, P < 0.0001), p-CREB and CREB (F (2.766, 24.89) = 3.690, P = 0.0278), and p-Syn-1 and Syn-1 (F (3, 27) = 12.49, P < 0.0001); *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Discussion
While the CaV1.3 channel is expressed in multiple organs, RNA editing of its IQ-domain is restricted to neurons of the central nervous system. Here, we provide evidence that mammalian RNA editing could be adaptive and that editing recoding has functional, physiological, and behavioral importance. This notion is supported by, first, the evolutionary conservation of CaV1.3 IQ-domain RNA editing across various mammalian species, from zebrafish to humans. Second, enhanced hippocampal plasticity, learning, and memory observed in mice with a total loss of CaV1.3 RNA editing suggest that a dynamic regulation of CaV1.3 RNA editing would affect spatial learning and memory. Moreover, these mice exhibited an unexpected gain of function because unedited CaV1.3 channels support a larger Ca2+ influx into the neurons. This result resolved the CaV1.3 editing paradox because RNA editing of the IQ-domain affects CDI and channel open probability, two calmodulin-dependent processes that elicit opposing effects on Ca2+ influx.
Functional Recoding of CaV1.3 RNA Editing Results in Decreased Ca2+ Influx.
The CaV1.3 RNA editing paradox revolves around the opposing effects of editing to slow Ca2+-dependent inactivation to increase Ca2+ influx (9), while on the contrary, editing lowers the affinity of the binding of apo-calmodulin to the CaV1.3 IQ-domain to lead to reduced open probability and less Ca2+ influx (10, 11). Here, we observed increased CaV1.3 current in CA1 pyramidal neurons in CaV1.3ΔECS mice, indicating that with the deletion of RNA editing, the unedited CaV1.3 channel allowed a larger entry of Ca2+. This result resolved the editing paradox, and although unedited CaV1.3 channels displayed more robust CDI, the effect of increased open probability was more dominant. The increased CaV1.3 Ca2+ currents, coupled with the more hyperpolarized activation of the unedited CaV1.3 channel, led to a more depolarized resting membrane potential, which served as the basis of the enhanced excitability of CA1 and CA3 neurons.
Close Coupling of CaV1.3 and BK Channel in CA1 Pyramidal Neurons Determines APD.
In this study, we demonstrated that mice with a deletion of RNA editing in the CaV1.3 channel exhibited enhanced excitability of CA1 and CA3 pyramidal neurons. Increased firing with current injection, depolarized RMP, and lower SFA were observed in both CA1 and CA3 neurons in the CaV1.3ΔECS hippocampus. We also found that narrowed APD was only present in CA1 pyramidal neurons but not in CA3 pyramidal neurons. Moreover, treatment using IbTX was able to broaden APD in CA1 neurons. This difference between CA1 and CA3 pyramidal neurons could be due to the different types of BK channels expressed in those neurons. In CA1 neurons, inactivating the BK channel with the accessory β2-subunit reveals fast gating kinetics, is IbTX-sensitive, and regulates repolarization of AP and spike duration (35, 36). For this type of BK channel, its effect on APD is strongly associated with the amount of Ca2+ influx. However, in CA3 neurons, high expression of the β4 subunit detected by in situ hybridization has been reported, and the data are corroborated in the Allan Brain Atlas (mouse.brain-map.org/gene/show/37365). These β4-containing noninactivating BK channels are expressed dominantly on the CA3 neuronal membrane, and they are slow-gating and IbTX-insensitive (37). It has also been reported that in CA3 neurons, BK channels modulate APD through the interaction of the β4 subunit and the Fragile X mental retardation protein (38). Therefore, an increased amount of inactivating BK current in CA1 neurons contributes to the narrowed APD and enhanced firing, whereas the increased excitability of CA3 pyramidal neurons is mainly associated with changes of membrane properties such as depolarized RMP. In addition, there are also multiple mechanisms that regulate BK channel activity such as alternative splicing, β-subunit composition, posttranslational modification, and the Ca2+ source that coalesce to underlie the differences in BK channel properties in various types of neurons (18, 39).
Enhanced Synaptic Activity and CB1R Homeostatic Feedback.
It is intriguing that on the one hand, both the frequency and amplitude of mEPSC were increased in CaV1.3ΔECS CA1 pyramidal neurons that are indicative of enhanced neurotransmitter release and response. On the other hand, the PPR was also higher with up-regulated activation of inhibitory CB1R in the CaV1.3ΔECS CA3–CA1 connection that would lead to a reduced release probability at the presynaptic terminal. Because the frequency of mEPSCs could be affected by multiple factors including the release probability of presynaptic terminals (40), the surface expression of AMPA receptors (23, 41), and the number and morphology of dendritic spines (42), we surmise that the increased frequency of mEPSC we observed here could be contributed by one factor or a combination of these factors. The up-regulation of endocannabinoid activity could be a postsynaptic homeostatic feedback signal to scale down synaptic neurotransmission for preventing hyperactivity. Nonetheless, the net effect we observed in CaV1.3ΔECS mice was enhanced synaptic transmission.
Functional Importance of CaV1.3 RNA Editing.
RNA editing, a posttranscriptional modification, could deaminate an adenosine in the noncoding RNA to affect transcript stability, binding affinity, or splicing and in coding exons to be recoded with synonymous or nonsynonymous change in the amino acid codon. Recoding of exons leads to diversification of protein function in response to various environmental or physiological conditions. The functional outcomes of CaV1.3 RNA editing provide evidence of its importance in response to physiological or pathological stimuli. Our results agree well with previous published reports on the functional importance of RNA editing, as shown in the conditional knockout mouse model of the glutamate recepor subunit B (GluR-B+/ΔECS) and the knock-in mouse models expressing an unedited /fully edited 5-HT2C receptor. However, because the glutamine/arginine (Q/R) editing site was almost fully edited, the homozygous deletion of GluR-B ECS led to early seizure-related death in mice. Therefore, the model used for functional study was the heterozygous GluR-B+/ΔECS or rescued ADAR2−/−/GluR-BR/R mice. Reduced Q/R editing in the hippocampus led to an epileptic phenotype due to the retained Ca2+ permeability of unedited GluR-B receptors (6), while a complete loss of GluR-B editing was a gain of function of Ca2+ permeability. While the 5-HT2C R-INI knockout mice did not show any obvious phenotype, the 5HT2C R-VGV knock-in mice showed a severe reduction in body fat as a result of the constitutive activation of the sympathetic nerve and increased energy expenditure (7). As for the GABAA receptor and KV1.1 channel, because their stem–loop structure that is formed between ECS and the editing site lies within the coding region, until now, transgenic mouse models specifically targeting RNA editing are not available (12, 13). As such, to the best of our knowledge, the CaV1.3ΔECS mice are the first surviving ECS knockout of ion channels or receptors that has a gain of function phenotype with enhanced spatial learning and memory and neuronal plasticity. To more precisely characterize other neurological functions, a CaV1.3 ECS conditional knockout model would have to be generated in the future. Nonetheless, the CaV1.3ΔECS mice uncovered a role of CaV1.3 channels in hippocampal-dependent learning and memory. Of note, the knockout mouse model of the CaV1.3 channel did not show any deficit in spatial learning (21). However, it seems that the roles of the CaV1.3 channel in neurocognitive functions were revealed through a gain of function of channel activity. Gain of function of the CaV1.3 channel has been associated with Parkinson’s disease (PD) and various neuropsychiatric disorders. The CaV1.3 channel serves an important role in supporting the spontaneous pace-making activity of substantia nigra pars compacta (SNc) neurons, which after degeneration and death lead to PD. L-type channel blocker DHPs have been found to be protective in PD mouse and nonhuman primate models (43–45). The stimulation of CaV1.3 channels by BayK8644 in vivo has induced depression-like behavior (46), while a lack of CaV1.3 leads to anti-depressive behavior (47). Multiple gain-of-function mutations in CACNA1D have also been reported in patients with sporadic autism and intellectual disability (48–50) as well as patients with primary aldosteronism and those with neurodevelopmental deficits and seizures at an early age (51). More intriguingly, recent studies have also reported significantly reduced A-to-I RNA editing of CaV1.3 channels from 14 to 8% in the hippocampus from patients with AD compared to healthy control samples (52, 53). This is likely associated with the down-regulation of ADAR expression in patients with AD. Moreover, the expression level of CaV1.3 channels has also been shown to be lower in patients with AD (54, 55). Due to the complexity of AD pathology, it is still largely unknown whether the down-regulation of editing is detrimental, compensatory, or neutral. Here, the enhanced hippocampal plasticity and spatial learning and memory observed from this gain-of-function model of CaV1.3ΔECS mice provide insights into the roles of the CaV1.3 channel and the importance of fine-tuning calcium channel activity to regulate Ca2+ influx for optimal functional outcomes. In this study, we have focused particularly on spatial learning and memory. Further investigations would be needed to reveal whether other memory systems such as fear memory and associative memory would be affected in CaV1.3ΔECS mice. The wider implications of CaV1.3 RNA editing in other brain functions await discovery.
The CaV1.3 channel is also known to be important for neurogenesis and the differentiation of the hippocampal dentate gyrus (DG) (56). The CaV1.3−/− mice exhibited impaired DG neurogenesis, reduced DG volume, and survival of newly differentiated neurons. As reported, the CaV1.3−/− mice failed to discriminate the differences in object location during the object location memory test, and the impairment of neurogenesis was reported to affect hippocampal-associated cognitive functions (56). Our findings on the functional effects of RNA editing of CaV1.3 channels on the hippocampal neurons provide insight to further investigate how a gain of function due to loss of CaV1.3 RNA editing may impact neuronal neurogenesis and differentiation.
Materials and Methods
Generation of Genetically Modified CaV1.3ΔECS/ΔECS Mice.
The strategy to generate an ECS targeting construct is summarized in SI Appendix, Fig. S1A. The embryonic stem cells that had undergone homologous recombination were injected into SV129 mouse blastocysts. Male and high agouti-color coated F0 mice were selected to mate with C57BL/6 female mice to generate F1 pups. Genotyping was performed using PCR and Southern blot analysis. Details are found in SI Appendix, Supplemental Experimental Procedures.
Evaluation of IQ-Domain RNA Editing in CaV1.3 Channels.
Total RNA was isolated from brain tissues using the TRIzol method (Invitrogen), and first-strand complementary DNA (cDNA) was synthesized with SuperScript II and oligo(dT)18 primers (Invitrogen). RNA was treated with DNase I (Invitrogen) before being subjected to reverse transcription. For tissues collected from mouse, rat, and Macaca fascicularis, all experimental procedures were approved by Institutional Animal Care and Use Committee (IACUC) at the National University of Singapore. For the postmortem brain samples from various animals from the Mandai Wildlife Group (MWG), all procedures were approved by IACUC at the MWG Research Panel. For humans, the Whole Brain Marathon-Ready cDNA library (Clontech, Takara) was used. PCR was performed with primers designed for different species (listed in SI Appendix, Table S1). Details are found in SI Appendix, Supplemental Experimental Procedures.
The level of RNA editing was calculated by measuring the heights of the peaks of A/G on the electropherograms obtained from DNA sequencing:
Immunoblotting.
To validate the expression level of targeted proteins, brain tissues or hippocampal slices were dissected on ice and were snap-frozen immediately. The CA1 region was then isolated carefully under a dissection microscope at 4 °C. Details are found in SI Appendix, Supplemental Experimental Procedures.
Hippocampal Slice Preparation.
CaV1.3ΔECS and WT mice maintained on a 12 h light/dark cycle were used in these studies. All experimental procedures were approved by IACUC at the National University of Singapore. For the whole-cell patch-clamp recordings, male mice (aged 5–7 wk) were anesthetized deeply with isoflurane (0.5 mL) before cervical dislocation. Mice were then decapitated; brains were removed quickly and placed in a dissection medium containing ice-cold sucrose–artificial cerebrospinal fluid (ACSF). Coronal slices (280–300 μm thickness) including the hippocampi were cut using a vibrating microtome (Leica 1200S). Slices were first incubated in a chamber containing ACSF for 30 to 45 min at 32 °C and then maintained at room temperature. Single slices were transferred to the recording chamber and superfused with ACSF bubbled with 95% O2–5% CO2. The composition of ACSF was as follows (in mM): 125 NaCl, 2.5 KCl, 1.25 Na2HPO4, 25 NaHCO3, 25 glucose, 1 MgSO4, and 2 CaCl2 (300–310 mosmol/L, pH 7.4). For the sucrose–ACSF solution, NaCl was substituted by sucrose (230 mM) and the concentrations of MgSO4 and CaCl2 were modified to 5 mM and 0.5 mM, respectively, and an additional 3 mM myo-inositol, 2 mM Na-pyruvate, and 0.4 mM ascorbic acid (320–330 mosmol/L, pH 7.3).
Whole-Cell Patch-Clamp Electrophysiological Recordings.
The soma of CA1 and CA3 pyramidal cells were visually identified using a Nikon Eclipse FN1 equipped with a 40× water immersion objective and a CoolSnap EZ camera. Brain slices were constantly perfused with the above-mentioned oxygenated ACSF at a flow rate of 1.5 to 2 mL/min at room temperature. Whole-cell patch-clamp electrophysiological recordings were conducted using an EPC-9 or EPC-10 USB amplifier controlled by Patchmaster (Heka Elektronik, Lambrecht, Germany). Patch pipettes of 3 to 5 MΩ were pulled from borosilicate glass capillaries using a vertical Narishige PC-10 puller (Japan). Whole-cell patch-clamp recordings were performed with CA1 and CA3 pyramidal neurons from both CaV1.3ECS and CaV1.3ΔECS mice. Details are found in SI Appendix, Supplemental Experimental Procedures.
Field Recording.
Transverse hippocampal slices of 400 μm thickness were prepared from the right and left hippocampus using a manual tissue chopper (Stoelting, Wood Dale, IL) and transferred onto a nylon net placed in an interface chamber (Scientific Systems Design, Ontario, Canada) and incubated at 32 °C at an ACSF flow rate of 1 mL/min and carbogen consumption of 16 L/h. The entire process of animal dissection, hippocampal slice preparation, and placement of slices on the chamber was completed within approximately 5 min to ensure that hippocampal slices were in good condition for electrophysiology studies. The slices were incubated for at least 3 h before starting the experiments.
In all the field electrophysiology recordings, single-pathway experiments were performed. A single monopolar lacquer-coated stainless-steel electrode (5MΩ; AM Systems, Sequim) was positioned in the stratum radiatum of the CA1 region for stimulating a neuronal population, thus evoking field excitatory postsynaptic potentials (fEPSP) from Schaffer collateral/commissural‐CA1 synapses (Fig. 1A). Details are found in SI Appendix, Supplemental Experimental Procedures.
Data Analysis.
The peak current of Ca2+ and K+ at different holding potentials was analyzed using Fit-master (HEKA Electronics). I-V curves of Ca2+ currents were fitted by
where Gmax is the maximum conductance of the cell, Erev is the reversal potential, V1/2 is the voltage for half-maximal activation, and k is the slope factor of Boltzmann function.
Action potential firing frequency, half-width, and threshold were measured by automatic detection and calculation using customized MATLAB scripts adapted from a published algorithm (57). Briefly, the action potential amplitude (baseline to action potential peak value) was measured and the time between the half-peak amplitude on the rising and falling phases was taken to be the action potential half-width. The spike threshold was defined as the time point when the membrane potential increasing slope was 5 mV/ms. The mEPSCs were analyzed using the Mini Analysis Program (Synaptosoft Inc), and AMPDAR/NMDAR ratios were analyzed using customized MATLAB scripts.
For field recordings, the fEPSP slope value, expressed as percentages of average baseline values per time point, was subjected to statistical analysis using GraphPad Prism (GraphPad, San Diego, CA). Nonparametric tests were selected because a normal distribution could not always be assumed with the sample size per series and analyses of prolonged recordings do not allow the use of parametric tests. Furthermore, the sample sizes did not always guarantee a Gaussian normal distribution of the data per series. The Wilcoxon signed rank test was used to compare fEPSP values within one group and the Mann–Whitney U test was used when data were compared between groups. P < 0.05 was considered as the cutoff for statistically significant difference, n represented the number of slices, and a minimum of five animals were used for each experiment.
Clearing of Hippocampal Slices.
To label the CA1 pyramidal neurons for examining morphological changes, 10 mM Alexa Fluor 488 hydrazide (A10436; Thermo Fisher Scientific) was loaded into the neurons under a whole-cell patch-clamp configuration with an internal solution used for mEPSC recording. The neurons were held at −70 mV for 15 min for the dye to diffuse first into the soma and then into the dendrites. Next, slices with labeled CA1 neurons were fixed in 4% PFA for 30 min at room temperature, followed by three washes in PBS before treatment in 0.5% PBST overnight. The slices were then transferred in RapiClear 1.47 (SunJin Lab) for at least 3 h and mounted in fresh RapiClear 1.47. All mounted samples were kept at 4 °C before confocal imaging.
Confocal Imaging and Image Analysis.
Images were taken with a laser scanning microscope (FV3000; Olympus) using an excitation wavelength of 488 nm and PlanApo (10×/0.40) and UPLSAPO (60×/1.35) objective lenses (Olympus). Dye-injected hippocampal pyramidal neurons were imaged using the multi-area time–lapse function comprising 4 × 3 tiles (each tile, 1024 × 1024 pixels), with the z axis range decided by manually adjusting the focal depth until no signal from the neuron could be observed.
Images were analyzed using a commercially available software, Filament Tracer (Imaris, Bitplane, Inc). Prior to neuron tracing, background fluorescent signals were removed using the mask function of Imaris. The neurons were traced using a semiautomated mode, Autopath, in the Imaris Filament Tracer. Traced neurons were then exported as images that were further used for Sholl analysis in ImageJ. Dendritic spines were semiautomatically traced single-blind in Imaris with auto-depth function on dendritic branches that were 100 to 200 μm from the soma. Dendritic spine numbers were subsequently obtained from the statistic tab in Imaris.
Morris Water Maze.
Spatial reference memory and insistence on sameness were tested in a circular tank (diameter, 183 cm) filled with water and surrounded by uniform curtains and visual cues. The water was made opaque by adding a sufficient amount of tempera paint. Animal movements were tracked using a ceiling camera and Topscan tracking software. Mice were introduced into the pool at pseudorandomized drop locations outside of the target quadrant. During the “hidden platform learning task,” an escape platform (15 cm) was placed in the middle of a designated target quadrant 1 cm below the water surface. Mice were trained to find the platform by four 60 s trials per day for 4 consecutive days. A trial ended either when a mouse rested on the hidden platform for 5 s or the end of the trial was reached. Mice who had failed to find the platform were then manually guided to it. On day 1, 30 min after completing 4 training trials, a short-term probe test was conducted with the escape platform removed. On day 5, a long-term probe test was conducted and a remote probe test was conducted on day 12. Latency to find the hidden platform was obtained during the training trials. An average latency of four trials was calculated for the learning curve. Time spent in quadrants was tracked during probe tests.
Supplementary Material
Acknowledgments
We thank Shun Qiang Lo and Qiang Yuan for help with MATLAB scripts and Thomas Behnisch (Fudan University) for suggestions on evoke EPSC and PPR experiments. We thank Thomas Sudhof for the gift of the p-Syn-1 and Syn-1 antibodies. We thank the Neuroscience Phenotyping Core for guidance and support for the behavioral tests, the Advance Imaging Laboratory for support for confocal imaging, and the Conservation, Research and Veterinary (CRV) and Animal Care teams of Mandai Wildlife Group for the postmortem brain samples from various animals. The generation of CaV1.3ΔECS mice in the paper was developed from Jing Zhai’s thesis.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2203883119/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Xu G., Zhang J., Human coding RNA editing is generally nonadaptive. Proc. Natl. Acad. Sci. U.S.A. 111, 3769–3774 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peng Z., et al. , Comprehensive analysis of RNA-seq data reveals extensive RNA editing in a human transcriptome. Nat. Biotechnol. 30, 253–260 (2012). [DOI] [PubMed] [Google Scholar]
- 3.O’Neil R. T., Wang X., Morabito M. V., Emeson R. B., Comparative analysis of A-to-I editing in human and non-human primate brains reveals conserved patterns and context-dependent regulation of RNA editing. Mol. Brain 10, 11 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liscovitch-Brauer N., et al. , Trade-off between transcriptome plasticity and genome evolution in cephalopods. Cell 169, 191–202.e11 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garrett S. C., Rosenthal J. J., A role for A-to-I RNA editing in temperature adaptation. Physiology (Bethesda) 27, 362–369 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krestel H. E., et al. , A genetic switch for epilepsy in adult mice. J. Neurosci. 24, 10568–10578 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawahara Y., et al. , Dysregulated editing of serotonin 2C receptor mRNAs results in energy dissipation and loss of fat mass. J. Neurosci. 28, 12834–12844 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin C. B., et al. , RNA splicing and editing modulation of 5-HT(2C) receptor function: Relevance to anxiety and aggression in VGV mice. Mol. Psychiatry 18, 656–665 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Huang H., et al. , RNA editing of the IQ domain in Ca(v)1.3 channels modulates their Ca2+-dependent inactivation. Neuron 73, 304–316 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bazzazi H., Ben Johny M., Adams P. J., Soong T. W., Yue D. T., Continuously tunable Ca(2+) regulation of RNA-edited CaV1.3 channels. Cell Rep. 5, 367–377 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams P. J., Ben-Johny M., Dick I. E., Inoue T., Yue D. T., Apocalmodulin itself promotes ion channel opening and Ca(2+) regulation. Cell 159, 608–622 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhalla T., Rosenthal J. J., Holmgren M., Reenan R., Control of human potassium channel inactivation by editing of a small mRNA hairpin. Nat. Struct. Mol. Biol. 11, 950–956 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Daniel C., Wahlstedt H., Ohlson J., Björk P., Ohman M., Adenosine-to-inosine RNA editing affects trafficking of the gamma-aminobutyric acid type A (GABA(A)) receptor. J. Biol. Chem. 286, 2031–2040 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang H., et al. , Tissue-selective restriction of RNA editing of CaV1.3 by splicing factor SRSF9. Nucleic Acids Res. 46, 7323–7338 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magee J. C., Avery R. B., Christie B. R., Johnston D., Dihydropyridine-sensitive, voltage-gated Ca2+ channels contribute to the resting intracellular Ca2+ concentration of hippocampal CA1 pyramidal neurons. J. Neurophysiol. 76, 3460–3470 (1996). [DOI] [PubMed] [Google Scholar]
- 16.Avery R. B., Johnston D., Multiple channel types contribute to the low-voltage-activated calcium current in hippocampal CA3 pyramidal neurons. J. Neurosci. 16, 5567–5582 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu W., Lipscombe D., Neuronal Ca(V)1.3alpha(1) L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J. Neurosci. 21, 5944–5951 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li B., et al. , Neuronal inactivity co-opts LTP machinery to drive potassium channel splicing and homeostatic spike widening. Cell 181, 1547–1565.e15 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vivas O., Moreno C. M., Santana L. F., Hille B., Proximal clustering between BK and CaV1.3 channels promotes functional coupling and BK channel activation at low voltage. eLife 6, 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodman T., et al. , Young hippocampal neurons are critical for recent and remote spatial memory in adult mice. Neuroscience 171, 769–778 (2010). [DOI] [PubMed] [Google Scholar]
- 21.McKinney B. C., Murphy G. G., The L-type voltage-gated calcium channel Cav1.3 mediates consolidation, but not extinction, of contextually conditioned fear in mice. Learn. Mem. 13, 584–589 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKinney B. C., Sze W., White J. A., Murphy G. G., L-type voltage-gated calcium channels in conditioned fear: A genetic and pharmacological analysis. Learn. Mem. 15, 326–334 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hiester B. G., et al. , L-type voltage-gated Ca2+ channels regulate synaptic-activity-triggered recycling endosome fusion in neuronal dendrites. Cell Rep. 21, 2134–2146 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawamura Y., et al. , The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J. Neurosci. 26, 2991–3001 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fletcher-Jones A., et al. , Protein interactors and trafficking pathways that regulate the cannabinoid type 1 receptor (CB1R). Front. Mol. Neurosci. 13, 108 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zamponi G. W., Currie K. P., Regulation of Ca(V)2 calcium channels by G protein coupled receptors. Biochim. Biophys. Acta 1828, 1629–1643 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mackie K., Hille B., Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proc. Natl. Acad. Sci. U.S.A. 89, 3825–3829 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu M., Yu B., Nakanishi O., Wieland T., Simon M., The Ca2+-dependent binding of calmodulin to an N-terminal motif of the heterotrimeric G protein beta subunit. J. Biol. Chem. 272, 18801–18807 (1997). [DOI] [PubMed] [Google Scholar]
- 29.Currie K. P., G protein modulation of CaV2 voltage-gated calcium channels. Channels (Austin) 4, 497–509 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bell T. J., Thaler C., Castiglioni A. J., Helton T. D., Lipscombe D., Cell-specific alternative splicing increases calcium channel current density in the pain pathway. Neuron 41, 127–138 (2004). [DOI] [PubMed] [Google Scholar]
- 31.Wheeler D. G., et al. , Ca(V)1 and Ca(V)2 channels engage distinct modes of Ca(2+) signaling to control CREB-dependent gene expression. Cell 149, 1112–1124 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen K., Meyer T., Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science 284, 162–166 (1999). [DOI] [PubMed] [Google Scholar]
- 33.Okamoto K., Narayanan R., Lee S. H., Murata K., Hayashi Y., The role of CaMKII as an F-actin-bundling protein crucial for maintenance of dendritic spine structure. Proc. Natl. Acad. Sci. U.S.A. 104, 6418–6423 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patzke C., et al. , Neuromodulator signaling bidirectionally controls vesicle numbers in human synapses. Cell 179, 498–513.e22 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLarnon J. G., Inactivation of a high conductance calcium dependent potassium current in rat hippocampal neurons. Neurosci. Lett. 193, 5–8 (1995). [DOI] [PubMed] [Google Scholar]
- 36.Shao L. R., Halvorsrud R., Borg-Graham L., Storm J. F., The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J. Physiol. 521, 135–146 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang B., Jaffe D. B., Brenner R., Current understanding of iberiotoxin-resistant BK channels in the nervous system. Front. Physiol. 5, 382 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng P. Y., et al. , FMRP regulates neurotransmitter release and synaptic information transmission by modulating action potential duration via BK channels. Neuron 77, 696–711 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harvey J. R. M., Plante A. E., Meredith A. L., Ion channels controlling circadian rhythms in suprachiasmatic nucleus excitability. Physiol. Rev. 100, 1415–1454 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han E. B., Stevens C. F., Development regulates a switch between post- and presynaptic strengthening in response to activity deprivation. Proc. Natl. Acad. Sci. U.S.A. 106, 10817–10822 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noel J., et al. , Surface expression of AMPA receptors in hippocampal neurons is regulated by an NSF-dependent mechanism. Neuron 23, 365–376 (1999). [DOI] [PubMed] [Google Scholar]
- 42.Ye Z., Cudmore R. H., Linden D. J., Estrogen-dependent functional spine dynamics in neocortical pyramidal neurons of the mouse. J. Neurosci. 39, 4874–4888 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan C. S., et al. , “Rejuvenation” protects neurons in mouse models of Parkinson’s disease. Nature 447, 1081–1086 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Ilijic E., Guzman J. N., Surmeier D. J., The L-type channel antagonist isradipine is neuroprotective in a mouse model of Parkinson’s disease. Neurobiol. Dis. 43, 364–371 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kupsch A., et al. , 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity in non-human primates is antagonized by pretreatment with nimodipine at the nigral, but not at the striatal level. Brain Res. 741, 185–196 (1996). [DOI] [PubMed] [Google Scholar]
- 46.Hetzenauer A., Sinnegger-Brauns M. J., Striessnig J., Singewald N., Brain activation pattern induced by stimulation of L-type Ca2+-channels: Contribution of Ca(V)1.3 and Ca(V)1.2 isoforms. Neuroscience 139, 1005–1015 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Busquet P., et al. , CaV1.3 L-type Ca2+ channels modulate depression-like behaviour in mice independent of deaf phenotype. Int. J. Neuropsychopharmacol. 13, 499–513 (2010). [DOI] [PubMed] [Google Scholar]
- 48.Iossifov I., et al. , De novo gene disruptions in children on the autistic spectrum. Neuron 74, 285–299 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Roak B. J., et al. , Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 485, 246–250 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pinggera A., et al. , CACNA1D de novo mutations in autism spectrum disorders activate Cav1.3 L-type calcium channels. Biol. Psychiatry 77, 816–822 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scholl U. I., et al. , Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat. Genet. 45, 1050–1054 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khermesh K., et al. , Reduced levels of protein recoding by A-to-I RNA editing in Alzheimer’s disease. RNA 22, 290–302 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu S., Yang M., Kim P., Zhou X., ADeditome provides the genomic landscape of A-to-I RNA editing in Alzheimer’s disease. Brief. Bioinform. 22, bbaa384 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mizuno Y., et al. , MUTYH actively contributes to microglial activation and impaired neurogenesis in the pathogenesis of Alzheimer’s disease. Oxid. Med. Cell. Longev. 2021, 8635088 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morabito S., Miyoshi E., Michael N., Swarup V., Integrative genomics approach identifies conserved transcriptomic networks in Alzheimer’s disease. Hum. Mol. Genet. 29, 2899–2919 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marschallinger J., et al. , The L-type calcium channel Cav1.3 is required for proper hippocampal neurogenesis and cognitive functions. Cell Calcium 58, 606–616 (2015). [DOI] [PubMed] [Google Scholar]
- 57.Sun A. X., et al. , Potassium channel dysfunction in human neuronal models of Angelman syndrome. Science 366, 1486–1492 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.