Abstract
Aims: Lipoprotein(a) [Lp(a)] is a plasma lipoprotein consisting of a low-density lipoprotein (LDL)–like particle with apolipoprotein (Apo)(a), attached via a disulfide bond to Apo B100. Previous studies have shown that high Lp(a) levels are associated with an increased risk of cardiovascular disease in patients with familial hypercholesterolemia (FH). To date, limited data are available as to distribution of Lp(a) in FH and associations of Lp(a) with other lipid profiles and cardiovascular disease. Our study aimed to investigate serum Lp(a) levels in relation to other lipid profiles and clinical conditions in the national largest-ever cohort of Japanese FH patients.
Methods: This study is a secondary analysis of the Familial Hypercholesterolemia Expert Forum (FAME) Study that includes a Japanese nationwide cohort of FH patients. In 399 patients under treatment for heterozygous FH who had a baseline measurement of serum Lp(a), the present study examined the distribution of Lp(a) levels and associations of Lp(a) with other lipid profiles and clinical conditions including coronary artery disease (CAD).
Results: The distribution of Lp(a) was skewed to the right with a median of 20.8 mg/dL, showing a log-normal distribution. Serum Apo B and Apo E levels were positively associated with Lp(a) levels. Age-adjusted mean of Apo B was 8.77 mg/dL higher and that of Apo E was 0.39 mg/dL higher in the highest category (40+ mg/dL) of Lp(a) than in the lowest category (<20 mg/dL). LDL-C levels did not show such an association with Lp(a) levels. A tendency towards a positive relationship between Lp(a) and prevalent CAD was observed in men.
Conclusion: Our study demonstrated a distribution pattern of Lp(a) in Japanese FH patients and positive relationships of Lp(a) with Apo B and Apo E levels.
Keywords: Familial hypercholesterolemia, Lipoprotein(a), Lipid-lowering therapy, Atherosclerotic disease
Introduction
Lipoprotein(a) [Lp(a)] is a plasma lipoprotein consisting of a low-density lipoprotein (LDL)–like particle with one additional protein, apolipoprotein(a), attached via a disulfide bond to apolipoprotein B100 1) . Lp(a) is a unique lipid profile in terms of its thrombogenic and atherogenic properties. Previous epidemiological and genetic studies have shown that high Lp(a) levels are associated with an increased risk of cardiovascular disease in both the general population and those with FH, independent of LDL cholesterol (LDL-C) levels 1 - 3) .
Familial hypercholesterolemia (FH) is a genetic disorder characterized by high serum levels of LDL-C, tendon and skin xanthomas and premature coronary artery disease (CAD) 4 , 5) . FH is caused by pathogenic mutations in genes of the LDL receptor, apolipoprotein (apo) B-100 and proprotein convertase subtilisin/kexin type 9 (PCSK9), which is involved in the LDL receptor pathway 6) .
Information is limited regarding the distribution of serum Lp(a) and its associations with other lipid parameters and cardiovascular disease in Japanese FH patients. A small Japanese study of 42 patients reported that serum levels of Lp(a) were significantly higher in patients with FH than in those without 7) . Our study aimed to investigate serum Lp(a) levels in relation to other lipid profiles and clinical conditions in the largest cohort of Japanese FH patients studied to date.
Methods
This study is a secondary analysis of the Familial Hypercholesterolemia Expert Forum (FAME) Study, which is a multi-center observational study to investigate the clinical features of FH patients and current real-world therapies for FH in Japan 8) . The study was registered with UMIN (UMIN000003211). While patients were largely enrolled during the period between June 2006 and December 2011 and followed up for 4 years, the recruitment was extended until the end of 2012, and the follow-up ended in 3 years for patients enrolled in 2012. A total of 803 patients were enrolled from 52 hospitals and clinics across Japan.
Patients were registered in the FAME study if they met all of the following 4 criteria: 1) they were diagnosed as probable or definite FH assessed by the clinical diagnostic criteria for heterozygous FH defined by the Research Committee of the Japanese Ministry of Health, Labour and Welfare 9) ; 2) Patients had serum LDL-C level >100 mg/dL; if patients had been taking ezetimibe, the pretreatment level of LDL-C should be >100 mg/dL; 3) they were outpatients of participating hospitals or clinics; and 4) they gave a written informed consent. Patients were excluded if they had serum triglycerides (TG) of >400 mg/dL; if they were suffering from severe liver dysfunction (acute phase and decompensated cirrhosis); if they had dyslipidemia secondary to hypothyroidism or pancreatitis; if they had uncontrolled diabetes mellitus (HbA1c >9%); if they were pregnant, potentially pregnant or lactating; or if they were inappropriate for enrollment as judged by study physicians.
Details of the diagnostic criteria for heterozygous FH have been described elsewhere 8) . In brief, the scoring was done on the basis of the 5 criterion items: untreated LDL-C level (1, 2, or 4 points), family history of FH (4 or 6 points), xanthoma (6 points), juvenile corneal arcus or premature CAD (4 points), and genetic mutations in the LDL receptor (8 points). Definite and probable FH were diagnosed for a total score of ≥ 8 points and 6-7 points, respectively. Described below are methodological issues relevant to the present study.
Study Population
The study subjects in the current analysis comprised 399 of the 762 heterozygous FH patients aged 15 years or older at baseline 8) . The 399 subjects were under medical treatment for heterozygous FH and had a baseline measurement of serum Lp(a).
Measurements
Laboratory measurements including serum Lp(a) were carried out at individual laboratories of the participating institutions. Commercially available immunoassays were used for measurement of Lp(a), the choice of which was at the discretion of each participating institution. In the present report, atherosclerotic disease was defined as CAD, cerebral infarction, and peripheral artery disease. CAD included ischemic heart diseases (I 20.0–I 25.9) of the 10th revision of the International Classification of Diseases), percutaneous coronary intervention, and coronary artery bypass grafting. Family history of CAD was defined as parental and/or sibling CAD.
Statistical Analysis
Proportions and means with standard deviation (SD) were calculated for categorical variables and for continuous variables, respectively, as descriptive statistics. The associations of Lp(a) with lipid-related and clinical variables were examined in terms of adjusted mean difference or odds ratio with 95% confidence interval according to Lp(a) categories. Cutoff points of Lp(a) were a priori determined at 20, 30, and 40 mg/dL with reference to the previous studies and guidelines 10 - 13) . The adjusted mean difference and odds ratios were estimated by means of multiple regression analysis and logistic regression analysis, respectively. Age and family history of CAD were found to be potential confounders in a preliminary analysis (see below). Age and family history of CAD (if necessary) were thus included as covariates in the analyses on Lp(a) in relation to lipid and clinical parameters. Those with missing values as to the covariates, family history of CAD excepted, were excluded in the analyses. A category of unknown history was created for the family history of CAD due to the large number of such patients. Serum TG and remnant lipoprotein cholesterol (RLP-C) were transformed to the natural logarithmic scale in the regression analysis because of the skewness to the right side. Lp(a) also showed a log-normal distribution (see below), and changes in Lp(a) from baseline according to the follow-up months were assessed by using the natural log-scale. Anti-logarithmic transformation of mean changes in the log-scale of Lp(a) necessarily leads to percent changes in the original scale of Lp(a). Statistical significance was declared if the two-sided P value was less than 0.05. Statistical analyses and graphical presentation were performed using Stata version 13 (StataCorp, College Station, TX).
Results
Baseline characteristics of the study participants are shown separately for males and females in Table 1 . Atherosclerotic diseases included CAD (n=99), cerebral infarction (n=11), and peripheral artery disease (n=1). Cholesterol-lowering treatments included statin, ezetimibe, apheresis, and other agents, with 87.4% of the study participants treated on statin-oriented therapy.
Table 1. Baseline Characteristics of the Study Patients.
| Variable | Both sexes | Male | Female | |||
|---|---|---|---|---|---|---|
| N* | Value | N* | Value | N* | Value | |
| Mean (SD) | ||||||
| Age (year) | 399 | 56.1 (15.4) | 159 | 53.3 (16.0) | 240 | 58.1 (14.8) |
| Heterozygous FH score | 399 | 13.8 (5.2) | 159 | 14.6 (5.4) | 240 | 13.4 (5.0) |
| Years from FH diagnosis | 388 | 10.3 (9.1) | 155 | 10.5 (9.7) | 233 | 10.2 (8.8) |
| Height (cm) | 387 | 160 (9) | 155 | 168 (6) | 232 | 155 (6) |
| Body weight (kg) | 393 | 60 (12) | 157 | 69 (11) | 236 | 54 (8) |
| Body mass index (kg/m2) | 384 | 23.2 (3.5) | 155 | 24.4 (3.5) | 229 | 22.3 (3.3) |
| LDL cholesterol (mg/dL) | 391 | 142 (42) | 154 | 136 (44) | 237 | 146 (41) |
| HDL cholesterol (mg/dL) | 399 | 54 (17) | 159 | 47 (14) | 240 | 59 (17) |
| Triglycerides (mg/dL) | 399 | 107 (68) | 159 | 112 (67) | 240 | 103 (68) |
| Apolipoprotein AI (mg/dL) | 352 | 137 (31) | 140 | 126 (29) | 212 | 144 (31) |
| Apolipoprotein B (mg/dL) | 353 | 110 (27) | 140 | 111 (30) | 213 | 110 (25) |
| Apolipoprotein E (mg/dL) | 343 | 4.3 (1.4) | 136 | 4.0 (1.3) | 207 | 4.5 (1.4) |
| RLP cholesterol (mg/dL) | 312 | 5.3 (4.4) | 129 | 5.4 (4.3) | 183 | 5.2 (4.5) |
| Lp(a) (mg/dL) | 399 | 31.6 (34.5) | 159 | 30.3 (29.1) | 240 | 32.5 (37.7) |
| Rf | 149 | 0.34 (0.04) | 62 | 0.35 (0.04) | 87 | 0.34 (0.05) |
| Maximal IMT (mm) | 359 | 1.63 (0.85) | 139 | 1.69 (0.86) | 220 | 1.59 (0.84) |
| Mean IMT (mm) | 324 | 0.91 (0.34) | 125 | 0.97 (0.35) | 199 | 0.87 (0.33) |
| Achilles tendon thickness (mm) | 347 | 11.5 (4.2) | 140 | 12.6 (4.7) | 207 | 10.9 (3.6) |
| Number (%) | ||||||
| Atherosclerotic disease | 399 | 104 (26.1) | 159 | 60 (37.7) | 240 | 44 (18.3) |
| Coronary artery disease | 399 | 99 (24.8) | 159 | 57 (35.8) | 240 | 42 (17.5) |
| Hypertension | 399 | 121 (30.3) | 159 | 55 (34.6) | 240 | 66 (27.5) |
| Diabetes mellitus | 399 | 68 (17.0) | 159 | 33 (20.8) | 240 | 35 (14.6) |
| Family history of coronary artery disease | 292 | 188 (64.4) | 112 | 69 (61.6) | 180 | 119 (66.1) |
| Smoking | 390 | 154 | 236 | |||
| Never | 278 (71.3) | 76 (49.4) | 202 (85.6) | |||
| Past | 82 (21.0) | 60 (39.0) | 22 (9.3) | |||
| Current | 30 (7.7) | 18 (11.7) | 12 (5.1) | |||
| Cholesterol-lowering drug | 399 | 159 | 240 | |||
| Statin only | 131 (32.8) | 53 (33.3) | 78 (32.5) | |||
| Ezetimibe only | 12 (3.0) | 3 (1.9) | 9 (2.5) | |||
| Statin + ezetimibe only | 98 (24.6) | 43 (27.0) | 55 (22.9) | |||
| Statin + others except ezetimibe | 58 (14.5) | 21 (13.2) | 37 (15.4) | |||
| Ezetimibe + others except statin | 9 (2.3) | 3 (1.9) | 6 (2.5) | |||
| Statin +ezetimibe + others | 62 (15.5) | 28 (17.6) | 34 (14.2) | |||
| Other drugs | 12 (3.0) | 0 (0.0) | 12 (5.0) | |||
| Apheresis only | 2 (0.5) | 2 (1.3) | 0 (0.0) | |||
| Apheresis + drug | 10 (2.5) | 5 (3.1) | 5 (2.1) | |||
| Unknown | 5 (1.3) | 1 (0.6) | 4 (1.7) | |||
*Numbers of the subjects were not uniform because of missing information.
The distribution of Lp(a) was highly skewed to the right side ( Fig.1A ) ; the median was 20.8 mg/dL, and the first and third quartiles were 11.3 and 38 mg/dL, respectively. The 90th and 95th percentiles were 73.3 mg/dL and 100 mg/dL, respectively. The natural logarithm of Lp(a) showed an almost normal distribution ( Fig.1B ) , with neither substantial skewness (P=0.27) nor kurtosis (P=0.22).
Fig.1.
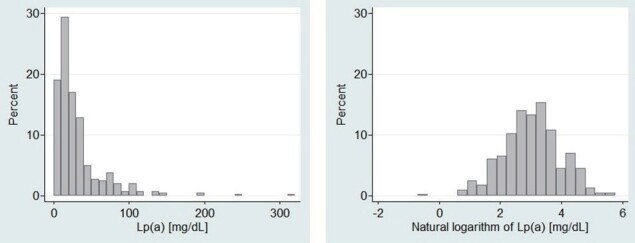
Histograms of Lp(a) concentrations (1A) and natural logarithm of Lp(a) (1B) at baseline in heterozygous FH patients under medical treatment
A preliminary analysis showed that age and family history of CAD were significantly related to serum Lp(a) levels, while sex, body mass index, and smoking were unrelated to serum Lp(a) levels ( Supplementary Table 1 ) . Therefore, age and family history of CAD were taken into account in the subsequent analyses.
Supplementary Table 1. Multiple regression analysis on serum Lp(a) levels (natural logarithm) in relation to sex, age, body mass index, smoking habit, and family history of coronary artery disease (CAD) at baseline (n = 375)* .
| Variable | Comparison | Regression coefficient | Standard error | P-value | Standardized regression coefficient |
|---|---|---|---|---|---|
| Sex | Female (referent) | ||||
| Male | −0.007 | 0.112 | 0.95 | −0.004 | |
| Age | Per 10 years | 0.110 | 0.032 | <10-3 | 0.181 |
| Body mass index | Per kg/m2 | −0.018 | 0.014 | 0.20 | −0.068 |
| Smoking | Never (referent) | ||||
| Past smoking | 0.090 | 0.131 | 0.49 | 0.039 | |
| Current smoking | 0.233 | 0.181 | 0.20 | 0.067 | |
| Family history of CAD | None (referent) | ||||
| Present | 0.263 | 0.115 | 0.02 | 0.140 | |
| Unknown | 0.164 | 0.133 | 0.22 | 0.076 |
*All the listed variables were included as explanatory variables in the multiple regression analysis. Patients with missing information on body mass index (n = 15) and smoking (n = 9) were excluded. Patients with information missing on family history of CAD were included in the “unknown” category. Two indicator variables were created for categorical variables of three categories, and regression coefficients for the indicator variables represent differences in Lp(a) levels (natural logarithm) in comparison with the referent category.
Lp(a) in Relation to other Lipid Profiles
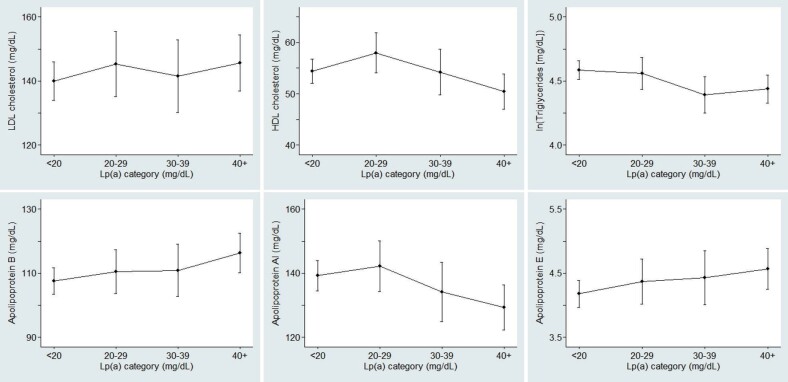
Age-adjusted mean differences (95% confidence intervals) of lipid and clinical parameters according to serum Lp(a) categories at baseline are shown in Table 2 . Additional adjustment for family history of CAD did not appreciably change the relationships of serum Lp(a) with the other lipid parameters ( Supplementary Table 2 ) . While LDL-C did not measurably vary with Lp(a) category, serum Apo B and Apo E levels were positively associated with Lp(a) levels. Apo AI levels were 10 mg/dL lower in the highest category, and high-density lipoprotein (HDL) cholesterol (HDL-C) also showed a similar pattern although the decrease was not statistically significant. TG levels were significantly lower in the highest two categories as compared with the referent category (<20 mg/dL), but the decreases in the two categories were of almost the same magnitude ( Fig.2 ) . Neither carotid intima-media thickness nor Achilles tendon thickness showed a notable variation according to Lp(a) categories.
Table 2. Age-adjusted mean differences (95% confidence intervals) of lipid and clinical parameters according to serum Lp(a) categories at baseline* .
| Parameter | N† | Lp(a) category (mg/dL) | Trend P | |||
|---|---|---|---|---|---|---|
| <20 (n = 192) | 20–29 (n = 67) | 30–39 (n = 52) | 40+ (n = 88) | |||
| LDL cholesterol (mg/dL) | 391 | 0.00 (referent) | 5.31 (−6.45; 17.1) | 1.48 (−11.4; 14.4) | 5.69 (−5.02; 16.4) | 0.34 |
| HDL cholesterol (mg/dL) | 399 | 0.00 (referent) | 3.57 (−0.99; 8.13) | −0.23 (−5.28; 4.82) | −4.02 (−8.20; 0.16) | 0.08 |
| ln (Triglycerides [mg/dL]) | 399 | 0.00 (referent) | −0.03 (−0.17; 0.12) | −0.19 (−0.35; −0.04) | −0.15 (−0.28; −0.02) | 0.008 |
| Apolipoprotein AI (mg/dL) | 352 | 0.00 (referent) | 2.88 (−6.33; 12.1) | −5.08 (−15.5; 5.32) | −9.99 (−18.5; −1.49) | 0.02 |
| Apolipoprotein B (mg/dL) | 353 | 0.00 (referent) | 2.99 (−5.02; 11.0) | 3.40 (−5.70; 12.5) | 8.77 (1.33; 16.2) | 0.02 |
| Apolipoprotein E(mg/dL) | 343 | 0.00 (referent) | 0.19 (−0.22; 0.60) | 0.25 (−0.22; 0.72) | 0.39 (0.01; 0.77) | 0.04 |
| ln (RLPC [mg/dL]) | 312 | 0.00 (referent) | −0.02 (−0.22; 0.18) | −0.06 (−0.30; 0.18) | −0.09 (−0.28; 0.09) | 0.31 |
| Rf | 149 | 0.00 (referent) | −0.02 (−0.04; −0.01) | −0.01 (--0.03; 0.02) | 0.01 (−0.01; 0.02) | 0.61 |
| Maximal IMT (mm) | 359 | 0.00 (referent) | 0.10 (−0.11; 0.31) | −0.08 (−0.31; 0.15) | 0.12 (−0.07; 0.31) | 0.39 |
| Mean IMT (mm) | 324 | 0.00 (referent) | −0.00 (−0.09; 0.09) | 0.00 (−0.10; 0.10) | 0.04 (−0.04; 0.12) | 0.36 |
| Achilles tendon thickness (mm) | 347 | 0.00 (referent) | 0.81 (−0.42: 2.04) | −0.53 (−1.91; 0.85) | 0.58 (−0.52; 1.69) | 0.51 |
*Based on multiple regression analyses including age and indicator variables for Lp(a) category in the model.
†Numbers of patients were not uniform due to missing information.
Supplementary Table 2. Adjusted mean differences (95% confidence intervals) of lipid and clinical parameters according to serum Lp(a) categories at baseline* .
| Parameter | N† | Lp(a) category (mg/dL) | Trend P | |||
|---|---|---|---|---|---|---|
| <20 (n = 192) | 20–29 (n = 67) | 30–39 (n = 52) | 40+ (n = 88) | |||
| LDL cholesterol (mg/dL) | 391 | 0.00 (referent) | 5.59 (−6.19;17.4) | 1.39 (−11.5; 14.3) | 4.70 (−6.12; 15.5) | 0.43 |
| HDL cholesterol (mg/dL) | 399 | 0.00 (referent) | 3.62 (−0.95; 8.19) | −0.24 (−5.30; 4.83) | −4.24 (−8.46; −0.01) | 0.07 |
| ln (Triglycerides [mg/dL]) | 399 | 0.00 (referent) | −0.03 (−0.17; 0.12) | −0.19 (−0.35; −0.03) | −0.14 (−0.28; −0.01) | 0.01 |
| Apolipoprotein AI (mg/dL) | 352 | 0.00 (referent) | 2.92 (−6.31; 12.2) | −4.88 (−15.3; 5.56) | −9.81 (−18.5; −1.15) | 0.02 |
| Apolipoprotein B (mg/dL) | 353 | 0.00 (referent) | 3.19 (−4.81; 11.2) | 3.54 (−5.57; 12.6) | 7.92 (0.36; 15.5) | 0.04 |
| Apolipoprotein E(mg/dL) | 343 | 0.00 (referent) | 0.20 (−0.22; 0.61) | 0.25 (−0.22; 0.73) | 0.36 (−0.03; 0.75) | 0.06 |
| ln (RLPC [mg/dL]) | 312 | 0.00 (referent) | −0.01 (−0.21; 0.19) | −0.04 (−0.28; 0.20) | −0.09 (−0.28; 0.09) | 0.33 |
| Rf | 149 | 0.00 (referent) | −0.02 (−0.04; −0.00) | −0.00 (--0.03; 0.02) | 0.01 (−0.01; 0.03) | 0.50 |
| Maximal IMT (mm) | 359 | 0.00 (referent) | 0.10 (−0.11; 0.31) | −0.08 (−0.31; 0.15) | 0.10 (−0.10; 0.30) | 0.51 |
| Mean IMT (mm) | 324 | 0.00 (referent) | 0.00 (−0.09; 0.09) | 0.00 (−0.10; 0.10) | 0.03 (−0.05; 0.11) | 0.47 |
| Achilles tendon thickness (mm) | 347 | 0.00 (referent) | 0.83 (−0.39: 2.06) | −0.65 (−2.03; 0.73) | 0.45 (−0.66; 1.56) | 0.70 |
*Based on multiple regression analyses including age, familial coronary artery disease, and indicator variables for Lp(a) category in the model.
†Numbers of patients were not uniform due to missing information.
Fig.2. Age-adjusted mean concentrations of LDL cholesterol (upper left), HDL cholesterol (upper middle), triglycerides (upper right), apolipoprotein B (lower left), apolipoprotein AI (lower middle), and apolipoprotein E (lower right).
The values are expressed according to Lp(a) categories at baseline in heterozygous FH patients under medical treatment.
Lp(a) and Prevalent CAD
The age-adjusted prevalence odds of CAD and atherosclerotic disease were relatively high in the highest category (40+ mg/dL) of Lp(a), but the elevation did not achieve statistical significance with both sexes combined ( Table 3 ) . Further adjustment for family history of CAD attenuated the elevation in odds ratio in the highest category. In the sex-specific analysis ( Supplementary Table 3 ) , the prevalence odds of atherosclerotic disease were positively related to Lp(a) levels in males, but not females, although the odds ratios did not progressively increase with increasing levels of Lp(a). The increasing trend was statistically significant in the age-adjusted analysis (P=0.02), but not in the analysis adjusting for age and family history of CAD (P=0.08). A similar pattern was also observed for CAD in males although the trend was not statistically significant in either analysis. However, the interaction between sex and Lp(a) category (ordinary variable) was not statistically significant for either atherosclerotic disease (interaction P=0.11) or CAD (interaction P=0.25) even in the analysis controlling for age alone. It has been suggested that a cutoff of 50 mg/dL be chosen with respect to the risk of CAD 10 , 11) . There was no further increase in the odds ratio of CAD in the category of 50+ mg/dL; however, the age-adjusted odds ratios of prevalent CAD were 1.73 (95% CI 0.62–4.80) for 40–49 mg/dL and 1.31 (95% CI 0.68–2.52) for 50+ mg/dL as compared with the referent category of <20 mg/dL.
Table 3. Adjusted odds ratios (OR) and 95% confidence intervals (CI) of atherosclerotic disease, coronary artery disease, hypertension, and diabetes mellitus according to serum Lp(a) categories at baseline (N = 399)* .
| Disease | Lp(a) category (mg/dL) | Trend P | |||
|---|---|---|---|---|---|
| <20 (n = 192) | 20–29 (n = 67) | 30–39 (n = 52) | 40+ (n = 88) | ||
| Atherosclerotic disease | |||||
| No.† | 42/150 | 17/50 | 15/37 | 30/58 | |
| OR (95% CI)‡ | 1.00 (referent) | 1.04 (0.53; 2.06) | 1.12 (0.55; 2.31) | 1.47 (0.82; 2.63) | 0.21 |
| OR (95% CI)§ | 1.00 (referent) | 1.08 (0.54; 2.16) | 1.11 (0.53; 2.30) | 1.30 (0.71; 2.36) | 0.41 |
| Coronary artery disease | |||||
| No.† | 40/152 | 16/51 | 15/37 | 28/60 | |
| OR (95% CI)‡ | 1.00 (referent) | 1.01 (0.51; 2.03) | 1.19 (0.58; 2.47) | 1.40 (0.77; 2.54) | 0.26 |
| OR (95% CI)§ | 1.00 (referent) | 1.06 (0.52; 2.15) | 1.18 (0.57; 2.48) | 1.22 (0.66; 2.24) | 0.50 |
| Hypertension | |||||
| No.† | 53/139 | 18/49 | 19/33 | 31/57 | |
| OR (95% CI)‡ | 1.00 (referent) | 0.73 (0.36; 1.47) | 1.05 (0.51; 2.14) | 0.99 (0.54; 1.80) | 0.94 |
| OR (95% CI)§ | 1.00 (referent) | 0.74 (0.36; 1.51) | 1.04 (0.50; 2.14) | 0.87 (0.47; 1.60) | 0.76 |
| Diabetes mellitus | |||||
| No.† | 35/157 | 6/61 | 11/41 | 16/72 | |
| OR (95% CI)‡ | 1.00 (referent) | 0.32 (0.12; 0.85) | 0.87 (0.39; 1.94) | 0.72 (0.36; 1.43) | 0.44 |
| OR (95% CI)§ | 1.00 (referent) | 0.32 (0.12; 0.84) | 0.89 (0.40; 1.98) | 0.67 (0.33; 1.36) | 0.37 |
*Based on multiple logistic regression analyses including age, familial coronary artery disease (if necessary), and indicator variables for Lp(a) categories in the model.
†Number of patients with/without the specified disease.
‡Adjusted for age.
§Adjusted for age and familial coronary artery disease.
Supplementary Table 3. Sex-specific adjusted odds ratios (OR) and 95% confidence intervals (CI) of prevalent atherosclerotic disease and coronary artery disease according to serum Lp(a) categories at baseline (N = 399)* .
| Sex | Lp(a) category (mg/dL) | Trend P | |||
|---|---|---|---|---|---|
| <20 | 20–29 | 30–39 | 40+ | ||
| Atherosclerotic disease | |||||
| Males | |||||
| No.† | 23/62 | 10/13 | 10/8 | 17/16 | |
| OR (95% CI)‡ | 1.00 (referent) | 1.84 (0.67; 5.10) | 3.37 (1.09; 10.4) | 2.35 (0.97; 5.65) | 0.02 |
| OR (95% CI)§ | 1.00 (referent) | 1.79 (0.60; 5.29) | 2.96 (0.91; 9.61) | 1.93 (0.77; 4.81) | 0.08 |
| Females | |||||
| No.† | 19/88 | 7/37 | 5/29 | 13/42 | |
| OR (95% CI)‡ | 1.00 (referent) | 0.68 (0.24; 1.92) | 0.52 (0.17; 1.60) | 1.03 (0.44; 2.43) | 0.87 |
| OR (95% CI)§ | 1.00 (referent) | 0.76 (0.27; 2.15) | 0.53 (0.17; 1.64) | 0.97 (0.40; 2.31) | 0.75 |
| Coronary artery disease | |||||
| Males | |||||
| No.† | 22/63 | 10/13 | 10/8 | 15/18 | |
| OR (95% CI)‡ | 1.00 (referent) | 1.97 (0.71; 5.50) | 3.63 (1.16; 11.4) | 1.91 (0.79; 4.64) | 0.06 |
| OR (95% CI)§ | 1.00 (referent) | 2.00 (0.65; 6.16) | 3.32 (0.99; 11.2) | 1.50 (0.59; 3.80) | 0.21 |
| Females | |||||
| No.† | 18/89 | 6/38 | 5/29 | 13/42 | |
| OR (95% CI)‡ | 1.00 (referent) | 0.60 (0.20; 1.76) | 0.56 (0.18; 1.73) | 1.11 (0.47; 2.64) | 0.95 |
| OR (95% CI)§ | 1.00 (referent) | 0.67 (0.22; 2.00) | 0.56 (0.18; 1.77) | 1.06 (0.44; 2.55) | 0.94 |
*Based on multiple logistic regression analyses including age, familial coronary artery disease (if necessary), and indicator variables for Lp(a) categories in the model.
†Number of patients with/without the specified disease.
‡Adjusted for age.
§Adjusted for age and familial coronary artery disease.
Changes in Lp(a) during the Follow-Up
Lp(a) levels tended to be slightly lower at 2–4 years of follow-up as compared with the baseline values ( Fig.3 ) .
Fig.3. Percent changes of Lp(a) from baseline at specified months of the follow-up in heterozygous FH patients under medical treatment.
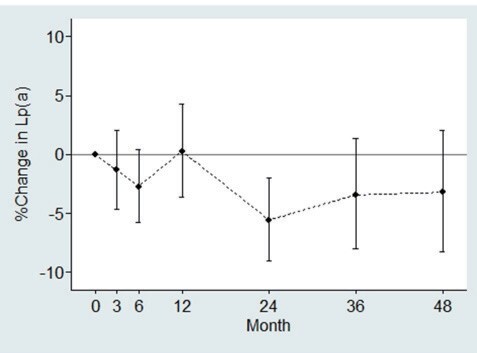
Vertical lines indicate 95% confidence intervals. Month-specific numbers of the patients were: baseline (n=399), 3 months (n=282), 6 months (n=270), 12 months (n=305), 24 months (n=295), 36 months (n=235), and 48 months (n=165).
Discussion
The main findings of this study are: 1) distribution of Lp(a) was skewed to the right in Japanese FH patients under medical treatment; 2) Apo B and Apo E levels were significantly elevated in patients with high Lp(a) values while LDL-C did not show a similar correlation with Lp(a); 3) Lp(a) was not measurably associated with prevalent CAD; and 4) serum Lp(a) levels were slightly lower at 2-4 years of follow-up than at baseline.
Serum Lp(a) Levels in Japanese FH
The distribution pattern of Lp(a) in our sample was right-skewed, as previously reported in Asian populations and Japanese FH patients 7 , 14) . The median Lp(a) in this study of 20.8 mg/dL seems to be higher than reported in previous studies on Japanese FH patients (12.1 mg/dL) and Japanese healthy individuals (12.3–14.1 mg/dL) 15 , 16) . Previous studies reported that serum Lp(a) levels were higher in FH than non-FH subjects 17 , 18) while other reports failed to corroborate such a finding 19 , 20) . To date, it has not been confirmed that LDL-receptor mutations resulting in heterozygous FH affect Lp(a).
It may be of interest whether Lp(a) levels differ in homozygotes and heterozygotes of FH. There were 7 cases of homozygous FH patients in the FAME study 8) , and only four of them had Lp(a) measurements at baseline, with a median of 27.3 mg/dL and a range of 5–128 mg/dL. There was no evidence that the distribution of Lp(a) in homozygous FH differed from that of heterozygous FH (Wilcoxson rank sum test P=0.99).
Lp(a) in Relation to other Lipid Profiles
Our study showed that Apo B and Apo E levels were positively associated with Lp(a) levels while LDL-C did not show such an association. Another interesting finding was that Apo AI was lowered in those with high Lp(a). A previous study reported positive correlations of Lp(a) with the Apo B100/Apo AI ratio, total cholesterol (TC)/HDL-C ratio, and LDL-C/HDL-C ratio 21) . Lp(a) showed significant negative correlations with HDL-C and Apo AI. Relationships between Lp(a) and other lipid profiles were not consistent among different studies. In a French cross-sectional study, a positive correlation between Lp(a) levels and Apo B100/Apo AI ratio was observed while no correlation was found between Lp(a) and Apo B100 22) . A U.S. population-based study reported that no correlation was found between Lp(a) and Apo B100 whereas they found a correlation between Lp(a) and LDL-C in men 23) . A multicenter study of U.S. diabetic patients also reported no correlation between Apo B100 and Lp(a) 24) . Further studies are needed to clarify the relationships of Lp(a) with Apo B, Apo AI, LDL-C, and HDL-C. The observed relationship between Apo E and Lp(a) in this study is in agreement with the previous report which has suggested that Apo E influences Lp(a) production, synthesis, or assembly 25) , although exact mechanisms explaining the relationship remain to be uncovered.
Theoretically, Lp(a) has a metabolism independent of LDL and Apo B100, although it is basically an LDL with Apo(a) as an extra apoprotein 26) . Since the production of Lp(a) is regulated at the genetic level, Lp(a) concentrations highly significantly correlate with its own production rate 27) , and lifestyle modifications such as diet and physical activity are unlikely to substantively affect the levels 1 , 28 , 29) .
Lp(a) and CAD
A positive association between serum Lp(a) levels and prevalent CAD was not substantiated in our study subjects under cholesterol-lowering therapy. A significant association between Lp(a) and CAD incidence was not demonstrated in the present study probably because the number of cases was small. It has been documented that Lp(a) was positively related to atherosclerotic disease including CAD 30) , via several mechanisms including the LDL component, increasing endothelial cell permeability, increased expression of adhesion molecule 31 , 32) and antifibrinolytic effects 32) . A recent meta-analysis revealed an odds ratio of 2.57 and a hazard ratio of 1.91 for the risk of cardiovascular disease (CVD) in relation to Lp(a) (high Lp(a) over low Lp(a) with varying cut-off values (30-60 mg/dL) depending on studies included) 33) . In another meta-analysis on the relationship of Lp(a) with CVD in a general population, a relative risk of 1.57 for CAD events was observed for the group with the high Lp(a) level as compared to the low Lp(a) group 34) . The fact that our study subjects were under cholesterol-lowering treatment of undefined duration should be taken into account when interpreting the nonsignificant positive relationship between Lp(a) levels and CAD prevalence in this study.
Sex-specific analyses in our study showed that the association between Lp(a) levels and CAD prevalence and atherosclerotic disease were stronger in men than in women. However, sex-specific risk differences in Lp(a)’s connection to cardiovascular disease were inconsistent among prior studies 35 - 37) . Furthermore, it has been suggested that there may be no threshold in the relationship between Lp(a) and CVD 1 , 2) . The present findings in males appear to be in agreement rather than disagreement with the lack-of-threshold hypothesis. Further studies are needed regarding this issue as well as any sex difference in the risk of CAD associated with Lp(a).
Temporal Change in Follow-Up Lp(a) Levels
Of note, serum levels of Lp(a) were slightly lower at 2–4 years of follow-up although PCSK9 inhibitors that can reduce Lp(a) 38) were not yet used during the study period. To date, evidence of Lp(a)-lowering therapy is scarce prior to the development of PCSK9 inhibitors. Statins able to lower LDL-C by upregulating LDL receptor expression increase LDL-C clearance and potentially lower Lp(a) via a hitchhiking-like process due to the structural similarities between Lp(a) and LDL. However, no clear evidence exists for Lp(a)-lowering effects of statins 39 - 42) . Niacin, which can raise HDL-C and lower TG, has been demonstrated to reduce Lp(a) through reduced apo(a) transcription or reduced Apo B secretion via inhibition of TG synthesis 43 , 44) . Previous clinical trials have reported a 20% reduction in Lp(a) by niacin 45 - 47) . As for Lp(a)-lowering effects of ezetimibe, a meta-analysis of randomized controlled trials reported that ezetimibe monotherapy demonstrated a 7.1% reduction in Lp(a) with statistical significance 48) . Although we do not have information on changes in Lp(a) in relation to individual lipid-lowering agents, statin-oriented medical therapy may contribute to a small reduction in Lp(a) in Japanese FH patients. However, caution should be exercised in interpretation because attrition in the number of patients increased year-by-year during cohort follow-up.
Limitations
There were several limitations in the present study. First, the diagnosis of FH was based on the scoring system of the Annual Report of the Research Committee on Primary Hyperlipidemia of the Ministry of Health and Welfare of Japan reported by Harada-Shiba et al. 9) . Therefore, there may be differences in FH diagnosis and clinical indices when compared to the guidelines currently in use 49) . Second, the data were collected from facilities throughout Japan, suggesting that variations may exist in the data due to different testing equipment and examiners. Especially, commercially-available immunoassays for measurement of Lp(a) were not harmonized across the participating institutions, which might have affected the results of this study. Despite a Lp(a) standardization working group supported by the WHO and an establishment of the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC), systematic comparison of six commercial immunological assays for measuring Lp(a) concentrations showed that the results of the different assays as compared to an IFCC reference, varied by 8% to 22% 50) . Unfortunately, we do not have data comparing Lp(a) levels measured by different methods, and are unable to evaluate the size of measurement errors. Third, an association between Lp(a) and CAD cannot be confirmed in this study with a cross-sectional study design. The non-significant association observed in this study may have been due to the small number of study subjects. Fourth, since our study participants were already treated with cholesterol-lowering agents, lipid profiles in the present study should not be taken at face value. Lipid treatment might have potentially affected the association between Lp(a) and CAD. Fifth, the observed temporal changes in Lp(a) might be biased because several cases whose Lp(a) values did not change might might have been disproportionally dropped from the analyses through fatal events.
Conclusion
The present study demonstrated a distribution pattern of Lp(a) in Japanese FH patients and positive relationships of Lp(a) with serum Apo B and Apo E levels. A tendency towards a positive relationship between Lp(a) and the prevalence of CAD was observed in male FH patients on cholesterol-lowering therapy. Further studies are needed to clarify Lp(a) lowering effects on atherosclerotic disease incidence including CAD in FH patients.
Acknowledgement
The authors are grateful to Dr. Suminori Kono (MedStat Corporation, Fukuoka), Professor Emeritus at Kyushu University, for his technical support in data management and statistical analysis. La Neuvelle Place (Tokyo) supported the registration of patients.
Conflict of Interest
Hidenori Arai has received honoraria from Sanofi, Daiichi-Sankyo Co., Ltd., MSD K.K., Kowa Pharmaceutical Co., Ltd., and Pfizer Co., Ltd. Hideaki Bujo has nothing to disclose. Hiroyuki Daida has received honoraria from Amgen Inc., Daiichi-Sankyo Co., Ltd., Kowa Pharmaceutical Co., Ltd., and MSD K.K., and received clinical research funding from Canon Medical Systems Corporation, Philips Japan, Ltd., Toho Holdings Co., Ltd., Asahi Kasei Corporation, and Inter Reha Co., Ltd. HD has also received scholarship grants from Nippon Boehringer Ingelheim Co., Ltd., Otsuka Pharmaceutical Company, Ltd., Sanofi K.K., MSD K.K., Daiichi-Sankyo Co., Ltd., Pfizer Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Astellas Pharma Inc., Takeda Pharmaceutical Co., Ltd., Teijin Pharma, Ltd., Shionogi & Co., Ltd., Actelion Pharmaceuticals, Ltd., Actelion Ltd., Kowa Pharmaceutical Co., Ltd., Bayer Yakuhin, Ltd. HD has also courses endowed by companies, including Philips Japan, Ltd., ResMed, Fukuda Denshi Co., Ltd., and Paramount Bed Co., Ltd. Mariko Harada-Shiba has received stock holdings or options from Liid Pharma, honoraria from Amgen Inc., Astellas Pharma Inc., Sanofi K.K., and scholarship grants from Aegerion Pharmaceuticals, Inc., Recordati Rare Diseases Japan, and Kaneka Cooperation. Shun Ishibashi has received honoraria from Kowa Pharmaceutical Co., Ltd., and a scholarship grant from Ono Pharmaceutical Co., Ltd. Nobuhiko Koga has nothing to disclose. Daisaku Masuda has received clinical research funding from MSD K.K., Takeda Pharmaceutical Co., Ltd., Daiichi-Sankyo Co., Ltd., Kowa Company, Ltd., Otsuka Pharmaceutical Co., Ltd., and scholarship grants from Skylight Biotec, Inc., Pfizer Japan Inc., Amgen Astellas Biopharma K.K., and Sanofi K.K. Masatsune Ogura has received honoraria from Amgen and Astellas Pharma Inc. Shinichi Oikawa has nothing to disclose. Shizuya Yamashita has received honoraria from Amgen Astellas BioPharma K.K., Kowa Pharmaceutical Co. Ltd., Sanofi K.K., MSD K.K., Bayer Yakuhin, Ltd., clinical research fundings from Ono Pharmaceutical Co., Ltd., Hitachi Chemical Diagnostics Systems Co., Ltd., Takeda Pharmaceutical Company Ltd, Mitsubishi Tanabe Pharma Corporation, Rohto Pharmaceutical Co., Ltd., Astellas Pharma Inc., Nippon Boehringer Ingelheim Co., Ltd., MSD K.K., Bayer Yakuhin, Ltd., scholarship grants from Astellas Pharma Inc., Nippon Boehringer Ingelheim Co., Ltd., MSD K.K., Bayer Yakuhin, Ltd., and Courses endowed by Izumisano City.
References
- 1).Nordestgaard BG, Chapman MJ, Ray K, Borén J, Andreotti F, Watts GF, Ginsberg H, Amarenco P, Catapano A, Descamps OS, Fisher E, Kovanen PT, Kuivenhoven JA, Lesnik P, Luis Masana, Reiner Z, Taskinen M-R, Tokgözoglu L, Tybjærg-Hansen A, European Atherosclerosis Society Consensus Panel. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J, 2010; 31: 2844-2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Emerging Risk Factors Collaboration; Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, White IR, Marcovina SM, Collins R, Thompson SG, Danesh J. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA, 2009; 302: 412-423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Kamstrup PR, Benn M, Tybjærg-Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and risk of myocardial infarction in the general population: the Copenhagen City Heart Study. Circulation, 2008; 117: 176-184 [DOI] [PubMed] [Google Scholar]
- 4).McGowan MP, Dehkordi SHH, Moriarty PM, Duell PB. Diagnosis and treatment of heterozygous familial hypercholesterolemia. J Am Heart Assoc, 2019; 8: e013225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Bujo H, Takahashi K, Saito Y, Maruyama T, Yamashita S, Matsuzawa Y, Ishibashi S, Shionoiri F, Yamada N, Kita T, Research Committee on Primary Hyperlipidemia of the Ministry of Health, Labour, and Welfare of Japan. Clinical features of familial hypercholesterolemia in Japan in a database from 1996−1998 by the Research Committee of the Ministry of Health, Labour and Welfare of Japan. J Atheroscler Thromb, 2004; 11: 146-151 [DOI] [PubMed] [Google Scholar]
- 6).Khera AV, Won H-H, Peloso GM, Lawson KS, Bartz TM, Deng X, van Leeuwen EM, Natarajan P, Emdin CA, Bick AG, Morrison AC, Brody JA, Gupta N, Nomura A, Kessler T, Duga S, Bis JC, van Duijn CM, Cupples LA, Psaty B, Rader DJ, Danesh J, Schunkert H, McPherson R, Farrall M, Watkins H, Lander E, Wilson JG, Correa A, Boerwinkle E, Merlini PA, Ardissino D, Saleheen D, Gabriel S, Kathiresan S. Diagnostic yield and clinical utility of sequencing familial hypercholesterolemia genes in patients with severe hypercholesterolemia. J Am Coll Cardiol, 2016; 67: 2578-2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Tada H, Kawashiri M, Yoshida T, Teramoto R, Nohara A, Konno T, Inazu A, Mabuchi H, Yamagishi M, Hayashi K. Lipoprotein(a) in familial hypercholesterolemia with proprotein convertase subtilisin/kexin type 9 (PCSK9) gain-of-function mutations. Circ J, 2016; 80: 512-518 [DOI] [PubMed] [Google Scholar]
- 8).Yamashita S, Masuda D, Harada-Shiba M, Arai H, Bujo H, Ishibashi S, Daida H, Koga N, and Oikawa S, on Behalf of the FAME Study Group. Effectiveness and Safety of Lipid-lowering Drug Treatments in Japanese Patients with Familial Hypercholesterolemia: Familial Hypercholesterolemia Expert Forum (FAME) Study. J Atheroscler Thromb, 2021; Advanced publication. doi: http://doi.org/10.5551/jat.62764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Harada-Shiba M. Evaluation of clinical management guidelines for familial hypercholesterolemia. Research Committee of Primary Hyperlipidemia supported by Grants-in-Aid for Scientific Research from the Japanese Ministry of Health, Labor and Welfare. Study Report in 2008, 2009; 50-55 (in Japanese) [Google Scholar]
- 10).Tsimikas S, Fazio S, Ferdinand KC, Ginsberg HN, Koschinsky ML, Marcovina SM, Moriarty PM, Rader DJ, Remaley AT, Reyes-Soffer G, Santos RD, Thanassoulis G, Witztum JL, Danthi S, Olive M, Liu L. NHLBI Working Group recommendations to reduce lipoprotein(a)-mediated risk of cardiovascular disease and aortic stenosis. J Am Coll Cardiol, 2018; 71: 177-192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Kinoshita M, Yokote K, Arai H, Iida M, Ishigaki Y, Ishibashi S, Umemoto S, Egusa G, Ohmura H, Okamura T, Kihara S, Koba S, Saito I, Shoji T, Daida H, Tsukamoto K, Deguchi J, Dohi S, Dobashi K, Hamaguchi H, Hara M, Hiro T, Biro S, Fujioka Y, Maruyama C, Miyamoto Y, Murakami Y, Yokode M, Yoshida H, Rakugi H, Wakatsuki A, Yamashita S, Committee for Epidemiology and Clinical Management of Atherosclerosis. Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2017. J Atheroscler Thromb, 2018; 25: 846-984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Tsimikas S. A test in context: Lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol, 2017; 69: 692-711 [DOI] [PubMed] [Google Scholar]
- 13).Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC Jr, Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation, 2019; 139: e1082-1143 [Google Scholar]
- 14).Cobbaert C, Kesteloot H. Serum lipoprotein(a) levels in racially different populations. Am J Epidemiol, 1992; 136: 441-449 [DOI] [PubMed] [Google Scholar]
- 15).Sakurabayashi I, Saito Y, Kita T, Matsuzawa Y, Goto Y. Reference intervals for serum apolipoproteins A-I, A-II, B, C-II, C-III, and E in healthy Japanese determined with a commercial immunoturbidimetric assay and effects of sex, age, smoking, drinking, and Lp(a) level. Clin Chim Acta, 2001; 312: 87-95 [DOI] [PubMed] [Google Scholar]
- 16).Aono H, Ito M, Ozawa H, Waki T, Magari Y, Bello MC, Rodoriguez AG. Lipoprotein(a) concentrations in healthy subjects in the Dominican Republic. Comparison with Japanese. Jpn Heart J, 1999; 40: 65-70 [DOI] [PubMed] [Google Scholar]
- 17).Mbewu AD, Bhatnagar D, Durrington PN, Hunt L, Ishola M, Arrol S, Mackness M, Lockley P, Miller JP. Serum lipoprotein(a) in patients heterozygous for familial hypercholesterolemia, their relatives, and unrelated control populations. Arterioscler Thromb, 1991; 11: 940-946 [DOI] [PubMed] [Google Scholar]
- 18).Friedlander Y, Leitersdorf E. Segregation analysis of plasma lipoprotein(a) levels in pedigrees with molecularly defined familial hypercholesterolemia. Genet Epidemiol, 1995; 12: 129-143 [DOI] [PubMed] [Google Scholar]
- 19).Soutar AK, McCarthy SN, Seed M, Knight BL. Relationship between apolipoprotein(a) phenotype, lipoprotein(a) concentration in plasma, and low density lipoprotein receptor function in a large kindred with familial hypercholesterolemia due to the pro6643leu mutation in the LDL receptor gene. J Clin Invest, 1991; 88: 483-492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Defesche JC, van de Ree MA, Kastelein JJ, van Diermen DE, Janssens NW, van Doormaal JJ, Hayden MR. Detection of the Pro664-Leu mutation in the low-density lipoprotein receptor and its relation to lipoprotein(a) levels in patients with familial hypercholesterolemia of Dutch ancestry from The Netherlands and Canada. Clin Genet, 1992; 42: 273-280 [PubMed] [Google Scholar]
- 21).Patel JV, Vyas A, Cruickshank JK, Prabhakaran D, Hughes E, Reddy KS, Mackness MI, Bhatnagar D, Durrington PN. Impact of migration on coronary heart disease risk factors: Comparison of Gujaratis in Britain and their contemporaries in villages of origin in India. Atherosclerosis, 2006; 185:297-306 [DOI] [PubMed] [Google Scholar]
- 22).Agoston-Coldea L, Mocan T, Gatfossé M, Dumitrascu DL. The correlation of apolipoprotein B, apolipoprotein B/apolipoprotein A-I ratio and lipoprotein(a) with myocardial infarction. Cent Eur J Med, 2008; 3: 422-429 [Google Scholar]
- 23).Kamboh M, Rewers M, Aston CE, Hamman RF. Plasma apolipoprotein A-I, apolipoprotein B, and lipoprotein(a) concentrations in normoglycemic Hispanics and non-Hispanic whites from the San Luis Valley, Colorado. Am J Epidemiol, 1997; 146: 1011-1018 [DOI] [PubMed] [Google Scholar]
- 24).Albers JJ, Marcovina SM, Imperatore G, Snively BM, Stafford J, Fujimoto WY, Mayer-Davis EJ, Petitti DB, Pihoker C, Dolan L, Dabelea DM. Prevalence and determinants of elevated apolipoprotein B and dense low-density lipoprotein in youths with type 1 and type 2 diabetes. J Clin Endocrinol Metab, 2008; 93: 735-742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Croyal M, Blanchard V, Ouguerram K, Chétiveaux M, Cabioch L, Moyon T, Billon-Crossouard S, Aguesse A, Bernardeau K, May CL, Flet L, Lambert G, Hadjadj S, Cariou B, Krempf M, Nobécourt-Dupuy E. VLDL (very-low-density lipoprotein)-apo E (apolipoprotein E) may influence Lp(a) (lipoprotein [a]) synthesis or assembly. Arterioscler Thromb Vasc Biol, 2020; 40: 819-829 [DOI] [PubMed] [Google Scholar]
- 26).Kostner GM, Wo X, Frank S, Kostner K, Zimmermann R, Steyrer E. Metabolism of Lp(a): assembly and excretion. Clin Genet, 2008; 52: 347-354 [DOI] [PubMed] [Google Scholar]
- 27).Kostner KM, Kostner GM. Factors affecting plasma lipoprotein(a) levels: role of hormones and other nongenetic factors. Semin Vasc Med, 2004; 4: 211-214 [DOI] [PubMed] [Google Scholar]
- 28).Mackinnon LT, Hubinger L, Lepre F. Effects of physical activity and diet on lipoprotein(a). Med Sci Sports Exerc, 1997; 29: 1429-1436 [DOI] [PubMed] [Google Scholar]
- 29).Tziomalos K, Athyros VG, Wierzbicki AS, Mikhailidis DP. Lipoprotein a: where are we now? Curr Opin Cardiol, 2009; 24: 351-357 [DOI] [PubMed] [Google Scholar]
- 30).Takami S, Kubo M, Yamashita S, Kameda-Takemura K, Kawasaki T, Kanbayashi J, Nakamura Y, Yokoi Y, Ohnishi K, Matsuzawa Y. High levels of serum lipoprotein(a) in patients with ischemic heart disease with normal coronary angiogram and thromboangiitis obliterans. Atherosclerosis, 1995; 112: 253-260 [DOI] [PubMed] [Google Scholar]
- 31).Takami S, Yamashita S, Kihara S, Ishigami M, Takemura K, Kume N, Kita T, Matsuzawa Y. Lipoprotein(a) enhances the expression of intercellular adhesion molecule-1 in cultured human umbilical vein endothelial cells. Circulation, 1998; 97: 721-728 [DOI] [PubMed] [Google Scholar]
- 32).Tsimikas S, Hall JL. Lipoprotein(a) as a potential causal genetic risk factor of cardiovascular disease. J Am Coll Cardiol, 2012; 60: 716-721 [DOI] [PubMed] [Google Scholar]
- 33).Watanabe J, Hamasaki M, Kotani K. Risk of cardiovascular disease with lipoprotein(a) in familial hypercholesterolemia: a review. Arch Med Sci Atheroscler Dis, 2020; 5: e148-e152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Genser B, Dias KC, Siekmeier R, Stojakovic T, Grammer T, Maerz W. Lipoprotein (a) and risk of cardiovascular disease; a systematic review and meta analysis of prospective studies. Clin Lab, 2011; 57: 143-156 [PubMed] [Google Scholar]
- 35).Ariyo AA, Thach C, Tracy R. Lp(a) Lipoprotein, vascular disease, and mortality in the elderly. N Engl J Med, 2003; 349: 2108-2115 [DOI] [PubMed] [Google Scholar]
- 36).Nguyen TT, Ellefson RD, Hodge DO, Bailey KR, Kottke TE, Abu-Lebdeh HS. Predictive value of electrophoretically detected lipoprotein(a) for coronary heart disease and cerebrovascular disease in a community-based cohort of 9936 men and women. Circulation, 1997; 96: 1390-1397 [DOI] [PubMed] [Google Scholar]
- 37).Nave AH, Lange KS, Leonards CO, Siegerink B, Doehner W, Landmesser U, Steinhagen-Thiessen E, Endres M, Ebinger M. Lipoprotein (a) as a risk factor for ischemic stroke: a meta-analysis. Atherosclerosis, 2015; 242: 496-503 [DOI] [PubMed] [Google Scholar]
- 38).Kotani K. Lipoprotein(a) in the advent of a PCSK9 world. Ann Clin Biochem, 2020; 57: 102 [DOI] [PubMed] [Google Scholar]
- 39).Arsenault BJ, Barter P, DeMicco DA, Bao W, Preston GM, LaRosa JC, Grundy SM, Deedwania P, Greten H, Wenger NK, Shepherd J, Waters DD, Kastelein JJP, Treating to New Targets (TNT) Investigators. Prediction of cardiovascular events in statin-treated stable coronary patients of the Treating to New Targets Randomized Controlled Trial by lipid and non-lipid biomarkers. PLoS ONE, 2014; 9: e114519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Khera AV, Everett BM, Caulfield MP, Hantash FM, Wohlgemuth J, Ridker PM, Mora S. Lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: an analysis from the JUPITER Trial (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin). Circulation, 2014; 129: 635-642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Fraley AE, Schwartz GG, Olsson AG, Kinlay S, Szarek M, Rifai N, Libby P, Ganz P, Witztum JL, Tsimikas S, MIRACL Study Investigators. Relationship of oxidized phospholipids and biomarkers of oxidized low-density lipoprotein with cardiovascular risk factors, inflammatory biomarkers, and effect of statin therapy in patients with acute coronary syndromes. J Am Coll Cardiol, 2009; 53: 2186-2196 [DOI] [PubMed] [Google Scholar]
- 42).Takagi H, Umemoto T. Atorvastatin decreases lipoprotein(a): a meta-analysis of randomized trials. Int J Cardiol, 2012; 154: 183-186 [DOI] [PubMed] [Google Scholar]
- 43).Chennamsetty I, Kostner KM, Claudel T, Vinod M, Frank S, Weiss TS, Trauner M, Kostner GM. Nicotinic acid inhibits hepatic APOA gene expression: studies in humans and in transgenic mice. J Lipid Res, 2012; 53: 2405-2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Kamanna VS, Kashyap ML. Mechanism of action of niacin. Am J Cardiol, 2008; 101: S20-S26 [DOI] [PubMed] [Google Scholar]
- 45).AIM-HIGH Investigators, Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med, 2011; 365: 2255-2267 [DOI] [PubMed] [Google Scholar]
- 46).HPS2-THRIVE Collaborative Group; Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, Wallendszus K, Craig M, Jiang L, Collins R, Armitage J. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med, 2014; 371: 203-212 [DOI] [PubMed] [Google Scholar]
- 47).Anderson TJ, Boden WE, Desvigne-Nickens P, Fleg JL, Kashyap ML, McBride R, Probstfield JL, AIM-HIGH Investigators. Safety profile of extended-release niacin in the AIM-HIGH Trial. N Engl J Med, 2014; 371: 288-290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48).Awad K, Mikhailidis DP, Katsiki N, Muntner P, Banach M, Lipid and Blood Pressure Meta-Analysis Collaboration (LBPMC) Group. Effect of ezetimibe monotherapy on plasma lipoprotein(a) concentrations in patients with primary hypercholesterolemia: a systematic review and meta-analysis of randomized controlled trials. Drugs, 2018; 78: 453-462 [Google Scholar]
- 49).Harada-Shiba M, Arai H, Ishigaki Y, Ishibashi S, Okamura T, Ogura M, Dobashi K, Nohara A, Bujo H, Miyauchi K, Yamashita S, Yokote K, Working Group by Japan Atherosclerosis Society for Making Guidance of Familial Hypercholesterolemia. Guidelines for diagnosis and treatment of familial hypercholesterolemia 2017. J Atheroscler Thromb, 2018; 25: 751-770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50).Scharnagla H, Stojakovica T, Dieplingerb B, Dieplingerc H, Erhartc G, Kostnerd GM, Herrmanna M, Märza W, Grammerga T. Comparison of lipoprotein (a) serum concentrations measured by six commercially available immunoassays. Atherosclerosis, 2019; 289: 206-213 [DOI] [PubMed] [Google Scholar]