Abstract
Aim: We used a dataset from a cross-sectional survey conducted in China to determine which of the anthropometric indices of obesity are important in terms of carotid atherosclerosis free of cardiovascular and cerebrovascular diseases.
Methods: A total of 5,245 participants who were volunteering for carotid ultrasound unit in this cross-sectional survey were included in the present analysis. All subjects were free of angina, myocardial infarction, heart failure and stroke, and cancer. A low-risk subgroup was defined as people free of hypertension, diabetes, and hyperlipidemia. All analyses based on logistic regression were gender-specific.
Results: The present study consisted of 2,501 males and 2,744 females, with 776 (31.03%) diagnosed as carotid artery plaque in males and 550 (20.04%) in females. Univariable analyses in unadjusted logistic model showed significant associations between disease presence and all central obesity indices. After adjusting for more variables, only a body shape index (ABSI) was associated with the presence of disease in both males and females. Moreover, stepwise regression approaches revealed that ABSI was always an independent determinant of the presence of subclinical carotid plaque. Multiple regression shows a linear and significant increase in the prevalence of atherosclerosis in males and females as ABSI decile levels increased. Similar results were obtained when the association between ABSI and carotid plaque was studied in this low-risk subgroup.
Conclusions: ABSI, as a novel anthropometric indicator compared with traditional indices, was found to have a closer relationship with subclinical carotid atherosclerosis, even in populations free of hypertension, diabetes, and hyperlipidemia.
Keywords: A body shape index, Obesity indices, Subclinical carotid atherosclerosis, Cross-sectional study
See editorial vol. 29: 1136-1137
Introduction
Atherosclerosis is a chronic progressive vascular disease, which is a dominant cause of cardiovascular and cerebrovascular diseases (CCVds) such as coronary heart disease and cerebral infarction 1 , 2) . The process of atherosclerosis begins in adolescence. There is no typical clinical manifestation for a long time before the occurrence of CCVds; that is, it is in the risk stage of subclinical atherosclerosis. When the plaque develops and evolves into high-risk plaque phenotype, it may lead to cerebrovascular events 2) . Cerebral atherosclerosis and carotid atherosclerosis are serious stages of atherosclerosis development and are important risk factors of ischemic stroke. Stroke with cerebral and carotid artery stenosis is the main type of severe disability and death 3) . A multicenter prospective study published in 2014 4) pointed out that the prevalence of intracranial atherosclerosis in patients with acute cerebral ischemia within 7 days after symptoms was 46.6%. At the same time, compared with patients without intracranial stenosis, stroke patients with intracranial stenosis had more severe clinical manifestations, more hospitalization days, and more recurrence. Therefore, it is critically important to identify and control atherosclerosis earlier. In particular, patients with subclinical carotid atherosclerosis should be found as soon as possible.
Obesity is a chronic metabolic disease, which is widely believed to be independently associated with cardiovascular and cerebrovascular risk. Body mass index (BMI) has been widely used in the evaluation of obesity. However, there is no consensus on the relationship between BMI and the prevalence and prognosis of CCVds 5 , 6) . BMI is not an appropriate indicator to assess fat distribution and diagnose visceral obesity; that is, it is unable to distinguish central obesity from systemic obesity. It is worth noting that central obesity seems more important to predict the risk of CCVds and deaths 7) . Waist circumference (WC, cm) is regarded as a simple physical measure to assess central obesity, but it does not take into account differences in height and overall body shape. Waist-to-hip ratio (WHR) and waist-to-height ratio (WHtR) have also been associated with increased arterial stiffness, but the relationship is not always significant 8 - 10) . A body shape index (ABSI) and the body roundness index (BRI) are newly developed anthropometric indices. Krakauer et al. 11) proposed ABSI in 2012, which combines height, BMI, and WC, and Thomas et al. 12) developed the BRI in 2013, which combines height and WC. Several studies 13 - 15) have raised the potential of these two indices to identify metabolic syndrome and cardiovascular health status; meanwhile, other studies 16 - 18) have looked at their association with atherosclerosis and subclinical vascular damage. However, these relationships have not been consistently recognized.
Aim
In this study, we aimed to explore the associations between obesity anthropometric indices and subclinical carotid atherosclerosis in rural adults in northwest China. Further, we analyzed whether this association persisted in populations at low risk of CCVds.
Methods
Study Design
This study was derived from a multi-stage sampling cross-sectional survey conducted in Jingyuan County, Ningxia Hui Autonomous Region, China, from January 2014 to April 2015 19) . All the respondents had lived in Jingyuan County for more than 5 years and were at least 18 years old. Pregnant women and patients with mental illness were excluded. Participants underwent clinical interviews, laboratory analysis, and imaging studies. The study protocol, which conforms to the ethical guidelines of Helsinki Declaration, was approved by the ethics committee of the sixth people’s hospital affiliated to Shanghai Jiao Tong University (approval no. 2014-18). All the subjects signed informed consent forms before the investigation.
This study includes residents between the ages of 18 and 80 who volunteered for carotid ultrasound unit. The main exclusion criteria in this study were known CCVds such as angina, myocardial infarction, heart failure and stroke, or cancer. At the same time, those who did not record gender, age (year), height (cm), weight (kg), WC (cm), hip circumference (HC, cm), systolic blood pressure (SBP, mmHg), diastolic blood pressure (DBP, mmHg), smoking status, and laboratory test results, such as fasting plasma glucose (FPG, mmol/L), serum total cholesterol (TC, mmol/L), serum total triglycerides (TG, mmol/L), high-density lipoprotein cholesterol (HDL-C, mmol/L), low-density lipoprotein cholesterol (LDL-C, mmol/L), and estimated glomerular filtration rate (eGFR, ml/min), were excluded.
Assessment of Physical Examination Indices and Blood Biochemical Parameters
Physical indicators were measured with participants in the fasted state, light clothing, and barefoot. Height and weight were measured to the nearest 0.1 cm and 0.1 kg, respectively. WC was measured using an inelastic tape at the level of the midpoint between the top edge of iliac crest and the bottom edge of ribs to the nearest 1 cm. HC was measured horizontally at the greatest level of protrusion of the buttocks to the nearest 1 cm. Blood pressure was measured by using a standard cuff sphygmomanometer in the same arm for three times after the subjects sat quietly for 10 min, each time with an interval of 2 min. Then, take the average of the three readings. Subjects were seated and measured twice, 30 s apart. The average of the two measurements was taken as the blood pressure. eGFR were calculated according to Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula 20) .
.
Venous blood was collected after 8 h of fasting for measurements of glucose and lipids using standard techniques. TC and TG were determined by oxidase method. HDL-C and LDL-C were measured by direct method.
Assessment of Atherosclerosis
Carotid color Doppler ultrasound was performed in all participants to assess the presence of carotid artery atherosclerotic (CAA) plaques. The presence of carotid plaques by ultrasound was assessed by cross-sectional sweep of bilateral common carotid artery, bilateral internal carotid artery extracranial segment, external carotid artery, and subclavian artery. Plaques were defined as an intravascular diffuse intima-media thickness greater than 1.5 mm, or a focal protrusion greater than 0.5 mm or 1/2 of the surrounding intima-media thickness 21) . The ultrasound examination was performed by an experienced, professional clinician and recorded and checked by another one. Finally, the two of them checked the results of the final images.
Definition of the CCVd-Free Population and the Low-Risk Subgroup
Participants in the study, who did not have angina, myocardial infarction, heart failure, or stroke, were defined as CCVd-free population. The history of CCVds was confirmed by personal disease history, medication history, and surgical history in questionnaire. Within the CCVd-free population, we also defined a subgroup with low risk of CCVds, in which individuals were free of hypertension, diabetes, and hyperlipidemia ( Fig.1 ) . Hypertension was defined as a mean SBP ≥ 140 mm Hg or a mean DBP ≥ 90 mm Hg or use of antihypertensive medication; diabetes was defined as FPG ≥ 7 mmol/L; and hyperlipidemia was defined as an elevated level of TC (>200 mg/dL), LDL-C (≥ 4.1 mmol/L), or HDL-C (<1 mmol/L).
Fig.1.
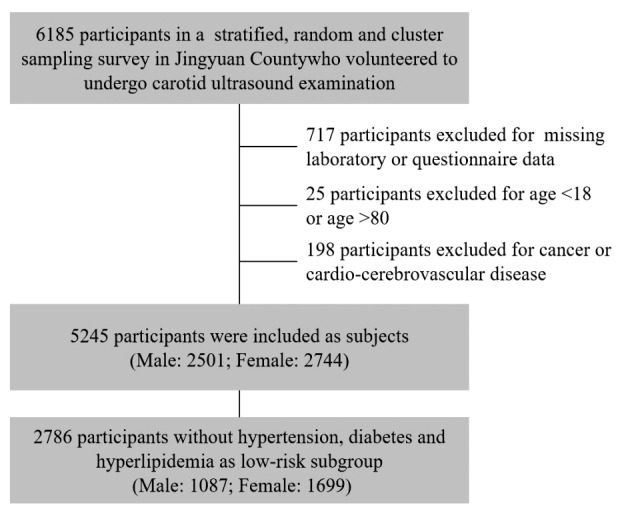
The flowchart of study population inclusion and exclusion
Statistics
The continuous variables of normal distribution or near normal distribution were described by mean and standard deviation ( ±s), and Student’s t-test was used for comparison. The classification variables were described by the number of cases and composition ratio [n (%)], and the chi-square test was used for comparison. Binary logistic regression was used to evaluate the associations between anthropometric variables and carotid atherosclerosis. Adjustments were made for age in adjusted model 1 and for age, race, smoking status, SBP, LDL-C, FBG, and eGFR in adjusted model 2. Explanatory variables in adjusted models were selected from past literature 22 - 25) , and race was also considered as an adjustment variable due to the different characteristics of Hui and Han 26 , 27) . Odds ratios (ORs) and 95% confidence intervals (CI) were calculated. The ORs examined the change in odds per unit increase in variables, except for WHR, WHtR, and ABSI, which were scaled by a factor of 10 owing to its small range. Stepwise multiple regression analysis was used to evaluate the relationship among the factors. The incidence of carotid atherosclerosis plaque in each ABSI decile level and the OR compared with the first decile level were described graphically. All analyses were gender-specific due to differences in the incidence of carotid plaque between the genders. We used R 3.5.2 (for Windows, 64 bit) for statistical analysis. α=0.05 was considered the statistical significance level.
Results
Characterization of the CCVd-Free Population and the Low-Risk Subgroup
The physical examination indices and serum biomarkers of the CCVd-free population stratified by CAA are shown in Table 1 . Our CCVd-free population consisted of 2,501 males and 2,744 females, with 776 (31.03%) diagnosed as CAA in males and 550 (20.04%) in females. In both males and females, all physical examination indices except for HC and BMI significantly differed according to atherosclerosis status. Meanwhile, significant differences were found in all serum biomarkers including blood glucose and blood lipids.
Table 1. The general demographic and clinical characteristics of the CCVd-Free population according to CAA.
| Male | Female | |||||
|---|---|---|---|---|---|---|
| CAA (N = 776) | Non-CAA (N = 1725) | p | CAA (N = 550) | Non-CAA (N = 2194) | p | |
| Age (years) | 58.23±10.60 | 44.62±11.78 | <0.001 | 58.01±9.27 | 42.51±10.91 | <0.001 |
| Race[n(%)] | <0.001 | <0.001 | ||||
| Han | 227 (29.25) | 348 (20.17) | 181 (32.91) | 450 (20.51) | ||
| Hui | 549 (70.75) | 1377 (79.83) | 369 (67.09) | 1744 (79.49) | ||
| Farmer[n(%)] | <0.001 | <0.001 | ||||
| Yes | 611 (78.74) | 1168 (67.71) | 486 (88.36) | 1542 (70.28) | ||
| No | 165 (21.26) | 557 (32.29) | 64 (11.64) | 652 (29.72) | ||
| Smoking[n(%)] | <0.001 | 0.037 | ||||
| Yes | 259 (33.38) | 731 (42.38) | 8 (1.45) | 11 (0.50) | ||
| No | 517 (66.62) | 994 (57.62) | 542 (98.55) | 2183 (99.50) | ||
| SBP (mmHg) | 126.00±16.31 | 120.48±14.71 | <0.001 | 127.90±17.08 | 116.64±16.28 | <0.001 |
| DBP (mmHg) | 82.55±12.12 | 79.87±11.03 | <0.001 | 82.76±12.12 | 77.07±11.05 | <0.001 |
| FBG (mmol/l) | 5.48±1.35 | 5.30±1.03 | 0.001 | 5.63±1.63 | 5.27±1.08 | <0.001 |
| TG (mmol/l) | 1.42±0.86 | 1.55±1.15 | 0.001 | 1.47±0.81 | 1.33±0.94 | <0.001 |
| TC (mmol/l) | 3.60±0.67 | 3.47±0.65 | <0.001 | 3.82±0.65 | 3.48±0.64 | <0.001 |
| HDL-C (mmol/l) | 1.17±0.27 | 1.13±0.26 | 0.002 | 1.33±0.32 | 1.28±0.27 | <0.001 |
| LDL-C (mmol/l) | 2.51±0.70 | 2.37±0.64 | <0.001 | 2.63±0.64 | 2.33±0.64 | <0.001 |
| eGFR (ml/min) | 80.85±10.92 | 88.21±12.02 | <0.001 | 71.99±9.95 | 81.14±11.29 | <0.001 |
| Height (cm) | 164.30±6.99 | 167.22±6.91 | <0.001 | 153.93±6.11 | 157.56±6.13 | <0.001 |
| Weight (kg) | 64.22±10.08 | 66.76±10.45 | <0.001 | 56.33±9.64 | 58.60±9.01 | <0.001 |
| WC (cm) | 81.59±9.00 | 80.50±9.19 | 0.005 | 79.97±10.03 | 76.13±9.22 | <0.001 |
| HC (cm) | 93.65±6.19 | 93.75±6.27 | 0.724 | 92.92±7.25 | 92.26±6.24 | 0.052 |
| BMI (kg/m2) | 23.74±3.05 | 23.84±3.21 | 0.447 | 23.71±3.42 | 23.59±3.26 | 0.455 |
| WHR | 0.87±0.07 | 0.86±0.06 | <0.001 | 0.86±0.07 | 0.82±0.07 | <0.001 |
| WHtR | 0.50±0.06 | 0.48±0.06 | <0.001 | 0.52±0.07 | 0.48±0.06 | <0.001 |
| ABSI | 0.77±0.07 | 0.75±0.06 | <0.001 | 0.78±0.08 | 0.74±0.07 | <0.001 |
| BRI | 3.36±1.08 | 3.09±1.03 | <0.001 | 3.83±1.36 | 3.13±1.16 | <0.001 |
CAA, carotid artery atherosclerosis; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose; TG, serum total triglycerides; TC, serum total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; WC, waist circumference; HC, hip circumference; BMI, body mass index; WHR, waist-hip ratio; WHtR, waist-to-height ratio; ABSI, a body shape index; BRI, the body roundness index.
Supplementary Table 1 shows the characteristics of the low-risk subgroup stratified by CAA. Similarly, all physical examination indices and serum biomarkers except for HC and BMI significantly differed according to atherosclerosis status in females. In males, there were no significant differences in BMI, HC, DBP, TG, and HDL-C between CAA and non-CAA populations.
Supplementary Table 1. The general demographic and clinical characteristics of the low-risk subgroup according to CAA.
| Male | Female | |||||
|---|---|---|---|---|---|---|
| CAA (N = 297) | Non-CAA (N = 790) | p | CAA (N = 242) | Non-CAA (N = 1457) | p | |
| Age (years) | 58.73±10.77 | 43.34±12.07 | <0.001 | 56.60±9.04 | 40.59±10.58 | <0.001 |
| Race[n(%)] | 0.037 | <0.001 | ||||
| Han | 82 (27.61) | 169 (21.39) | 79 (32.64) | 314 (21.55) | ||
| Hui | 215 (72.39) | 621 (78.61) | 163 (67.36) | 1143 (78.45) | ||
| Farmer[n(%)] | <0.001 | <0.001 | ||||
| Yes | 245 (82.49) | 573 (72.53) | 214 (88.43) | 1003 (68.84) | ||
| No | 52 (17.51) | 217 (27.47) | 28 (11.57) | 454 (31.16) | ||
| Smoking[n(%)] | 0.004 | 0.027 | ||||
| Yes | 97 (32.66) | 335 (42.41) | 5 (2.07) | 8 (0.55) | ||
| No | 200 (67.34) | 455 (57.59) | 237 (97.93) | 1449 (99.45) | ||
| SBP (mmHg) | 116.12±10.79 | 114.39±10.82 | 0.019 | 118.00±11.64 | 111.02±11.48 | <0.001 |
| DBP (mmHg) | 74.85±7.53 | 74.60±7.30 | 0.636 | 75.00±7.04 | 72.91±7.20 | <0.001 |
| FBG (mmol/l) | 5.27±0.55 | 5.12±0.51 | <0.001 | 5.26±0.55 | 5.12±0.49 | <0.001 |
| TG (mmol/l) | 1.18±0.63 | 1.24±0.76 | 0.19 | 1.31±0.60 | 1.13±0.60 | <0.001 |
| TC (mmol/l) | 3.55±0.59 | 3.43±0.57 | 0.005 | 3.81±0.62 | 3.41±0.57 | <0.001 |
| HDLC (mmol/l) | 1.29±0.22 | 1.26±0.21 | 0.074 | 1.39±0.28 | 1.34±0.24 | 0.014 |
| LDLC (mmol/l) | 2.40±0.59 | 2.28±0.58 | 0.002 | 2.58±0.62 | 2.25±0.58 | <0.001 |
| eGFR (ml/min) | 81.50±11.09 | 89.70±12.12 | <0.001 | 74.33±9.77 | 82.78±10.93 | <0.001 |
| Height (cm) | 163.43±6.97 | 167.05±6.83 | <0.001 | 153.82±5.94 | 157.63±6.11 | <0.001 |
| Weight (kg) | 60.70±9.30 | 63.75±9.33 | <0.001 | 54.08±8.82 | 57.09±8.42 | <0.001 |
| WC (cm) | 79.08±7.98 | 77.43±8.34 | 0.003 | 77.90±10.65 | 74.02±8.39 | <0.001 |
| HC (cm) | 91.90±5.76 | 91.82±5.76 | 0.834 | 91.98±7.77 | 91.06±5.73 | 0.081 |
| BMI (kg/m2) | 22.65±2.61 | 22.83±2.99 | 0.336 | 22.79±3.05 | 22.96±3.01 | 0.425 |
| WHR | 0.86±0.06 | 0.84±0.06 | <0.001 | 0.84±0.07 | 0.81±0.06 | <0.001 |
| WHtR | 0.48±0.05 | 0.46±0.05 | <0.001 | 0.51±0.07 | 0.47±0.06 | <0.001 |
| ABSI | 0.78±0.07 | 0.75±0.06 | <0.001 | 0.78±0.09 | 0.73±0.06 | <0.001 |
| BRI | 3.11±0.89 | 2.76±0.92 | <0.001 | 3.58±1.45 | 2.88±1.03 | <0.001 |
CAA, carotid artery atherosclerosis; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose; TG, serum total triglycerides; TC, serum total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; WC, waist circumference; HC, hip circumference; BMI, body mass index; WHR, waist-hip ratio; WHtR, waist-to-height ratio; ABSI, a body shape index; BRI, the body roundness index.
Association Between ABSI and Subclinical Carotid Atherosclerosis
As shown in Table 2 , we performed whether obesity indices that showed statistically significant differences above such as ABSI, BRI, WHR, WHtR, WC, and BMI are associated with CAA in CCVd-free population. Univariable analyses in unadjusted model showed significant associations between disease presence and all obesity indices except for BMI. In adjusted model 1, taking age into account, all central obesity indices were significantly associated with CAA in males, while only ABSI and WHR were in females. In adjusted model 2, after adjusting for more variables including age, race, smoking status, SBP, LDL-C, FBG, and eGFR, only ABSI was associated with the presence of disease in both males and females. BMI was statistically significant as a protective factor for CAA in females.
Table 2. Correlations between obesity anthropometric indices with CAA in CCVd-Free population.
| Unadjusted Model | Adjusted Model 1 | Adjusted Model 2 | ||||
|---|---|---|---|---|---|---|
| OR(95%CI) | p | OR(95%CI) | p | OR(95%CI) | p | |
| Male | ||||||
| ABSI | 1.58 (1.39, 1.81) | <0.001 | 1.31 (1.12, 1.53) | <0.001 | 1.29 (1.10, 1.51) | 0.002 |
| BRI | 1.28 (1.18, 1.39) | <0.001 | 1.14 (1.04, 1.25) | 0.006 | 1.09 (0.98, 1.20) | 0.099 |
| WHR | 1.35 (1.19, 1.54) | <0.001 | 1.33 (1.14, 1.55) | <0.001 | 1.20 (1.02, 1.41) | 0.025 |
| WHtR | 1.62 (1.39, 1.88) | <0.001 | 1.28 (1.07, 1.54) | 0.007 | 1.17 (0.97, 1.42) | 0.106 |
| WC | 1.01 (1.00, 1.02) | 0.006 | 1.01 (1.00, 1.02) | 0.028 | 1.01 (1.00, 1.02) | 0.210 |
| BMI | 0.99 (0.96, 1.02) | 0.455 | 1.00 (0.97, 1.03) | 0.837 | 0.97 (0.94, 1.01) | 0.149 |
| Female | ||||||
| ABSI | 2.23 (1.96, 2.56) | <0.001 | 1.42 (1.21, 1.66) | <0.001 | 1.45 (1.24, 1.71) | <0.001 |
| BRI | 1.53 (1.42, 1.65) | <0.001 | 1.09 (0.99, 1.19) | 0.063 | 1.10 (1.00, 1.21) | 0.053 |
| WHR | 2.16 (1.88, 2.49) | <0.001 | 1.20 (1.01, 1.42) | 0.033 | 1.17 (0.98, 1.39) | 0.081 |
| WHtR | 2.36 (2.04, 2.74) | <0.001 | 1.18 (0.98, 1.41) | 0.080 | 1.19 (0.98, 1.44) | 0.072 |
| WC | 1.04 (1.03, 1.05) | <0.001 | 1.00 (0.99, 1.02) | 0.471 | 1.00 (0.99, 1.02) | 0.461 |
| BMI | 1.01 (0.98, 1.04) | 0.442 | 0.95 (0.92, 0.99) | 0.080 | 0.95 (0.91, 0.98) | 0.003 |
Adjusted model 1: adjusted for age. Adjusted model 2: adjusted for age, race, smoking status, SBP, LDL-C, FBG and eGFR. WHR, WHtR and ABSI were scaled by a factor of 10 owing to its small range.
CAA, carotid artery atherosclerosis; CCVd, cardiovascular and cerebrovascular diseases; ABSI, a body shape index; BRI, the body roundness index; WHR, waist-hip ratio; WHtR, waist-to-height ratio; WC, waist circumference; BMI, body mass index.
Moreover, stepwise regression approaches revealed that ABSI was always an independent determinant of the presence of subclinical carotid plaque in both males and females ( Table 3 ) . Age, race, SBP, and eGFR were all associated with the presence of disease in both genders. Additional independent predictors included LDL-C in males and farmer in females.
Table 3. Multivariable logistic regression analyses for CAA in CCVd-Free population.
| Estimate | SE | Z | OR(95%CI) | p | |
|---|---|---|---|---|---|
| Male | |||||
| ABSI | 0.2557 | 0.0802 | 3.19 | 1.29 (1.10, 1.51) | 0.001 |
| Age | 0.1135 | 0.0062 | 18.27 | 1.12 (1.11, 1.13) | <0.001 |
| Race(Hui) | -0.6504 | 0.1151 | -5.65 | 0.52 (0.42, 0.65) | <0.001 |
| SBP | 0.0081 | 0.0033 | 2.48 | 1.01 (1.00, 1.01) | 0.013 |
| LDL-C | 0.3734 | 0.0778 | 4.80 | 1.45 (1.25, 1.69) | <0.001 |
| eGFR | 0.0210 | 0.0060 | 3.52 | 1.02 (1.01, 1.03) | <0.001 |
| Female | |||||
| ABSI | 0.3573 | 0.0822 | 4.35 | 1.43 (1.22, 1.68) | <0.001 |
| Age | 0.1325 | 0.0082 | 16.18 | 1.14 (1.12, 1.16) | <0.001 |
| Race(Hui) | -0.6836 | 0.1284 | -5.32 | 0.50 (0.39, 0.65) | <0.001 |
| Farmer(No) | -0.591 | 0.1651 | -3.58 | 0.55 (0.40, 0.76) | <0.001 |
| SBP | 0.0141 | 0.0035 | 4.01 | 1.01 (1.01, 1.02) | <0.001 |
| eGFR | 0.0182 | 0.0074 | 2.48 | 1.02 (1.00, 1.03) | 0.013 |
ABSI, a body shape index; SBP, systolic blood pressure; LDL-C, low-density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate. ABSI were scaled by a factor of 10 owing to its small range.
The relationship between ABSI levels and atherosclerosis in CCVd-free population was illustrated in Fig.2 . The ABSI deciles of males and females were calculated separately to generate ten levels. As ABSI levels increased, there was a linear and significant increase in the prevalence of CAA in males, ranging from 19.60% in the lowest level to 43.43% in the highest level (p for trend <0.001). This progressive increase was also noted in females, with the prevalence of CAA ranging from 6.18% in the lowest level to 38.55% in the highest level (p for trend <0.001). Multivariate analysis showed that those with higher ABSI levels had a higher risk of suffering from carotid plaque than those in the lowest level group, which were the ninth and tenth decile levels for males and the fourth, seventh, eighth, ninth, and tenth decile levels for females. Compared with the lowest decile level, the OR of the highest decile level was 1.87 (95% CI 1.17–3.00, p=0.009) for males and 2.83 (95% CI 1.52–5.50, p=0.001) for females, respectively.
Fig.2. Prevalence and adjusted odds ratios for the association of CAA with ABSI decile levels for both men and women in CCVd-free population.
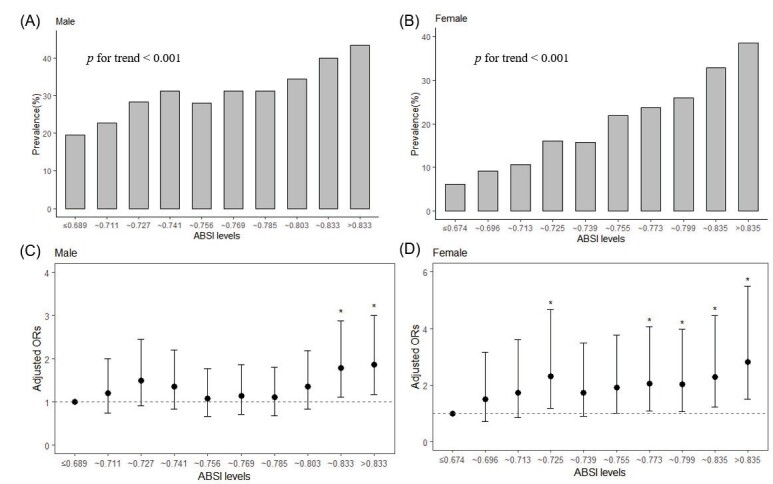
(A) As ABSI decile levels rise, there is a linear and significant increase in the prevalence of CAA in males, ranging from 19.60% in the lowest level to 43.43% in the highest decile level (p<0.001). (B) A similar pattern is observed for females, with the prevalence of CAA ranging from 6.18% in the lowest level to 38.55% in the highest level (p<0.001). (C and D) Adjusted odds ratios for the association of CAA with ABSI decile levels for males and females, respectively. Compared with the lowest decile level, the OR of the highest decile level was 1.87 (95% CI 1.17–3.00, p=0.009) for males and 2.83 (95% CI 1.52–5.50, p=0.001) for females, respectively.
*means the OR compared with the first decile level were statistical significance (p<0.05).
The lowest decile served as the reference category. Vertical bars represent 95 percent confidence intervals.
Association Between ABSI and Atherosclerosis in Low-Risk Subgroup
Individuals who were free of hypertension, diabetes, and hyperlipidemia were involved in low-risk subgroup. Similar results were obtained when the association between ABSI and carotid plaque was studied in this low-risk subgroup ( Fig.3 ) . In all models for both males and females, ABSI consistently represented a risk factor for CAA. Meanwhile, as ABSI levels increased, there was also a linear and significant increase in the prevalence of atherosclerosis (p for trend <0.001 for both males and females) that was revealed in Fig. 4 . The ORs for the highest deciles in males and females were 5.02 (95% CI 2.18–12.41, p <0.001) and 3.34 (95% CI 1.37–9.26, p=0.012), respectively. Supplementary Table 2 displayed adjusted ORs for the association of CAA with ABSI grouped by smoking and eGFR. In the non-smoking subgroup of low-risk individuals, the ORs were 1.82 (1.32~2.52) for males and 1.6 (1.27~2.01) for females. Similar results were observed in subgroups with normal renal function (eGFR ≥ 60 ml/min). What’s more, ABSI still associated with CAA in a low-risk group without smoking and kidney diseases, with ORs of 1.9 (1.37, 2.66) for males and 1.58 (1.25, 2.01) for females.
Fig.3. Correlations between ABSI and CAA in low-risk subgroup.
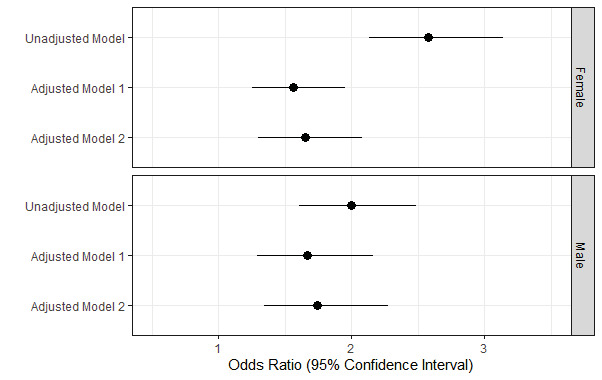
Adjusted model 1: adjusted for age. Adjusted model 2: adjusted for age, race, smoking status, SBP, LDL-C, FBG, and eGFR.
Fig.4. Prevalence and adjusted odds ratios for the association of CAA with ABSI decile levels for both men and women in low-risk subgroup.
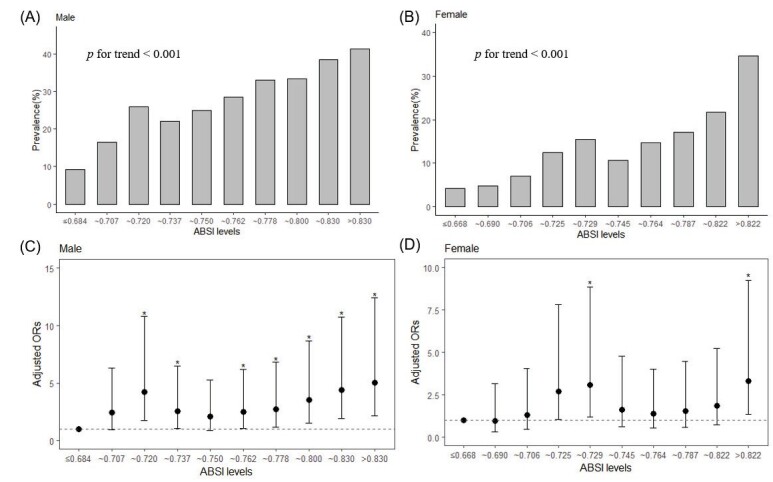
(A and B) As ABSI decile levels rise, there is a linear and significant increase in the prevalence of CAA in both males and females (p<0.001). (C and D) Adjusted odds ratios for the association of CAA with ABSI decile levels for males and females, respectively. Compared with the lowest decile level, the ORs for the highest deciles in males and females were 5.02 (95% CI 2.18–12.41, p<0.001) and 3.34 (95% CI 1.37–9.26, p=0.012), respectively.
*means the OR compared with the first decile level were statistical significance (p<0.05).
The lowest decile served as the reference category. Vertical bars represent 95 percent confidence intervals.
Supplementary Table 2. Adjusted ORs for the association of CAA with ABSI grouped by smoking and eGFR.
| Male | Female | |||
|---|---|---|---|---|
| OR(95%CI) | p | OR(95%CI) | p | |
| Smoking[n(%)] | ||||
| Yes | 1.60 (1.01, 2.57) | 0.046 | - | - |
| No | 1.82 (1.32, 2.52) | <0.001 | 1.60 (1.27, 2.01) | <0.001 |
| eGFR (ml/min) | ||||
| <60 | - | - | 2.14 (0.87, 6.03) | 0.114 |
| 60~89 | 1.78 (1.30, 2.45) | <0.001 | 1.43 (1.11, 1.84) | 0.006 |
| > 90 | 1.73 (1.02, 2.91) | 0.039 | 4.32 (2.16, 11.02) | <0.001 |
The number of subgroups is too small to fit the regression equation, which is expressed by “-”. eGFR, estimated glomerular filtration rate.
Discussion
Cerebrovascular disease has the characteristics of high morbidity, mortality, and disability, which has brought a heavy burden of public health 28) . Acute ischemic stroke is the most common type of stroke, accounting for about 70% to 80% of all strokes 29) . Approximately 20% of all strokes are attributable to thromboembolism due to atherosclerotic disease 30) . It is generally believed that people can reduce the risk of CCVds and complications by preventing and treating obesity. Guidelines recommend BMI and WC as valid indicators of obesity 31 , 32) ; however, a number of studies have indicated that they may not be accurate measures of body fatness distribution 33) . To overcome the limitations of traditional obesity indicators, previous studies have developed new indicators such as WHR, WHtR, ABSI, and BRI to measure central obesity. Results reported in this study support the role of ABSI, rather than other indicators, as an independent risk factor for the weight loss due to sarcopenia with age subclinical carotid atherosclerosis in adults, even in people with low risk of CCVds, which is defined as those without hypertension, diabetes, and hyperlipidemia.
Obesity is often seen as a risk factor for many diseases. BMI is the most commonly used parameter of generalized obesity, but not of central obesity 33) . There is no consensus on the relationship between BMI and carotid plaque 34) . Similar to the findings of atherosclerosis from Salari et al. 35) and Geraci et al. 36) , in the present study, BMI showed no statistically significant correlation with the presence of subclinical carotid atherosclerosis in unadjusted model in females and all models in males. Brodsky et al. 37) and Tang et al. 16) suggested BMI as a protective factor for atherosclerosis, and we also found this relationship after multivariate adjustment in females. A number of studies have suggested an irrelevant or negative association between BMI and cerebrovascular disease and its complications, which is reported as the “obesity paradox” 38) . Several hypotheses have been proposed to explain it, including the inability of BMI to recognize visceral fat deposition 39) , the possibility that endothelial function may remain normal in individuals with a high BMI 40) , and the weight loss due to sarcopenia with age 41) .
Previous studies have indicated that abdominal obesity seems more strongly associated with carotid atherosclerosis than general obesity 8 , 42 , 43) . As a traditional anthropometric parameter of central obesity, WC has been suggested as an alternative to BMI 32) . However, our study found that WC could not be an appropriate indicator to predict subclinical carotid atherosclerosis. WC does not take into account that the distribution of abdominal fat is inconsistent among people of the same abdominal circumference and different heights. Moreover, WHR and WHtR, the commonly used parameters of central obesity based on WC, are suitable for diagnosing central obesity and have better predictive value for obesity-related disease than WC 44) . Our findings support that WHR was weakly significantly associated with subclinical carotid atherosclerosis only in the men. In the women, WHR was negatively associated with subclinical carotid atherosclerosis. WHtR was not statistically significant for both men and women.
Thomas et al. 12) developed the BRI combining height and WC. BRI was claimed to be a better predictor of visceral fat than BMI and WC; however, the findings of studies about the relationship between BRI and atherosclerosis are not consistent. Li G et al. 18) found that BRI have a close relationship with arterial stiffness in overweight and obesity people. In other studies, BRI was not or only had a weak association with atherosclerosis or arterial stiffness after adjusting for covariates 36 , 45) . In this study, BRI did not show a better predictive power than traditional indicators in predicting subclinical carotid atherosclerosis.
ABSI, a novel proposed obesity indicator, was also positively associated with visceral fat 11) . The findings of previous studies suggested that ABSI appears to reflect visceral adiposity independently of BMI and significantly correlate with arterial stiffness in adults 45 , 46) and subjects with type 2 diabetes 47) . The arterial stiffness of these studies was mostly represented by the mean ba-PWV. Moreover, Geraci G et al. 36) and Nimkuntod P et al. 48) have concentrated on carotid atherosclerosis and presented that ABSI was closely associated with carotid plaques. In these two studies, the relationship between ABSI and carotid plaques was explored in perimenopausal and menopausal women and in patients with arterial hypertension, respectively. Therefore, we attempted to elucidate the relationship between ABSI and subclinical carotid plaques in a larger adult population. At the present study, ABSI was independently associated with CAA in both males and females of adult population without CCVds. Furthermore, it is worth noting that ABSI also showed an independent association with CAA in males and females in a low-risk subgroup who was free of hypertension, diabetes, and hyperlipidemia of CCVd-free population. These results suggested that ABSI may be convenient and useful to apply as simple assessment indicator for CAA risk in adult population without CCVds. It is believed that ABSI can identify visceral fat and thus predict the occurrence and development of CAA and other CCVds 11) . Recent studies 49 , 50) indicated that ABSI may represent not only be a marker of visceral obesity but also an index of muscle mass. Cho et al. 51) expressed that ABSI is a useful clinical approach to identify sarcopenia. The accumulation of fat in the abdomen can stimulate muscle protein breakdown and inhibit protein synthesis through an inflammatory response 52) . Aging and a sedentary lifestyle may lead to loss of muscle mass and, at the same time, promote abdominal fat deposition 53) . The interaction of these two mechanisms may represent a vicious cycle leading to the occurrence and adverse outcomes of cardiovascular and cerebrovascular diseases 50) .
It is known that age, SBP, and eGFR are important risk factors for atherosclerosis 22 - 24) , which we confirmed in stepwise multivariate regressions in both males and females. LDL-C is also commonly regarded as an independent risk factor for CAA 25) , and we demonstrated such association in multivariate regression in males. LDL-C showed a “scissor-shaped” pattern between genders, rising with age in females but remaining stable or even declining in males 54) . Although attempts have been made to explain sex differences by studying the role of sex hormones in metabolism and inflammation, studies that directly compare the mechanisms and factors of plaque between genders are limited and require stronger explanations 55) .
In our study, Hui nationality was protectively associated with CAA. Previous studies have also shown significant differences in the prevalence of stroke and atherosclerosis between Hui and Han populations 26) . The influence of race on atherosclerosis may be caused by the difference of pathogenic genes, living habits, and the composition of chronic diseases between Hui and Han 26 , 27) . Moreover, farmer was founded as a risk factor for CAA in females in the present study. Wang X et al. 56) also reported that prevalence of CAA was higher among participants living in rural areas than in urban areas in China. This may be due to the lower education level and poor eating habits of rural residents 56 , 57) . M Rosvall et al. 58) noted that women with low education levels and low occupational status had a higher risk of carotid artery stenosis. Although possible mechanisms have been reported, more precisely designed studies are needed in the future.
This work was the first study to examine the relationship between obesity anthropometric indices and CAA in a predominantly ethnic minority population in western China. Jingyuan County in Ningxia is a region with a majority of ethnic minorities and a unique dietary structure and lifestyle. The prevalence of diabetes in this region is lower than the average level in China, but the prevalence of obesity, hypertension, and hypertriglyceridemia are higher 19) . Our results demonstrate a high prevalence of subclinical carotid plaque in both males and females. These indicate a potential burden of stroke among the population in this region. Therefore, our work may help in understanding the association between central obesity indices, especially ABSI, and subclinical carotid atherosclerosis and aid in more effective community and clinical management.
This study has some limitations. First, this study cannot establish a cause-effect relationship due to its cross-sectional design. Second, our study is based on participants who lived in a predominantly ethnic minority county in western China. Caution is needed when applying the results of our study to other regions and ethnics.
Conclusion
In summary, ABSI, as a novel anthropometric indicator compared with traditional indices, was found to have a closer relationship with subclinical carotid plaque in rural adults in northwest China, even in populations free of hypertension, diabetes, and hyperlipidemia. In addition, the role of this association in prospective plaque development and unstable plaque subtypes deserves further investigation.
Acknowledgements and Notice of Grant Support
We kindly thank all the staff contributed to Jingyuan cross-sectional survey.
COI
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author Contributions
Xiaotian Ma performed statistical analysis, and wrote the manuscript. Lihong Chen contributed to standardised the database and interpreted the data. Wenchao Hu interpreted the data and edited the manuscript. Lanjie He contributed to the conception of the study and reviewed the manuscript.
References
- 1).Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, Alla F, Alvis-Guzman N, Amrock S, Ansari H, Ärnlöv J, Asayesh H, Atey TM, Avila-Burgos L, Awasthi A, Banerjee A, Barac A, Bärnighausen T, Barregard L, Bedi N, Belay Ketema E, Bennett D, Berhe G, Bhutta Z, Bitew S, Carapetis J, Carrero JJ, Malta DC, Castañeda-Orjuela CA, Castillo-Rivas J, Catalá-López F, Choi JY, Christensen H, Cirillo M, Cooper L Jr, Criqui M, Cundiff D, Damasceno A, Dandona L, Dandona R, Davletov K, Dharmaratne S, Dorairaj P, Dubey M, Ehrenkranz R, El Sayed Zaki M, Faraon E, Esteghamati A, Farid T, Farvid M, Feigin V, Ding EL, Fowkes G, Gebrehiwot T, Gillum R, Gold A, Gona P, Gupta R, Habtewold TD, Hafezi-Nejad N, Hailu T, Hailu GB, Hankey G, Hassen HY, Abate KH, Havmoeller R, Hay SI, Horino M, Hotez PJ, Jacobsen K, James S, Javanbakht M, Jeemon P, John D, Jonas J, Kalkonde Y, Karimkhani C, Kasaeian A, Khader Y, Khan A, Khang YH, Khera S, Khoja AT, Khubchandani J, Kim D, Kolte D, Kosen S, Krohn KJ, Kumar GA, Kwan GF, Lal DK, Larsson A, Linn S, Lopez A, Lotufo PA, El Razek H, Malekzadeh R, Mazidi M, Meier T, Meles KG, Mensah G, Meretoja A, Mezgebe H, Miller T, Mirrakhimov E, Mohammed S, Moran AE, Musa KI, Narula J, Neal B, Ngalesoni F, Nguyen G, Obermeyer CM, Owolabi M, Patton G, Pedro J, Qato D, Qorbani M, Rahimi K, Rai RK, Rawaf S, Ribeiro A, Safiri S, Salomon JA, Santos I, Santric Milicevic M, Sartorius B, Schutte A, Sepanlou S, Shaikh MA, Shin MJ, Shishehbor M, Shore H, Silva D, Sobngwi E, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadele Atnafu N, Tesfay F, Thakur JS, Thrift A, Topor-Madry R, Truelsen T, Tyrovolas S, Ukwaja KN, Uthman O, Vasankari T, Vlassov V, Vollset SE, Wakayo T, Watkins D, Weintraub R, Werdecker A, Westerman R, Wiysonge CS, Wolfe C, Workicho A, Xu G, Yano Y, Yip P, Yonemoto N, Younis M, Yu C, Vos T, Naghavi M, Murray C. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J Am Coll Cardiol, 2017; 70: 1-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Rothwell PM. Atherothrombosis and ischaemic stroke. BMJ, 2007; 334: 379-380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Cerebrovascular Division, Neurology Society of Chinese Medical Association. Consensus on diagnosis and treatment of head and neck atherosclerosis in China. Chin J Neurol, 2017; 572-578 [Google Scholar]
- 4).Wang Y, Zhao X, Liu L, Soo YO, Pu Y, Pan Y, Wang Y, Zou X, Leung TW, Cai Y, Bai Q, Wu Y, Wang C, Pan X, Luo B, Wong KS, CICAS Study Group. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke, 2014; 45: 663-669 [DOI] [PubMed] [Google Scholar]
- 5).Wessel TR, Arant CB, Olson MB, Johnson BD, Reis SE, Sharaf BL, Shaw LJ, Handberg E, Sopko G, Kelsey SF, Pepine CJ, Merz NB. Relationship of physical fitness vs body mass index with coronary artery disease and cardiovascular events in women. JAMA, 2004; 292: 1179-1187 [DOI] [PubMed] [Google Scholar]
- 6).Shen Y, Shi L, Nauman E, Katzmarzyk PT, Price-Haywood EG, Bazzano AN, Nigam S, Hu G. Association between Body Mass Index and Stroke Risk Among Patients with Type 2 Diabetes. J Clin Endocrinol Metab, 2020; 105: 96-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Sahakyan KR, Somers VK, Rodriguez-Escudero JP, Hodge DO, Carter RE, Sochor O, Coutinho T, Jensen MD, Roger VL, Singh P, Lopez-Jimenez F. Normal-Weight Central Obesity: Implications for Total and Cardiovascular Mortality. Ann Intern Med, 2015; 163: 827-835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Raele R, Lotufo PA, Bittencourt MS, de Jesus M Fonseca M, Goulart AC, Santos IS, Bensenor IM. The association of waist-to-height ratio and other anthropometric measurements with subclinical atherosclerosis: Results from the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Nutr Metab Cardiovasc Dis, 2020; 30: 1989-1998 [DOI] [PubMed] [Google Scholar]
- 9).Logan JG, Kang H, Kim S, Duprez D, Kwon Y, Jacobs DR Jr, Forbang N, Lobo JM, Sohn MW. Association of obesity with arterial stiffness: The Multi-Ethnic Study of Atherosclerosis (MESA). Vasc Med, 2020; 25: 309-318 [DOI] [PubMed] [Google Scholar]
- 10).Nabati M, Moosazadeh M, Soroosh E, Shiraj H, Gholami M, Ghaemian A. Correlation between overweightness and the extent of coronary atherosclerosis among the South Caspian population. BMC Cardiovasc Disord, 2020; 20: 257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS One, 2012; 7: e39504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Thomas DM, Bredlau C, Bosy-Westphal A, Mueller M, Shen W, Gallagher D, Maeda Y, McDougall A, Peterson CM, Ravussin E, Heymsfield SB. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity (Silver Spring), 2013; 21: 2264-2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Ramírez-Vélez R, Pérez-Sousa MÁ, Izquierdo M, Cano-Gutierrez CA, González-Jiménez E, Schmidt-RioValle J, González-Ruíz K, Correa-Rodríguez M. Validation of Surrogate Anthropometric Indices in Older Adults: What Is the Best Indicator of High Cardiometabolic Risk Factor Clustering. Nutrients, 2019; 11: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Wen WL, Wang CW, Wu DW, Chen SC, Hung CH, Kuo CH. Associations of Heavy Metals with Metabolic Syndrome and Anthropometric Indices. Nutrients, 2020; 12: 2666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Stefanescu A, Revilla L, Lopez T, Sanchez SE, Williams MA, Gelaye B. Using A Body Shape Index (ABSI) and Body Roundness Index (BRI) to predict risk of metabolic syndrome in Peruvian adults. J Int Med Res, 2020; 48: 0300060519848854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Tang J, Zhao S, Yu S, Chi C, Ji H, Xiong J, Teliewubai J, Fan X, Maimaitiaili R, Xu Y, Zhang Y. Association between hypertension-mediated organ damage and obesity defined by novel anthropometric indices in community-dwelling elderly individuals. Clin Nutr, 2021; 40: 4473-4480 [DOI] [PubMed] [Google Scholar]
- 17).Haraguchi N, Koyama T, Kuriyama N, Ozaki E, Matsui D, Watanabe I, Uehara R, Watanabe Y. Assessment of anthropometric indices other than BMI to evaluate arterial stiffness. Hypertens Res, 2019; 42: 1599-1605 [DOI] [PubMed] [Google Scholar]
- 18).Li G, Yao T, Wu XW, Cao Z, Tu YC, Ma Y, Li BN, Peng QY, Wu B, Hou J. Novel and traditional anthropometric indices for identifying arterial stiffness in overweight and obese adults. Clin Nutr, 2020; 39: 893-900 [DOI] [PubMed] [Google Scholar]
- 19).Wang T, Zhang HD, Lu QL, Xue HL, Wang FX, Ma Z, Wang JL, Li XW, Yu XF, Hou XH, Sun QY, Jia WP, He LJ. Prevalence of metabolic syndrome in adults in Jingyuan county, Ningxia. Chin J Intern Med, 2017; 56: 409-413 [DOI] [PubMed] [Google Scholar]
- 20).Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med, 2009; 150: 604-612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol, 1990; 15: 827-832 [DOI] [PubMed] [Google Scholar]
- 22).Wang JC, Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res, 2012; 111: 245-259 [DOI] [PubMed] [Google Scholar]
- 23).Whelton SP, McEvoy JW, Shaw L, Psaty BM, Lima J, Budoff M, Nasir K, Szklo M, Blumenthal RS, Blaha MJ. Association of Normal Systolic Blood Pressure Level With Cardiovascular Disease in the Absence of Risk Factors. JAMA Cardiol, 2020; 5: 1011-1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Manjunath G, Tighiouart H, Coresh J, Macleod B, Salem DN, Griffith JL, Levey AS, Sarnak MJ. Level of kidney function as a risk factor for cardiovascular outcomes in the elderly. Kidney Int, 2003; 63: 1121-1129 [DOI] [PubMed] [Google Scholar]
- 25).Fernández-Friera L, Fuster V, López-Melgar B, Oliva B, García-Ruiz JM, Mendiguren J, Bueno H, Pocock S, Ibáñez B, Fernández-Ortiz A, Sanz J. Normal LDL-Cholesterol Levels Are Associated With Subclinical Atherosclerosis in the Absence of Risk Factors. J Am Coll Cardiol, 2017; 70: 2979-2991 [DOI] [PubMed] [Google Scholar]
- 26).Liu JX, Li JS, Niu Y, Du XL, Chu GQ, Wang MY, Liu HX. A comparative study of stroke risk factors in Hui and Han communities in Ningxia. Lishizhen Medicine and Materia Medica Research, 2013; 24: 2963-2965 [Google Scholar]
- 27).Chen JK, Zhang MH, Zhou LX, Zhao L, Liu XM, Kong FQ, Wang YH, Ma WB, Jiang YD. Diagnostic Value and Variations both ABCA1 DNA Methylation and Its Expression between Hui and Han Patients with AS in Ningxia. J Ningxia Med Univ, 2015; 37: 1138-1142 [Google Scholar]
- 28).Feigin VL, Norrving B, Mensah GA. Global Burden of Stroke. Circ Res, 2017; 120: 439-448 [DOI] [PubMed] [Google Scholar]
- 29).Wang W, Jiang B, Sun H, Ru X, Sun D, Wang L, Wang L, Jiang Y, Li Y, Wang Y, Chen Z, Wu S, Zhang Y, Wang D, Wang Y, Feigin VL, NESS-China Investigators. Prevalence, Incidence, and Mortality of Stroke in China: Results from a Nationwide Population-Based Survey of 480 687 Adults. Circulation, 2017; 135: 759-771 [DOI] [PubMed] [Google Scholar]
- 30).Leys D. Atherothrombosis: a major health burden. Cerebrovasc Dis, 2001; 11 Suppl 2: 1-4 [DOI] [PubMed] [Google Scholar]
- 31).Bray GA, Heisel WE, Afshin A, Jensen MD, Dietz WH, Long M, Kushner RF, Daniels SR, Wadden TA, Tsai AG, Hu FB, Jakicic JM, Ryan DH, Wolfe BM, Inge TH. The Science of Obesity Management: An Endocrine Society Scientific Statement. Endocr Rev, 2018; 39: 79-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Guidelines for Primary Obesity Treatment (2019). Chin J Gen Pract, 2020; 19: 95-101 [Google Scholar]
- 33).Gómez-Ambrosi J, Silva C, Galofré JC, Escalada J, Santos S, Millán D, Vila N, Ibañez P, Gil MJ, Valentí V, Rotellar F, Ramírez B, Salvador J, Frühbeck G. Body mass index classification misses subjects with increased cardiometabolic risk factors related to elevated adiposity. Int J Obes (Lond), 2012; 36: 286-294 [DOI] [PubMed] [Google Scholar]
- 34).Zhang J, Fang L, Qiu L, Huang L, Zhu W, Yu Y. Comparison of the ability to identify arterial stiffness between two new anthropometric indices and classical obesity indices in Chinese adults. Atherosclerosis, 2017; 263: 263-271 [DOI] [PubMed] [Google Scholar]
- 35).Salari A, Shakiba M, Mahdavi-Roshan M, Gholipour M, Naghshbandi M, Rajabi R. The association between various indices of obesity and severity of atherosclerosis in adults in the north of Iran. Medicine (Baltimore), 2016; 95: e5670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Geraci G, Zammuto M, Gaetani R, Mattina A, D’Ignoto F, Geraci C, Noto D, Averna M, Cottone S, Mulè G. Relationship of a Body Shape Index and Body Roundness Index with carotid atherosclerosis in arterial hypertension. Nutr Metab Cardiovasc Dis, 2019; 29: 822-829 [DOI] [PubMed] [Google Scholar]
- 37).Brodsky SV, Barth RF, Mo X, Yildiz V, Allenby P, Ivanov I, Moore S, Hitchcock CL, Smith S, Sachak T, Yao K, Ball M, Rosborough K, Olson Z, Kiehl M, Muni N, Virmani R. An obesity paradox: an inverse correlation between body mass index and atherosclerosis of the aorta. Cardiovasc Pathol, 2016; 25: 515-520 [DOI] [PubMed] [Google Scholar]
- 38).Forlivesi S, Cappellari M, Bonetti B. Obesity paradox and stroke: a narrative review. Eat Weight Disord, 2021; 26: 417-423 [DOI] [PubMed] [Google Scholar]
- 39).Neeland IJ, Poirier P, Després JP. Cardiovascular and Metabolic Heterogeneity of Obesity: Clinical Challenges and Implications for Management. Circulation, 2018; 137: 1391-1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Fahs CA, Smith DL, Horn GP, Agiovlasitis S, Rossow LM, Echols G, Heffernan KS, Fernhall B. Impact of excess body weight on arterial structure, function, and blood pressure in firefighters. Am J Cardiol, 2009; 104: 1441-1445 [DOI] [PubMed] [Google Scholar]
- 41).Wannamethee SG, Atkins JL. Muscle loss and obesity: the health implications of sarcopenia and sarcopenic obesity. Proc Nutr Soc, 2015; 74: 405-412 [DOI] [PubMed] [Google Scholar]
- 42).Imahori Y, Mathiesen EB, Morgan KE, Frost C, Hughes AD, Hopstock LA, Johnsen SH, Emaus N, Leon DA. The association between anthropometric measures of adiposity and the progression of carotid atherosclerosis. BMC Cardiovasc Disord, 2020; 20: 138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Ge W, Parvez F, Wu F, Islam T, Ahmed A, Shaheen I, Sarwar G, Demmer RT, Desvarieux M, Ahsan H, Chen Y. Association between anthropometric measures of obesity and subclinical atherosclerosis in Bangladesh. Atherosclerosis, 2014; 232: 234-241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).See R, Abdullah SM, McGuire DK, Khera A, Patel MJ, Lindsey JB, Grundy SM, de Lemos JA. The association of differing measures of overweight and obesity with prevalent atherosclerosis: the Dallas Heart Study. J Am Coll Cardiol, 2007; 50: 752-759 [DOI] [PubMed] [Google Scholar]
- 45).Choi HS, Cho YH, Lee SY, Park EJ, Kim YJ, Lee JG, Yi YH, Tak YJ, Hwang HR, Lee SH. Association between new anthropometric parameters and arterial stiffness based on brachial-ankle pulse wave velocity. Diabetes Metab Syndr Obes, 2019; 12: 1727-1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Nagayama D, Watanabe Y, Yamaguchi T, Maruyama M, Saiki A, Shirai K, Tatsuno I. New index of abdominal obesity, a body shape index, is BMI-independently associated with systemic arterial stiffness in real-world Japanese population. Int J Clin Pharmacol Ther, 2020; 58: 709-717 [DOI] [PubMed] [Google Scholar]
- 47).Bouchi R, Asakawa M, Ohara N, Nakano Y, Takeuchi T, Murakami M, Sasahara Y, Numasawa M, Minami I, Izumiyama H, Hashimoto K, Yoshimoto T, Ogawa Y. Indirect measure of visceral adiposity ‘A Body Shape Index’ (ABSI) is associated with arterial stiffness in patients with type 2 diabetes. BMJ Open Diabetes Res Care, 2016; 4: e000188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48).Nimkuntod P, Tongdee P. A Body Shape Index versus Traditional Anthropometric Parameters to Identify Subclinical Atherosclerosis in Perimenopausal/Menopausal Women. J Med Assoc Thai, 2016; 99 Suppl 7: S81-86 [PubMed] [Google Scholar]
- 49).Krakauer NY, Krakauer JC. Association of Body Shape Index (ABSI) with Hand Grip Strength. Int J Environ Res Public Health, 2020; 17: 6797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50).Biolo G, Di Girolamo FG, Breglia A, Chiuc M, Baglio V, Vinci P, Toigo G, Lucchin L, Jurdana M, Pražnikar ZJ, Petelin A, Mazzucco S, Situlin R. Inverse relationship between “a body shape index” (ABSI) and fat-free mass in women and men: Insights into mechanisms of sarcopenic obesity. Clin Nutr, 2015; 34: 323-327 [DOI] [PubMed] [Google Scholar]
- 51).Cho HW, Chung W, Moon S, Ryu OH, Kim MK, Kang JG. Effect of Sarcopenia and Body Shape on Cardiovascular Disease According to Obesity Phenotypes. Diabetes Metab J, 2021; 45: 209-218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52).Zamboni M, Mazzali G, Fantin F, Rossi A, Di Francesco V. Sarcopenic obesity: a new category of obesity in the elderly. Nutr Metab Cardiovasc Dis, 2008; 18: 388-395 [DOI] [PubMed] [Google Scholar]
- 53).Olsen RH, Krogh-Madsen R, Thomsen C, Booth FW, Pedersen BK. Metabolic responses to reduced daily steps in healthy nonexercising men. JAMA, 2008; 299: 1261-1263 [DOI] [PubMed] [Google Scholar]
- 54).Wang M, Liu M, Li F, Guo C, Liu Z, Pan Y, Liu Y, Liu F, Cai H, Wu Y, He Z, Ke Y. Gender heterogeneity in dyslipidemia prevalence, trends with age and associated factors in middle age rural Chinese. Lipids Health Dis, 2020; 19: 135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55).Man JJ, Beckman JA, Jaffe IZ. Sex as a Biological Variable in Atherosclerosis. Circ Res, 2020; 126: 1297-1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).Wang X, Li W, Song F, Wang L, Fu Q, Cao S, Gan Y, Zhang W, Yue W, Yan F, Shi W, Wang X, Zhang H, Zhang H, Wang Z, Lu Z. Carotid Atherosclerosis Detected by Ultrasonography: A National Cross-Sectional Study. J Am Heart Assoc, 2018; 7: e008701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57).Yuan YQ, Li F, Dong RH, Chen JS, He GS, Li SG, Chen B. The Development of a Chinese Healthy Eating Index and Its Application in the General Population. Nutrients, 2017; 9: 977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58).Rosvall M, Ostergren PO, Hedblad B, Isacsson SO, Janzon L, Berglund G. Occupational status, educational level, and the prevalence of carotid atherosclerosis in a general population sample of middle-aged Swedish men and women: results from the Malmö Diet and Cancer Study. Am J Epidemiol, 2000; 152: 334-346 [DOI] [PubMed] [Google Scholar]


