Highlights
-
•
Alveolar bone loss is positively associated with the acquisition of COVID-19 disease.
-
•
Number of missing teeth is positively associated with severity of COVID-19 disease.
-
•
COVID-19 cases demonstrated fewer missing teeth and lack of smoking history vs controls.
-
•
Hospitalization among COVID-19 cases was positively associated with number of missing teeth.
Abbreviations: COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome virus 2; ACE2, angiotensin-converting enzyme 2; TMPRSS2, transmembrane serine protease 2; ACH, alveolar crestal height; CEJ, cemento-enamel junction; AC, alveolar crest
Keywords: COVID-19, Coronavirus, Bone loss, Periodontal disease(s)/periodontitis, Periodontal tissues/periodontium
Abstract
Objective
Studies have shown that gingival crevices may be a significant route for SARS-CoV-2 entry. However, the role of oral health in the acquisition and severity of COVID-19 is not known.
Design
A retrospective analysis was performed using electronic health record data from a large urban academic medical center between 12/1/2019 and 8/24/2020. A total of 387 COVID-19 positive cases were identified and matched 1:1 by age, sex, and race to 387 controls without COVID-19 diagnoses. Demographics, number of missing teeth and alveolar crestal height were determined from radiographs and medical/dental charts. In a subgroup of 107 cases and controls, we also examined the rate of change in alveolar crestal height. A conditional logistic regression model was utilized to assess association between alveolar crestal height and missing teeth with COVID-19 status and with hospitalization status among COVID-19 cases.
Results
Increased alveolar bone loss, OR = 4.302 (2.510 – 7.376), fewer missing teeth, OR = 0.897 (0.835–0.965) and lack of smoking history distinguished COVID-19 cases from controls. After adjusting for time between examinations, cases with COVID-19 had greater alveolar bone loss compared to controls (0.641 ± 0.613 mm vs 0.260 ± 0.631 mm, p < 0.01.) Among cases with COVID-19, increased number of missing teeth OR = 2.1871 (1.146– 4.174) was significantly associated with hospitalization.
Conclusions
Alveolar bone loss and missing teeth are positively associated with the acquisition and severity of COVID-19 disease, respectively.
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome virus 2 (SARS-CoV-2), overwhelmed New York City health care systems, filling intensive care units to capacity (Narula & Singh, 2020), and resulted in over 1.6 million infections and 35,000 deaths. SARS-CoV-2 was transmitted by aerosol (cough, sneeze, droplet inhalation) and contact methods (contact with oral, nasal, ocular mucous membranes) (Peng et al., 2020). As one of the first viral entry points into the body, the oral cavity may play a critical role at the onset of COVID-19 (Sawa et al., 2021).
SARS-CoV-2 enters cells through the human angiotensin-converting enzyme 2 (ACE2) receptor. ACE2 positive cells are abundantly present throughout the respiratory tract and in cells compatible with the salivary gland duct epithelium, tongue, buccal mucosa, and gingiva (Huang et al., 2021). Populations with higher expressions of ACE2 may be more susceptible to SARS-CoV-2 (Cao et al., 2020). Transmembrane serine protease 2 (TMPRSS2) aids viral spread through spike protein priming (Hoffmann et al., 2020). Periodontal disease was associated with altered expression of ACE2 and TMPRSS2 and the expression of TMPRSS2 increases during gingivitis induction (Jonsson et al., 2011). Therefore, improving oral hygiene may help prevent COVID-19 infection (Addy, 2020). A link between periodontitis and more severe complications of COVID-19 was observed via cross-sectional study (Gupta et al., 2021b), but commonalities between risk factors for periodontal disease and poor outcomes from COVID-19 infection complicate the potential causal link between the conditions (Basso et al., 2021).
Risk factors for severe COVID-19 include age over 65 years, male sex, and pre-existing comorbidities (hypertension, diabetes, and cardiovascular disease) (Zheng et al., 2020). The same comorbidities are also associated with imbalances in the oral microbiome and increased risk of periodontal disease (Sampson et al., 2020). When controlling for common risk factors, periodontitis was associated with a higher risk of COVID-19 complications (Shamsoddin, 2021). Periodontal disease can cause increases in both local and systemic levels of cytokines, which may potentiate the cytokine storm in COVID-19 patients (Sampson et al., 2020). Periodontal disease in combination with obesity resulted in increased odds of hospitalization for COVID-19 compared to obesity alone (Larvin et al., 2021). Therefore, improving oral health may help reduce the severity of COVID-19 (Botros et al., 2020). The existing literature presents with limitations to study design and recruitment, however biological plausibility and preliminary retrospective and cross-sectional reports indicate that periodontal disease may be directly linked to COVID-19 infection severity.
This study aims to assess the plausibility of a history of periodontal disease as a risk for COVID-19 infection, the rate of periodontal deterioration as a proximal risk for COVID-19 infection, and severity of periodontal disease as a correlative with the likelihood of COVID-19 disease requiring hospitalization. We utilized a convenience sample of age, sex and race matched COVID-19 patients and uninfected COVID-19 controls with recent dental records and COVID-19 hospitalization records from patients evaluated at Columbia Irving Medical Center between 12/1/2019 and 8/24/2020 during the first wave of COVID-19 infection in New York City.
2. Materials & methods
2.1. Study population
This study was approved by the International Review Board (IRB), reference number AAAT2272. Dental records and radiographs and medical records are all on the same electronic health records platform (EPIC) at Columbia Irving Medical Center. We identified 387 patients with dental visit records at the institution between 12/1/2019 and 8/24/2020 (n = 22,112) and an ICD-10-CM code for COVID-19 (U07.01) after their dental visit and 387 age, sex, and race matched controls without a COVID-19 diagnosis code. Among the case-control pairs, a subgroup of 50 cases and 57 controls had radiographs before and after 2/1/20—when the first case of COVID-19 was diagnosed at the institution—and were included in the subgroup analysis of change in alveolar crestal height after COVID-19 infection.
2.2. Medical and dental chart review
Medical and dental data from EPIC was manually extracted for all 774 cases and controls. Age, sex, race, ethnicity, diabetes diagnosis, hypertension diagnosis, hospital admission status due to COVID-19 and smoking status were recorded from demographics, medical history, problem list, and substance abuse chart sections in EPIC. Smoking status was coded as never, past, or current smoker. Hospital admission status was coded as hospitalized for cases with admissions attributed to COVID-19 or COVID-19 symptoms such as shortness of breath, pneumonia, or respiratory distress with a concurrent diagnosis code.
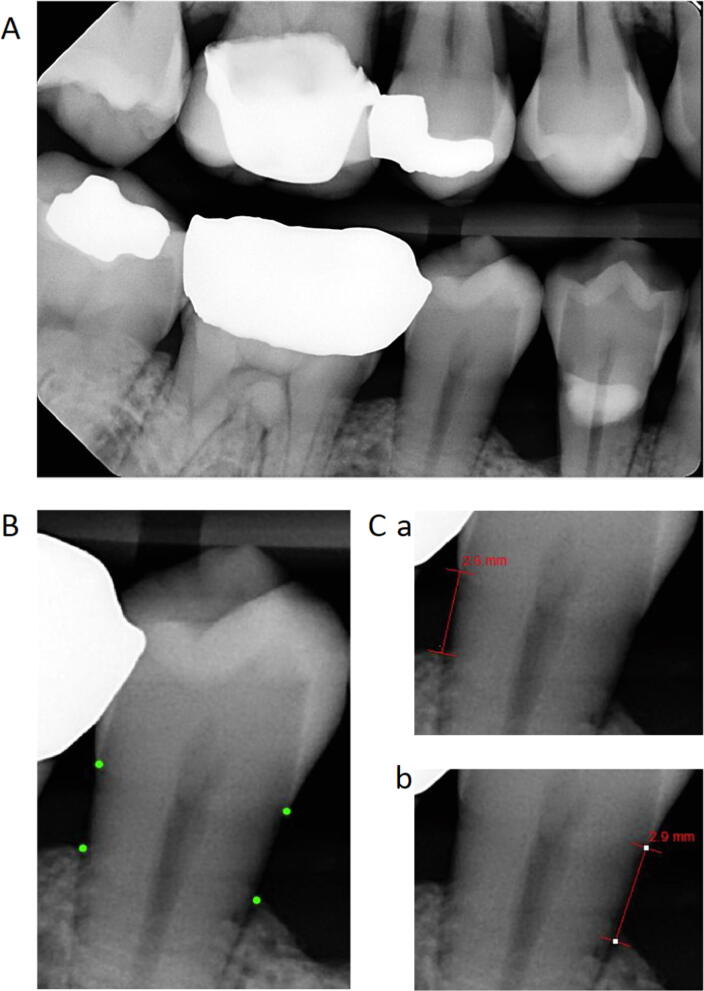
Data for number of missing teeth excluding third molars (maximum number = 28) was obtained using the most recent tooth charts in EPIC, full mouth series of dental X-rays, or panoramic dental X-rays. Alveolar crestal height (ACH) measurements for each patient with a full mouth series of radiographs were made by one of four investigators using the image analysis tools within Medcore Imaging MiPACS Dental Enterprise Viewer software (Fig. 1). ACH was measured as the distance between the cemento-enamel junction (CEJ) and the alveolar crest (AC) on the mesial and distal of teeth present on bitewing radiographs. The investigator identified the CEJ as the interface of the higher contrast enamel and the lower contrast dentin and the AC as the interface between the lower density of the bone-free region and the alveolar bone (Zaki et al., 2015). An average of all measurements was taken to determine the patient’s average alveolar crestal height (Wactawski-Wende et al., 2005). Agreement between investigators was confirmed by calculating Pearson correlation coefficient between all four investigators for a random sample of radiographs (n = 13) (Table 1).
Fig. 1.
Alveolar Crestal Height Measurements. A. Alveolar crestal height (ACH) measurements were obtained from bitewing radiographs. B. Investigators visually located the cemento-enamel junction (CEJ) and alveolar crest (AC) on the mesial and distal of teeth present on bitewings, demonstrated by the dot markings on tooth #29. C. Measurements were obtained using the image analysis tools within Medcore Imaging MiPACS Dental Enterprise Viewer software. ACH was measured as the distance between the CEJ and the AC on the distal (a) and mesial (b) of each tooth captured on bitewing. An average of all measurements was taken to determine the patient’s average alveolar crestal height.
Table 1.
Interrater reliability.
| Pearson correlation coefficient | ||||
|---|---|---|---|---|
| Researcher 1 | Researcher 2 | Researcher 3 | Researcher 4 | |
| Researcher 1 | 1 | 0.77 | 0.71 | 0.87 |
| Researcher 2 | 0.77 | 1 | 0.93 | 0.96 |
| Researcher 3 | 0.71 | 0.93 | 1 | 0.92 |
| Researcher 4 | 0.87 | 0.96 | 0.92 | 1 |
2.3. Statistical approach
Overall case-control group differences were evaluated with Chi-square or Fisher’s Exact Test for categorical variables and T-tests or Wilcoxon Sign Rank tests for continuous variables. Paired T-tests for continuous variables and conditional logistic regressions of paired case-control categorical data were performed to estimate univariable associations between risks (ethnicity, hypertension, diabetes smoking category and current smoking status) and COVID-19 case status. A missing value for race, ethnicity, hypertension, or diabetes was represented in the reference coding and analysis. Results were reported for the non-missing coding against the reference group: white, non-Hispanic, non-hypertensive and non-diabetic, respectively. Alveolar crestal height measures were available for 234 cases and 266 controls. A conditional logistic regression stepwise selection model, p-value <0.20 to enter and <0.05 to stay, assessed the above risks. The resultant model was tested forcing alveolar crestal height and number of missing teeth into the model to assess the influence of alveolar crestal height and missing teeth on risk estimates. Next, cases and controls with dental radiographs before and after 2/1/20 date were compared on change in alveolar crestal height unadjusted by T-test and adjusted for the number of years between radiographs by analysis of covariance. Lastly, we assessed risks for hospitalization in the case cohort using a similar method as our initial approach with the exception that age, sex, and race were included in the vector of risks. No missing data were imputed and no adjustment for multiple comparisons was implemented. All data management and statistical analyses used SAS (SAS Institute, Cary, NC).
3. Results
3.1. Dental measures in COVID-19 cases and controls
COVID-19 cases were well matched to controls with regards to age, sex, and race. Compared to controls, COVID-19 cases had a higher proportion of Hispanics/LatinX, hypertension and diabetes and a lower proportion of “ever” smokers. COVID-19 cases also had greater alveolar crestal heights (2.42 mm ± 0.90 vs 1.82 mm ± 0.73, p < 0.001) (Table 2) in comparison to controls, indicating more alveolar bone loss. Interestingly, COVID-19 cases had fewer number of missing teeth than controls (3.68 teeth ± 5.77 vs 4.67 teeth ± 7.02, p < 0.04) (Table 2).
Table 2.
Demographics, comorbidities, and dental measures in patients with and without COVID-19 diagnosis.
| Covid-19+ (n = 387) |
Covid-19− (n = 387) |
p value | |
|---|---|---|---|
| Demographics | mean ± SD or n (%) | mean ± SD or n (%) | |
| Age (years old) | 47.1 ± 19.7 | 47.0 ± 19.6 | N/A |
| Sex | N/A | ||
| Males | 130 (34 %) | 130 (34 %) | |
| Females | 257 (66 %) | 257 (66 %) | |
| Race | N/A | ||
| Asian American/Native Hawaiian/Pacific Islander | 22 (5 %) | 22 (5 %) | |
| Black or African American | 56 (14 %) | 56 (14 %) | |
| Other | 163 (42 %) | 163 (42 %) | |
| White | 146 (38 %) | 146 (38 %) | |
| Ethnicity | 0.001 | ||
| Hispanic or Latino or Spanish Origin | 149 (39 %) | 95 (25 %) | |
| Not Hispanic or Latino or Spanish Origin | 163 (42 %) | 195 (50 %) | |
| Unknown | 75 (19 %) | 97 (25 %) | |
| Co-morbidities | n (%) with condition | n (%) with condition | |
| Hypertension | 120 (31 %) | 68 (18 %) | <0.001 |
| Diabetes | 50 (13 %) | 24 (6 %) | 0.01 |
| Smoking status | |||
| Ever | 348 (90 %) | 375 (97 %) | 0.001 |
| Current | 6 (2 %) | 6 (2 %) | 1.00 |
| Dental measurements | mean ± SD | mean ± SD | |
| Alveolar crestal height (mm) | 2.42 ± 0.90 | 1.82 ± 0.73 | <0.001 |
| Missing Teeth (number) | 3.68 ± 5.77 | 4.67 ± 7.02 | 0.04 |
Multivariate logistic regression models for COVID-19 status were performed on 147 case-controls pairs with data for both alveolar crestal height and number of teeth missing available. Greater alveolar crestal height, OR = 4.302 (2.510–7.376), fewer missing teeth, OR = 0.897 (0.835–0.965), and lack of a history of smoking distinguished COVID-19 cases from controls (Table 3).
Table 3.
Multivariate logistic regression models for COVID-19 status.
| COVID-19 status | |
|---|---|
| n = 147 case-control pairs | |
| Characteristic | Adjusted Odds Ratios |
| History of smoking (ever) | 0.222 (0.079–0.621) |
| Alveolar crestal height (mm) | 4.302 (2.510–7.376) |
| Missing teeth (number) | 0.897 (0.835–0.967) |
* with alveolar crestal height and missing teeth as forced variables.
Variables included in univariate analysis: race, ethnicity, HTN, diabetes, smoking (ever vs never).
3.2. Association of the rate of alveolar bone loss with Covid-19 diagnosis
Change in alveolar crestal height was evaluated in a subgroup of 50 COVID-19 cases and 57 controls that had a dental visit with radiographs before and after 2/1/20, when the first cases of COVID-19 were diagnosed in New York City. Alveolar bone loss was 0.650 ± 0.085 mm in the COVID-19 cases over 2.54 ± 1.99 years and 0.266 ± 0.079 mm in the controls over 1.97 ± 1.21 years and remained significant after adjusting for the time difference between scans, p = 0.0014.
3.3. Dental measures and severity of COVID-19 disease
Among the 387 COVID-19 cases, 48 required hospitalization (Table 4). The hospitalized COVID-19 patients were older, more likely to be Hispanic and more likely to have hypertension or diabetes than non-hospitalized COVID-19 patients. In addition, COVID-19 patients requiring hospitalization had greater alveolar crestal height (2.86 mm ± 1.08 vs 2.37 mm ± 0.87, p < 0.01) and greater number of missing teeth (7.00 ± 8.27 vs 3.26 +_/- 5.24, p < 0.01) than non-hospitalized COVID-19 patients (Table 4). The multivariable assessment of risk of COVID-19 hospitalization on the 231 cases with complete records (216 non-hospitalized and 15 hospitalized) showed that greater number of missing teeth OR = 1.113 (1.034–1.197), was statistically significant compared to non-hospitalized COVID-19 patients. P < 0.0001, (Table 5).
Table 4.
Demographics, comorbidities, and dental measures in COVID-19 patients with and without hospitalization.
| Hospitalized (n = 48) |
Non-Hospitalized (n = 338) |
p value | |
|---|---|---|---|
| Demographics | mean ± SD or n (%) | mean ± SD or n (%) | |
| Age (years old) | 53.4 ± 20.4 | 46.0 ± 19.1 | 0.02 |
| Sex | 0.21 | ||
| Males | 20 (42 %) | 110 (32 %) | |
| Females | 28 (58 %) | 229 (68 %) | |
| Race | 0.87 | ||
| Asian American/Native Hawaiian/Pacific Islander | 2 (4 %) | 20 (6 %) | |
| Black or African American | 8 (17 %) | 48 (14 %) | |
| Other | 22 (46 %) | 141 (42 %) | |
| White | 16 (33 %) | 130 (38 %) | |
| Ethnicity | 0.01 | ||
| Hispanic or Latino or Spanish Origin | 29 (60 %) | 120 (35 %) | |
| Not Hispanic or Latino or Spanish Origin | 13 (27 %) | 150 (44 %) | |
| Unknown | 6 (13 %) | 69 (21 %) | |
| Co-morbidities | n (%) with condition | n (%) with condition | |
| Hypertension | 23 (48 %) | 97 (29 %) | 0.01 |
| Diabetes | 13 (27 %) | 37 (11 %) | 0.01 |
| Smoking status | |||
| Ever | 40 (83 %) | 308 (91 %) | 0.11 |
| Current | 1 (2 %) | 5 (1 %) | 0.94 |
| Dental measurements | mean ± SD | mean ± SD | |
| Alveolar crestal height (mm)* | 2.87 ± 1.08 | 2.37 ± 0.87 | 0.01 |
| Missing Teeth (number)# | 7.00 ± 8.27 | 3.26 ± 5.24 | 0.0001 |
Alveolar crestal height data available in 25 cases and 209 controls.
Missing teeth data available in 39 cases and 209 controls.
Table 5.
Multivariate logistic regression models for hospitalization among COVID-19 patients.
| Hospitalization status | |
|---|---|
| n = 231 COVID-19 cases- n = 25 hospitalized and n = 216 non-hospitalized | |
| Characteristic | Adjusted Odds Ratios |
| Alveolar crestal height (mm) | 1.313 (0.812–2.124) p = 0.27 |
| Missing teeth (number) | 1.113 (1.034–1.197) p = 0.005 |
* with ACH and missing teeth as forced variables.
Variables included in univariate analysis: Age, Sex, race, ethnicity, HTN, diabetes, smoking (ever vs never).
4. Discussion
In this retrospective study of data from a single institution, historical measures of periodontal disease are differentially present in COVID-19 cases. When controlling for other risk factors for COVID-19 disease, dental patients with a COVID-19 diagnosis had evidence of greater alveolar bone loss by dental radiographs than matched controls. Numerous researchers have hypothesized that periodontal disease may cause an increase in the likelihood of SARS-CoV-2 infection (Kara et al., 2020, Pitones-Rubio et al., 2020); however, existing literature on the topic was inconclusive and contradictory. A large study of extracted data from UK biobank participants that found that self-reported painful or bleeding gums and loose teeth were not associated with an increased likelihood in COVID-19 infection (Larvin et al., 2020). This finding differs from what our data shows, possibly due to the inaccuracies of self-reporting as a surrogate for the mild/moderate stages of periodontal disease (Deng et al., 2021). Interestingly, our data shows COVID-19 infection was associated with the presence of fewer missing teeth (not including third molars.) Others have demonstrated that periodontal pockets present suitable conditions for viral replication, infection, and spread to gingival capillaries and may serve as reservoir for SARS-CoV-2 (Badran et al., 2020, Gupta et al., 2021a, Gupta et al., 2021b). Our finding that COVID-19 cases had more teeth present than controls may be explained by the presence of more teeth allowing for more periodontal pockets and points of viral entry.
Through examination of the rate of change in ACH in a subgroup of cases and controls with radiographs before and after the first cases of COVID-19 were diagnosed in New York City, we found an association between the rate of periodontal disease progression and COVID-19 disease. Cases with COVID-19 had greater alveolar bone loss over this period than controls, indicating that more rapidly deteriorating oral health co-occurs with COVID-19 disease. There are two possible explanations for this finding. First, people with active periodontal disease are more likely to become infected with COVID-19. In support, a case control study found by clinical examination that people who recovered from Covid-19 had more severe periodontal disease than patients who never had Covid-19 (Patel et al., 2021). Second, COVID-19 infection itself induces alveolar bone loss. In support, SARS-CoV-2 binds and causes alterations of the ACE2 receptor pathways (Patel et al., 2021) and the ACE2 pathway promotes an anabolic pathway in alveolar bone (Queiroz-Junior et al., 2019). In addition, SARS-CoV-2 infection causes morphological alterations in the junctional epithelium in post-mortem periodontal tissue (Fernandes Matuck et al., 2020).
Lastly, we found that a more compromised periodontium was associated with COVID-19 severity. In the absence of viral load data or other measures of COVID-19 severity, hospitalization due to COVID-19 infection was used as a proxy for disease severity. Increased alveolar bone loss and more missing teeth were associated with greater likelihood of hospitalization in COVID-19 cases. This was in agreement with other studies that found periodontitis, painful and/or bleeding gums, and dental damage to be associated with more severe COVID-19 infection outcomes (Larvin et al., 2020, Sirin and Ozcelik, 2021) and that the negative effect of obesity on Covid-19 outcomes was exacerbated by periodontal disease (Shah & Badner, 2020). Our multivariable assessment of risk of COVID-19 hospitalization showed that increased number of missing teeth was associated with increased risk of COVID-19 hospitalization. Increased tooth loss was also associated with chronic obstructive pulmonary disease (Cunningham et al., 2016), asthma (Shah & Badner, 2020), and pneumonia mortality (Gomes-Filho et al., 2020, Suma et al., 2018), which may be due to aspiration into the lung of oral pathogens (Bansal et al., 2013) and/or periodontal disease induced systemic cytokines causing exacerbation of respiratory disease activity (Wahaidi et al., 2011). It was surprising that more teeth present are associated with increased likelihood of COVID-19 infection whereas fewer teeth present are associated with more severe outcomes among the infected population. We plan to examine data from other institutions to see if these findings are unique to our dataset or institution.
This study had several limitations. Based on COVID-19 statistics from the New York City Department of Health, at least 1 out of every 11 people in Washington Heights, the catchment area of the medical center, was diagnosed with COVID-19. Based on a total patient population of 22,112 in our dental clinic, we expected over 2000 cases of COVID-19 instead of only 387. Our low case number was likely due to undertesting during the early stages of the epidemic in New York City, when testing was restricted to hospitalized patients and diagnoses made in other facilities. We considered adding self-reported COVID-19 data to mitigate this, however two recent studies suggested that <50 % of people with self-reported COVID-19 can be confirmed by serological testing (Matta et al., 2022, Mulchandani et al., 2021). Another limitation was the lack of complete data (dental chart and radiographs) to evaluate alveolar crestal height and missing teeth on every patient. Additionally, the longitudinal sample was limited in size. Lastly, the effects of Covid-19 infection on endodontic disease was not investigated despite a recent report showing that the SARS-CoV2 entry molecules, TMPRSS-2 and ACE2, are expressed in the dental pulp and that Covid-19 infection may potentiate pulpitis (Galicia et al., 2020).
5. Conclusions
COVID-19 cases demonstrated lower alveolar bone levels (more bone loss) and a faster rate of alveolar bone loss, measured as the difference in bone level between radiographs taken before and after the first cases of COVID-19 were diagnosed in New York City, than controls. Patients with more severe COVID-19 disease, as indicated by hospitalization due to COVID-19 infection, were more likely to have more missing teeth than non-hospitalized COVID-19 patients. Additional studies are needed to confirm the role of periodontal disease in risk and severity of COVID-19 infection and the role of COVID-19 infection on accelerating alveolar bone loss.
Ethical statement
The work presented in this manuscript has been approved by the appropriate ethical committees related to the institution; this study was approved by the International Review Board (IRB), reference number AAAT2272.
CRediT authorship contribution statement
S. Wadhwa: Conceptualization, Methodology, Writing – original draft. S. Dave: Data curation, Writing – original draft. M.L. Daily: Data curation, Writing – original draft. A. Nardone: Data curation, Writing – review & editing. R. Li: Data curation, Writing – review & editing. J. Rosario: Data curation, Writing – review & editing. A. Cantos: Writing – review & editing. J. Shah: Writing – review & editing. H.H. Lu: Conceptualization, Methodology, Writing – review & editing. D.J. McMahon: Conceptualization, Methodology, Writing – review & editing. M.T. Yin: Conceptualization, Methodology.
Declaration of Competing Interest
S.W. and M.T.Y. currently receive NIDCR funding (R01 DE026924), which partially supported this study.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
S. Wadhwa, Email: sw2680@cumc.columbia.edu.
S. Dave, Email: svd2117@cumc.columbia.edu.
M.L. Daily, Email: md3667@cumc.columbia.edu.
A. Nardone, Email: an2861@cumc.columbia.edu.
R. Li, Email: rl3141@cumc.columbia.edu.
J. Rosario, Email: jr3677@cumc.columbia.edu.
A. Cantos, Email: ac4314@cumc.columbia.edu.
J. Shah, Email: jgs2142@cumc.columbia.edu.
H.H. Lu, Email: hhlu@columbia.edu.
D.J. McMahon, Email: djm6@cumc.columbia.edu.
M.T. Yin, Email: mty4@cumc.columbia.edu.
References
- Addy M. Toothbrushing against coronavirus. Br. Dent. J. 2020;228(7):487. doi: 10.1038/s41415-020-1450-9. [DOI] [PubMed] [Google Scholar]
- Badran Z., Gaudin A., Struillou X., Amador G., Soueidan A. Oct). Periodontal pockets: a potential reservoir for SARS-CoV-2? Med. Hypotheses. 2020;143:109907. doi: 10.1016/j.mehy.2020.109907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal M., Khatri M., Taneja V. Sep). Potential role of periodontal infection in respiratory diseases – a review. J. Med. Life. 2013;6(3):244–248. https://www.ncbi.nlm.nih.gov/pubmed/24155782 [PMC free article] [PubMed] [Google Scholar]
- Basso L., Chacun D., Sy K., Grosgogeat B., Gritsch K. Periodontal diseases and COVID-19: a scoping review. Eur. J. Dent. 2021;15(4):768–775. doi: 10.1055/s-0041-1729139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botros N., Iyer P., Ojcius D.M. Is there an association between oral health and severity of COVID-19 complications? Biomed. J. 2020;43(4):325–327. doi: 10.1016/j.bj.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Li L., Feng Z., Wan S., Huang P., Sun X., Wen F., Huang X., Ning G., Wang W. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020;6:11. doi: 10.1038/s41421-020-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham T.J., Eke P.I., Ford E.S., Agaku I.T., Wheaton A.G., Croft J.B. Cigarette smoking, tooth loss, and chronic obstructive pulmonary disease: findings from the behavioral risk factor surveillance system. J. Periodontol. 2016;87(4):385–394. doi: 10.1902/jop.2015.150370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng K., Pelekos G., Jin L., Tonetti M.S. Diagnostic accuracy of self-reported measures of periodontal disease: a clinical validation study using the 2017 case definitions. J. Clin. Periodontol. 2021 doi: 10.1111/jcpe.13484. [DOI] [PubMed] [Google Scholar]
- Fernandes Matuck B., Dolhnikoff M., Maia G.V.A., Isaac Sendyk D., Zarpellon A., Costa Gomes S., Duarte-Neto A.N., Rebello Pinho J.R., Gomes-Gouvêa M.S., Sousa S.C.O.M., Mauad T., Saldiva P.H.D.N., Braz-Silva P.H., da Silva L.F.F. Periodontal tissues are targets for Sars-Cov-2: a post-mortem study. J. Oral. Microbiol. 2020;13(1):1848135. doi: 10.1080/20002297.2020.1848135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galicia J.C., Guzzi P.H., Giorgi F.M., Khan A.A. Predicting the response of the dental pulp to SARS-CoV2 infection: a transcriptome-wide effect cross-analysis. Genes Immun. 2020;21(5):360–363. doi: 10.1038/s41435-020-00112-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes-Filho I.S., Cruz S.S.D., Trindade S.C., Passos-Soares J.S., Carvalho-Filho P.C., Figueiredo A.C.M.G., Lyrio A.O., Hintz A.M., Pereira M.G., Scannapieco F. Periodontitis and respiratory diseases: a systematic review with meta-analysis. Oral Dis. 2020;26(2):439–446. doi: 10.1111/odi.13228. [DOI] [PubMed] [Google Scholar]
- Gupta S., Mohindra R., Singla M., Khera S., Sahni V., Kanta P., Soni R.K., Kumar A., Gauba K., Goyal K., Singh M.P., Ghosh A., Kajal K., Mahajan V., Bhalla A., Sorsa T., Raisanen I. The clinical association between Periodontitis and COVID-19. Clin. Oral Investig. 2021 doi: 10.1007/s00784-021-04111-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Mohindra R., Chauhan P.K., Singla V., Goyal K., Sahni V., Gaur R., Verma D.K., Ghosh A., Soni R.K., Suri V., Bhalla A., Singh M.P. SARS-CoV-2 detection in gingival crevicular fluid. J. Dent. Res. 2021;100(2):187–193. doi: 10.1177/0022034520970536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280 e278. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang N., Pérez P., Kato T., Mikami Y., Okuda K., Gilmore R.C., Conde C.D., Gasmi B., Stein S., Beach M., Pelayo E., Maldonado J.O., Lafont B.A., Jang S.I., Nasir N., Padilla R.J., Murrah V.A., Maile R., Lovell W., Wallet S.M., Bowman N.M., Meinig S.L., Wolfgang M.C., Choudhury S.N., Novotny M., Aevermann B.D., Scheuermann R.H., Cannon G., Anderson C.W., Lee R.E., Marchesan J.T., Bush M., Freire M., Kimple A.J., Herr D.L., Rabin J., Grazioli A., Das S., French B.N., Pranzatelli T., Chiorini J.A., Kleiner D.E., Pittaluga S., Hewitt S.M., Burbelo P.D., Chertow D., Frank K., Lee J., Boucher R.C., Teichmann S.A., Warner B.M., Byrd K.M., Consortium, N. C.-A., Network, H. O. a. C. B. SARS-CoV-2 infection of the oral cavity and saliva. Nat. Med. 2021;27(5):892–903. doi: 10.1038/s41591-021-01296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson D., Ramberg P., Demmer R.T., Kebschull M., Dahlen G., Papapanou P.N. Gingival tissue transcriptomes in experimental gingivitis. J. Clin. Periodontol. 2011;38(7):599–611. doi: 10.1111/j.1600-051X.2011.01719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara C., Çelen K., Dede F., Gökmenoğlu C., Kara N.B. Is periodontal disease a risk factor for developing severe Covid-19 infection? The potential role of Galectin-3. Exp. Biol. Med. (Maywood) 2020;245(16):1425–1427. doi: 10.1177/1535370220953771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larvin H., Wilmott S., Wu J., Kang J. The impact of periodontal disease on hospital admission and mortality during COVID-19 pandemic. Front Med. (Lausanne) 2020;7:604980. doi: 10.3389/fmed.2020.604980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larvin H., Wilmott S., Kang J., Aggarwal V.R., Pavitt S., Wu J. Additive effect of periodontal disease and obesity on COVID-19 outcomes. J. Dent. Res. 2021;100(11):1228–1235. doi: 10.1177/00220345211029638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta J., Wiernik E., Robineau O., Carrat F., Touvier M., Severi G., de Lamballerie X., Blanche H., Deleuze J.F., Gouraud C., Hoertel N. Association of self-reported COVID-19 infection and SARS-CoV-2 serology test results with persistent physical symptoms among french adults during the COVID-19 pandemic. JAMA Int. Med. 2022;182(1):19–25. doi: 10.1001/jamainternmed.2021.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulchandani R., Taylor-Philips S., Jones H.E., Ades A.E., Borrow R., Linley E., Kirwan P.D., Stewart R., Moore P., Boyes J., Hormis A., Todd N., Colda A., Reckless I., Brooks T., Charlett A., Hickman M., Oliver I., Wyllie D. Association between self-reported signs and symptoms and SARS-CoV-2 antibody detection in UK key workers. J. Infect. 2021;82(5):151–161. doi: 10.1016/j.jinf.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narula N., Singh H.S. NYC innocence lost: cardiology in the COVID-19 pandemic. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S.K., Juno J.A., Lee W.S., Wragg K.M., Hogarth P.M., Kent S.J., Burrell L.M. Plasma ACE2 activity is persistently elevated following SARS-CoV-2 infection: implications for COVID-19 pathogenesis and consequences. Eur. Respir. J. 2021;57(5) doi: 10.1183/13993003.03730-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X., Xu X., Li Y., Cheng L., Zhou X., Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int. J. Oral. Sci. 2020;12(1):9. doi: 10.1038/s41368-020-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitones-Rubio V., Chávez-Cortez E.G., Hurtado-Camarena A., González-Rascón A., Serafín-Higuera N. Is periodontal disease a risk factor for severe COVID-19 illness? Med. Hypotheses. 2020;144:109969. doi: 10.1016/j.mehy.2020.109969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queiroz-Junior C.M., Santos A.C.P.M., Galvão I., Souto G.R., Mesquita R.A., Sá M.A., Ferreira A.J. The angiotensin converting enzyme 2/angiotensin-(1–7)/Mas Receptor axis as a key player in alveolar bone remodeling. Bone. 2019;128 doi: 10.1016/j.bone.2019.115041. [DOI] [PubMed] [Google Scholar]
- Sampson V., Kamona N., Sampson A. Could there be a link between oral hygiene and the severity of SARS-CoV-2 infections? Br. Dent J. 2020;228(12):971–975. doi: 10.1038/s41415-020-1747-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa Y., Ibaragi S., Okui T., Yamashita J., Ikebe T., Harada H. Expression of SARS-CoV-2 entry factors in human oral tissue. J. Anat. 2021;238(6):1341–1354. doi: 10.1111/joa.13391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P.D., Badner V.M. Dec). Association between asthma and severe tooth loss in the adult population of the United States. J. Asthma. 2020:1–12. doi: 10.1080/02770903.2020.1856868. [DOI] [PubMed] [Google Scholar]
- Shamsoddin E. Is periodontitis associated with the severity of COVID-19? Evid. Based Dent. 2021;22(2):66–68. doi: 10.1038/s41432-021-0179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirin D.A., Ozcelik F. The relationship between COVID-19 and the dental damage stage determined by radiological examination. Oral Radiol. 2021 doi: 10.1007/s11282-020-00497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suma S., Naito M., Wakai K., Naito T., Kojima M., Umemura O., Yokota M., Hanada N., Kawamura T. Tooth loss and pneumonia mortality: a cohort study of Japanese dentists. PLoS ONE. 2018;13(4):e0195813. doi: 10.1371/journal.pone.0195813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wactawski-Wende J., Hausmann E., Hovey K., Trevisan M., Grossi S., Genco R.J. The association between osteoporosis and alveolar crestal height in postmenopausal women. J. Periodontol. 2005;76(11 Suppl):2116–2124. doi: 10.1902/jop.2005.76.11-S.2116. [DOI] [PubMed] [Google Scholar]
- Wahaidi V.Y., Kowolik M.J., Eckert G.J., Galli D.M. Endotoxemia and the host systemic response during experimental gingivitis. J. Clin. Periodontol. 2011;38(5):412–417. doi: 10.1111/j.1600-051X.2011.01710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki H.A.M., Hoffmann K.R., Hausmann E., Scannapieco F.A. Is radiologic assessment of alveolar crest height useful to monitor periodontal disease activity? Dent. Clin. North Am. 2015;59(4):859–872. doi: 10.1016/j.cden.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z., Peng F., Xu B., Zhao J., Liu H., Peng J., Li Q., Jiang C., Zhou Y., Liu S., Ye C., Zhang P., Xing Y., Guo H., Tang W. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J. Infect. 2020;81(2):e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]