Abstract
Background:
Proton beam radiation therapy (PBRT) has dosimetric advantages compared to photon radiation therapy for the treatment of major salivary gland tumors (MSGTs).
Methods:
Patients with non-metastatic MSGTs treated at a single proton therapy center from October 2013 to October 2018 were retrospectively reviewed.
Results:
Sixty-Eight patients with MSGTs were included and the most common site and histology were the parotid gland (75.0%) and adenoid cystic carcinoma (22.1%), respectively. The 3-year rates of locoregional control, progression-free survival, and overall survival were 95.1% (95% CI: 89.9 – 100.0%), 80.7% (70.2 –92.7%), and 96.1% (95% CI: 90.9 – 100.0%), respectively.
Conclusion:
In a large cohort of MSGTs treated with PBRT, the rates of locoregional control were high in short term follow up and treatment was well tolerated.
Keywords: Proton therapy, Radiation, Salivary gland, Cancer, Survival
Introduction
Surgery is the mainstay of treatment for major salivary gland tumors (MSGTs), and adjuvant radiation therapy is often added to improve local control in advanced disease [1–4]. Indications for post-operative radiotherapy often include advanced tumors, close or positive margins, node-positive disease, recurrent disease, or other adverse pathological features such as high grade, perineural invasion, or lymphovascular invasion [5–10]. When surgery is not feasible, definitive radiation therapy is appropriate.
Proton beam radiation therapy (PBRT) provides several potential radiobiologic and dosimetric advantages to photon radiation therapy. The Bragg peak of PBRT allows for a sharper dose fall-off with the absence of exit dose, which can reduce radiation dose to nearby critical organs [11–13]. Increasing the accuracy of dose deposition can expand the therapeutic window of PBRT [13]. Ipsilateral head and neck irradiation with PBRT reduces radiation doses to normal tissues and results in low acute dysgeusia, mucositis, and nausea [14–15].
Despite the potentially improved therapeutic ratio of proton therapy compared to photons, there is limited clinical data on the outcomes of proton therapy for MSGTs. The purpose of this study was to review a large clinical experience with the management of MSGTs using PBRT in the adjuvant setting. We aimed to evaluate the tumor control, survival, and toxicity of PBRT.
Methods
Patient Sample
Patients with MSGTs treated with PBRT from October 2013 to October 2018 were included in this retrospective study. All patients were treated at the ProCure Proton Therapy Center in Somerset, New Jersey. Patients were excluded if they had primary tumors of the skin or minor salivary glands. Patients receiving bilateral neck radiation or palliative radiation therapy were also excluded.
Data was collected from electronic medical records, including patient and tumor characteristics. Pathological information was acquired from diagnostic biopsies or surgeries and was reviewed at Memorial Sloan Kettering Cancer Center. The details of treatment delivery were reviewed including radiation planning and chemotherapy. Data on treatment toxicity was reviewed from patient medical records and graded according to the Common Terminology Criteria for Adverse Events (version 4.03), with acute toxicity defined as an adverse event occurring within 3 months of PBRT completion.
Treatment Details
The Proteus 235 system (Ion Beam Applications, Belgium) was used to deliver proton therapy with either uniform scanning (US) or pencil beam scanning (PBS) techniques. PBS consisted of either single field uniform dose or multi-field optimization treatment planning. Robustness analysis with range uncertainty of ± 3.5% was used to ensure good planning target coverage and safety of organs at risk. A relative biological effectiveness of 1.1 was used for proton dose calculations.
Patients underwent CT simulation with PET/CT and MRI fusion when available. All patients included received only ipsilateral neck radiation therapy. The clinical target volume (CTV) included the post-operative bed with or without inclusion of the ipsilateral neck depending on the tumor histology and pathology. The skull base was included at the discretion of the treating physician. A planning margin of 3 to 5 mm was added to the CTV for treatment planning.
Patients with positive margins were treated with a dose of 66 to 70 Cobalt Gray Equivalent (CGE) to the post-operative tumor bed at the discretion fo the treating physician and depending on the level of concern for potential residual disease. Close margins and clear margins were typically treated to 60 to 66 CGE and 50 to 60 CGE to the post-operative bed, respectively. Elective coverage of the ipsilateral neck and skull base was treated to a dose of 50 to 60 CGE.
Elective nodal volumes commonly included the ipsilateral levels IB–IV. Elective nodal coverage was commonly included for poorly differentiated carcinoma, salivary ductal carcinoma, and squamous cell carcinoma. Elective nodal coverage was not routinely delivered for pleomorphic adenoma, acinic cell carcinoma, or adenoid cystic carcinoma in the absence of involved nodes. Coverage of the skull base was routinely included for adenoid cystic tumors and for poorly differentiated carcinoma, salivary ductal carcinoma, or squamous cell carcinoma with perineural invasion.
Statistical Analysis
Locoregional control, distant metastases, progression-free survival (PFS) and overall survival (OS) were evaluated. Locoregional control was defined as first recurrence in the primary site or regional lymph nodes. Distant metastases were defined as first recurrence in distant organs. PFS was defined as local or distant recurrence or death from any cause. OS was defined as death from any cause.
The Kaplan-Meier method was used to estimate time-to-event endpoints. The Cox proportional hazards model was used for univariable and multivariable analysis of the effects of coviarates on time-to-event endpoints. Statistical analysis was conducted in the R software, version 3.2.2.
Results
Patient and Tumor Characteristics
We identified 68 patients with pathologically confirmed MSGTs treated with PBRT (Table 1). The median age was 53 years and the majority of patients (95.6%) had a Karnofsky Performance Status of 80 or higher at the time of treatment. The most common site was the parotid gland (75.0%) followed by the submandibular gland (25.0%). The most common pathology was adenoid cystic carcinoma (22.1%) followed by acinic cell carcinoma (14.7%) and mucoepidermoid carcinoma (11.8%).
Table 1.
Patient and Tumor Characteristics.
| Characteristic | No. (%) |
|---|---|
|
| |
| Median Age (range) | 53 (13–88) |
| Sex | |
| Male | 32 (47.1) |
| Female | 36 (52.9) |
| KPS | |
| ≤70 | 3 (4.4) |
| >70 | 65 (95.6) |
| Primary Site | |
| Parotid gland | 51 (75.0) |
| Submandibular gland | 17 (25.0) |
| Primary or Recurrent | |
| Primary | 56 (82.4) |
| Recurrent | 12 (17.6) |
| Pathology | |
| Adenoid cystic carcinoma | 15 (22.1) |
| Acinic cell carcinoma | 10 (14.7) |
| Mucoepidermoid carcinoma | 8 (11.8) |
| Carcinoma ex-pleomorphic adenoma | 7 (10.3) |
| Salivary duct carcinoma | 7 (10.3) |
| Pleomorphic adenoma | 6 (8.8) |
| Myoepithelial carcinoma ex-pleomorphic adenoma | 5 (7.4) |
| Myoepithelial carcinoma | 3 (4.4) |
| Liposarcoma | 2 (2.9) |
| Squamous cell carcinoma | 2 (2.9) |
| Poorly differentiated carcinoma | 1 (1.5) |
| Secretory carcinoma | 1 (1.5) |
| Basal cell carcinoma | 1 (1.5) |
| Grade | |
| High | 18 (26.5) |
| Intermediate | 9 (13.2) |
| Low | 9 (13.2) |
| Benign | 6 (8.8) |
| Not stated | 26 (38.2) |
| T classification | |
| T1 | 33 (48.5) |
| T2 | 23 (33.8) |
| T3 | 8 (11.8) |
| T4 | 4 (5.9) |
| N classification | |
| N0 | 55 (80.9) |
| N1 | 7 (10.3) |
| N2 | 6 (8.8) |
| M classification | |
| M0 | 68 (100) |
| M1 | 0 (0) |
| Margin | |
| Clear | 12 (17.6) |
| Close (<1 mm) | 20 (29.4) |
| Involved | 36 (52.9) |
| Lymphovascular Invasion | |
| Present | 6 (8.8) |
| Absent | 62 (91.2) |
| Perineural Invasion | |
| Present | 20 (29.4) |
| Absent | 48 (70.6) |
KPS = Karnofsky Performance Status
The majority of patients had T1 or T2 tumors (82.4%) and most patients did not have nodal metastases (80.9%). Only 17.6% presented with a recurrence of MSGT treated with prior surgery and without prior adjuvant radiation. Perineural invasion was more common than lymphovascular invasion (29.4 vs 8.8%). Over half of the patients (52.9%) had involved surgical margins.
Treatment Details
Patients were treated with PBRT to a median dose of 66.07 CGE (mean: 64.92 CGE; range: 54.05 – 70.46 CGE) (Table 2). No patient was prescribed a dose less than 60 CGE, although one patient only received 54.05 CGE after deciding to discontinue treatment due to skin pruritus and dry desquamation consistent with grade 2 dermatitis. PBRT was most often directed to the primary site and neck, followed by the primary site alone, and the primary site and skull base. Less than a fifth (19.1%) of patients received concurrent chemotherapy, with cisplatin being the most common drug.
Table 2.
Treatment Details.
| Characteristic | No. (%) |
|---|---|
|
| |
| Concurrent chemotherapy | |
| Yes | 13 (19.1) |
| No | 55 (80.9) |
| Regimen of concurrent chemotherapy | |
| Cisplatin | 12 (92.3) |
| Carboplatin | 1 (7.7) |
| Dose of PBRT (median 66.07 CGE) | |
| > 50, ≤60 | 1 (1.5) |
| > 60, ≤67 | 55 (80.9) |
| ≥ 67 | 12 (17.6) |
| Median dose of PBRT by histology (CGE) | |
| Adenoid cystic carcinoma | 66.07 |
| Acinic cell carcinoma | 60.15 |
| Mucoepidermoid carcinoma | 66.07 |
| Carcinoma ex-pleomorphic adenoma | 66.07 |
| Salivary duct carcinoma | 70.07 |
| Pleomorphic adenoma | 66.07 |
| Myoepithelial carcinoma ex-pleomorphic adenoma | 66.07 |
| Myoepithelial carcinoma | 66.07 |
| Liposarcoma | 70.07 |
| Squamous cell carcinoma | 63.05 |
| Poorly differentiated carcinoma | 66.07 |
| Secretory carcinoma | 70.46 |
| Basal cell carcinoma | 60.05 |
| Treatment Site | |
| Primary | 30 (44.1) |
| Primary and skull base | 19 (27.9) |
| Primary and neck | 15 (22.1) |
| Primary, neck, and skull base | 4 (5.9) |
Tumor Control and Survival
Median follow up for all patients was 36.4 months (range: 1.4 – 67.9). In total, there were 3 locoregional failures, 10 distant failures, and 3 deaths. Two of the 3 patients with locoregional failure had nodal metastases at the time of surgery with lymphovascular invasion on pathology. The remaining patient had a mixed liposarcoma and pleomorphic adenoma with involved margins and perineural invasion.
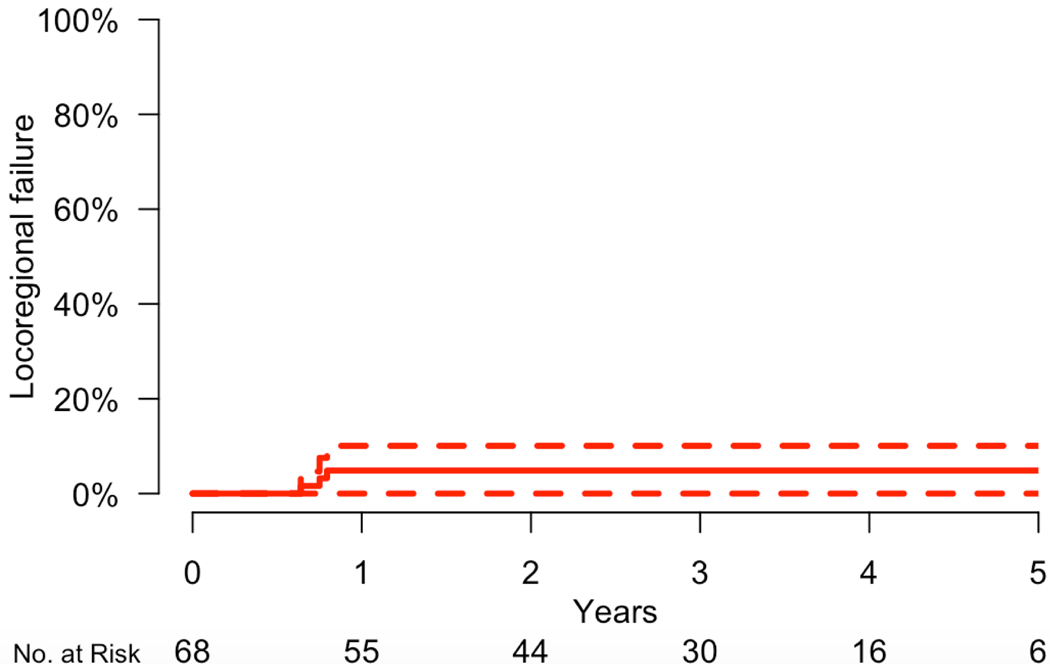
For the total cohort, the 3-year rate of locoregional control was 95.1% (95% CI: 89.9 – 100.0%) (Figure 1). The rates of 3-year progression-free survival and overall survival were 80.7% (70.2 – 92.7%), and 96.1% (95% CI: 90.9 – 100.0%), respectively (Figure 2). Distant failure was the predominant mode of failure, and the 3-year rate of freedom from distant failure were 79.9% (69.3 – 92.3%). There were no cases of isolated locoregional failure in the absence of distant metastatic disease. All three patients who died had distant metastases.
Figure 1.
Incidence of locoregional failure.
Figure 2.
Kaplan-Meier plots of progression-free survival (a) and overall survival (b).
On univariable analysis, factors associated with worse PFS in the whole cohort were involved nodes, lymphovascular invasion, perineural invasion, and high grade, while age, gender, advanced T classification, involved margins, and recurrent disease were not (Table 3). Age, involved nodes, and perineural invasion were statistically significant in the multivariable analysis.
Table 3.
Univariable and Multivariable Analysis of factors associated with progression-free survival.
| Univariable | Multivariable | |||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age | 1.03 (0.99 – 1.07) |
0.15 | 1.14 (1.01 – 1.28) |
0.033 |
| Male gender | 1.60 (0.45 – 5.69) |
0.46 | 2.55 (0.21 – 31.25) |
0.46 |
| T classification 3/4 | 1.29 (0.27 – 6.11) |
0.75 | 0.13 (0.01–1.83) |
0.13 |
| N classification 1/2 | 9.02 (2.50 – 32.57) |
<0.001 | 528.65 (4.32 – 64,628.15) |
0.011 |
| Lymphovascular invasion | 19.93 (4.71 – 84.44) |
<0.001 | 4.64 (0.32 – 66.92) |
0.26 |
| Perineural invasion | 10.61 (2.25 – 50.01) |
0.0028 | 28.45 (2.03 – 398.57) |
0.013 |
| Margins involved | 1.98 (0.51 – 7.68) |
0.32 | 3.03 (0.25 – 36.42) |
0.38 |
| High grade |
4.60 (1.28 – 16.46) |
0.019 | 0.15 (0.01–4.56) |
0.28 |
| Recurrent | 2.66 (0.68 – 10.41) |
0.16 | 64.62 (0.83 – 5030.45) |
0.061 |
HR = hazard ratio; CI = confidence interval.
Toxicity
The most common acute grade 1–2 toxicities were fatigue, dermatitis, mucositis, and dysphagia (Table 4). Dermatitis was the only acute grade 2 or higher toxicity that occurred in more than 10% of patients. Grade 3 acute toxicity occurred in 9 (13.2%) of patients and was due to grade 3 dermatitis in all cases. There were no grade 4 or 5 acute toxicities.
Table 4.
Acute toxicity.
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | |
|---|---|---|---|---|
| Fatigue | 35 (51.5%) | 30 (44.1%) | 3 (4.4%) | 0 (0.0%) |
| Dermatitis | 0 (0.0%) | 21 (30.9%) | 38 (55.9%) | 9 (13.2%) |
| Mucositis | 46 (67.6%) | 18 (26.5%) | 4 (5.9%) | 0 (0.0%) |
| Dysphagia | 55 (80.9%) | 9 (13.2%) | 4 (5.9%) | 0 (0.0%) |
| Dysgeusia | 61 (89.7%) | 5 (7.4%) | 2 (2.9%) | 0 (0.0%) |
| Nausea | 62 (91.2%) | 6 (8.8%) | 0 (0.0%) | 0 (0.0%) |
There was one case of grade 3 late toxicity involving osteoradionecrosis of the mandible. The case of radiation necrosis occurred in a 29 year old male with mucoepidermoid carcinoma of the left submandibular gland. The tumor was resected with positive margins and the patient received adjuvant proton radiation to dose of 70.24 CGE with weekly cisplatin 40 mg/m2. The max dose to the mandible was 74.39 CGE. At 50.3 months of follow up, he presented with left jaw swelling and pain. He was diagnosed with a periodontal infection with underlying osteoradionecrosis. He was managed conservatively as an inpatient with chlorhexidine rinses and intravenous antibiotics. At last follow up of 61.5 months, he was doing well with no evidence of disease and no toxicity aside from mild xerostomia.
Discussion
To the best of our knowledge, this study is the largest report of treatment outcomes for MSGTs treated with PBRT. The following table lists the outcomes of MSGTs in articles published in last decade (Table 5) [16–20]. Our results compare favorably in terms of locoregional control and survival, especially when considering over half the patients in our study had positive margins. The high rate of positive margins in this cohort is a reflection of this being a common referral to our center for adjuvant proton therapy.
Table 5.
Results and prognostic factors of MSGTs in last decade
| No. of Pts | Radiation Technique | Median Follow-up | Locoregional Control (LRC) | Distant Control (DC) | Survival | Prognostic Factors | |
|---|---|---|---|---|---|---|---|
|
Tam
(2013) |
200 | IMRT, 3D-CRT, or conventional |
50 months |
2y LC 91% 5y LC 88% 2y RC 96% 5y RC 94% |
2y DMFS 84% 5y DMFS 73% |
5y 76.6% | T classification, N classification associated with DC; N classification associated with survival |
|
Jensen
(2015) |
53 | IMRT and Carbon Ion Boost | 42 months |
2y LC 84.3% 3y LC 81.9% |
3y PFS 57.9% | 3y 78.4% | - |
|
Jegadeesh
(2015) |
112 | - | 55.1 months |
5y LRC stages I/II 94.6 stages III/IV 60.4% |
5y DC stages I/II 93.0% stages III/IV 56.9% |
5y 76.0% | CNI, age, tumor size associated with LRC; site, FNP, high grade, T classification, tumor size associated with DC |
|
Hosni
(2016) |
304 | 3D-CRT or IMRT |
82 months |
5y LC 96% 5y RC 95% |
5y DC 80% | 5y 78% | Stage III–IVB, LVI, surgical margin, high risk pathology associated with DC; Stage III–IVB, LVI associated with survival |
|
Haderlein
(2019) |
65 | 3D-CRT or IMRT |
45 months |
3y LC 93% 3y LRC 90.3% |
3y DC 64% | 3y 74.9% | Macroscopic resection margin (R2) associated with LC |
Intensity modulated radiation therapy (IMRT); 3-dimensional conformal radiation therapy (3D-CRT); Local control (LC); Regional control (RC); Distant metastasis-free survival (DMFS); Progression-free survival (PFS); Clinical nerve involvement (CNI), facial nerve paralysis (FNP).
The high locoregional control with PBRT may translate to benefits on distant disease spread and survival. Local failure has been shown to be predictive of distant metastasis for MSGTs [16]. We found that distant recurrence was the predominant mode of failure, which is consistent with other studies in patients with MSGTs [16–20]. There were also no cases of isolated locoregional recurrence, indicating that more effective systemic therapy is needed.
PBRT was well tolerated with low rates of grade 3 toxicity and no cases of acute or late grade 4–5 toxicity. Dermatitis represented the only acute grade 3 toxicity, and there was only one case of late grade 3 toxicity which involved radionecrosis of the mandible. This case of radionecrosis was managed conservatively without the need for surgical intervention. The overall toxicity profile is favorable considering the median dose of PBRT was 66.07 CGE and that one-third of patients had coverage of the skull base.
This study has several limitations including retrospective data collection, heterogeneity of tumor histologies, and median follow up of 3 years. The variety of tumor histologies included in this study poses a challenge for assessing treatment outcomes. For instance, adenoid cystic carcinoma can recur years after initial treatment, and these late recurrences may not be captured in our follow up time. However due to the rariety of MSGTs, it is not feasible to report separate outcomes for each histology with proton beam radiation. It is important to note that there is a paucity of evidence on outcomes with proton bream radiation therapy for MSGTs and this study includes patients treated at a large proton center.
In summary, PBRT results in excellent locoregional control with low toxicity in patients with MSGTs. Distant metastases remain the predominant mode of failure and improvements in systemic therapy are needed. PBRT should be considered for patients with MSGTs at high risk of locoregional failure.
References
- 1.Adelstein DA, Koyfman SA, El-Naggar AK, Hanna EY. Biology and management of salivary gland cancers. Semin Radiat Oncol. 2012; 22:245–253. [DOI] [PubMed] [Google Scholar]
- 2.North CA, Lee DJ, Piantadosi S, et al. Carcinoma of the major salivary glands treated by surgery or surgery plus postoperative radiotherapy. Int J Radiat Oncol Biol Phys 1990;18:1319–1326. [DOI] [PubMed] [Google Scholar]
- 3.Garden AS, el-Naggar AK, Morrison WH, et al. Postoperative radiotherapy for malignant tumors of the parotid gland. Int J Radiat Oncol Biol Phys 1997;79–85. [DOI] [PubMed] [Google Scholar]
- 4.Ali S, Palmer FL, Katabi N, et al. Long-term local control rates of patients with adenoid cystic carcinoma of the head and neck managed by surgery and postoperative radiation. Laryngoscope. 2017. Oct;127(10):2265–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terhaard CHJ, Lubsen H, Van Der Tweel, et al. Salivary gland carcinoma: independent prognostic factors for locoregional control, distant metastases, and overall survival: results of the Dutch Head and Neck Oncology Cooperative Group. Head Neck 2004;26:681–693. [DOI] [PubMed] [Google Scholar]
- 6.Vander Poorten VLM, Balm AJM, Hilgers FJM, et al. The development of a prognostic score for patients with parotid carcinoma. Cancer 1999;85:2057– 2067. [PubMed] [Google Scholar]
- 7.Ali S, Palmer FL, Yu C, et al. A predictive nomogram for recurrence of carcinoma of the major salivary glands. JAMA Otolaryngol Head Neck Surg. 2013;139(7):698–705. [DOI] [PubMed] [Google Scholar]
- 8.Ali S, Palmer F, Yu C, et al. Postoperative nomograms predictive of survival after surgical management of malignant tumors of the major salivary glands. Ann Surg Oncol 21(2):637–642, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Chen AM, Bucci MK, Weinberg V, et al. Adenoid cystic carcinoma of the head and neck treated by surgery with or without postoperative radiation therapy: Prognostic features of recurrence. Int J Radiat OncolBiol Phys. 2006; 66:152–159. [DOI] [PubMed] [Google Scholar]
- 10.Hay A, Migliacci J, Zanoni DK, Patel S, Yu C, Kattan MW, Ganly I. Validation of nomograms for overall survival, cancer-specific survival, and recurrence in carcinoma of the major salivary glands. Head Neck. 2018. May;40(5):1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orlandi E, Iacovelli NA, Bonora M, Cavallo A, Fossati P. Salivary Gland. Photon beam and particle radiotherapy: Present and future. Oral Oncol. 2016. Sep;60:146–56. [DOI] [PubMed] [Google Scholar]
- 12.Blanchard P, Gunn GB, Lin A, Foote RL, Lee NY, Frank SJ. Proton Therapy for Head and Neck Cancers. Semin Radiat Oncol. 2018. Jan;28(1):53–63. [DOI] [PubMed] [Google Scholar]
- 13.Leeman JE, Romesser PB, Zhou Y, McBride S, Riaz N, Sherman E, et al. Proton therapy for head and neck cancer: expanding the therapeutic window. Lancet Oncol. 2017. May;18(5):e254–e265. [DOI] [PubMed] [Google Scholar]
- 14.Swisher-McClure S, Teo BKK, Kirk M, Chang C, Lin A. Comparison of Pencil Beam Scanning Proton- and Photon-Based Techniques for Carcinoma of the Parotid. International Journal of Particle Therapy 2016. 2:4, 525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romesser PB, Cahlon O, Scher E, et al. Proton beam radiation therapy results in significantly reduced toxicity compared with intensity-modulated radiation therapy for head and neck tumors that require ipsilateral radiation. Radiother Oncol. 2016. Feb;118(2):286–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tam M, Riaz N, Salgado LR, et al. Distant metastasis is a critical mode of failure for patients with localized major salivary gland tumors treated with surgery and radiation. J Radiat Oncol. 2013. Sep;2(3):285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen AD, Nikoghosyan AV, Lossner K, et al. COSMIC: a regimen of intensity-modulated radiotherapy plus dose-escalated, raster-scanned carbon ion boost for malignant salivary gland tumors (MSGTs). Results of the prospective phase II trial. Int J Radiat Oncol Biol Phys. 2015. Sep 1;93(1):37–46. [DOI] [PubMed] [Google Scholar]
- 18.Jegadeesh N, Liu Y, Prabhu RS, et al. Outcomes and prognostic factors in modern era management of major salivary gland cancer. Oral Oncol. 2015. Aug;51(8):770–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosni A, Huang SH, Goldstein D, et al. Outcomes and prognostic factors for major salivary gland carcinoma following postoperative radiotherapy. Oral Oncol. 2016. Mar;54:75–80. [DOI] [PubMed] [Google Scholar]
- 20.Haderlein M, Scherl C, Semrau S, et al. Salivary gland carcinoma (SGC) with perineural spread and/or positive resection margin – high locoregional control rates after photon (chemo) radiotherapy - experience from a monocentric analysis. Radiat Oncol. 2019. Apr 23;14(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]