Abstract
BACKGROUND
Pregnant women less frequently receive COVID-19 vaccination and are at increased risk for adverse pregnancy outcomes from COVID-19.
OBJECTIVE
This study aimed to first, describe the vaccination status, treatment, and outcomes of hospitalized, symptomatic pregnant women with COVID-19, and second, estimate whether treatment differs by pregnancy status among treatment-eligible (ie, requiring supplemental oxygen per National Institutes of Health guidelines at the time of the study) women.
STUDY DESIGN
From January to November 2021, the COVID-19-Associated Hospitalization Surveillance Network completed medical chart abstraction for a probability sample of 2715 hospitalized women aged 15 to 49 years with laboratory-confirmed SARS-CoV-2 infection. Of these, 1950 women had symptoms of COVID-19 on admission, and 336 were pregnant. We calculated weighted prevalence estimates of demographic and clinical characteristics, vaccination status, and outcomes among pregnant women with symptoms of COVID-19 on admission. We used propensity score matching to estimate prevalence ratios and 95% confidence intervals of treatment-eligible patients who received remdesivir or systemic steroids by pregnancy status.
RESULTS
Among 336 hospitalized pregnant women with symptomatic COVID-19, 39.6% were non-Hispanic Black, 24.8% were Hispanic or Latino, and 61.9% were aged 25 to 34 years. Among those with known COVID-19 vaccination status, 92.9% were unvaccinated. One-third (32.7%) were treatment-eligible. Among treatment-eligible pregnant women, 74.1% received systemic steroids and 61.4% received remdesivir. Among those that were no longer pregnant at discharge (n=180), 5.4% had spontaneous abortions and 3.5% had stillbirths. Of the 159 live births, 29.0% were preterm. Among a propensity score–matched cohort of treatment-eligible hospitalized women of reproductive age, pregnant women were less likely than nonpregnant women to receive remdesivir (prevalence ratio, 0.82; 95% confidence interval, 0.69–0.97) and systemic steroids (prevalence ratio, 0.80; 95% confidence interval, 0.73–0.87).
CONCLUSION
Most hospitalized pregnant patients with symptomatic COVID-19 were unvaccinated. Hospitalized pregnant patients were less likely to receive recommended remdesivir and systemic steroids compared with similar hospitalized nonpregnant women. Our results underscore the need to identify opportunities for improving COVID-19 vaccination, implementation of treatment of pregnant women, and the inclusion of pregnant women in clinical trials.
Key words: remdesivir, SARS-CoV-2 steroids, stillbirth, surveillance, vaccination
AJOG MFM at a Glance.
Why was this study conducted?
The extent to which COVID-19 treatment guidelines in hospitalized pregnant patients are followed is not known. In addition, the vaccination rate of hospitalized pregnant women is not well-described.
Key findings
One-fifth of treatment-eligible hospitalized pregnant patients did not receive recommended treatment, and most symptomatic pregnant women hospitalized with COVID-19 were unvaccinated.
What does this add to what is known?
This study confirms undertreatment of hospitalized pregnant patients with COVID-19 and underscores the importance of COVID-19 vaccination in pregnant patients to prevent hospitalization.
Introduction
Pregnant women with COVID-19 are at increased risk for adverse pregnancy outcomes including preterm birth and stillbirth.1 , 2 The National Institutes of Health (NIH) provides COVID-19 treatment guidelines for hospitalized adults.3 At the time of this study, these guidelines recommend dexamethasone or alternate systemic steroids and remdesivir to decrease disease severity among hospitalized patients who require supplemental oxygen or mechanical ventilation.3 , 4 The Society for Maternal-Fetal Medicine supports the NIH COVID-19 treatment guidelines and recommends that remdesivir and dexamethasone be offered to pregnant patients with COVID-19 who require supplemental oxygen.5 However, the extent to which these guidelines are followed in hospitalized pregnant patients with COVID-19 is unknown.
Our objectives were to first, describe the demographic and clinical characteristics, vaccination status, and in-hospital outcomes of hospitalized pregnant patients with symptomatic laboratory-confirmed SARS-CoV-2 infection from January to November 2021 using data from the COVID-19-Associated Hospitalization Surveillance Network (COVID-NET). Second, in a propensity score–matched cohort of treatment-eligible pregnant and nonpregnant women of reproductive age, we compared the prevalence of receiving remdesivir or systemic steroids.
Materials and Methods
Data source and study design
COVID-NET conducts population-based surveillance of laboratory-confirmed COVID-19–associated hospitalizations in 99 counties across 14 states (CA, CO, CT, GA, IA, MD, MI, MN, NM, NY, OH, OR, TN, and UT).6 , 7 The COVID-NET case definition includes all hospitalizations with a positive real-time reverse transcription-polymerase chain reaction or rapid antigen detection test result for SARS-CoV-2 during hospitalization or within the 14 days preceding admission among patients residing in the COVID-NET catchment area.8 Using previously described methods,6 each month data were collected from a random sample of COVID-19–associated hospitalizations, stratified by age and site. Some sites used modified procedures to identify and abstract information for all pregnant patients, including those not sampled by COVID-NET. Sampling weights were based on the probability of selection; sample sizes varied by surveillance month, site, and age group, and were based on the total number of patients identified in each of these strata.
Detailed demographic and clinical data on sampled patients were abstracted from patient medical records by trained surveillance officers using a standardized case report form. This activity was reviewed, considered exempt from institutional review board approval, and was conducted in accordance with applicable federal law and Centers for Disease Control and Prevention policy (45 C.F.R. part 46, 21 C.F.R. part 56; 42 U.S.C. Sect. 241(d); 5 U.S.C. Sect. 552a; 44 U.S.C. Sect. 3501 et seq.).
Inclusion and exclusion criteria
For the first objective, we included both sampled and nonsampled pregnant patients aged 15 to 49 years with complete medical chart abstraction who were admitted from January 1 to November 30, 2021. We included nonsampled pregnant patients from a specific site if full medical chart information was collected from all pregnant women in that site for that month. If a site did not collect all pregnancy information from nonsampled patients, their original sample weight was applied, and only sampled patients were included in analyses. The inclusion of nonsampled patients allowed COVID-NET to retain a representative sample while allowing for more precise estimates regarding pregnancy data.
We excluded individuals who did not have complete medical chart abstraction. We also excluded women who did not have COVID-19 symptoms recorded at admission to limit the scope of this analysis to women with at least mild disease; COVID-NET patients can include asymptomatic infections detected through routine laboratory testing of pregnant women at admission. Women were considered symptomatic for COVID-19 if their medical chart documented symptoms at admission (Table S1) or they developed clinical manifestations of COVID-19 during hospitalization as indicated by a discharge diagnosis of pneumonia, acute respiratory failure, or acute respiratory distress syndrome.
For the second objective, we included all symptomatic women aged 15 to 49 years who were hospitalized from January to November 2021. We then further restricted our sample to those who were eligible for treatment with remdesivir and systemic steroids per NIH criteria.3 Being “treatment-eligible” was defined as having an oxygen saturation of <94% on admission or receiving supplemental oxygen on admission (nasal cannula, face mask, continuous positive airway pressure [CPAP] or bilevel positive airway pressure [BIPAP], high-flow nasal cannula, invasive mechanical ventilation, nonrebreather mask) or during the hospital stay (mechanical ventilation, extracorporeal membrane oxygenation, BIPAP/CPAP, or high-flow nasal cannula). Because COVID-NET does not collect data on nasal cannula or face mask use in the intensive care unit (ICU), we additionally classified 3 patients (2 pregnant, 1 nonpregnant) who were admitted to the ICU as treatment-eligible, even if higher-level oxygen support was not specifically noted.
Variable specification
Socio-demographic characteristics
COVID-NET collects data on age, race and Hispanic ethnicity group, and smoking status. All demographic data were primarily self-reported, and were obtained from multiple sources, including notifiable disease, laboratory, and hospital databases.9 Race and ethnicity were based on National Center for Health Statistics bridged race categories.10 We also included a category for people who identified as more than one or unknown race and ethnicity.
Underlying medical conditions and pregnancy characteristics
COVID-NET abstracted information on underlying medical conditions for all sampled patients (Table S2). Among pregnant patients, COVID-NET collected gestational age in weeks at the time of hospital admission based on the medical record, which was used to classify patients into first (<14 weeks), second (14–27 weeks), and third (≥28 weeks) trimester. Information on pregnancy-associated conditions and plurality (singleton, multiple, or unknown) was also collected. Among women who were no longer pregnant at discharge, COVID-NET ascertained mode of delivery and the following birth outcomes: live birth, fetal loss, or unknown. A spontaneous abortion and stillbirth were defined as intrauterine death at <20 or ≥20 gestational weeks, respectively. Live-born infants were further classified as preterm (<37 weeks of gestation) or term (≥37 weeks).
Vaccination status
COVID-19 vaccination status (doses, dates administered, and product) was determined from state immunization information systems for all sampled COVID-NET patients. Fully vaccinated adults with a COVID-19–associated hospitalization were persons who had received the second dose of a 2-dose COVID-19 vaccine series or a single dose of a 1-dose product ≥14 days before the specimen collection date of the positive SARS-CoV-2 test result associated with their hospitalization. Adults whose positive SARS-CoV-2 test date was ≥14 days after the first dose of a 2-dose series and <14 days after receipt of the second dose were considered partially vaccinated. If the SARS-CoV-2 test date was not available, hospital admission date was used. Adults without documented receipt of any COVID-19 vaccine dose before the test date were considered unvaccinated. One site did not collect vaccination information and was excluded from analysis including vaccination status. COVID-NET methods for determining vaccination status have been described previously.11
In-hospital clinical interventions and outcomes
Information on oxygen saturation and supplemental oxygen received at admission, highest level of respiratory support received during hospitalization, other clinical interventions (vasopressor, renal replacement therapy/dialysis), ICU admission, and in-hospital death was collected. Information on in-hospital COVID-19 treatment with remdesivir, systemic steroids, tocilizumab, casirivimab/imdevimab, convalescent plasma, and baricitinib was also collected. Treatment with remdesivir and systemic steroids among treatment-eligible women of reproductive age by pregnancy status was a primary outcome of this study.
Statistical analysis
Analyses were conducted using SAS statistical software (version 9.4; SAS Institute, Cary, NC). For the first objective, unweighted sample size (n), weighted percentages, and 95% confidence intervals (CIs) accounting for the age- and site-stratified sampling were used to describe demographic and clinical characteristics, interventions, and in-hospital clinical outcomes among all symptomatic pregnant patients hospitalized with COVID-19 in our sample. We compared characteristics between included symptomatic and excluded asymptomatic pregnant patients using bivariate log-linked Poisson generalized estimating equations that accounted for clustering by COVID-NET site and complex sample weights. Variances were estimated using the Taylor series linearization method.
For the second objective, to compare prevalence of treatment with remdesivir and systemic steroids between treatment-eligible pregnant and nonpregnant women, we conducted propensity score matching to balance pregnant and nonpregnant women on demographic and underlying medical conditions.12 , 13 First, we calculated propensity scores for each patient using multivariable logistic regression to estimate the probability of pregnancy on the basis of baseline covariates, among all women regardless of pregnancy status. The model included the following covariates: age group, site, race and Hispanic ethnicity group, underlying medical conditions, and complex sampling weight. Second, to match pregnant and nonpregnant women, we used a SAS macro to do nearest-neighbor 1-to-1 matching without replacement in which the algorithm matches a pregnant woman to the nonpregnant woman with the closest propensity score.14 To assess balance between pregnant and nonpregnant women before and after propensity score matching, we calculated standardized differences.12 Finally, using the matched dataset, we estimated the prevalence ratio of COVID-19 treatment comparing treatment-eligible pregnant and nonpregnant women using log-linked binomial generalized estimating equations to account for clustering of hospitalizations within COVID-NET sites. In these models we adjusted for month and accounted for complex sample weights. To determine the robustness of our findings, we also conducted sensitivity analyses using two alternate methods (multivariable regression and inverse probability treatment weights12) to assess the association between pregnancy status and COVID-19 treatment (Table S5).
Results
Characteristics of hospitalized symptomatic pregnant patients with COVID-19
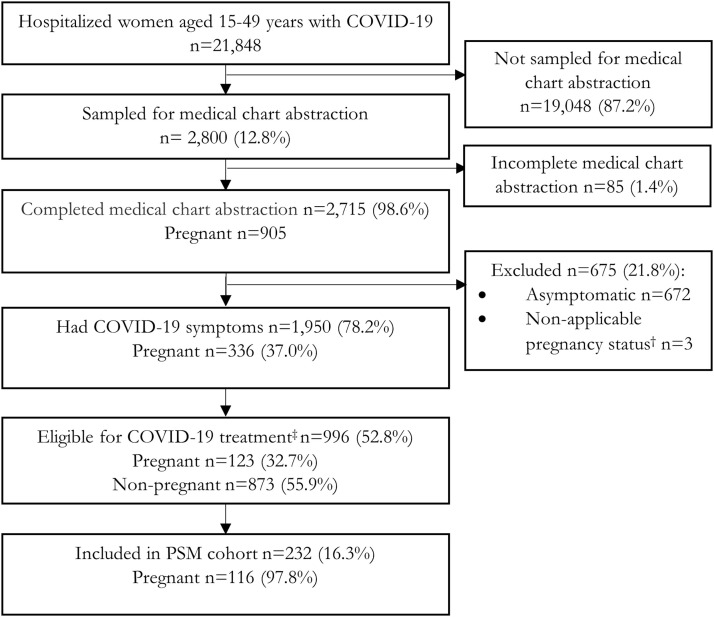
Among 21,848 hospitalized women aged 15 to 49 years with COVID-19 identified from January to November 2021, medical chart abstraction was completed for 2715 of 2800 sampled patients, of which 905 were pregnant (Figure 1 ). Of these pregnant women, 336 (37.0%) had symptoms of COVID-19. Of the symptomatic women, 123 (32.7%) were treatment-eligible (Figure 1).
Figure 1.
Flow chart for hospitalized, symptomatic women
COVID-19-Associated Hospitalization Surveillance Network (COVID-NET), 14 states, January to November 2021. All percentages are weighted, except for the percent sampled and not sampled for medical chart abstraction. All percentages are percent included or excluded from previous denominator.
† Represents postpartum (n=2) or post-termination (n=1).
‡ Denotes being treatment-eligible was defined as an oxygen saturation of <94% on admission, receiving supplemental oxygen on or during the hospital stay. Because COVID-NET does not collect data on nasal cannula or face mask use in the ICU, we additionally classified 3 pregnant patients who were admitted to the ICU as treatment-eligible, even if they did not receive higher-level oxygen support.
ICU, intensive care unit; PSM, propensity score–matched.
Sekkarie. Hospitalized pregnant women with COVID-19. Am J Obstet Gynecol MFM 2022.
Pregnant women of American Indian/Alaska Native or Black non-Hispanic race with COVID-19 were more likely to be symptomatic compared with White non-Hispanic pregnant women with COVID-19 (Table S3). Women in the first and third trimester were more and less likely to be symptomatic, respectively, than those in the second trimester. Women with liver disease were more likely to be symptomatic compared with those without liver disease.
Of the 336 symptomatic pregnant women, the median age was 27.9 years (interquartile range, 23.0–33.0). The largest race or ethnicity group was non-Hispanic Black (39.6%) followed by Hispanic or Latino (24.8%) (Table 1 ). One-third (32.6%) had an underlying medical condition; asthma (12.5%) and hypertension (8.9%) were the most prevalent underlying conditions. Most (70.7%) were hospitalized during the third trimester. The most common pregnancy-associated conditions were hypertensive disorders of pregnancy (10.3%) and gestational diabetes mellitus (5.9%). The median hospital length of stay was 3 days (interquartile range, 2–5) overall and 4 days for patients in the ICU (interquartile range, 3–16) (data not shown).
Table 1.
Characteristics of hospitalized pregnant women with symptomatic COVID-19a
| Variable | Unweighted n | Weighted % | (95% CI) |
|---|---|---|---|
| Overall n=336 | |||
| Age group (y) | |||
| 15-24 | 100 | 20.6 | (14.9–27.4) |
| 25-34 | 179 | 61.9 | (53.0–70.2) |
| 35-49 | 57 | 17.5 | (11.6–24.9) |
| Race/ethnicity | |||
| American Indian or Alaska Native, non-Hispanic | 8 | 2.4 | (0.6–6.3) |
| Asian or Pacific Islander, non-Hispanic | 26 | 5.2 | (2.8–8.7) |
| Black, non-Hispanic | 90 | 39.6 | (29.8–50.1) |
| Hispanic or Latino | 87 | 24.8 | (17.9–32.8) |
| White, non-Hispanic | 102 | 22.3 | (15.7–30.1) |
| None of the aboveb | 23 | 5.6 | (2.8–10.0) |
| Vaccine statusc | |||
| Unvaccinated | 322 | 92.9 | (83.2–98.0) |
| Partially vaccinated | 2 | 2.2 | (0.1–9.9) |
| Fully vaccinated | 9 | 4.9 | (0.9–14.0) |
| Underlying medical conditionsd | |||
| Any condition or conditions | 123 | 32.6 | (24.7–41.3) |
| Asthma | 38 | 12.5 | (7.6–19.2) |
| Hypertension | 30 | 8.9 | (4.5–15.4) |
| Chronic metabolic diseasee | 21 | 4.7 | (1.8–9.7) |
| Cardiovascular diseasee | 15 | 3.0 | (1.3–5.7) |
| Diabetes mellitus | 10 | 2.6 | (0.5–7.9) |
| Neurologic condition | 13 | 2.5 | (1.0–5.0) |
| Thyroid dysfunction | 13 | 2.1 | (0.7–4.9) |
| Other disease | 10 | 1.8 | (0.6–4.0) |
| Liver disease | 7 | 0.9 | (0.2–2.6) |
| Chronic lung diseasee | 2 | 0.1 | (0.0–1.3) |
| Smoking | |||
| Current smoker | 19 | 7.7 | (3.4–14.6) |
| Former smoker | 43 | 8.9 | (5.3–13.8) |
| Not a smoker/unknown smoking history | 274 | 83.4 | (76.1–89.2) |
| Pregnancy trimester at hospital admission | |||
| First | 30 | 10 | (5.3–16.7) |
| Second | 65 | 19.3 | (12.6–27.7) |
| Third | 241 | 70.7 | (61.5–78.8) |
| Current pregnancy plurality | |||
| Singleton pregnancy | 309 | 90.7 | (82.7–95.8) |
| Multiple pregnancy | 7 | 1.1 | (0.2–3.3) |
| Unknown | 20 | 8.2 | (3.3–16.4) |
| Pregnancy-associated conditionsd | |||
| Any condition or conditions | 70 | 19.0 | (12.1–27.7) |
| Hypertensive disorders of pregnancy | 44 | 10.3 | (6.2–15.9) |
| Unknown | 22 | 6.8 | (3.0–13.0) |
| Gestational diabetes mellitus | 25 | 5.9 | (3.2–9.6) |
| Intrauterine growth restriction | 9 | 4.5 | (0.5–15.4) |
CI, confidence interval.
Data are from the COVID-19-Associated Hospitalization Surveillance Network (COVID-NET), 14 states, January to November 2021
None of the above included multiracial, non-Hispanic, or unknown categories. Individuals without ethnicity information were categorized as non-Hispanic15
Vaccination status missing for 3 patients because one site did not collect that information
Underlying medical conditions and pregnancy-associated conditions percentage columns do not add up to 100% because multiple options could be chosen per patient
Chronic metabolic disease does not include diabetes mellitus or thyroid dysfunction; cardiovascular disease does not include hypertension; and chronic lung disease does not include asthma (Table S2).
Sekkarie. Hospitalized pregnant women with COVID-19. Am J Obstet Gynecol MFM 2022.
Of the 336 symptomatic pregnant women, 333 (98.5%) had known COVID-19 vaccination status; 92.9% (n=322) were unvaccinated, 2.2% (n=2) were partially vaccinated, and 4.9% (n=9) were fully vaccinated (Table 1). Among the 9 fully vaccinated pregnant women, 3 had >1 underlying medical condition (including 1 immunocompromised). The patient with an immunocompromizing condition was the only fully vaccinated patient to require oxygen but did not receive remdesivir or systemic steroids. No fully vaccinated pregnant women were admitted to the ICU. Eight of the 9 fully vaccinated women gave birth during their hospitalization; 7 were term live births, 1 was a preterm live birth, and 1 pregnancy ended by induced abortion. In sensitivity analysis using data only from May to November 2021, when vaccinations were more widely available to pregnant women, 89.3% were unvaccinated.
Approximately half of the symptomatic pregnant women were no longer pregnant at discharge (n=180; 51.9%). Of these pregnancies, 88.2% ended in live births, 3.5% ended in stillbirth, and 5.4% ended in spontaneous abortion (Table 2 ). Of the 159 live births, 29.0% were preterm. There were no in-hospital maternal deaths. Of pregnant women, 12.6% were admitted to the ICU and 6.6% required invasive mechanical ventilation. Over one-third (38.2%) of all symptomatic pregnant women in the sample received systemic steroids (36.1%) or remdesivir (27.4%). Among those symptomatic pregnant women receiving systemic steroids, the most frequently administered was dexamethasone (90.1%). Among those who received dexamethasone and had a live birth (n=39), 55.0% had preterm births (data not shown).
Table 2.
Symptoms, interventions, and outcomes of hospitalized pregnant women with symptomatic COVID-19a
| Variable | Unweighted n | Weighted % | (95% CI) | ||
|---|---|---|---|---|---|
| Overall n=336 | |||||
| Symptoms on admission | |||||
| Cough | 179 | 59.9 | (50.7–68.6) | ||
| Shortness of breath | 147 | 49.3 | (39.8–59.0) | ||
| Fever | 123 | 36.2 | (27.4–45.9) | ||
| Congestion/rhinorrhea | 58 | 18.5 | (12.2–26.2) | ||
| Loss of taste/smell | 56 | 16.9 | (10.4–25.3) | ||
| Abdominal pain | 70 | 15.8 | (10.6–22.3) | ||
| Sore throat | 34 | 8.8 | (5.3–13.7) | ||
| New clinical discharge diagnosis | |||||
| Acute respiratory distress syndrome | 11 | 2.3 | (0.9–4.7) | ||
| Acute respiratory failure | 61 | 19.8 | (12.5–28.9) | ||
| Pneumonia | 89 | 31.8 | (22.7–42.1) | ||
| Sepsis | 11 | 2.0 | (0.7–4.5) | ||
| Interventions | |||||
| High-flow nasal cannulab | 24 | 7.5 | (4.0–12.8) | ||
| BIPAP/CPAPb | 7 | 1.4 | (0.4–3.6) | ||
| Invasive mechanical ventilationb,c | 22 | 6.6 | (3.2–11.8) | ||
| Vasopressor | 34 | 11.1 | (5.0–20.5) | ||
| Renal replacement therapy or dialysis | 1 | 0.3 | (0.0–1.8) | ||
| COVID-19 treatments | |||||
| Remdesivir | 94 | 27.4 | (19.7–36.2) | ||
| Systemic steroids | 137 | 36.1 | (27.6–45.2) | ||
| Dexamethasone | 120 | 91.0 | (81.2–96.7) | ||
| Hydrocortisone | 4 | 6.2 | (1.3–16.9) | ||
| Methylprednisolone | 14 | 7.1 | (3.0–13.8) | ||
| Prednisolone | 1 | 0.4 | (0.0–3.5) | ||
| Prednisone | 3 | 1.3 | (0.1–5.0) | ||
| Betamethasone | 4 | 0.9 | (0.0–4.2) | ||
| Tocilizumab | 12 | 4.0 | (1.5–8.6) | ||
| Casirivimab/imdevimab | 7 | 1.3 | (0.4–3.3) | ||
| Convalescent plasma | 2 | 1.3 | (0.1–5.7) | ||
| Baricitinib | 2 | 0.5 | (0.1–2.0) | ||
| Severe outcomes | |||||
| ICU admission | 44 | 12.6 | (7.8–19.0) | ||
| In-hospital maternal death | 0 | 0 | |||
| Pregnancy status at discharged | |||||
| Still pregnant | 155 | 48.1 | (38.5–57.8) | ||
| No longer pregnant | 180 | 51.9 | (42.2–61.5) | ||
| Live birth | 159 | 88.2 | (89.1–94.3) | ||
| Term | 114 | 69.4 | (53.9–82.3) | ||
| Preterm (<37 wk of gestation) | 36 | 29.0 | (16.2–44.8) | ||
| Unknown | 9 | 1.6 | (0.3–5.0) | ||
| Induced abortion | 1 | 0.3 | (0.0–2.6) | ||
| Stillbirth | 11 | 3.5 | (1.3–7.3) | ||
| Spontaneous abortion | 5 | 5.4 | (1.1–15.2) | ||
| Unknown | 4 | 2.6 | (0.5–7.6) | ||
| Mode of deliverye | |||||
| Vaginal | 105 | 56.5 | (43.4–68.9) | ||
| Cesarean delivery | 68 | 36.8 | (25.2–49.5) | ||
| Unknown | 7 | 6.8 | (1.8–16.5) |
BIPAP/CPAP, bilevel positive airway pressure/continuous positive airway pressure; CI, confidence interval; ICU, intensive care unit.
Data are from the COVID-19-Associated Hospitalization Surveillance Network (COVID-NET), 14 states, January to November 2021
Mutually exclusive of other oxygen support categories. The highest level of oxygen support was chosen for each patient (invasive mechanical ventilation>BiPAP/CPAP>high-flow nasal cannula)
Five (0.9%, 95% CI, 0.2–2.6) patients that received mechanical ventilation also received extracorporeal membrane oxygenation
One missing pregnancy status at discharge
Among the 180 that gave birth.
Sekkarie. Hospitalized pregnant women with COVID-19. Am J Obstet Gynecol MFM 2022.
Receipt of remdesivir or systemic steroids among treatment-eligible pregnant women hospitalized with COVID-19
Among the 123 pregnant women who were treatment-eligible, 22.2% did not receive either remdesivir or systemic steroids (Table 3 ). There was a higher number of women aged 35 to 49 years, those with underlying medical conditions, and those with non-Hispanic Black or other race who received treatment compared with women in the youngest age category, those without underlying medical conditions, and those of non-Hispanic White ethnicity, respectively (Table 3).
Table 3.
Characteristics of treatment-eligible hospitalized pregnant women with COVID-19 by receipt of treatmenta
| Overall | Received treatmentb | No treatment | ||||
|---|---|---|---|---|---|---|
| (n=123, 32.7%) |
(n=87, 77.8%) |
(n=36, 22.2%) |
||||
| Variable | n | Weighted % | n | Weighted % | n | Weighted % |
| Age group (y) | ||||||
| 15-24 | 28 | 17.4 | 14 | 11.2 | 14 | 39.2 |
| 25-34 | 71 | 64.3 | 53 | 68.3 | 18 | 50.4 |
| 35-49 | 24 | 18.2 | 20 | 20.5 | 4 | 10.4 |
| Race/ethnicity | ||||||
| Black, non-Hispanic | 25 | 26.0 | 19 | 27.8 | 6 | 19.8 |
| Hispanic | 24 | 23.0 | 16 | 21.7 | 8 | 27.6 |
| White, non-Hispanic | 44 | 26.1 | 25 | 20.7 | 19 | 44.8 |
| None of the abovec | 30 | 24.9 | 27 | 29.7 | 3 | 7.8 |
| Underlying medical conditions | ||||||
| Yes | 50 | 29.5 | 38 | 32.2 | 12 | 19.8 |
| No | 73 | 70.5 | 49 | 67.8 | 24 | 80.2 |
Data are from the COVID-19-Associated Hospitalization Surveillance Network (COVID-NET), 14 states, January to November 2021
Treatment included receipt of remdesivir (n=63, 61.4%) or systemic steroids (n=83, 74.1%)
None of the above categories included American Indian/Alaska Native, non-Hispanic; Asian/Pacific Islander, non-Hispanic; multiracial, non-Hispanic; and unknown racial categories. Individuals without ethnicity information were categorized as non-Hispanic.15 These categories were collapsed in models because of small cell sizes.
Sekkarie. Hospitalized pregnant women with COVID-19. Am J Obstet Gynecol MFM 2022.
Differential receipt of remdesivir or systemic steroids among treatment-eligible women of reproductive age hospitalized with COVID-19 by pregnancy status
After propensity score matching, the distribution of characteristics between treatment-eligible pregnant and nonpregnant women, was better balanced, except in the 15-to-24-years age group, Black non-Hispanic, and liver disease categories (Table S4).
In the matched cohort, remdesivir was administered to 61.9% (95% CI, 47.5–75.0) and 80.6% (95% CI, 69.9–88.7) of pregnant and nonpregnant women, respectively. Systemic steroids were administered to 74.3% (95% CI, 61.3–84.8) of pregnant women (Table 4 ). The most frequently administered systemic steroid was dexamethasone (91.5%). Among nonpregnant women, 94.0% (95% CI, 88.0–97.6) received systemic steroids, of which dexamethasone was most commonly administered (97.7%). Results from adjusted models showed that pregnant women had lower prevalence of treatment with remdesivir (prevalence ratio [PR], 0.82; 95% CI, 0.69–0.97) and systemic steroids (PR, 0.80; 95% CI, 0.73–0.87) compared with nonpregnant women (Table 4). All sensitivity analyses with various methods for adjustment had prevalence ratios of similar direction and magnitude, although the precision varied (Table S5).
Table 4.
| Prevalence |
Multivariable modelc |
||||||
|---|---|---|---|---|---|---|---|
| Outcome | Pregnant (n=116, 50%) |
Nonpregnant (n=116, 50%) |
|||||
| n | Weighted % (95% CI) | n | Weighted % (95% CI) | PR | 95% CI | P value | |
| Remdesivir | |||||||
| No | 56 | 38.1 (25.0–52.5) | 33 | 19.4 (11.3–30.1) | ref | ||
| Yes | 60 | 61.9 (47.5–75.0) | 83 | 80.6 (69.9–88.7) | 0.82 | (0.69–0.97) | .024 |
| Systemic steroids | |||||||
| No | 37 | 25.7 (15.2–38.7) | 17 | 6.0 (2.4–12.0) | ref | ||
| Yes | 79 | 74.3 (61.3–84.8) | 99 | 94.0 (88.0–97.6) | 0.80 | (0.73–0.87) | <.001 |
CI, confidence interval; PR, prevalence ratio; ref, reference group.
Propensity score models to create propensity score adjusted for age group, race and ethnic group, site, asthma, chronic lung disease not including asthma, cardiovascular disease, diabetes mellitus, thyroid dysfunction, hypertension, liver disease, and neurologic disease, and complex sample weights. To match pregnant and nonpregnant women, we used nearest-neighbor 1-to-1 matching without replacement
Data are from the COVID-19-Associated Hospitalization Surveillance Network (COVID-NET), 14 states, January to November 2021
Generalized estimating equation model to estimate PR comparing pregnant with nonpregnant women, adjusted for month, and accounting for clustering by site and complex sample weights.
Sekkarie. Hospitalized pregnant women with COVID-19. Am J Obstet Gynecol MFM 2022.
Comment
Principal findings
In this investigation of hospitalized, symptomatic pregnant women with COVID-19, we identified adverse outcomes including ICU admission, stillbirths, and spontaneous abortions. Nearly all (>90%) were unvaccinated. In addition, approximately 1 in 5 treatment-eligible pregnant patients did not receive remdesivir or systemic steroids.3 Among our propensity score–matched cohort of treatment-eligible women, pregnant women were 18% less likely to receive remdesivir and 21% less likely to receive systemic steroids than nonpregnant women.
Results
Although clinical presentation, in-hospital disease outcomes, and severity among hospitalized pregnant patients are well documented,1 , 16 , 17 most previous studies have not described vaccination status or treatment in hospitalized pregnant patients. The vast majority (>90%) of symptomatic pregnant women who were hospitalized for COVID-19 in 2021 in this network were unvaccinated, and those with breakthrough infections (n=9) experienced milder disease. No fully vaccinated pregnant women were admitted to the ICU. This is consistent with multiple studies that have shown that COVID-19 vaccines are highly effective in preventing hospitalization in pregnant women.18, 19, 20, 21 In addition, vaccination is not associated with pregnancy loss, preterm birth, or small-for-gestational-age infants.22 Furthermore, maternal COVID-19 vaccination has been associated with a reduced risk of COVID-19 hospitalization among infants aged <6 months.23 , 24 Low coverage of vaccination in pregnant women (11.1%) compared with nonpregnant women (24.9%) has been shown in nonhospitalized women; vaccination completion was even lower in our sample of hospitalized pregnant patients.25 Although vaccination coverage in pregnant women is increasing, it remains low, with an estimated 67.8% of pregnant women fully vaccinated as of February 5, 2022.26 Low vaccination coverage in pregnant women could be owing to a variety of factors, including theoretical concerns about safety stemming from the lack of inclusion in clinical trials and potential for greater vaccine hesistancy among healthcare providers and pregnant women.
This study was conducted after the effectiveness and safety of treatments for COVID-19 were established.27 Previous studies have shown remdesivir to be effective in preventing severe disease in pregnant patients.28 , 29 However, very little is known about treatment patterns in hospitalized pregnant women. We found that not all treatment-eligible women received remdesivir or systemic steroids and that there is evidence of differential use of these treatments in pregnant and nonpregnant women. The reasons for differential treatment practices by pregnancy status are unknown but may be related to severity of disease, lack of availability of treatment protocols during pregnancy, lack of familiarity of providers with initiation of treatment during pregnancy, potential concerns about fetal safety by providers or patients leading them to decline recommended therapy, or perceived risks owing to pregnant women generally being excluded from clinical trials of new treatment protocols.
Clinical implications
There is a need for patient and clinical education and targeted communication about and improved processes for the vaccination and treatment of pregnant women.
Research implications
Additional qualitative or quantitative research exploring factors that influence healthcare systems, provider practice patterns, and hospital protocols specific to the treatment of hospitalized pregnant women with COVID-19 would be informative. Additional studies focused on the safety and efficacy of COVID-19 treatment during pregnancy and the potential maternal and infant benefits would be critically important. In the future, the voluntary inclusion of pregnant patients in clinical studies of new treatment protocols could prevent disparities in access to recommended care for pregnant patients.30
Strengths and limitations
Our observational study required robust methods to limit biases given that pregnant women hospitalized with COVID-19 are systematically different from nonpregnant women. To minimize confounding, we limited our sample to symptomatic women that required supplemental oxygen and for whom treatment was thus recommended. We also used propensity score matching to balance comparison groups. Despite these methods, there may have been residual confounding. Reassuringly, sensitivity analysis with a variety of regression and propensity score methods to adjust for confounding yielded similar results. A second limitation is that COVID-19 cases might have been missed because of testing practices and test availability. Third, information on obesity as an underlying prepregnancy condition was not available, thus this underlying health condition could not be described. Fourth, information was abstracted from medical charts and might not have been complete. For example, only one oxygen saturation value and corresponding support were provided per hospitalization outside of ICU stays. Information on previous pregnancies was not available. Fifth, we could not establish the indications for the use of systemic steroids that are also used to promote fetal lung maturity in the management of preterm labor; thus, the actual use rates of systemic steroids for COVID-19 treatment may have been even lower. Finally, any maternal deaths after discharge were not captured.
Conclusions
Despite current recommendations, most symptomatic pregnant women hospitalized with COVID-19 were unvaccinated, and one-fifth of treatment-eligible hospitalized pregnant patients did not receive recommended treatment, underscoring the need for increased targeted communication about and improved processes for the vaccination and treatment of pregnant women.
Acknowledgments
The COVID-NET Surveillance Team group authorship includes the following authors: Pam Daily Kirley, MPH, California Emerging Infections Program, Oakland, CA; Nisha B. Alden, MPH, Colorado Department of Public Health Environment, Denver, CO; Kimberly Yousey-Hindes, MPH, Connecticut Emerging Infections Program, Yale School of Public Health, New Haven, CT; Emily Fawcett, MPH, Division of Infectious Diseases, Emory University School of Medicine, Atlanta, GA, Georgia Emerging Infections Program, Georgia Department of Public Health, Atlanta, GA, and Foundation for Atlanta Veterans Education and Research, Atlanta Veterans Affairs Medical Center, Decatur, GA; Kenzie Teno, MPH, Iowa Department of Public Health, Des Moines, IA; Chloe Brown, MPH, Michigan Department of Health and Human Services, Lansing, MI; Erica Bye, MPH, Minnesota Department of Health, Saint Paul, MN; Yadira Salazar-Sánchez, MPH, New Mexico Emerging Infections Program, University of New Mexico Health Sciences Center, Albuquerque, NM; Nancy Spina, MPH, New York State Department of Health, Albany, NY; Christina B. Felson, MS, University of Rochester School of Medicine and Dentistry, Rochester, NY; Eli Shiltz, MPH, Ohio Department of Health, Columbus, OH; Nasreen Abdullah, MD, MPH, Public Health Division, Oregon Health Authority, Portland, OR; William Schaffner, MD, Vanderbilt University Medical Center, Nashville, TN; Andrea George, MPH, Salt Lake County Health Department, Salt Lake City, UT.
We acknowledge with gratitude the clinicians and surveillance officers at the COVID-NET sites included in this study. We also wish to acknowledge the following people: Onika Anglin, MPH (COVID-NET Surveillance Team, Centers for Disease Control and Prevention; General Dynamics Information Technology); Jennifer L. Milucky, MSPH (COVID-NET Surveillance Team, Centers for Disease Control and Prevention); Sherry Quach, BA, Ashley O. Coates, MPH, Monica J. Napoles, BA (California Emerging Infections Program); Jordan Surgnier, MPH, Sarah McLafferty, MPH, Millen Tsegaye, MHA, Madelyn Lensing, MPH, Isaac Armistead, MD, MPH (Colorado Department of Public Health and Environment); Ann Basting, BS, Tessa Carter, MPH, Maria Correa, MPH, Daewi Kim, MBS, Carol Lyons, MPH, Amber Maslar, MPA, Julie Plano, MS (Connecticut Emerging Infections Program, Yale School of Public Health); Katelyn Ward, MPH, Allison Roebling, DVM, MPH, Chandler Surell, MPH, Jana Manning, MPH, Asmith Joseph, MPH, Marina Bruck, MPH, Grayson Kallas, MPH, Rayna Ceaser, BS, Stephanie Lehman, RN, BSN, Hope Wilson, MPH, Johanna Hernandez, MPH, Sabrina Hendrick, MPH, Annabel Patterson, MPH (Division of Infectious Diseases, School of Medicine, Emory University; Georgia Emerging Infections Program, Georgia Department of Public Health; Foundation for Atlanta Veterans Education and Research, Veterans Affairs Medical Center); Jim Collins, MPH, RS, Shannon Johnson, MPH, Justin Henderson, MPH, Sue Kim, MPH, Libby Reeg, MPH, Alexander Kohrman, MPH, Val Tellez Nunez, MPH, Sierra Peguies-Khan, MPH (Michigan Department of Health and Human Services); Kayla Bilski, MPH, Ruth Lynfield, MD, Erica Mumm, MPH (Minnesota Department of Health); Kathy M. Angeles, MPH, Molly Bleecker, MA, Nancy Eisenberg, MPH, Emily B. Hancock, MS, Sarah A. Khanlian, MPH, Sarah Lathrop, DVM, PhD, Wickliffe Omondi, MPH, Francesca Pacheco, BS, Mayvilynne Poblete, MA, MPH, Dominic Rudin, BS, Sarah Shrum Davis, MA, MPH (New Mexico Emerging Infections Program); Cory Cline, MPH, Melissa Judson, MPH, Sunshine Martinez, CBCS, CMAA, EHR, Mark Montoya, MPH, Florent Nkouaga, MA, Jasmyn Sanchez, Daniel M. Sosin, MD, MPH, Kelly Plymesser, RN (New Mexico Department of Health); Jennifer Akpo, MPH, Celina Chavez, BSN, RN, Murtada Khalifa, MBBS, Alesia Reed, BSN, RN, Yassir Talha, MBBS, (CDC Foundation/New Mexico Department of Health); Grant Barney, MPH, Adam Rowe, BA, Kerianne Engesser, MPH (New York State Department of Health); Sophrena Bushey, MHS, Christine Long, MPH, Kevin Popham, MPH, Maria Gaitan, BS, Virginia Cafferky, BS, Thomas Peer, DrPH (University of Rochester School of Medicine and Dentistry); Ann Salvator, MS, Julie Freshwater, PhD, MPH, Denise Ingabire-Smith, MPH, CLS (ASCP), Rebekah Sutter, BSN, RN (Ohio Department of Health); Sam Hawkins, MPH (Public Health Division; Oregon Health Authority); Tiffanie Markus, PhD, Karen Leib, RN, Katie Dyer, Terri McMinn, Danielle Ndi, MPH, Anise Elie, MPH, RN, BSN, Kathy Billings, MPH, Manideepthi Pemmaraju, BBS, MPH, Bentley Akoko, MD, MPH, Victoria Umutoni, MPH (Vanderbilt University Medical Center); Amanda Carter, BS, Andrea Price, LPN, Ashley Swain, CHES, Caitlin Shaw, BS, Laine McCullough, MPH, Melanie Crossland, MPH, Ryan Chatelain, MPH (Salt Lake County Health Department); Keegan McCaffrey, BS (Utah Department of Health).
Footnotes
Group authors have been listed in the Acknowledgments.
The authors report no conflict of interest.
This work was supported by the Centers for Disease Control and Prevention through an Emerging Infections Program cooperative agreement (CK17-1701) and through a Council of State and Territorial Epidemiologists cooperative agreement (NU38OT000297-02-00).
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the United States Department of Health and Human Services, the United States Public Health Service Commissioned Corps, the Centers for Disease Control and Prevention, or the authors’ institutions.
Cite this article as: Sekkarie A, Woodruff R, Whitaker M, et al. Characteristics and treatment of hospitalized pregnant women with COVID-19. Am J Obstet Gynecol MFM 2022;XX:x.ex–x.ex.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajogmf.2022.100715.
Appendix. Supplementary materials
References
- 1.Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeSisto CL, Wallace B, Simeone RM, et al. Risk for stillbirth among women with and without COVID-19 at delivery hospitalization - United States, March 2020-September 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1640–1645. doi: 10.15585/mmwr.mm7047e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Institutes of Health. Therapeutic management of hospitalized adults with COVID-19. 2022. Available at: https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/hospitalized-adults–therapeutic-management/. Accessed February 16, 2022.
- 4.United States Food & Drug Administration. Coronavirus (COVID-19) update: FDA issues emergency use authorization for potential COVID-19 treatment. 2020. Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment. Accessed March 8, 2022.
- 5.Society for Maternal-Fetal Medicine. COVID-19 task force. 2021. Available at: https://www.smfm.org/covidclinical. Accessed February 16, 2022.
- 6.Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 - COVID-NET, 14 states, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim L, Garg S, O'Halloran A, et al. Risk factors for Intensive Care Unit admission and in-hospital mortality among hospitalized adults identified through the US coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-NET) Clin Infect Dis. 2021;72:e206–e214. doi: 10.1093/cid/ciaa1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Coronavirus disease. 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET) purpose and methods. 2022. Available at: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covid-net/purpose-methods.html. Accessed February 16, 2022.
- 9.Acosta AM, Garg S, Pham H, et al. Racial and ethnic disparities in rates of COVID-19-associated hospitalization, intensive care unit admission, and in-hospital death in the United States From March 2020 to February 2021. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.30479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ingram DD, Parker JD, Schenker N, et al. United States Census 2000 population with bridged race categories. Vital Health Stat. 2003;2:1–55. [PubMed] [Google Scholar]
- 11.Havers FP, Pham H, Taylor CA, et al. COVID-19-associated hospitalizations among vaccinated and unvaccinated adults ≥18 years – COVID-NET, 13 states, January 1 – July 24, 2021. medRxiv. 2021 doi: 10.1001/jamainternmed.2022.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karabon P. Applying propensity score methods to complex survey data using. 2019. Available at: https://www.sas.com/content/dam/SAS/support/en/sas-global-forum-proceedings/2019/3634-2019.pdf. Accessed February 16, 2022.
- 14.Parsons L. SAS Institute Inc; Cary, NC: 2001. Reducing bias in a propensity score matched-pair sample using greedy matching techniques. [Google Scholar]
- 15.Yoon P, Hall J, Fuld J, et al. Alternative methods for grouping race and ethnicity to monitor COVID-19 outcomes and vaccination coverage. MMWR Morb Mortal Wkly Rep. 2021;70:1075–1080. doi: 10.15585/mmwr.mm7032a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savasi VM, Parisi F, Patanè L, et al. Clinical findings and disease severity in hospitalized pregnant women with coronavirus disease 2019 (COVID-19) Obstet Gynecol. 2020;136:252–258. doi: 10.1097/AOG.0000000000003979. [DOI] [PubMed] [Google Scholar]
- 17.Pierce-Williams RAM, Burd J, Felder L, et al. Clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnancies: a United States cohort study. Am J Obstet Gynecol MFM. 2020;2 doi: 10.1016/j.ajogmf.2020.100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273–2282. doi: 10.1056/NEJMoa2104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zauche LH, Wallace B, Smoots AN, et al. Receipt of mRNA Covid-19 vaccines and risk of spontaneous abortion. N Engl J Med. 2021;385:1533–1535. doi: 10.1056/NEJMc2113891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dagan N, Barda N, Biron-Shental T, et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat Med. 2021;27:1693–1695. doi: 10.1038/s41591-021-01490-8. [DOI] [PubMed] [Google Scholar]
- 21.Morgan JA, Biggio JR, Jr Martin JK, et al. Maternal outcomes after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in vaccinated compared with unvaccinated pregnant patients. Obstet Gynecol. 2022;139:107–109. doi: 10.1097/AOG.0000000000004621. [DOI] [PubMed] [Google Scholar]
- 22.Lipkind HS, Vazquez-Benitez G, DeSilva M, et al. Receipt of COVID-19 vaccine during pregnancy and preterm or small-for-gestational-age at birth - eight integrated health care organizations, United States, December 15, 2020-July 22, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:26–30. doi: 10.15585/mmwr.mm7101e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halasa NB, Olson SM, Staat MA, et al. Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19-associated hospitalization in infants aged <6 months - 17 states, July 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:264–270. doi: 10.15585/mmwr.mm7107e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halasa NB, Olson SM, Staat MA, et al. Maternal vaccination and risk of hospitalization for COVID-19 among infants. N Engl J Med. 2022;387:109–119. doi: 10.1056/NEJMoa2204399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Razzaghi H, Meghani M, Pingali C, et al. COVID-19 vaccination coverage among pregnant women during pregnancy - eight integrated health care organizations, United States, December 14, 2020-May 8, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:895–899. doi: 10.15585/mmwr.mm7024e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. COVID-19 vaccination among pregnant people aged 18-49 years overall, by race/ethnicity, and date reported to CDC - Vaccine Safety Datalink,* United States. 2022. Available at: https://covid.cdc.gov/covid-data-tracker/#vaccinations-pregnant-women. Accessed February 16, 2022.
- 27.RECOVERY Collaborative Group. Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eid J, Abdelwahab M, Colburn N, et al. Early administration of remdesivir and intensive care unit admission in hospitalized pregnant individuals with coronavirus disease 2019 (COVID-19) Obstet Gynecol. 2022;139:619–621. doi: 10.1097/AOG.0000000000004734. [DOI] [PubMed] [Google Scholar]
- 29.Burwick RM, Yawetz S, Stephenson KE, et al. Compassionate use of remdesivir in pregnant women with severe coronavirus disease 2019. Clin Infect Dis. 2021;73:e3996–e4004. doi: 10.1093/cid/ciaa1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor MM, Kobeissi L, Kim C, et al. Inclusion of pregnant women in COVID-19 treatment trials: a review and global call to action. Lancet Glob Health. 2021;9:e366–e371. doi: 10.1016/S2214-109X(20)30484-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.