Abstract
As the COVID-19 pandemic continues into its third year, emerging data indicates increased risks associated with SARS-CoV-2 infection during pregnancy, including pre-eclampsia, intrauterine growth restriction, preterm birth, stillbirth, and risk of developmental defects in neonates. Here, we review clinical reports to date that address different COVID-19 pregnancy complications. We also document placental pathologies induced by SARS-CoV-2 infection, entry mechanisms in placental cells, and immune responses at the maternal-fetal interface. Since new variants of SARS-CoV-2 are emerging with characteristics of higher transmissibility and more effective immune escape strategies, we also briefly highlight the genomic and proteomic features of SARS-CoV-2 investigated to date. Vector and mRNA-based COVID-19 vaccines continue to be rolled out globally. However, because pregnant individuals were not included in the vaccine clinical trials, some pregnant individuals have safety concerns and are hesitant to take these vaccines. We describe the recent studies that have addressed the effectiveness and safety of the current vaccines during pregnancy. This review also sheds light on important areas that need to be carefully or more fully considered with respect to understanding SARS-CoV-2 disease mechanisms of concern during pregnancy.
Introduction
The COVID-19 pandemic caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) that emerged in late 2019 continues to create havoc globally and has claimed at least 6.3 million lives by midway through 2022.1 SARS-CoV-2 continuously acquires new mutations as it replicates, resulting in a series of several variants of concern (VOCs) that thus far includes B.1.1.7 (Alpha), B.1.351 (Beta), P.1 (Gamma), B.1.617.2 (Delta), and most recently, the B.1.1.529 (Omicron) variant that emerged in late 2021. The Omicron variant accumulated a total of fifty mutations across its genome, 32 of which were in the spike protein, making it the most modified of these 5 VOCs.2 The highly transmissible Omicron rapidly supplanted the more pathogenic Delta variant,3 , 4 , 5 and is currently circulating globally with increased resistance to neutralization by monoclonal antibodies, vaccinated sera, and convalescent sera.6, 7, 8 Although approved vaccines are currently available against SARS-CoV-2, the emerging variants have shown immune escape characteristics that are effective enough to cause concern. This has created a demand for continued study on the efficacy of vaccines against new variants.9
SARS-CoV-2 is in the betacoronavirus genus of the Coronaviridae family of viruses that constitutes a divergent group of enveloped viruses with a genome of single-stranded positive-sense (+) RNA. Coronavirus tropism primarily extends to humans and other mammals (including many of economic importance), and avian species. The alpha- and betacoronavirus genus of Coronaviridae include all human coronaviruses and many mammalian coronaviruses, and are mostly associated with respiratory and enteric infections.10, 11, 12 In addition to the less severe ‘common cold’ causing coronaviruses that were already endemic, SARS-CoV-2 is the third highly pathogenic respiratory disease-causing human coronavirus to emerge in the past 20 years; following the highly-pathogenic severe acute respiratory syndrome coronavirus (SARS-CoV) outbreaks of 2002–2003, and the Middle East respiratory syndrome coronavirus (MERS-CoV) outbreaks since its emergence in 2012.12 The RNA genome of SARS-CoV-2 shares a 79% sequence similarity with SARS-CoV and nearly 50% similarity with MERS-CoV.13
Although SARS-CoV-2 infections are associated with a lower mortality rate in comparison to SARS-CoV and MERS, a significant proportion of people in intensive care due to SARS-CoV-2 are reported to develop acute respiratory distress syndrome (ARDS) that is associated with coronavirus disease 2019 (COVID-19).14 SARS-CoV-2 disease severity varies in populations, and it has been observed that the elderly, men, and individuals with other cardiovascular comorbidities disproportionately develop a critical illness.15 , 16
There is growing evidence that SARS-CoV-2 infections also pose a higher risk of severe illness in pregnant individuals, and risks to this population can, unfortunately, be overlooked. In the early months of the COVID-19 pandemic, studies from New York City and China suggested that the risk for severe disease in pregnant individuals was not significantly greater than in nonpregnant individuals.17, 18, 19 However, pregnancy is considered a time of greater susceptibility to pathogens, and recent studies indicate that SARS-CoV-2 infection is associated with development of severe disease in pregnant individuals and risks for the fetus.20, 21, 22 The major pregnancy-related complications that have been reported include: a higher risk of preterm birth, stillbirth, pre-eclampsia, intrauterine growth restriction (IUGR), and developmental defects in neonates.20 , 23 , 24
SARS-CoV-2 disease progression is mostly reliant on virus entry into host cells after binding to angiotensin-converting enzyme 2 (ACE2). This can be a factor in making pregnant individuals more susceptible to COVID-19 because ACE2 is expressed on cellular membranes and it is abundantly expressed in the placenta throughout gestation.25 It is considered to be a crucial component of the renin-angiotensin system (RAS), which is well known to regulate maternal hemodynamic adaptations.25 , 26 Though vertical transmission of SARS-CoV-2 from mother to fetus is reported to be rare or inconclusive, the interaction of SARS-CoV-2 with RAS pathway components could have severe effects on maternal-fetal health.25 , 27 Deciphering in-depth molecular mechanisms of SARS-CoV-2 pathogenesis in pregnant individuals continues to be an important field of investigation with many more questions to be investigated.
This review will briefly discuss clinical reports to date that investigate pregnancy-related complications and major risk factors associated with SARS-CoV-2 infections, including placental pathology and immunology in response to the infection. Also, we summarize the most recent data on genomic and proteomic features of SARS-CoV-2, possible placental cell entry pathways, the efficacy and safety of currently available vaccines in the context of pregnancy, and the effect of the Omicron variant on the pregnant population.
SARS-CoV-2 viral genomic structure, organization, and function
Genome
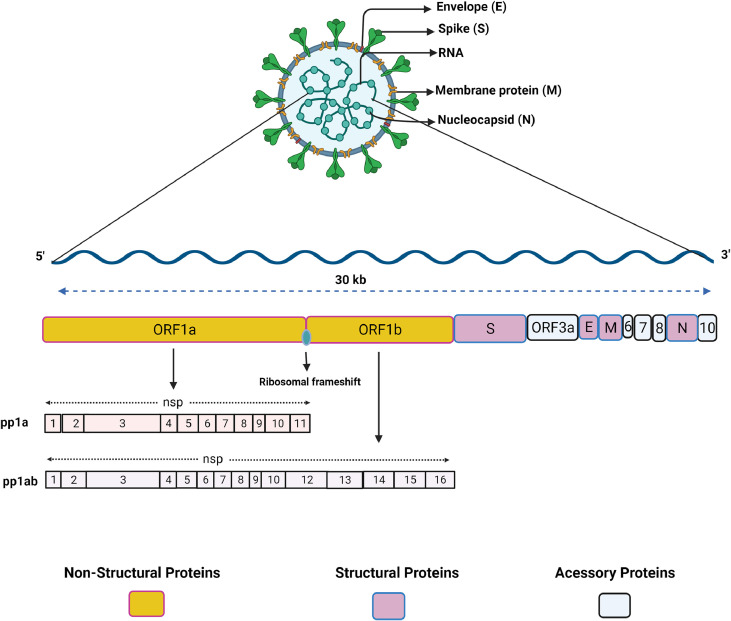
Coronaviruses have ∼30kb positive-sense single stranded RNA that mimics mRNA with 5′-capping and a 3′-polyadenylated tail. The genome is generally translated into 15–16 nonstructural proteins (nsp), 4 structural proteins (envelope (E), spike (S), membrane (M) and Nucleocapsid (N)), and a few accessory proteins. The SARS-CoV-2 genome is organized into 14 open reading frames (ORFs) where the largest ORFs include ORF1a and 1b, which are translated into polyprotein 1a (pp1a) and polyprotein 1ab (pp1ab) (Fig 1 ). These 2 polyproteins originate during translation from a ribosomal frameshift overlap between ORF1a and ORF1b.28 The efficiency of the frameshift was reported to lie between 45% and 70% in SARS-CoV-2, where pp1a expresses nearly 2 times more than pp1ab.28 The maturation of pp1a and pp1ab is carried out by viral protease nsp3 (papain-like protease) and nsp5 (chymotrypsin-like protease) that cleave the polyproteins into the nonstructural proteins, where pp1a contributes to the formation of nsp1 to 11, and pp1ab contributes to nsp1 to 16.29 The subsequent ORFs at the 3′ end of the genome code for structural proteins (S, E, M, and N) as well as accessory proteins (ORF3a, ORF6, ORF7a, ORF7b, ORF8, and ORF10).
Fig 1.
Schematic organization of SARS-CoV-2 genome and proteome. SARS-CoV-2 possesses a positive-sense single-stranded RNA (ssRNA) genome of nearly 30kb in size. The ssRNA is translated into a polypeptide which is further cleaved by viral proteases into individual proteins. ORF1a and ORF1b encode nonstructural proteins (nsp1-nsp16), the other ORFs encode structural proteins spike (S), membrane (M), envelope (E), and nucleocapsid (N). Accessory proteins are also encoded in the genome such as: ORF3, ORF6, ORF7, ORF8, and ORF10. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Nonstructural proteins
The interaction among coronavirus NSPs and host proteins is mainly required for the initiation and formation of replication compartments, whereas structural proteins mediate genome packaging, entry into host cells and maturation of the virion.30, 31, 32, 33 Since the maturation of most of the nonstructural proteins is carried out at least in part by the nsp5 protease, this protease is considered to be a crucial target for the design of antiviral drug therapies.34 , 35 The nsp1 protein is processed faster than the others, and when released it binds to the host 40s ribosomal complex, ultimately shutting off the host translational machinery and overcoming the innate immune responses.12 , 36 Nsp2 to 16 are responsible for preparing and coordinating the replication/transcription complex. Nsp3, nsp4, and nsp6 are known to induce intracellular membrane rearrangements that form compartments for organizing the replication/transcription complex, which may play a role in evading host immune recognition.37 Nsp7 and nsp8 act as cofactors for nsp12 (the RNA-dependent RNA polymerase, RdRP) to form a polymerase complex and synthesize viral RNA.38 Nsp10 is reported to perform a cofactor function for nsp14 and nsp16. Nsp14 possesses proofreading and guanine-N7-methyltransferase (N7-MTase) activities to further assist in the efficient replication of a large genome.12 Nsp10 enhances the proofreading function of nsp14, and in SARS-CoV-2, nsp14 forms a complex with nsp10 to carry out the host translational inhibition that further leads to the blockage of type I interferon (IFN-I)-dependent responses.39 Nsp13 acts as an RNA helicase and performs nucleoside triphosphate hydrolase (NTPase) and RNA unwinding activities.40 The complex of nsp16 (2′-O-methyltransferase) and nsp10 is involved in capping the viral RNA with a cap1 type of structure, which is similar to host mRNA and enables the viral RNA to evade the host immune system.41
Structural proteins
Although the molecular mechanisms and functions have not been fully elucidated for all SARS-CoV-2 structural proteins, these proteins are generally understood to execute major functions involved in formation, assembly, and maturation of viral particles.12 , 42 The S glycoprotein of coronaviruses is an indispensable determining factor in the viral attachment to host cell surface receptors. The S protein has 2 subunits (S1 and S2), where S1 binds to ACE2 receptor through specific receptor binding sequences and the S2 subunit interacts with cellular membranes to facilitate the virus-cell fusion.43 The E protein of coronaviruses is an essential component of the virion which is reported to form cationic channels (viroporins) inside endoplasmic reticulum-Golgi intermediate compartment (ERGIC) membranes while also interacting with the viral M protein as it carries out virus particle assembly and release.44 , 45 The E protein is also reported to interact with host epithelial cell tight junction regulator protein Zona Occludens-1 (ZO1), where this interaction was speculated to disrupt the epithelial barrier thereby facilitating the spread of virus.46 In addition to contributing to viral assembly, the M protein of SARS-CoV-2 has shown inhibition of type I and type III interferon mediated antiviral immunity.47 The N protein is also multifunctional, carrying out functions such as binding to the viral RNA, regulating viral transcription, genome packaging, and modulating host immune responses.12 Recently, the SARS-CoV-2 N protein was reported to also regulate the innate immune responses in host cells by interfering with type-I IFN and RIG-1 signaling.48
Accessory proteins
Several the accessory proteins have functions that are not yet deciphered completely. The general understanding for coronaviruses is that the accessory proteins are not involved in replication mechanisms directly, but play an essential role in pathogenesis.49 The accessory proteins show high sequence variability even among individual coronavirus species and are suspected to regulate host cell response to infection. Due largely to this lack of sequence similarity among these accessory proteins, the exact role of these proteins continues to be difficult to identify.49 , 50 It has been reported that currently circulating strains of SARS-CoV-2 contain mutations in their accessory genes that may play a role in transmissivity and pathogenicity.49 , 51 Some support for this has been found as the functions of a few of the accessory proteins of SARS-CoV-2 have been shown to be associated with viral pathogenesis. For instance, the ORF3a accessory protein of coronavirus was shown to act as viroporin that modulated the NF-kB signaling and NLRP3 inflammasome pathway that resulted in cytokine storms.49 And in SARS-CoV-2, ORF3a was reported to interfere with autophagic activity of host cells by blocking the degradation of autophagosomes in lysosomes.52 Another accessory protein, ORF3b, is a very small (22 amino acids), prematurely truncated protein in SARS-CoV-2 in comparison to its homolog in SARS-CoV (153 amino acids), and it was reported to antagonize type-I interferon signaling.53 Similarly, the ORF6 and ORF7 proteins of SARS-CoV-2 were also reported to antagonize and block the interferon responses.54 , 55 The ORF8 gene of SARS-CoV-2 has shown higher variability among coronavirus species and functional studies are thus limited.56 But, ORF8 has shown impairment of antigen presentation by interacting with class-I MHC molecules.57 The exact number of accessory proteins expressed for SARS-CoV-2 is uncertain. For example, the ORF10 protein of SARS-CoV-2 has not been reported in proteomics data of infected cells and the corresponding transcriptomic profiling is questionable in supporting the expression of ORF10 sub genomic RNA.12 , 32 In functional studies, the role of ORF10 in SARS-CoV-2 replication and human infections was reported to be nonsignificant.49 , 58
Vertical transmission risk
At the beginning of the COVID-19 pandemic, there were significant concerns that SARS-CoV-2 could be vertically transmitted as observed with the Zika virus (ZIKV) outbreaks only a few years prior.59 This has prompted several observational studies seeking to address this question, with differences in approaches and findings. In a study conducted by Edlow et al with a cohort of 127 pregnant individuals in 2020, no evidence of placental infection or definitive vertical transmission of SARS-CoV-2 was found in the 64 patients who tested positive for SARS-CoV-2.60 The transplacental transfer of anti-SARS-CoV-2 antibodies was found to be inefficient. Decreased viral load and co-expression of ACE2 and TMPRSS2 were proposed as defenses against vertical transmission. An acknowledged flaw of this early study was that it relied on a convenience sample for its controls, which could reflect differences in demographics between the cases and the controls.
In a larger cohort study from 2020 by Adhikari, et al among pregnant women screened in both inpatient and outpatient settings at a large county medical center, 252 tested positive for SARS-CoV-2 and 3122 tested negative.61 Similar adverse pregnancy outcomes were seen among both groups, and 3% of newborns tested for SARS-CoV-2 were infected with the virus. These infections were reported to occur in situations where the mother was asymptomatic or mildly symptomatic. There was no correlation between the presence of placental abnormalities and the severity of disease.
A retrospective cohort analysis by Dumitriu, et al reported data for 101 newborns from mothers that tested positive for SARS-CoV-2 in March or April of 2020. During the first 25 days of life, there was no indication of any vertical transmission, despite the newborns rooming-in with mothers and direct breastfeeding practices.62 A similar analysis by Flaherman, et al of data from 2020 on 263 infants from the Pregnancy Coronavirus Outcomes Registry (PRIORITY) database reported the incidence of a positive infant SARS-CoV-2 test at 1.1%, and the infants had minimal symptoms.63 These findings offer some reassurance in indicating that infants born to infected mothers fare well in the first 6–8 weeks of life.
Kotlyar et al conducted a systematic review of 68 case reports or cohort studies from 2020, in which they pooled information from 936 newborns whose mothers were infected with SARS-CoV-2. Within 48 hours of birth, nasopharyngeal swabs were used to test neonates for SARS-CoV-2 viral RNA, and 3.2% of the pooled population tested positive, particularly those where infection was experienced during the third trimester.64
A systematic review by Robaina-Castellanos, et al of 87 published or prepublished studies and articles from 2020 investigated possible reports of congenital/intrapartum/postpartum infection of SARS-CoV-2 during pregnancy. Investigators identified 53 reported neonates who tested positive for SARS-CoV-2 in the first 48 hours of life either showing presence of the virus by qPCR or IgM tests. The timing of infection corresponded to congenital or intrapartum transmission in 39.6% (21 of 53) of the COVID-19-positive newborns, and to postpartum transmission in 15.1% (8 of 53), with the remaining 45.3% (24 of 53) being unspecified. The investigators estimated that congenital or intrapartum infections occur in only 1.8%–8.0% of newborns born to individuals with COVID-19 at the end of their pregnancies, leading to the conclusion that such infections in the fetus/newborn are possible, but rare.65 Another systematic review was conducted by Bwire, et al, which identified 33 studies from 2020 that met inclusion criteria such as infection prevention and other control measures at birth. Out of 205 infants reported in those studies to be born to COVID-19 positive mothers, 13 (6.3%) tested positive for COVID-19 at birth. Six (18.8%) of the 33 studies reported on IgG/IgM levels against SARS-CoV-2, and in these studies IgG/IgM were detected in about 90% of newborns (10 of 11; 95% CI: 73.9%–107.9%) who tested negative for COVID-19, which supports the possibility of conferred immunity.66 Similarly, another observational study by Zimmerman et al reported on those who tested positive for COVID-19 during pregnancy and gave birth in 2020 under conditions of strict prevention and infection control, including separation of mothers and neonates, and reported that 4 of 67 (6.0%) newborns tested positive for COVID-19 despite those measures.
Adhikari, et al published a more recent study that compared disease severity in pregnancy and neonatal positivity during the pre-Delta, Delta, and Omicron eras.67 Of the 1919 infants born to patients with COVID-19 during the study period, 32 (3.1%) were found to have SARS-CoV-2 (13 in the pre-Delta period, 8 in the Delta period, and 11 in the Omicron period), but none of them were seriously ill. This rate corresponds well with what Adhikari reported in 2020.61 Early neonatal positivity did not vary significantly across the pre-Delta, Delta, and Omicron eras. The study's limitations include its reliance on cases from a single institution and the potential absence of data on vaccination or positive test results obtained outside the health care system.67
Although studies to date have not established common occurrence of maternal-fetal transmission for SARS-CoV-2,60, 61, 62, 63, 64, 65, 66, 67, 68 there does appear to be some evidence of vertical transmission with rates of 1%–3% or higher consistently reported across multiple reports and meta-analyses. The rare possibility of in utero transmission cannot be ruled out.61 , 64 , 68 , 71, 72, 73 Although neonatal infections of SARS-CoV-2 have been reported postpartum in these studies, discerning the exact route of infection and whether SARS-CoV-2 is vertically transmitted or transmitted during or after delivery can be challenging due to limitations with the data accuracy as a result of self-reporting and the possibility of false-positive and false-negative results.74 , 75 Definitive proof of SARS-CoV-2 transplacental transmission will require carefully designed studies with proper control measures and inclusion/exclusion criteria, in addition to in vivo experimental work that moves beyond observational reports. Future studies should also seek to understand the magnitude of natural passive immunity conferred from COVID-19 infected mother to fetus.
Pre-eclampsia
Pre-eclampsia (PE) is a condition where endothelial cells stop functioning due to failing vascular protection, giving rise to a hypertensive maternal phenotype.76 SARS-CoV-2 infections are known to induce pulmonary endothelium dysfunction, and this presents a major risk factor for pre-eclamptic maternal phenotypes when infection occurs during pregnancy.77 Unexpectedly, PE, a complex multifactorial obstetric syndrome, has several pathologies in common with COVID-19. For instance, in PE, the placental ischemia is caused by ineffective placentation and intraplacental malperfusion, which results in a hypoxic placenta secreting inflammatory cytokines and bioactive mediators like soluble FMS-like tyrosine kinase 1 (sFlt-1), which ultimately cause endothelial destruction.78 In COVID-19, alveolar hypoxia is caused by interstitial pneumonia, which then leads to a severe respiratory distress syndrome characterized by endothelial dysfunction and multiple organ dysfunction due to an inflammatory cytokine storm.79 Overall it appears that that both PE and COVID-19 cause an angiogenic imbalance, which in turn activates systemic inflammatory pathways associated with RAS activation.80 The angiogenic imbalance in PE is caused by a reduced level of the proangiogenic molecule placental growth factor (PlGF) and an elevated level of the anti-angiogenic factor sFlt-1, with RAS being one of the pathways that influences the regulation of these molecules.81 Since the RAS pathway is central to SARS-CoV-2 pathology, PE or similar observations were reported in both COVID-19 patients with pneumonia and pregnant individuals where angiogenic imbalance was found distributed between PIGF and sFLT1.27 , 82 , 83
According to a prospective observational study, pregnant individuals with severe SARS-CoV-2 infections may develop a PE-like syndrome.84 Similarly, a multinational cohort study reported a higher risk of developing pre-eclamptic phenotype in SARS-CoV-2 infected pregnant individuals (n = 706) compared to uninfected pregnant population (n = 1424).85 , 86
Recent research in Brazil indicated that the frequency of PE was high but did not vary significantly between women with and without confirmed cases of COVID-19 (nearly 10%).87 Clinical and laboratory manifestations at the appearance of illness did not accurately predict the occurrence of PE, and the incidence of PE was higher in COVID-19-positive pregnant women with chronic hypertension and obesity. These findings also extend support to the theory that COVID-19 with PE is associated with worse maternal and perinatal outcomes than COVID-19 alone.88 Few reports have been made public so far on the incidence of newly diagnosed hypertension among COVID-19 participants. When comparing COVID-19 patients admitted to the hospital, the incidence of new-onset hypertension was found to be significantly higher in those with more severe disease in a single-center, retrospective observational study.80 , 88 Further research is required to differentiate between PE and COVID-19 pathologies so that appropriate medical treatments can be provided.
Preterm births and stillbirths
There is evidence that COVID-19 also increases the risk of preterm birth, and other different types of developmental defects in neonates.17 , 89 One case study from early in the pandemic with 116 pregnant individuals ruled out the possibility of preterm birth or abortions associated with SARS-CoV-2 infected individuals,19 however this stands in contrast to other more recent reports. Data collected from individuals (n = 342,080) who gave birth between May 2020 and January 2021 in England reported that those who experienced a SARS-CoV-2 infection (n = 3,527) had a higher incidence of adverse pregnancy outcomes including PE, preterm birth, emergency cesarean delivery, and fetal death.90 This correlates with a large cohort study on 759 Swedish individuals that clearly indicated higher risk of preterm birth in COVID-19 affected pregnancies,91 and data from other studies also indicating a significant increased risk of preterm deliveries in SARS-CoV-2 infected pregnant individuals.92 , 93 In the United States, COVID-NET data from 2020 has shown a higher prevalence (nearly 12.6%) in preterm deliveries with pregnant individuals that had COVID-19 compared to the general population in 2018 (10.0%). From this data, the symptomatic individuals also showed increased risk (23.1%) of preterm birth.94 And according to a national study conducted in the United Kingdom, symptomatic SARS-CoV-2 was found to be associated with an increase in iatrogenic preterm births in various ethnic groups such as Black, Asian, and other minorities.95 An increasing incidence of preterm deliveries has also been reported in SARS-CoV-2 infected pregnancies.96 And in line with these reports, a systematic review shows that reported rates of preterm births in COVID-19 patients varies greatly between studies, from 14.3% to 61.2%.97 Despite the variety of available clinical reports, there is some consensus among them that there is a strong link between premature birth and symptomatic COVID-19 during pregnancy. Recently, a large cohort study indicated that the SARS-CoV-2 infection during third trimester is significantly associated with preterm births as compared to matched noninfected controls.98 First and second trimester infections did not cause any adverse pregnancy outcomes when compared to noninfected controls. This study emphasizes the importance of gestational age at SARS-CoV-2 infection in determining the outcome of the pregnancy. However, these reports raise unanswered questions about underlying mechanisms that may lead to preterm birth during SARS-CoV-2 infections. For instance, there is evidence from one small study that shows that immune cells expressing ACE2 are able to infiltrate the placenta, which may further enhance SARS-CoV-2 infection and increase risk of preterm deliveries as well as placental infection.99
The question of whether COVID-19 exposure during pregnancy increases the risk of stillbirth has been similarly fraught with mixed results. Stillbirth rates were reported to be higher during the early stages of the pandemic, but later a few of studies have found no evidence that they are significantly higher in COVID-19-infected pregnancies compared to uninfected pregnancies (0.2% ).100 , 101 For instance, a retrospective cohort study (2017–2020) in France reported that the rate of stillbirths during the pandemic has not increased in comparison to prepandemic levels.102 However, a set of more recent studies have again linked COVID-19 to an increase in stillbirths.103, 104, 105, 106, 107 This is corroborated by a CDC report that compared the number of stillbirths in the US before and after the Delta time period. The data indicated that stillbirth rates were nearly 4 times higher during the Delta phase than pre-Delta phase.103 Many of these studies did not explore in detail the biological mechanisms connecting stillbirth and COVID-19, but abnormalities in the placenta are the most common cause of stillbirth.108 Severe placental damage due to placental malperfusion was found to be strongly associated with fetal deaths in 1 study from Ireland that linked 6 stillbirths to SARS-CoV-related placentitis.107 Similarly, another study that looked into the pathological mechanism linked 5 stillbirths to COVID-19-induced placentitis.109 A larger study that included data from 12 nations reported that SARS-CoV-2 has been linked to 68 stillbirths and/or neonatal deaths, and documented a common pattern of abnormalities among these placentas such as trophoblast necrosis, fibrin deposition, and chronic histiocytic intervillositis as the most prominent pathology lesions.104 And in a recent case study, 2 stillbirths were linked to severe placentitis induced by SARS-CoV-2 Delta variant infection.106 In summary, evidence associating maternal SARS-CoV-2 infection with an increased risk of stillbirth is accumulating and should now be considered with particular regard to the emergence of recent VOCs. Mechanistic research and sustained observation will be required to establish whether stillbirths are the primary result of SARS-CoV-2 infection or a defensive mechanism adopted by the placenta to dispose of virus-infected cells.
Neonatal, infant, and child outcomes following SARS-CoV-2 infection
Limited data is available on neonatal outcomes of maternal COVID-19. Several studies report neonates that tested positive for SARS-CoV-2 RNA had no observed adverse outcomes,96 , 110 and others report that the majority of newborns and infants with SARS-CoV-2 infection appear to have mild disease.111 , 96 However, a review gathered and reported data for 629 neonates that were tested for the virus after birth with 35 positive cases an reported that some adverse effects were observed. A common symptom was respiratory distress in the symptomatic cases. Other described symptoms were gastrointestinal, lethargy and temperature instability, although it is challenging to attribute all these symptoms to COVID-19 because some virus negative neonates also developed similar symptoms.111 Another recent report has identified late onset neonatal sepsis as a possible severe outcome of SARS-CoV-2 infection, which necessitated mechanical ventilation.112
A recent retrospective cohort study that included 222 births to SARS-CoV-2 positive mothers attempted to answer the question of whether or not prenatal exposure to SARS-CoV-2 causes neurodevelopmental delay or impairment during the first year of life.113 Results indicated that the neonates exposed during third trimester experienced substantial neurodevelopment abnormalities linked to motor function, speech and language.113 Other preliminary studies have assessed different time periods following delivery, such as 3, 6, and 12 months, and also suggested that SARS-CoV-2 infection during pregnancy can have detrimental impacts on the brain development of the fetus.114, 115, 116 Emerging data suggests the Delta variant of SARS-CoV-2 is increasingly associated with severe illness in young children.117 A large multicenter investigation indicated that COVID-19 was associated with widespread, temporary brain impairment in children and adolescents hospitalized with the virus.118 Studies such as these raise significant concern about early effects of COVID-19 exposure during pregnancy that may not be immediately apparent and indicate the need for continued investigation with larger cohorts, longer follow-ups, and more focus on the recent variants to determine the impact of SARS-CoV-2 exposure on children's health.
It is known that substantial neurodevelopmental impairment in offspring can be caused by the activation of the maternal immune system pathway, regardless of whether the infection was caused by a virus or bacteria.116 , 119 , 120 Extreme cases with widespread neurological problems and mortality in children exposed to SARS-CoV-2 infection have been connected to elevated levels of inflammation.118 Preliminary investigations have also shown that pregnant women infected with SARS-CoV-2 had highly elevated levels of proinflammatory cytokines such as IL-6, IFN-γ, and IL-1β.121 , 122 Enhanced evidence from transcriptome analyses of placental villi and single-cell RNA sequencing of cord blood mononuclear cells has shown that SARS-CoV-2 infection during pregnancy activates the maternal immune system, with considerable overexpression of interferon stimulated genes (ISGs).123 , 124 These, and other possible underlying causes of adverse developmental outcomes in neonates and young children, need further investigation, particularly as more is learned about Omicron and other emerging variants that present new potential dangers.24 , 111 Several unanswered questions remain, including the following: how does infection timing (first vs third trimester) affect overall neurological development; what types of changes are associated with infection; and whether these changes are persistent or transient; and finally, a robust cell model needs to be developed to understand various signaling pathways.
Placental histopathology
Early in the pandemic, no unique histopathological signature was seen with maternal SARS-CoV-2 infection. However VOCs such as Alpha and Delta were found to be associated with a distinct SARS-CoV-2 generated placentitis that comprises histiocytic intervillosititis, perivillous fibrin, and necrotic villous trophoblasts.60 , 104 , 106 , 125 The infection of SARS-CoV-2 in the placenta produces a higher rate of decidual arteriopathy and other maternal malperfusion features compared to the normal placenta. This suggests that the infection causes inflammation and changes in the placenta that can lead to long-term multisystemic defects in exposed infants.126 A review of 40 studies from COVID-19 pregnancies examined clinical samples including vaginal secretions (22.5%), amniotic fluid (35%), breast milk (22.5%), and umbilical cord blood. Quantitative real time polymerase chain reaction (qRT-PCR) was also performed on all neonates by swabbing. In 8 of these studies, the neonates tested positive for SARS-CoV-2, with 2 studies showing presence of IgM antibody in neonatal blood. The histopathological analysis of the placenta in individuals with COVID-19 showed the presence of viral particles, inflammation and vascular malperfusion. In 17 of the studies, placenta, breast milk, umbilical cord, and amniotic fluid were tested, and only 1 amniotic fluid sample tested positive.127 In another study, there was evidence of defects in the placenta upon SARS-CoV-2 infection due to the deposition of fibrin that hampers the maternal-fetal gas exchange, leading to increased fetal distress and possibly resulting in emergency Caesarean-section delivery.128 These studies provide compelling evidence that SARS-CoV-2 can colonize different placenta compartments (Fig 2 ), and so the possibility of related complications occurring during pregnancy upon infection cannot be overlooked.70
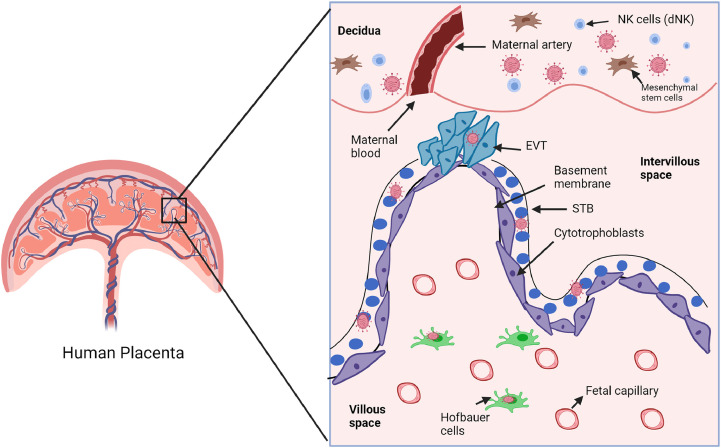
Fig 2.
Schematic representation of different compartments of the placenta colonized with SARS-CoV-2. The placenta comprises different types of cells that form a strong physiological and immune barrier around the fetus. The outer trophoblast layer is a physical barrier divided into 2 layers: the inner cytotrophoblasts (purple) lining the basement membrane and outer multi nucleated (blue color lined with black solid line) syncytiotrophoblasts (STBs). The decidua is the maternal part containing blood vessels and different types of cells such as mesenchymal stem cells and decidual natural killer cells (dNKs). The intervillous space is filled with maternal blood. Based upon multiple studies, SARS-CoV-2 was shown to colonize mainly in STBs, extravillous trophoblasts (EVTs), cytotrophoblasts maternal decidua and fetal macrophages (Hofbauer cells). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Additionally, the different variants of SARS-CoV-2 may have varying impacts on the placenta. After the Alpha wave hit the United Kingdom, researchers published a significant case series of placentas infected with SARS-CoV-2.129 This study also found that placentas from asymptomatic individuals had placentitis signatures identical to those of persons with severe disease and other comorbidities, such as diabetes or obesity.129 According to a more recent report the Delta variant has shown severe placentitis and greater fetal demise or distress.106 , 107 Another CDC study shows that the Delta period had a greater rate of stillbirth and serious placentitis.103 In a study involving researchers from 12 countries, researchers found severe placentitis with persistent histiocytic intervillositis and elevated fibrin deposition in 68 stillbirths who tested positive for SARS-CoV-2.104 SARS-CoV-2-associated placentitis was also observed in 6 patients in a Chicago-based cohort investigation.105 Two cases of viremia were also reported in this investigation, an occurrence not previously documented for SARS-CoV-2 infections. Histiocytic intervillositis, syncytial node enlargement, and increased fibrin deposition were all observed at higher rates in SARS-CoV-2 infected placentas, according to a retrospective study conducted in Italy.130 As a whole, these studies point to a distinctive form of placentitis caused by SARS-CoV-2 infections, which causes extensive placental destruction and increases the risk to the developing fetus. The effect of the recent Omicron variant on pregnancy related complications remains to be determined in ensuing months
SARS-CoV-2 entry into placental cells
It is evident that the placenta is susceptible to SARS-CoV-2 infections due to various factors related to virus entry.70 As noted, SARS-COV-2 prefers to bind to the human ACE2 cell receptor via the viral spike (S) proteins to initiate entry into the host cell.12 , 31 , 131 , 132 The next crucial step in coronavirus pathogenesis after attachment of S protein onto ACE2 is the cleavage of S by the cellular protease Transmembrane Serine Protease 2 (TMPRSS2).43 , 133 The simultaneous expression of ACE2 and TMPRSS2 in respiratory cells assists in the pathogenesis of SARS-CoV-2, as it also does in case of SARS-CoV. Notably, the inhibition of TMPRSS2 significantly decreases SARS-CoV-2 virus entry into lung cell lines.132 , 134 An alternate endosomal cathepsin protease (CatB and CatL)-dependent pathway for S protein priming and cell entry has also been reported in older research on other coronaviruses.12 , 135 For SARS-CoV-2, the major pathway required for entry was reported to be TMPRSS2-dependent, since inhibition of this protease significantly reduced the virus entry in lung cells.26 Although TMPRSS2-dependent cleavage is most prevalent, S protein cleavage can be primed by multiple cellular host proteases such as furin, trypsin, and cathepsins.26 Despite showing similarity in receptor binding and cleavage requirements, the pathogenesis mechanisms of SARS-CoV and SARS-CoV-2 differ greatly in terms of rate and site of replication. Incidentally, the SARS-CoV outbreak of 2003–2004 was more limited and had a greater rate of mortality, whereas SARS-CoV-2 infection is highly contagious and more efficient in evading immune surveillance.136 These differences within the same group of beta-coronaviruses highlight the importance of in-depth investigation of the structure and related functions and mechanisms performed by different proteins of these viruses. It is clear that the extent of coronavirus infection and tissue tropism depends upon multiple factors including, ACE2 and other receptor expression profiles, TMPRSS2 availability, and other proteases required for S protein priming such as cathepsins and furin.25
The higher ACE2 expression in uteroplacental area during gestation is clearly responsible for the higher susceptibility of the placenta to infection. ACE2 expression levels and distribution during pregnancy are dynamic. During early pregnancy, a higher ACE2 expression was reported in decidua and near villi, whereas ACE2 expression was reduced at the maternal-fetal interface.137 As pregnancy advances, the syncytiotrophoblast (STB) layer, cytotrophobasts (CTBs) and villous endothelial cells begin to show higher ACE2 expression.138 These dynamic ACE2 expression patterns during pregnancy are directly correlated with varying degrees of SARS-CoV-2 infection and disease severity in pregnant individuals.138
Along with ACE2, TMPRSS2 expression has also been reported in the uteroplacental area during different stages of gestation.139 This was shown in RNA-based expression studies that were carried out mainly on frozen placenta samples, although these studies lacked specific information regarding ACE2 receptor expression at the membrane level and its enzymatic functions in the placenta.139 However, another study has described an important mechanism of ACE2 shedding by metalloproteinase ADAM17 in placental cells, which could serve as an additional protective pathway against SARS-CoV-2 infection by reducing the availability of membrane bound ACE2.140 SARS-CoV-2 entry into placental cells could also be governed by recently described alternative entry routes that are TMPRSS2-independent in that they rely on other human proteases such as cathepsin L and furin.140 , 141
Recently the cluster of differentiation 147 (CD147) receptor or the novel SARS-CoV-2 entry receptor basigin (BSG) has been shown to bind to S protein, and the higher expression of these receptors in placentas and chorioamniotic membranes has been described in reports.25 , 142 The maternal peripheral immune cells are also reported to express ACE2, and these cells play an essential role during placentation and labor.141 The adhesion molecule CD169, which is expressed on macrophages, was reported to increase the SARS-CoV-2 infection by an unknown mechanism.140 Macrophages and other immune cells are known to infiltrate into placenta during chorioamnionitis, which seems to induce the expression of ACE2.141 These studies emphasize the fact that immune cells could also act as an alternative route of SARS-CoV-2 entry into placentas under inflammatory conditions like chorioamnionitis.
It should also be noted that in recent studies, a reduced expression of ACE2 was reported upon SARS-CoV-2 infection in placentas.27 Since ACE2 is an essential member of renin-angiotensin system (RAS) pathway, its depletion could adversely affect pregnancy outcomes.25 SARS-CoV-2 infected placentas with depleted ACE2 were reported to show a phenotype similar to what has been observed in PE and IUGR.25 , 84 , 143 Higher expression of PE markers, fms-like tyrosine kinase-1 (s-Flt-1) and AngII receptor type-1 (AT1) autoantibodies, were identified in SARS-CoV-2 infected pregnant individuals.27 It has been shown through in vitro investigations of placental cell lines and primary trophoblast cells that SARS-CoV-2 is more infective to cytotrophoblasts, trophoblast stem cells, and precursor cells than STBs and Hofbauer cells.124 According to a separate study, isolated differentiated primary trophoblasts from term placentas were not infected with SARS-CoV-2 and did not produce a significant titer after 4 days of examination.144 Authors note that SARS-CoV-2 was not able to infect isolated primary trophoblasts because of moderate ACE2 expression and substantial lack of TMPRSS2 expression at term.144 This work further highlights the importance of ACE2 and TMPRSS2 expression dynamics throughout pregnancy as a possible determinant of infection timing and outcome.
The placenta as a barrier during viral infections
During pregnancy, the placenta is the strongest barrier in protecting the fetus from different pathogens, including bacteria and viruses. The fetal immune system is underdeveloped and not able to efficiently combat pathogens.145 , 146 The placenta is a complex organization of multiple cell types, however trophoblasts are considered as the most crucial functional and architectural elements in forming a strong barrier to pathogens.147 Placental villi are composed of trophoblast cells, which form a polarized epithelium. Trophoblast cells are organized into different layers of floating and anchoring villi with high proliferative and migratory potential, which allows cells to migrate from the basal membrane and their fusion further gives rise to multinucleated cells known as syncytiotrophoblasts (STBs).148 The STB layer of the placenta maintains a strong barrier to protect the fetus from invading pathogens. Also, these trophoblast cells have been associated with essential immune regulation mechanisms in the placenta during infections.149 However, the viruses have developed several host cell-evading strategies along with escape mechanisms from immune surveillance. Moreover, the trophoblast cells are reported to express receptors which are utilized by viruses to make entry.150 Viruses such as Cytomegalovirus (CMV), Herpes Simplex Virus (HSV) and Zika virus (ZIKV) utilize different strategies to invade through the trophoblast layer and cross the placental barrier.
The SARS-CoV-2 virus has been reported in different compartments of the placenta at different stages of pregnancy (Fig 1).27 , 151 , 152 For example, a recent study identified SARS-CoV-2 in maternal decidua, Hofbauer cells, fetal trophoblasts, and premature placenta.27 The virus colonization in term placenta does not show major histopathological changes as reported in other studies.69 , 153 However, in preterm placentas, significant inflammation has been reported.154 Also, the virus presence during the second trimester of pregnancy was associated with increased hospitalization and higher risks of preterm births.155 Since the SARS-CoV-2 virus is known to hijack multiple cell survival pathways, it could interfere with normal trophoblast function and lead to detrimental effects on fetal health. Thus, there remains a great need to identify the mechanisms in the placenta in response to SARS-CoV-2 infection.155
SARS-CoV-2 and placental immunity
A complex and enigmatic immunological balance is maintained during pregnancy in which a temporary state of immunosuppression in the mother is needed for the implantation event to be successful, while at the same time strong defenses against various infections must also be retained.156 The maternal immune responses follow a precise “immune clock” dynamic throughout gestation that shifts between anti-inflammatory (first and third trimester) and proinflammatory (second trimester) phenotypes, eventually maintaining the maternal tolerance to the fetus.157 , 158 The innate immune mechanisms predominate in early pregnancy (first trimester) and subsequently with advancing pregnancy. This includes a depletion of peripheral B-cells, reduced buildup of effector T cells in decidua, and the development of a tolerogenic phenotype as described in previous reports.157 All of these changes are tightly coordinated by maternal decidua that comprise a diverse population of immune cells such as natural killer (NK) cells, dendritic cells (DC), macrophages, and T cells.157 , 159 Decidual immunity is crucial for maintaining the pregnancy-specific immunological state, as it performs several functions such as training adaptive cells, recruiting more leukocytes to decidua, and regulating peripheral immune cells.160 The communication between these different immune cells is critical for a healthy placentation, therefore dysregulation of these interactions has been observed in various pregnancy related complications such as PE, recurrent miscarriage, fetal growth restrictions, preterm birth, and chorioamnionitis.157 , 161 In normal pregnancy, the global activation of innate immunity further regulates several mechanisms such as increased complement activity, increase in type I interferon (IFN) producing DC, activation of antiviral IFN-α-induced STAT1 signaling pathways, and increased expression of NK cells, myeloid DC, and monocytes.157 The hyperactivation or attenuated immune responses during acute viral infections could lead to the various adverse pregnancy related complications that have been observed.157
In recent studies, the SARS-CoV-2 accessory proteins have shown essential roles in immune evasion pathways such as inhibition of cytokine secretion and antagonizing IFN responses.49 SARS-CoV-2 infection appears to reduce the lymphocyte count in comparison to inflammatory monocytes, and could contribute to the development of the cytokine storm that leads to multisystem organ failure.20 The higher expression of different cytokines was quantified from the plasma of COVID-19 patients and includes interleukins, interferon-γ-inducible protein 10 (IP-10 or CXCL10), monocyte chemoattractant protein 1 (MCP-1), granulocyte-colony stimulating factor (GCSF), and tumor necrosis factor α (TNF-α).162 Also, in COVID-19 positive pregnant individuals, a higher level of C-reactive protein was reported.89 Notably the SARS-CoV-2 induced cytokine storm may lead to higher risk of adverse pregnancy outcomes by stabilizing a pro-inflammatory state that could greatly affect fetal neural development.20 , 163 In a single cell transcriptomic study, SARS-CoV-2 infection was reported to generate a strong immunological response with upregulated interferon pathways, T cell activation and NK cell proliferation.124 These studies contribute to evidence that inflammatory responses to SARS-CoV-2 infection could lead to a higher risk of adverse pregnancy outcomes.
A study of SARS-CoV-2-exposed placenta explants showed that infectious virus particles were produced despite eliciting cytotoxicity or a proinflammatory cytokine response.164 Recent research has demonstrated that SARS-CoV-2 is able to replicate effectively in placenta ex-vivo cultures, and that STBs are a suitable host for the virus.165 Significant activation of pro-inflammatory cytokines and chemokines, typically associated with "cytokine storm," has also been found in an in vitro cell line investigation of SARS-Cov-2 spike protein.166 A robust cell line or organoid model may support precise understanding of SARS-CoV-2 pathogenesis during pregnancy.167 From the above reports, it is evident that SARS-CoV-2 infections in the placenta could lead to adverse effects by impairing the essential physiological balance maintained through the RAS pathway. More in-depth mechanistic studies are needed to understand the range of possible long-term developmental defects on maternal and fetal health due to dysregulation of RAS pathways.25
Currently, studies are lacking that investigate interactions of SARS-CoV-2 with decidual immune cells, although the SARS-CoV-2 effect on peripheral blood leukocytes has been reported in severely affected COVID-19 patients where a significant decrease in IFN-γ and TNF-α levels in CD4+ T cells was observed.168 Being the first-line of defense against various infections, the placenta protects the fetus by supporting different immunological tools such as: expression of toll-like receptors (TLRs) at different gestational stages to recognize the pathogen, a diverse population of immune cells in decidua, caspase activation pathways, production of cytokines and inflammatory responses, and release of antimicrobial peptides into amniotic fluid.74 , 159 A second line of defense in the form of NOD-like receptors (NLRs) has also been reported in trophoblasts. Additional fundamental pathways of placental defense can include: type III IFN signaling, secreted miRNAs activating autophagy, and the NF-κB pathway.74
Bordt et al recently revealed fetal sex-dependent differential immune responses across the maternal-fetal interface in response to SARS-CoV-2 infections.169 Mothers with male fetuses exhibited lower maternal titers of SARS-CoV-2 IgG antibodies. This finding suggests that fetal sex alters maternal antibody responses during pregnancy. Pregnancies carrying male fetuses had a lower ratio of SARS-CoV-2 antibodies in cord blood. Epidemiological studies have found that males are generally more likely to become infected with and suffer more severely from COVID-19 than females of any age group.170, 171, 172, 173 The study by Bordt et al sheds light on an important aspect of fetal sex-dependent immune responses in the placenta, which may have broader implications for our ability to comprehend the effects of the SARS-CoV-2 vaccine during pregnancy. Although it is evident that SARS-CoV-2 can infect the placenta, there is minimal information regarding how SARS-CoV-2 proteins interact with different placental defense pathways. Further investigation into the molecular interactions of SARS-CoV-2 at the maternal-fetal interface is needed to fill this important knowledge gap.
Safety and effectiveness of COVID-19 vaccines during pregnancy
The global collaboration of multiple pharmaceutical companies and researchers in conjunction with government agencies has actively pursued the development of safe and effective vaccines against COVID-19.174 , 175 In addition to the traditional vaccine platforms such as those based on viruses or proteins, novel platforms based on nucleic acids or viral vectors were actively taken into consideration early in the pandemic.176 As a result, the mRNA-based COVID-19 vaccines were developed as new generation vaccine platforms and therefore some concerns were raised regarding their safety.177 However, the safety and mechanism of these vaccines have been corroborated by clinical trials and scientific evidence. Following the clinical trials, the Pfizer-BioNTech mRNA vaccine was fully approved by the Food & Drug Administration (FDA) in August of 2021 for use in the United States for adults 16 years of age and older, including pregnant individuals.178 The safety and mechanism of mRNA nanoparticle and adenovirus vector based vaccines has also been developed and established through decades of prior work.179 , 180
A large retrospective cohort study carried out in Israel adds some recent evidence of COVID-19 vaccine efficacy in pregnant individuals. In this study, the Pfizer-BioNTech mRNA vaccine in pregnant individuals (n = 7,530) reduced the risk of SARS-CoV-2 infection compared to the nonvaccinated group (n = 7530).181 To gather self-reported surveillance on postvaccination safety, the US Centers for Disease Control and Prevention (CDC) established the V-safe After Vaccination Health Checker and Vaccine Adverse Event Reporting System (VAERS), which includes a record of health outcomes for pregnant individuals who are vaccinated for COVID-19. A study used data collected from 35,691 participants in this surveillance system and reported that adverse pregnancy outcomes for those who had been vaccinated with the Pfizer-BioNTech or Moderna mRNA vaccines during pregnancy occurred at a rate similar to what has been observed prior to the pandemic.182 The CDC has reported on additional analysis of the V-safe surveillance data, which found no increased risk of miscarriage among pregnant individuals who received an mRNA COVID-19 vaccine.183 , 184 Recently, an observational cohort study of 10,861 pregnant individuals vaccinated with BNT162b2 mRNA vaccine suggested that this vaccine is also highly protective and effective in pregnant individuals, just as it is in the general population.185 It has also been reported that the vaccinated mother can transfer antibodies to the fetus, with a higher persistence of anti-Spike antibodies in infants seen in comparison to infants whose mother got natural immunity to SARS CoV2 infection.186
These preliminary findings offer encouraging evidence that these vaccines are safe and beneficial for pregnant individuals, and further follow-up is continuing to evaluate the longitudinal outcome of maternal pregnancy and infant health.182 More rigorous studies are also underway to rule out any detrimental effects on fetal health. The continued surges of COVID-19 infections due largely to highly virulent Delta variant and highly transmissible Omicron variant of SARS-CoV-2 present a substantial risk for pregnant individuals, and so there will be a continued demand for more safety data for those who are pregnant.
The need for more inclusive data on COVID-19, vaccines, and pregnancy
Despite being a higher risk group, pregnant individuals have not been included in any of the trials for vaccines approved for use in the US and UK Since pregnancy is considered an immunosuppressed state already, excluding this population from SARS-CoV-2 vaccine trials causes a notable limitation in the available safety data that may influence a pregnant woman's decision to receive a COVID-19 vaccine.187, 188, 189 As noted, several studies report evidence of a higher risk of adverse outcomes for newborns in SARS-CoV-2 affected pregnancies, thus a more comprehensive vaccine trial dataset that includes pregnant individuals can benefit fetal/neonatal health as well.73 , 190, 191, 192
Including pregnancy data will also address some public concerns about harmful effects of mRNA platform-based vaccines in relation to pregnancy, not all of which are supported by reliable evidence. For instance, concerns have been raised about the effect on fertility and cross reactivity of the anti-spike antibody with syncytin-1, a human placental protein that is required for human placental morphogenesis and expressed in placental syncytiotrophoblasts.193 , 194 , 195 However, a number of reports have illustrated that the amino acid sequences of syncytin-1 and SARS-CoV-2 spike protein are quite different, and the serum from COVID-19 patients does not show any cross reactivity with syncytin-1.194 , 196 , 197 Some clinical reports have also suggested that SARS-CoV-2 infection does not interfere with early pregnancy and most complications have been observed in second or third trimester.198, 199, 200, 201 However, these reports clearly conflict with other concerns about interference of vaccine elicited antibodies with fertility and conceiving pregnancy. Thus, concerns based on unsubstantiated or conflicting data can generate further COVID-19 vaccine hesitancy among pregnant individuals, but may be alleviated by ongoing studies that address the outcome of COVID-19 vaccines in pregnant individuals and help to establish their effects on the fetus and newborns.194
The emerging SARS-CoV-2 variants further emphasize the immediate need for more vaccine data in the context of pregnancy. Compared to the parent SARS-CoV-2 strain, new variants have demonstrated more pathogenic traits such as higher transmissibility in variant Alpha, the ability to escape natural immunity and neutralizing antibodies in variant Beta, and increased infectivity in variant Delta.202, 203, 204, 205, 206 Some work has begun to address questions surrounding these variants and pregnancy. A recent explorative cohort study has shown that mRNA vaccine administration in pregnant individuals generated significant antibodies against SARS-CoV-2 VOCs Alpha and Beta, and these antibodies were also detected in breast milk and newborn cord blood.188 , 207 Another study reported that maternal vaccination resulted in significant detection of anti-SARS-CoV-2 antibodies in breast milk, and no adverse effect was reported on the infant health.208 Atyeo et al found that pregnant and lactating women need at least 2 doses to reach immune levels similar to those seen in nonpregnant women.209 In this study, it was found that Fc receptor binding and other antibody effector functions were delayed in pregnant and lactating women after a single dose of vaccination compared to nonpregnant women, but were restored later following a second dose.209 Antibody generation in response to an mRNa-based vaccine was reported to have begun 5 days after the initial dosage, and transplacental transfer was recorded 16 days later, thus timing of vaccination is an additional crucial consideration.210
The Omicron variant of SARS-CoV-2 is the most altered, and as a result, it has developed superior immunological escape capabilities.8 In a study by Sievers et al, the ability of serum or plasma from SARS-CoV-2 infected or vaccinated patients to neutralize pseudoviruses producing spike proteins was evaluated.211 When compared to the improved neutralization activity, the investigators discovered that the Beta and Omicron variants neutralized poorly. The results strengthen the case for using booster vaccines and increase our knowledge of the extent to which a particular variation can dodge immune responses. Two doses of the mRNA vaccine during pregnancy and a booster shot afterward were most effective.211 Overall, the results imply that immunizations offer enhanced protection during pregnancy if timely boosters are administered, and that the severity of disease is also mitigated in pregnant women.
Conclusion and future perspectives
There is now accumulating evidence that SARS-CoV-2 infection during pregnancy leads to a higher risk of adverse outcomes for maternal, fetal, and neonatal health. Although SARS-CoV-2 transplacental vertical transmission appears to be rare based on limited data of neonatal disease cases, other risks remain. Some increased risks may stem from the ability of SARS-CoV-2 to colonize in different placental compartments such as STB, CTB, EVT, and even in Hofbauer cells. Moreover, the dynamic expression of ACE2 in the placenta throughout gestation makes it more vulnerable to SARS-CoV-2 infection, although there are protective mechanisms in the placenta operating actively during infections to protect the fetus. However, these mechanisms are not fully understood and still needed to be explored. For instance, ACE2 is a key binding receptor for the virus, but also an important part of the RAS pathway that regulates maternal hemodynamics during pregnancy through various direct and indirect mechanisms. The poor placentation observed in PE and IUGR because of ACE2 dysfunction has been described in literature but there is still a need to elucidate the exact role of ACE2 in pregnancy and in the context of COVID-19 disease development. Current data shows ACE2 receptor downregulation with SARS-CoV-2 internalization, which may further affect the placenta vascularization and healthy development, and this is supported by the reports of PE-like phenotype development displayed in COVID-19 infections during pregnancy. Immune cells also have ACE2 receptors expressed on their membranes that could lead to another entry route for SARS-CoV-2. Since SARS-CoV-2 viral accessory proteins are reported to antagonize various interferon responses in the host cells, the immunological responses need to be more fully characterized in the placenta during SARS-CoV-2 infection at different stages of gestation. A significant barrier to this work is the lack of a well-developed human placenta animal model. Alternatively, organoid models may be the most optimal current means of characterizing the SARS-CoV-2 pathogenesis in pregnant individuals. Although current vaccines against COVID-19 seem to be safe and effective during pregnancy, the emerging new variants of SARS-CoV-2 with higher transmissibility and better immune escape abilities raise new questions about effectiveness. The ever-changing Omicron variants have been recently identified to produce a new sub-lineage, BA.5, which is increasingly replacing the older Omicron lineages.212 A high degree of infectivity, similar to that of Delta, and increased transmissibility, like that of an older version of Omicron, have been attributed to this BA.5 variation.212 Thus, it is crucial to continue vaccine development that includes pregnant individuals in the clinical trials to better cope with the severity of disease, along with continuing further investigation into unique features of SARS-CoV-2 pathogenesis in pregnant individuals.
Key points
-
•
Pregnant individuals are at greater risk of SARS-CoV-2 infection that could lead to adverse pregnancy outcomes.
-
•
Vertical transmission of SARS-CoV-2 appears to be rare based upon all the reports to date, but other risks to fetal health remain.
-
•
SARS-CoV-2 entry into placental cells could be governed by alternate endocytic pathways other than ACE2 and TMPRSS2.
-
•
SARS-CoV-2 presence in the placenta could lead to various histopathological changes.
-
•
SARS-CoV-2 accessory proteins play a major role in antagonizing host immune responses.
-
•
The current COVID-19 vaccines are encouraged and declared safe by the US Food and Drug Administration for use in pregnant individuals to reduce the serious pregnancy related complications due to SARS-CoV-2 infection.
Acknowledgments
Conflict of interest: All authors have read the journal's policy on disclosure of potential conflicts of interest and report the following: IUM serves on the Scientific Advisory Board of Luca Biologics. No other conflict of interest exists.
This work was funded in part by NIH / NICHD grant R01 HD091218 and 3R01HD091218-04S1(RADx-UP Supplement) (To IUM). All authors have read the journal's authorship agreement, and the manuscript has been reviewed by and approved by all named authors. We thank Dr. Robert Lawrence for their valuable comments on the manuscript. Figures were generated in part using BioRender.
References
- 1.WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard With Vaccination Data. 2022 Available at: https://covid19.who.int/.
- 2.Tian D., Sun Y., Xu H., Ye Q. The emergence and epidemic characteristics of the highly mutated SARS-CoV-2 Omicron variant. J. Med. Virol. 2022;94:2376–2383. doi: 10.1002/jmv.27643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan Y., Li X, Zhang L., et al. SARS-CoV-2 Omicron variant: recent progress and future perspectives. Signal Transduct Target Ther. 2022;7:141. doi: 10.1038/s41392-022-00997-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattacharyya R.P., Hanage W.P. Challenges in inferring intrinsic severity of the SARS-CoV-2 Omicron variant. N Engl J Med. 2022;386:e14. doi: 10.1056/NEJMp2119682. [DOI] [PubMed] [Google Scholar]
- 5.Long B., et al. Clinical update on COVID-19 for the emergency clinician: presentation and evaluation. Am J Emerg Med. 2022;54:46–57. doi: 10.1016/j.ajem.2022.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh J., Pandit P., McArthur A.G., Banerjee A., Mossman K. Evolutionary trajectory of SARS-CoV-2 and emerging variants. Virol J. 2021;18:1–21. doi: 10.1186/s12985-021-01633-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harvey W.T., et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19:409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ledford H. How severe are Omicron infections. Nature. 2021;600:577–578. doi: 10.1038/d41586-021-03794-8. [DOI] [PubMed] [Google Scholar]
- 9.Hossain M.K., Hassanzadeganroudsari M., Apostolopoulos V. The emergence of new strains of SARS-CoV-2. What does it mean for COVID-19 vaccines? Expert Rev Vacc. 2021;20:1. doi: 10.1080/14760584.2021.1915140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corman VM, Muth D, Niemeyer D, Drosten C. Hosts and Sources of Endemic Human Coronaviruses. Adv Virus Res. 2018;100:163–188. doi: 10.1016/bs.aivir.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.V'kovski P., Kratzel A., Steiner S., Stalder H., Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. 2021;19:1. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu R., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.PG G., L Q., SH P. COVID-19 acute respiratory distress syndrome (ARDS): clinical features and differences from typical pre-COVID-19 ARDS. Med J Aust. 2020;213:54–56. doi: 10.5694/mja2.50674. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.M B, et al. Inflamm-aging: why older men are the most susceptible to SARS-CoV-2 complicated outcomes. Cytokine Growth Factor Rev. 2020;53:33–37. doi: 10.1016/j.cytogfr.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.M M., P S.-N., SD S., O V. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5:831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 17.F E, et al. A systematic scoping review of COVID-19 during pregnancy and childbirth. Int. J. Gynaecol. Obstet. 2020;150:47–52. doi: 10.1002/ijgo.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.C F, et al. Perinatal transmission of 2019 coronavirus disease-associated severe acute respiratory syndrome coronavirus 2: should we worry? Clin. Infect. Dis. 2021;72:862–864. doi: 10.1093/cid/ciaa226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.J Y, et al. Coronavirus disease 2019 in pregnant women: a report based on 116 cases. Am. J. Obstet. Gynecol. 2020;223 doi: 10.1016/j.ajog.2020.04.014. 111.e1-111.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verma S., Carter E.B., Mysorekar I.U. SARS-CoV2 and pregnancy: An invisible enemy? Am. J. Reprod. Immunol. 2020;84 doi: 10.1111/aji.13308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cosma S., et al. The “scar” of a pandemic: cumulative incidence of COVID-19 during the first trimester of pregnancy. J. Med. Virol. 2021;93:537–540. doi: 10.1002/jmv.26267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patberg E.T., et al. Coronavirus disease 2019 infection and placental histopathology in women delivering at term. Am. J. Obstet. Gynecol. 2021;224 doi: 10.1016/j.ajog.2020.10.020. 382.e1-382.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasmussen S.A., Smulian J.C., Lednicky J.A., Wen T.S., Jamieson D.J. Coronavirus Disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am. J. Obstet. Gynecol. 2020;222:415–426. doi: 10.1016/j.ajog.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Girardelli S., Mullins E., Lees C.C. COVID-19 and pregnancy: lessons from 2020. Early Hum. Dev. 2021;105460 doi: 10.1016/J.EARLHUMDEV.2021.105460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.N A.N.C., D S., DE C., M B. Role of ACE2 in pregnancy and potential implications for COVID-19 susceptibility. Clin. Sci. (Lond). 2021;135:1805–1824. doi: 10.1042/CS20210284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shang J., et al. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verma S., et al. SARS-CoV-2 colonization of maternal and fetal cells of the human placenta promotes alteration of local renin-angiotensin system. Med (New York, N.y.) 2021;2:575. doi: 10.1016/j.medj.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finkel Y., et al. The coding capacity of SARS-CoV-2. Nature. 2020;589:125–130. doi: 10.1038/s41586-020-2739-1. [DOI] [PubMed] [Google Scholar]
- 29.Romano M., Ruggiero A., Squeglia F., Maga G., Berisio R. A Structural View of SARS-CoV-2 RNA replication machinery: RNA synthesis, proofreading and final capping. Cells. 2020;9(5):1–22. doi: 10.3390/cells9051267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D B, et al. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature. 2020;583:469–472. doi: 10.1038/s41586-020-2332-7. [DOI] [PubMed] [Google Scholar]
- 31.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Coronaviruses. 2015;1282:1. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D K, et al. The architecture of SARS-CoV-2 transcriptome. Cell. 2020;181:914–921. doi: 10.1016/j.cell.2020.04.011. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.S S, et al. The intracellular sites of early replication and budding of SARS-coronavirus. Virology. 2007;361:304–315. doi: 10.1016/j.virol.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang L., et al. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved a-ketoamide inhibitors. Science. 2020;368:409–412. doi: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ullrich S., Nitsche C. The SARS-CoV-2 main protease as drug target. Bioorg. Med. Chem. Lett. 2020;30 doi: 10.1016/j.bmcl.2020.127377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schubert K., et al. SARS-CoV-2 Nsp1 binds the ribosomal mRNA channel to inhibit translation. Nat. Struct. Mol. Biol. 2020;27:959–966. doi: 10.1038/s41594-020-0511-8. [DOI] [PubMed] [Google Scholar]
- 37.Santerre M., Arjona S.P., Allen C.N., Shcherbik N., Sawaya B.E. Why do SARS-CoV-2 NSPs rush to the ER? J. Neurol. 2020;268:2013–2022. doi: 10.1007/s00415-020-10197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hillen H.S., et al. Structure of replicating SARS-CoV-2 polymerase. Nature. 2020;584:154–156. doi: 10.1038/s41586-020-2368-8. [DOI] [PubMed] [Google Scholar]
- 39.Hsu J.C.-C., Laurent-Rolle M., Pawlak J.B., Wilen C.B., Cresswell P. Translational shutdown and evasion of the innate immune response by SARS-CoV-2 NSP14 protein. Proc. Natl. Acad. Sci. 2021;118 doi: 10.1073/pnas.2101161118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shu T., et al. SARS-Coronavirus-2 Nsp13 Possesses NTPase and RNA helicase activities that can be inhibited by bismuth salts. Virol. Sin. 2020;35:321. doi: 10.1007/s12250-020-00242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin S., et al. Crystal structure of SARS-CoV-2 nsp10/nsp16 2′-O-methylase and its implication on antiviral drug design. Signal Transduct. Target. Ther. 2020;5:1–4. doi: 10.1038/s41392-020-00241-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klein S., et al. SARS-CoV-2 structure and replication characterized by in situ cryo-electron tomography. Nat. Commun. 2020;11:1–10. doi: 10.1038/s41467-020-19619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ou X., et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11:1–12. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Troyano-Hernáez P., Reinosa R., Holguín Á. Evolution of SARS-CoV-2 envelope, membrane, nucleocapsid, and spike structural proteins from the beginning of the pandemic to September 2020: a global and regional approach by epidemiological week. Viruses. 2021;13:243. doi: 10.3390/v13020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.B B, et al. The SARS-CoV-2 envelope and membrane proteins modulate maturation and retention of the spike protein, allowing assembly of virus-like particles. J. Biol. Chem. 2021;296:1–13. doi: 10.1074/jbc.RA120.016175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shepley-McTaggart A., et al. SARS-CoV-2 Envelope (E) protein interacts with PDZ-domain-2 of host tight junction protein ZO1. PLoS One. 2021;16 doi: 10.1371/journal.pone.0251955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng Y., et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) membrane (M) protein inhibits type I and III interferon production by targeting RIG-I/MDA-5 signaling. Signal Transduct. Target. Ther. 2020;5:1–13. doi: 10.1038/s41392-020-00438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao Y., et al. A dual-role of SARS-CoV-2 nucleocapsid protein in regulating innate immune response. Signal Transduct. Target. Ther. 2021;6:1–14. doi: 10.1038/s41392-021-00742-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Redondo N., Zaldívar-López S., Garrido J.J., Montoya M. SARS-CoV-2 Accessory Proteins in Viral Pathogenesis: knowns and unknowns. Front. Immunol. 2021;12:2698–2706. doi: 10.3389/fimmu.2021.708264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu D.X., Fung T.S., Chong K.K.L., Shukla A., Hilgenfeld R. Accessory proteins of SARS-CoV and other coronaviruses. Antiviral Res. 2014;109:97–109. doi: 10.1016/j.antiviral.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jungreis I., Sealfon R., Kellis M. SARS-CoV-2 gene content and COVID-19 mutation impact by comparing 44 Sarbecovirus genomes. Nat. Commun. 2021;12:1–20. doi: 10.1038/s41467-021-22905-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miao G., et al. ORF3a of the COVID-19 virus SARS-CoV-2 blocks HOPS complex-mediated assembly of the SNARE complex required for autolysosome formation. Dev. Cell. 2021;56:427–442.e5. doi: 10.1016/j.devcel.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Y K, et al. SARS-CoV-2 ORF3b is a potent interferon antagonist whose activity is increased by a naturally occurring elongation variant. Cell Rep. 2020;32:1–11. doi: 10.1016/j.celrep.2020.108185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.L M, et al. SARS-CoV-2 Orf6 hijacks Nup98 to block STAT nuclear import and antagonize interferon signaling. Proc. Natl. Acad. Sci. U. S. A. 2020;117:28344–28354. doi: 10.1073/pnas.2016650117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Z C, et al. Ubiquitination of SARS-CoV-2 ORF7a promotes antagonism of interferon response. Cell. Mol. Immunol. 2021;18:746–748. doi: 10.1038/s41423-020-00603-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.F P. Evolutionary dynamics of the SARS-CoV-2 ORF8 accessory gene. Infect. Genet. Evol. 2020;85:1–10. doi: 10.1016/j.meegid.2020.104525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y., et al. The ORF8 protein of SARS-CoV-2 mediates immune evasion through down-regulating MHC-Ι. Proc. Natl. Acad. Sci. 2021;118:1–12. doi: 10.1073/pnas.2024202118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.K P, et al. The SARS-CoV-2 ORF10 is not essential in vitro or in vivo in humans. PLoS Pathog. 2020;16:1–8. doi: 10.1371/journal.ppat.1008959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liang B., Guida J.P., Costa M.L., Mysorekar I.U. Host and viral mechanisms of congenital Zika syndrome. Virulence. 2019;10:768–775. doi: 10.1080/21505594.2019.1656503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Edlow A.G., et al. Assessment of maternal and neonatal SARS-CoV-2 viral load, transplacental antibody transfer, and placental pathology in pregnancies during the COVID-19 pandemic. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.30455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.EH A., et al. Pregnancy outcomes among women with and without severe acute respiratory syndrome coronavirus 2 infection. JAMA Netw. open. 2020;3:1–11. doi: 10.1001/jamanetworkopen.2020.29256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.D D, et al. Outcomes of neonates born to mothers with severe acute respiratory syndrome coronavirus 2 infection at a large medical center in New York City. JAMA Pediatr. 2021;175:157–167. doi: 10.1001/jamapediatrics.2020.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Flaherman V.J., et al. Infant outcomes following maternal infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): first report from the pregnancy coronavirus outcomes registry (PRIORITY) study. Clin. Infect. Dis. 2021;73:e2810–e2813. doi: 10.1093/cid/ciaa1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.AM K., et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2021;224:35–53. doi: 10.1016/j.ajog.2020.07.049. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.GR R.-C., SC R.-R. Congenital and intrapartum SARS-CoV-2 infection in neonates: hypotheses, evidence and perspectives. MEDICC Rev. 2021;23:72–83. doi: 10.37757/MR2021.V23.N1.13. [DOI] [PubMed] [Google Scholar]
- 66.GM B., BJ N., DL M., D S., BF S. Possible vertical transmission and antibodies against SARS-CoV-2 among infants born to mothers with COVID-19: a living systematic review. J. Med. Virol. 2021;93:1361–1369. doi: 10.1002/jmv.26622. [DOI] [PubMed] [Google Scholar]
- 67.Adhikari E.H., et al. COVID-19 cases and disease severity in pregnancy and neonatal positivity associated with Delta (B.1.617.2) and Omicron (B.1.1.529) variant predominance. JAMA. 2022;327:1500–1502. doi: 10.1001/jama.2022.4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.H C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet (London, England) 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.S C, et al. [Pregnancy with new coronavirus infection: clinical characteristics and placental pathological analysis of three cases] Zhonghua Bing li xue za zhi = Chinese J. Pathol. 2020;49:418–423. doi: 10.3760/cma.j.cn112151-20200225-00138. [DOI] [PubMed] [Google Scholar]
- 70.M M., A S., N R. A systematic review of pregnant women with COVID-19 and their neonates. Arch. Gynecol. Obstet. 2021;304:5–38. doi: 10.1007/s00404-021-06049-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kelly J.C., et al. False-negative testing for severe acute respiratory syndrome coronavirus 2: consideration in obstetrical care. Am. J. Obstet. Gynecol. MFM. 2020;2 doi: 10.1016/j.ajogmf.2020.100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.R Y, et al. Pregnant women with COVID-19 and risk of adverse birth outcomes and maternal-fetal vertical transmission: a population-based cohort study in Wuhan, China. BMC Med. 2020;18:1–7. doi: 10.1186/s12916-020-01798-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Woodworth K.R. Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 Infection in Pregnancy — SET-NET, 16 Jurisdictions, March 29–October 14, 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020;69:1635–1640. doi: 10.15585/mmwr.mm6944e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kreis N.-N., Ritter A., Louwen F., Yuan J. A Message from the human placenta: structural and immunomodulatory defense against SARS-CoV-2. Cells. 2020;9:1777. doi: 10.3390/cells9081777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blumberg D.A., Underwood M.A., Hedriana H.L., Lakshminrusimha S. Vertical transmission of SARS-CoV-2: what is the optimal definition? Am. J. Perinatol. 2020;37:769–772. doi: 10.1055/s-0040-1712457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ahmed A., Rezai H., Broadway-Stringer S. Evidence-based revised view of the pathophysiology of preeclampsia. Adv. Exp. Med. Biol. 2016;956:355–374. doi: 10.1007/5584_2016_168. [DOI] [PubMed] [Google Scholar]
- 77.K D., E A., E G. The effect of coronavirus infection (SARS-CoV-2, MERS-CoV, and SARS-CoV) during pregnancy and the possibility of vertical maternal-fetal transmission: a systematic review and meta-analysis. Eur. J. Med. Res. 2020;25:1–14. doi: 10.1186/s40001-020-00439-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Staff, A.C. The two-stage placental model of preeclampsia: an update. J. Reprod. Immunol.134–135, 1–10 (2019). [DOI] [PubMed]
- 79.Lippi G., Sanchis-Gomar F., Henry B.M. COVID-19: unravelling the clinical progression of nature’s virtually perfect biological weapon. Ann. Transl. Med. 2020;8:1–6. doi: 10.21037/atm-20-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Giardini V., Gambacorti-Passerini C., Casati M., Carrer A., Vergani P. Can similarities between the pathogenesis of preeclampsia and COVID-19 increase the understanding of COVID-19? Int J Transl Med. 2022;2:186–197. [Google Scholar]
- 81.Stepan H., Hund M., Andraczek T. Combining biomarkers to predict pregnancy complications and redefine preeclampsia: the angiogenic-placental syndrome. Hypertension. 2020;75:918–926. doi: 10.1161/HYPERTENSIONAHA.119.13763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Giardini V., et al. Increased sFLT1/PlGF ratio in COVID-19: a novel link to Angiotensin II-mediated endothelial dysfunction. Am. J. Hematol. 2020;95(8):E188–E191. doi: 10.1002/ajh.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Giardini V., et al. Letter to the Editor: SFlt-1 and PlGF levels in pregnancies complicated by SARS-CoV-2 infection. Viruses. 2021;13(12):1–5. doi: 10.3390/v13122377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.M M, et al. Pre-eclampsia-like syndrome induced by severe COVID-19: a prospective observational study. BJOG. 2020;127:1374–1380. doi: 10.1111/1471-0528.16339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Villar J., et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175:817–826. doi: 10.1001/jamapediatrics.2021.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jung E., et al. The etiology of preeclampsia. Am. J. Obstet. Gynecol. 2022;226:S844–S866. doi: 10.1016/j.ajog.2021.11.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guida J.P., et al. Preeclampsia among women with COVID-19 during pregnancy and its impact on maternal and perinatal outcomes: results from a national multicenter study on COVID in Brazil, the REBRACO initiative. Pregnancy Hypertens. 2022;28:168–173. doi: 10.1016/j.preghy.2022.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xiong S., et al. Clinical characteristics of 116 hospitalized patients with COVID-19 in Wuhan, China: a single-centered, retrospective, observational study. BMC Infect. Dis. 2020;20:787. doi: 10.1186/s12879-020-05452-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.J J, et al. Effect of coronavirus disease 2019 (COVID-19) on maternal, perinatal and neonatal outcome: systematic review. Ultrasound Obstet. Gynecol. 2020;56:15–27. doi: 10.1002/uog.22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.I G.-U, et al. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection at the time of birth in England: national cohort study. Am. J. Obstet. Gynecol. 2021 doi: 10.1016/J.AJOG.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.M A, et al. Association of SARS-CoV-2 test status and pregnancy outcomes. JAMA. 2020;324:1782–1785. doi: 10.1001/jama.2020.19124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li N., et al. Maternal and neonatal outcomes of pregnant women with coronavirus disease 2019 (COVID-19) pneumonia: a case-control study. Clin. Infect. Dis. 2020;71:2035–2041. doi: 10.1093/cid/ciaa352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sentilhes L., et al. Coronavirus disease 2019 in pregnancy was associated with maternal morbidity and preterm birth. Am. J. Obstet. Gynecol. 2020;223:914.e1–914.e15. doi: 10.1016/j.ajog.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Delahoy M.J. Characteristics and maternal and birth outcomes of hospitalized pregnant women with laboratory-confirmed COVID-19 — COVID-NET, 13 States, March 1–August 22, 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020;69:1347–1354. doi: 10.15585/mmwr.mm6938e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vousden N., et al. The incidence, characteristics and outcomes of pregnant women hospitalized with symptomatic and asymptomatic SARS-CoV-2 infection in the UK from March to September 2020: a national cohort study using the UK Obstetric Surveillance System (UKOSS) PLoS One. 2021;16 doi: 10.1371/journal.pone.0251123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chae S.Y., Bhattacharyya A., Mendoza R. COVID-19 in pregnancy: a current review of global cases. Obstet. Gynecol. Surv. 2021;76:504–513. doi: 10.1097/OGX.0000000000000925. [DOI] [PubMed] [Google Scholar]
- 97.Vergara-Merino L., et al. Maternal and perinatal outcomes related to COVID-19 and pregnancy: an overview of systematic reviews. Acta Obstet. Gynecol. Scand. 2021;100:1200–1218. doi: 10.1111/aogs.14118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fallach N., et al. Pregnancy outcomes after SARS-CoV-2 infection by trimester: a large, population-based cohort study. PLoS One. 2022;17 doi: 10.1371/journal.pone.0270893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lye P., et al. ACE2 Is expressed in immune cells that infiltrate the placenta in infection-associated preterm birth. Cells. 2021;10:1724. doi: 10.3390/cells10071724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mullins E., et al. Pregnancy and neonatal outcomes of COVID-19: coreporting of common outcomes from PAN-COVID and AAP-SONPM registries. Ultrasound Obstet. Gynecol. 2021;57:573–581. doi: 10.1002/uog.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Khalil A., et al. Change in the incidence of stillbirth and preterm delivery during the COVID-19 pandemic. JAMA. 2020;324:705–706. doi: 10.1001/jama.2020.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Simon E., et al. Impact of the COVID-19 pandemic on preterm birth and stillbirth: a nationwide, population-based retrospective cohort study. Am. J. Obstet. Gynecol. 2021;225:347–348. doi: 10.1016/j.ajog.2021.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.DeSisto C.L., et al. Risk for stillbirth among women with and without COVID-19 at delivery hospitalization—United States, March 2020–September 2021. Morb. Mortal. Wkly. Rep. 2021;70:1640. doi: 10.15585/mmwr.mm7047e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schwartz D.A., et al. Placental tissue destruction and insufficiency from COVID-19 causes stillbirth and neonatal death from hypoxic-ischemic injury: a study of 68 cases with SARS-CoV-2 placentitis from 12 countries. Arch. Pathol. Lab. Med. 2022;146:660–676. doi: 10.5858/arpa.2022-0029-SA. [DOI] [PubMed] [Google Scholar]
- 105.Mithal L.B., et al. Low-level SARS-CoV-2 viremia coincident with COVID placentitis and stillbirth. Placenta. 2022;121:79–81. doi: 10.1016/j.placenta.2022.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shook L.L., et al. SARS-CoV-2 placentitis associated with B.1.617.2 (Delta) variant and fetal distress or demise. J. Infect. Dis. 2022;225:754–758. doi: 10.1093/infdis/jiac008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fitzgerald B., et al. Fetal deaths in Ireland due to SARS-CoV-2 placentitis caused by SARS-CoV-2 Alpha. Arch. Pathol. Lab. Med. 2022 doi: 10.5858/arpa.2021-0586-SA. [DOI] [PubMed] [Google Scholar]
- 108.Gibbins K.J., et al. Findings in stillbirths associated with placental disease. Am. J. Perinatol. 2020;37:708–715. doi: 10.1055/s-0039-1688472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schwartz D.A., et al. Chronic histiocytic intervillositis with trophoblast necrosis is a risk factor associated with placental infection from coronavirus disease 2019 (COVID-19) and intrauterine maternal-fetal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transm. Arch. Pathol. Lab. Med. 2021;145:517–528. doi: 10.5858/arpa.2020-0771-SA. [DOI] [PubMed] [Google Scholar]
- 110.F D.T, et al. Impact of COVID-19 on maternal and neonatal outcomes: a systematic review and meta-analysis. Clin. Microbiol. Infect. 2021;27:36–46. doi: 10.1016/j.cmi.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.V V., A P., A P., SK B. Perinatal COVID-19: review of current evidence and practical approach towards prevention and management. Eur. J. Pediatr. 2021;180:1009–1031. doi: 10.1007/s00431-020-03866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.A C.M, et al. Late-onset neonatal sepsis in a patient with covid-19. N. Engl. J. Med. 2020;382:e49. doi: 10.1056/NEJMc2010614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Edlow A.G., Castro V.M., Shook L.L., Kaimal A.J., Perlis R.H. Neurodevelopmental outcomes at 1 year in infants of mothers who tested positive for SARS-CoV-2 during pregnancy. JAMA Netw. Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.15787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Huang P., et al. Association between the COVID-19 pandemic and infant neurodevelopment: a comparison before and during COVID-19. Front. Pediatr. 2021;1087:1–11. doi: 10.3389/fped.2021.662165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Deoni, S., Beauchemin, J., Volpe, A. & D'Sa, V. Impact of the COVID-19 pandemic on early child cognitive development: initial findings in a longitudinal observational study of child health. MedRxiv (2021).
- 116.Shook L.L., Sullivan E.L., Lo J.O., Perlis R.H., Edlow A.G. COVID-19 in pregnancy: implications for fetal brain development. Trends Mol. Med. 2022;28(4):319–330. doi: 10.1016/j.molmed.2022.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Delahoy, M.J. et al.Hospitalizations associated with COVID-19 among children and adolescents — COVID-NET, 14 States, March 1, 2020–August 14, 2021. [DOI] [PMC free article] [PubMed]
- 118.LaRovere K.L., et al. Neurologic involvement in children and adolescents hospitalized in the United States for COVID-19 or multisystem inflammatory syndrome. JAMA Neurol. 2021;78:536–547. doi: 10.1001/jamaneurol.2021.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Careaga M., Murai T., Bauman M.D. Maternal immune activation and autism spectrum disorder: from rodents to nonhuman and human primates. Biol. Psychiatry. 2017;81:391–401. doi: 10.1016/j.biopsych.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Haddad F.L., Patel S.V, Schmid S. Maternal immune activation by Poly I: C as a preclinical model for neurodevelopmental disorders: a focus on autism and schizophrenia. Neurosci. Biobehav. Rev. 2020;113:546–567. doi: 10.1016/j.neubiorev.2020.04.012. [DOI] [PubMed] [Google Scholar]
- 121.Tanacan A., et al. The impact of COVID-19 infection on the cytokine profile of pregnant women: A prospective case-control study. Cytokine. 2021;140 doi: 10.1016/j.cyto.2021.155431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sherer M.L., et al. Pregnancy alters interleukin-1 beta expression and antiviral antibody responses during severe acute respiratory syndrome coronavirus 2 infection. Am. J. Obstet. Gynecol. 2021;225:301. doi: 10.1016/j.ajog.2021.03.028. -e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Matute J.D., et al. Single-cell immunophenotyping of the fetal immune response to maternal SARS-CoV-2 infection in late gestation. Pediatr. Res. 2022;91:1090–1098. doi: 10.1038/s41390-021-01793-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lu-Culligan A., et al. Maternal respiratory SARS-CoV-2 infection in pregnancy is associated with a robust inflammatory response at the maternal-fetal interface. Med. 2021;2:591–610.e10. doi: 10.1016/j.medj.2021.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shanes E.D., et al. Placental pathology in COVID-19. Am. J. Clin. Pathol. 2020;154:23–32. doi: 10.1093/ajcp/aqaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.E P., M J., I B. COVID-19 in pregnancy: placental and neonatal involvement. Am. J. Reprod. Immunol. 2020;84(5):1–9. doi: 10.1111/aji.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.AP M., et al. Mechanisms and evidence of vertical transmission of infections in pregnancy including SARS-CoV-2s. Prenat. Diagn. 2020;40:1655–1670. doi: 10.1002/pd.5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.S S, et al. Severe acute respiratory syndrome coronavirus 2 placental infection and inflammation leading to fetal distress and neonatal multi-organ failure in an asymptomatic woman. J. Pediatric Infect. Dis. Soc. 2021;10:556–561. doi: 10.1093/jpids/piaa153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stenton S., et al. SARS-COV2 placentitis and pregnancy outcome: A multicentre experience during the Alpha and early Delta waves of coronavirus pandemic in England. eClinicalMedicine. 2022;47 doi: 10.1016/j.eclinm.2022.101389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Moresi S., et al. SARS-CoV-2 infection in pregnancy: clinical signs, placental pathology, and neonatal outcome—implications for clinical care. Front. Med. 2021;8:1–8. doi: 10.3389/fmed.2021.676870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li W., et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shang J., et al. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gierer S., et al. The spike protein of the emerging Betacoronavirus EMC Uses a novel coronavirus receptor for entry, can be activated by TMPRSS2, and is targeted by neutralizing antibodies. J. Virol. 2013;87:5502–5511. doi: 10.1128/JVI.00128-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hoffmann, M. et al.SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell181, 271-280.e8 (2020). [DOI] [PMC free article] [PubMed]
- 135.Murgolo N., et al. SARS-CoV-2 tropism, entry, replication, and propagation: considerations for drug discovery and development. PLOS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.GA R., O S., E M., L C., F M. Differences and similarities between SARS-CoV and SARS-CoV-2: spike receptor-binding domain recognition and host cell infection with support of cellular serine proteases. Infection. 2020;48:665–669. doi: 10.1007/s15010-020-01486-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.G V, et al. Distribution of angiotensin-(1-7) and ACE2 in human placentas of normal and pathological pregnancies. Placenta. 2006;27:200–207. doi: 10.1016/j.placenta.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 138.E T, et al. Consistent localization of SARS-CoV-2 spike glycoprotein and ACE2 over TMPRSS2 predominance in placental villi of 15 COVID-19 positive maternal-fetal dyads. Placenta. 2020;100:69–74. doi: 10.1016/j.placenta.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.E B, et al. Expression of severe acute respiratory syndrome coronavirus 2 cell entry genes, angiotensin-converting enzyme 2 and transmembrane protease serine 2, in the placenta across gestation and at the maternal-fetal interface in pregnancies complicated by preterm birth or preeclampsia. Am. J. Obstet. Gynecol. 2021;224 doi: 10.1016/j.ajog.2020.08.055. 298.e1-298.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.R P.-R, et al. Does the human placenta express the canonical cell entry mediators for SARS-CoV-2? Elife. 2020;9:1–15. doi: 10.7554/eLife.58716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lye, P. et al.SARS-CoV-2 cell entry gene ACE2 expression in immune cells that infiltrate the placenta in infection-associated preterm birth. medRxiv (2020) doi:10.1101/2020.09.27.20201590. [DOI] [PMC free article] [PubMed]
- 142.Wang K., et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct. Target. Ther. 2020;5:1–10. doi: 10.1038/s41392-020-00426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.S T, et al. Angiotensin converting enzyme 2 (ACE2) in pregnancy: preeclampsia and small for gestational age. Front. Physiol. 2020;11:1–10. doi: 10.3389/fphys.2020.590787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Colson A., et al. Clinical and in vitro evidence against placenta infection at term by severe acute respiratory syndrome coronavirus 2. Am. J. Pathol. 2021;191:1610–1623. doi: 10.1016/j.ajpath.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Heerema-McKenney A. Defense and infection of the human placenta. APMIS. 2018;126:570–588. doi: 10.1111/apm.12847. [DOI] [PubMed] [Google Scholar]
- 146.E D.-A., Y S., CB C. The placenta as a barrier to viral infections. Annu. Rev. Virol. 2014;1:133–146. doi: 10.1146/annurev-virology-031413-085524. [DOI] [PubMed] [Google Scholar]
- 147.Benirschke K., Driscoll S.G. The pathology of the human placenta. Placenta. 1967:97–571. doi: 10.1007/978-3-662-38455-8_2. [DOI] [Google Scholar]
- 148.Ji L., et al. Placental trophoblast cell differentiation: Physiological regulation and pathological relevance to preeclampsia. Mol. Aspects Med. 2013;34:981–1023. doi: 10.1016/j.mam.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 149.K K., G I., G M., T F., Y O. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy complications. Am. J. Reprod. Immunol. 2014;72:192–205. doi: 10.1111/aji.12258. [DOI] [PubMed] [Google Scholar]
- 150.León-Juárez M., et al. Cellular and molecular mechanisms of viral infection in the human placenta. Pathog. Dis. 2017;75:93. doi: 10.1093/femspd/ftx093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Facchetti F., et al. SARS-CoV2 vertical transmission with adverse effects on the newborn revealed through integrated immunohistochemical, electron microscopy and molecular analyses of Placenta. EBioMedicine. 2020;59 doi: 10.1016/j.ebiom.2020.102951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Penfield C.A., et al. Detection of severe acute respiratory syndrome coronavirus 2 in placental and fetal membrane samples. Am. J. Obstet. Gynecol. MFM. 2020;2 doi: 10.1016/j.ajogmf.2020.100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hecht J.L., et al. SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: a series of 19 placentas from COVID-19-positive mothers. Mod. Pathol. 2020;33:2092–2103. doi: 10.1038/s41379-020-0639-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wong Y.P., Khong T.Y., Tan G.C. The effects of COVID-19 on placenta and pregnancy: what do we know so far? Diagnostics. 2021;11:94. doi: 10.3390/diagnostics11010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Komine-Aizawa S., Takada K., Hayakawa S. Placental barrier against COVID-19. Placenta. 2020;99:45. doi: 10.1016/j.placenta.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mor G., Aldo P., Alvero A.B. The unique immunological and microbial aspects of pregnancy. Nat. Rev. Immunol. 2017;17:469–482. doi: 10.1038/nri.2017.64. [DOI] [PubMed] [Google Scholar]
- 157.Cornish E.F., Filipovic I., Åsenius F., Williams D.J., McDonnell T. Innate immune responses to acute viral infection during pregnancy. Front. Immunol. 2020;11:1–24. doi: 10.3389/fimmu.2020.572567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Aghaeepour N., et al. An immune clock of human pregnancy. Sci. Immunol. 2017;2:1–11. doi: 10.1126/sciimmunol.aan2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.I B.-O., M P., P K. The immunological role of the placenta in SARS-CoV-2 infection-viral transmission, immune regulation, and lactoferrin activity. Int. J. Mol. Sci. 2021;22:1–26. doi: 10.3390/ijms22115799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kwan M., et al. Dynamic changes in maternal decidual leukocyte populations from first to second trimester gestation. Placenta. 2014;35:1027–1034. doi: 10.1016/j.placenta.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 161.Yang F., Zheng Q., Jin L. Dynamic function and composition changes of immune cells during normal and pathological pregnancy at the maternal-fetal interface. Front. Immunol. 2019;10:1–15. doi: 10.3389/fimmu.2019.02317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tetro J.A. Is COVID-19 receiving ADE from other coronaviruses? Microbes Infect. 2020;22:72–73. doi: 10.1016/j.micinf.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chudnovets A., et al. Dose-dependent structural and immunological changes in the placenta and fetal brain in response to systemic inflammation during pregnancy. Am. J. Reprod. Immunol. 2020;84:e13248. doi: 10.1111/aji.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fahmi A., et al. SARS-CoV-2 can infect and propagate in human placenta explants. Cell Reports Med. 2021;2 doi: 10.1016/j.xcrm.2021.100456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Argueta L.B., et al. Inflammatory responses in the placenta upon SARS-CoV-2 infection late in pregnancy. iScience. 2022;25 doi: 10.1016/j.isci.2022.104223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Guo X., et al. Regulation of proinflammatory molecules and tissue factor by SARS-CoV-2 spike protein in human placental cells: implications for SARS-CoV-2 pathogenesis in pregnant women. Front. Immunol. 2022;13:1–12. doi: 10.3389/fimmu.2022.876555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Karvas R.M., et al. Stem-cell-derived trophoblast organoids model human placental development and susceptibility to emerging pathogens. Cell Stem Cell. 2022;29:810–825. doi: 10.1016/j.stem.2022.04.004. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zheng H.-Y., et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell. Mol. Immunol. 2020;17:541–543. doi: 10.1038/s41423-020-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.A B.E, et al. Maternal SARS-CoV-2 infection elicits sexually dimorphic placental immune responses. Sci. Transl. Med. 2022;13(617):1–15. doi: 10.1126/scitranslmed.abi7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Petrilli C.M., et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:1–15. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yonker L.M., et al. Pediatric severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): clinical presentation, infectivity, and immune responses. J. Pediatr. 2020;227:45–52. doi: 10.1016/j.jpeds.2020.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Godfred-Cato S., et al. COVID-19–associated multisystem inflammatory syndrome in children—United States, March–July 2020. Morb. Mortal. Wkly. Rep. 2020;69:1074. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Abate B.B., Kassie A.M., Kassaw M.W., Aragie T.G., Masresha S.A. Sex difference in coronavirus disease (COVID-19): a systematic review and meta-analysis. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-040129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Slaoui, M. & Hepburn, M. Developing safe and effective covid vaccines — operation warp speed's strategy and approach. https://doi.org/10.1056/NEJMp2027405 383, 1701–1703 (2020). [DOI] [PubMed]
- 175.Ho R.J.Y. Warp-speed covid-19 vaccine development: beneficiaries of maturation in biopharmaceutical technologies and public-private partnerships. J. Pharm. Sci. 2021;110:615–618. doi: 10.1016/j.xphs.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.van Riel D., de Wit E. Next-generation vaccine platforms for COVID-19. Nat. Mater. 2020;19:810–812. doi: 10.1038/s41563-020-0746-0. [DOI] [PubMed] [Google Scholar]
- 177.Dodd R.H., et al. Concerns and motivations about COVID-19 vaccination. Lancet. Infect. Dis. 2021;21:161. doi: 10.1016/S1473-3099(20)30926-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.FDA Approves First COVID-19 Vaccine | FDA. 2022 Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine.
- 179.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines — a new era in vaccinology. Nat. Rev. Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kremer E.J. Pros and cons of adenovirus-based SARS-CoV-2 vaccines. Mol. Ther. 2020;28:2303–2304. doi: 10.1016/j.ymthe.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Goldshtein I., et al. Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. JAMA. 2021 doi: 10.1001/JAMA.2021.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Shimabukuro, T.T. et al.Preliminary findings of mRNA covid-19 vaccine safety in pregnant persons. https://doi.org/10.1056/NEJMoa2104983 384, 2273–2282 (2021). [DOI] [PMC free article] [PubMed]
- 183.New CDC Data: COVID-19 Vaccination Safe for Pregnant People | CDC Online Newsroom CDC. Available at: https://www.cdc.gov/media/releases/2021/s0811-vaccine-safe-pregnant.html.
- 184.Wilcox, A.J., Rose, C.E., Meaney-Delman, D. & Ellington, S.R. Receipt of mRNA COVID-19 vaccines preconception and during pregnancy and risk of self-reported spontaneous abortions, CDC v-safe COVID-19 Vaccine Pregnancy Registry 2020-21. (2021) doi:10.21203/RS.3.RS-798175/V1.
- 185.Dagan N., et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat. Med. 2021;27:1693–1695. doi: 10.1038/s41591-021-01490-8. [DOI] [PubMed] [Google Scholar]
- 186.Shook L.L., et al. Durability of anti-spike antibodies in infants after maternal COVID-19 vaccination or natural infection. JAMA. 2022;327:1087–1089. doi: 10.1001/jama.2022.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bardají A., et al. The need for a global COVID-19 maternal immunisation research plan. Lancet. 2021;397:e17–e18. doi: 10.1016/S0140-6736(21)00146-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.KJ G., et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am. J. Obstet. Gynecol. 2021 doi: 10.1016/J.AJOG.2021.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Klein S.L., Creisher P.S., Burd I. COVID-19 vaccine testing in pregnant females is necessary. J. Clin. Invest. 2021;131 doi: 10.1172/JCI147553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Pirjani R., et al. Maternal and neonatal outcomes in COVID-19 infected pregnancies: a prospective cohort study. J. Travel Med. 2020;27:1–7. doi: 10.1093/jtm/taaa158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zambrano L.D. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status — United States, January 22–October 3, 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020;69:1641–1647. doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Heath P.T., Doare K.Le, Khalil A. Inclusion of pregnant women in COVID-19 vaccine development. Lancet Infect. Dis. 2020;20:1007–1008. doi: 10.1016/S1473-3099(20)30638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Beleche, T. et al.May 2021 ISSUE BRIEF 1 ISSUE BRIEF COVID-19 vaccine hesitancy: demographic factors, geographic patterns, and changes over time key points. (2021) doi:10.1016/j.vaccine.2015.09.035.
- 194.Male V. Are COVID-19 vaccines safe in pregnancy? Nat. Rev. Immunol. 2021;21:200–201. doi: 10.1038/s41577-021-00525-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mi S., et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature. 2000;403:785–789. doi: 10.1038/35001608. [DOI] [PubMed] [Google Scholar]
- 196.Opinion | False Rumors About Covid-19 Vaccines Are Scaring Women - The New York Times. 2022 Available at: https://www.nytimes.com/2021/01/26/opinion/covid-vaccine-rumors.html.
- 197.Mattar, C.N. et al.Addressing anti-syncytin antibody levels, and fertility and breastfeeding concerns, following BNT162B2 COVID-19 mRNA vaccination. medRxiv 2021.05.23.21257686 (2021) doi:10.1101/2021.05.23.21257686.
- 198.Cosma S., et al. Coronavirus disease 2019 and first-trimester spontaneous abortion: a case-control study of 225 pregnant patients. Am. J. Obstet. Gynecol. 2021;224:391.e1–391.e7. doi: 10.1016/j.ajog.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Khalil A., et al. SARS-CoV-2 infection in pregnancy: a systematic review and meta-analysis of clinical features and pregnancy outcomes. EClinicalMedicine. 2020;25:1–12. doi: 10.1016/j.eclinm.2020.100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Fda & Cber. Vaccines and Related Biological Products Advisory Committee December 17, 2020 Meeting Briefing Document - FDA. (2020).
- 201.Pierce-Williams R.A.M., et al. Clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnancies: a United States cohort study. Am. J. Obstet. Gynecol. MFM. 2020;2 doi: 10.1016/j.ajogmf.2020.100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Korber B., et al. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182:812. doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kemp S.A., et al. Recurrent emergence and transmission of a SARS-CoV-2 spike deletion H69/V70. SSRN Electron. J. 2021 doi: 10.2139/SSRN.3780277. [DOI] [Google Scholar]
- 204.Wibmer C.K., et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Nat. Med. 2021;27:622–625. doi: 10.1038/s41591-021-01285-x. [DOI] [PubMed] [Google Scholar]
- 205.Wu, K. et al.Serum neutralizing activity elicited by mRNA-1273 vaccine. https://doi.org/10.1056/NEJMc2102179 384, 1468–1470 (2021). [DOI] [PMC free article] [PubMed]
- 206.Lapinsky, S.E. & Adhikari, N.K. COVID-19, variants of concern and pregnancy outcome: https://doi.org/10.1177/1753495×211028499 14, 65–66 (2021). [DOI] [PMC free article] [PubMed]
- 207.Collier A.Y., et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA. 2021;325:2370–2380. doi: 10.1001/jama.2021.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Perl S.H., et al. SARS-CoV-2–specific antibodies in breast milk after COVID-19 vaccination of breastfeeding women. JAMA. 2021;325:2013–2014. doi: 10.1001/jama.2021.5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Caroline A., et al. COVID-19 mRNA vaccines drive differential antibody Fc-functional profiles in pregnant, lactating, and nonpregnant women. Sci. Transl. Med. 2022;13:eabi8631. doi: 10.1126/scitranslmed.abi8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Prabhu M., et al. Antibody response to coronavirus disease 2019 (COVID-19) messenger RNA vaccination in pregnant women and transplacental passage into cord blood. Obstet. Gynecol. 2021;138:278. doi: 10.1097/AOG.0000000000004438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.L S.B, et al. Antibodies elicited by SARS-CoV-2 infection or mRNA vaccines have reduced neutralizing activity against Beta and Omicron pseudoviruses. Sci. Transl. Med. 2022;14:eabn7842. doi: 10.1126/scitranslmed.abn7842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Aggarwal, A. et al.SARS-CoV-2 Omicron BA.5: Evolving tropism and evasion of potent humoral responses and resistance to clinical immunotherapeutics relative to viral variants of concern. medRxiv 2022.07.07.22277128 (2022) doi:10.1101/2022.07.07.22277128. [DOI] [PMC free article] [PubMed]