Abstract
Alcohol use disorder (AUD) is a leading cause of death and disability worldwide. Genome-wide association studies (GWAS) have identified ~30 AUD risk genes in European populations, but many fewer in East Asians. We conducted GWAS and genome-wide meta-analysis of AUD in 13,551 subjects with East Asian ancestry, using published summary data and newly genotyped data from five cohorts: (1) electronic health record (EHR)-diagnosed AUD in the Million Veteran Program (MVP) sample; (2) DSM-IV diagnosed alcohol dependence (AD) in a Han Chinese–GSA (array) cohort; (3) AD in a Han Chinese–Cyto (array) cohort; and (4) two AD Thai cohorts. The MVP and Thai samples included newly genotyped subjects from ongoing recruitment. In total, 2254 cases and 11,297 controls were analyzed. An AUD polygenic risk score was analyzed in an independent sample with 4464 East Asians (Genetic Epidemiology Research in Adult Health and Aging (GERA)). Phenotypes from survey data and ICD-9-CM diagnoses were tested for association with the AUD PRS. Two risk loci were detected: the well-known functional variant rs1229984 in ADH1B and rs3782886 in BRAP (near the ALDH2 gene locus) are the lead variants. AUD PRS was significantly associated with days per week of alcohol consumption (beta = 0.43, SE = 0.067, p = 2.47 × 10−10) and nominally associated with pack years of smoking (beta = 0.09, SE = 0.05, p = 4.52 × 10−2) and ever vs. never smoking (beta = 0.06, SE = 0.02, p = 1.14 × 10−2). This is the largest GWAS of AUD in East Asians to date. Building on previous findings, we were able to analyze pleiotropy, but did not identify any new risk regions, underscoring the importance of recruiting additional East Asian subjects for alcohol GWAS.
Subject terms: Addiction, Genetics
Introduction
Globally, alcohol use disorders (AUD) are among the top causes of morbidity and mortality [1]. Numerous factors predispose to the risk of developing AUD. Genetic factors contribute substantial risk to the etiology of AUD [2], and the heritability has been estimated to be ~0.5 in twin studies [3]. Genome-wide association studies (GWAS) of AUD have been completed in multiple populations including European (EUR), African, Latin American and Asians [4–13]. To date, the largest GWAS of problematic alcohol use (PAU, a proxy for AUD) in 435,563 EUR subjects identified 29 independent risk variants [14]. In contrast, the largest GWAS of AUD in East Asians included less than 1% of this number: 3381 subjects (533 cases) [12]. Genetic architecture often differs between populations; polygenic risk prediction between populations, though sometimes useful, often is not transferable [15]. Thus, it is critically important that non-EUR populations be investigated to permit inferences to be made about these ancestral populations, which represent the majority of the world’s people [16, 17].
Because of the limited sample available and consequent lack of power for GWAS, little is known about the genetic architecture of AUD in East Asians. The most consistent loci identified are ADH1B (alcohol dehydrogenase 1b) and ALDH2 (aldehyde dehydrogenase). Candidate studies of ADH1B*rs1229984 and ALDH2*rs671 in East Asians showed strong associations between these functional variants and alcohol dependence (AD) [18, 19]. The first GWAS of AD in a Chinese sample was conducted in 102 male cases and 212 male controls; rs3782886 in the ALDH2 region was genome-wide significant [5] despite the very small sample size. The first GWAS of AD in Thai samples included 1045 subjects and identified rs149212747 in the ALDH2 region as the lead variant [6]. The latest GWAS of AUD in a Chinese cohort identified both ADH1B and ALDH2 genes as risk loci [12]. However, only a small proportion of the variance was explicable by variants in these genes. Larger samples are required to identify more risk variants to provide a better understanding of the genetic architecture in Asian populations.
Here we conducted a GWAS that combined five datasets from previously published cohorts and newly genotyped subjects from Thai and MVP cohorts. In total, 13,551 subjects of East Asian ancestry were analyzed, including 2254 AUD cases. We then analyzed the resulting AUD PRS in an independent East Asian sample for associations with 26 phenotypes from surveys or ICD diagnoses. This GWAS of AUD is the largest to date in East Asians.
Methods and materials
Datasets
Thai METH–GSA
As described previously [6], subjects were recruited in two stages for studies of the genetics of methamphetamine dependence (Thai METH). For both stages, subjects were recruited in Bangkok and assessed using the Thai version of the Semi-Structured Assessment for Drug Dependence and Alcoholism [20]. The IRB protocols were approved by both the Chulalongkorn University (Thailand) IRB and the Yale University Human Research Protection Program. All subjects provided written informed consent prior to their research participation.
The first stage included methamphetamine users hospitalized between 2007 and 2011 for 4 months of residential drug treatment (Thai METH–GSA, Table 1) [21]. DNA samples were genotyped on the Illumina (San Diego, CA) Global Screening Array (GSA) which includes ~640 K SNPs. Among the 863 genotyped subjects, we removed those with sample genotype call rate <0.9, mismatched genotypic and phenotypic sex, or excess heterozygosity rate [6]. Unlike for our prior report, here we retained related subjects and applied linear mixed models (LMM) to correct for relatedness (see below). SNPs with genotype call rate ≥0.95, minor allele frequency (MAF) ≥0.01, and Hardy–Weinberg equilibrium (HWE) p value >10−6 were kept for imputation. Imputation was done by IMPUTE2 [22] with 1000 Genome project phase 3 (1KG) data [23] as reference. SNPs with imputation INFO score ≥0.8, best-guess genotype call rate ≥0.95, MAF ≥0.01and HWE p value >10−6 were retained for association analyses. Principal component analysis (PCA) was performed for the remaining subjects using EIGENSOFT [24, 25]. In contrast with our previous study, here we used DSM-IV AD to define case status, rather than the DSM-IV AD criterion count to match the design in other cohorts. This yielded 127 cases and 405 were exposed controls. LMM implemented in GEMMA [26] were used to test association, with age, sex, and the first ten PCs as covariates.
Table 1.
Sample characteristics.
| Cohorts | Traits | N (%female) | # Cases | Mean (SD), Age | Country of recruitment |
|---|---|---|---|---|---|
| Thai METH–GSA | DSM-IV AD | 532 (49.4) | 127 | 26.6 (6.9) | Thailand |
| Thai METH–MEGA | DSM-IV AD | 2370 (42.5) | 794 | 34.7 (10.1) | Thailand |
| MVP–EAA | ICD-9/10 AUD | 6955 (10.7) | 701 | 53.4 (17.1) | United States |
| Han Chinese–GSA | DSM-IV AD | 3381 (29.9) | 533 | 34.2 (8.3) | China |
| Han Chinese–Cyto | DSM-IV AD | 313 (0) | 99 | 49.6 (14.7) | China |
| Total | 13,551 | 2254 |
Thai METH studies of the genetics of methamphetamine dependence in Thailand, GSA Global Screening Array, MEGA Multi-Ethnic Global Array, EAA East Asian American, Cyto Cyto12 array, AD alcohol dependence, AUD alcohol use disorder.
Thai METH–MEGA
Second-stage subjects (N = 3,161; the Thai METH-MEGA sample, Table 1) were recruited from 2015 to 2020 [6]. DNA samples were genotyped using the Illumina Multi-Ethnic Global Array (MEGA) which includes ~1.78 M SNPs. We removed subjects with sample genotype call rate <0.95, sex mismatch, excess heterozygosity rate, or that were duplicates. SNPs with genotype call rate ≥0.95, or MAF ≥0.01, or HWE p value >10−6 were retained for imputation as with the Thai METH–GSA sample. The same imputation processes and post-imputation quality controls (QC) were applied. We included 794 cases and 1576 alcohol-exposed controls in the association analysis, which used GEMMA and age, sex and the first ten PCs as covariates.
MVP–EAA
The Million Veteran Program (MVP) is an ongoing observational cohort study and mega-biobank supported by the U.S. Department of Veterans Affairs [27, 28]. In October 2020, MVP released the latest genotype data (Release 4), which included 658,582 subjects. MVP subjects were genotyped using an Affymetrix Axiom Biobank Array with ~687 K markers. QC was first done by the MVP Release 4 Data Team and included the removal of duplicate DNA samples and those with sex mismatch, excessive heterozygosity, or a genotype call rate <0.985. We ran PCA for the MVP subjects with 1KG as the reference, Euclidean distances between each participant and the centers of the five reference populations were calculated using the first ten PCs, with each participant assigned to the nearest reference population. For subjects grouped as East Asian Americans (EAA), we ran a second PCA and removed outliers with PC scores >6 standard deviations from the mean on any of the ten PCs (as we did before [8]), yielding in 7364 EAAs. Imputation [22] was performed specifically for the EAAs using the 1KG as reference. SNPs with genotype call rate ≥0.95, MAF ≥ 0.01, HWE > 1 × 10−6, and imputation INFO ≥ 0.8 were retained for analysis. As for our prior study in EUR [14], subjects with ≥2 outpatient or ≥1 inpatient International Classification of Diseases (ICD) codes for AUD were defined as cases (N = 701, Table 1) and subjects with no AUD ICD code as controls (N = 6254). BOLT-LMM [29] was used to correct for relatedness, with age, sex, and the first ten PCs as covariates.
Han Chinese–Cyto
This first GWAS of AD, flushing response, and maximum daily drinks consumed in a Han Chinese family sample [5] used the Illumina Cyto12 array containing ~300 K SNPs (Table 1). Whereas the cohort was not imputed in the original report, we re-analyzed the data and imputed the SNPs for an AD GWAS. Subjects with genotype call rate <0.95, duplicated DNA samples, mismatched sex, or excessive heterozygosity were removed, resulting in 511 subjects for imputation. Imputation used IMPUTE2 and 1KG reference, SNPs with MAF <0.01, genotype call rate <0.95, HWE p value <1 × 10−6, or imputation INFO < 0.8 were removed from further analyses. Due to the drinking practices and characteristic of this particular population that result in few AD cases in females, only males were included in this analysis. After QC, 99 DSM-IV-diagnosed male AD cases and 214 male alcohol-exposed controls were analyzed using GEMMA to correct for relatedness, with age, sex and ten PCs as covariates.
Han Chinese–GSA
The second case-control AD GWAS in Han Chinese [12] included 533 cases and 2848 alcohol-exposed controls who were genotyped using the GSA array (Table 1). Here we used the summary statistics from previous study.
Meta-analysis
Using association analyses or summary statistics for each of the five cohorts, effective sample-size-weighted meta-analysis was performed using METAL [30]. SNPs present in only one cohort or in less than 15% of the total samples were removed (6.8 million SNPs remained). To define lead variants, the meta-analysis summary data were clumped by LD with r2 < 0.1 in a 2500-kb window, using 1KG East Asians as the LD reference. For the two lead SNPs in the ADH1B gene region (rs1229984 and rs1814125), we performed conditional analysis [31] for rs1814125 conditioning on rs1229984 to test if its association is independent from rs1229984. Regional association plots were generated using LocusZoom v1.4 [32] with reference LD calculated from corresponding 1KG populations. We converted the effect sizes of lead SNPs from the LMM to odds ratios (OR) for comparison and further investigation of cohort heterogeneity [33]. This method takes sample prevalence, effect size from LMM, and allele frequency as input. We also did meta-analyses for the lead SNPs using inverse variance-weighted meta-analysis using METAL and the converted log(OR) as input, for comparison.
Polygenic risk scores
Target dataset
We requested and downloaded dbGaP (phs000788.v2.p3) data from the Kaiser Permanente Research Program on Genes, Environment, and Health Genetic Epidemiology Research on Adult Health and Aging (GERA) cohort. This large and ethnically diverse cohort contains genotype data from 5182 self-reported Asians using a custom Affymetrix Axiom array [34]. All subjects completed a broad written consent.
Imputation
Subjects with mismatched sex or genotype call rate <0.95 were removed. The genomic build was transferred from 36 to 37 using LiftOver [35]. As we did for MVP, we ran PCA for the 5182 Asian subjects using the 1KG as reference, clustering them into different groups. A second PCA among Asians was used to remove outliers, resulting in 4464 genetically classified East Asians for imputation. Imputation was performed using IMPUTE2 and 1KG reference, SNPs with MAF <0.01, genotype call rate <0.95, HWE p value <1 × 10−6, or imputation INFO < 0.8 were removed from further analysis.
Target phenotypes
Two sources of phenotypes are included in this study. The first is survey data on physical observations, lifestyle and environment, including phenotypes such as BMI, general health, physical activity, alcohol use, smoking status and pack years. The second is ICD-9-CM disease and conditions measures. Participant were coded as cases if there were at least two diagnoses in a disease category. Binary phenotypes with less than 100 cases were removed from analyses. See Table 2 for details of the target phenotypes.
Table 2.
Tested phenotypes in GERA and association results with AUD PRS.
| Traits | Distribution | Beta (SE) | p value |
|---|---|---|---|
| Alcohol use in days per week | 1 = 2757, 2 = 603, 3 = 503, 4 = 159, 5 = 267c | 0.43 (0.07) | 2.47 × 10−10 |
| Smoking in pack years | 0 = 3232, 1 = 530, 2 = 306, 3 = 128, 4 = 44d | 0.09 (0.05) | 4.52 × 10−2 |
| Ever vs. never smoked | 1 = 1055, 0 = 3232 | 0.06 (0.02) | 1.14 × 10−2 |
| Former vs. current smoker | 1 = 924, 0 = 131 | −0.04 (0.04) | 2.50 × 10−1 |
| Physical activity | 1 = 897, 2 = 958, 3 = 1181, 4 = 1323e | 0.02 (0.06) | 7.97 × 10−1 |
| Health status | 1 = 740, 2 = 1506, 3 = 1625, 4 = 476 f | 0.03 (0.05) | 5.23 × 10−1 |
| Disease or conditions | |||
| Acute reaction to stress | 1 = 275, 0 = 4189 | −0.01 (0.01) | 5.22 × 10−1 |
| Allergic rhinitis | 1 = 1307, 0 = 3157 | 0.01 (0.03) | 5.62 × 10−1 |
| Asthma | 1 = 654, 0 = 3810 | −0.01 (0.02) | 7.21 × 10−1 |
| Cancer: anya | 1 = 529, 0 = 3935 | −0.00 (0.02) | 9.36 × 10−1 |
| Cardiovascular disease: anyb | 1 = 688, 0 = 3776 | −0.03 (0.02) | 1.48 × 10−1 |
| Major depressive disorder | 1 = 262, 0 = 4202 | 0.01 (0.01) | 3.66 × 10−1 |
| Dermatophytosis | 1 = 374, 0 = 4090 | −0.01 (0.02) | 4.87 × 10−1 |
| Type II diabetes | 1 = 729, 0 = 3735 | 0.03 (0.02) | 9.65 × 10−2 |
| Dyslipidaemia | 1 = 2192, 0 = 2272 | −0.02 (0.03) | 5.17 × 10−1 |
| Hemorrhoids | 1 = 716, 0 = 3748 | 0.01 (0.02) | 6.19 × 10−1 |
| Hernia abdominopelvic cavity | 1 = 177, 0 = 4287 | 0.00 (0.01) | 7.54 × 10−1 |
| Hypertensive disease | 1 = 2028, 0 = 2436 | −0.00 (0.02) | 9.52 × 10−1 |
| Insomnia | 1 = 185, 0 = 4279 | −0.01 (0.01) | 2.30 × 10−1 |
| Iron deficiency anemias | 1 = 118, 0 = 4346 | 0.00 (0.01) | 9.14 × 10−1 |
| Irritable bowel syndrome | 1 = 103, 0 = 4361 | 0.00 (0.01) | 7.05 × 10−1 |
| Macular degeneration | 1 = 130, 0 = 4334 | −0.00 (0.01) | 7.94 × 10−1 |
| Osteoarthritis | 1 = 941, 0 = 3523 | −0.01 (0.02) | 6.72 × 10−1 |
| Osteoporosis | 1 = 392, 0 = 4072 | 0.01 (0.01) | 6.46 × 10−1 |
| Psychiatric disorder: any | 1 = 433, 0 = 4031 | −0.00 (0.02) | 9.93 × 10−1 |
| Peripheral vascular disease | 1 = 160, 0 = 4304 | 0.01 (0.01) | 3.81 × 10−1 |
If not specified for distribution, 1 is case and 0 is control. Traits with p value < 0.05 are labled in bold font..
aCancer: includes malignant tumors, neoplasms, lymphoma and sarcoma.
bHeart disease: includes ischemic heart disease, cardiac arrest, congestive health failure, dysrhythmias, cardiomyopathy, aortic aneurysm, and cerebrovascular disease, but excludes PVD which is encompassed by the PVD variable.
cDays of alcohol intake per week, 1 is no days, 2 is 1 day, 3 is 2–4 days, 4 is 5–6 days, 5 is every day.
dPack years for former or current smoker, 0 = 0, 1 < 10, 2 = 10–20, 3 = 20–30, 4 ≥ 30.
ePhysical activity total metabolic equivalency of task (MET), 1 = first quartile, 0–173 for males and 0–74 for females, 2 = second quartile, 174–600 for males and 75–344 for females, 3 = third quartile, 601–1380 for males and 345–983 for females, 4 = fourth quartile, 1381+ for males and 984+ for females.
fHealth status, 1 = excellent, 2 = very good, 3 = good, 4 = fair.
Polygenic risk scoring and association
PRS-CS [36] was used to infer posterior effect sizes of SNPs using GWAS summary statistics for AUD from this study, and an external East Asian LD reference panel (generated by the authors of PRS-CS using the 1KG East Asian reference). We used PLINK v1.9 [37] for polygenic risk scoring in the GERA East Asian samples. GEMMA was used to analyze associations between the PRS and target phenotypes, accounting for relatedness and correcting for age (in 5-year categories), sex and the first ten PCs. Bonferroni correction was applied such that associations with p value <0.05/26 = 1.92 × 10−3 are considered significant.
Additional downstream analyses
We used LD score regression [38] to estimate the SNP-based observed scale heritability of AUD using 1KG East Asians as the LD reference. We also investigated the trans-ancestry genetic correlation between this study sample and PAU in EUR populations using Popcorn, a method that uses only summary-level data from GWAS while accounting for LD [39]. Trans-population meta-analysis between this study and PAU in EUR was conducted using METAL. Multi-trait analysis [40] was performed, which combined data from this study with excessive alcohol consumption defined as weekly intake >150 ml of alcohol for ≥6 months from the Taiwan Biobank [41].
Results
Genome-wide association and meta-analyses
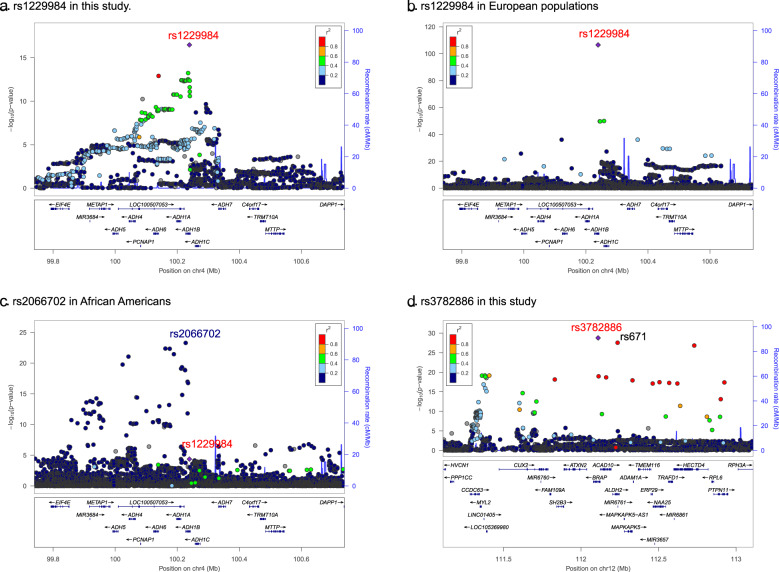
As in our previous study of AUD in East Asians [12], in a meta-analysis here of 2254 cases and 11,297 controls, we confirmed two loci that were significantly associated with AUD (Table 1 and Fig. 1). One locus is on chromosome 4q23 and includes multiple alcohol dehydrogenase genes. After LD clumping, there are two lead SNPs in this locus. The first is rs1229984 (Arg48His, p = 3.35 × 10−17, Fig. 2a) in ADH1B (Alcohol Dehydrogenase 1B (Class I), Beta Polypeptide), the second is rs1814125 (p = 2.14 × 10−10) near ADH1C. Conditional analysis indicated that rs1814125 is not independent from rs1229984. For comparison, we also looked up the association of rs1229984 in other populations. Rs1229984 is also associated with PAU in European populations [14] (Fig. 2b). In African Americans from MVP, rs122994 is nominally significantly associated with AUD while another coding variant, rs2066702, is the lead SNP [8] at ADH1B (Fig. 2c). Another locus is a long region with high LD on chromosome 12 for which there is positive selection in East Asians [42], which includes ALDH2 (Aldehyde Dehydrogenase 2) and BRAP (BRCA1 associated protein) genes. The lead SNP is rs3782886 (p = 1.68 × 10−29), a coding variant in the BRAP gene. The previously reported functional coding variant rs671 in ALDH2 is the second most significant SNP (p = 2.70 × 10−28); it is not independent from rs3782886. No other independent associations were detected in this study. There are allele frequency differences among cohorts for these two lead SNPs, and moderate heterogeneity of effect sizes (the converted ORs) detected by the IVW meta-analyses (Fig. S1). The heterogeneity p values are 6.17 × 10−13 for rs1229984 and is 2.63 × 10−5 for rs3782886 by IVW meta-analysis, justifying the use of effective sample size-weighted meta-analysis.
Fig. 1. Association results for AUD meta-analyses.
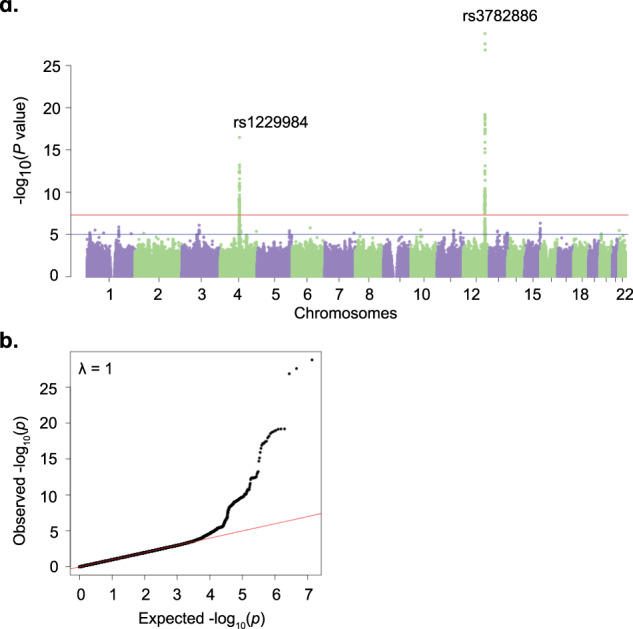
a Manhattan plot for AUD, ncase = 2254, ncontrol = 11,297. Effective sample size-weighted meta-analyses were performed using METAL. Red line indicates genome-wide significant after correction for multiple testing (p < 5 × 10–8), blue line indicates suggestive significant (p < 1 × 10–5). b QQ plot for AUD.
Fig. 2. Regional Manhattan plots for the top SNPs.
a Regional plot for rs1229984 in East Asians. b Regional plot for rs1229984 in European populations in a previous study (Zhou et al. [14]). c Regional plot for rs1229984 in African Americans from a previous MVP study (Kranzler et al. [8]) where rs1229984 is nominally significant, rs2066702 is the lead variant. In total, 500 kb in the upstream and downstream of rs1229984 were presented in a–c. d Regional plot for rs3782886 in East Asians. Given the high LD in this region, 1 Mb from both sides were extended.
Polygenic risk score for AUD
We calculated PRS for AUD in an independent East Asian cohort from the GERA cohort. We tested 26 phenotypes from survey and ICD-9-CM diagnosed conditions for association with the AUD PRS (Table 2). As expected, AUD PRS is significantly associated with alcohol consumption as measured in days per week of drinking (beta = 0.43, SE = 0.067, p = 2.47 × 10−10). Also, AUD PRS is nominally significantly associated with pack years of smoking (beta = 0.09, SE = 0.05, p = 4.52 × 10−2) and ever vs. never smoking (beta = 0.06, SE = 0.02, p = 1.14 × 10−2), but these associations did not survive Bonferroni correction. None of the other traits in this small target sample were associated with AUD PRS.
Additional downstream analyses
SNP-based heritability of AUD was estimated to be 0.11 (SE = 0.07), which is not a significant estimate (probably due to the limited sample size). Genetic correlation between AUD in East Asian and PAU in European samples is 0.62 (SE = 0.23, p = 8.42 × 10−3), showing moderate trans-ancestry genetic correlation. None of the trans-population meta-analysis between this study and PAU in EUR or multi-trait analysis with excessive alcohol consumption from Taiwan Biobank identified additional association signals.
Discussion
We collected data from 13,551 subjects with East Asian ancestry to conduct the largest meta-analysis to date for an alcohol-related trait in this population (quadruple the previous largest reported sample). We detected association signals at the ADH1B and ALDH2 loci with substantially stronger statistical significance than has been seen previously, but did not identify any novel risk loci. This is mostly consistent with observations from other studies, where GWAS of alcohol-related traits with sample sizes in this range are generally underpowered to detect multiple replicable variants [4, 7,43–46]. In EUR and African-Ancestry populations, the first and strongest associations detected have been at ADH1B. The ALDH2 association is, to this date, unique to East Asians (rs671, a well-known functional ALDH2 variant, is apparently unique to certain Asian populations [19]). It is a common issue for complex traits like AUD that many variants contribute to the heritability, each with a small effect size [47, 48]. Missing ancestral diversity in human genetic studies is a critical issue and recruitment of non-EUR subjects is crucial to addressing this disparity [16, 17]. The identification of ALDH2, which as noted is unique to Asians, exemplifies that there are differences in the genetic architecture of AUD between Asians and, for example, EUR, making well-powered investigations in this population an important scientific issue. Beyond identifying ADH1B and ALDH2 with greater statistical significance than previous studies, the present investigation extends prior findings in several ways, including by examining the utility of the AUD PRS derived from this meta-analysis in an independent cohort of 4,464 East Asians and testing the association between the AUD PRS and alcohol, smoking, and other traits.
The two genes implicated—ADH1B and ALDH2—are involved in ethanol metabolism [48]. ADH1B encodes an alcohol dehydrogenase that oxidizes alcohol to acetaldehyde, which is then oxidized to acetate by aldehyde dehydrogenases, including that encoded by ALDH2. This is the major metabolic pathway for ethanol metabolism but other genes are involved as well. For example, in the first step, ADH1C, ADH4 and ADH7, which map to the same chromosome 4 gene cluster as ADH1B, encode proteins that perform similar biological functions under certain conditions, ALDH1A1 and ALDH1B1 similarly encode proteins with roles that are sometimes overlapping with that of ALDH2 [49]. Given the importance of other genes in the metabolic pathway, lead variants in genes other than ADH1B*rs1229984 (EUR and Asian) and ALDH2*rs671 (Asian) have been reported [6, 8, 12, 14]. Some of these associations are supported by conditional analyses [8, 14], and some appear to be variants that “hitchhike” with rs1229984 or rs671 due to their strong LD. Here, conditional analyses identified only one lead variant at each locus: rs1229984 (p = 3.35 × 10−17) in the AHD1B region and rs3782886 (p = 1.68 × 10−29) in the ALDH2 region. The high LD between rs3782886 and rs671 (r2 = 0.98) makes it difficult to distinguish the real causal variant, though biochemical analysis favors rs671 (reviewed in [48]), which is nearly a null variant. A single copy of the rs671*T allele renders the aldehyde dehydrogenase protein product nearly inactive and it is also more rapidly degraded, which causes flushing in East Asians and other associated symptoms that are protective against heavy drinking and AUD [50].
Rs3782886 in the BRAP gene (breast cancer suppressor protein (BRCA1)-associated protein) has been associated with many traits in East Asians, include alcohol-related traits [41, 51], myocardial infarction [52], and a biochemical trait—alanine aminotransferase level [53]. Some or all these associations could be due to the high LD with rs671 (as in this study), or reflect effects on activity of the metabolic pathway or cerebral cortical neurogenesis (argued in [41]). We would suggest that the different lead variants (rs671 or rs3782886) in this high LD region could reflect uncertainty introduced by different SNP arrays, imputation processes, association analyses, or random variation in comparatively small samples. More data are needed to ascertain the true causal variant (or variants) despite the previous support and mechanistic appeal of rs671.
We used additional analyses to explore the genetic architecture of AUD in East Asians. The SNP-based heritability estimate was very low with a large standard error (SE), indicating a lack of statistical power. Moderate genetic correlation (rg = 0.61, SE = 0.23, p = 8.42 × 10−3) was detected between the main meta-analysis of this study and PAU in EUR populations, indicating shared genetic architecture across ancestries. However, the trans-population meta-analysis in which PAU in EUR was added did not detect any novel signals, probably due to the limited power in this study. Multi-trait analysis combining this study sample and excessive alcohol consumption from the Taiwan Biobank also identified no novel variants. Thus, additional study samples of East Asian ancestry are needed to provide adequate power for GWAS of AUD in East Asians.
Since it is a genetically complex trait, we expect that there are many variants that contribute to the genetic risk of AUD, consistent with findings in EUR [14]. Polygenic risk score analysis is a powerful tool for the application of GWAS results to investigate associations with traits of interest, which has been used widely in studies to test the association with AUD or related phenotypes in target cohorts [7, 8, 12, 14]. Here, we analyzed AUD PRS from our meta-analysis in an independent East Asian cohort from GERA, a US cohort collected to facilitate research on the genetic and environmental factors that affect health and disease [34]. We tested the association between AUD PRS and 26 phenotypes in 4464 subjects of East Asian ancestry. AUD PRS was significantly associated with alcohol consumption as measured using days of drinking per week (see Table 2), and nominally significantly associated with pack years of smoking and ever vs. never smoking, consistent with the shared genetic architecture of AUD and alcohol and (possibly) smoking traits in East Asians. These same, or closely similar, associations, have been well established in EUR [8, 14]. These was no association detected between AUD PRS and other diseases or conditions in this study.
This study has limitations, the most important of which is the sample size, which despite being the largest reported so far for East Asian provides limited statistical power. Second, the phenotypes among the different study samples are not identical, with AUD diagnosed as ICD-9/10 codes in MVP and DSM-IV AD in other cohorts. This analytic approach is supported by the high genetic correlation between AUD and AD in EUR, which is estimated to approach 1.0 [14]. Third, some cohorts used alcohol-exposed controls, and others used unscreened controls (i.e., the MVP). Controls with demonstrated exposure to alcohol are ideal, but such exposure is commonplace in all the populations studied. Finally, although all of the cohorts are of East Asian ancestry, there are population differences among cohorts that increase heterogeneity and reduce power [54, 55]. These include cultural or environmental differences that affect trait prevalence (e.g., drinking practices), and geographical differences that introduce genetic differences (Fig. S1).
In conclusion, we conducted a GWAS of AUD in 13,551 East Asian subjects, in which we confirmed the two previously known risk loci and applied the AUD PRS in an independent cohort. Despite a large increment in sample size over the previous largest Asian-population GWAS, the power remains an important limitation. Accordingly, we will continue to recruit more East Asian subjects for alcohol studies and urge other investigators to do the same.
Supplementary information
Acknowledgements
This research used data from the Million Veteran Program, which was supported by funding from the Department of Veterans Affairs Office of Research and Development, Million Veteran Program Grant #I01BX003341 (ACJ, HRK). This publication does not represent the views of the Department of Veterans Affairs or the United States Government. Supported also by NIH (NIDA) R01 DA037974 (JG, RTM), (NCI) R21 CA252916 (HZ), and a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation (HZ). Data from the Kaiser Permanente Research Program on Genes, Environment, and Health (RPGEH) were accessed from dbGaP (phs000788.v2.p3). We thank the authors of the Taiwan Biobank excessive alcohol consumption study for sharing summary statistics.
Author contributions
HZ and JG take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: HZ and JG. Acquisition of data: all authors. Analysis, or interpretation of data: HZ and JG. Drafting of the manuscript: HZ. Critical revision of the manuscript for important intellectual content: HZ, JG, and HRK. Obtained funding: HRK, ACJ, JG, RTM, and HZ. Final approval of the version to be published: all authors. Administrative, technical, or material support: YZN and JG. Study supervision: JG.
Data availability
Summary statistics are available on dbGaP (https://www.ncbi.nlm.nih.gov/gap) under study accession (phs001672).
Competing interests
HRK is a member of a scientific advisory board for Dicerna Pharmaceuticals and a consultant to Sophrosyne Pharmaceuticals and Sobrera Pharmaceuticals and is a member of the American Society of Clinical Psychopharmacology’s Alcohol Clinical Trials Initiative, which was supported in the last 3 years by AbbVie, Alkermes, Ethypharm, Indivior, Lilly, Lundbeck, Otsuka, Pfizer, Arbor, and Amygdala Neurosciences. HRK and JG are named as inventors on PCT patent application #15/878,640 entitled: “Genotype-guided dosing of opioid agonists,” filed January 24, 2018. The remaining authors have nothing to disclose.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Deceased: Robert T. Malison.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-022-01265-w.
References
- 1.GBD Causes of Death Collaborators Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hart AB, Kranzler HR. Alcohol dependence genetics: lessons learned from genome-wide association studies (GWAS) and post-GWAS analyses. Alcohol Clin Exp Res. 2015;39:1312–27. doi: 10.1111/acer.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verhulst B, Neale MC, Kendler KS. The heritability of alcohol use disorders: a meta-analysis of twin and adoption studies. Psychol Med. 2015;45:1061–72. doi: 10.1017/S0033291714002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gelernter J, Kranzler HR, Sherva R, Almasy L, Koesterer R, Smith AH, et al. Genome-wide association study of alcohol dependence:significant findings in African- and European-Americans including novel risk loci. Mol Psychiatry. 2014;19:41–9. doi: 10.1038/mp.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quillen EE, Chen XD, Almasy L, Yang F, He H, Li X, et al. ALDH2 is associated to alcohol dependence and is the major genetic determinant of “daily maximum drinks” in a GWAS study of an isolated rural Chinese sample. Am J Med Genet B Neuropsychiatr Genet. 2014;165B:103–10. doi: 10.1002/ajmg.b.32213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gelernter J, Zhou H, Nuñez YZ, Mutirangura A, Malison RT, Kalayasiri R. Genomewide association study of alcohol dependence and related traits in a Thai population. Alcohol Clin Exp Res. 2018;42:861–8. doi: 10.1111/acer.13614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walters RK, Polimanti R, Johnson EC, McClintick JN, Adams MJ, Adkins AE, et al. Transancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. Nat Neurosci. 2018;21:1656–69. doi: 10.1038/s41593-018-0275-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kranzler HR, Zhou H, Kember RL, Vickers Smith R, Justice AC, Damrauer S, et al. Genome-wide association study of alcohol consumption and use disorder in 274,424 individuals from multiple populations. Nat Commun. 2019;10:1499. doi: 10.1038/s41467-019-09480-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bierut LJ, Goate AM, Breslau N, Johnson EO, Bertelsen S, Fox L, et al. ADH1B is associated with alcohol dependence and alcohol consumption in populations of European and African ancestry. Mol Psychiatry. 2012;17:445–50. doi: 10.1038/mp.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Treutlein J, Cichon S, Ridinger M, Wodarz N, Soyka M, Zill P, et al. Genome-wide association study of alcohol dependence. Arch Gen Psychiatry. 2009;66:773–84. doi: 10.1001/archgenpsychiatry.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank J, Cichon S, Treutlein J, Ridinger M, Mattheisen M, Hoffmann P, et al. Genome-wide significant association between alcohol dependence and a variant in the ADH gene cluster. Addict Biol. 2012;17:171–80. doi: 10.1111/j.1369-1600.2011.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Y, Chang S, Wang F, Sun H, Ni Z, Yue W, et al. Genome-wide association study of alcohol dependence in male Han Chinese and cross-ethnic polygenic risk score comparison. Transl Psychiatry. 2019;9:249. doi: 10.1038/s41398-019-0586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gelernter J, Polimanti R. Genetics of substance use disorders in the era of big data. Nat Rev Genet. 2021;22:712–29. doi: 10.1038/s41576-021-00377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou H, Sealock JM, Sanchez-Roige S, Clarke TK, Levey DF, Cheng Z, et al. Genome-wide meta-analysis of problematic alcohol use in 435,563 individuals yields insights into biology and relationships with other traits. Nat Neurosci. 2020;23:809–18. doi: 10.1038/s41593-020-0643-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin AR, Gignoux CR, Walters RK, Wojcik GL, Neale BM, Gravel S, et al. Human demographic history impacts genetic risk prediction across diverse populations. Am J Hum Genet. 2017;100:635–49. doi: 10.1016/j.ajhg.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peterson RE, Kuchenbaecker K, Walters RK, Chen CY, Popejoy AB, Periyasamy S, et al. Genome-wide association studies in ancestrally diverse populations: opportunities, methods, pitfalls, and recommendations. Cell. 2019;179:589–603. doi: 10.1016/j.cell.2019.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sirugo G, Williams SM, Tishkoff SA. The missing diversity in human genetic studies. Cell. 2019;177:26–31. doi: 10.1016/j.cell.2019.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li D, Zhao H, Gelernter J. Strong association of the alcohol dehydrogenase 1B gene (ADH1B) with alcohol dependence and alcohol-induced medical diseases. Biol Psychiatry. 2011;70:504–12. doi: 10.1016/j.biopsych.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li D, Zhao H, Gelernter J. Strong protective effect of the aldehyde dehydrogenase gene (ALDH2) 504lys (*2) allele against alcoholism and alcohol-induced medical diseases in Asians. Hum Genet. 2012;131:725–37. doi: 10.1007/s00439-011-1116-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pierucci-Lagha A, Gelernter J, Feinn R, Cubells JF, Pearson D, Pollastri A, et al. Diagnostic reliability of the semi-structured assessment for drug dependence and alcoholism (SSADDA) Drug Alcohol Depend. 2005;80:303–12. doi: 10.1016/j.drugalcdep.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Kalayasiri R, Verachai V, Gelernter J, Mutirangura A, Malison RT. Clinical features of methamphetamine-induced paranoia and preliminary genetic association with DBH-1021C→T in a Thai treatment cohort. Addiction. 2014;109:965–76. doi: 10.1111/add.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howie B, Fuchsberger C, Stephens M, Marchini J, Abecasis GR. Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nat Genet. 2012;44:955–9. doi: 10.1038/ng.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.1000 Genomes Project Consortium. Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–9. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 25.Patterson N, Price AL, Reich D. Population structure and eigenanalysis. PLoS Genet. 2006;2:e190. doi: 10.1371/journal.pgen.0020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou X, Stephens M. Efficient multivariate linear mixed model algorithms for genome-wide association studies. Nat Methods. 2014;11:407–9. doi: 10.1038/nmeth.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaziano JM, Concato J, Brophy M, Fiore L, Pyarajan S, Breeling J, et al. Million Veteran Program: a mega-biobank to study genetic influences on health and disease. J Clin Epidemiol. 2016;70:214–23. doi: 10.1016/j.jclinepi.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 28.Hunter-Zinck H, Shi Y, Li M, Gorman BR, Ji SG, Sun N, et al. Measuring genetic variation in the multi-ethnic Million Veteran Program (MVP) bioRxiv. 2020 doi: 10.1101/2020.01.06.896613. [DOI] [Google Scholar]
- 29.Loh PR, Kichaev G, Gazal S, Schoech AP, Price AL. Mixed-model association for biobank-scale datasets. Nat Genet. 2018;50:906–8. doi: 10.1038/s41588-018-0144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–1. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88:76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, Gliedt TP, et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010;26:2336–7. doi: 10.1093/bioinformatics/btq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lloyd-Jones LR, Robinson MR, Yang J, Visscher PM. Transformation of summary statistics from linear mixed model association on all-or-none traits to odds ratio. Genetics. 2018;208:1397–408. doi: 10.1534/genetics.117.300360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Banda Y, Kvale MN, Hoffmann TJ, Hesselson SE, Ranatunga D, Tang H, et al. Characterizing race/ethnicity and genetic ancestry for 100,000 subjects in the genetic epidemiology research on adult health and aging (GERA) cohort. Genetics. 2015;200:1285–95. doi: 10.1534/genetics.115.178616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hinrichs AS, Karolchik D, Baertsch R, Barber GP, Bejerano G, Clawson H, et al. The UCSC Genome Browser Database: update 2006. Nucleic Acids Res. 2006;34:D590–8. doi: 10.1093/nar/gkj144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ge T, Chen CY, Ni Y, Feng YA, Smoller JW. Polygenic prediction via Bayesian regression and continuous shrinkage priors. Nat Commun. 2019;10:1776. doi: 10.1038/s41467-019-09718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4:7. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bulik-Sullivan BK, Loh PR, Finucane HK, Ripke S, Yang J, Schizophrenia Working Group of the Psychiatric Genomics Consortium et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47:291–5. doi: 10.1038/ng.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown BC. Asian Genetic Epidemiology Network Type 2 Diabetes Consortium, Ye CJ, Price AL, Zaitlen N. Transethnic genetic-correlation estimates from summary statistics. Am J Hum Genet. 2016;99:76–88. doi: 10.1016/j.ajhg.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turley P, Walters RK, Maghzian O, Okbay A, Lee JJ, Fontana MA, et al. Multi-trait analysis of genome-wide association summary statistics using MTAG. Nat Genet. 2018;50:229–237. doi: 10.1038/s41588-017-0009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen IC, Kuo PH, Yang AC, Tsai SJ, Liu TH, Liu HJ, et al. CUX2, BRAP and ALDH2 are associated with metabolic traits in people with excessive alcohol consumption. Sci Rep. 2020;10:18118. doi: 10.1038/s41598-020-75199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oota H, Pakstis AJ, Bonne-Tamir B, Goldman D, Grigorenko E, Kajuna SL, et al. The evolution and population genetics of the ALDH2 locus: random genetic drift, selection, and low levels of recombination. Ann Hum Genet. 2004;68:93–109. doi: 10.1046/j.1529-8817.2003.00060.x. [DOI] [PubMed] [Google Scholar]
- 43.Schumann G, Coin LJ, Lourdusamy A, Charoen P, Berger KH, Stacey D, et al. Genome-wide association and genetic functional studies identify autism susceptibility candidate 2 gene (AUTS2) in the regulation of alcohol consumption. Proc Natl Acad Sci USA. 2011;108:7119–24. doi: 10.1073/pnas.1017288108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanchez-Roige S, Fontanillas P, Elson SL, 23andMe Research Team. Gray JC, de Wit H, et al. Genome-wide association study of alcohol use disorder identification test (AUDIT) scores in 20 328 research participants of European ancestry. Addict Biol. 2019;24:121–31. doi: 10.1111/adb.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jorgenson E, Thai KK, Hoffmann TJ, Sakoda LC, Kvale MN, Banda Y, et al. Genetic contributors to variation in alcohol consumption vary by race/ethnicity in a large multi-ethnic genome-wide association study. Mol Psychiatry. 2017;22:1359–67. doi: 10.1038/mp.2017.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schumann G, Liu C, O'Reilly P, Gao H, Song P, Xu B, et al. KLB is associated with alcohol drinking, and its gene product beta-Klotho is necessary for FGF21 regulation of alcohol preference. Proc Natl Acad Sci USA. 2016;113:14372–7. doi: 10.1073/pnas.1611243113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Qi G, Park JH, Chatterjee N. Estimation of complex effect-size distributions using summary-level statistics from genome-wide association studies across 32 complex traits. Nat Genet. 2018;50:1318–26. doi: 10.1038/s41588-018-0193-x. [DOI] [PubMed] [Google Scholar]
- 48.Edenberg HJ, McClintick JN. Alcohol dehydrogenases, aldehyde dehydrogenases, and alcohol use disorders: a critical review. Alcohol Clin Exp Res. 2018;42:2281–97. doi: 10.1111/acer.13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vasiliou V, Pappa A, Estey T. Role of human aldehyde dehydrogenases in endobiotic and xenobiotic metabolism. Drug Metab Rev. 2004;36:279–99. doi: 10.1081/DMR-120034001. [DOI] [PubMed] [Google Scholar]
- 50.Crabb DW, Edenberg HJ, Bosron WF, Li TK. Genotypes for aldehyde dehydrogenase deficiency and alcohol sensitivity. The inactive ALDH2(2) allele is dominant. J Clin Invest. 1989;83:314–6. doi: 10.1172/JCI113875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim JW, Choe YM, Shin JG, Park BL, Shin HD, Choi IG, et al. Associations of BRAP polymorphisms with the risk of alcohol dependence and scores on the alcohol use disorders identification test. Neuropsychiatr Dis Treat. 2019;15:83–94. doi: 10.2147/NDT.S184067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ozaki K, Sato H, Inoue K, Tsunoda T, Sakata Y, Mizuno H, et al. SNPs in BRAP associated with risk of myocardial infarction in Asian populations. Nat Genet. 2009;41:329–33. doi: 10.1038/ng.326. [DOI] [PubMed] [Google Scholar]
- 53.Kamatani Y, Matsuda K, Okada Y, Kubo M, Hosono N, Daigo Y, et al. Genome-wide association study of hematological and biochemical traits in a Japanese population. Nat Genet. 2010;42:210–5. doi: 10.1038/ng.531. [DOI] [PubMed] [Google Scholar]
- 54.Hugo Pan-Asian SNP, Consortium, Abdulla MA, Ahmed I, Assawamakin A, Bhak J, Brahmachari SK, et al. Mapping human genetic diversity in Asia. Science. 2009;326:1541–5. doi: 10.1126/science.1177074. [DOI] [PubMed] [Google Scholar]
- 55.Xu S, Yin X, Li S, Jin W, Lou H, Yang L, et al. Genomic dissection of population substructure of Han Chinese and its implication in association studies. Am J Hum Genet. 2009;85:762–74. doi: 10.1016/j.ajhg.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Summary statistics are available on dbGaP (https://www.ncbi.nlm.nih.gov/gap) under study accession (phs001672).