Abstract
Aggression is an evolutionarily conserved, adaptive component of social behavior. Studies in male mice illustrate that aggression is influenced by numerous factors including the degree to which an individual finds aggression rewarding and will work for access to attack and subordinate mice. While such studies have expanded our understanding of the molecular and circuit mechanisms of male aggression very little is known about female aggression, within these established contexts. Here we use an ethologically relevant model of male vs. female aggression by pair housing adult male and female outbred CFW mice with opposite sex cage mates. We assess reactive (defensive) aggression in the resident intruder (RI) test and appetitive (rewarding) aggression in the aggression conditioned place preference (CPP) and operant self-administration (SA) tests. Our results show dramatic sex differences in both qualitative and quantitative aspects of reactive vs. appetitive aggression. Males exhibit more wrestling and less investigative behavior during RI, find aggression rewarding, and will work for access to a subordinate to attack. Females exhibit more bites, alternate between aggressive behaviors and investigative behaviors more readily during RI, however, they do not find aggression to be rewarding or reinforcing. These results establish sex differences in aggression in mice, providing an important resource for the field to better understand the circuit and molecular mechanisms of aggression in both sexes.
Subject terms: Motivation, Social neuroscience
Introduction
Aggression exists along a spectrum from adaptive to maladaptive and is governed by both reactive (defensive) and appetitive (rewarding) drives. The transition away from an adaptive state can be associated with neuropsychiatric conditions and presents a challenge to patients and caregivers. Modeling and understanding the behavioral etiology of aggressive behavior is therefore a health priority with the potential to guide therapeutic interventions across a number of neuropsychiatric diseases. In mice, aggressive behavior serves as an evolutionary adaptation for survival [1] and engages highly conserved neural mechanisms [2]. However, while aggression is often a focus of both popular and scientific inquiry and highly evolutionarily conserved, very little is known about the neural and behavioral mechanisms controlling aggression-related sex differences.
Preclinical behavioral models developed in males have allowed for direct comparison of reactive and appetitive aggression [3–5]. Typically, reactive aggression is investigated using the resident intruder (RI) test in which a male intruder is introduced to the home cage of a male resident and they are allowed to freely interact [6]. To assess aggression reward, aggression conditioned place preference (CPP) can be used where male mice will display a preference for contexts previously associated with opportunities to attack a naïve conspecific [7–9]. However, like RI testing, this procedure uses forced involuntary social interactions and therefore cannot fully dissociate reactive from appetitive components. To overcome this, several groups have developed social operant tasks that measure voluntary appetitive aggression seeking in male mice [10–13], and established that appetitive aggression can transition to compulsive addiction-like behavior in some mice [8].
While these procedures have proven effective for studying aggressive behavior and the underlying neurobiology in animal models, studies have focused nearly entirely on males. Male aggression is typically studied in the context of isolated housing to enhance aggression, yet under similar conditions, naïve female mice do not show comparable intruder-directed aggression. To overcome this, alternative model organisms can be used such as Syrian hamsters [14] and California mice [15], or with mouse models of gestational aggression [16–18]. However, there are limitations to these alternate models: (i) only mouse models can presently exploit the broad transgenic toolbox available to understand circuit and molecular mechanisms, and (ii) gestational aggression models are not ideal for evaluating sex differences in aggressive behavior since these behaviors are linked to hormonal changes specifically associated with pregnancy, parturition, and lactation.
Two recent studies have revisited female aggression during RI tests using outbred mouse strains, as opposed to the more typically used inbred strains, and have found that naïve outbred CFW mice display similar levels of aggression as males. Outbred mouse strains such as CD1 and CFW have gained popularity in aggression-related studies due to their high individual variability in innate aggressive behavior (Chia et al., 2005, [9]). Similarly, isolated sex-naive female outbred CFW, but not inbred C57BL6/J, mice will attack juvenile male or adult female C57BL6/J intruders in the home cage [19], and female CFW mice pair-housed with a castrated male partner will attack adult female C57BL6/J mice, with similar but non-identical behavioral strategies to males [20]. The establishment of a female RI procedure opens the door for sex comparisons of the neurobiological substrates of reactive aggression, but currently, there are no direct comparisons of appetitive aggression in males versus females.
To further develop preclinical models of aggression, we directly compared adult male and female CFW mice in reactive and appetitive aggression procedures to evaluate sex as a biological variable. The evaluation of sex differences in aggression are compounded by the need for detailed annotation of behavioral actions and sequences. Typically, only gross measures of aggression, are reported. This lack of in-depth behavioral analysis obscures potential differences in specific behaviors and their sequences. Building upon a recent study in male mice [21], we utilized a discrete state hidden Markov model (HMM) to define sex differences in aggression. HMMs analyze the ordering, clustering, and transitions between actions and are able to cluster time-series of behavior into distinct hidden states. These models have been used extensively in analyzing sequences of speech, gestures, animal behavior, and the analysis of gene and protein sequences [22–25]. The discrete state HMM allowed us to examine the sequential composition of social behaviors and the hidden states which contribute to male versus female aggression. We also tested whether there are differences in reactive or appetitive aggression between male and female mice.
We therefore set out in this study to accomplish two goals: (i) to elaborate upon a mouse model of aggressive behavior encompassing a broad suite of ethologically relevant and reward-related behavioral metrics, and (ii) to compare reactive and appetitive aggression between male and female mice. Our findings demonstrate clear sex differences in the behavioral sequences that make up bouts of aggressive and investigative behavior. Further, using both aggression CPP and operant social self-administration (SA) procedures, we show that female CFW mice in these contexts do not exhibit context-dependent aggression reward nor do they exhibit appetitive aggression-seeking behavior. To our knowledge, this is the first report of sex differences in appetitive vs. reactive aggression in mus musculus. Together these data support distinct patterns of aggressive behavior between males and female outbred mice and underscore the importance of future research to identify detailed mechanisms by which different sexes express aggressive behavior.
Methods
Mice
10-week-old CFW mice (Charles River Laboratories) were used for all studies. Females were pair-housed with a castrated male for the study duration [20] and males were pair-housed for 48-hr with stimulus females prior to isolate housing. Subject males were separated from group-housed cage mates and paired with a female for 2-d, then singly housed for an additional 10-d before all protocols. This procedure was used in order to acquire roughly equal amounts of aggressors (AGG) and non-aggressors (NON) without affecting the amount of aggression observed in AGGs. Females were housed with surgically castrated male CFW mice (see supplemental methods) for at least 14-d before all protocols. 12-week-old C57BL/6J mice were used as intruders for all social interactions. All studies were conducted during the light cycle. Procedures were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Mice and approved by the Icahn School of Medicine at Mount Sinai Institutional Animal Care and Use Committee. To assess the role of traditional isolate housing versus the modified pair housing condition introduced in [20], additional control groups of two-week isolated males and females were used as subjects at the University of Washington. All studies were conducted during the dark cycle. Procedures were performed in accordance with the National Institutes of Health (NIH) Guide for Care Use of Laboratory Mice and approved by the University of Washington Institutional Animal Care and Use Committee.
Aggressor Screening and Resident-Intruder (RI) Test
Mice were screened using protocols adapted from previous studies [6, 9]. Briefly, cage tops were removed and replaced with Plexiglas covers to monitor trials. Before initiating trials with paired female mice, the cohabiting male mouse was removed to a holding cage until completion of test. A novel C57BL/6J mouse matching the sex of the resident was introduced into each cage and mice were allowed to freely interact for 5-min. After 5-min elapsed, intruder mice were returned to their home cages and, in the case of female resident-intruder trials, cohabiting male mice were returned to their home cages. All videos were recorded for later analysis. Resident behaviors from the Mount Sinai videos were manually annotated using Observer XT 11.5 (Noldus Interactive Technologies).
Aggression Conditioned Place Preference (CPP)
CPP testing was conducted in three phases as previously reported [7, 9]: pre-test, acquisition, and post-test. Mice were habituated to test rooms 1 h before acquisition or test trials. All phases were conducted under red light and in sound-attenuated conditions. The CPP apparatus (Med Associates) has a neutral middle zone that allowed for unbiased entry and two conditioning chambers with different walls and floors.
Appetitive aggression SA
Following RI training, each group was further separated into mice who were either AGG or NON during RI testing. Only one male was NON, and as such only AGG males were tested in operant SA. While all CFW mice in this group underwent RI testing, previous work demonstrates that male CD1 mice that have not undergone previous RI testing acquire aggression self-administration at rates ranging from 57–81% [8]. These results suggest that prior resident intruder experience is not necessary to elicit appetitive aggression self-administration.
3-d following RI testing, mice underwent 1-d of magazine training, in which they were exposed to operant cues (house light and a two-second tone) in addition to a same-sex intruder mouse entering the operant chamber (3 times each). On the following day, mice underwent SA training every other day for 9-d as previously described [8]. Researchers were present throughout all aggression testing to ensure that no mice were injured. Mice with an average of 3 presses or less across days 4–8 of training were considered non-acquirers.
See supplemental material for a full description of the methods.
Results
Gross characterization of social behavior in male and female mice
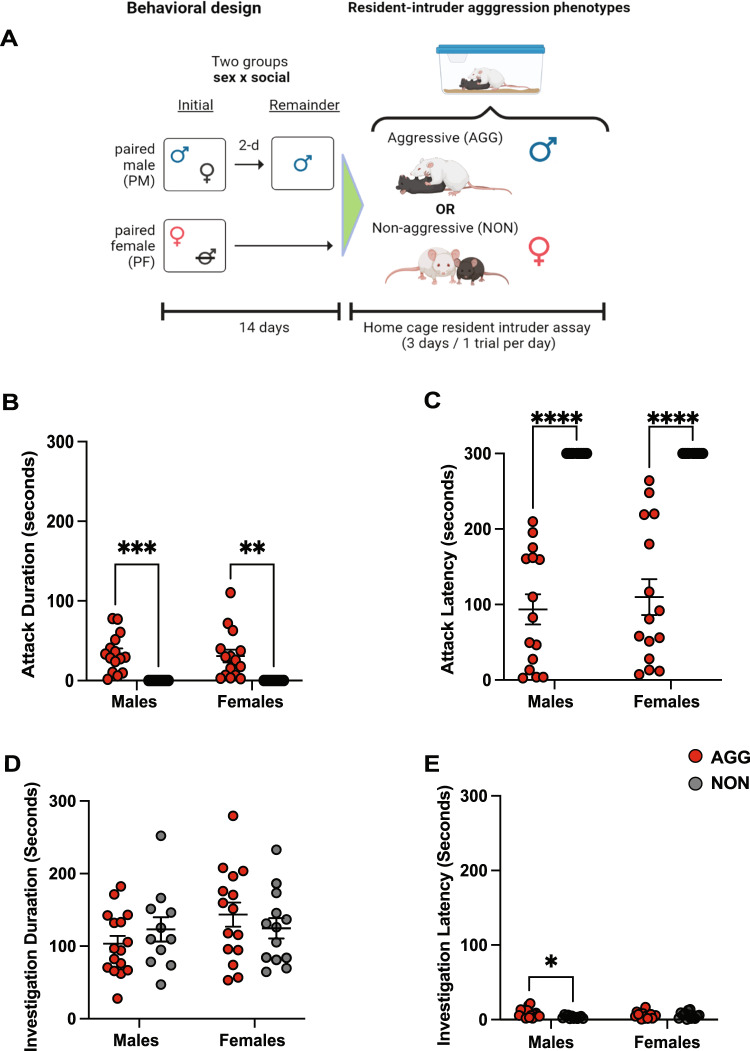
We focused our analysis on Day 3 of the RI test given that this when aggressive behavior is at its highest [6, 9]. On the third day of RI, both male and female AGGs engaged in more aggressive behavior than NONs (Phenotype F(1, 51) = 36.20, p < 0.0001, Male AGG vs. Male NON, p = 0.0002, Female AGG vs Female NON, p = 0.001), and there were no differences in duration or latency to attack between male and female AGGs (p > 0.05) (Fig. 1B, C). There were no differences between groups in investigative behavior (Sex: F(1, 51) = 2.947, p = 0.0920. Phenotype: F(1, 51) = 0.6242 Fig. 1D). Male NONs displayed a shorter latency to investigate intruders (Phenotype × Sex interaction F(1, 51) = 5.003, p = 0.0297, Male NON vs Male AGG, p = 0.0139, Fig. 1E).
Fig. 1. Male and Female CFW mice engage in similar amounts of aggressive and investigative social behavior.
A Schematic illustrating the housing conditions prior to the resident intruder test. B Total attack duration (B) and latency (C) did not significantly differ in male and female AGGs. D Total investigation. All groups show similar levels of social investigation E Investigation latency. Male NONs had a significantly shorter latency to investigate the intruder than male AGGs.
Male and female mice display distinct suites of social behavior
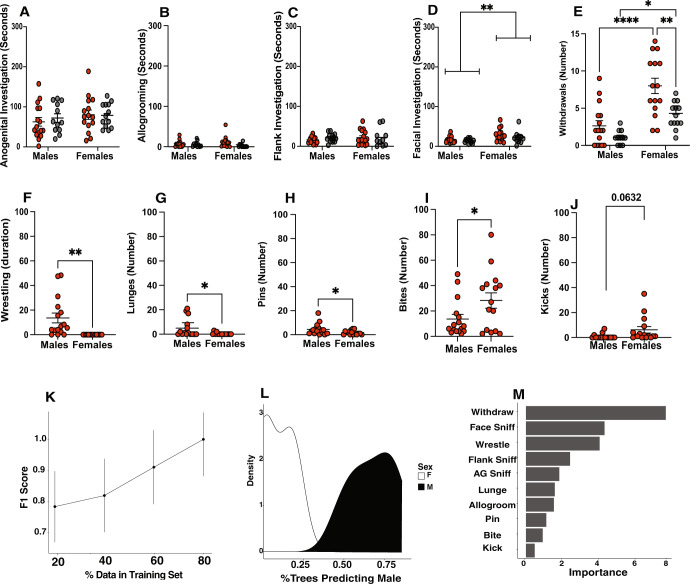
We next investigated whether male and female AGGs/NONs displayed distinct aggressive and/or investigative behaviors. We did not find any differences in anogenital (Sex: F(1, 51) = 0.4110, p = 0.5243 Phenotype: F(1, 51) = 1.141, p = 0.2905, Interaction: (1, 51) = 0.2701, p = 0.605) or flank investigation (Sex: F(1, 51) = 0.4182 p = 0.4551, Phenotype: F(1, 51) = 0.0577, p = 0.8904, Interaction: F(1, 51) = 0.3814, p = 0.490) between any of the four groups (Fig. 2A, C). Interestingly, we found that females engaged in significantly more facial investigation than males, regardless of their phenotype (F(1, 51) = 9.54, p = 0.0032, Interaction: F(1, 51) = 0.03007, p = 0.8630, Fig. 2D). We also observed that AGGs, regardless of their sex, engaged in more allogrooming than NONs (Phenotype: F(1, 51) = 4.574, p = 0.0373), although only female AGGs were significantly different from female NONs (p = 0.0412, Fig. 2B). We also found a main effect of both sex (F(1, 51) = 31.46, p < 0.0001) and phenotype (F(1, 51) = 13.40, p = 0.006) with no interaction (F(1, 51) = 3.690, p = 0.06) on the number of withdrawals observed (Fig. 3E). female AGGs displayed a higher number of withdrawals than male AGGs (p < 0.0001) and female NONs (p = 0.0022).
Fig. 2. Male and female mice display distinct investigative and aggressive behaviors.
For investigative behaviors, there were no group differences in anogenital investigation (A) or flank investigation (C). AGGs regardless of sex spent more time allogrooming (B). Females regardless of phenotype engaged in more facial investigation (D) and withdrew from interactions more frequently (E). For aggressive behaviors, males engaged in more wrestling (F), lunges (G), and pinned (H) the intruder more than females. Females delivered more bites (I) and kicks (J) K Learning curves from Random Forrest classifier. Curves were created using 1 K trees, 4 data splits (20–80%), and with shuffled 10-fold cross-validation at each data split. Errors represent ± SEM. L Density plot demonstrating probability of being classified as M or F as a function of the number of trees predicting male. M Variable importance plot for the random forest classifier.
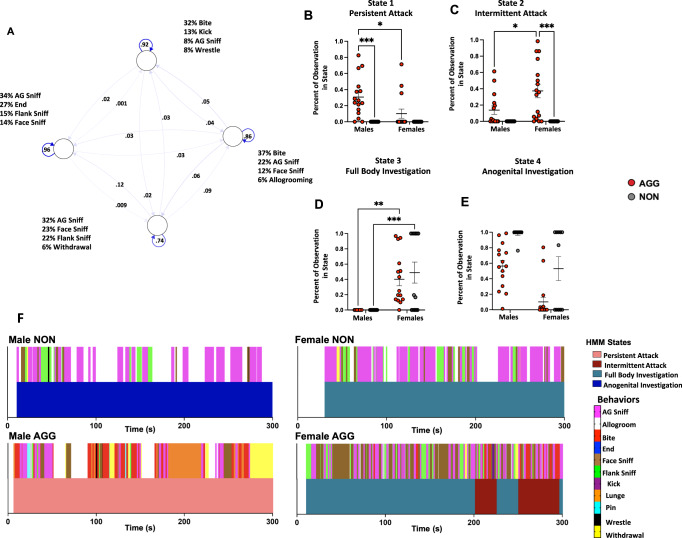
Fig. 3. Hidden Markov model of social behavior in the resident intruder paradigm.
A Schematic of HMM. Each node represents a hidden state. Numbers along the arrows indicate the probabilities of transitioning between states. Listed behaviors indicate the probability of occurrence during each state. Male AGGs were more likely to be in a state of persistent aggression B while female AGGs were more likely to be in a state of intermittent aggression (C). Females regardless of phenotype were more likely to be in the full-body investigation state than males (D). NON’s regardless of sex and males regardless of phenotype were more likely to be in the anogenital investigation state (E). F Representative examples of behavioral sequences (top) and predicted state (bottom) for all four groups.
When examining aggression, we observed that male and female mice engage in qualitatively distinct behaviors on day 3. Male AGGs engaged in wrestling behavior, in which the resident male lunges at the intruder and tumbles around the home cage, while female AGGs did not engage in this behavior at all (t(15) = 3.571, p = 0.0034, Fig. 2F). Although some females did engage in lunging behavior, it was to a lesser extent than male AGGs (t(15) = 2.070, p = 0.054, Fig. 2G). Females delivered more bites than males (t(15) = 2.104, p = 0.046, Fig. 2I) with a and there was a trend for females to exhibit more kicks than males (t(15) = 2.005, p = 0.0632, Fig. 2J). These single kicks were usually delivered following a single bite. In contrast, males were more likely to pin the intruder (t(15) = 2.151, p = 0.0442, Fig. 2H) prior to delivering a bite.
Given that male and female mice display distinct sets of investigative and aggressive behavior, we used a random forest classifier to determine whether trials involving a male or a female as the resident were distinguishable based on the metrics quantified in Fig. 2. Trials from day 3 were included in the model. We tested models in which 20, 40, 60, or 80 percent of the data was used for training the model (see Methods for details). We found that when 80% of the data was used to train the model, an F score of 1 was achieved, indicating a perfect classification of the remaining 20% of the trials (Fig. 2K, L). We extracted the gini impurity metric to determine which variables were important for classifying males vs. females. The analysis indicated that withdrawals, facial investigation, and wrestling were important in classifying male vs. females (Fig. 2M).
Male and female mice display distinct sequences of social behavior
For the HMM, we found that a 4-state model best fit the sequences of observations (see Methods for details) when males and females were analyzed together. When males and females were analyzed separately a two-state model fit the data best (Supplemental Fig. 4). Inspection of the emission probabilities (Supplementary Table 1) suggests that states 1 and 2 (Persistent Attack & Intermittent Attack listed below as A1-A2 or I1-I2) were predominantly associated with aggressive actions, with bite being the most likely behavior to occur when the animal was in these states. Interestingly, state A2 was also characterized by a relatively high probability of investigation occurring, while state A1 was associated with relatively low probabilities of investigation (39% for state 2, 19% for state 1). Conversely, States 3 and 4 (Full Body Investigation & Anogenital Investigation) were predominantly associated with investigative behaviors, with aggressive behaviors being highly unlikely to occur (6 and 0.06% respectively). These investigative states were differentiated by the probability of specific investigative behaviors occurring. While in state I1, there was a roughly equal probability of anogenital (32%), facial (23%), and flank investigation (22%) (Supplementary Table 1). However, while in the anogenital investigation state, the mice were much more likely to engage in anogenital investigation (34%) rather than facial (14%) and or flank investigation (15%) (Supplementary Table 1). To determine whether certain groups were more likely to be in a particular state, we calculated the percentage of behavioral observations that occurred in each state for each mouse. We found that male AGGs had a significantly higher percentage of their observations in the persistent attack state than female AGGs (Sex × Phenotype interaction F(1, 51) = 4.556, p = 0.0376, Male AGG vs. Female AGG, p = 0.0111, Fig. 3B).
Conversely, female AGGs had a significantly higher percentage of their observations in the intermittent attack state compared to male AGGs (Sex × Phenotype interaction F(1, 51) = 4.451, p = 0.0398, Male AGG vs. Female AGG, p = 0.0206,Fig. 3C). The difference between male and female AGGs is likely due to the fact that females are more likely to investigate the intruder before or after delivering a bite (36%) compared to males (14%) (Supplementary Table 3A, B) With regard to the full body investigation state, there was a striking sex difference, with none of the males showing any observations in this state (F(1, 51) = 30.77 p < 0.0001, Male AGG vs. Female AGG, p = 0.0010. Female NON vs Male NON p = 0.0023, Fig. 3E). This phenomenon is due to the fact that females were more likely to string together multiple investigatory actions than males (Fig. 3F, Supplementary Table 3A, B). Lastly, NON mice were more likely than AGGs to be in state I2 (phenotype F(1, 51) = 25.85, p < 0.0001, Fig. 3E). Furthermore, Males were more likely to be in state I2 than females (sex F(1, 51) = 30.02, p < 0.0001, Fig. 3E).
Males, but not females, display aggression reward and appetitive aggression
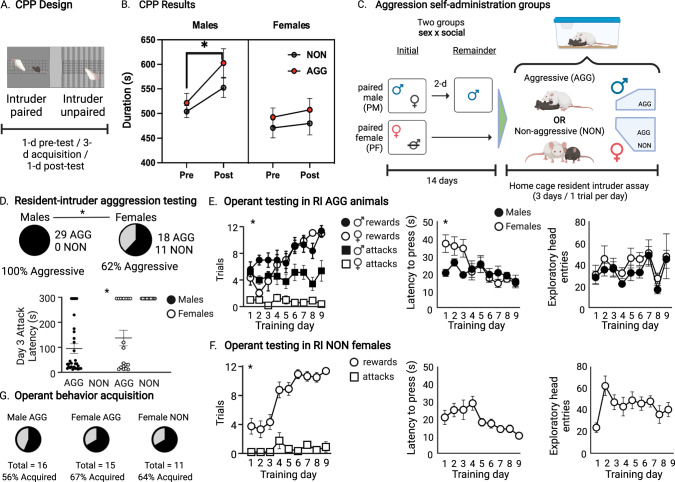
In the CPP assay (Fig. 4A), there was a significant effect of time (F (1,38) = 8.269, p = 0.006), sex (F (1, 38) = 9.952, p = 0.003), and a time × sex interaction (F (1, 38) = 3.899, p = 0.055). Post hoc analysis revealed that only male AGGs spent more time in the paired chambered in the post-test relative to the pre-test (p < 0.05, Fig. 4B).
Fig. 4. Males and females are similar in reactive but not appetitive aggression.
A Schematic of CPP paradigm. B Male AGGs but not NONs develop a CPP to the paired chamber. Neither female AGGs or NONs developed a CPP to the paired chamber. C Schematic of social housing paradigm for self-administration animals. All males tested were aggressive during at least one trial of resident intruder screening, while the females separated into aggressive (AGG) and non-aggressive (NON) phenotypes. D Latency to attack in the resident intruder assay differed significantly between groups, with female NONs having significantly higher latency to attack than the male or female AGGs. E Females show slightly slower learning curves than males in acquiring the aggression self-administration task. Additionally, females show almost no attacks once they have self-administered a same-sex conspecific, while male aggression was steady across days. Females are initially slower than males to lever press, but both groups decrease latency over time. There were no differences in exploratory head entries across days or sex. F Females who were not aggressive in the resident intruder screening show increasing rewards over time with steady attacks and decreasing latency to lever press. They show an increase in exploratory head entries initially which is steady thereafter. G Similar percentages acquired operant self-administration across groups.
RI screening of mice used for appetitive aggression test
All 29 males were aggressive during at least one RI trial, while 18 of 29 females were aggressive. The three resulting groups (male AGG, female AGG, female NON) differed significantly in latency to attack, with NON-females showing significantly longer latency to attack when compared to AGG male or female mice (p < 0.0001, F (2, 56) = 14.47) (Fig. 4D).
Males and females learn to self-administer intruders similarly, but vary in attack behavior
Between male and females AGGs, there was a significant sex × day interaction in reward and attack behavior (interaction F24,306 = 3.327, p < 0.001, day F8,306 = 6.787, p < 0.001, sex F3,306 = 108.2, p < 0.001) with females showing significantly fewer attacks than males (p < 0.001, df = 306 Tukey’s). Latency to press for an intruder significantly decreased over days in both males and females (p = 0.0151, F (8, 148) = 2.475), but there was no difference in exploratory head entry activity across days or sex. (p = 0.9963, F (8, 153) = 0.1520) (Fig. 4E).
Female NONs showed significantly more rewards over time, but near zero attacks across training days (Interaction F8,108 = 9.277, p < 0.001, Day F8,108 = 13.02, p < 0.001, Attack v Reward F1,108 = 490.9, p < 0.001). There were no significant differences across days in latency to press (F(1.942,11.65) = 2.658, p = 0.11) or exploratory head entries (F(3.977,23.86) = 2.46, p = 0.07). (Fig. 4F).
When compared directly, female NONs and AGGS both showed increasing rewards over time, with stable but very low attack frequencies (phenotype × day Interaction F24,270 = 6.225, p < 0.001, day F8,270 = 18.71, p < 0.0001, phenotype F3,270 = 238.4, p < 0.0001). Tukey’s multiple comparisons test revealed that NON females showed slightly higher rewards than AGG females (p = 0.0248, means 7.857 and 6.833 respectively), with no differences in attack behavior (p = 0.9309).
RI aggression phenotype does not predict operant self-administration acquisition
There was no significant difference in the proportion of mice per group that acquired operant SA, as evidenced by an average of >3 presses per day for the last five days of training (male AGG = 9/16, female AGG = 10/15, female NON = 7/11, Chi-square, df = 0.3752, 2, p = 0.829 Fig. 4G). All mice that did not acquire self-administration were excluded from analysis.
Housing condition does not appear to impact aggression
Isolated males showed a trend toward increasing rewards over time (p = 0.057), as well as stable attack frequency (p = 0.701), latency to press (p = 0.514), and exploratory head entries (p = 0.197) over time (Figure S5B). Separately, isolated females showed increasing rewards (p < 0.001), stable but low attack frequency (p = 0.0763), decreased latency to press (p = 0.009), and steady exploratory head entries (p = 0.251) over time (Fig. S5C).
Food training data
A subset of male and females that did not acquire aggression self-administration were tested for learning capability via food self-administration testing. There were no sex or housing differences in food self-administration performance (p = 0.6169, F (6, 49) = 0.7440), and we, therefore, collapse housing conditions across sexes for analysis. There were no differences between sexes in the amount of food (g/kg) self-administered (p = 0.8402, F (6, 96) = 0.4544), though rewards per day similarly increased over time in both sexes (Fig. S5D).
Discussion
We sought to characterize differences in aggressive and investigative social behavior in outbred male and female CFW mice. To this end, we adopted the protocol of Newman et al. [20] to quantify aggressive social behavior in females. Until now, most female aggression studies in laboratory mice have resorted to using lactating females during the postpartum period [17–19, 26]. This is not ideal for evaluating sex differences in aggressive behavior since these behaviors are linked to hormonal changes specifically associated with pregnancy, parturition, and lactation. Utilizing this protocol, we found that when grossly measured as “aggressive” or “investigative” males and females are largely similar. Although females tended to engage in more investigation than males, this effect was only significant on day 1 and waned with successive bouts of the RI test.
When rodents approach and contact a conspecific they engage in sniffing behavior of distinct body parts such as the face, anogenital, and flank regions [27]. We observed that females, regardless of their RI phenotype, engaged in facial investigation for longer durations than males. The facial area contains different excretory glands that give off distinct signals to the investigating animal. The Harderian glands are located near the eyes and excrete a lipid-containing porphyrins [28] and have been shown to provide information about the sex and reproductive status of the individual, which can influence social behavior in males [29, 30]. Whether certain facial cues more significantly impact female-female social interaction is unknown and requires further study.
Males and females also displayed distinct attacking behaviors. When male residents attacked the intruder, they displayed full-body lunges and wrestling behaviors that involved the two mice tumbling around the cage at very high speeds. This is in contrast to females, who were more likely to deliver a series of bites followed by a single kick with their hindlimbs. Male aggression thus seems much more explosive and offensive whereas female aggression seems tamer and possibly defensive in nature. This is in line with a previous study [18] which found that male bouts were more contact-oriented with the male intruder having a higher chance of getting wounded from the bout relative to female intruders. In contrast, females were more likely to attack with a single bite or “jump-attack” followed by the resident withdrawing from the encounter.
As mentioned above, we employed a discrete state HMM. Although Markov chains have been used to examine aggressive behavior in males in the past [31, 32] this is the first instance of a hidden state model being used to compare aggressive behavior in male and female mice. We found that a 4-state model best fit our behavioral observations. Of these 4 states, states 1 and 2 were dominated by aggressive behaviors while states 3 and 4 were dominated by investigative behaviors. Interestingly, the “aggressive states” could be further differentiated by the probabilities of particular behaviors occurring. Although both states 1 and 2 were associated with a high level of aggression occurring, only state 2 was associated with a high level of investigation also occurring. This suggests that state 1 is characterized by persistent attacking for prolonged periods of time, while state 2 is characterized by a mix of both investigative and aggressive actions. Given the above discussion regarding the qualitative differences in attack behavior in males and females, it is not surprising that male AGGs had a greater proportion of their behaviors in state 1 whereas female AGGs had a greater proportion of their behaviors in state 2.
As with states 1 and 2, states 3 and 4 can also be further differentiated based on which particular behaviors were more likely to occur. State 3 was characterized by a roughly equal probability of any of the three main investigatory behaviors occurring, while state 4 was also characterized by a relatively high probability of AG investigation occurring relative to other modes of investigation. Strikingly, none of the behavioral sequences demonstrated by males were characterized as being in state 3. This is likely due to the fact that females were more likely to string together multiple investigative behaviors in succession, while males predominantly engaged in AG investigation or ended the interaction and then re-engaged in AG investigation during a separate bout. In contrast males tend to engage in interaction bouts that consist solely of one of the two types of social behavior (aggressive or investigative), terminate the bout, and then re-engage in a separate bout.
Although male and female AGGs displayed robust levels of reactive aggression, they differed with regard to aggression reward and the acquisition of appetitive aggression. The CPP experiment revealed that only male AGGs developed a preference for the side paired with aggressive experience, suggesting they find it to be rewarding or reinforcing. In line with these findings, while both males and females acquired SA behavior, only males attacked during the subsequent social interaction bout with the intruders. We can speculate that the robust female social self-administration may be affiliative, rather than aggressive, when social interactions are volitional rather than forced. These data agree with recently published work using outbred CD1 female mice, where female mice readily lever press for sensory contact to female partner mice [33]. However, our data also caution against the use of purely barrier-based social self-administration procedures in males and females due to the potential incongruence in aggressive behavior between RI and SA testing. Use of a barrier and purely sensory contact may mask the ultimate motivation of the resident mouse. Further, these results indicate that female CFW mice are a valid model for studying reactive aggression. Female CFW mice cannot, however, be used to examine appetitive aggression behavior using operant self-administration procedures.
These results indicate that female CFW mice are a valid model for studying reactive aggression, which is a departure from the historical narrative that female mice are only maternally aggressive and can therefore be excluded under the NIH sex as a biological variable initiative. In striking opposition to our reactive aggression observations, we find that female CFW mice that exhibit strong reactive aggression do not exhibit appetitive aggression-seeking behavior under these housing and testing conditions. This mimics the sex difference observed using aggression CPP, and suggests a significant behavioral sex difference between male and female CFW mice regarding the reinforcing effects of aggression and aggression-seeking behavior.
This work highlights the limitations of developing preclinical models entirely in males, and highlights the need for a more parametric exploration of female aggression. While our study demonstrates that female CFW mice do not demonstrate aggression reward under currently established male models, there may be additional manipulations, which could elicit aggression reward in female mice. Additionally, species in which females more typically show non-maternal aggression, including California mice, Syrian hamsters, and prairie voles may all provide opportunities to directly compare aggression reward across sexes.
Of note, we saw differences in the percentage of males that were NON versus AGG in RI testing between Mount Sinai and the University of Washington. Outbred lines, while helpful in studying individual differences in aggression, can exhibit batch differences due to the nature of their genetic variability, as has been seen in CD1 outbred mouse aggression testing [21]. As such, variation between sites in percentage of aggressive CFW mice during RI testing is not unexpected.
In summary, we show that despite similar levels of aggression and investigation, the actions displayed by male and female residents—which make up the gross measures of social behavior—are both qualitatively and quantitatively distinct. Our HMM revealed that females are more likely to switch between aggressive and investigative behaviors within a given interaction bout, while males typically engage in only one of these behaviors per bout. Furthermore, while female outbred CFW mice exhibit reactive aggression, only male outbred CFW mice displayed robust levels of appetitive aggression in CPP and SA experiments. Thus, future studies to disentangle the underlying biology driving these sex differences are critical.
Supplementary information
Author contributions
AVA, CJB, NLG, SAG & SJR designed experiments. AVA, CJB, NLG, LL, JN, YZ, VT, & RDC performed experiments. AVA, CJB, & NLG analyzed data. AVA, CJB, NLG, SAG & SJR wrote and edited the manuscript.
Funding
The research was supported R01MH127820 (SJR), R01MH114882 (SJR), R01MH104559 (SJR), R01MH120514 (SJR), R01MH120637 (SJR), R00DA045662 (SAG), P30DA048736 (SAG), NARSAD Young Investigator Award 27082 (SAG), and F31MH125587-01 (NLG). Some figures created with BioRender.com.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Antonio V. Aubry, C. Joseph Burnett, Nastacia L. Goodwin.
Contributor Information
Sam A. Golden, Email: sagolden@uw.edu
Scott J. Russo, Email: scott.russo@mssm.edu
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-022-01375-5.
References
- 1.Kravitz EA, Huber R. Aggression in invertebrates. Curr Opin Neurobiol. 2003;13:736–43. doi: 10.1016/j.conb.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Lischinsky JE, Lin D. Neural mechanisms of aggression across species. Nat Neurosci. 2020;23:1317–28. doi: 10.1038/s41593-020-00715-2. [DOI] [PubMed] [Google Scholar]
- 3.Aleyasin H, Flanigan ME, Golden SA, Takahashi A, Menard C, Pfau ML, et al. Cell-type-specific role of DeltaFosB in nucleus accumbens in modulating intermale aggression. J Neurosci. 2018;38:5913–24. doi: 10.1523/JNEUROSCI.0296-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flanigan ME, Russo SJ. Recent advances in the study of aggression. Neuropsychopharmacology. 2019;44:241–44. doi: 10.1038/s41386-018-0226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golden SA, Jin M, Shaham Y. Animal Models of (or for) aggression reward, addiction, and relapse: behavior and circuits. J Neurosci. 2019;39:3996–4008. doi: 10.1523/JNEUROSCI.0151-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golden SA, Covington HE, III, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–91. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flanigan ME, Aleyasin H, Li L, Burnett CJ, Chan KL, LeClair KB, et al. Orexin signaling in GABAergic lateral habenula neurons modulates aggressive behavior in male mice. Nat Neurosci. 2020;23:638–50. doi: 10.1038/s41593-020-0617-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golden SA, Aleyasin H, Heins R, Flanigan M, Heshmati M, Takahashi A, et al. Persistent conditioned place preference to aggression experience in adult male sexually-experienced CD-1 mice. Genes Brain Behav. 2017;16:44–55. doi: 10.1111/gbb.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golden SA, Heshmati M, Flanigan M, Christoffel DJ, Guise K, Pfau ML, et al. Basal forebrain projections to the lateral habenula modulate aggression reward. Nature. 2016;534:688–92. doi: 10.1038/nature18601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fish EW, De Bold JF, Miczek KA. Aggressive behavior as a reinforcer in mice: activation by allopregnanolone. Psychopharmacology. 2002;163:459–66. doi: 10.1007/s00213-002-1211-2. [DOI] [PubMed] [Google Scholar]
- 11.Fish EW, DeBold JF, Miczek KA. Escalated aggression as a reward: corticosterone and GABA(A) receptor positive modulators in mice. Psychopharmacology. 2005;182:116–27. doi: 10.1007/s00213-005-0064-x. [DOI] [PubMed] [Google Scholar]
- 12.Bannai M, Fish EW, Faccidomo S, Miczek KA. Anti-aggressive effects of agonists at 5-HT1B receptors in the dorsal raphe nucleus of mice. Psychopharmacology. 2007;193:295–304. doi: 10.1007/s00213-007-0780-5. [DOI] [PubMed] [Google Scholar]
- 13.Falkner AL, Grosenick L, Davidson TJ, Deisseroth K, Lin D. Hypothalamic control of male aggression-seeking behavior. Nat Neurosci. 2016;19:596–604. doi: 10.1038/nn.4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grieb ZA, Ross AP, McCann KE, Lee S, Welch M, Gomez MG, et al. Sex-dependent effects of social status on the regulation of arginine-vasopressin (AVP) V1a, oxytocin (OT), and serotonin (5-HT) 1A receptor binding and aggression in Syrian hamsters (Mesocricetus auratus) Horm Behav. 2021;127:104878. doi: 10.1016/j.yhbeh.2020.104878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva AL, Fry WH, Sweeney C, Trainor BC. Effects of photoperiod and experience on aggressive behavior in female California mice. Behav Brain Res. 2010;208:528–34. doi: 10.1016/j.bbr.2009.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashikawa K, Hashikawa Y, Tremblay R, Zhang JX, Feng JE, Sabol A, et al. Esr1(+) cells in the ventromedial hypothalamus control female aggression. Nat Neurosci. 2017;20:1580–+. doi: 10.1038/nn.4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Unger EK, Burke KJ, Jr, Yang CF, Bender KJ, Fuller PM, Shah NM. Medial amygdalar aromatase neurons regulate aggression in both sexes. Cell Rep. 2015;10:453–62. doi: 10.1016/j.celrep.2014.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blanchard DC, Fukunaga-Stinson C, Takahashi LK, Flannelly KJ, Blanchard RJ. Dominance and aggression in social groups of male and female rats. Behav Process. 1984;9:31–48. doi: 10.1016/0376-6357(84)90006-8. [DOI] [PubMed] [Google Scholar]
- 19.Hashikawa K, Hashikawa Y, Tremblay R, Zhang J, Feng JE, Sabol A, et al. Esr1(+) cells in the ventromedial hypothalamus control female aggression. Nat Neurosci. 2017;20:1580–90. doi: 10.1038/nn.4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newman EL, Covington HE, III, Suh J, Bicakci MB, Ressler KJ, DeBold JF, et al. Fighting females: Neural and behavioral consequences of social defeat stress in female mice [Peer Reviewed] Biol Psychiatry. 2019;86:31255250. doi: 10.1016/j.biopsych.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwiatkowski CC, Akaeze H, Ndlebe I, Goodwin N, Eagle AL, Moon K, et al. Quantitative standardization of resident mouse behavior for studies of aggression and social defeat. Neuropsychopharmacology. 2021;46:1584–93. doi: 10.1038/s41386-021-01018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee W, Fu J, Bouwman N, Farago P, Curley JP. Temporal microstructure of dyadic social behavior during relationship formation in mice. PLoS One. 2019;14:e0220596. doi: 10.1371/journal.pone.0220596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rabiner LR. A tutorial on hidden Markov-models and selected applications in speech recognition. Proc IEEE. 1989;77:257–86. doi: 10.1109/5.18626. [DOI] [Google Scholar]
- 24.Stanke M, Waack S. Gene prediction with a hidden Markov model and a new intron submodel. Bioinformatics. 2003;19:ii215–25. doi: 10.1093/bioinformatics/btg1080. [DOI] [PubMed] [Google Scholar]
- 25.Carola V, Mirabeau O, Gross CT. Hidden Markov model analysis of maternal behavior patterns in inbred and reciprocal hybrid mice. PLoS One. 2011;6:e14753. doi: 10.1371/journal.pone.0014753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parmigiani S, Brain PF, Mainardi D, Brunoni V. Different patterns of biting attack employed by lactating female mice (Mus domesticus) in encounters with male and female conspecific intruders. J Comp Psychol. 1988;102:287–93. doi: 10.1037/0735-7036.102.3.287. [DOI] [PubMed] [Google Scholar]
- 27.Arakawa H, Cruz S, Deak T. From models to mechanisms: Odorant communication as a key determinant of social behavior in rodents during illness-associated states [Peer Reviewed] Neurosci Biobehav Rev. 2011;35:21414355. doi: 10.1016/j.neubiorev.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Chen WB, Kelly MA, OpitzAraya X, Thomas RE, Low MJ, Cone RD. Exocrine gland dysfunction in MC5-R-deficient mice: Evidence for coordinated regulation of exocrine gland function by melanocortin peptides. Cell. 1997;91:789–98. doi: 10.1016/S0092-8674(00)80467-5. [DOI] [PubMed] [Google Scholar]
- 29.Hattori T, Osakada T, Matsumoto A, Matsuo N, Haga-Yamanaka S, Nishida T, et al. Self-exposure to the male pheromone ESP1 enhances male aggressiveness in mice. Curr Biol. 2016;26:1229–34. doi: 10.1016/j.cub.2016.03.029. [DOI] [PubMed] [Google Scholar]
- 30.Cavaliere RM, Silvotti L, Percudani R, Tirindelli R. Female mouse tears contain an anti-aggression pheromone. Sci Rep. 2020;10:59293-9. [DOI] [PMC free article] [PubMed]
- 31.Haccou P, Kruk MR, Meelis E, Van Bavel ET, Wouterse KM, Meelis W, (1988). Markov models for social interactions: Analysis of electrical stimulation in the hypothalamic aggression area of rats [Peer Reviewed]. Animal Behav. 36, 10.1016/S0003-3472%2888%2980074-5
- 32.Natarajan D, de Vries H, Saaltink D-J, de Boer SF, Koolhaas JM. Delineation of violence from functional aggression in mice: An ethological approach [Peer Reviewed] Behav Genet. 2009;39:18972199. doi: 10.1007/s10519-008-9230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramsey LA, Holloman FM, Hope BT, Shaham Y, Venniro M, Id, Holloman FMOHOO, et al. (2021). Waving through the window: A model of volitional social interaction in female mice [Peer Reviewed]. Biol. Psychiatry . 10.1016/j.biopsych.2021.10.023 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.