Abstract
There is considerable interest in the therapeutic potential of psychedelic drugs. Dimethyltryptamine (DMT) is a potent, rapid-onset, and short-acting psychedelic drug that has not yet been independently tested for the treatment of depression. The safety, tolerability, and efficacy of intravenous DMT were investigated in treatment-resistant individuals with major depressive disorder (MDD) and healthy controls (HC) in an open-label, fixed-order, dose-escalation (0.1 mg/kg followed by 0.3 mg/kg) exploratory phase 1 study that was conducted in a typical hospital setting with strategic psychoeducation/support, but minimal psychotherapy. Tolerability, safety, cardiovascular function, abuse liability, psychedelic, and psychotomimetic effects, mood, and anxiety were assessed at each dosing session. In addition, depression was measured using the HAMD-17 in MDD participants 1 day after each dosing session. DMT was tolerated by both HC (n = 3) and MDD participants (n = 7) studied; there were no dropouts. HAMD-17 scores decreased significantly (p = 0.017) compared to baseline in MDD participants the day after receiving 0.3 mg/kg DMT (mean difference −4.5 points, 95% CI: −7.80 to −1.20, Hedge’s g = 0.75). Adverse events were mostly mild with one self-limited serious event. DMT increased blood pressure, heart rate, anxiety, psychedelic effects, and psychotomimetic effects, which resolved within 20–30 min of injection. There were no dose-related differences in measures of drug reinforcement and abuse liability. In this small exploratory pilot study, intravenous DMT at doses of 0.1 and 0.3 mg/kg was mostly safe and tolerated and may have next-day (rapid) antidepressant effects in patients with treatment-resistant MDD. Further rigorous trials are warranted to replicate these findings and to determine the durability of antidepressant effects.
Subject terms: Outcomes research, Depression
Introduction
There is burgeoning interest in the therapeutic potential of psychedelic compounds. Psilocybin, a prototypical serotonergic hallucinogen, has shown tantalizing therapeutic effects in several neuropsychiatric disorders [1–6].
The proliferating research with psilocybin has generated several important questions. For example, whether the promising therapeutic potential of psilocybin extends to other serotonergic hallucinogens remains unclear. Furthermore, the extent to which psychedelic effects are necessary for later therapeutic effects needs further study. Currently, psilocybin is administered orally, and thus, there is a latency to the onset of its psychedelic effects, which then last for several hours. Whether shorter psychedelic experiences suffice to elicit clinically meaningful benefit is not clear. Current models require the presence of two therapists and a specific psychotherapy protocol for the entire dosing session [7]. The extent to which multiple therapists and integrative psychotherapy are necessary for the beneficial effects of psilocybin needs to be determined given that such a resource-intensive treatment model is challenging to implement on a wide scale and for the overwhelming majority of patients.
Like psilocybin, N,N-dimethyltryptamine (DMT) is a serotonergic hallucinogen. DMT is present in certain plants used to make ayahuasca, a South American psychoactive brew [8] used socially and as a ceremonial medicine by some indigenous peoples in the Amazon. Ayahuasca contains DMT and other compounds including monoamine oxidase inhibitors (MAOIs). When consumed orally, DMT is rapidly deaminated by MAOs to an inactive metabolite [8]. However, when administered intravenously, DMT bypasses enteric metabolism and produces effects which are brief in contrast to orally administered psilocybin or ayahuasca.
The effects of DMT have been extensively studied in humans for more than 50 years [9–13]. In healthy volunteers, intravenous DMT produces a range of psychedelic effects including profound dose-related alterations in perceptions, emotion, and thought. The effects include visual hallucinations, altered reality, “spiritual insights”, distortion of body perception, and mood alterations or anxiety [11, 12, 14]. Psychedelic effects emerge at doses higher than 0.2 mg/kg. The effects emerge rapidly (within 2 min) after intravenous administration and fully resolve within about 30 min. At doses ranging from 0.05 to 0.4 mg/kg, the effects are well tolerated in healthy controls (HC) [12].
The precise mechanisms underlying the psychedelic effects of DMT are not clear [15]. DMT has agonist activity at serotonin 5-HT2A receptors. Other identified binding sites include various serotonin, dopamine, and adrenergic receptors, serotonin uptake transporters, trace amine-associated receptors, and sigma-1 receptors [16–19]. In contrast to other psychedelics, DMT is also present at low concentrations in the brains of animals and humans [20] at low concentrations. Because DMT activates trace amine-associated receptor 1 and may be stored in vesicles at concentrations sufficient to activate receptors [18] DMT may have a role in normal physiology and pathology. However, the extent to which interactions between exogenous DMT and endogenous DMT contribute to its overall effects is unknown.
We are unaware of any reports on the therapeutic effects of pure DMT in depression. In a small (n = 6) open-label study, Osorio et al. found that a single dose of ayahuasca significantly reduced depression scores in major depressive disorder (MDD) patients without inducing mania or psychosis [21]. More recently, Palhano-Fontes et al. reported that ayahuasca reduced depression scores in patients with treatment-resistant depression (n = 29) in a parallel-arm, double-blind, randomized, placebo-controlled trial [22]. However, while ayahuasca contains DMT, it contains numerous other potentiating constituents, including MAOIs such as harmaline that may have effects on depression independent of DMT. Furthermore, the pharmacological profile of all of ayahuasca’s constituents is poorly understood [23].
The slow onset and long duration of orally administered psilocybin prompted us to study the effects of DMT, given its near-instantaneous onset, yet short duration, of psychedelic effects. As the first clinical trial utilizing DMT in MDD, we first studied its dose-related safety, tolerability, and potential efficacy in individuals with MDD and HC as a prelude to a larger trial.
Materials and methods (see Supplementary materials for details)
Regulatory
This study was conducted at the Neurobiological Studies Unit (VA Connecticut Healthcare System [VACHS], West Haven, CT) with approval from the Institutional Review Boards of VACHS and Yale University School of Medicine and was monitored by an independent Data Safety Monitoring Board. The study was registered on clinical trials.gov (NCT04711915) on January 15, 2021, and conducted under an approved IND (DCD # 146,883). All participants provided written informed consent.
General study design
This was an exploratory, open-label, fixed-order, dose-escalation (0.1 and 0.3 mg/kg intravenous DMT) study involving two dosing sessions at least 48 h apart. DMT hemifumarate was synthesized at the University of Wisconsin-Madison. Participants included patients with treatment-resistant MDD and HC. Participants, investigators, and raters were not masked to treatment assignment, and all participants received the study intervention.
Participants (Supplementary S1)
Inclusion criteria for MDD participants: DSM5 MDD with ≥17 on HAMD-17, engaged in treatment, but treatment resistant as defined as a minimum of two prior treatment failures and confirmation of prior adequate dose and duration [24] and at least one failed antidepressant trial in the current depressive episode. MDD participant exclusion criteria: psychotic disorder, unstable medical co-morbidities, history of mania, and recent high risk for suicide or homicide. Exclusion criteria common to HC and MDD groups were pregnancy, current or recent drug dependence, lifetime history of hallucinogen use disorder, regular use of psychedelics, and current use of over-the-counter products with serotoninergic properties.
Recruitment
The HC group was recruited from the community using advertisements, online postings, and word of mouth. MDD participants were recruited from the community using clinician referrals, advertisements, online postings, and word of mouth.
Screening (Supplementary S2)
Eligible participants completed a thorough psychiatric and medical history and examination including comprehensive laboratory testing. For MDD participants, collateral information and support from the participant’s primary mental health clinician was required.
Preparation
If eligible, participants met with the study psychiatrists for a preparatory session of about 45 min, during which participants were provided information about DMT’s psychological effects, and approaches to navigate the experience itself. Furthermore, MDD participants were invited to discuss their mood symptoms and their depression history. Participants were told that they would receive a 0.1 mg/kg followed by a 0.3 mg/kg dose of DMT. Furthermore, they were told that while they may not benefit from study participation, their participation may lead to knowledge that may help others.
Drugs
Since freebase DMT is not water soluble, water-soluble DMT hemifumarate was prepared that was minimally 99.9% pure for intravenous injection. Detailed synthetic and analytic procedures have been previously published [25]. DMT hemifumarate was compounded into a sterile investigational product (20 mg/mL injection, 1 mL vial). The doses were chosen based on previous work [26] showing that 0.1 mg/kg is sub-psychedelic while 0.3 mg/kg DMT reliably induced psychedelic effects. Only if participants tolerated the first dosing (0.1 mg/kg DMT) session, were willing to continue, and the research team approved, was the next dose (0.3 mg/kg DMT) session scheduled.
Test sessions (Supplementary S3)
On arrival, participants were first checked for recent alcohol and drug use and completed pre-dose/baseline assessments (Supplementary Table S1). Participants were dosed in a booth containing a medical-grade reclining chair and desk that was lit with overhead fluorescent lighting. There was no art adorning the room and no music was played. Participants were provided pillows and hospital-issued linens. The two psychiatrists stood on either side of the medical chair, with a research nurse and research assistants stood immediately behind the subject. After connecting the DMT-containing syringe to the intravenous port, the impending administration of DMT was announced. Participants were administered DMT by intravenous push over 30–60 seconds. Blood pressure was measured at baseline and 5, 10, 15, 20, 30, 45, 60, and 120 min after drug administration. Heart rate and pulse oximetry were measured continuously. Subjective drug effects were measured 60 min before, as well as 30 and 120 min after drug administration.
Given the intensity and brevity of DMT effects, participants were allowed a mostly uninterrupted experience with psychiatrists utilizing a non-directive and supportive approach. Participants were asked how they were feeling at the same time points as the physiological recordings; no psychotherapy was provided. Rescue medications for psychological distress (lorazepam and risperidone) and hypertension (labetalol) were available. Prior to discharge, a field sobriety test and mini-mental status examination were conducted to confirm return to baseline.
Debriefing
For the approximately 2 h between the waning of DMT effects and the time of discharge, participants were debriefed by a psychiatrist (SAS). The day after each session, participants were contacted via telephone to check on their well-being, to monitor for any adverse events (AEs), and, in MDD participants, to administer the HAMD-17.
Outcomes (Supplementary S4)
Tolerability defined by the US Food and Drug Administration (FDA) as “the degree to which overt adverse effects can be tolerated” by a subject was assessed [27]. At the end of the test day, after all drug effects had worn off, participants were asked to score (1) the overall experience on a visual analog scale [VAS] (0 = intolerable to 100 = well tolerated), (2) the likelihood of using the drug again (0 = not at all to 100 = most of all), and (3) how much they were willing to pay for the experience ($0–100). Similarly, all participants were asked to rate anxiety on a VAS (0 = not more than usually to 100 = much more than usual); MDD participants also rated depression. Safety defined by the FDA as “the risk to the subject or patient from a drug or biologic assessed by tests” was assessed by monitoring vital signs and recording AEs [27]. The quality and intensity of psychedelic effects were captured using a 23-item Psychedelic Effects Visual Analog Scale [14]. To capture the effects of DMT on perception, thought, and sensory processing, participants were administered the Psychotomimetic States Inventory (PSI) [28]. Shortly after the resolution of effects, participants were instructed to retroactively rate the effects experienced during the peak effects. Depression was measured using the clinician-administered HAMD-17 at baseline (at screening) and one day after each dosing session. The HAMD-17 was chosen as the primary efficacy measure due to its high reliability and validity [29]. We chose the day after each dose session as the primary efficacy point because we did not want the acute psychedelic effects to obscure any meaningful changes in mood and to coincide with the effects of the next neuroplastic changes induced by psychedelics.
Statistical analysis (Supplementary S5)
This exploratory–feasibility study was conducted to investigate the tolerability, safety, and potential efficacy of two doses of DMT to inform and power a larger, double-blind, randomized, placebo-controlled, crossover study. For subjective measures including the psychedelic effects VAS, PSI, and VAS (anxiety and depression), peak change from baseline was calculated and analyzed using paired t-test, when data met parametric assumptions, or nonparametrically, using two-tailed Wilcoxon signed-rank tests. Cardiovascular parameters were analyzed using repeated-measures ANOVA with dose as a between-subject factor and time as the within-subject factor. HAMD-17 scores for each dose were compared to baseline using paired t-tests. Mean differences are presented with 95% confidence intervals and effect size is reported using Hedge’s g formula. Multiple comparison correction was performed for PSI subscales (Bonferroni) and VAS psychedelic effect items (FDR), associated with reduced power, in order to minimize type I errors [30].
Results
Enrollment occurred between March 17 and October 12, 2021. Of the 52 individuals who were initially considered for the study and for a telephone screen (see Supplementary CONSORT diagram), 14 were selected for an in-person screening visit, and 12 (3 HC and 9 with current MDD) were enrolled in the trial. 2 MDD participants dropped out before randomization. Amongst the 7 MDD participants who received DMT, 4 were self-referred and 3 were clinician-referred. Participants’ demographic and clinical characteristics are presented in Table 1. Three of 7 MDD participants met the criteria for severe depression at baseline (HAMD-17 score ≥24) while the remaining 4 met criteria for moderate severity depression (HAMD-17 score 17–<24). As a prerequisite to participation, all MDD participants were engaged in treatment including psychotherapy and were not taking any antidepressant medication that could interfere with DMT effects. Except for one participant (1 month), all the others had last taken antidepressants more than 3 months before their first test day. Only one MDD participant withdrew from taking antidepressants in order to participate. The study ended on October 31, 2021 when the batch of DMT expired.
Table 1.
Demographics.
| Group | Sex | Age (years) | Race | Ethnic origin | Employment status | Marital/relationship status | Estimated illness duration (years) | Baseline HAMD-17 item | Baseline PHQ-9 | Past antidepressant trials | Past psychotherapy | Education (years) | Weekly alcohol intake (units) | Previous psychedelic use (time since last use) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | HC | M | 58 | Asian | Non-Hispanic | Employed | Married | N/A | N/A | N/A | N/A | N/A | >17 | 7 | None |
| 2 | HC | M | 24 | Asian | Non-Hispanic | Student | Single | N/A | N/A | N/A | N/A | N/A | 17 | 0 | Psilocybin (3 years ago) |
| 3 | HC | M | 24 | White | Non-Hispanic | Employed | Domestic partnership | N/A | N/A | N/A | N/A | N/A | 16 | 2 | None |
| 4 | MDD | F | 43 | White | Non-Hispanic | Employed | Single | 22 | 22 | 22 |
SSRI × 3 SNRI × 2 Atypical antidepressant × 1 |
Yes | >17 | 0 | MDMA (10+ years ago) |
| 5 | MDD | F | 59 | White | Non-Hispanic | Unemployed | Married | 20+ | 25 | 22 |
SSRI × 3 SNRI × 3 TCA × 2 Atypical antidepressant × 2 |
Yes | >17 | 0 | None |
| 6 | MDD | M | 31 | White | Non-Hispanic | Employed | Married | 25 | 18 | 19 |
SSRI × 1 Atypical antidepressant × 1 |
Yes | >17 | 0 | Psilocybin (6 weeks ago) |
| 7 | MDD | M | 59 | White | Non-Hispanic | Employed | Married | 20+ | 28 | 14 |
SSRI × 4 SNRI × 2 SGA × 1 Atypical antidepressant × 2 Mood stabilizer × 1 Ketamine × 1 ECT × 1 |
Yes | >17 | 0 | None |
| 8 | MDD | F | 46 | White | Non-Hispanic | Unemployed | Married | 20+ | 22 | 21 |
SSRI × 2 Atypical antidepressant × 1 |
Yes | 16 | 2 | Psilocybin (>20 years) |
| 9 | MDD | M | 38 | Asian | Non-Hispanic | Employed | Single | 29 | 31 | 22 |
SSRI × 5 TCA × 1 Atypical antidepressant × 2 Stimulant × 2 SGA × 4 Mood stabilizer × 2 Ketamine × 1 ECT × 1 |
Yes | >17 | 1 | Psilocybin (10 years) LSD (5 years) |
| 10 | MDD | M | 53 | White | Non-Hispanic | Employed | Married | 20+ | 21 | 18 |
SSRI × 3 SNRI × 1 Mood stabilizer × 2 Atypical antidepressant × 2 |
Yes | 12 | 0 | LSD (30 years) |
N/A not applicable, HC healthy control, MDD major depressive disorder, M male, F female, SSRI selective serotonin reuptake inhibitor, SNRI serotonin norepinephrine reuptake inhibitor, TCA tricyclic antidepressant, ECT electro convulsive treatment, SGA second-generation antipsychotic, LSD lysergic acid diethylamide.
Tolerability
Participants reported overall tolerability (0 = intolerable to 100 = well tolerated) as 89.80 (SD 12.95) for the 0.1 mg/kg dose session and 71.11 (SD 24.52) for the 0.3 mg/kg dose session (difference −17.78 [95% CI −32.81 to −2.75] t = −2.72, p = 0.026) (Supplementary Fig. S1).
After completion of the 0.1 mg/kg dose session, all participants reported willingness to return for the second dosing session (0.3 mg/kg). Furthermore, on completion of the first dosing session (0.1 mg/kg), the study psychiatrists determined that all the participants who completed the first dosing session were appropriate to continue to the second dose day based on a review of the self-reported effects, study-clinician observed effects, cardiovascular parameters, participants debriefing, and clearance testing.
Safety
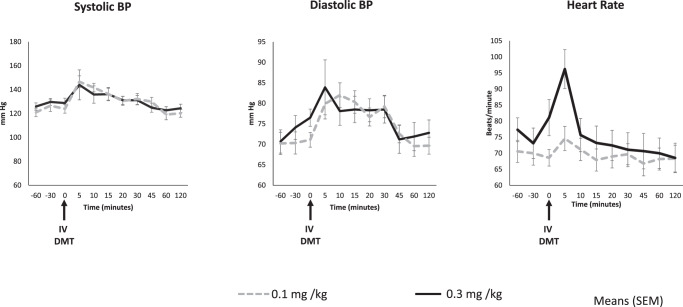
DMT increased systolic and diastolic blood pressure, and heart rate (Fig. 1); while there was a significant time effect for both doses, there were no significant dose or dose-time effects. Post-hoc analyses revealed statistically significant increases in systolic pressure at the +5, +10, and +15 min time points relative to baseline; significant increases in diastolic pressure at +5, +10, +15, +20, and +30 min relative to baseline; and significant increases in heart rate at the +5 min time point relative to baseline (Supplementary Table S2).
Fig. 1. Blood pressure and heart rate.
Figure shows changes in means (error bars indicate standard error of mean) of systolic and diastolic blood pressure (mm Hg), and heart rate (beats/min) over time on the 0.1 and 0.3 mg/kg DMT. Arrow indicates the time at which DMT was administered. n = 10 for 0.1 mg/kg (n = 3 healthy, n = 7 depressed) and n = 9 for 0.3 mg/kg (n = 3 healthy, n = 6 depressed).
Adverse events
The most common AEs were transient anxiety prior to administration and drug onset (n = 6), transient headache (n = 2) during onset and after resolution of drug effects, and transient hypertension (n = 2) before dose and during onset of effects (Table 2). Some of the AEs were deemed definitely related to DMT effects while others were deemed unrelated. There was one serious adverse event (SAE) on a 0.3 mg/kg dosing session in a female participant who had significant asymptomatic bradycardia and hypotension. The latter was addressed by placing the participant in Trendelenburg position and increasing intravenous saline without sequelae (Supplementary S6). None of the participants experienced any clinically relevant psychotic symptoms. All participants passed a standard field sobriety test and pre-dose baseline-matched Mini-Mental Status Exam prior to discharge. No rescue medications were used during any dosing session to address psychological or physiologic AEs reported (except for increased intravenous fluids to address hypotension -S6).
Table 2.
Adverse events.
| Subject no. | Group | DMT dose (mg/kg) | Adverse event | Severity | Onset relative to DMT administration (min) | Duration | Comment |
|---|---|---|---|---|---|---|---|
| 1 | Healthy | 0.1 | Headache | Mild | +45 | 5 hours | |
| 0.3 | Hypertension | Moderate | +5 | 5 minutes | |||
| 2 | 0.1 | – | – | – | – | ||
| 0.3 | Ringing sensation in the ears | Mild | +3 | 5 minutes | |||
| 3 | 0.1 | Anxiety | Mild | –30 | 40 minutes | Anticipatory | |
| Lightheadedness | Mild | +1 | 10 minutes | ||||
| 0.3 | Lightheadedness | Mild | +1 | 15 minutes | |||
| Tachycardia and subjective feeling of palpitation | Mild | +1 | 5 minutes | ||||
| 4 | Major depressive disorder | 0.1 | Anxiety | Mild | 0 | 10 minutes | |
| 0.3 | Back pain | Mild | The day after the test day, right after a moderately intense physical activity, improved with rest and nonsteroidal anti-inflammatory use | Significantly improved within 24 h, completely subsided within a week. | History of back pain | ||
| 5 | 0.1 | Headache | Mild | +1 | 20 seconds | ||
| 0.3 |
Hypotension (asymptomatic) See supplementary for details |
Severea | +2 | 5 minutes | History of reflex syncope | ||
|
Bradycardia (asymptomatic) See supplementary for details |
Severea | +2 | 5 minutes | History of reflex syncope | |||
| 6 | 0.1 | Nausea | Mild | +4 | 5 minutes | ||
| 0.3 | Nausea | Mild | +2 | 5–10 min | |||
| 7 | 0.1 | – | – | – | – | ||
| 0.3 | Anxiety | Mild | +2 | 60 minutes | |||
| Dysphoria | Mild | +5 | 24 hours | ||||
| 8 | 0.1 | Tingling | Mild | +3 | 15 minutes | ||
| 0.3 | – | – | – | – | |||
| 9 | 0.1 | Anxiety | Mild | +2 | 30 minutes | ||
| 0.3 | Anxiety | Mild | −20 | 60 minutes | Anticipatory | ||
| 10 | 0.1 | Anxiety | Mild | −30 | 30 minutes | Anticipatory | |
| Hypertension | Mild | −30 | 20 minutes |
aSee Supplementary S6 for details.
Subjective effects
DMT’s acute psychedelic effects typically became detectable between 2 and 5 min of administration, peaked between 5 and 10 min after dosing, and completely subsided by 30 min. While there were no significant scores on any of the 23-item VAS psychedelic effects at baseline, scores increased with both doses at the +30 min rating. Peak change in VAS psychedelic composite score was 13.16 (SD 11.91) for the 0.1 mg/kg session and 55.83 (SD 23.89) for the 0.3 mg/kg dose (difference 42.67 [95% CI 33.30 to 52.03]; z = 4.11; p < 0.0001; Hedge’s g = 2.2) (Table 3). There were significant dose-related differences on 16 of the 23-items (FDR correction, q = 0.05) (Supplementary Table S3). The top five ranked items with significant dose-related differences (p adjusted ≤0.01) were “intensity of experience” (p = 0.002), “complex visual images” (p = 0.004), “experienced different reality/dimension” (p = 0.006), “things looked strange” (p = 0.008), and “imagination was very vivid” (p = 0.01) (Supplementary Table S3 and Supplementary Fig. S2).
Table 3.
Dose-related peak change in subjective effects.
| 0.1 mg/kg, mean (SD) | 0.3 mg/kg, mean (SD) | Statistic | Significance | Effect (Hedge’s g) | ||
|---|---|---|---|---|---|---|
| VAS psychedelic effects | Composite | 13.51 (11.75) | 53.46 (25.95) | z = 4.05 | p < 0.0001 | 2.20 |
| PSI | Total | 7.33 (7.62) | 31.67 (16.57) | t = 4.84 | p = 0.001 | 1.89 |
| VAS depression and anxiety | Anxiety | 2.56 (31.8) | 29.67 (39.96) | z = 1.13 | p = 0.26 | – |
| Depression | 6.83 (28.9) | 14.83 (37.8) | z = 0.11 | p = 0.92 | – |
VAS visual analog scale, PSI Psychotomimetic States Inventory.
Mean peak change in PSI scale total score was 7.33 (SD 7.62) for the 0.1 mg/kg session and 31.67 (16.57) for the 0.3 mg/kg session (difference 24.33; 95% CI 12.74 to 35.94: t = 4.84: p = 0.001; Hedge’s g = 1.89) (Table 3 and Supplementary Fig. S3). The dose-related difference on peak change in PSI subscales was significant only for the perceptual disturbance subscale (p < 0.005) (Supplementary Table S4).
Peak change in VAS anxiety scale administered to all participants was 2.56 (SD 31.8) on the 0.1 mg/kg session and 29.67 (SD 39.96) on the 0.3 mg/kg session (difference 27.11 [95% CI −14.30 to 68.53] z = 1.1, p = 0.26). Peak change in VAS depression administered only to MDD participants was 6.83 (SD 28.94) for the 0.1 mg/kg session and 14.83 (SD 37.79) on the 0.3 mg/kg session (difference 8.00 [95% CI −41.47 to 57.47] z = 0.11, p = 0.92 (Table 3 and Supplementary Fig. S5).
On measures of drug desirability, participants reported overall willingness to use DMT as 24.00 (SD 17.91) for the 0.1 mg/kg session and 22.44 (SD 22.72) for the 0.3 mg/kg session (difference −1.56 [95% CI: −16.74 to 13.62] z = −0.14, p = 0.90). Participants monetized the value of drug effects (scale from $0 to 100) as $25.56 (SD 29.07) for the 0.1 mg/kg session and $24.78 (SD 33.36) for the 0.3 mg/kg session; (difference −0.78 [95% CI −32.38 to 33.94] z = −0.14, p = 0.89) (Supplementary Table S5).
Next-day depression ratings
Most MDD participants showed a nominal reduction in depression severity on the HAMD-17 after the 0.1 mg/kg session (Fig. 2 and Supplementary Table S6). HAMD-17 depression scores were significantly reduced from baseline (23.86 (SD 4.45)) to post-0.3 mg/kg session (20.20 (SD 7.82)) (difference −4.5 [95% CI: −7.80 to −1.20] t = −3.50, p = 0.017; Hedge’s g = 0.75). Difference in HAMD-17 was also significant between the 0.1 and 0.3 mg/kg sessions (Fig. 2) (difference −3.5 [95% CI: −6.87 to −0.013] t = −2.67, p = 0.044). One treatment-resistant MDD participant who experienced a significant improvement in depression, requested additional dosing.
Fig. 2. Change in depression.
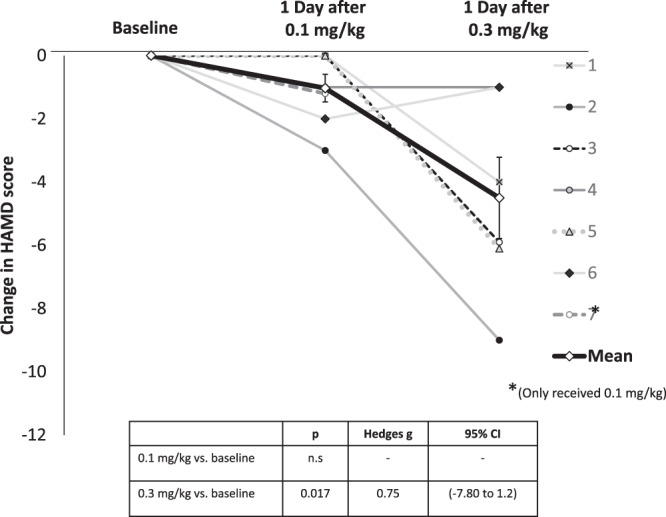
Figure shows changes in HamD-17 total scores of individual participants the day after 0.1 and 0.3 mg/kg DMT relative to baseline. Each subject is represented by a line in a different color. The group mean is shown in black. *Note that subject 7 completed the 0.1 mg/kg dosing session before being withdrawn for administrative reasons.
Discussion
This is the first report to our knowledge of the safety, tolerability, and efficacy of DMT in MDD. Intravenous DMT can be safely administered to and tolerated by individuals with moderate to severe, treatment-resistant MDD, and it may have next-day antidepressant effects.
The most novel finding of this study was the significant reduction in HAMD-17 scores one day after receiving DMT 0.3 mg/kg in MDD patients who had failed several previous antidepressant trials and who had been chronically ill (average duration of illness was 27 years). The mean reduction in HAMD-17 score was about 4.5 points the day after receiving 0.3 mg/kg, a medium to large effect size (Hedge’s g = 0.75). Of note, HAMD-17 scores changed nominally the day after the 0.1 mg/kg dose, and the difference between the two doses was significant, findings that will inform dose-selection for future studies. However, because of the fixed order and the lack of any measure of depression immediately prior to the 0.3 mg/kg DMT session, it is not possible to determine the contribution of carryover effects from the 0.1 mg/kg DMT session. Consistent with the known heterogeneity of MDD, some participants had greater reductions in HAMD-17 scores than others. Identifying predictors of response will be an important subject of future investigations. One MDD participant had a profound and sustained improvement in depression (corroborated by her partner) and requested receiving additional doses.
The magnitude of antidepressant effects with DMT was smaller than those reported by Carhart-Harris et al., with psilocybin [31]. A number of differences between the two studies including the samples studied, the setting, augmentation with psychotherapy, the duration of psychedelic effects, the timing of assessments and the route of administration of the drugs, may have accounted for the differences in the magnitude of effects. A direct head-to-head comparison of the two drugs will be necessary to determine their differences and similarities.
Considerable importance has been given to the centrality of set and setting in psychedelic treatment models. Recent psychedelic studies are typically conducted in settings that are adorned with art, plants, flowers, and homey furnishings, painted in warm colors, and lit with muted lighting. Studies often include specific music. Furthermore, psychotherapy is considered a fundamental part of psychedelic treatment. In contrast, this DMT study was conducted in a typical hospital setting, and participants received strategic education and psychological support but minimal psychotherapy, similar to ketamine. Despite the hospital setting and minimal psychotherapy provided in this study, depression scores decreased with DMT. This raises the question of whether psychotherapy and a nonhospital setting may have enhanced the reduction in depression scores in this study, or alternatively, how critical setting and psychotherapy are necessary for the effects of DMT. Perhaps unsurprisingly, neither psychedelic/psychotomimetic effects (ASC/PSI) significantly correlated with changes in depression scores from baseline to post-0.3 mg/kg dose.
The study provides the first information on the safety and tolerability of DMT in depressed individuals. The physiological and psychedelic effects of DMT observed were overall concordant with previous work in nonclinical populations. DMT produced transient dose-related increases in perceptual alterations (Supplementary Figs. S2–S4 and Supplementary Table S4). The largest changes were on items of intensity, visual imagery, and alternate reality experience. Participants reported transient increases in anxiety. There were no serious psychiatric AEs. Consistent with the literature, DMT produced transient increases in blood pressure and heart rate. However, there was one SAE of one participant who had precipitous hypotension and bradycardia lasting 5 min after receiving 0.3 mg/kg DMT; there were no sequelae. Given that DMT typically increases blood pressure, the SAE was determined to be an interaction between the participant’s (undisclosed) history of autonomic instability and DMT effects. The cardiovascular effects of DMT warrant careful screening for cardiovascular risk factors, and for implementing procedures in place to manage cardiovascular events.
Participants reported that the experience was intense and challenging (ASC scale items), and transiently increased anxiety. Yet participants reported that the experience was meaningful and pleasurable (modified ASC scale items); all participants were willing to return to receive IV DMT 0.3 mg/kg. Participants rated the tolerability of the 0.1 and 0.3 mg/kg doses as 89.80 (SD 12.95) and 71.11 (SD 24.52), respectively. Furthermore, no participants dropped out from our study. Collectively taken, while intense and challenging, IV DMT was mostly safe and tolerated. Larger studies may be necessary to more fully evaluate the safety and tolerability of IV DMT.
The fact that immediately after the dosing session, participants were willing to pay only $25 for the experience ($0–100), and reported being less likely to use DMT, suggests that intravenous DMT has low abuse potential.
The small sample size, open-label, and fixed-order design are limitations of the study. The brief follow-up period of mood symptoms does not inform whether, like psilocybin, DMT has longer-lasting antidepressant effects. Longer, more rigorous trials are needed to explore this further. Future studies need to use assessments of psychedelic and other effects that have consensus and have been well-validated.
The strengths of the study include the use of a sub-psychedelic and psychedelic dose, the inclusion of both HC and MDD participants, and the study of treatment-resistant MDD.
While the logical next step would be to conduct a randomized, double-blind, placebo-controlled trial of intravenous DMT with standardized and minimal psychological support, our findings raise a number of questions that warrant further study. How long do the antidepressant effects of DMT observed a day after dosing, last? Is the 0.3 mg/kg dose, which produced robust psychedelic effects in this study, the optimal dose? Would a slower infusion that produces less intense effects be safer? A slower and longer infusion may allow participants to engage in psychotherapy. Do sub-psychedelic doses, which might be better tolerated and more acceptable to patients, still have antidepressant effects? In this regard, it will be critical to determine the optimal level of psychedelic effects and their duration that are necessary for therapeutic effects. Once that is determined, it would be possible to design studies aimed at reaching, but not exceeding, the target intensity and duration of psychedelic effects. The rapid and profound effects of DMT especially when administered intravenously make blinding a significant challenge. The effectiveness of blinding needs to be estimated in future studies. Future studies might consider using an active control or using sub-psychedelic doses of DMT for comparison. The measurement of expectancy and the potential manipulation of expectancy need to be studied. Furthermore, recruiting a balance of self-referred and clinician-referred participants, as in this study, would help to control for the likely strong expectancy effects seemingly intrinsic to psychedelic effects on depression. Whether the intensive psychotherapy that has been proposed as integral to clinical trials with psychedelics would enhance the putative antidepressant effects of DMT needs further study. For this to occur, the intensity of psychedelic effects needs to be reduced and the duration of effects needs to be prolonged to allow for more therapeutic engagement. Whether the intravenous DMT paradigm is less or more resource-intensive compared to oral psychedelic treatments will need to be studied. Lastly, to what extent differences in mechanism (e.g., TAAR-1 and sigma-1) contribute to DMT’s effects and distinguish it from other 5-HT2A psychedelics needs further study.
In conclusion, the findings of this exploratory study provide support for DMT’s tolerability, safety, and potential rapid antidepressant effect. In contrast to other psychedelic treatment models, reductions in depression were observed the day after DMT dosing, and occurred within a typical hospital setting, and without intensive psychotherapy. This intriguing finding suggests it may be easier to implement DMT for the treatment of MDD. Future studies are warranted to replicate the findings and inform the therapeutic potential of DMT.
Supplementary information
Acknowledgements
The expertise of Rick Strassman who served as a paid consultant. The expertise of Dr Paul Daley of the Alexander Shulgin Research Institute in performing GC/MS analysis of some of the intermediates in the DMT synthesis procedure. Dr Raja at Hybrid Pharma for the sterile compounding of DMT. The research nursing staff of the Neurobiological Studies Unit and the research pharmacists of VA Connecticut Healthcare System.
Author contributions
DCD designed the study and NVC synthesized the DMT hemifumarate. DCD and SAS wrote the report. LTF, HS-A, and SAS coordinated the study and collected the data. SAS and HS-A analyzed the data. DCD was the lead psychiatrist on the trial. DCD, SAS, and MR provided psychological support for the participants. All authors contributed important intellectual content and approved the final version to be submitted.
Funding
This work was supported by Henry Wallace Foundation. DCD receives research funding administered through Yale University from the US National Institute of Health, US Department of Veteran Affairs, Takeda, Biogen, Boehringer Ingelheim, Ceruvia, Heffter Institute, and Wallace Foundation. DCD has served as a paid consultant to Jazz Pharmaceuticals, Biohaven, and Abide. None of these represent a conflict of interest.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-022-01344-y.
References
- 1.Grob CS, Danforth AL, Chopra GS, Hagerty M, McKay CR, Halberstadt AL, et al. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch Gen Psychiatry. 2011;68:71–8. doi: 10.1001/archgenpsychiatry.2010.116. [DOI] [PubMed] [Google Scholar]
- 2.Griffiths RR, Johnson MW, Carducci MA, Umbricht A, Richards WA, Richards BD, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol. 2016;30:1181–97. doi: 10.1177/0269881116675513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schindler EA, Sewell RA, Gottschalk CH, Luddy C, Flynn LT, Lindsey H, et al. Exploratory controlled study of the migraine-suppressing effects of psilocybin. Neurotherapeutics. 2021;18:534–43. doi: 10.1007/s13311-020-00962-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogenschutz MP. It’s time to take psilocybin seriously as a possible treatment for substance use disorders. Am J Drug Alcohol Abus. 2017;43:4–6. doi: 10.1080/00952990.2016.1200060. [DOI] [PubMed] [Google Scholar]
- 5.Johnson MW, Garcia-Romeu A, Cosimano MP, Griffiths RR. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol. 2014;28:983–92. doi: 10.1177/0269881114548296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nichols DE, Johnson MW, Nichols CD. Psychedelics as medicines: an emerging new paradigm. Clin Pharmacol Therapeutics. 2017;101:209–19. doi: 10.1002/cpt.557. [DOI] [PubMed] [Google Scholar]
- 7.Davis AK, Barrett FS, May DG, Cosimano MP, Sepeda ND, Johnson MW, et al. Effects of psilocybin-assisted therapy on major depressive disorder: a randomized clinical trial. JAMA Psychiatry. 2021;78:481–9. doi: 10.1001/jamapsychiatry.2020.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.SzARA S. Dimethyltryptamin: its metabolism in man; the relation of its psychotic effect to the serotonin metabolism. Experientia. 1956;12:441–2. doi: 10.1007/BF02157378. [DOI] [PubMed] [Google Scholar]
- 9.Dittrich A, Bickel P, Schopf J, Zimmer D. [Comparison of altered states of consciousness induced by the hallucinogens (–)-delta9-trans-tetrahydrocannabinol (delta9-THC) and N,N-dimethyltryptamine (DMT) (author’s transl)] Arch Psychiatr Nervenkr. 1976;223:77–87. doi: 10.1007/BF00367455. [DOI] [PubMed] [Google Scholar]
- 10.Gouzoulis-Mayfrank E, Heekeren K, Neukirch A, Stoll M, Stock C, Obradovic M, et al. Psychological effects of (S)-ketamine and N,N-dimethyltryptamine (DMT): a double-blind, cross-over study in healthy volunteers. Pharmacopsychiatry. 2005;38:301–11. doi: 10.1055/s-2005-916185. [DOI] [PubMed] [Google Scholar]
- 11.Strassman RJ, Qualls CR, Uhlenhuth EH, Kellner R. Dose-response study of N,N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry. 1994;51:98–108. doi: 10.1001/archpsyc.1994.03950020022002. [DOI] [PubMed] [Google Scholar]
- 12.Strassman RJ. Human psychopharmacology of N,N-dimethyltryptamine. Behav Brain Res. 1996;73:121–4. [DOI] [PubMed]
- 13.Szára S. DMT at fifty. Neuropsychopharmacol Hung. 2007;9:201–5. [PubMed] [Google Scholar]
- 14.Timmermann C, Roseman L, Schartner M, Milliere R, Williams LT, Erritzoe D, et al. Neural correlates of the DMT experience assessed with multivariate EEG. Sci Rep. 2019;9:1–13. doi: 10.1038/s41598-019-51974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keiser MJ, Setola V, Irwin JJ, Laggner C, Abbas AI, Hufeisen SJ, et al. Predicting new molecular targets for known drugs. Nature. 2009;462:175–81. doi: 10.1038/nature08506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ray TS. Psychedelics and the human receptorome. PLoS One. 2010;5:1–17. doi: 10.1371/annotation/e580a864-cf13-40c2-9bd9-b9687a6f0fe4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science. 2009;323:934–7. doi: 10.1126/science.1166127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cozzi NV, Gopalakrishnan A, Anderson LL, Feih JT, Shulgin AT, Daley PF, et al. Dimethyltryptamine and other hallucinogenic tryptamines exhibit substrate behavior at the serotonin uptake transporter and the vesicle monoamine transporter. J Neural Transm. 2009;116:1591–9. doi: 10.1007/s00702-009-0308-8. [DOI] [PubMed] [Google Scholar]
- 19.Bunzow JR, Sonders MS, Arttamangkul S, Harrison LM, Zhang G, Quigley DI, et al. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol Pharm. 2001;60:1181–88. doi: 10.1124/mol.60.6.1181. [DOI] [PubMed] [Google Scholar]
- 20.Saavedra JM, Axelrod J. Psychotomimetic N-methylated tryptamines: formation in brain in vivo and in vitro. Science. 1972;175:1365–6. doi: 10.1126/science.175.4028.1365. [DOI] [PubMed] [Google Scholar]
- 21.Osório FdL, Sanches RF, Macedo LR, Dos Santos RG, Maia-de-Oliveira JP, Wichert-Ana L, et al. Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a preliminary report. Braz J Psychiatry. 2015;37:13–20. doi: 10.1590/1516-4446-2014-1496. [DOI] [PubMed] [Google Scholar]
- 22.Palhano-Fontes F, Barreto D, Onias H, Andrade KC, Novaes MM, Pessoa JA, et al. Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: a randomized placebo-controlled trial. Psychological Med. 2019;49:655–63. doi: 10.1017/S0033291718001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brito-da-Costa AM, Dias-da-Silva D, Gomes NG, Dinis-Oliveira RJ, Madureira-Carvalho Á. Toxicokinetics and toxicodynamics of ayahuasca alkaloids N, N-dimethyltryptamine (DMT), harmine, harmaline and tetrahydroharmine: clinical and forensic impact. Pharmaceuticals. 2020;13:334. doi: 10.3390/ph13110334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaynes BN, Lux L, Gartlehner G, Asher G, Forman‐Hoffman V, Green J, et al. Defining treatment‐resistant depression. Depression Anxiety. 2020;37:134–45. doi: 10.1002/da.22968. [DOI] [PubMed] [Google Scholar]
- 25.Cozzi NV, Daley PF. Synthesis and characterization of high-purity N,N-dimethyltryptamine (DMT) hemifumarate for human clinical trials. Drug Test Anal. 2020;12:1483–93. [DOI] [PubMed]
- 26.Strassman RJ. Human psychopharmacology of N, N-dimethyltryptamine. Behav Brain Res. 1995;73:121–4. doi: 10.1016/0166-4328(96)00081-2. [DOI] [PubMed] [Google Scholar]
- 27.Shader RI. Safety versus tolerability. Clin Therapeutics. 2018;40:672–3. doi: 10.1016/j.clinthera.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Mason OJ, Morgan CJ, Stefanovic A, Curran HV. The Psychotomimetic States Inventory (PSI): measuring psychotic-type experiences from ketamine and cannabis. Schizophrenia Res. 2008;103:138–42. doi: 10.1016/j.schres.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 29.Williams JB, Kobak KA, Bech P, Engelhardt N, Evans K, Lipsitz J, et al. The GRID-HAMD: standardization of the Hamilton depression rating scale. Int Clin Psychopharmacol. 2008;23:120–9. doi: 10.1097/YIC.0b013e3282f948f5. [DOI] [PubMed] [Google Scholar]
- 30.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc: Ser B (Methodol) 1995;57:289–300. [Google Scholar]
- 31.Carhart-Harris RL, Bolstridge M, Rucker J, Day CM, Erritzoe D, Kaelen M, et al. Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry. 2016;3:619–27. doi: 10.1016/S2215-0366(16)30065-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.