Abstract
The current opioid epidemic has dramatically increased the number of children who are prenatally exposed to opioids, including oxycodone. A number of social and cognitive abnormalities have been documented in these children as they reach young adulthood. However, little is known about the mechanisms underlying developmental effects of prenatal opioid exposure. Microglia, the resident immune cells of the brain, respond to acute opioid exposure in adulthood. Moreover, microglia are known to sculpt neural circuits during typical development. Indeed, we recently found that microglial phagocytosis of dopamine D1 receptors (D1R) in the nucleus accumbens (NAc) is required for the natural developmental decline in NAc-D1R that occurs between adolescence and adulthood in rats. This microglial pruning occurs only in males, and is required for the normal developmental trajectory of social play behavior. However, virtually nothing is known as to whether this developmental program is altered by prenatal exposure to opioids. Here, we show in rats that maternal oxycodone self-administration during pregnancy leads to reduced adolescent microglial phagocytosis of D1R and subsequently higher D1R density within the NAc in adult male, but not female, offspring. Finally, we show prenatal and adult behavioral deficits in opioid-exposed offspring, including impaired extinction of oxycodone-conditioned place preference in males. This work demonstrates for the first time that microglia play a key role in translating prenatal opioid exposure to changes in neural systems and behavior.
Subject terms: Neuroimmunology, Addiction
Introduction
In the past decade, rates of opioid use disorder (OUD) have increased to epidemic proportions (Haight et al., 2018), including a dramatic increase in OUD among pregnant women. Between 1999 and 2014 the incidence of OUD at delivery increased by 333% [1]. Infants exposed to opioids during gestation often develop neonatal opioid withdrawal syndrome, which is characterized by tremors, difficulty feeding, high-pitched crying, inconsolability, and diarrhea [2, 3]. While these symptoms can be acutely managed with opioid replacement therapies, little is known about long-term consequences of prenatal opioid exposure for brain development and behavior.
The literature on the long-term effects of prenatal opioid exposure, while sparse, suggests enduring consequences. At school-age, differences have been found between opioid-exposed and un-exposed children in outcomes including cognitive performance [4, 5], visual acuity [6], language [7, 8], and attention-deficit disorder [9–11]. However, interpretation of the human literature is confounded by several factors, including retrospective chart review, small sample sizes, and sociodemographic variables [2, 12]. Interestingly, boys, but not girls exposed to opioids in utero had poorer language and cognitive scores [4, 13]. These findings may suggest a male bias in susceptibility to prenatal opioid exposure.
Behavioral and neurobiological changes have also been identified in animal models of prenatal opioid exposure [14–18]. Male rats exposed to gestational morphine exhibit decreased extinction of methamphetamine-conditioned place preference (CPP) and increased drug-primed reinstatement [19]. Similarly, prenatal methadone increased behavioral sensitization to methamphetamine in adolescent male rats [20]. Endogenous opioids and dopamine act in the nucleus accumbens (NAc; a key part of the mesolimbic reward system) to facilitate the hedonic and motivational aspects of drug-seeking behavior, respectively. While prenatal opioid effects on the endogenous opioid system have been well-studied [16], less is known about how prenatal opioid exposure influences the dopamine system during development. Both dopamine D1 and D2 receptor (D1R and D2R) densities in the NAc peak during adolescence (postnatal day [P]30) in male rats, and then decline to adult levels by P55 [21, 22]. Microglia, the resident immune cells of the brain, play a key role in the developmental sculpting of neural circuits. Microglial pruning of D1Rs is responsible for the developmental decline following the peak at P30, but only in males [21]. Early work has begun to explore the impact of prenatal opioid exposure on microglia. Prenatal methadone exposure alters microglial morphology in the cortex at P10, increases toll-like receptor signaling pathways, and increases serum concentrations of several proinflammatory cytokines, e.g., interleukin 1ß (IL-1ß) and tumor necrosis factor-alpha (TNFα), some of which persisted until P21 [23].
Based on these findings, we hypothesized that prenatal opioid exposure would impact microglial function and disrupt D1R pruning, leading to persistent alterations in the NAc dopamine system, in a sex-specific manner. To test our hypothesis, we used intravenous oxycodone self-administration in rats to approximate a real-world scenario in which women are chronically self-administering opioids—due either to an untreated opioid use disorder or to medication-assisted treatment for an opioid use disorder. Female rats self-administered the prescription opioid, oxycodone, for 3 weeks prior to mating to account for the fact that women do not initiate opioid use upon pregnancy, but rather become pregnant in the context of opioid use. Female rats continue to self-administer oxycodone throughout pregnancy until parturition, when access to drug was stopped.
Methods
Animals
Adult Sprague-Dawley rats (Charles River Laboratory, Wilmington, MA) were group-housed under standard laboratory conditions (12-h light-dark cycle [lights on at 7:00 am], food and water ad libitum). All experiments were conducted during the light phase and approved by/in accordance with the Animal Care and Use Committee at McLean Hospital and the National Institutes of Health guide for the care and use of Laboratory animals.
Intravenous oxycodone self-administration
Adult female rats underwent jugular catheter implantation surgery and were trained to self-administer oxycodone hydrochloride (NIDA Drug Supply) prior to mating. Briefly, rats were implanted with chronic, indwelling silastic (0.51 mm internal diameter) jugular catheters [24]. One week after surgery, females underwent oxycodone self-administration training in operant conditioning chambers (Med Associates, 30.5 × 24.1 × 29.2 cm). Operant chambers were enclosed in sound-attenuated cubicles with ventilation fans and contained 2 retractable response levers with cue lights, a house light, a counterbalanced fluid swivel and tether, and an infusion pump. A syringe containing oxycodone solution was located outside the chamber and connected to the rat’s catheter via a spring-covered Tygon tube connected to a fluid swivel. Rats self-administered oxycodone (0.1 mg/kg/infusion) for 4-h/d on a Fixed Ratio 1 (FR1) schedule of reinforcement, with one press on the active lever resulting in a 4-second infusion (0.1 ml/infusion), the concentration of which was adjusted to the rat’s body weight every 3 days. Infusions were followed by a 6-second time-out period in which rats could press the levers, but no drug infusion occurred. Prior to mating, females were given access to self-administration for 4 h/day, 5 days/week for 3 weeks. Control dams were implanted with jugular catheters and allowed to self-administer oxycodone for 5 days in the first week, after which they remained in their home cages with no access to oxycodone. Next, all females were time-mated, and pregnancy was determined by confirmation of sperm in the vaginal canal. The oxycodone treatment group continued to self-administer oxycodone 7-days/week until giving birth. Control dams remained in their home cages. In a small additional cohort, cross-fostering was used to assess maternal care in dams of both treatments raising pups of each treatment condition (see Supplementary Fig. 1 and Supplementary Table 1 for full details and results). Briefly, cross-fostering was conducted on P0 and the % of pups isolated from the litter was quantified on P1, P3, and P6 as a measure of maternal care.
Neonatal pup outcomes and behavioral quantification
Number of pups in each litter was counted on P1, P3, and P6 to assess neonatal mortality. Milk band size was assessed between 9:00 am–10:00 am on P1 to assess how much milk the pups were consuming and categorized according to a 1–4 rating scale (1: no band, 2: small band, 3: medium band, 4: large band). Body weight was assessed in offspring at P1, P3, P6, and then again at P21, P26, P36, P46, and P50 to determine long-term changes in weight. Neonatal behavioral tests were conducted to assess motor capacities (righting reflex and incline plane). In the righting reflex test (P1 and P3), pups were placed on their backs on a flat surface, and latency for each pup to right itself was recorded with a stopwatch. Latency to right, number of pups, and % pups/litter unable to right were quantified. In the incline plane test (P6), pups were placed facing downward on a 45° angle plane, and latency to pivot 180o (facing upwards) was recorded with a stopwatch. Pups were scored based on the final angle reached (1: 0–90°, 2: 90–179°, 3: 180°) as well on latency to reach 180° (if attained). Both the righting reflex and incline tests had a maximum cut-off time of 60 s. Ultrasonic vocalizations (USVs) were measured at P3 and P6 using Avisoft Bioacoustic recorder and software. Pups were removed from the dam and placed into a 1 pint plastic cup on a heating pad (low setting), inside a sound-attenuated Styrofoam box. USVs were recorded for 3 min. and pups were returned to their home cages immediately after. Data for male and female pups were combined for measurements, as our ability to accurately sex the pups on P1-P6 was not reliable.
Brain tissue collection & sectioning
At P20, P30, and P50, offspring of both treatments and sexes were anesthetized using ketamine/xylazine and transcardially perfused using ice-cold saline followed by 4% Paraformaldehyde (PFA). Brains were postfixed in 4% PFA for 48 h followed by 30% sucrose for 48 h and then frozen at −80 °C before cryosectioning at 40 µm. Brain sections were stored in cryoprotectant at −20 °C until IHC staining. Ages for assessment were chosen based on our previous work [21] to span the adolescent period and to capture the peak timepoint for microglial pruning of D1R (P30).
Immunohistochemistry (IHC)
IHC staining was conducted according to Kopec et al. (2018; [21]). Briefly, sections were rinsed 5 times in 1 x PBS and incubated at 80 °C for 30 min in 10 mM sodium citrate (pH 9.0) for epitope retrieval. Next, sections were incubated in 1 mg/ml sodium tetraborate in 0.1 M PB and then 50% methanol in PBS to quench background fluorescence for 1 h each. Sections were blocked for 1 h in 10% goat normal serum with 0.3% Triton-x100 and 3% H202 in 1x PBS before primary antibody incubation. Two separate IHCs were conducted in separate NAc brain sections to label for D1R + microglia (using Iba1) and for dopamine D2 receptor (D2R) and tyrosine hydroxylase (Th; labels dopamine fibers). For D1R and Iba1 staining, sections were incubated for 48 h at 4 °C with a mouse D1R antibody (Novus Biologicals #NB110–60017; 1:1500) and a chicken Iba1 antibody (Synaptic Systems, 1:1500). We previously validated the specificity of the Novus D1R antibody in our own hands using D1R knock-out mouse tissue [21]. For D2R and Th staining, sections were incubated for 24 h at RT with a rabbit D2R antibody (Milllipore, 1:1500) and a mouse Th antibody (Immunostar, 1:1000). These were followed by secondary antibody incubation with goat anti-mouse Alexa-Fluor (AF)488 (D1R or D2R) and goat anti-chicken AF568 (Iba1 or Th; Thermofisher Scientific, each 1:500) for 2 h at room temperature (RT; Supplementary Fig. 3). Following IHC, all sections were mounted, coverslipped with vectashield antifade mounting media w/DAPI (Vector Labs), and stored at −20 °C until imaging.
Imaging and analysis of D1R, D2R, and Th immunofluorescence
To quantify immunofluorescent intensity in the NAc, 40x magnification Z-stacks were taken on a Zeiss AxioImager microscope. 10 step Z-stacks were taken with a step-size of 0.65 µm measured from the center of the focal Z-plane. Images were taken medial to the anterior commissure (AC) using the AC as a landmark. A total of 3–6 images were taken between Bregma +2.76 and +2.28 for each animal (based on the Paxinos and Watson Rat Brain Atlas [25]). For D1R, D2R, and Th, we quantified mean grey value as a measure of immunofluorescent intensity and, therefore, density of signal using FIJI (JWatcher). First, Z-stacks were converted to maximum projection images, and mean grey value was measured using the measure function with the entire image as a region of interest. These values were then normalized to background (defined as the mean grey value of the AC). The values of all images taken for a given animal were averaged to provide a single measurement per rat at each age. Higher ‘density’ as used throughout the manuscript is defined as a higher mean grey value.
IMARIS quantification of microglial engulfment of D1R and microglial morphology
To quantify microglial engulfment of D1R at P30, 63X magnification Z-stacks were taken on a Zeiss Airyscan 880 Confocal Microscope (step size: 0.3 µm; 60–100 steps per microglia). Imaris 9.5.1 (Bitplane Scientific Software) was used to create surface renderings of individual microglia (Iba1 labeling) and D1R (D1R labeling) within the microglial surface. Volume of engulfed D1R was then quantified and normalized to total cell volume. 4–5 cells were reconstructed per animal from 3–5 separate brain sections between Bregma distances of +2.76 and +2.28 within the NAc. For assessment of microglial morphology, IMARIS neurite tracer was used to create a skeletonization of individual microglia on which Sholl analyses were conducted.
Place conditioning with oxycodone in adult offspring
An unbiased, three-compartment place-conditioning apparatus (Med Associates) was used [26] to test oxycodone-conditioned place preference in adulthood. Within the apparati, each chamber differed in lighting (bright vs. dim), floor texture (mesh vs. metal rods), and wall coloring (black vs. white). A screening session was conducted in which rats were placed into the apparatus and given 20 min to freely explore all three compartments. Rats that showed any bias (i.e., preference for one of the compartments ≥14 min) would have been eliminated from further testing, however, no animals showed such a bias in this experiment. On the second day, rats underwent two place-conditioning sessions. In the morning (10:00 am) conditioning session, all experimental rats were injected with saline and confined to one side compartment within the apparatus for 30 min, counter balanced across sides and groups. In the afternoon (2:00 pm) conditioning session, all experimental rats were injected with oxycodone (3 mg/kg) and confined to the opposite side compartment for 30 min. On the third day, experimental rats were given 20 min to freely explore all 3 compartments in a single test session. This was followed by 2 days of additional test sessions (extinction sessions 1 and 2) in which subjects were exposed to the apparatus without any treatments and allowed to freely explore for 20 min. Total activity counts were measured by the infrared photo beams in the place conditioning chambers and quantified as a measure of locomotor activity.
Statistics
Graphpad Prism version 9.2.1 was used for all statistics. A one-way ANOVA was used to assess oxycodone self-administration in dams. 2-way mixed-effects ANOVAs (age x treatment) were used to assess number of pups/litter, avg. body weight/litter, body weight per animal, righting reflex outcomes (% of litter unable to right and avg. latency to right/litter), and USVs. Milk band size (%/litter) and inclined plane outcomes (avg./litter) were compared using un-paired t-tests. For D1R and Th, 2-way ANOVAs (age x treatment) were used, with Bonferroni post-hoc tests, in each sex separately (tissues from males and females were processed separately and thus, could not be compared directly). For D2R mean grey values at P55, t-tests were used to compare treatment groups. For microglial engulfment analyses and Sholl analyses, nested t-tests were used to compare treatment groups. 2-way mixed effects ANOVAs (session x treatment) were used to assess conditioned place preference. All data are represented as mean+/− SEM and significance was set at p < 0.05.
Results
Prenatal oxycodone exposure impacts neonatal outcomes in offspring
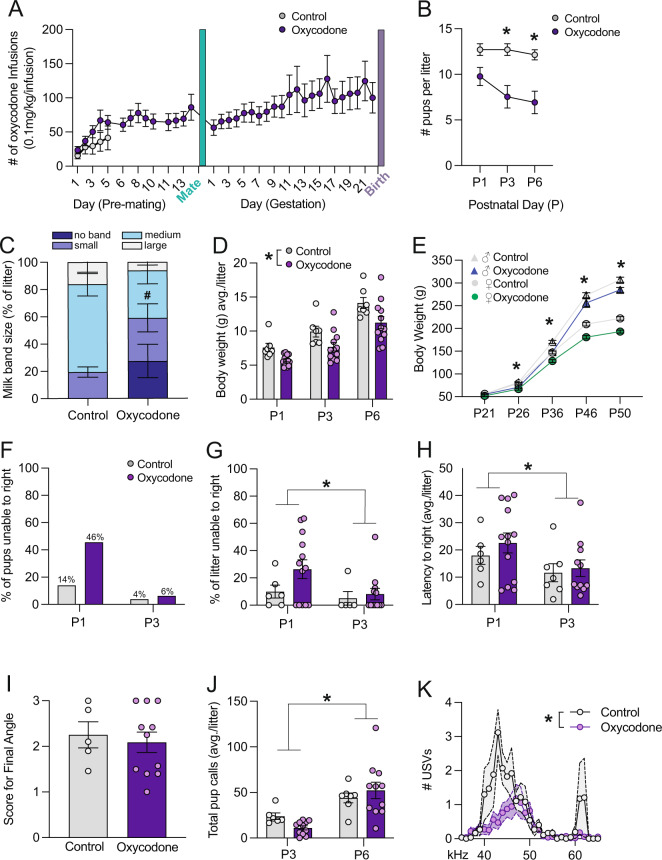
Experimental dams self-administered progressively more oxycodone across pregnancy (F(35,499) = 1.66, p = 0.01, Fig. 1A). In offspring, prenatal opioid exposure significantly decreased the number of surviving pups per litter at P3 and P6 (F(1, 18) = 8.66, p < 0.01, Fig. 1B, posthoc P3: p < 0.01, P6: p < 0.01). ~25% of oxycodone pups had no visible milk band, and there was a trend towards a smaller proportion of pups with milk bands classified as medium-sized (t(1,18) = 1.961, p = 0.066, Fig. 1C). Pup body weight was also significantly lower during both the perinatal period (F(1,18) = 7.276, p < 0.05, Fig. 1D), and adulthood (F(3,131) = 67.63, p < 0.0001, Fig. 1E). No significant correlations were observed between maternal infusions during pregnancy and litter size or offspring body weight during the perinatal period (Supplementary Fig. 2). In a separate cohort in which cross-fostering was performed on P0 such that control and oxycodone-exposed pups were cross-fostered to control and oxycodone self-administering dams, we assessed the % of pups isolated from the nest on P1, P3, and P6. The greatest % of isolated pups was observed in the oxycodone-exposed pups cross-fostered to oxycodone dams, suggesting that both maternal and offspring exposures contribute to maternal care (Supplementary Fig. 1 and Table 1).
Fig. 1. Prenatal opioid exposure impacts neonatal outcomes in offspring.
A Rat dams self-administered oxycodone for either 5 days pre-pregnancy (control) or throughout gestation. B Number of pups per litter was significantly decreased at P3 and P6 following oxycodone exposure. C ~ 20% of oxyocodone exposed pups lacked a detectable milk band and oxycodone pups tended to have fewer medium-size milk bands. Data are presented as % in each rating category per litter. D We observed a main effect of treatment such that the average body weight of oxycodone exposed litters was lower than that of control litters. E Both male and female oxycodone-exposed offspring were persistently lower in body weight into adulthood. A–D N = 7 litters (CON), 12 litters (Oxycodone), male and female offspring are combined. F 46% of oxycodone exposed pups were unable to right themselves in 60 s on P1, as compared to only 14% of control pups, N = 79–127 pups per group. G There was no significant treatment effect on the % of each litter unable to right or on the latency to right (H), N (litters) = 6 (P1 control), 5 (P3 control), 13 (P1 oxycodone), 12 (P3 oxycodone). However, both measures were significantly lower at P3 as compared to P1. I There was a significant main effect of age, but no significant main effect of treatment on total USVs at P3 and P6 (J). Analysis of USVs by frequency at P3 revealed significantly more USVs in control pups as compared to oxycodone-exposed pups, particularly at lower frequencies (K). Data represent Mean +/− SEM, *p = 0.05.
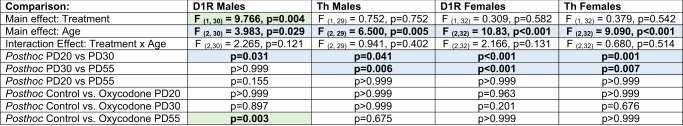
Table 1.
Complete results of 2-way ANOVAs (treatment x age) for D1R and Th in male and female offspring with Bonferroni posthoc tests.
Values represent p values, bolded if significant at p < 0.05. Color coding indicates significant age comparisons (blue) and treatment comparisons (green).
In the righting reflex test, 45% of oxycodone pups were unable to right themselves within 60 s at P1, as compared to 14% of control pups—suggesting motor impairments (Fig. 1F). However, the percentage of pups in each litter that were unable to right in 60 s only trended towards significance at P1 (t(1,17) = 1.528, p = 0.14; Fig. 1G). The average latency to right (per litter) was higher at P1 as compared to P3 (Age effect: F(1,33) = 4.302, p < 0.05, Fig. 1H) but did not differ between treatment groups (Treatment effect: F(1,33) = 0.689, p = 0.413, Fig. 1H). In the inclined plane test, there was no effect of oxycodone on either the final angle reached on average/litter (t(1,14) = 0.428, p = 0.675, Fig. 1I) or on the average latency to reach 180o (t(1,12) = 0.2064, p = 0.95). There was no significant main effect of treatment for total USVs (avg./litter; F(1,16) = 0.07, p = 0.80; Fig. 1J). However, there was a significant main effect of age (F(1,15) = 25.63, p < 0.001) and a trend towards an interaction effect (F(1,15) = 2.77, p < 0.12). Analysis of USVs by frequency at P3 revealed significant effects of treatment and frequency, and an interaction effect, with fewer USVs in oxycodone-exposed pups between 40–50 Hz (avg./litter; treatment: F(1,15) = 8.98, p < 0.01; frequency: F(4.6,69.3) = 7.40, p < 0.001; interaction: F(30,450) = 2.70, p < 0.001 Fig. 1K).
Prenatal oxycodone exposure increases D1R density in the NAc in adulthood in males but not females
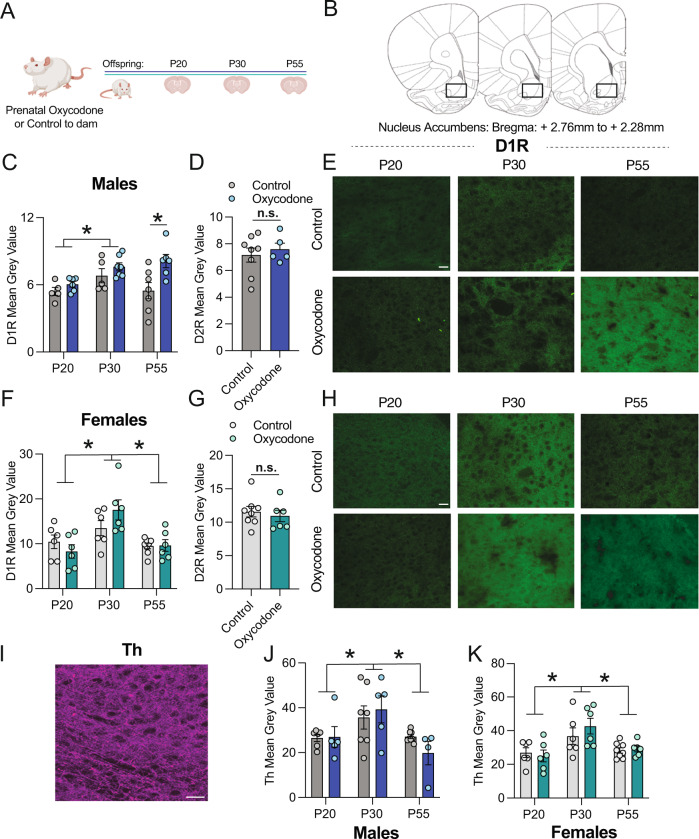
Based on our previous work, we hypothesized that prenatal oxycodone exposure might alter the developmental trajectory of D1R in the NAc between P30 and P55. Thus, we assessed D1R immunofluoresent intensity in the NAc at P20, 30, and 55 (Fig. 2A, B). We found a significant effect of age (p < 0.05) and a significant age x treatment interaction effect (p < 0.05) for D1R (Table 1). Posthoc testing revealed a significant increase in D1R at P55 in oxycodone-treated males relative to controls (Fig. 2C, E, p < 0.05). This increase was male-specific as there was a main effect of age for D1R in the females (Fig. 2F, H, p < 0.001), but no treatment or interaction effects. At P55, there was no difference in D2R between control and oxycodone exposed males (Fig. 2D, t(11) = 0.563; p = 0.585) or females (Fig. 2G, t(11) = 0.536; p = 0.602), demonstrating that D1R, but not D2R, is persistently increased in the NAc by prenatal opioid exposure. To determine whether changes in D1R were driven by changes in dopaminergic input to the NAc, we also assessed the density of Th fibers within the NAc at the same timepoints (Fig. 2i). We found significant effects of age in both males (Fig. 2J, p < 0.01) and females (Fig. 2K, p < 0.001), but no treatment or interaction effects. Hence, we hypothesized that decreased microglial phagocytosis of D1R, and not changes in DA fiber input to the NAc, is responsible for higher NAc-D1R following prenatal oxycodone exposure.
Fig. 2. Prenatal oxycodone exposure increases D1R density in the NAc in adulthood in males but not females.
A Schematic of timeline for treatment and tissue collection. B Rat brain atlas images delineating NAc region where D1R, D2R, and Th were quantified (based on Paxinos and Watson Rat Brain Atlas). C In males, D1R mean grey value differed significantly with age, and was higher at P55 in oxycodone-exposed males as compared to control. D D2R did not differ with treatment at P55 in males. E Representative 20x images of D1R staining in the NAc of males, scale bar = 27 microns. F D1R peaked at P30 in both control and oxycodone-exposed females, but did not differ with treatment. G D2R did not differ with treatment at P55 in females. H Representative 20x images of D1R staining in the NAc of females, scale bar = 27 microns. I Representative 20x image of Th staining in the NAc of males, scale bar = 27 microns. J Th peaked at P30 in both control and oxycodone exposed males and females (K), but did not differ with treatment. Data represent Mean +/− SEM, *p = 0.05.
Prenatal opioid exposure decreases microglial engulfment of NAc-D1R during adolescence in males
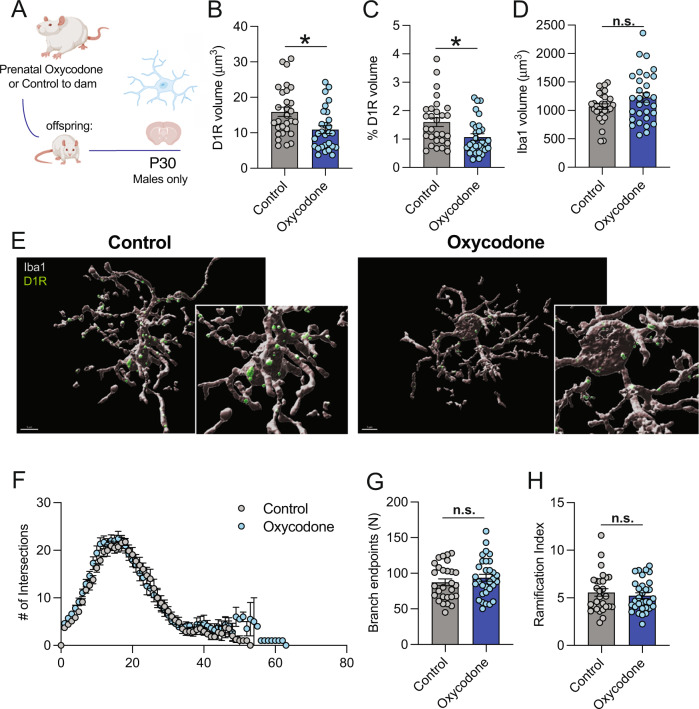
We hypothesized that higher NAc-D1R following prenatal oxycodone exposure might be due to decreased microglial phagocytosis of D1R at P30 (Fig. 3A). In line with this hypothesis, we found that D1R volume within microglia at P30 was significantly lower following prenatal oxycodone exposure (Fig. 3B, C, % D1R volume/microglia, t(11) = 2.36; p < 0.05), while microglia volume did not differ (Fig. 3D, E, t(11) = 0.97; p = 0.36). This effect was not due to gross changes in microglial morphology, as Sholl analysis revealed no significant differences in number of branch endpoints in microglia (Fig. 3F,G, t(11) = 0.96; p = 0.39) or in Schoenen’s Ramification Index (Fig. 3H, t(11) = 0.68; p = 0.51). In oxycodone-exposed males, there were no significant correlations between maternal infusions and offspring D1R densities or microglial phagocytosis (Supplementary Table 2), suggesting that these effects do not depend on the amount of oxycodone exposure in utero.
Fig. 3. Prenatal opioid exposure decreases microglial engulfment of NAc-D1R during adolescence in males.
A Schematic of timeline for exposure and P30 engulfment assessment. B D1R volume within microglia was significantly decreased following oxycodone exposure in male offspring at P30. C % D1R volume of total microglial volume was significantly decreased following oxycodone exposure in male offspring at P30. D There was no treatment effect on microglial volume. E Representative 60x images of microglial D1R engulment: grey: Iba1, green: D1R, scale bar = 5 microns. F Scholl analysis revealed no differences in # of intersections between control and oxycodone males, or in branch endpoints (G) or Ramification Index (H). B–H Dots represent individual microglia, N = 29 (control), 30 (oxycodone) nested per animal 6 and 7, respectively for analysis (nested t-tests). Data represent Mean +/− SEM, *p = 0.05.
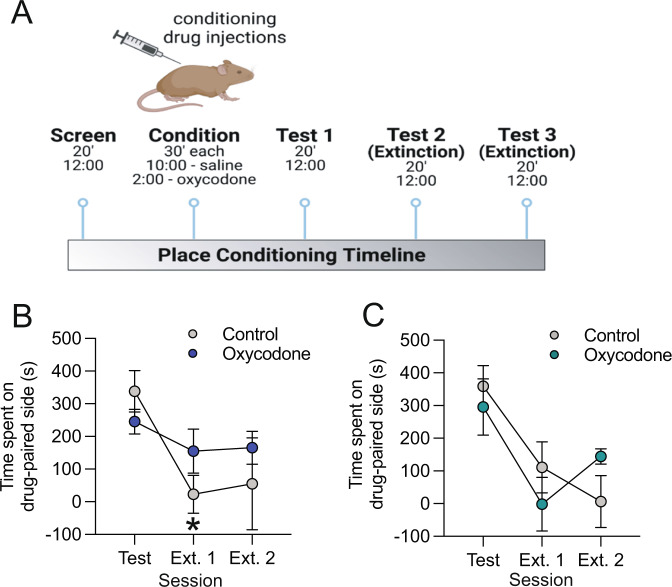
Prenatal opioid exposure reduces extinction, but not acquisition, of oxycodone-induced place conditioning in adult male but not female offspring
To assess potential enduring effects of prenatal opioid exposure on adult behavior, we measured both the acquisition and extinction of oxycodone-induced conditioned place preferences in male and female offspring (Fig. 4A). No significant group differences were observed in locomotor activity (Supplementary Fig. 4). There was a significant preference for the oxycodone-paired side of the chamber during the test, regardless of prenatal oxycodone exposure (Supplementary Fig. 4). In males, we found that a significant main effect of session (F(2,22) = 12.24, p < 0.01, Fig. 4B) as well as significant session x treatment interaction effect (F(2,22) = 3.79), p < 0.05, but no significant main effect of treatment (F(1,11) = 0.30, p = 0.596). Posthoc testing revealed that while expression of oxycodone-induced conditioned place preferences decreased significantly between test and extinction day 1 in control males (p < 0.01), oxycodone-exposed males failed to extinguish their preference for the oxycodone-paired compartment—as evidenced by no significant differences between test day and either extinction day 1 or 2. Interestingly, in oxycodone-exposed males, there were no significant relationships between maternal oxycodone infusions and offspring place preferences on either test or extinction days (Supplementary Table 2), suggesting that behavioral effects do not titrate with amount of prenatal opioid exposure. In females, there was a significant main effect of session (F(2,20) = 11.88, p < 0.01, Fig. 4C), but no significant main effect of treatment (F(1,10) = 0.030, p = 0.865) or session x treatment interaction effect (F(2,20) = 2.271, p = 0.129) indicating that both control and oxycodone-exposed female offspring demonstrated normal extinction of oxycodone-induced conditioned place preferences.
Fig. 4. Prenatal opioid exposure prevents extinction of oxycodone-conditioned place preference in adult male, but not female, offspring.
A Schematic of conditioned place preference procedure. B In males, a significant interaction effect was observed between treatment and session. Posthoc testing revealed significant group differences such that control male offspring showed a significantly lower preference for the drug-paired compartment on extinction session 1 as compared to the test session, while no decrease between test and extinction 1 was observed in oxycodone-exposed males. C Both control and oxycodone-exposed females showed extinction of conditioned place preference. Data represent Mean +/− SEM, *p = 0.05 relative to Test session.
Discussion
Our results demonstrate that prenatal oxycodone exposure increases D1R density in the NAc of young adult male rats (P55) compared to controls. Notably, this effect is sex-specific as no increase was observed in females. Neither D2R density nor Th-immunoreactive (ir) fiber density in the NAc differed between control and prenatal oxycodone-exposed groups, suggesting that higher D1R density is not driven by greater Th inputs to the NAc. Rather, microglial engulfment of D1R is reduced at P30 following prenatal oxycodone exposure in males—a critical age at which microglia phagocytose NAc-D1Rs [21]. Together, these findings suggest that prenatal opioid exposure impairs microglial pruning of D1R during adolescence in males. Consequently, the normal developmental decline in NAc-D1R between adolescence and adulthood fails to occur, resulting in increased D1R density in males only.
Microglia play a critical role in the sculpting of neural circuits in the brain, by phagocytosing synapses and even whole newborn neurons [27, 28]. Human studies have implicated microglia as a key cell type in substance use disorders. RNA sequencing of postmortem NAc from individuals with a history of substance use revealed changes in microglial gene sets [29]. However, virtually nothing is known about how prenatal exposure to drugs of abuse alters microglial phagocytic processes. To our knowledge, our findings are the first demonstration that prenatal opioid exposure disrupts a normal microglial developmental neural circuit pruning program.
The latent mechanisms by which prenatal opioid exposure alters adolescent microglial pruning remain to be elucidated. However, studies have investigated the mechanisms by which opioids impact microglial biology later in life (i.e., in adolescent and/or adult animals or cell cultures). The chemokines CCL4 and CCL17, as well as their receptor CCR4, are upregulated in NAc microglia following adolescent morphine [30]. Similarly, repeated morphine during adolescence (but not young adulthood) persistently changes microglial function and increases reinstatement of morphine CPP as adults. Pre-treatment with a glial modulator prevents this increased reinstatement, implicating a critical role for microglia [30]. Rivera et al. (2019; [31]) found that microglia-specific knock-out of MyD88 increased microglial phagocytosis of newborn (doublecortin positive) neurons in the hippocampus following morphine exposure in adult male mice. Rivera et al. [31]. also showed that male mice lacking microglial-MyD88 showed prolonged extinction of morphine-conditioned place preference and increased drug reinstatement. Together, these findings suggest that microglia are an essential mediator between prenatal opioid exposure and the reward-related effects of drugs of abuse in adulthood.
One outstanding question is what role perinatal opioid withdrawal plays in the lasting effects that we observe. In humans, tapering doses of opioids such as morphine are used to treat neonatal opioid withdrawal syndrome (NOWS; [32]). We find that opioid-exposed pups have smaller milk bands and lower body weights than control-exposed pups. This could be due to changes in maternal care or the ability of the pups to nurse effectively—a known component of NOWS [2]. Exploring opioid replacement therapy in this model is an exciting avenue for future investigation. Still, our preliminary maternal care data shed some light on this question. Only oxycodone-exposed dams rearing oxycodone-exposed pups had a significantly higher proportion of isolated pups upon inspection of their litters. This suggests that behavioral changes in both the mother and offspring contribute to perinatal outcomes. Another question is whether there is a critical window during gestation in which opioid exposure has the most impact. Rats are altricial, and their brain development at birth is likely similar to that of a human fetus at around 6 months gestational age [33]. Therefore, our model likely recapitulates opioids during the first 2 trimesters of pregnancy.
We find that prenatal oxycodone exposure decreases microglial pruning of D1R in male, but not female offspring. The sex-specificity of this effect is in line with our previous finding that microglial pruning of D1R is required for adolescent development in males but not females [21], as well as a large body of literature demonstrating sex differences in microglial biology (for review see [34, 35]). Furthermore, across species, males are more sensitive to a variety of early life insults including stress, infection, and toxicant exposures (for review see [36]). However, the precise mechanisms that render males more vulnerable to prenatal opioids remain unknown and are an important avenue for future investigation.
In conclusion, our results provide novel evidence that prenatal opioid exposure disrupts the development of the dopamine system, at least in part by altering microglial engulfment of D1R during adolescence. Further work is needed to shed light on the sex-specific molecular mechanisms by which these changes occur and their long-term implications for behavior.
Supplementary information
Acknowledgements
We thank all the members of the Bilbo lab for their critical reading of the manuscript, as well as Dr. Ravikiran Raju for providing his clinical insight as a neonatologist. We thank the Animal Care staff at McLean Hospital for providing excellent animal care and the staff of the Duke Light Microscopy Core for assistance with learning and trouble-shooting confocal microscopy.
Author contributions
EHC, SDB, CJS, and TL designed the study. CJS, TL, MJC, KEM, NC, AA, YAC, VJK, and YCJ conducted experiments. CJS, EHC, and SDB wrote the manuscript. All authors approved the final version of the manuscript.
Funding
This work was supported by NIH R21DA048399 to EHC and SDB, NIH F32ES029912 to CJS, and by a Harvard University Mind, Brain, and Behavior Faculty Research Award to EHC and SDB.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Caroline J. Smith, Tania Lintz, Staci D. Bilbo, Elena H. Chartoff.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-022-01376-4.
References
- 1.Haight SC, Ko JY, Tong VT, Bohm MK, Callaghan WM. Opioid use disorder documented at delivery hospitalization—United States, 1999–2014. MMWR Morb Mortal Wkly Rep. 2018;67:845–9. doi: 10.15585/mmwr.mm6731a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conradt E, Flannery T, Aschner JL, Annett RD, Croen LA, Duarte CS.et al. Prenatal opioid exposure: neurodevelopmental consequences and future research priorities. Pediatrics. 2019;144: 10.1542/peds.2019-0128. [DOI] [PMC free article] [PubMed]
- 3.Arter S, Lambert J, Brokman A, Fall N. Diagnoses during the first three years of life for children with prenatal opioid exposure and neonatal abstinence syndrome using a large maternal-infant data hub. J Pediatr Nurs. 2021;61:34–9. doi: 10.1016/j.pedn.2021.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Nygaard E, Moe V, Slinning K, Walhovd KB. Longitudinal cognitive development of children born to mothers with opioid and polysubstance use. Pediatr Res. 2015;78:330–5. doi: 10.1038/pr.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Baar A, de Graaff BM. Cognitive development at preschool-age of infants of drug-dependent mothers. Dev Med Child Neurol. 1994;36:1063–75. doi: 10.1111/j.1469-8749.1994.tb11809.x. [DOI] [PubMed] [Google Scholar]
- 6.Moe V. Foster-placed and adopted children exposed in utero to opiates and other substances: prediction and outcome at four and a half years. J Dev Behav Pediatr. 2002;23:330–9. doi: 10.1097/00004703-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Hunt RW, Tzioumi D, Collins E, Jeffery HE. Adverse neurodevelopmental outcome of infants exposed to opiate in-utero. Early Hum Dev. 2008;84:29–35. doi: 10.1016/j.earlhumdev.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Benninger KL, Borghese T, Kovalcik JB, Moore-Clingenpeel M, Isler C, Bonachea EM, et al. Prenatal exposures are associated with worse neurodevelopmental outcomes in infants with neonatal opioid withdrawal syndrome. Front Pediatr. 2020;8:462. doi: 10.3389/fped.2020.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandtorv LB, Fevang SKE, Nilsen SA, Bøe T, Gjestad R, Haugland S, et al. Symptoms associated with attention-deficit/hyperactivity disorder and autism spectrum disorders in school-aged children prenatally exposed to substances. Subst Abus. 2018;12:1178221818765773. doi: 10.1177/1178221818765773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Cubas MM, Field T. Children of methadone-dependent women: developmental outcomes. Am J Orthopsychiatry. 1993;63:266–76. doi: 10.1037/h0079429. [DOI] [PubMed] [Google Scholar]
- 11.Nygaard E, Slinning K, Moe V, Walhovd KB. Cognitive function of youths born to mothers with opioid and poly-substance abuse problems during pregnancy. Child Neuropsychol. 2017;23:159–87. doi: 10.1080/09297049.2015.1092509. [DOI] [PubMed] [Google Scholar]
- 12.Singer LT, Chambers C, Coles C, Kable J. Fifty years of research on prenatal substances: lessons learned for the opioid epidemic. Advers Resil Sci. 2020;1:223–34. doi: 10.1007/s42844-020-00021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skumlien M, Ibsen IO, Kesmodel US, Nygaard E. Sex differences in early cognitive development after prenatal exposure to opioids. J Pediatr Psychol. 2020;45:475–85. doi: 10.1093/jpepsy/jsaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grecco GG, Atwood BK, Prenatal Opioid exposure enhances responsiveness to future drug reward and alters sensitivity to pain: a review of preclinical models and contributing mechanisms. eNeuro. 2020;7. 10.1523/ENEURO.0393-20.2020. [DOI] [PMC free article] [PubMed]
- 15.Minakova E, Sarafinovska S, Mikati MO, Barclay KM, McCullough KB, Dougherty JD, et al. Ontogenetic oxycodone exposure affects early life communicative behaviors, sensorimotor reflexes, and weight trajectory in mice. Front Behav Neurosci. 2021;15:615798. doi: 10.3389/fnbeh.2021.615798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byrnes EM, Vassoler FM. Modeling prenatal opioid exposure in animals: Current findings and future directions. Front Neuroendocrinol. 2018;51:1–13. doi: 10.1016/j.yfrne.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abu Y, Roy S. Prenatal opioid exposure and vulnerability to future substance use disorders in offspring. Exp Neurol. 2021;339:113621. doi: 10.1016/j.expneurol.2021.113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vassoler FM, Oranges ML, Toorie AM, Byrnes EM. Oxyxodone self-administration during pregnancy disrupts the maternal-infant dyad and decreases midbrain OPRM1 expression during early postnatal development in rats. Pharm Biochem Behav. 2018;173:74–83. doi: 10.1016/j.pbb.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen Y-L, Chen S-T, Chan T-Y, Hung T-W, Tao P-L, Liao R-M, et al. Delayed extinction and stronger drug-primed reinstatement of methamphetamine seeking in rats prenatally exposed to morphine. Neurobiol Learn Mem. 2016;128:56–64. doi: 10.1016/j.nlm.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Wong C-S, Lee Y-J, Chiang Y-C, Fan L-W, Ho I-K, Tien L-T. Effect of prenatal methadone on reinstated behavioral sensitization induced by methamphetamine in adolescent rats. Behav Brain Res. 2014;258:160–5. doi: 10.1016/j.bbr.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 21.Kopec AM, Smith CJ, Ayre NR, Sweat SC, Bilbo SD. Microglial dopamine receptor elimination defines sex-specific nucleus accumbens development and social behavior in adolescent rats. Nat Commun. 2018;9:3769. doi: 10.1038/s41467-018-06118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tarazi FI, Baldessarini RJ. Comparative postnatal development of dopamine D(1), D(2), and D(4) receptors in rat forebrain. Int J Dev Neurosci. 2000;18:29–37. doi: 10.1016/S0736-5748(99)00108-2. [DOI] [PubMed] [Google Scholar]
- 23.Jantzie LL, Maxwell JR, Newville JC, Yellowhair TR, Kitase Y, Madurai N, et al. Prenatal opioid exposure: The next neonatal neuroinflammatory disease. Brain Behav Immun. 2020;84:45–58. doi: 10.1016/j.bbi.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mavrikaki M, Pravetoni M, Page S, Potter D, Chartoff E. Oxycodone self-administration in male and female rats. Psychopharmacology. 2017;234:977–87. doi: 10.1007/s00213-017-4536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paxinos G, Watson C, The rat brain in stereotaxic coordinates: Hard Cover Edition. Elsevier; 2006.
- 26.Russell SE, Puttick DJ, Sawyer AM, Potter DN, Mague S, Carlezon WA, Jr, et al. Nucleus accumbens AMPA receptors are necessary for morphine-withdrawal-induced negative-affective states in rats. J Neurosci. 2016;36:5748–62. doi: 10.1523/JNEUROSCI.2875-12.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faust TE, Gunner G, Schafer DP. Mechanisms governing activity-dependent synaptic pruning in the developing mammalian CNS. Nat Rev Neurosci. 2021;22:657–73. doi: 10.1038/s41583-021-00507-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.VanRyzin JW, Marquardt AE, Argue KJ, Vecchiarelli HA, Ashton SE, Arambula SE, et al. Microglial phagocytosis of newborn cells is induced by endocannabinoids and sculpts sex differences in juvenile rat social play. Neuron. 2019;102:435–449. doi: 10.1016/j.neuron.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seney ML, Kim S-M, Glausier JR, Hildebrand MA, Xue X, Zong W, et al. Transcriptional alterations in dorsolateral prefrontal cortex and nucleus accumbens implicate neuroinflammation and synaptic remodeling in opioid use disorder. Biol Psychiatry. 2021;90:550–62. doi: 10.1016/j.biopsych.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwarz JM, Smith SH, Bilbo SD. FACS analysis of neuronal-glial interactions in the nucleus accumbens following morphine administration. Psychopharmacology. 2013;230:525–35. doi: 10.1007/s00213-013-3180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rivera PD, Hanamsagar R, Kan MJ, Tran PK, Stewart D, Jo YC, et al. Removal of microglial-specific MyD88 signaling alters dentate gyrus doublecortin and enhances opioid addiction-like behaviors. Brain Behav Immun. 2019;76:104–15. doi: 10.1016/j.bbi.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patrick SW, Barfield WD, Poindexter BB. Neonatal opioid withdrawal syndrome. Pediatrics. 2020;146:1–18. doi: 10.1542/peds.2020-0818B. [DOI] [PubMed] [Google Scholar]
- 33.Chini M, Hanganu-Opatz IL. Prefrontal cortex development in health and disease: lessons from rodents and humans. Trends Neurosci. 2021;44:227–40. doi: 10.1016/j.tins.2020.10.017. [DOI] [PubMed] [Google Scholar]
- 34.Bordt EA, Ceasrine AM, Bilbo SD. Microglia and sexual differentiation of the developing brain: A focus on ontogeny and intrinsic factors. Glia. 2020;68:1085–99. doi: 10.1002/glia.23753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.VanRyzin JW, Marquardt AE, Pickett LA, McCarthy MM. Microglia and sexual differentiation of the developing brain: A focus on extrinsic factors. Glia. 2020;68:1100–13. doi: 10.1002/glia.23740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–38. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.