Abstract
Chronic and harmful substance use is associated with a cluster of harms to health, including micronutrient deficiencies. Maintaining adequate levels of vitamin D is important for musculoskeletal and other aspects of health. In this prospective longitudinal cohort study, 666 participants drawn from outpatient opioid agonist therapy (OAT) clinics and community care clinics for substance use disorder in Western Norway were assessed annually for determination of serum 25-hydroxyvitamin D [s-25(OH)D] levels. Fifty-seven percent were deficient at baseline (s-25(OH)D < 50 nmol/l), and 19% were severely deficient (s-25(OH)D < 25 nmol/l). Among those deficient/severely deficient at baseline, 70% remained deficient/severely deficient at the last measurement (mean duration 714 days). Substance use patterns and dosage of opioids for OAT were not associated with vitamin D levels. One exception was found for cannabis, where consumption on a minimum weekly basis was associated with lower levels at baseline (mean difference: −5.2 nmol/l, 95% confidence interval [CI]: −9.1, − 1.3), but without clear time trends (mean change per year: 1.4 nmol/l, CI: − 0.86, 3.7). The high prevalence of sustained vitamin D deficiency in this cohort highlights the need for targeted monitoring and supplementation for this and similar at-risk populations.
Subject terms: Nutrition, Medical research
Introduction
Substance use disorder (SUD) contributes to 18 million healthy years of life lost annually worldwide, and opioids are the leading contributors to this adversity1. People with SUDs, including opioid use disorder (OUD), face many harms to health, one of which is poor nutrition and development of micronutrient malnutrition2. Micronutrient deficiencies are prevalent in SUD, in part owing to suboptimal diets and adverse dietary behaviors3–5. People with sustained and harmful substance use often experience economic difficulties and unstable housing, resulting in limited access to adequate nutrition4,5. Their intake of nutrient-dense foods, such as fresh produce, dairy, and sea food, is often limited and in many cases replaced by a high consumption of simple carbohydrates, high-sugar foods, and processed meats4–7. While enrollment in treatment programs for SUD, including opioid agonist therapy (OAT) for OUD, is known to improve anthropometric indices of nutritional status, adverse dietary patterns and micronutrient imbalances seem to persist7–9. Among the micronutrients, vitamin D is one that is well-studied, yet heavily debated; and it is one of the most frequently measured nutritional parameters in blood10. The current evidence on vitamin D status in SUD and OUD populations is conflicted. Whereas some studies report of poor vitamin D status among people with SUD5 and OUD11, others have found favorable levels among those with SUD compared to healthy controls12,13. Moreover, little is known about the substance-use or treatment-related risk factors of vitamin D deficiency in populations with severe SUD.
Vitamin D consists of a group of fat-soluble steroid-based prohormones essential for health through their role in the uptake of calcium and other micronutrients14,15. Vitamin D is available to humans in the form of vitamin D2 (ergocalciferol) and D3 (cholecalciferol) which are obtained from food ingestion or ultraviolet ray-induced dermal synthesis. Irrespective of source, vitamin D2 and D3 are converted to 25-hydroxyvitamin D [25(OH)D)] in the liver, which is converted in the kidneys to produce 1,25-dihydroxyvitamin D [1,25(OH)2D]—the biologically active metabolite of vitamin D15. Due to its structural stability and long half-time in circulation, serum or plasma concentration of 25(OH)D is the preferred indicator of vitamin D status16. The optimal blood concentration of vitamin D is debated in literature and integrally dependent on the metabolic needs of the individual, however there is a current consensus that serum 25(OH)D levels of 50 nmol/l or greater are representative of sufficient intake for most17,18. Vitamin D has an integral role in regulating calcium homeostasis and bone tissue turnover, and sustained and severe deficiency is known to cause rickets in children and osteomalacia in adults19. Beyond this, vitamin D is claimed to have an immunomodulating and neuroprotective role, and poor vitamin D status is associated with the onset and progression of autoimmune and neurodegenerative diseases including rheumatoid arthritis, multiple sclerosis and Parkinson disease19,20. Although inadequately studied, there is some preliminary evidence pointing to an association of poor vitamin D status with acquisition of SUD21–23.
Vitamin D deficiency could pose an additional, yet potentially mendable, burden to the health of people with sustained and severe substance use. The objective of this study was to assess the vitamin D status of an SUD, and primarily OUD, cohort drawn from outpatient clinics for OAT and community care clinics for SUD in Western Norway. We aimed to measure the prevalence of vitamin D deficiency in this population, and to assess to what extent deficiency was sustained through follow-up. Additionally, we aimed to determine whether factors related to substance use severity or OAT were associated with vitamin D levels in this population.
Methods
Study characteristics; design, population, data collection and study sample
This is a prospective longitudinal cohort study presenting data drawn from the multicenter INTRO-HCV and ATLAS4LAR studies24,25. The study was approved by the Regional Ethical Committee for Health Research, Norway (REK no: 155386 and REK Vest 2017/51), and conducted in accordance with the declaration of Helsinki and relevant guidelines and regulations. Participants provided written informed consent prior to enrolling in the study. Participants were recruited from a population of outpatients visiting OAT clinics or municipal community care clinics for SUD in Bergen and Stavanger, Norway. The cohort consisted mainly of individuals whose lives and health are severely impacted by SUD mostly with dependance to a range of substances including opioids, thus the descriptive term “severe SUD” is used. Participants were assessed yearly with a research nurse-led and questionnaire-based interview focused on somatic and mental health, psychosocial aspects and substance use patterns. Data was collected using the software CheckWare. Clinical data was obtained from the electronic medical record. The data presented was collected between March 2016 and June 2020 and includes a total of 1887 serum 25-hydroxy-vitamin D [25(OH)D] measurements from 666 participants. In total, 491 of the participants had at least two serum 25(OH)D assessments during the study period. Mean time elapsed between the first and last serum 25(OH)D assessment for participants with ≥ 2 blood samplings was 714 days.
Measuring serum vitamin D; laboratory assays and definitions
Venous blood samples were collected and sent to the Department of Medical Biochemistry and Pharmacology at Haukeland University Hospital and the Department of Medical Biochemistry at Stavanger University Hospital (both accredited by ISO-standard 15189) for analysis of 25(OH)D concentration in serum samples. The former used a liquid chromatography-mass spectrometry (LC-MS) assay for assessment of serum 25(OH)D3 concentration for samples analyzed between March 2016 and November 2018, and thereafter the electrochemiluminescence immunoassay (ECLIA) was used to determine the pooled concentration of 25(OH)D2 and 25(OH)D3 (10% analytical variation). The latter laboratory used the LC-MS method for determining the pooled concentration of 25(OH)D2 and 25(OH)D3 (5% analytical variation). The difference in mean serum 25(OH)D in samples that were analyzed using these two methods was < 5 nmol/l. Data on the participants´ serum 25(OH)D concentration was obtained from the electronic medical record. The unit used was nanomoles per liter (nmol/l), and serum 25(OH)D levels ≥ 5 nmol/l were specified. In accordance with laboratory procedures the following cut-offs were used for describing vitamin D status: deficiency was defined as serum 25(OH)D < 50 nmol/l and severe deficiency was defined as serum 25(OH)D < 25 nmol/l26,27.
Study variables; baseline, opioid agonist therapy, clinical and sociodemographic factors
Baseline was defined as the serum 25(OH)D measurement performed in closest proximity in time to the first annual health assessment for each individual. Subsequent serum 25(OH)D measurements were listed chronologically and included as follow-up, and time was defined as years from baseline. OAT was defined as receiving methadone or buprenorphine-based medication. We calculated an OAT dose ratio corresponding to the prescribed daily dose of medication divided by the expected mean dose (90 mg for methadone and 18 mg for buprenorphine) according to the World Health Organization28. As for the clinical factors, injecting substances was defined as having injected any substance within the prior 6 months. Frequent substance consumption was defined as consuming any of the following substances on a minimum weekly basis during the 12 months leading up to the annual health assessment: alcohol, cannabis, benzodiazepines, stimulants (amphetamine, methamphetamine, and cocaine), non-OAT opioids (e.g., heroin) and tobacco (smoking or consuming snuff products). Hepatitis C virus (HCV) status was determined by means of a quantitative polymerase chain reaction assay, where non-zero values were defined as HCV infection. Regarding sociodemographic factors, housing conditions for the last 30 days leading up to the annual health assessment were defined as stable (living in owned or rented home or at an institution) or unstable (being homeless, living at shelter or with friends and family). Source of income was presented as a dichotomous variable consisting of paid labor (full or part-time employment) and receiving social benefits (unemployment, disability or disease benefits and work assessment allowance). Age was categorized into the following groups: < 30 years, 30–39 years, 40–49 years, 50–59 years and ≥ 60 years. Seasons were defined as summer (June–August), autumn (September–November), winter (December–February) and spring (March–May).
Statistical analyses
Stata/SE 16.0 (Stata Corporation) was used for the generation of descriptive data and a linear mixed model. SPSS version 26.0 (IBM) was used for expectation maximization imputation. The software R version 4.0.3 (R foundation for Statistical Computing) with the package mgcv was used for the preparation of generalized additive models and their graphical presentations. The website Sankeymatic (sankeymatic.com/build) was used for the generation of a Sankey diagram. The threshold of statistical significance was set to p < 0.05 for all analyses. Nine percent of values were missing across the sociodemographic and clinical variables. These were assumed to be missing and random and expectation maximization imputation was performed to replace them with estimates. Descriptive data is presented with total numbers and percentages, only including valid values. Median vitamin D levels for different seasons are presented with interquartile range (IQR). The prevalence of deficiency and severe deficiency during different seasons of the year is presented with 95% confidence intervals. Generalized additive models and plots were generated to visualize the nonlinear associations of substance use severity with serum vitamin D concentration, adjusted for gender and age. To do this, a substance use severity index29 was generated based on the type, frequency and number of substances used (for details see Supplementary table S1). A generalized additive model of the association of weeks of the year with vitamin D concentration was generated to visualize the seasonality of vitamin D. A Sankey diagram was generated to display the flow between vitamin D status categories (i.e., vitamin D replete, deficiency and severe deficiency) between the first and last vitamin D assessment for participants with at least two measurements (n = 491). A linear mixed model was performed in order to estimate associations of clinical and sociodemographic factors with serum 25(OH)D concentration at baseline, as well as the time interactions of these associations. Clinical factors included injection of substances, OAT dose ratio and frequent consumption of substances. A variable for season was added to adjust for seasonal fluctuations in serum 25(OH)D concentration. The model was random intercept fixed slope with the estimator set to restricted maximum likelihood. Time was defined as years from baseline, and predictor variables were kept constant to baseline values. A time interaction was added to the variables frequent substance consumption, injecting substances and OAT dose ratio to investigate the impact of time on their associations with vitamin D. Partial adjusted models predicting associations of individual clinical factors with serum 25(OH)D concentration (adjusted for gender and age) are presented in addition to the adjusted model including all variables.
Results
Baseline characteristics of the cohort
The median age at baseline was 44 (IQR: 16), and 70% of participants were male (Table 1). Eighty-nine percent were enrolled in OAT, and of these 60% were prescribed buprenorphine-based medications and 39% were prescribed methadone. Twelve percent lived under unstable housing conditions and 53% had injected a substance at least once during the past six months. Frequent consumption of substances (on a minimum weekly basis) was reported by 77%, and the most common substances consumed were cannabis (50%) and benzodiazepines (38%), followed by alcohol (25%), stimulants (26%) and finally non-OAT opioids (14%).
Table 1.
The table presents baseline characteristics of the cohort (N = 666).
| Characteristic | n/N (%) |
|---|---|
| Gender | |
| Male | 465/666 (70) |
| Female | 201/666 (30) |
| Age group | |
| < 30 years | 79/666 (11) |
| 30–39 years | 188/666 (28) |
| 40–49 years | 205/666 (31) |
| 50–59 years | 155/666 (23) |
| ≥ 60 years | 39/666 (6) |
| Education level | |
| Not completed primary school | 36/666 (5) |
| Primary school (9 years) | 300/666 (45) |
| High school (12 years) | 267/666 (40) |
| ≤ 3 years higher education | 52/666 (8) |
| > 3 years higher education | 11/666 (2) |
| Source of income | |
| Paid labor | 50/666 (8) |
| Social benefits1 | 616/666 (92) |
| Housing condition2 | |
| Unstable | 79/666 (12) |
| Stable | 587/666 (88) |
| HCV infection3 | 310/595 (52) |
| Injecting substances4 | 321/609 (53) |
| Opioid agonist therapy | 594/666 (89) |
| Buprenorphine | 356/594 (60) |
| Methadone | 229/594 (39) |
| Other opioids | 9/594 (2) |
| Weekly substance use5 | |
| Alcohol | 151/607 (25) |
| Cannabis | 304/607 (50) |
| Stimulants6 | 159/607 (26) |
| Benzodiazepines | 230/607 (38) |
| Non-OAT opioids | 83/607 (14) |
| No weekly substance use | 142/607 (23) |
| Weekly tobacco use7 | 564/607 (85) |
1Social benefits include disability, disease and unemployment benefits and work assessment allowance.
2Stable housing included living in owned or rented housing or at an institution, unstable housing included homelessness, living at temporary camping sites or with friends or family.
3Hepatitis C virus infection, defined as non-zero values on a quantitative HCV-RNA assay at baseline.
4Self-reported injection of any substance during the 6 months prior to the first health assessment.
5Self-reported substance use on a minimum weekly basis during the 12 months prior to the first health assessment.
6Amphetamine, methamphetamine or cocaine, 7Self-reported tobacco use (smoking or snuff) on a minimum weekly basis during the 12 months prior to the first health assessment.
Vitamin D status and seasonality
The median serum 25(OH)D concentration of the cohort was 45 nmol/l (IQR: 36) overall, ranging from 56 nmol/l (IQR: 37) in summer to 39 nmol/l (IQR: 34) in winter season (Table 2). Fifty-seven percent (CI: 54–59) had serum 25(OH)D levels < 50 nmol/l overall, ranging from 42% (CI: 40–50) in the summer season to 66% (CI: 58–67) in the winter and 66% (CI) in spring season. The corresponding numbers for serum 25(OH)D < 25 nmol/l were 19% (CI: 16–19) overall, 8.4% (CI: 7–13) in summer season and 27% (CI: 22–30) in winter season. Supplementary table S2 displays the association of serum 25(OH)D concentration with weeks of the year. The strongest positive associations were seen for week number 25–40, corresponding to mid-June to early October (summer and early autumn season in the Northern Hemisphere).
Table 2.
The table presents median serum 25(OH)D levels and the prevalence of deficiency and severely deficiency for different seasons of the year.
| Total | Autumn | Winter | Spring | Summer | |
|---|---|---|---|---|---|
| Vit. D, median (IQR), nmol/l | 45 (36) | 46 (39) | 39 (34) | 40 (30) | 56 (37) |
| Deficiency, n/N (%) | 380/666 (57) | 96/180 (53) | 106/160 (66) | 114/172 (66) | 64/154 (42) |
| Severe deficiency, n/N (%) | 123/666 (19) | 29/180 (16) | 43/160 (27) | 38/172 (22) | 13/154 (8.4) |
The table is based on the baseline measurement of all included participants (n = 666).
IQR 25–75 interquartile range; deficiency, serum 25(OH)D < 50 nmol/l; severe deficiency, serum 25(OH)D < 25 nmol/l.
Changes in Vitamin D status over time
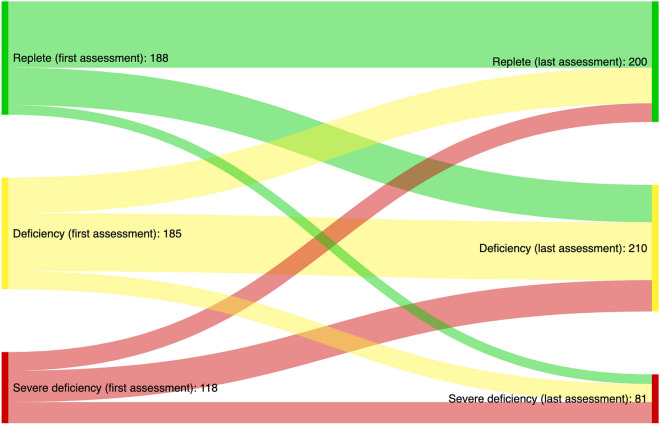
Among the 303 participants who were deficient or severely deficient at baseline, 213 (70%, CI: 65–75) remained deficient or severely deficient while 90 (30%, CI: 25–35) had sufficient levels at the last assessment (Fig. 1). Reversely, 78 of the 188 participants (41%, CI: 35–49) with sufficient levels at baseline had deficient or severely deficient levels at the last assessment and 110 (59%, CI: 51–65) remained vitamin D replete. The linear mixed model predicted a significant increase in serum 25(OH)D levels for the cohort over time (change per year: 5.19 nmol/l, CI: 0.33, 10.0) (Table 3).
Figure 1.
The figure displays changes in vitamin D status categories from the first (left) to the last (right) assessment for participants with at least two vitamin D measurements (n = 491). Definitions: vitamin D replete = serum 25(OH)D > 50 nmol/l, deficiency = serum 25(OH)D > 50 nmol/l severe deficiency = serum 25(OH)D < 25 nmol/l.
Table 3.
The table displays the results of a linear mixed model (restricted maximum likelihood regression) estimating associations of serum 25(OH)D concentration (nmol/l) with sociodemographic and clinical predictor variables at baseline (effect estimates), as well as the impact of predictors on changes in serum vitamin D concentrations over time (time trends per year).
| Partly adjusted* | Adjusted | |||
|---|---|---|---|---|
| Effect estimate | Time trend (per year) | effect estimate | Time trend (per year) | |
| Estimate (CI) | Slope (CI) | Estimate (CI) | Slope (CI) | |
| Serum 25(OH)D | 57.2 (47.8, 66.6) | 5.19 (0.33, 10.0) | ||
| Gender | ||||
| Male | 0.00 (reference) | |||
| Female | 0.81 (−2.73, 4.34) | |||
| Age | ||||
| < 30 | 0.00 (reference) | |||
| 30–39 | −1.57 (−6.43, 3.29) | |||
| 40–49 | −2.50 (−7.60, 2.61) | |||
| 50–59 | −3.15 (−8.55, 2.26) | |||
| ≥ 60 | −0.62 (−8.42, 7.17) | |||
| Season | ||||
| Summer | 0.00 (reference) | |||
| Autumn | −6.06 (−8.89, −3.23) | |||
| Winter | −11.4 (−14.4, −8.53) | |||
| Spring | −10.8 (−13.7, −7.97) | |||
| Source of income | ||||
| Social benefits1 | 0.00 (reference) | 0.00 (reference) | 0.00 (reference) | 0.00 (reference) |
| Paid labor | 8.73 (1.64, 15.8) | 0.42 (−3.99, 4.83) | 8.74 (1.52, 16.0) | −1.52 (−6.10, 3.06) |
| OAT dose ratio2 | −0.90 (−4.73, 2.94) | −0.11 (−2.61, 2.38) | −1.33 (−5.18, 2.52) | 0.17 (−2.38, 2.72) |
| Frequent consumtion3 | ||||
| Alcohol | 0.85 (−3.50, 5.19) | −1.91 (−4.42, 0.59) | 0.86 (−3.44, 5.16) | −1.62 (−4.18, 0.94) |
| Cannabis | −4.88 (−8.63, −1.14) | 0.35 (−1.77, 2.47) | −5.20 (−9.11, −1.29) | 1.41 (−0.86, 3.68) |
| Non−OAT opioids | 1.94 (−3.62, 7.51) | −0.97 (−4.37, 2.43) | 0.68 (−5.05, 6.40) | 0.31 (−3.26, 3.87) |
| Stimulants4 | −0.86 (−5.19, 3.46) | −2.92 (−5.52, −0.32) | −0.97 (−5.48, 3.53) | −2.17 (−4.94, 0.60) |
| Benzodiazepines | 1.01 (−2.85, 4.88) | −2.47 (−4.61, −0.33) | 3.47 (−0.70, 7.65) | −2.43 (−4.87, 0,01) |
| Tobacco5 | 0.40 (−7.01, 7.81) | −3.14 (−7.28, 1.00) | 1.31 (−6.04, 8.65) | −3.82 (−8.01, 0.38) |
Significant results are shown in italics (p < 0.05). CI, 95% confidence interval.
*Adjusted for gender and age.
1Social benefits include disability, disease and unemployment benefits and work assessment allowance.
2The prescribed daily dosage of opioid agonist divided by the WHO mean recommended dosage (90 mg for methadone, 18 mg for buprenorphine). In this variable, zero represents no prescribed OAT medication.
3Self-reported consumption of a substance at a minimum weekly basis during the 12 months prior to the first assessment.
4Amphetamine, methamphetamine and cocaine.
5Self-reported tobacco use (smoking or snuff) on a minimum weekly basis during the 12 months prior to the first health assessment.
Associations of substance use patterns with Vitamin D at baseline and over time
Lower serum 25(OH)D concentration at baseline was found for those with a frequent consumption of cannabis, compared to those with less or no use (mean difference: − 5.20 nmol/l, CI: − 9.11, − 1.29), however the time trend was not significant (mean change per year: 1.41 nmol/l, CI: − 0.86, 3.68) (Table 3). There were no significant associations with serum 25(OH)D levels at baseline or with changes in serum 25(OH)D levels over time for frequent consumption of alcohol, benzodiazepines, non-OAT opioids or stimulants. Similarly, no significant associations were found for the OAT dose ratio variable. A graphical representation of the non-linear association of serum 25(OH)D levels with the substance use severity index is presented in the as figure S3 in the Supplementary information. Substance use severity was not significantly associated with serum 25(OH)D concentration at baseline (p = 0.41). Due to strong correlations between frequent consumption of stimulants and injecting drug use (correlation coefficient = 0.42), injecting drug use was omitted from the linear mixed model. When including the variable for injecting substances in the model, similar findings were observed (see Supplementary table S4). An additional, sensitivity analysis with a linear mixed model of serum 25(OH)D concentration without the variable “source of income” provided relatively similar estimates as the model presented in Table 3 (see Supplementary table S5).
Discussion
This study revealed an overall poor vitamin D status in this cohort drawn from an SUD, and predominantly OUD, population in Western Norway. Fifty-seven percent of the cohort had serum vitamin D concentrations below 50 nmol/l corresponding to deficiency, and 19% were severely deficient with serum levels below 25 nmol/l. Although vitamin D deficiency is not uncommon in Norway, the numbers presented in our study are markedly higher than those reported in studies on the general Norwegian population (57% vs. < 40%)30–32. In particular, the prevalence of severe deficiency is ~ 5 times higher in our study cohort compared to numbers presented in studies on the general Norwegian population (19% vs. ~ 4%)30–32. These finding are in line with another Norwegian study among heavy substance users reporting a mean serum vitamin D concentration of < 40 nmol/l, well below the threshold of deficiency5. Similarly, a descriptive study on methadone-users in the Boston-region of the US found that 36% were deficient in late summer-fall season11. In contrast, case–control studies from respectively Turkey and Australia have reported of more favorable serum concentrations of vitamin D among people with SUD and OUD compared to healthy controls12,13.
We did not find that substance use patterns, namely severity of substance use nor the consumption of most individual substance classes, were associated vitamin D levels. One exception was found for cannabis where frequent consumption was associated with lower vitamin D levels at baseline (however, without significant time trends). This finding is in line with another study describing lower serum vitamin D levels among heavy cannabis users compared to non-users33. Additionally, we did not find support of an association of opioid consumption with vitamin D levels in this population. Neither enrollment in OAT, the dose of opioid agonist prescribed, nor the use of non-OAT opioids were associated with vitamin D levels. The absence of consistent associations between substance use or treatment-related factors and vitamin D levels in our analyses suggests that there could be other factors contributing. A multitude of adversities are known to accompany sustained and severe substance use, including a high burden of disease, disability and economic disadvantage. In light of this, we found that the subset of participants in our cohort (8%) whose income stemmed from paid labor had markedly higher serum vitamin D levels compared to those receiving benefits. Employed individuals feasibly have a higher income and lower health impairment compared to the majority that rely on social benefits for income linked to disorders, disability, disease, or unemployment. The overall high prevalence of subjective health-complaints in this population, including fatigue34 and psychological complaints35 could contribute to behaviors that impact vitamin D status negatively, such as inactivity36 and less time spent outdoors exposed to ultraviolet radiation37.
Maintaining adequate vitamin D levels is important for many aspects of health38, and the high prevalence of deficiency poses a potential additional burden to the health of people with severe SUD. Addressing this could have positive outcomes on their health and well-being. In line with this, a randomized controlled trial on supplementation with 50,000 IU vitamin D every other week for 12 weeks showed promising results for several aspects of the health and well-being of people receiving methadone for OAT, including improved sleep quality, reduced psychological symptoms and blood markers of systemic inflammation and glucose tolerance39. As the prevalence of vitamin D deficiency, and in particular severe deficiency, was substantial in our study cohort, we suggest that the time has come to consider implementing targeted policies, including monitoring and/or supplementation in this and similar at-risk populations40.
Strengths and limitations
Strengths of this study include its relatively large sample size among a “hard-to-reach” and “hard-to-treat” population. We managed to reach the majority of the target population, thereby minimizing the potential of a selection bias. Although participation in the study was offered to all eligible candidates, a possible minor selection bias related to sampling could be present due to the requirement of consenting in written form and in Norwegian language. This may have led to a skewed selection of participants with Norwegian language literacy, and thereby exclusion of some at-risk persons. A major strength of this study is its longitudinal design. Each participant, on average, had more than two successive vitamin D measurements. This allowed us to follow patterns in vitamin D and predictor variables over time, adding strength to the descriptive analyses and associations presented in this study. A limitation of this study is the absence of indicators of cerebral vitamin D status and variability (namely genetic variants of intracerebral 1, 25(OH)2D forming enzymes and effectors). A methodological limitation of this study is related timing of health assessments and blood samplings. Although health assessments were performed annually, the blood samples were not necessarily drawn in exact concurrence with these assessments. The two laboratories that analyzed serum samples did not employ identical techniques for determining 25(OH)D concentration over the entire study period, although the reference ranges as well as the measured levels were consistent. An important limitation on the interpretation of our analysis is related to the lack of information regarding the skin type of participants, as well as information regarding the adherence to treatment and treatment responses. As our design was an observational epidemiological study, confounders are a threat. Thus, identifying key confounders and adjusting properly for these is essential. It could be argued that our variable for economic conditions (i.e., source of income) was suboptimal. Some caution in interpretation of this is thus warranted, and we did also present a sensitivity analysis without this that was generally similar for other variables. Although associations between vitamin D and several different substance classes were assessed, most participants were people with opioid dependence enrolled in OAT. Our findings may therefore not be generalized to different SUDs or to those not receiving comparable treatment or follow-up. Additionally, our cohort is drawn from Western Norway in a northern latitude setting, and our findings are likely not generalizable to southern latitude settings.
Conclusions
The vitamin D status of people visiting outpatient opioid agonist therapy clinics or community care clinics for SUD in Western Norway was inadequate. More than half were deficient and nearly a fifth were severely deficient. A majority of those deficient at baseline, remained deficient through follow-up. Frequent consumption of cannabis was associated with lower levels at baseline, but without consistent time trends. There were no clear associations for other individual substance classes, substance use severity or for the dosage of opioids consumed for opioid agonist therapy. These findings emphasize the need to address the vitamin D status of people with severe substance use disorders through targeted monitoring and/or supplementation. Further research is needed to establish more reliable causation of events.
Supplementary Information
Acknowledgements
This study was nested to the INTRO-HCV study. The INTRO-HCV study group is comprised by the following investigators (according to location and alphabetical order of surnames): Bergen: Christer Frode Aas, Vibeke Bråthen Buljovcic, Fatemeh Chalabianloo, Jan Tore Daltveit, Silvia Eiken Alpers, Lars T. Fadnes (principal investigator), Trude Fondenes Eriksen, Per Gundersen, Velinda Hille, Kristin Holmelid Håberg, Kjell Arne Johansson, Rafael Alexander Leiva, Siv-Elin Leirvåg Carlsen, Martine Lepsøy Bonnier, Lennart Lorås, Else-Marie Løberg, Mette Hegland Nordbotn, Cathrine Nygård, Maria Olsvold, Christian Ohldieck, Lillian Sivertsen, Hugo Torjussen, Jørn Henrik Vold, Jan-Magnus Økland. Stavanger: Tone Lise Eielsen, Nancy Laura Ortega Maldonado, Ewa Joanna Wilk. proLAR: Ronny Bjørnestad, Ole Jørgen Lygren, Marianne Cook Pierron. Oslo: Olav Dalgard, Håvard Midgard, Svetlana Skurtveit. Bristol: Aaron G. Lim, Peter Vickerman. We thank Kåre Johan Sønstabø for contributing to data processing of blood samples.
Author contributions
L.T.F., M.B., J.H.V. and K.A.J. conceptualized the study. L.T.F., J.H.V. and C.F.A. supervised data collection and curation. L.T.F., J.H.V., R.C. and M.B. analyzed the data. M.B. drafted the manuscript with input from K.S. and R.C. All authors contributed to the editing and finalization of the manuscript. All authors have read and approved the final manuscript.
Funding
Open access funding provided by University of Bergen. This work was supported with funding by The Norwegian Research Council (BEHANDLING, contract no. 269855) and the Western Norway Regional Health Authority («Åpen prosjektstøtte» for INTRO-HCV and «Strategiske forskningsmidler» for ATLAS4LAR) with the Department of Addiction Medicine, Haukeland University Hospital, Bergen, Norway as responsible institution. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. Authors are funded by their respective affiliations.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to data protection requirements but are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-17804-w.
References
- 1.United Nations Office on Drugs and Crime. World Drug Report 2021; Booklet 1; Executive summary Policy implicationshttps://www.unodc.org/res/wdr2021/field/WDR21_Booklet_1.pdf (2021).
- 2.Mahboub N, Rizk R, Karavetian M, de Vries N. Nutritional status and eating habits of people who use drugs and/or are undergoing treatment for recovery: a narrative review. Nutr. Rev. 2020;79:627–635. doi: 10.1093/nutrit/nuaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.el-Nakah A, Frank O, Louria DB, Quinones MA, Baker H. A vitamin profile of heroin addiction. Am. J. Public Health. 1979;69:1058–1060. doi: 10.2105/AJPH.69.10.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Himmelgreen DA, et al. A comparison of the nutritional status and food security of drug-using and non-drug-using hispanic women in hartford connecticut. Am. J. Phys. Anthropol. 1998;107:351–361. doi: 10.1002/(SICI)1096-8644(199811)107:3<351::AID-AJPA10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Saeland M, et al. High sugar consumption and poor nutrient intake among drug addicts in Oslo Norway. Br. J. Nutr. 2011;105:618–624. doi: 10.1017/S0007114510003971. [DOI] [PubMed] [Google Scholar]
- 6.Neale J, Nettleton S, Pickering L, Fischer J. Eating patterns among heroin users: a qualitative study with implications for nutritional interventions. Addiction. 2012;107:635–641. doi: 10.1111/j.1360-0443.2011.03660.x. [DOI] [PubMed] [Google Scholar]
- 7.Zador D, Lyons Wall PM, Webster I. High sugar intake in a group of women on methadone maintenance in south western Sydney Australia. Addiction. 1996;91:1053–1061. doi: 10.1111/j.1360-0443.1996.tb03601.x. [DOI] [PubMed] [Google Scholar]
- 8.Kheradmand A, Kheradmand A. Nutritional status in patients under methadone maintenance treatment. J Subst Use. 2020;25:173–176. doi: 10.1080/14659891.2019.1672817. [DOI] [Google Scholar]
- 9.Nolan LJ, Scagnelli LM. Preference for sweet foods and higher body mass index in patients being treated in long-term methadone maintenance. Subst. Use Misuse. 2007;42:1555–1566. doi: 10.1080/10826080701517727. [DOI] [PubMed] [Google Scholar]
- 10.LeFevre ML, LeFevre NM. Vitamin D screening and supplementation in community-dwelling adults: common questions and answers. Am. Fam. Physician. 2018;97:254–260. [PubMed] [Google Scholar]
- 11.Kim TW, Alford DP, Holick MF, Malabanan AO, Samet JH. Low vitamin D status of patients in methadone maintenance treatment. J. Addict. Med. 2009;3:134–138. doi: 10.1097/ADM.0b013e31819b736d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reece AS, Hulse GK. What are the characteristics of vitamin D metabolism in opioid dependence? An exploratory longitudinal study in Australian primary care. BMJ Open. 2018;8:016806. doi: 10.1136/bmjopen-2017-016806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yazici AB, et al. Comparison of vitamin B12, vitamin D and folic acid blood levels in patients with schizophrenia, drug addiction and controls. J. Clin. Neurosci. 2019;65:11–16. doi: 10.1016/j.jocn.2019.04.031. [DOI] [PubMed] [Google Scholar]
- 14.Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am. J. Clin Nutr. 2008;88:491s–499s. doi: 10.1093/ajcn/88.2.491S. [DOI] [PubMed] [Google Scholar]
- 15.Holick MF. Vitamin D deficiency. N. Engl. J. Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 16.Zerwekh JE. Blood biomarkers of vitamin D status. Am. J. Clin. Nutr. 2008;87:1087S–1091S. doi: 10.1093/ajcn/87.4.1087S. [DOI] [PubMed] [Google Scholar]
- 17.Ross AC, Taylor CL, Yaktine AL, Del Valle HB; Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academies Press (2011). [PubMed]
- 18.Holick MF, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 19.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am. J. Clin. Nutr. 2008;87:1080s–1086s. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 20.Rimmelzwaan LM, van Schoor NM, Lips P, Berendse HW, Eekhoff EM. Systematic review of the relationship between vitamin D and parkinson's disease. J. Parkinsons. Dis. 2016;6:29–37. doi: 10.3233/JPD-150615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galyuk TM, Loonen AJM. Putative role of vitamin D in the mechanism of alcoholism and other addictions: a hypothesis. Acta. Neuropsychiatr. 2021;33:1–8. doi: 10.1017/neu.2020.41. [DOI] [PubMed] [Google Scholar]
- 22.Kemény LV, et al. Vitamin D deficiency exacerbates UV/endorphin and opioid addiction. Sci. Adv. 2021;7:4577. doi: 10.1126/sciadv.abe4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim Y, et al. Perioperative serum 25-hydroxyvitamin d levels as a predictor of postoperative opioid use and opioid use disorder: a cohort study. J. Gen. Intern. Med. 2020;35:2545–2552. doi: 10.1007/s11606-020-06001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fadnes LT, et al. Integrated treatment of hepatitis C virus infection among people who inject drugs: study protocol for a randomised controlled trial (INTRO-HCV) BMC Infect. Dis. 2019;19:943. doi: 10.1186/s12879-019-4598-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helse Bergen. ATLAS4LAR: Kartlegging og behandling av lungesykdom i legemiddelassistert behandlinghttps://helse-bergen.no/avdelinger/rusmedisin/rusmedisin-seksjon-forsking/bar/atlas4lar-kartlegging-og-behandling-av-lungesykdom-i-legemiddelassistert-behandling (2019).
- 26.Haukeland universitetssjukehus; Helse Bergen. Analyseoversikten: 25(OH)vitamin D, https://analyseoversikten.no/analyse/11 (2021).
- 27.Stavanger Universitetssjukehus; Helse Stavanger. Laboratoriehåndbok: Vitamin D (25-OH-vitamin D, 25-hydroksyvitamin D i serum)https://labhandbok.sus.no/docs/doc_28356/index.html (2020).
- 28.World Health Organization. Guidelines for the psychosocially assisted pharmacological treatment of opioid dependence https://www.who.int/publications/i/item/9789241547543 (2009). [PubMed]
- 29.Vold JH, et al. Changes in substance use during outpatient treatment for substance use disorders: a prospective Norwegian cohort study from 2016 to 2020. Subst. Abuse Treat Prev. Policy. 2021;16:67. doi: 10.1186/s13011-021-00403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christensen MH, Lien EA, Hustad S, Almås B. Seasonal and age-related differences in serum 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D and parathyroid hormone in patients from Western Norway. Scand. J. Clin. Lab. Invest. 2010;70:281–286. doi: 10.3109/00365511003797172. [DOI] [PubMed] [Google Scholar]
- 31.Larose TL, et al. Factors associated with vitamin D deficiency in a Norwegian population: the HUNT Study. J. Epidemiol. Community Health. 2014;68:165–170. doi: 10.1136/jech-2013-202587. [DOI] [PubMed] [Google Scholar]
- 32.Petrenya N, Lamberg-Allardt C, Melhus M, Broderstad AR, Brustad M. Vitamin D status in a multi-ethnic population of northern Norway: the SAMINOR 2 clinical Survey. Public Health Nutr. 2020;23:1186–1200. doi: 10.1017/S1368980018003816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sophocleous A, et al. Heavy cannabis use is associated with low bone mineral density and an increased risk of fractures. Am. J. Med. 2017;130:214–221. doi: 10.1016/j.amjmed.2016.07.034. [DOI] [PubMed] [Google Scholar]
- 34.Vold JH, et al. Impact of clinical and sociodemographic factors on fatigue among patients with substance use disorder: a cohort study from Norway for the period 2016–2020. Subst. Abuse Treat Prev. Policy. 2020;15:93. doi: 10.1186/s13011-020-00334-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aas CF, et al. Substance use and symptoms of mental health disorders: a prospective cohort of patients with severe substance use disorders in Norway. Subst. Abuse Treat Prev. Policy. 2021;16:20. doi: 10.1186/s13011-021-00354-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brock K, et al. Low vitamin D status is associated with physical inactivity, obesity and low vitamin D intake in a large US sample of healthy middle-aged men and women. J. Steroid. Biochem. Mol. Biol. 2010;121:462–466. doi: 10.1016/j.jsbmb.2010.03.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr. 2004;80:1678s–1688s. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 38.Hossein-nezhad A, Holick MF. Vitamin D for health: a global perspective. Mayo. Clin. Proc. 2013;88:720–755. doi: 10.1016/j.mayocp.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghaderi A, et al. Clinical trial of the effects of vitamin D supplementation on psychological symptoms and metabolic profiles in maintenance methadone treatment patients. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2017;79:84–89. doi: 10.1016/j.pnpbp.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 40.Roth DE, et al. Global prevalence and disease burden of vitamin D deficiency: a roadmap for action in low- and middle-income countries. Ann. N Y Acad. Sci. 2018;1430:44–79. doi: 10.1111/nyas.13968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to data protection requirements but are available from the corresponding author on reasonable request.