Abstract
The korAB operon of broad-host-range plasmid RK2 encodes five genes, two of which, incC and korB, belong to the parA and parB families, respectively, of genome partitioning functions. Both korB and a third gene, korA, are responsible for coordinate regulation of operons encoding replication, transfer, and stable inheritance functions. Overexpression of incC alone caused rapid displacement of RK2. Using two different reporter systems, we show that incC modulates the action of KorB. Using promoter fusions to the reporter gene xylE, we show that incC potentiates the repression of transcription by korB. This modulation of korB activity was only observed with incC1, which encodes the full-length IncC (364 amino acids [aa]), whereas no effect was observed with incC2, which encodes a polypeptide of 259 aa that lacks the N-terminal 105 aa. Using bacterial extracts with IncC1 and IncC2 or IncC1 purified through the use of a His6 tail and Ni-agarose chromatography, we showed that IncC1 potentiates the binding of KorB to DNA at representative KorB operators. The ability of IncC to stabilize KorB-DNA complexes suggests that these two proteins work together in the global regulation of many operons on the IncP-1 genomes, as well in plasmid partitioning.
IncP plasmids transfer between and replicate in most gram-negative bacterial species (36). They are very stably maintained under a range of environmental conditions. A key part of their genetic organization is what we have termed the “central control operon” which coordinates the expression of genes for replication, stable inheritance, and transfer (Fig. 1; see also review in reference 28). Two of the genes in this operon, incC and korB, encode proteins which are related to the products of the parA (incC) and parB (korB) gene families (25), respectively, found on many other plasmids and near the replication origins of most bacterial chromosomes sequenced to date. These proteins play a vital role in the active partitioning of bacterial plasmids (40), and recent studies of their chromosomal homologues in Bacillus subtilis (8, 20, 30) and Caulobacter crescentus (22) suggest that they are important for a process which ensures proper genome segregation during both vegetative growth and differentiation.
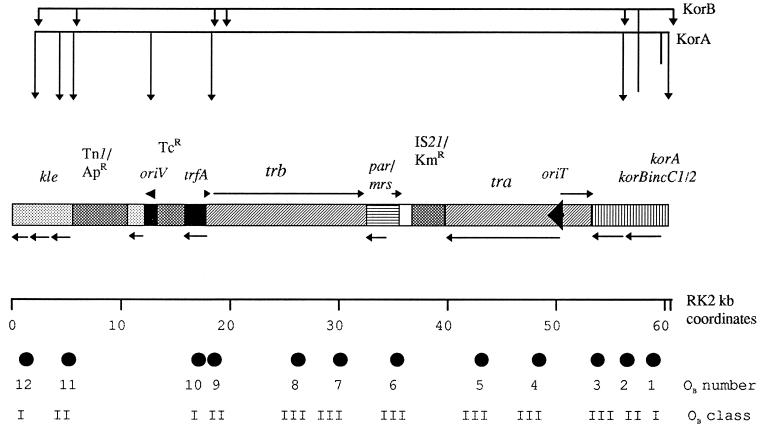
FIG. 1.
Summary of the organization of the RK2 genome, the central control region, and the global circuits that control expression of the replication and transfer genes. Gene designations are as follows: kle, genes that are coregulated by the central control region and are implicated in stable inheritance; oriV, vegetative replication origin; trfA, gene encoding the activator of oriV; tra and trb, blocks of transfer genes; oriT, origin of transfer replication. korA, korB, and incC1/2 are as defined in the text. Replication functions are shown as solid black, transfer functions as diagonal hatching, partitioning and control regions as horizontal and vertical hatching, auxilliary maintenance functions as light gray, and phenotypic markers and transposable elements as dark gray. korA, korB, and incC are shown in the central control region, with incC1 and korA overlapping. General transcriptional organization of the replication, transfer, and stable inheritance functions are shown by arrows above and below the lines. The location of the KorB binding sites are shown as black filled circles, which are labelled in order starting with OB1 associated with the korA promoter. OB class refers to location very close to a promoter (I), up to 190 bp away from a promoter (II) or more than 1 kb from a promoter. The global regulatory circuits provided by KorB and KorA are indicated by the lines and arrowheads above the diagram.
The central control regions of RK2 and R751, archetypes of the IncPα and IncPβ subfamilies, express an active partitioning phenotype, showing better than random segregation when joined to an unstable low-copy-number plasmid (21, 25). This phenotype depends on both incC and korB. KorB (358 amino acids [aa] [18, 32]) is a global regulator that binds to 12 operators, OB1 to OB12, on the plasmid genome (Fig. 1) (3, 41). For RK2 we have shown that the cis-acting sequence necessary for the active partitioning phenotype involves OB3, one of the three korB operators in or close to the central control region (42). IncC (364 aa) belongs to the ParA family of proteins, which share an ATP binding motif (25). ParA of P1 and SopA of F have been shown to have ATPase activity (4, 39) and are DNA binding proteins with an autoregulatory function (1, 7, 24). The ATPase activity is necessary for DNA binding and autoregulation (4). However, by replacing the normal parA promoter in the P1 system with a weak constitutive promoter it was possible to demonstrate that the ATPase activity is also necessary for partitioning (5). Stimulation of this ATPase activity by ParB indicates an interaction between ParA and ParB. However, the relationship between the members of the ParA-ParB pairs does not appear to be consistent. Studies of the chromosomal homologues suggest that they are not equally necessary for genome inheritance (22), and there is no reported direct evidence of interactions between them.
There are a number of points which justify the study of the incC-korB gene pair in addition to the well-characterized P1 and F systems. First, examination of the aligned polypeptide sequences shows that of all the known plasmid-encoded ParA and ParB homologues, those of the IncP families (IncC and KorB) have the highest sequence similarity to the chromosomal members of these families. Second, KorB is clearly not just a partitioning protein since it plays a key role in global regulation on the IncP genome (26, 34, 37). A third interesting feature of these genes is that incC contains two translational start codons (35). The first start codon yields IncC1, which is 364 aa in length, with an approximately 100-aa N-terminal region like ParA of P1 and SopA of F. The second start codon gives IncC2, which is 259 aa and starts just before the first ATP binding motif as found for some plasmid- and all chromosomally encoded homologues studied to date. Significantly, we found that IncC2 is sufficient for the active partitioning phenotype of the central control region in both RK2 (42) and the related IncP-1 plasmid R751 (21). However, before the involvement of incC in partitioning was discovered we had made various observations concerning its activities. Initially, we implicated incC in the temperature-sensitive phenotype of a mini-IncP-1 plasmid with a defect in korA which resulted in derepression of the korA-incC operon (33); the temperature-sensitive phenotype could be suppressed by a deletion in incC. korB was not present in this plasmid. We subsequently found that the effect of the cloned wild-type korA-incC region in displacing a resident IncP-1 plasmid in an incC-dependent manner was dependent on the presence of korB, suggesting that incC normally works through korB or affects a process that is regulated by korB (34). However, these conclusions were complicated by the overlap of korA and incC: the 101-codon open reading frame (ORF) for korA starts 11 bp downstream of the incC1 start codon and is thus included entirely within the incC1 ORF (34). The korA stop codon overlaps the start codon for incC2. Therefore, the fragment containing incC always also produced the transcriptional repressor KorA, which acts on its own promoter (korAp) as well as on the promoter for the replication operon, trfAp.
To remove this complication, we have generated a site-directed mutant which no longer produces KorA but which still expresses IncC normally. We have used this to examine the effects of incC on the stable inheritance of RK2 and on transcriptional repression by korB. In addition, we have used purified IncC1 to study KorB binding to DNA. The results show that incC1 modulates the action of korB both in vivo and in vitro, providing evidence for direct interaction between these proteins and indicating that IncC adds another layer to the global regulation circuits that control and coordinate the majority of the genes of this plasmid system.
MATERIALS AND METHODS
Bacterial strains and growth.
The Escherichia coli strains used were the K-12 strains C600K (thr-1 leu-6 thi-1 lacY1 supE44 ton21 galK) and BL21 [F− ompT hsdSB(rB− mB−) gal dcm] (phage DE3) (Novagen, Inc.). Bacteria were generally grown in L broth (17) at 37°C or on L agar (L broth with 1.5% [wt/vol] agar) supplemented with the following antibiotics as appropriate: benzyl penicillin, sodium salt (100 μg ml−1 in liquid medium and 300 μg ml−1 in agar plates) or ampicillin (100 μg ml−1) for penicillin resistance, kanamycin sulfate (50 μg ml−1) for kanamycin resistance, tetracycline hydrochloride (20 μg ml−1) for tetracycline resistance, and streptomycin sulfate (50 μg ml−1) for streptomycin resistance.
Plasmids.
Descriptions of the standard vectors can be found in Sambrook et al. (29). RK2 (60 kb; Pnr Kmr Tcr) and pUB307 (53 kb; Kmr Tcr) are natural isolates of IncP-1 plasmids which have been described previously (10, 28). The constructed plasmids more specific to this work are listed in Table 1. All tacp expression plasmids also carry the lacIq gene to allow regulated expression controlled by the addition of IPTG (isopropyl-β-d-thiogalactopyranoside). Details of the construction of the plasmids not previously described are given below.
TABLE 1.
Plasmids used in this study
| Plasmid | Resistance determinantsa | Replicon | Size (kb) | Key genetic properties | Reference |
|---|---|---|---|---|---|
| pCT800 | Pnr | pMB1 | 7.2 | tacp-His6incC1 | This work |
| pDM1.1 | Smr | IncQ | 11.6 | tacp | 21 |
| pDM1.12 | Smr Tcr | IncQ | 13.0 | tacp-korB | 21 |
| pDM3.1 | Kmr | pSC101 | 10.4 | korAp-xylE | 21 |
| pDM300 | Kmr | pSC101 | 10.2 | kfrAp-xylE | 21 |
| pGBT30 | Pnr | pMB1 | 6.0 | tacp | 16 |
| pGBT36 | Pnr | pMB1 | 7.1 | tacp-incC1 | This work |
| pGBT37 | Pnr | pMB1 | 6.4 | tacp-korA | 12 |
| pGBT58 | Kmr | pSC101 | 10.2 | trfAp-xylE | 12 |
| pGBT59 | Kmr | pSC101 | 10.2 | trbAp-xylE | 13 |
| pGBT63 | Kmr | pSC101 | 10.5 | trbBp-xylE | 12 |
| pGBT70 | Kmr | pSC101 | 10.2 | trfAp-1-xylE | 15 |
| pGBT71 | Kmr | pSC101 | 10.2 | trbAp-xylE | 15 |
| pGBT302 | Pnr | pMB1 | 6.8 | tacp-incC2 | This work |
| pGBT400 | Smr | IncQ | 11.6 | tacp | This work |
| pGBT401 | Smr | IncQ | 11.9 | tacp-korA | This work |
| pKK1 | Tcr | IncP | 9.6 | trfAp-1 | 19 |
| pOLE1 | Smr | IncQ | 12.7 | tacp-incC1 | This work |
Pnr, Kmr, Smr, and Tcr, resistance to penicillin, kanamycin, streptomycin, and tetracycline, respectively.
pCT800 is a derivative of pGBT30 carrying the incC1 ORF amplified by PCR so as to create a His6 tail at the N terminus and was cloned as an EcoRI-SalI fragment.
pGBT36 was created by inserting the PCR product of the incC1 ORF as an EcoRI-SalI fragment into pGBT30. The upstream primer changes the internal ATG initiation codon for korA from ATG to ACG without altering the amino acid sequence of IncC1 (5′-CGGAATTCATGGGTGTTATCCACGAAGA-3′). pGBT302 was created by inserting the PCR product of the incC2 ORF as an EcoRI-SalI fragment into pGBT30.
pGBT400 is a lacIq tacp expression vector based on the Strr IncQ replicon, which was derived from pDM1.1 by inserting a SalI linker (BRL) into the EcoRI site, which had been treated with deoxynucleoside triphosphates and DNA PolI Klenow fragment. pGBT401 is a pGBT400 derivative with the korA ORF from pGBT37 inserted under tacp.
pOLE1 was constructed by inserting the BamHI-SalI incC1 fragment from pGBT36 into the vector pGBT400 to create an IncQ Smr plasmid with incC1 under the control of tacp.
Plasmid DNA isolation, analysis, cloning, and manipulation of DNA.
Plasmid DNA was isolated by standard procedures (29). Large-scale plasmid purification was carried out by using alkaline sodium dodecyl sulfate (SDS), followed by CsCl-ethidium bromide density gradient centrifugation. Digestion of plasmid DNA with restriction enzymes was carried out under the conditions recommended by the suppliers on 0.8 to 2.0% (wt/vol) agarose gels. These and all other methods were carried out by standard protocols (29). DNA sequencing was performed by AltaBioscience by the dye-terminator method with an ABI 373 automated DNA sequencer. Sequences were aligned and analyzed with programs of the Wisconsin package (6). Standard PCRs (27) were performed as described previously (12). Fragments were amplified on a pCT690 (14) template for RK2. All PCR-derived clones were analyzed by DNA sequencing for verification. DNA PCR-amplified fragments were labelled with terminal transferase and [α-32P]ddATP. Restriction fragments were isolated from the agarose gel, purified by using GeneClean extraction (Bio 101), and radioactively labelled with [γ-32P]ATP and T4 polynucleotide kinase.
Determination of XylE activity.
Catechol 2,3-oxygenase (the product of xylE) activity was assayed in logarithmically growing cells (optical density at 600 nm of 0.6) as described by Zukowski et al. (43). The protein concentration was determined by the biuret method (9).
Purification of His6-tailed polypeptide.
Exponentially growing C600K(pCT800) (ptac-his6incC1) was induced with 0.5 mM IPTG at a cell density of approximately 4 × 107 CFU/ml, grown for 4 h with shaking at 37°C, harvested by centrifugation, and then purified as recommended by Qiagen for soluble native proteins on Ni-agarose columns with an imidazole gradient in phosphate buffer at pH 6. IncC1 eluted between 0.15 and 0.20 M imidazole. Polypeptides during purification were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) by using the Pharmacia PHAST gel system.
Analysis of protein-DNA interactions by gel retardation and DNase I footprinting.
For gel retardation experiments, radioactive fragments were incubated either with purified RK2 KorB (41) or with wild-type His6IncC1 and then separated by PAGE under conditions described previously (14). DNase I footprinting was performed as outlined earlier (14). After a standard binding reaction at 37°C for 30 min, an equal volume of 2× DNase I buffer plus DNase I was added, and the mixture was incubated at room temperature for 1 min before the addition of stop buffer, precipitation, washing, and loading onto 6% urea–6% PAGE gels.
RESULTS
incC alone can efficiently displace a resident IncP plasmid.
We previously showed that incC is necessary for the central control region of IncP plasmids to be able to displace a resident IncP plasmid when introduced in trans on a compatible replicon. To determine whether incC alone is sufficient to express incompatibility, we amplified the incC gene by using a primer which changed the korA start codon (internal to incC) from ATG to ACG (see Materials and Methods) and inserted the fragment downstream of tacp (pGBT3; Table 1). The sequence change in incC (CAT to CAC) caused no alteration in the amino acid sequence since both triplets code for histidine. We determined that the induction of transcription from tacp did not result in the expression of any repressor active directly against a korA-regulated promoter by using xylE reporter fusions with trfAp and trbAp (data not shown). This result confirmed that we had inactivated korA. Induction with IPTG gave the expected products of incC: an IncC1 of ca. 39,000 Mr and an IncC2 of ca. 28,000 Mr. We also subcloned this incC gene into pDM1.1, which has the same tacp expression system controlled by lacIq but is based on a lower-copy-number Smr IncQ replicon, resulting in pOLE1 (Table 1). This allowed us to select for the incC expression plasmid in the presence of RK2, which confers resistance to kanamycin, penicillin, and tetracycline. After the introduction of both pDM1.1 (vector alone) and pOLE1 into a strain with RK2, we induced transcription from tacp by the addition of different levels of IPTG and monitored the loss of RK2 over a period of 20 generations in the absence of selection for RK2. Table 2 shows that while pDM1.1 had no effect on RK2, a major loss of RK2 occurred when pOLE1 was present after the addition of 0.1 and 1.0 mM IPTG. Since pOLE1 should produce both IncC1 and IncC2, it was pertinent to ask whether incC2 alone was sufficient to mediate the displacement. Plasmid pGBT302 has incC2 under the control of tacp. Production of IncC2 was confirmed by SDS-PAGE analysis of the bacterial extracts after standard induction by IPTG (1 mM). The production of active IncC2 was also confirmed by the ability to complement an incC mutant derivative of an active partitioning test plasmid that is unstable due to its loss of the incC gene (data not shown). To increase the chances of seeing even a weak incompatibility, we used pGBT302 directly rather than subcloning into a lower-copy-number PolA-independent IncQ vector. However, this meant that we had to use pUB307 as the test Aps plasmid to be displaced. Comparison of the effects of the high-copy-number plasmids pGBT30 (no RK2 DNA), pGBT36 (incC1/2), and pGBT302 (incC2) showed that pGBT36 caused dramatic displacement of pUB307 (<0.01% survival after 20 generations), while no effect was found for pGBT30 or pGB302. We concluded that the incompatibility towards effectively complete IncP-1 plasmids is associated with incC1.
TABLE 2.
Loss of RK2 from E. coli C600K in response to the expression of incCa
| lacIqtacp plasmid (no. of generations) | CFU on L agar + Sm + Km (pDM1.1 or pOLE1 plus RK2)/CFU on L agar + Sm (pDM1.1 or pOLE1) with:
|
|||
|---|---|---|---|---|
| 0 mM IPTG | 0.02 mM IPTG | 0.1 mM IPTG | 0.5 mM IPTG | |
| pDM1.1 (0) | 1.0 | 1.0 | 1.0 | 1.0 |
| pDM1.1 (20) | 0.88 | 0.95 | 0.93 | 1.16 |
| pOLE1 (0) | 0.95 | 0.95 | 0.95 | 0.95 |
| pOLE1 (20) | 0.62 | 0.73 | 2.9 × 10−2 | 8.7 × 10−6 |
Sm, streptomycin; Km, kanamycin.
IncC modulates the effect of KorB on the inheritance of the mini-IncP-1 plasmid pKK1.
Our previous results suggested that the incompatibility of incC is dependent on the presence of korB. Since further analysis of the interplay of incC and korB depended on the controlled production of IncC and KorB in the same bacterial strain, it was first necessary to determine whether it was feasible to express both genes simultaneously. We therefore studied the growth of E. coli C600K carrying only the compatible vectors (pGBT30 and pDM1.1), a combination of the vector and an overproducing plasmid for single proteins (pGBT30 and pDM1.12; pDM1.1 and pGBT36), and combinations of the two compatible plasmids overproducing KorB and IncC (pDM1.12 and pGBT36, respectively). This analysis showed that when korB was induced with IPTG at concentrations of ≥0.2 mM, there was growth retardation and that the additional presence of induced incC allowed this effect to be seen at 0.1 mM IPTG, although incC alone had no effect. The estimation of CFU on L agar and microscopic examination of the cultures indicated that the effect of KorB on apparent culture density was due to clumping. However, when incC was also present with korB the number of CFU per milliliter was reduced up to 10-fold when the IPTG concentration was ≥0.2 mM, and there appeared to be a 60 to 70% loss of both plasmids from the bacteria in the culture. Consequently, only IPTG concentrations up to 0.1 or 0.2 mM were used for routine assays.
To assess the effect of incC on korB, the first strategy we used was to determine its influence on the replication of mini-IncP plasmid pKK1 (Tcr), which depends on oriV and TrfA and does not carry either incC or korB. Transcription of trfA in pKK1 (19) is from trfAp-1, which carries a promoter-down mutation (31). This leaves trfAp-1 strong enough to synthesize enough TrfA for efficient replication only when no repressors of trfAp are present. The presence of korA, whose product represses trfAp-1, strongly deprives the cells of TrfA initiator and causes a loss of the plasmid DNA (31). We expected that korB would act in the same way.
We examined the pattern of pKK1 segregation in the presence of korB or korA with or without incC. After 5 to 7 h of logarithmic growth in L broth with different concentrations of IPTG (0.01 to 0.5 mM) as well as with streptomycin (which selects for the korB plasmid) and penicillin (which selects for the incC plasmid) but not tetracycline (which selects for pKK1), the cultures were diluted and spread onto L agar with or without tetracycline. The culture grown on 0.01 mM IPTG showed a 90% loss of pKK1 when pDM1.12 (tacp-korB) was present compared to only a 20% loss of pKK1 in the presence of vectors or pGBT36 (tacp-incC) (Fig. 2). In the same strain, exposure to 0.02 mM IPTG led to a more than 99% loss of pKK1. Interestingly, over the IPTG concentration range from 0.01 to 0.05 mM, the simultaneous induction of korB and incC halved the rate of pKK1 segregation, whereas concentrations of IPTG of >0.1 mM with this strain led to a 10-fold-greater rate of plasmid loss compared to when incC was absent.
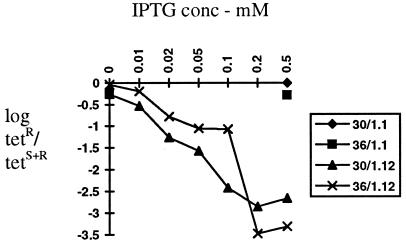
FIG. 2.
Loss of resident mini-IncP plasmid pKK1 (Tcr) indicated as log the ratio of tetracycline-resistant bacteria to total (tetracycline-resistant plus tetracycline-sensitive) bacteria when grown in the presence of tacp-korB (pDM1.12) without or with tacp-incC (pGBT36) induced with increasing concentrations of IPTG. Controls lacking a regulatory gene were provided by pDM1.1 (Smr IncQ vector control for pDM1.12) or pGBT30 (Pnr pMB1-based vector control for pGBT36). Only in the presence of korB is any loss seen, and the effect of incC is to relieve the korB effect at ≤0.1 mM IPTG but to potentiate this effect at higher concentrations.
To extend these observations, we made use of the fact that we had previously established that the transformation of C600K(pKK1) with plasmid DNA carrying tacp-korA also resulted in the loss of pKK1 in the presence of IPTG (19). This was indicated by inhibition of the growth of transformants when tetracycline resistance was selected as well as the resistance of the incoming plasmid. Loss of pKK1 was verified by the isolation and characterization of plasmid DNA. We constructed C600K(pKK1) with pGBT30 (vector) and pGBT36 (tacp-incC) and then transformed it with pDM1.1 (vector), pDM1.12 (tacp-korB), or pGBT401 (tacp-korA) (as a control). To test the effect of these incoming plasmids on pKK1 survival we spread the bacteria after transformation on L agar with streptomycin, penicillin, and tetracycline. Whereas 0.01 mM IPTG had no effect on the ability of C600K(pKK1) to form transformants with any of the incoming plasmids, for pGBT401 (ptac-korA), 0.02 mM IPTG present in the agar gave either none or tiny transformants, indicating severe inhibition of pKK1 survival. With pDM1.12 (ptac-korB), transformants were tiny at 0.02 and 0.05 mM IPTG and completely inhibited at IPTG concentrations of 0.1 mM and above. When incC was induced together with korB, transformants were still obtained at 0.1 mM IPTG (and tiny transformant colonies were even obtained with 0.2 mM IPTG). When the same experiment was performed with plasmids overproducing KorA and IncC, no effect of IncC on KorA repression of trfAp was observed.
Enhancement of KorB repression by IncC1 protein.
Since the apparent IncC counteraction of the KorB effect on pKK1 was unexpected, we used a second reporter system based on transcriptional fusions to the xylE gene. KorB regulates the expression of seven promoters in the RK2 genome by binding to six OBs (binding of KorB to OB10 in the divergent trfAp/trbAp region simultaneously downregulates both promoters [15]). In relation to three of the KorB-repressed promoter regions (trfAp, korAp, and klaAp), OB is located immediately upstream of the −35 sequence (proximal position), while in relation to the other four (trbAp, trbBp, kfrAp, and kcrAp) KorB acts at a distance (OB is located 80 to 180 bp from the tsp). We chose a representative selection of transcriptional fusions between RK2 promoters and the xylE cassette to determine the effect of IncC on KorB-mediated repression. The presence of incC (induced from tacp-incC in pGBT36) in the absence of KorB had no effect on gene expression (data not shown), but it potentiated the effect of KorB repression (expressed from tacp-korB in pDM1.12) with all of the promoters tested (Table 3) and therefore is independent of the position of OB (proximal or distal to promoter sequences). We also carried out the experiment with a range of IPTG concentrations. The results, as presented for trbBp in Fig. 3, show that the stimulation of the korB effect by incC is seen over the whole range of IPTG concentrations tested. We also determined the effect of overproducing IncC2 alone (pGBT302) on the repression by KorB (pDM1.12). The results showed no effect of IncC2 on repression by KorB, suggesting that the N-terminal 105 aa are necessary for the modulation of KorB repressor function by IncC1.
TABLE 3.
Effect of overexpression of incC on repression by korB
| Promoter and KorB operator | xylE reporter plasmid | Locationa | XylE activity (standard units)b for:
|
||
|---|---|---|---|---|---|
| pDM1.1/pGBT30 (−/−) | pDM1.12/pGBT30 (korB/−) | pDM1.12/pGBT36 (korB/incC) | |||
| korAp OB1 | pDM 3.1 | p/u | 0.85 | 0.3 | 0.15 |
| trfAp OB10 | pGBT58 | p/u | 3.12 | 0.25 | 0.1 |
| trfAp-1 OB10 | pGBT70 | p/u | 0.26 | 0.10 | 0.03 |
| trbAp* OB10 | pGBT71 | d/d | 0.09 | <0.01 | <0.01 |
| trbBp OB9 | pGBT63 | d/u | 1.50 | 0.25 | 0.05 |
| kfrAp OB2 | pDM300 | d/u | 0.60 | 0.15 | 0.05 |
Location is indicated as proximal (p [close to]) or distal (d [well removed from]) and upstream (u) or downstream (d).
Activities are representative results from at least three experiments. The effect of pGBT36 (tacp-incC) in the presence of pDM1.1 (tacp control) showed no repression on any of the promoters. The level of IPTG used in this experiment to induce repressor expression was 0.5 mM.
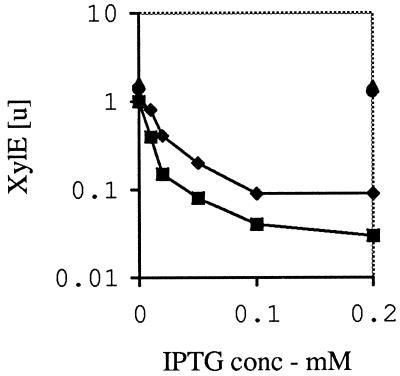
FIG. 3.
Effect of IncC on repression of trbBp by KorB. XylE was assayed as described in Materials and Methods and as presented in Table 3 except that the level of IPTG was varied as shown. The plasmid combinations were as in Table 3, with the addition that pGBT36 was included with pGBT30 so as to have a strain with IncC but without KorB. The controls with neither KorB nor IncC (circles) or just IncC (triangles) were assayed only either without IPTG or at the highest IPTG concentration. KorB alone and KorB plus IncC are indicated by diamonds and squares, respectively. The log scale is used to make the difference between the latter two lines clearer.
IncC potentiates in vitro binding of KorB to DNA.
The above in vivo experiments raised the possibility that IncC directly modulates the binding of KorB to its target DNA. To initiate studies on the effect of IncC in vitro, we produced soluble cell extracts from bacteria with pGBT30 (vector alone) or pGBT36 (vector with tacp-incC) after induction with IPTG and then determined their effect on the retardation of a radioactively labelled DNA fragment with the trfAp/trbAp region whose in vivo activity was described above. Increasing concentrations of KorB, purified as previously described (41), were titrated with a range of concentrations of these extracts. It was clear that while the extracts of bacteria carrying the expression vector without IncC had no effect on the mobility of the labelled fragment, the presence of IncC in cell extracts after the induction of bacteria with pGBT36 promoted retardation when KorB was present, even at concentrations of KorB which showed negligible retardation on their own (data not shown). These results were important since they indicate that native IncC with no modifications at either end does modulate KorB DNA binding activity.
To facilitate the purification, we constructed a plasmid with incC1 modified so that the protein product would have a His6 tail at the N terminus (pCT800). Induction with IPTG gave a clear band corresponding to IncC1 at a level of approximately 1% total soluble protein. After breakage by lysozyme treatment, sonication, and clearing, all of the IncC1 appeared to remain in the supernatant. IncC1 was purified with nickel-agarose as described in Materials and Methods. It eluted over a concentration range from 100 to 150 mM imidazole, yielding fractions that when pooled were calculated to represent more than a 50% yield of the overproduced protein and which was judged to be >95% pure. The identity of the protein was confirmed by N-terminal sequencing.
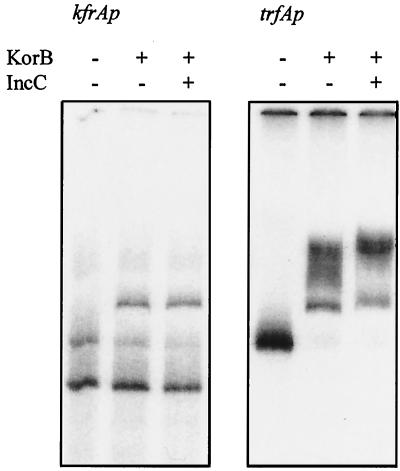
Since His6IncC1 modulated KorB activity in vivo at all of the promoters tested, we selected a further subset of these promoters for study in vitro: trfAp/trbAp, korAp, and kfrAp. We found that these OBs do not have identical affinities for KorB: OB10 binds KorB more strongly than OB1, which in turn binds about 10-fold more strongly than OB2. This lower affinity for OB2 correlates with the single mismatch from the consensus shown by this operator (Table 3). At each of these promoter regions His6IncC1 stimulated the in vitro binding of KorB, whereas it had no effect on the mobility of the fragments when present alone, as illustrated in Fig. 4 for trfAp and korAp. By running this sort of gel over a wide range of concentrations, the Kapp for each of these fragments in the absence or presence of IncC was estimated (Table 4). We decreased the amount of IncC added in order to determine how much was needed to potentiate KorB binding. A Kapp of 100 nM was calculated.
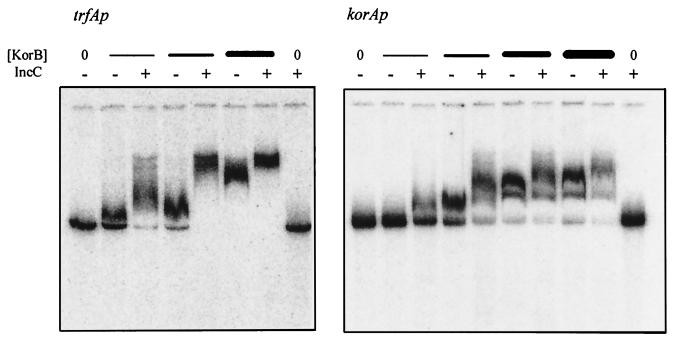
FIG. 4.
Effect of purified IncC on the ability of KorB to retard radioactively labelled fragments with the trfAp and korAp regions. For trfAp/trbAp, the 300-bp region previously described (13) was amplified by PCR and then labelled with [α-32P]ddATP and terminal transferase. For korAp PCR, primers 5′-GGGATCCTCCTGAACTGGCTTTCGG-3′ (RK2 coordinates 59306 to 59325) and 5′-GGGAATTCTTGTTGGGCTGGCAGTGTCG-3′ (RK2 coordinates 59578 to 59597) were used to amplify a 300-bp fragment, which was labelled with [α-32P]ddATP and terminal transferase. The extra non-RK2 bases correspond to the restriction sites added to allow subcloning of the products, and the extra bases were added to allow efficient cutting by BamHI and EcoRI. The fragments were incubated under conditions described previously (13) with the combinations of IncC and KorB indicated. IncC was present at 150 nM when it was added. KorB was added at 2.5, 7.5, 25, and 75 nM (the highest concentration was applied to korAp only).
TABLE 4.
Apparent binding constants (Kapp) for the three KorB operators studied in vitro in the absence or presence of IncC at 150 nM
| Operator (name/location) | Sequence of operator plus flanking sequencesa |
Kappb (nM) for:
|
|
|---|---|---|---|
| KorB | KorB plus IncC | ||
| OB1 (korAp) | ACACCTTTAGCCGCTAAAACTCGTCC | 7.5 | 2.5 |
| OB2 (kfrAp) | GGTTTTTTAGCGGCTGAAGGGCAGGC | 100 | 20 |
| OB10 (trfAp/trbAp) | AGAACTTTAGCGGCTAAAATTTTGCG | 2.5 | <1 |
Kapp was calculated as the protein concentration at which 50% of the radioactivity in the unretarded band has moved to a more slowly moving species as quantified by phosphorimager analysis. The protein concentration was calculated on the basis of KorB as a dimer but IncC as a monomer, since we have no information at present on the oligomeric state of IncC.
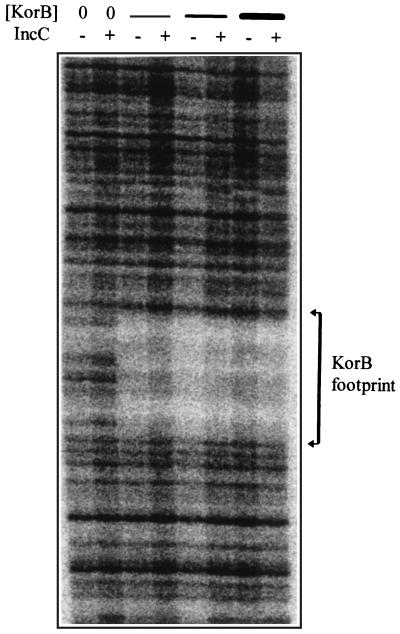
A striking feature of these gel mobility shift experiments was that at both korAp/OB1 and trfAp/OB10 KorB formed what appeared to be higher-order complexes as the KorB concentration was increased (Fig. 4). The primary complex formed with krfAp/OB2 had the same mobility as the primary complex for trfAp/OB10 (Fig. 5), suggesting that this result is due to simple binding of the dominant form of KorB, a tetramer (41), present in the solution. The facts that no higher-order complex was formed on the kfrAp fragment (nor the control fragment lacking an OB) despite the DNA concentration being similar to those of trfAp and korAp and that the higher-order complexes with trfAp/OB10 and korAp/OB1 had different mobilities suggested that these complexes are not simply due to nonspecific binding to the DNA fragments. This conclusion was reinforced by DNase I footprinting at increasing KorB concentrations on end-labelled DNA from the trfAp region (Fig. 6). A clear window of protection by KorB around OB10 was observed, and even at the highest concentration of KorB there was no evidence for nonspecific protection of the flanking DNA sequences.
FIG. 5.
Comparison of the effect of KorB on the retardation of radioactively labelled fragments kfrAp and trfAp regions without or with IncC. For trfAp, the PCR fragment described in the legend to Fig. 4 was used, whereas for kfrAp the 550-bp PpuMI fragment from pDM300 was labelled and recut with BsmAI, which generates two fragments of approximately 300 and 250 bp. KorB was added at 250 nM. IncC was added at 150 nM.
FIG. 6.
Effect of purified IncC on KorB DNase I footprints at trfAp region. Single end-labelled fragments carrying OB10 were incubated in 20-μl volumes with increasing concentrations of KorB, either without or with IncC as described in Materials and Methods. After DNase I treatment and separation of the fragments on a urea–6% PAGE gel, the image was visualized with a phosphorimager. The concentrations of KorB used were 7.5, 25, and 75 nM. IncC was used at 150 nM in all of the samples indicated prior to the addition of DNase I.
IncC potentiated the formation of the higher-order complexes, which formed on the trfAp and korAp fragments. In some gels it appeared that the most slowly moving species formed when IncC was added had a lower mobility than those formed in the absence of IncC (Fig. 4), which might be expected if IncC is physically part of the complexes formed. In the DNase I protection experiments the presence of IncC alone did not give a window of protection, although close comparison of tracks with or without IncC did show some minor differences. The presence of IncC did not significantly modify the KorB footprint. The results therefore suggest that IncC works through KorB and has little, if any, contact with the DNA itself.
DISCUSSION
We have extended our knowledge in this study of the in vivo biological activity associated with the incC gene, and we have shown that purified IncC1 can modulate KorB binding at a representative range of its operators in vitro. This helps to explain its identification as an incompatibility determinant and suggests that it adds an additional level of global regulation to that provided by KorB, which binds to 12 sites on the RK2 genome. Other members of the ParA proteins which share sequence motifs with IncC have been shown to be ATPases (4, 39). Therefore, it is possible that IncC provides a response to the energetic or physiological state of the bacteria and that this is relayed to the KorB regulon. This could be important in controlling expression of the plasmid replication functions as its bacterial host makes the transition from exponential growth to stationary phase and back again.
The function of some ParA and ParB protein pairs, such as those of F and P1, appear to be limited to partitioning, although the ParA protein in these systems does act in an autoregulatory capacity (2, 11, 40). On the other hand some ParB homologues are known exclusively as regulators, for example, as repressors of plasmid-encoded virulence genes (38), where they appear to work without a ParA homologue. The IncC-KorB pair clearly provides more than just active partitioning in the stable inheritance of IncP plasmids, so the IncP system may bridge these two extremes of function. Therefore, the study of IncC-KorB function in the context of the IncP plasmids is likely to provide deeper insights into the biological activities of the ParA-ParB families.
It may be significant that the modulation of korB activity by incC seems to be associated with IncC1 not IncC2. IncC1 has an N-terminal region of approximately 100 aa like ParA of P1 and SopA of F, which are associated with autoregulation (7, 23, 35). While IncC alone does not show any DNA binding activity in vitro or any regulation of gene expression on its own in our assays, it seems likely that this part of the protein is required for its role in modulating gene expression. Our studies on the partitioning activity of incC indicate that IncC2 is sufficient for partitioning activity (21, 42). Therefore this ability to modulate KorB transcriptional repressor activity is not necessary for the partitioning process. This dissection of activities may be of considerable significance, since all of the chromosomal homologues of IncC and some of the plasmid-encoded homologues lack this N-terminal region prior to the ATP binding motif.
It was also significant that in the plasmid displacement assay with low levels of induction, the expression of incC actually appeared to reduce the negative activity of korB. A possible trivial explanation for this is that incC expression reduces effective KorB levels either directly by affecting the availability and/or stability of KorB or indirectly by affecting the expression of korB. We do not think that these explanations are likely because with the same combination of expression plasmids in the promoter-xylE fusion system at similar IPTG concentrations we observed that IncC potentiated repression by KorB. This rules out the possibility that KorB levels have gone down or that KorB is sequestered into a less-active form. An alternative hypothesis is that at low levels IncC and KorB can form a complex on the DNA which counterbalances its inhibitory effect on transcription, possibly by giving better-than-random segregation due to provision of an active partitioning activity. This would be very interesting because it would suggest that OB10 could simultaneously participate in the regulation of trfA gene expression and active partitioning, a situation which would have an attractive symmetry to it since trfA is required for replication. While recent studies with a heterologous replicon and the central control region (42) indicate that OB3 has centromere-like activity, it is possible that a number of individual OBs could have this activity if present alone or in different contexts. Our current work on the sequence requirements for the cis-acting sequences which are necessary for centromere-like function should establish whether this explanation is plausible.
In studying the effect of IncC1 on binding of KorB, we made a number of novel observations. First, we found that there are significant differences in the affinity of KorB for different operators, suggesting a hierarchy of binding sites. These differences were not noticed when studies were first performed with purified KorB (3, 41), although reexamination of the data presented in those earlier studies indicates that our observations here do not contradict those studies. It will be important therefore to test all of the other operators to define their relative affinities and thus determine which ones are likely to be occupied first as the concentration of KorB rises in the cell. Second, we observed the formation of higher-order complexes of KorB-DNA at both OB1 (korAp) and OB10 (trfAp). Such complexes were not observed previously (3, 41), probably because of gel conditions. The absence of such complexes with OB2 appears to correlate with its lower affinity for KorB. DNase I footprinting indicated that the complexes are not the result of coating the DNA, since the footprint remains sharply focused around OB10. The nature of these higher-order complexes will be addressed in a separate paper. Third, we observed that IncC favored the formation of all observed KorB-DNA complexes, even those at OB2 where it does not form higher-order complexes. Since IncC itself does not cause any retardation of the fragments studied and does not show any change in the KorB DNase I footprint, it seems unlikely that IncC is making contact with an extended segment of DNA, although we cannot rule out small yet significant contacts. Even if these contacts do occur, the data suggest very strongly that IncC acts through KorB. We propose that IncC interacts with KorB and stabilizes the primary KorB-DNA complexes. The molecular details of these interactions will be explored in future work.
ACKNOWLEDGMENTS
G.J.-B. was supported by a project grant from the MRC (G9112613CB). G.J.-B. and K.K. were supported by a project grant from The Wellcome Trust (048040/Z/96). Automated DNA sequencing was performed by AltaBioscience with an ABI373 machine purchased with a grant from The Wellcome Trust (038654/Z/93). The phosphorimager was purchased by grants from The Wellcome Trust (037160/Z/92) and the MRC (G9216078MB).
REFERENCES
- 1.Abeles A L, Reaves L D, Austin S J. Protein-DNA interactions in regulation of P1 plasmid replication. J Bacteriol. 1989;171:43–52. doi: 10.1128/jb.171.1.43-52.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Austin S. Plasmid partitioning. Plasmid. 1988;20:1–9. doi: 10.1016/0147-619x(88)90001-7. [DOI] [PubMed] [Google Scholar]
- 3.Balzer D, Ziegelin G, Pansegrau W, Kruft V, Lanka E. KorB protein of promiscuous plasmid RK2 recognizes inverted sequence repetitions in regions essential for conjugative transfer. Nucleic Acids Res. 1992;20:1851–1858. doi: 10.1093/nar/20.8.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis M A, Martin K, Austin S. Biochemical activities of the ParA protein of the P1 plasmid. Mol Microbiol. 1992;6:1141–1147. doi: 10.1111/j.1365-2958.1992.tb01552.x. [DOI] [PubMed] [Google Scholar]
- 5.Davis M A, Radnedge L, Martin K A, Hayes F, Austin S J. The P1 ParA protein and its ATPase activity play a direct role in the segregation of plasmid copies to daughter cells. Mol Microbiol. 1996;21:1029–1036. doi: 10.1046/j.1365-2958.1996.721423.x. [DOI] [PubMed] [Google Scholar]
- 6.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–399. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman S A, Austin S J. The P1 plasmid-partition system synthesizes two essential proteins from an autoregulated operon. Plasmid. 1988;19:103–112. doi: 10.1016/0147-619x(88)90049-2. [DOI] [PubMed] [Google Scholar]
- 8.Glaser P, Sharpe M, Raether B, Perego M, Ohlsen K, Errington J. Dynamic mitotic-like behavior of a bacterial protein required for accurate chromosome partitioning. Genes Dev. 1997;11:1160–1168. doi: 10.1101/gad.11.9.1160. [DOI] [PubMed] [Google Scholar]
- 9.Gornall A G, Bardwell C J, David M M. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177:751–766. [PubMed] [Google Scholar]
- 10.Grinsted J, Bennett P M, Richmond M H. A restriction enzyme map of R-plasmid RP1. Plasmid. 1977;1:34–37. doi: 10.1016/0147-619x(77)90006-3. [DOI] [PubMed] [Google Scholar]
- 11.Hiraga S. Chromosome and plasmid partitioning in E. coli. Annu Rev Biochem. 1992;61:283–306. doi: 10.1146/annurev.bi.61.070192.001435. [DOI] [PubMed] [Google Scholar]
- 12.Jagura-Burdzy G, Thomas C M. Crosstalk between plasmid vegetative replication and conjugative transfer: repression of the trfA operon by trbA of broad host range plasmid RK2. Nucleic Acids Res. 1992;20:3939–3944. doi: 10.1093/nar/20.15.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jagura-Burdzy G, Thomas C M. KorA protein of promiscuous plasmid RK2 controls a transcriptional switch between divergent operons for plasmid replication and conjugative transfer. Proc Natl Acad Sci USA. 1994;91:10571–10575. doi: 10.1073/pnas.91.22.10571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jagura-Burdzy G, Thomas C M. Purification of KorA protein from broad-host-range plasmid RK2: definition of a hierarchy of operators. J Mol Biol. 1995;253:39–50. doi: 10.1006/jmbi.1995.0534. [DOI] [PubMed] [Google Scholar]
- 15.Jagura-Burdzy G, Thomas C M. Dissection of the switch between genes for replication and transfer of promiscuous plasmid RK2: basis of the dominance of trfAp over trbAp and specificity for KorA in controlling the switch. J Mol Biol. 1997;265:507–518. doi: 10.1006/jmbi.1996.0747. [DOI] [PubMed] [Google Scholar]
- 16.Jagura-Burdzy G, Ibbotson J P, Thomas C M. The korF region of broad-host-range plasmid RK2 encodes two polypeptides with transcriptional repressor activity. J Bacteriol. 1991;173:826–833. doi: 10.1128/jb.173.2.826-833.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahn M R, Kolter R, Thomas C M, Figurski D, Meyer R, Remault E, Helsinki D R. Plasmid cloning vehicles derived from plasmids ColE1, R6K and RK2. Methods Enzymol. 1979;68:268–280. doi: 10.1016/0076-6879(79)68019-9. [DOI] [PubMed] [Google Scholar]
- 18.Kornacki J A, Balderes P J, Figurski D H. Nucleotide sequence of korB, a replication control gene of broad host range plasmid RK2. J Mol Biol. 1987;198:211–222. doi: 10.1016/0022-2836(87)90307-x. [DOI] [PubMed] [Google Scholar]
- 19.Kostelidou K, Jagura-Burdzy G, Thomas C M. Mutational analysis of the global regulator KorA of broad-host-range plasmid RK2. J Mol Biol. 1998;181:453–463. doi: 10.1006/jmbi.1998.1956. [DOI] [PubMed] [Google Scholar]
- 20.Lin D, Levin P, Grossman A. Bipolar localization of a chromosome partition protein in Bacillus subtilis. Proc Natl Acad Sci USA. 1997;94:4721–4726. doi: 10.1073/pnas.94.9.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macartney D P, Williams D R, Stafford T, Thomas C M. Divergence and conservation of the partitioning and global regulation functions in the central control region of the IncP plasmids RK2 and R751. Microbiology. 1997;143:2167–2177. doi: 10.1099/00221287-143-7-2167. [DOI] [PubMed] [Google Scholar]
- 22.Mohl D A, Gober J W. Cell cycle-dependent polar localisation of chromosome partitioning proteins in Caulobacter crescentus. Cell. 1997;88:675–684. doi: 10.1016/s0092-8674(00)81910-8. [DOI] [PubMed] [Google Scholar]
- 23.Mori H, Kondo A, Oshima A, Ogura T, Hiraga S. Structure and function of the F plasmid genes essential for partitioning. J Mol Biol. 1986;192:1–15. doi: 10.1016/0022-2836(86)90459-6. [DOI] [PubMed] [Google Scholar]
- 24.Mori H, Mori Y, Ichinose C, Niki H, Ogura T, Kato A, Hiraga S. Purification and characterization of SopA and SopB proteins essential for F plasmid partitioning. J Biol Chem. 1989;264:15535–15541. [PubMed] [Google Scholar]
- 25.Motallebi-Veshareh M, Rouch D A, Thomas C M. A family of ATPases involved in active partitioning of diverse bacterial plasmids. Mol Microbiol. 1990;4:1455–1463. doi: 10.1111/j.1365-2958.1990.tb02056.x. [DOI] [PubMed] [Google Scholar]
- 26.Motallebi-Veshareh M, Balzer D, Lanka E, Jagura-Burdzy G, Thomas C M. Conjugative transfer functions of broad-host-range plasmid RK2 are coregulated with replication. Mol Microbiol. 1992;6:907–920. doi: 10.1111/j.1365-2958.1992.tb01541.x. [DOI] [PubMed] [Google Scholar]
- 27.Mullis K, Faloona F, Scharf S, Saiki R, Horn G, Erlich H. Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harbor Symp Quant Biol. 1986;51:263–273. doi: 10.1101/sqb.1986.051.01.032. [DOI] [PubMed] [Google Scholar]
- 28.Pansegrau W, Lanka E, Barth P T, Figurski D H, Guiney D G, Haas D, Helinski D R, Schwab H, Stanisich V A, Thomas C M. Complete sequence of Birmingham IncPα plasmids. Compilation and comparative analysis. J Mol Biol. 1994;239:623–663. doi: 10.1006/jmbi.1994.1404. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 30.Sharpe M, Errington J. The Bacillus subtilis soj-spo0J locus is required for a centromere-like function involved in prespore chromosome partitioning. Mol Microbiol. 1996;21:501–509. doi: 10.1111/j.1365-2958.1996.tb02559.x. [DOI] [PubMed] [Google Scholar]
- 31.Smith C A, Shingler V, Thomas C M. The trfA and trfB promoter regions of broad host range plasmid RK2 share common regulatory sequences. Nucleic Acids Res. 1984;12:36119–36130. doi: 10.1093/nar/12.8.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Theophilus B D M, Thomas C M. Nucleotide sequence of the key transcriptional repressor gene korB which plays a key role in regulation of the copy number of broad host range plasmid RK2. Nucleic Acids Res. 1987;15:7443–7450. doi: 10.1093/nar/15.18.7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas C M. Evidence for the involvement of the incC locus of broad host range plasmid RK2 in plasmid maintenance. Plasmid. 1986;16:15–29. doi: 10.1016/0147-619x(86)90075-2. [DOI] [PubMed] [Google Scholar]
- 34.Thomas C M, Hussain A A K. The korB gene of broad host range plasmid RK2 is a major copy number control element which may act together with trfB by limiting trfA expression. EMBO J. 1984;3:1513–1519. doi: 10.1002/j.1460-2075.1984.tb02004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas C M, Smith C A. The trfB region of broad host range plasmid RK2: the nucleotide sequence reveals incC and key regulatory gene trfB/korA/korD as overlapping genes. Nucleic Acids Res. 1986;14:4453–4469. doi: 10.1093/nar/14.11.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas C M, Smith C A. Incompatibility group P plasmids: genetics, evolution and use in genetic manipulation. Annu Rev Microbiol. 1987;41:77–101. doi: 10.1146/annurev.mi.41.100187.000453. [DOI] [PubMed] [Google Scholar]
- 37.Thomson V J, Jovanovic O S, Pohlman R F, Chang C-H, Figurski D H. Structure, function, and regulation of the kilB locus of promiscuous plasmid RK2. J Bacteriol. 1993;175:2423–2435. doi: 10.1128/jb.175.8.2423-2435.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watanabe H, Arakawa E, Ito K-I, Kato J-I, Nakamura A. Genetic analysis of an invasion region by use of a Tn3-lac transposon and identification of a second positive regulator gene, invE, for cell invasion of Shigella sonnei: significant homology of InvE with ParB of plasmid P1. J Bacteriol. 1990;172:619–629. doi: 10.1128/jb.172.2.619-629.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watanabe E, Wachi M, Yamasaki M, Nagai K. ATPase activity of SopA, a protein essential for active partitioning of F plasmid. Mol Gen Genet. 1992;234:249–352. doi: 10.1007/BF00538693. [DOI] [PubMed] [Google Scholar]
- 40.Williams D R, Thomas C M. Active partitioning of bacterial plasmids. J Gen Microbiol. 1992;138:1–16. doi: 10.1099/00221287-138-1-1. [DOI] [PubMed] [Google Scholar]
- 41.Williams D R, Motallebi-Veshareh M, Thomas C M. Multifunctional repressor KorB can block transcription by preventing isomerization of RNA polymerase-promoter complexes. Nucleic Acids Res. 1993;21:1141–1148. doi: 10.1093/nar/21.5.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams D R, Macartney D M, Thomas C M. The partitioning activity of the RK2 central control region requires only incC, korB and KorB binding site OB3 but other KorB binding sites form destabilizing complexes in the absence of OB3. Microbiology. 1998;144:3369–3378. doi: 10.1099/00221287-144-12-3369. [DOI] [PubMed] [Google Scholar]
- 43.Zukowski M M, Gaffney D F, Speck D, Kauffman M, Findeli A, Wisecup A, Lecoq J P. Chromogenic identification of genetic regulatory signals in Bacillus subtilis based on expression of a cloned Pseudomonas gene. Proc Natl Acad Sci USA. 1983;80:1101–1105. doi: 10.1073/pnas.80.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]