Abstract
Although tissue-resident-memory T (TRM) cells, a recently identified non-circulating memory T cell population, play a crucial role in mediating local immune responses and protect against pathogens upon local reinfection, the composition, effector function, and specificity of TRM cells in the kidney and their relevance for chronic kidney disease remain unknown. In this study, we found that renal tissue displayed high abundance of tissue-resident lymphocytes, and the proportion of CD8+ TRM cells was significantly increased in the kidney from patients and mice with focal segmental glomerulosclerosis (FSGS), diabetic kidney disease (DKD), and lupus nephritis (LN). Mechanistically, IL-15 significantly promoted CD8+ TRM cell formation and activation, thereby promoting podocyte injury and glomerulosclerosis. Interestingly, Sparsentan, the dual angiotensin II (Ang II) receptor and endothelin type A receptor antagonist, can also reduce TRM cell responses by intervening IL-15 signaling, exploring its new pharmacological functions. Mechanistically, Sparsentan inhibited Ang II or endothelin-1 (ET-1)-mediated IL-15 signaling, thereby further regulating renal CD8+ TRM cell fates. Collectively, our studies provide direct evidence for the pivotal role of renal CD8+ TRM cells in podocyte injury and further strengthen that targeting TRM cells represents a novel therapeutic strategy for patients with glomerular diseases.
Keywords: TRM cells, podocyte injury, IL-15, Sparsentan, FSGS, diabetes
Graphical abstract
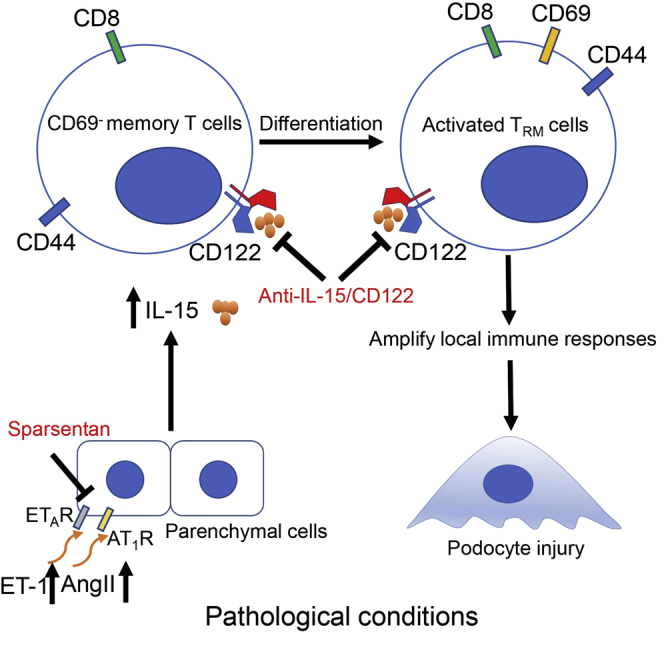
Yi and colleagues provide direct evidence for the pivotal role of renal CD8+ TRM cells in glomerulosclerosis and podocyte injury. Pharmacological targeting of IL-15-mediated CD8+ TRM cell formation and activation at multiple levels may represent a novel approach for the treatment of glomerular diseases.
Introduction
Tissue-resident-memory T (TRM) cells, a recently identified non-circulating memory T cell population mainly located in barrier tissues at interfaces with the environment, play a crucial role in mediating potent local immune responses and provide a long-term localized defense against pathogens.1, 2, 3 The formation and maintenance of TRM cell are influenced by numerous factors, including inflammation, antigen triggering, and tissue-specific cues.4,5 Emerging evidence has revealed that TRM cells are also present in the kidney and other non-barrier tissues.6 Recent studies have found a marked increase in CD4+ TRM cells in kidney biopsies from patients with antineutrophil cytoplasmic antibody (ANCA)-dependent glomerulonephritis and further confirmed that infection-induced CD4+ TRM cells may rapidly react to local inflammatory cytokines and aggravate renal autoimmune diseases, supporting a new concept for the predisposing role of microbial infections in aggravating autoimmune diseases.7 However, most studies so far mainly focus on TRM cells and their relevance for immediate protection against pathogens upon local reinfection; the composition, localization, effector function, and specificity of TRM cells in the kidney and their relevance for chronic kidney disease (CKD) remain unknown.
Podocytes are highly differentiated epithelial cells and are essential for the formation and maintenance of the glomerular filtration barrier.8 Despite compelling evidence identifying podocyte injury as the key mediator in the pathogenesis of glomerular diseases, such as focal segmental glomerulosclerosis (FSGS), minimal change disease (MCD), diabetic kidney disease (DKD), and lupus nephritis (LN), the delivery of efficient therapies targeting podocytes is still a great challenge. On the other hand, podocyte injuries are closely associated with disruption of immune homeostasis.9,10 Podocytes share many elements of the innate and adaptive immune system. They not only produce and express complement components and receptors but also express both class I and II major histocompatibility complex (MHC) molecules and co-stimulatory molecules that are involved in local immune responses. Recent studies have reported that antigen presentation by podocytes under inflammatory conditions plays an important role in activating T cell immune responses and facilitating immune-mediated glomerular disease development. Meanwhile, inflammatory cytokines produced by T cells, such as interferon-γ (IFN-γ), can also induce the expression of MHC-I, MHC-II, CD80, and CD86 on podocyte surface, which promotes podocyte present antigen and podocyte apoptosis.10 Therefore, a comprehensive understanding of the local immune responses associated with the activation and functions of TRM cells in the kidney and their relevance of podocyte injury is necessary for the development of tissue-resident, cell-based immunotherapies for glomerular diseases.
In this study, we found that renal tissue displayed high abundance of tissue-resident lymphocytes, and the proportion of CD8+ TRM cells was significantly increased in the kidney from patients and mice with glomerular diseases. Mechanistically, interleukin-15 (IL-15) significantly promoted CD8+ TRM cell formation and activation, thereby promoting podocyte injury and glomerulosclerosis. Interestingly, Sparsentan, the dual angiotensin II receptor and endothelin type A receptor antagonist, can also reduce TRM cell responses by intervening IL-15 signaling, exploring its new pharmacological functions. Collectively, our studies suggest pharmacological targeting of IL-15-mediated CD8+ TRM cell formation and activation at multiple levels may provide a novel approach for the treatment of glomerular diseases.
Results
The kidney displays high abundance of TRM cells
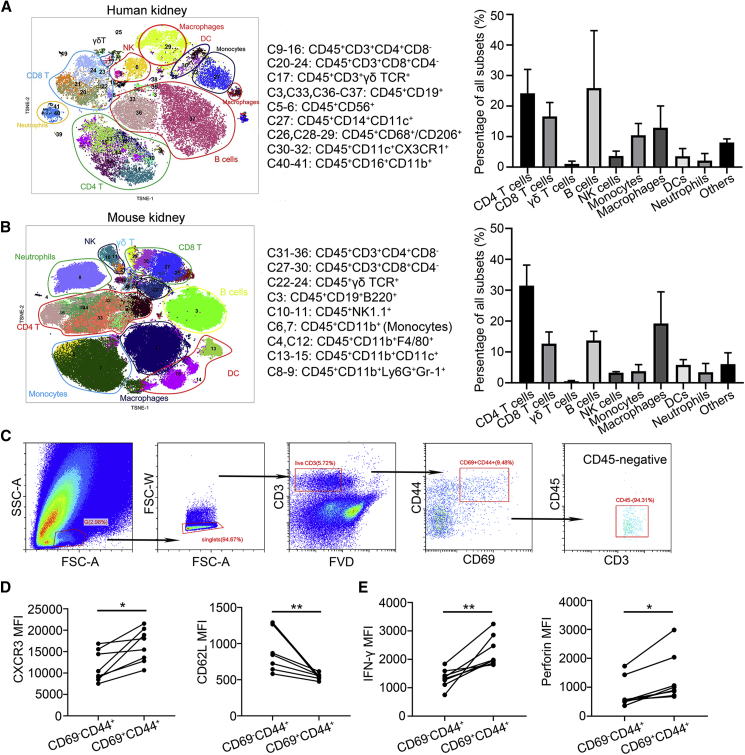
We performed a 42-antibody panel for mass cytometry to build a composite of human (Figures 1A and S1A) and murine (Figures 1B and S1B) renal leukocytes in the steady state. The immune landscape indicated that T lymphocytes were enriched in the kidney. CD69 expression on T cells is indicative of a tissue-resident phenotype11 and almost all of renal CD69+ T cells exhibited memory cell phenotypes: CD45RA− CCR7− in human (Figure S1C) and CD44+ in mouse (Figure S1D). Next, we examined whether these renal CD69+CD44+ T cells exhibited tissue-resident features in mouse. In vivo labeling assay showed that renal CD69+CD44+ T cells were protected from intravenous (i.v.) injection antibody labeling (CD45−) and they were bona fide resident population (Figure 1C). Phenotypic analysis showed that renal CD69+CD44+ T cells highly expressed tissue homing chemokine receptor CXCR3 but lowly expressed lymph node homing receptor CD62L (Figure 1D). Furthermore, they produced more effector molecular IFN-γ and perforin than renal CD69−CD44+ T cells (Figure 1E). Collectively, these data confirmed that the kidney harbored a large number of TRM cells in human and mouse under homeostatic conditions.
Figure 1.
The kidney displays high abundance of TRM cells
See also Figure S1. (A) Unsupervised cell cluster detection and quantification by t-distributed stochastic neighbor embedding (TSNE) and cytometry by time-of-flight (CyTOF) cluster detection algorithm on renal CD45+ cells from normal human kidneys (n = 6). (B) Unsupervised cell cluster detection and quantification by TSNE and CyTOF cluster detection algorithm on renal CD45+ cells from normal mouse kidneys (n = 6). (C) Gating strategy for renal intravascular staining after intravenous (i.v.) injection of allophycocyanin (APC) conjugated CD45 antibody. (D) The median fluorescence intensity (MFI) of CXCR3 and CD62L in renal CD69−CD44+ and CD69+CD44+ T cells individually (n = 8). (E) The MFI of IFN-γ and perforin in renal CD69−CD44+ and CD69+CD44+ T cells individually (n = 8). Data are represented as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01.
The proportion of CD8+ TRM cells is significantly increased in mice and patients with glomerular diseases
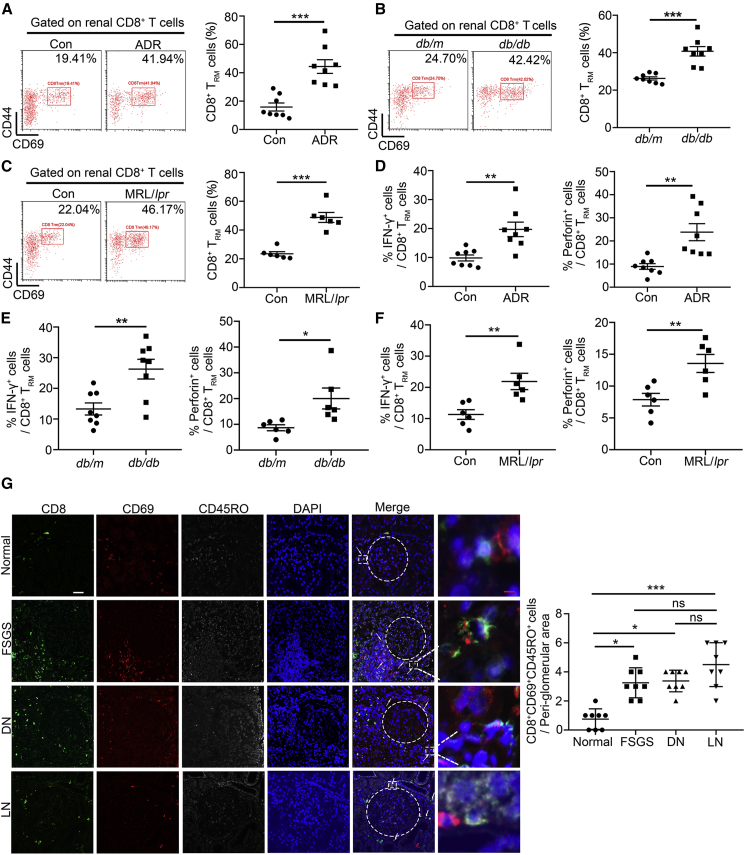
We found that the proportion of CD8+ TRM cells was significantly increased in the kidney in independent mouse models for different glomerular diseases, including Adriamycin (ADR)-induced FSGS mice (Figure 2A), db/db mice (30 weeks of age, a spontaneous type 2 diabetes mellitus model, Figure 2B), and MRL/lpr mice (a mouse model of systemic lupus erythematosus, Figure 2C).12 Among these animal models, the number of CD8+ TRM cells had no significant difference. Meanwhile, the proportion of CD4+ TRM cells in MRL/lpr mice was also increased (Figure S2C), but there were no changes in mice with ADR treatment and db/db mice compared with their normal controls (Figures S2A and S2B). We further measured cytokine production in isolated renal CD8+ TRM cells from mice under these pathological conditions and found that CD8+ TRM cells expressed more IFN-γ and perforin (Figures 2D–2F), indicating that TRM cells were activated.
Figure 2.
The proportion of CD8+ TRM cells is significantly increased in mice and patients with glomerular diseases
See also Figure S2. (A–C) Representative flow cytometric analyses and quantification of renal CD8+ TRM (CD8+CD69+CD44+) cells in ADR-treated mice (A), db/db mice (B), MRL/lpr mice, (C) and their controls (n = 8). (D) The frequency of IFN-γ and perforin in renal CD8+ TRM cells in control and ADR-treated mice (n = 8). (E) The frequency of IFN-γ (n = 8) and perforin (n = 6) in renal CD8+ TRM cells in db/m and db/db mice. (F) The frequency of IFN-γ and perforin in renal CD8+ TRM cells in control and MRL/lpr mice (n = 6). (G) Representative immunofluorescence staining for CD8 (green), CD69 (red), and CD45RO (gray) in human renal tissues from normal, subjects with FSGS diabetic nephropathy (DN) and LN. Left: representative images, white arrows highlight CD8+ TRM cells (CD8+CD69+CD45RO+); scale bars: white, 70 μm; red 5, μm; right: quantification of CD8+ TRM cells surrounding the glomeruli per high power field (HPF, n = 8). Data are represented as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ns, no significance.
Importantly, immunofluorescent (IF) results showed that the number of CD8+ TRM cells was increased in the tubulointerstitium surrounding the glomeruli in renal biopsies from patients with FSGS, DKD, and LN (Figure 2G). The number of CD4+ TRM cells in renal biopsies from patients with LN was also increased, but there were no changes from patients with FSGS and DKD compared with healthy controls (Figure S2D). Together, our results indicate that CD8+ TRM cells may play a universal role in different glomerular diseases.
The increased CD8+ TRM cells are derived from peripheric CD69− memory T cells
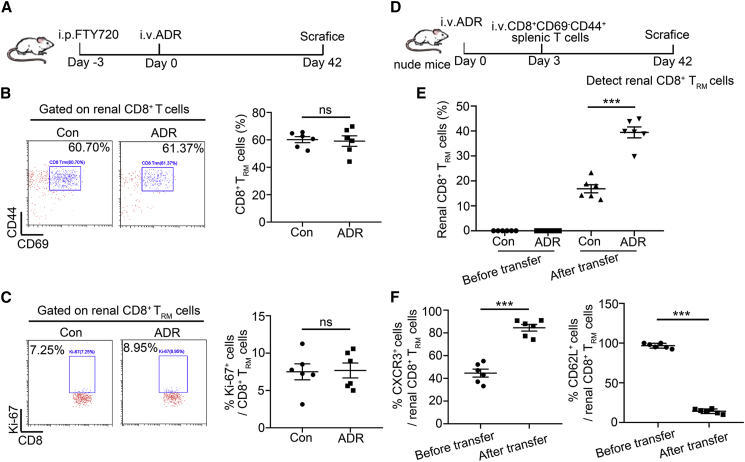
To identify whether renal TRM cells are derived from circulating CD69− memory T cells or the local proliferation of TRM cells under pathological conditions, FTY720, a sphingosine-1-phosphate (S1P) inhibitor that prevents lymphocyte circulation,13 was used for the pretreatment of mice (Figure 3A). There were no more proliferating renal TRM cells in mice with ADR treatment (Figures 3B and 3C). Next, we further determined whether peripheric CD69− memory T cells differentiated into TRM cells. We sorted CD8+CD69−CD44+ T cells from normal mice and then transfused them to normal or ADR-treated nude mice (Figure 3D). Peripheral-infiltrating CD8+ T cells were phenotyped by flow cytometry after 6 weeks of ADR treatment. It was found that more CD69+ T cells were present in the kidney from ADR-treated transfused nude mice (Figure 3E) and highly expressed TRM cells markers, such as CXCR3, and lowly expressed CD62L (Figure 3F). Therefore, we concluded that CD8+ TRM cells are derived from peripheral-infiltrating CD69− memory T cells under pathological conditions.
Figure 3.
The increased CD8+ TRM cells are derived from peripheric CD69− memory T cells
(A) A schematic diagram showing the usage of FTY720 in ADR-treated mice. (B) Representative flow cytometric analyses and quantification of renal CD8+ TRM cells (n = 6). (C) Representative flow cytometric analyses and the frequency of Ki-67+ cells in renal CD8+ TRM cells (n = 6). (D) A schematic diagram showing that nude mice were adoptive transferred with splenic CD8+CD69−CD44+ T cells after ADR injection. Mice were euthanized at day 42. (E) The percentage of renal CD8+ TRM cells from control or ADR-treated transfused nude mice (n = 6). (F) The percentage of CXCR3+ and CD62L+ cells in splenic CD8+CD69−CD44+ T cells (before transfer) and renal CD8+ TRM cells (after transfer, n = 6). Data are represented as mean ± SEM. ∗∗∗p < 0.001; ns, no significance.
IL-15 promotes CD8+ TRM cell formation and activation in the kidney
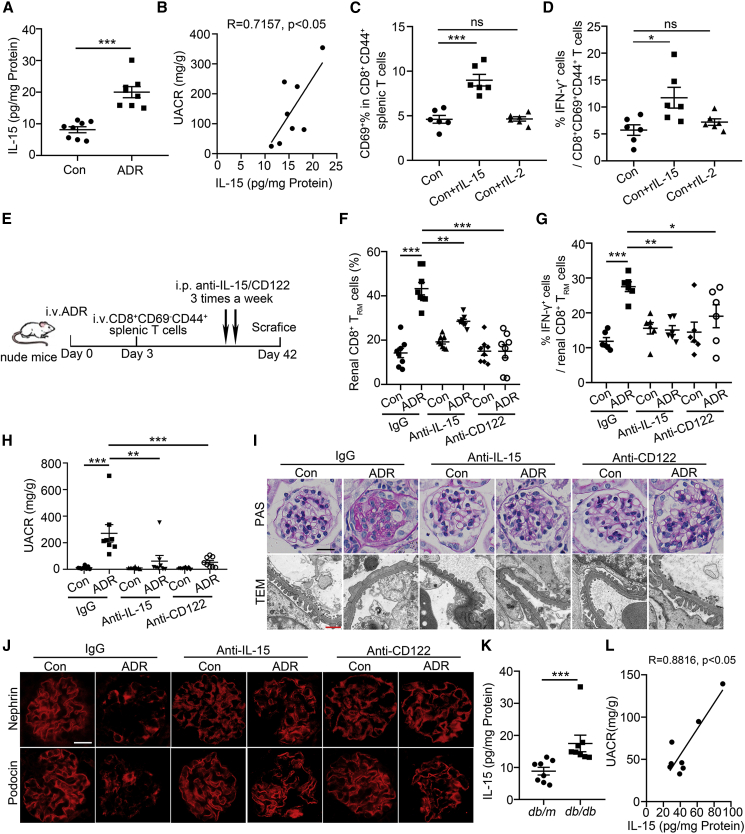
We found that the level of IL-15 in the kidney was significantly increased in ADR-treated mice (Figure 4A) and positively correlated with the urine-albumin-to-creatinine ratio (UACR, Figure 4B). We further investigated whether IL-15 also regulates CD8+ TRM cell formation and activation as reported.14 We isolated splenic mononuclear cells and sequential exposure to recombinant IL-15 (rIL-15). It was found that rIL-15 induced CD69+ T cell development. As a negative control, IL-2, one cytokine that shares the β-chain with the receptor for IL-1515 had no effects on CD69 expression (Figure 4C). Furthermore, IL-15 could directly promote IFN-γ production in CD8+ TRM cells (Figure 4D), indicating that IL-15 can activate renal CD8+ TRM cells.
Figure 4.
IL-15 signaling blockade alleviates glomerulosclerosis and podocyte injury by inhibiting renal CD8+ TRM cells formation and activation
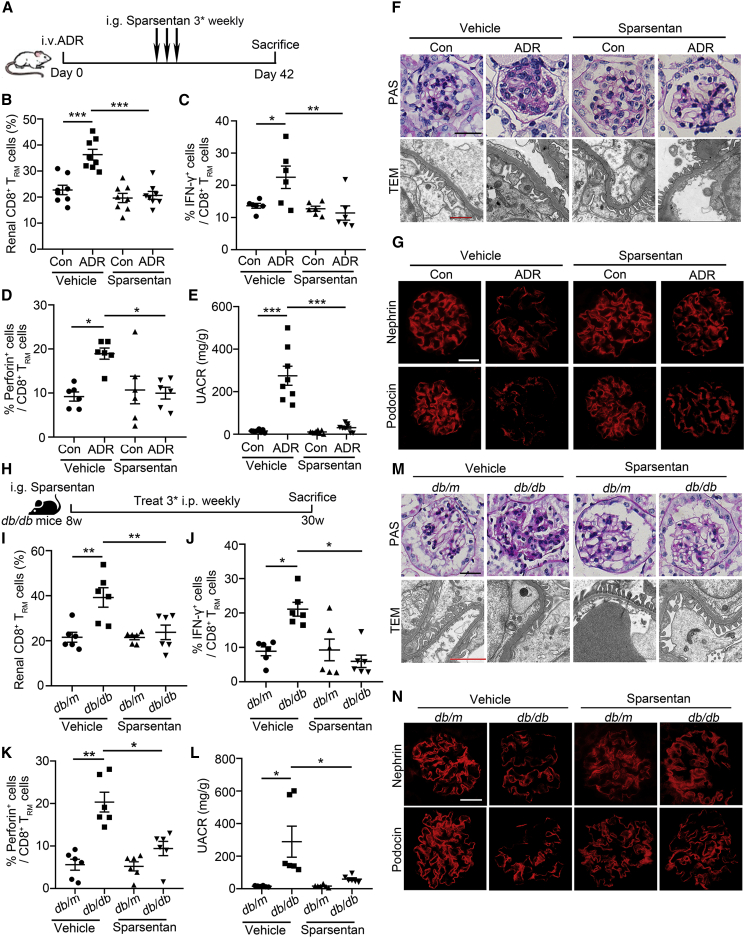
See also Figures S3–S5. (A) The level of IL-15 in renal cortex of ADR-treated mice (n = 8). (B) Correlation between IL-15 and urine-albumin-to-creatinine ratio (UACR) in ADR-treated mice (n = 8). (C) The percentage of CD69+ in splenic CD8+CD44+ T cells after 3-day culture in the presence of rIL-15 (50 ng/mL) or rIL-2 (50 ng/mL, n = 6). (D) The frequency of IFN-γ in splenic CD8+CD69+CD44+ T cells after 3-day culture in the presence of rIL-15 (50 ng/mL) or rIL-2 (50 ng/mL, n = 6). (E) A schematic diagram showing anti-IL-15/CD122 treatment in transfused nude mice after ADR injection. (F) The percentage of renal CD8+ TRM cells in IgG- or anti-IL-15/CD122-treated control or ADR-treated transfused nude mice (n = 8). (G) The frequency of IFN-γ in renal CD8+ TRM cells from IgG- or anti-IL-15/CD122-treated control or ADR-treated transfused nude mice (n = 6). (H) UACR in mice (n = 8). (I) Morphological examinations of glomerular changes by periodic acid–Schiff (PAS) and transmission electron microscopy (TEM) analyses in mice. Scale bars: black, 20 μm; red, 1 μm. (J) Representative immunofluorescence images about nephrin and podocin expressions in glomeruli from mice. Scale bar, 20 μm. (K) The level of IL-15 in renal cortex of db/db mice (n = 8). (L) Correlation between IL-15 and UACR in db/db mice. R, Pearson correlation coefficient (n = 8). Data are represented as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
IL-15 signaling blockade alleviates podocyte injury and glomerulosclerosis by inhibiting renal CD8+ TRM cell formation and activation
Considering that CD122 is an integral part of the receptor complex for IL-15,16 CD122 was highly expressed in renal CD8+ TRM cells (Figure S3A), and anti-CD122 antibody inhibited IL-15-induced CD8+ TRM cell formation and activation in vitro (Figure S3B), we therefore injected anti-IL-15 or anti-CD122 antibody to ADR-treated nude mice, which were transfused CD8+CD69−CD44+ T cells (Figure 4E). Anti-IL-15 or anti-CD122-antibody treatment significantly inhibited renal CD8+ TRM cell formation (Figure 4F) and activation (Figure 4G). Moreover, anti-IL-15- or anti-CD122-antibody-treated mice showed lower UACR (Figure 4H) and fewer glomerular and podocyte injuries (Figure 4I) compared with their controls. In addition, IL-15 signaling blockade recovered the expressions of nephrin and podocin (Figure 4J).
Furthermore, in db/db mice, the level of IL-15 was also increased (Figure 4K) and positively correlated with the proteinuria (Figure 4L). Consistently, in anti-IL-15- or anti-CD122-antibody-treated db/db mice (Figure S4A), the formation and activation of renal CD8+ TRM cell were markedly inhibited (Figures S4B and S4C). Lower urinary albumin excretion (Figure S4D) and less glomerulosclerosis and podocyte injury were also observed in db/db mice after anti-IL-15 or anti-CD122 antibody treatment (Figures S4E and S4F).
As a pleiotropic cytokine, IL-15 can also induce the proliferation of natural killer (NK) cells.17 To investigate whether blocking of IL-15 signaling could affect NK cells and thereby alleviate podocyte and glomerular injuries, we injected db/db mice or ADR-induced mice with anti-NK1.1 antibody for three weeks and found that podocyte and glomerular injuries were not alleviated in mice with deficiency of NK cells, as shown in Figure S5, indicating that blocking of IL-15 signaling protects against podocyte and glomerular injuries, which may not be relevant to NK cells under these pathogenetic conditions.
Sparsentan inhibits CD8+ TRM cell formation and activation
Sparsentan is a first-in-class, orally active, selective, and dual antagonist of the angiotensin II type 1 (AT1) receptor and the endothelin type A (ETA) receptor.18,19 Interestingly, we occasionally found that Sparsentan (Figure 5A) could also reduce the number of renal CD8+ TRM cells in mice with ADR treatment (Figure 5B). Moreover, Sparsentan reduced the production of IFN-γ (Figure 5C) and perforin (Figure 5D) in isolated renal CD8+ TRM cells and alleviated proteinuria (Figure 5E), mesangial matrix expansion (Figure 5F), and podocyte injury (Figure 5G). To further confirm the broad implications of Sparsentan in conferring renal protection, we sought to investigate whether Sparsentan also has beneficial effects in db/db mice (Figure 5H). Consistently, Sparsentan inhibited CD8+ TRM cell formation (Figure 5I) and activation (Figures 5J and 5K) and alleviated proteinuria (Figure 5L) and podocyte injury (Figures 5M–5N). These data provide direct evidence for the new pharmacological functions of Sparsentan. Targeting CD8+ TRM cells may further strengthen therapeutic effects of Sparsentan besides directly acting on podocytes.
Figure 5.
Sparsentan inhibits CD8+ TRM cell formation and activation and alleviates podocyte injury in ADR-treated mice and db/db mice
(A) A schematic diagram showing the treatment of Sparsentan in ADR-treated mice. (B) The percentage of renal CD8+ TRM cells in mice (n = 8). (C and D) The frequency of IFN-γ (C) and perforin (D) in renal CD8+ TRM cells (n = 6). (E) Urinary albumin-to-creatinine ratio (UACR) in mice (n = 8). (F) Morphological examinations of glomerular changes by periodic acid–Schiff (PAS) and transmission electron microscopy (TEM) analyses in mice. Scale bars: black 20, μm; red, 1 μm. (G) Representative immunofluorescence images about nephrin and podocin expressions in glomeruli from mice. Scale bar, 20 μm. (H) A schematic diagram showing the treatment of Sparsentan in db/db mice. (I) The percentage of renal CD8+ TRM cells in mice (n = 6). (J and K) The frequency of IFN-γ (J) and perforin (K) in renal CD8+ TRM cells (n = 6). (L) UACR in mice (n = 6). (M) Morphological examinations of glomerular changes by PAS and TEM analyses in mice. Scale bars: black, 20 μm; red, 1 μm. (N) Representative immunofluorescence images about nephrin and podocin expressions in glomeruli from mice. Scale bar, 20 μm. Data are represented as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Angiotensin II and endothelin-1 induce IL-15 signaling
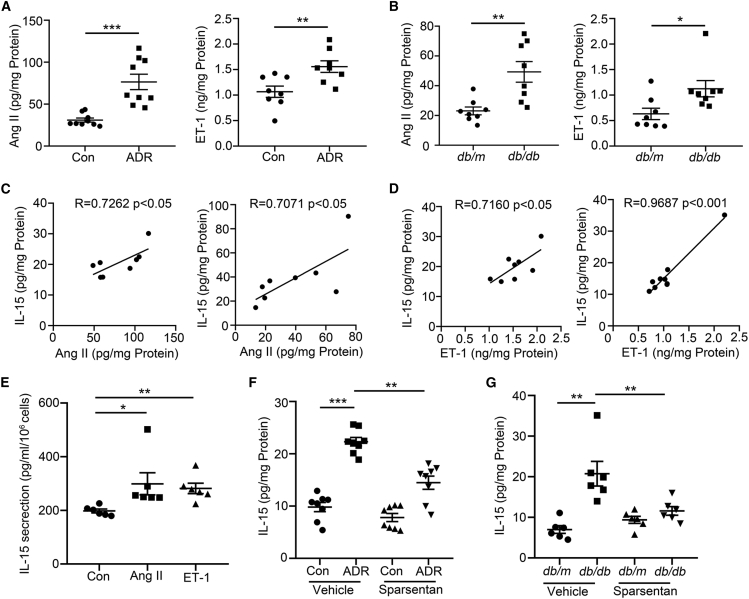
We found that the levels of angiotensin II (Ang II)20 and endothelin-1 (ET-1) were enhanced in the kidney from mice with ADR treatment (Figure 6A) and db/db mice (Figure 6B), and the levels of Ang II (Figure 6C) and ET-1 (Figure 6D) were positively correlated with the level of IL-15. We further found that primary renal parenchymal cells secreted more IL-15 after Ang II or ET-1 treatment (Figure 6E). In addition, Sparsentan inhibited IL-15 production in the kidney from mice with ADR treatment (Figure 6F) and db/db mice (Figure 6G). Our results suggest Sparsentan may inhibit IL-15 production, thereby further regulating renal CD8+ TRM cells fate under pathological conditions.
Figure 6.
Angiotensin II and endothelin-1 induce IL-15 signaling
(A and B) The levels of angiotensin II (Ang II) and endothelin-1 (ET-1) in the renal cortex from ADR-treated mice (A, n = 9) and db/db mice (B, n = 8).
(C) Correlation between Ang II and IL-15 in renal cortex of ADR-treated and db/db mice (n = 8). R, Pearson correlation coefficient. (D) Correlation between ET-1 and IL-15 in renal cortex of ADR-treated and db/db mice (n = 8). R, Pearson correlation coefficient. (E) The secretion of IL-15 in primary renal parenchymal cells after Ang II (10−6 mol/L) or ET-1 (10−6 mol/L) treatment (n = 6).
(F and G) The level of IL-15 in renal cortex of ADR-treated mice (F, n = 8) and db/db mice (G, n = 6). Data are represented as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Activated renal CD8+ TRM cells trigger podocyte injury
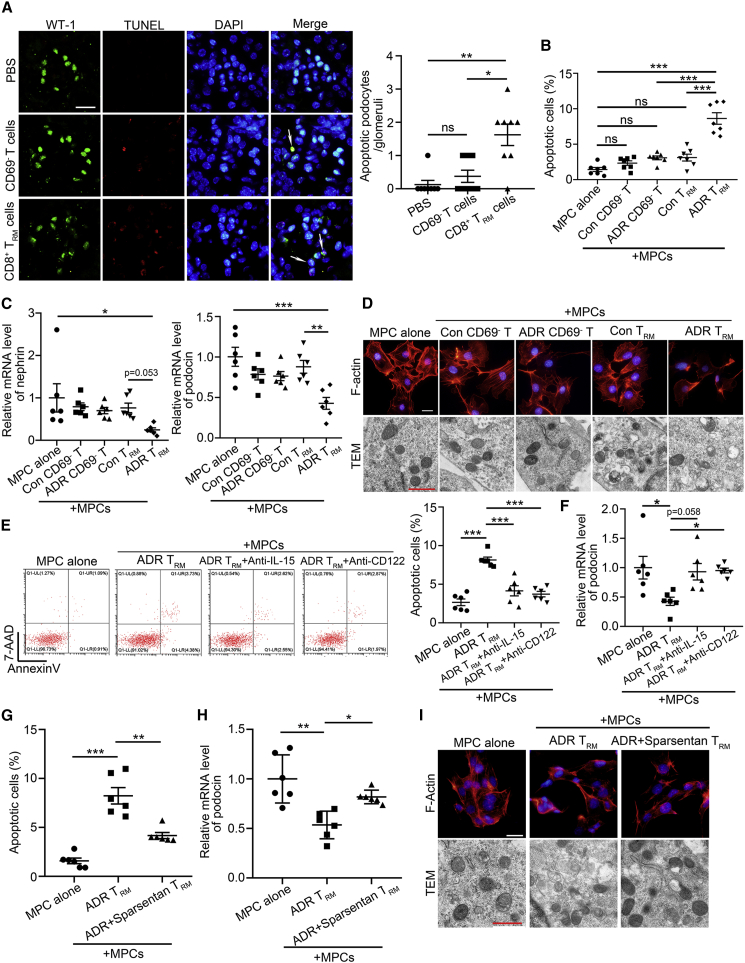
To address the effects of renal CD8+ TRM cells on the regulation of podocyte function and fate,10 we sorted renal CD8+CD69−CD44+ T cells and CD8+ TRM cells from ADR-treated mice and then transferred them to normal nude mice. It was found that only CD8+ TRM cells triggered podocyte apoptosis (Figure 7A). In vitro, we isolated different types of renal memory T cells and co-cultured with murine podocytes. Similarly, only renal CD8+ TRM cells from ADR-treated mice had cytotoxic effects on podocytes, including enhanced apoptosis (Figure 7B), reduced nephrin and podocin expression (Figure 7C), induced actin cytoskeleton derangement, and mitochondria damage (Figure 7D). However, activated CD8+ TRM cells with pretreatment with anti-IL-15 or anti-CD122 antibody had less cytotoxicity (Figure 7E) and recovered the expressions of podocin in podocytes (Figure 7F). To evaluate the effects of CD8+ TRM cells on other renal parenchymal cells, we then co-cultured the activated renal CD8+ TRM cells and glomerular cells, such as mesangial cells or glomerular endothelial cells. It was found that CD8+ TRM cells had no significant effect on the fates of mesangial cells and endothelial cells (Figure S6). Consistently, Sparsentan alleviated podocyte injury, as evidenced by reduced apoptosis (Figure 7G), recovered podocin expression (Figure 7H), attenuated cytoskeleton, and mitochondria injury (Figure 7I), when we sorted renal CD8+ TRM cells from Sparsentan-treated ADR-treated mice and co-cultured with podocytes.
Figure 7.
Activated renal CD8+ TRM cells trigger podocyte injury
See also Figure S6. (A) Representative immunofluorescence images and quantifications of apoptotic podocytes per glomeruli by TUNEL assay (10 areas per mouse were analyzed); white arrows highlight the apoptotic podocytes. Scale bars: black, 20 μm; red, 1 μm (n = 8). (B) The percentage of apoptotic murine podocytes (MPCs, n = 7). (C) Relative mRNA levels of nephrin and podocin in MPCs (n = 6). (D) Morphological examinations of cytoskeleton and mitochondria injury by F-actin staining and transmission electron microscopy (TEM) analyses in MPCs. Scale bars: black, 20 μm; red, 1 μm. (E) Representative flow cytometric analyze and quantification of apoptotic MPCs (n = 6). (F) Relative mRNA levels of podocin in MPCs (n = 6). (G) The percentage of apoptotic MPCs (n = 6).
(H) Relative mRNA levels of podocin in MPCs (n = 6). (I) Morphological examinations of cytoskeleton and mitochondria injury by F-actin staining and TEM analyses in MPCs. Scale bars: black, 20 μm; red, 1 μm. Data are represented as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Discussion
Podocyte dysfunction is central to the underlying pathophysiology of many common glomerular diseases, including DKD, FSGS, LN, and genetic forms of nephrotic syndrome.8 By detection of immune landscape of human and mice kidney, we found that tissue-resident lymphocytes were present in the kidney. Although the function of renal TRM cells under physiological conditions is still not very clear, previous studies have proposed that TRM cells could be the consequence of a subclinical renal inflammation or infection. Renal TRM cells might be involved in control of urogenital bacterial infection ascending to the kidney. It is also possible that TRM cells are required to control latent chronic infections (e.g., by polyoma viruses).21 More importantly, under pathogenetic conditions, the proportion of CD8+ TRM cells was substantially increased in the kidney from patients with glomerular diseases, such as FSGS, DKD, and LN, compared with healthy controls, as well as the kidney from experimental mouse models for these glomerular diseases. Meanwhile, we found that the number of CD4+ TRM cells was also increased in the kidney from mice and patients with LN, but not in FSGS and DKD, reflecting that CD8+ TRM cells but not CD4+ TRM cells may be a key and universal regulator contributing to glomerular injury. Therefore, considering that CD8+ TRM cells are undoubtedly attractive therapeutic targets with their distinct functions,22, 23, 24 we further explored their regulatory mechanisms in glomerular diseases, including FSGS and DKD.
In this study, we demonstrated for the first time that CD8+ TRM cells in the kidney were mainly derived from CD69− memory T cells and further explored a previously unappreciated role of renal CD8+ TRM cells in podocyte injury through their cytotoxic effects on podocytes. The principal hallmark of bona fide CD8+ TRM cells is their long-term persistence in non-lymphoid tissues without recirculation in the blood. Differentiation of TRM is controlled by various factors.25 Our data support that targeting IL-15 signaling pathways is a potential strategy to clear autoreactive memory cells from the kidney and protects against podocyte injury in mice. Mechanistically, IL-15 promoted CD8+ TRM cell formation and activation. IL-15 is a pluripotent cytokine from the IL-2 family that possesses many functions involved in regulating both adaptive and innate immune systems. IL-15 has been investigated for its therapeutic potential for the induction and maintenance of T cell responses because of its unique properties, such as wide expression, tightly regulated secretion, and trans-presentation.26 Studies have demonstrated that IL-15 is important for the generation of TRM in viral infections and in cutaneous lymphomas,27 and targeting IL-15 provides a durable treatment strategy for vitiligo.16 In this study, we found that IL-15 was highly expressed in the kidney and was required for the formation and activation of CD8+ TRM cell under pathological conditions. Furthermore, we demonstrated that blocking of IL-15 signaling alleviated podocyte and glomerular injuries by inhibiting renal CD8+ TRM cells rather than NK cells, which was consistent with previous studies showing that NK cells cannot mediate renal injury in mice with ADR nephropathy.28 Therefore, we suggest that inhibiting renal CD8+ TRM cells by targeting IL-15 signaling may provide a novel and durable treatment strategy for glomerular diseases.
In this study, another important finding is that Sparsentan can reduce TRM cell responses by intervening IL-15 signaling, exploring its new pharmacological functions. In FSGS, current treatment with corticosteroids or other immunosuppressive drugs is aimed at reducing proteinuria.29 These agents are routinely combined with renin-angiotensin system (RAS) inhibitors. Due to a spectrum of serious side effects of immunomodulating drugs, the availability of effective, safe, and well-tolerated drugs to protect renal function and reduce proteinuria is an unmet medical need in FSGS. Compared with RAS inhibitors, ETA receptor antagonists have shown a wide range of beneficial hemodynamic, anti-inflammatory, anti-fibrotic, and podocyte-protective effects in glomerular diseases.30,31 In particular, the combination of RAS inhibitors and ETA receptor antagonists has additional benefits in experimental models of kidney diseases and in patients with CKD.32 Sparsentan is a first-in-class, orally active, selective, and dual antagonist of the AT1 receptor and the ETA receptor. Since 2020, a multicenter, international, phase 3, randomized, double-blind, active-controlled study of sparsentan in patients with FSGS (DUPLEX; NCT0349368528) trial has been initiated to evaluate the long-term nephroprotective effects and safety of Sparsentan in patients with primary FSGS.33 In this study, we found that the levels of ET-1 and Ang II were significantly elevated and positively correlated with the level of IL-15 in the kidney from mice with FSGS or DKD. We further explored an unexpected role that Sparsentan can improve podocyte injury by inhibiting IL-15-mediated CD8+ TRM cell formation and activation, despite that we cannot exclude that Sparsentan may directly act on podocytes because Ang II and ET-1 have direct effect on podocyte function. Therefore, we propose that CD8+TRM-cell-dependent or -independent mechanisms may synergize together to protect against podocyte injury by Sparsentan. On the other hand, a feedforward mechanism may exist because the reduced proteinuria in glomerular diseases by Sparsentan may further decrease the CD8+ TRM cell formation and activation by the reduction of renal toxic local antigen.34,35 Further studies are needed to address this issue.
Although in this study we demonstrate a contribution of CD8+ TRM cells to podocyte injury, further studies are required to elucidate why podocytes are more susceptible to the pathogenicity of renal CD8+ TRM cells than other cell types in the kidney. Furthermore, CD8+ TRM cells are mostly accumulated surrounding the glomeruli; how cytotoxic CD8+ TRM cells influence podocytes remains unclear. Normally, podocytes are not accessible to CD8+ T cells. However, under some pathogenetic conditions, such as crescentic glomerulonephritis or the late stage of DKD, breaches in Bowman’s capsule can allow access of CD8+ T cells to the glomerular tuft and podocytes, resulting in their destruction.36 However, the breached Bowman’s capsule is not observed in FSGS.37 Therefore, the implication of periglomerular CD8+ TRM cells in podocyte injury is closely associated with different glomerular diseases or different stage of diseases. In this study, it is possible that IFN-γ produced by CD8+ TRM cells may potentiate robust local expression of chemokines and rapidly recruit other circulating inflammatory immune cells into glomeruli, which could amplify immune responses and result in podocyte injury. On the other hand, based on the results from in vitro studies by using a transwell co-culture system, renal CD8+ TRM cells from ADR-treated mice had cytotoxic effects on podocytes. Therefore, it is also possible that CD8+ TRM cells might release inflammatory cytokines, which get into glomeruli to injure podocytes directly. Of course, we cannot exclude that under some certain pathogenetic conditions, breaches in Bowman’s capsule allow access of CD8+ TRM cells to the glomerular tuft and podocytes, resulting in podocyte injury. Therefore, further studies for addressing this issue are also of great interest.
In conclusion, our studies for the first time provide direct evidence for the pivotal role of renal CD8+ TRM cells, suggesting that targeting TRM cells may represent a novel therapeutic strategy for patients with glomerular diseases.
Materials and methods
Human renal biopsy samples
Renal biopsies had been performed as part of routine clinical diagnostic investigation. The samples of renal biopsies were obtained from Department of Pathology, Shandong University School of Basic Medical Sciences. Normal controls were obtained from the healthy kidney poles of individuals who underwent tumor nephrectomies without other kidney diseases. The investigations were conducted in accordance with the principles of the Declaration of Helsinki and were approved by the Research Ethics Committee of Shandong University after informed consent was obtained from the patients.
Animal studies
All experimental protocols for animal studies were approved by the Institutional Animal Care and Use Committee of the School of Basic Medical Sciences, Shandong University (document no. ECSBMSSDU2018-2-074) and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Different groups were allocated in a randomized manner and investigators were blinded to the allocation of different groups when doing surgeries and doing outcome evaluations. The mouse models used in this study were described in the supplemental methods.
Cells isolation and sorting
The kidney was enzymatically digested with 400 μg/mL collagenase D (Roche, Mannheim, Germany) and 10 U/mL DNase I (Roche, Mannheim, Germany) for 45 min at 37°C. Subsequently, leukocytes were isolated by density gradient centrifugation using 40% Percoll (Merck Millipore, Darmstadt, Germany). CD8+CD69−CD44+ T cells or renal CD8+ TRM cells were purified with fluorescence-activated cell sorting (MoFlo Astrios EQ, Beckman Coulter).
Adoptive transfer
Splenic CD8+CD69−CD44+ cells were adoptively transferred to nude mice by a single tail-vein injection (106/head). Renal CD8+CD69−CD44+ T cells, and CD8+ TRM cells from ADR-treated mice were adoptively transferred to nude mice by a single tail-vein injection (106/head).
In vivo labeling assay
Intravascular injection of anti-CD45 mAb (clone: 30-F11, 2.5 μg per mouse) was administered to mice 5 min before sacrifice.38,39
Mass cytometry
The detailed methods are described in the supplemental method. A summary of the antibodies/clones used in the mass cytometry analysis is presented in the Tables S1 and S2.
Flow cytometry
Surface IF staining was performed at 4°C for 30 min. For intracellular (IC) cytokine staining, cells were stimulated with 50 ng/mL phorbol-12-myristate-13-acetate (PMA, MultiSciences), 1 μg/mL ionomycin (MultiSciences), and brefeldin A (5 μg/mL) for 5 h. Cells were fixed with IC fixation buffer (Invitrogen) after surface staining, permeabilized with permeabilization buffer (Invitrogen), and stained with IC antibodies cocktail for 30 min at 4°C. A summary of the antibodies/clones used in the flow cytometry analysis is presented in the Table S3. Cells were then washed and resuspended in phosphate buffer saline. Acquisition was performed on a CytoFLEX S Flow Cytometer, and data were analyzed using CytExpert software (both from Beckman Coulter).
Cell culture
The detailed cell preparations and cell culture conditions of murine podocyte cell line (MPC), glomerular mesangial cell line (MC), and glomerular endothelial cell line (GENC) were briefly described in the supplemental methods.
Transwell migration assay
In a transwell co-culture system, MPCs, MCs, or GENCs (5 ×104, lower) were seeded on a 0.8 mm Transwell insert (Corning, Corning, NY, USA) with medium and co-cultured with renal CD8+ memory T cells (5×103, upper) from normal or ADR-treated mice.
Enzyme-linked immunoabsorbent assay
According to the manufacturer’s instructions, renal cortex homogenates or cells supernatant samples were analyzed by mouse IL-15 (MultiSciences), ET-1 (OmnimAbs, CA, USA), and Ang II (OmnimAbs, CA, USA) ELISA kit.
Antibody and drug treatment
Anti-mouse IL-15 antibody (300 μg/head; Bio X Cell, clone AIO.3), anti-mouse CD122 antibody (100 μg/head; Bio X Cell, clone TM-beta 1), anti-mouse NK1.1 antibody (200 μg/head; BioLegend, clone PK136), or isotype controls (Bio X Cell, clone C1.18.4) were administered intraperitoneally three times weekly. The current concentrations of IL-15,40 CD122,16 and NK1.140 neutralizing antibodies were used, as previous studies indicated. Sparsentan (30 μmol/kg, MCE) was administered orally three times weekly. FTY720 (240 μg/kg, MCE) was administered intraperitoneally before ADR treatment.
Transmission electron microscopy
Electron microscopic sample handling and detection were performed by the electron microscopic core lab of Shandong University. Tissues and cells were collected and fixed with 2.5% glutaraldehyde at 4°C. Sections were washed 15 min for 3 times in 0.1 mol/L PBS and post-fixed in 1% osmium tetroxide at room temperature for 2 h. Specimens were then dehydrated using 30%, 50%, 70%, 80%, 90%, 95%, and 100% ethanol and 100% acetone in series. After dehydration, the sections were embedded in Pon 812 resin overnight at 37°C using acetone as a transitional solvent. The ultra-thin sections were cut and post-stained with 2% saturated uranyl acetate and lead citrate.
Multiplex immunohistochemistry staining
The detailed multiplex immunohistochemistry stain method descriptions are available in the supplemental methods. Details of antibodies are described in Table S4.
Immunofluorescence staining
Tissues were transferred to 4% paraformaldehyde (PFA) and fixed at 4°C overnight, followed by paraffin embedded and cross-sectioned (3 μm) for IF staining by using a modified protocol, as previously described.41 Details of antibodies are described in Table S4.
TUNEL assay
Cell death in the kidney section was detected by TUNEL assay following the manufacturer’s protocol (Roche Diagnostics, Mannheim, Germany).
RNA extraction and real-time quantitative PCR
Total RNA was isolated from cells using TRIzol reagent (Invitrogen). The mRNA expression levels were determined by real-time quantitative RT-PCR using a Bio-Rad iCycler system (Bio-Rad, Hercules, CA). The sequences of specific primers are listed in Table S5.
Statistics
Data are expressed as mean ± SEM. Statistical analyses were performed with GraphPad Prism (v.8.0, GraphPad Software, San Diego, CA). Normality assumption of the data distribution was assessed using Kolmogorov-Smirnov test. Comparisons between two groups were performed using two-tailed Student’s t test for normally distributed data and Mann-Whitney rank-sum test for non-normally distributed data. Differences between multiple groups with one variable were determined using one-way ANOVA followed by post hoc Tukey’s test. To compare multiple groups with more than one variable, two-way ANOVA followed by post hoc Tukey’s test was used. For correlation analysis, Pearson correlation coefficient was applied as appropriate.
Acknowledgments
We thank Translational Medicine Core Facility of Shandong University for consultation and instrument availability that supported this work. This study was supported by the National Nature Science Foundation of China (91949202, 82090020, 82090024, 81970580, 81800645, 81800643, 81873614, and 81900659); National Key R&D Program of China (2020YFC2005000); and Shandong Provincial Natural Science Foundation, China (ZR2019ZD40 and ZR2019BH030).
Author contributions
L.L., W.T., Y.Z., and M.J. conducted experiments, performed data analysis, and helped write the manuscript. L.W. helped analyze flow cytometry data. Q.L., Q.H., and X.P. contributed with cells isolation from animals. Y.Z. and J.W. performed in vivo animal studies. W.T. and Y.X. helped design experiments. Z.W., J.Z., X.W., M.L., Y.S., and C.Z. analyzed human renal biopsy samples. F.Y. designed the experiment, interpreted the data, wrote the manuscript, and approved the final version of the manuscript for publication.
Declaration of interests
All authors declare no conflict of interest.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2022.04.024.
Supplemental information
References
- 1.Schenkel J.M., Masopust D. Tissue-resident memory T cells. Immunity. 2014;41:886–897. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mueller S.N., Mackay L.K. Tissue-resident memory T cells: local specialists in immune defence. Nat. Rev. Immunol. 2016;16:79–89. doi: 10.1038/nri.2015.3. [DOI] [PubMed] [Google Scholar]
- 3.Mueller S.N., Gebhardt T., Carbone F.R., Heath W.R. Memory T cell subsets, migration patterns, and tissue residence. Annu. Rev. Immunol. 2013;31:137–161. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- 4.Takamura S. Niches for the long-term maintenance of tissue-resident memory T cells. Front. Immunol. 2018;9:1214. doi: 10.3389/fimmu.2018.01214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin H. Formation and function of tissue-resident memory T cells during viral infection. Curr. Opin. Virol. 2018;28:61–67. doi: 10.1016/j.coviro.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Shin H., Iwasaki A. Tissue-resident memory T cells. Immunol. Rev. 2013;255:165–181. doi: 10.1111/imr.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krebs C.F., Reimers D., Zhao Y., Paust H.J., Bartsch P., Nunez S., Rosemblatt M.V., Hellmig M., Kilian C., Borchers A., et al. Pathogen-induced tissue-resident memory TH17 (TRM17) cells amplify autoimmune kidney disease. Sci. Immunol. 2020;5 doi: 10.1126/sciimmunol.aba4163. [DOI] [PubMed] [Google Scholar]
- 8.Nagata M. Podocyte injury and its consequences. Kidney. Int. 2016;89:1221–1230. doi: 10.1016/j.kint.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Bhargava R., Tsokos G.C. The immune podocyte. Curr. Opin. Rheumatol. 2019;31:167–174. doi: 10.1097/bor.0000000000000578. [DOI] [PubMed] [Google Scholar]
- 10.Li S., Liu Y., He Y., Rong W., Zhang M., Li L., Liu Z., Zen K. Podocytes present antigen to activate specific T cell immune responses in inflammatory renal disease. J. Pathol. 2020;252:165–177. doi: 10.1002/path.5508. [DOI] [PubMed] [Google Scholar]
- 11.Cibrian D., Sanchez-Madrid F. CD69: from activation marker to metabolic gatekeeper. Eur. J. Immunol. 2017;47:946–953. doi: 10.1002/eji.201646837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou M., Guo C., Li X., Huang Y., Li M., Zhang T., Zhao S., Wang S., Zhang H., Yang N. JAK/STAT signaling controls the fate of CD8(+)CD103(+) tissue-resident memory T cell in lupus nephritis. J. Autoimmun. 2020;109:102424. doi: 10.1016/j.jaut.2020.102424. [DOI] [PubMed] [Google Scholar]
- 13.Wilk M.M., Misiak A., McManus R.M., Allen A.C., Lynch M.A., Mills K.H.G. Lung CD4 tissue-resident memory T cells mediate adaptive immunity induced by previous infection of mice with bordetella pertussis. J. Immunol. 2017;199:233–243. doi: 10.4049/jimmunol.1602051. [DOI] [PubMed] [Google Scholar]
- 14.Mackay L.K., Wynne-Jones E., Freestone D., Pellicci D.G., Mielke L.A., Newman D.M., Braun A., Masson F., Kallies A., Belz G., Carbone F. T-box transcription factors combine with the cytokines TGF-beta and IL-15 to control tissue-resident memory T cell fate. Immunity. 2015;43:1101–1111. doi: 10.1016/j.immuni.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Ikemizu S., Chirifu M., Davis S.J. IL-2 and IL-15 signaling complexes: different but the same. Nat. Immunol. 2012;13:1141–1142. doi: 10.1038/ni.2472. [DOI] [PubMed] [Google Scholar]
- 16.Richmond J.M., Strassner J.P., Zapata L., Jr., Garg M., Riding R.L., Refat M.A., Fan X., Azzolino V., Tovar-Garza A., Tsurushita N., et al. Antibody blockade of IL-15 signaling has the potential to durably reverse vitiligo. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aam7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X., Zhao X.Y. Transcription factors associated with IL-15 cytokine signaling during NK cell development. Front. Immunol. 2021;12:610789. doi: 10.3389/fimmu.2021.610789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davenport A.P., Kuc R.E., Southan C., Maguire J.J. New drugs and emerging therapeutic targets in the endothelin signaling pathway and prospects for personalized precision medicine. Physiol. Res. 2018;67:S37–S54. doi: 10.33549/physiolres.933872. [DOI] [PubMed] [Google Scholar]
- 19.Komers R., Gipson D.S., Nelson P., Adler S., Srivastava T., Derebail V.K., Meyers K.E., Pergola P., MacNally M.E., Hunt J.L., et al. Efficacy and safety of Sparsentan compared with Irbesartan in patients with primary focal segmental glomerulosclerosis: randomized, controlled trial design (DUET) Kidney. Int. Rep. 2017;2:654–664. doi: 10.1016/j.ekir.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang S.C., Leung J.C., Chan L.Y., Eddy A.A., Lai K.N. Angiotensin converting enzyme inhibitor but not angiotensin receptor blockade or statin ameliorates murine adriamycin nephropathy. Kidney. Int. 2008;73:288–299. doi: 10.1038/sj.ki.5002674. [DOI] [PubMed] [Google Scholar]
- 21.Turner J.E., Becker M., Mittrucker H.W., Panzer U. Tissue-resident lymphocytes in the kidney. J. Am. Soc. Nephrol. 2018;29:389–399. doi: 10.1681/asn.2017060599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu H., Liao W., Li Q., Long H., Yin H., Zhao M., Chan V., Lau C.S., Lu Q. Pathogenic role of tissue-resident memory T cells in autoimmune diseases. Autoimmun. Rev. 2018;17:906–911. doi: 10.1016/j.autrev.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 23.Dumauthioz N., Labiano S., Romero P. Tumor resident memory T cells: new players in immune surveillance and therapy. Front Immunol. 2018;9:2076. doi: 10.3389/fimmu.2018.02076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milner J.J., Toma C., He Z., Kurd N.S., Nguyen Q.P., McDonald B., Quezada L., Widjaja C.E., Witherden D.A., Crowl J.T., et al. Heterogenous populations of tissue-resident CD8(+) T cells are generated in response to infection and malignancy. Immunity. 2020;52:808–824.e7. doi: 10.1016/j.immuni.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang K., Kallies A. Tissue-specific differentiation of CD8(+) resident memory T cells. Trends. Immunol. 2021;42:876–890. doi: 10.1016/j.it.2021.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Patidar M., Yadav N., Dalai S.K. Interleukin 15: a key cytokine for immunotherapy. Cytokine Growth Factor Rev. 2016;31:49–59. doi: 10.1016/j.cytogfr.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Adachi T., Kobayashi T., Sugihara E., Yamada T., Ikuta K., Pittaluga S., Saya H., Amagai M., Nagao K. Hair follicle-derived IL-7 and IL-15 mediate skin-resident memory T cell homeostasis and lymphoma. Nat. Med. 2015;21:1272–1279. doi: 10.1038/nm.3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng G., Zheng L., Wang Y., Wu H., Kairaitis L., Zhang C., Tay Y.C., Alexander S., Harris D. NK cells do not mediate renal injury in murine adriamycin nephropathy. Kidney Int. 2006;69:1159–1165. doi: 10.1038/sj.ki.5000244. [DOI] [PubMed] [Google Scholar]
- 29.Campbell K.N., Tumlin J.A. Protecting podocytes: a key target for therapy of focal segmental glomerulosclerosis. Am. J. Nephrol. 2018;47:14–29. doi: 10.1159/000481634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barton M., Sorokin A. Endothelin and the glomerulus in chronic kidney disease. Semin. Nephrol. 2015;35:156–167. doi: 10.1016/j.semnephrol.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barton M. Endothelin antagonism and reversal of proteinuric renal disease in humans. Contrib. Nephrol. 2011;172:210–222. doi: 10.1159/000328702. [DOI] [PubMed] [Google Scholar]
- 32.Komers R., Plotkin H. Dual inhibition of renin-angiotensin-aldosterone system and endothelin-1 in treatment of chronic kidney disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016;310:R877–R884. doi: 10.1152/ajpregu.00425.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Komers R., Diva U., Inrig J.K., Loewen A., Trachtman H., Rote W.E. Study design of the phase 3 sparsentan versus irbesartan (DUPLEX) study in patients with focal segmental glomerulosclerosis. Kidney. Int. Rep. 2020;5:494–502. doi: 10.1016/j.ekir.2019.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crowley S.D., Rudemiller N.P. Immunologic effects of the renin-angiotensin system. J. Am. Soc. Nephrol. 2017;28:1350–1361. doi: 10.1681/asn.2016101066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan T.N., Mooster J.L., Kilgore A.M., Osborn J.F., Nolz J.C. Local antigen in nonlymphoid tissue promotes resident memory CD8+ T cell formation during viral infection. J. Exp. Med. 2016;213:951–966. doi: 10.1084/jem.20151855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen A., Lee K., D'Agati V.D., Wei C., Fu J., Guan T.J., He J.C., Schlondorff D., Agudo J. Bowman's capsule provides a protective niche for podocytes from cytotoxic CD8+ T cells. J. Clin. Invest. 2018;128:3413–3424. doi: 10.1172/jci97879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuppe C., Grone H.J., Ostendorf T., van Kuppevelt T.H., Boor P., Floege J., Smeets B., Moeller M.J. Common histological patterns in glomerular epithelial cells in secondary focal segmental glomerulosclerosis. Kidney. Int. 2015;88:990–998. doi: 10.1038/ki.2015.116. [DOI] [PubMed] [Google Scholar]
- 38.Anderson K.G., Sung H., Skon C.N., Lefrancois L., Deisinger A., Vezys V., Masopust D. Cutting edge: intravascular staining redefines lung CD8 T cell responses. J. Immunol. 2012;189:2702–2706. doi: 10.4049/jimmunol.1201682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson K.G., Mayer-Barber K., Sung H., Beura L., James B.R., Taylor J.J., Qunaj L., Griffith T.S., Vezys V., Barber D.L., Masopust D. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat. Protoc. 2014;9:209–222. doi: 10.1038/nprot.2014.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koda Y., Teratani T., Chu P.S., Hagihara Y., Mikami Y., Harada Y., Tsujikawa H., Miyamoto K., Suzuki T., Taniki N., et al. CD8(+) tissue-resident memory T cells promote liver fibrosis resolution by inducing apoptosis of hepatic stellate cells. Nat. Commun. 2021;12:4474. doi: 10.1038/s41467-021-24734-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fu Y., Sun Y., Wang M., Hou Y., Huang W., Zhou D., Wang Z., Yang S., Tang W., Zhen J., et al. Elevation of JAML promotes diabetic kidney disease by modulating podocyte lipid metabolism. Cell. Metab. 2020;32:1052–1062.e8. doi: 10.1016/j.cmet.2020.10.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.