Abstract
Efforts to prolong thoracic paravertebral block (TPVB) analgesia include local anesthetic adjuvants, such as dexamethasone (Dex). Previous studies showed that both perineural (PN) and intravenous (i.v.) routes could prolong analgesia. As PN Dex is an off‐label use, anesthesiologists should be fully informed of the clinical differences, if any, on block duration. This study was designed to evaluate the two administration routes of Dex for duration of analgesia in TPVB. Ninety‐five patients scheduled for Ivor‐Lewis esophagectomy were randomized to receive TPVB (0.5% ropivacaine 15 ml), PN or i.v. Dex 8 mg. The primary end point was the duration of analgesia. The secondary end points included pain scores, analgesic consumption, adverse effects rate, and incidence of chronic pain at 3 months postoperatively. The PN‐Dex group showed better analgesic effects than the i.v.‐Dex group (p < 0.05). Similarly, the visual analogue scale scores in patients at 2, 4, 8, and 12 h postoperatively were lower in the PN‐Dex group than the i.v.‐Dex group (p < 0.05). The analgesic consumption in both the PN‐Dex and i.v.‐Dex groups was significantly lower than that in the control group (p < 0.05). Regarding the incidence of chronic pain, regardless of route, Dex decreased the incidence of chronic postsurgical pain and neuropathic pain at 3 months after surgery (p < 0.05), but there were no clinical differences between the i.v.‐Dex and PN‐Dex groups. Perineural dexamethasone improved the magnitude and duration of analgesia compared to that of the i.v.‐Dex group in TPVB in Ivor‐Lewis esophagectomy. However, there were no clinically significant differences between the two groups in the incidence of chronic pain.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Both perineural (PN) and intravenous (i.v.) dexamethasone (Dex) could prolong the duration of a nerve block, but the superiority of either route is still inconclusive.
WHAT QUESTION DID THIS STUDY ADDRESS?
The study investigated the effects of the two routes of Dex added to ropivacaine on analgesic effects of thoracic paravertebral block in patients undergoing Ivor‐Lewis esophagectomy.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
These results extend the knowledge of the superior analgesic effect of Dex for the management of perioperative pain in the setting of Ivor‐Lewis Esophagectomy.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Because PN Dex is an off‐label use, our study conformed the safety of Dex as PN adjuvants and extended its application field in clinical work.
INTRODUCTION
Ivor‐Lewis esophagectomy is a major complex palliative or curative operation for patients with esophageal cancer; however, the rate of perioperative morbidity is up to 60%. 1 , 2 Severe pain, which is a common issue following esophagectomy, may occur in the early postoperative phase, resulting in changes in respiratory function and pulmonary mechanisms, and further affecting the quality of life and survival rate of patients. 3 Therefore, adequate pain management has an important role in relieving anxiety, enhancing early mobilization and recovery, and thereby reducing postoperative complications and duration of hospital stay. 4 , 5 , 6
Recently, thoracic paravertebral block (TPVB) was introduced as an appropriate option for patients undergoing Ivor‐Lewis esophagectomy, allowing patients to achieve superior pain relief and reduction of opioid consumption. 7 However, TPVB still has some problems, such as the limited duration of block by local anesthetics (LAs). Efforts to prolong analgesic duration by the continuous catheter block technique are limited by catheter displacement. 8
Dexamethasone (Dex) is a representative adjunct for antiemesis as well as postoperative pain control in multimodal strategies. 7 , 9 , 10 A recent study confirmed that perineural (PN) Dex with ropivacaine prolonged the effects of TPVB. 7 However, the PN administration of Dex is an “off‐label” use, and there are no pharmacokinetic data on the use of Dex via the PN route. In addition, the optimal method of administration remains unknown. In a randomized trial that compared PN and i.v. Dex (8 mg) for axillary block, the PN modality provided longer analgesia. 11 A review also showed that PN Dex prolonged analgesia by 3 h compared with i.v. administration. 12 However, similar results were not found in other studies. 13 , 14 We speculated that these contradictory findings in the literature may stem from differences in the doses of Dex and LA used as well as insufficient statistical power due to small sample sizes. In addition, different nerve blocks may respond differently to i.v. and PN Dex. 15
Accordingly, the Dex dose chosen in this study was 8 mg, which was consistent with the dose used in the study by Julian Aliste et al. 11 The present study was designed to investigate whether PN Dex provides analgesia for a longer duration than i.v. Dex or a saline control in patients receiving TPVB undergoing Ivor‐Lewis esophagectomy.
METHODS
The study was a prospective single‐center, double‐blinded, randomized, controlled study conducted in Xuzhou Central Hospital. The study protocol was approved by the local ethics committee (Approval No. XZXY‐LJ‐20210526‐076). Written, informed consent was obtained from all subjects. The study was registered at clinicaltrials.gov (ChiCTR2100044278).
Patients
Ninety‐five patients aged 40–75 years old, American Society of Anesthesiology (ASA) physical status I–III, 18.5 kg/m2 less than body mass index less than or equal to 30 kg/m2, and scheduled to undergo Ivor‐Lewis esophagectomy were eligible for enrollment. The exclusion criteria were as follows: allergy or intolerance to study medications; contraindication to Dex (peptic ulcer disease, systemic infection, glaucoma, active varicella/herpetic infections, and diabetes mellitus); contraindication to TPVB (severe spinal or thoracic deformity, coagulopathy, and local infection), chronic opioid use (>30 mg oral morphine or equivalents), or failure to provide a pain score using the visual analog scale (VAS).
Study design
The subjects were randomized into three groups using a computer‐generated random number table. The group assignments were sealed in sequentially numbered opaque envelopes that would not be opened until informed consent was obtained. Study medications were identical in appearance and prepared by an investigator who was not involved with patient care or data collection. The flow chart of the study protocol is shown in Figure 1.
FIGURE 1.
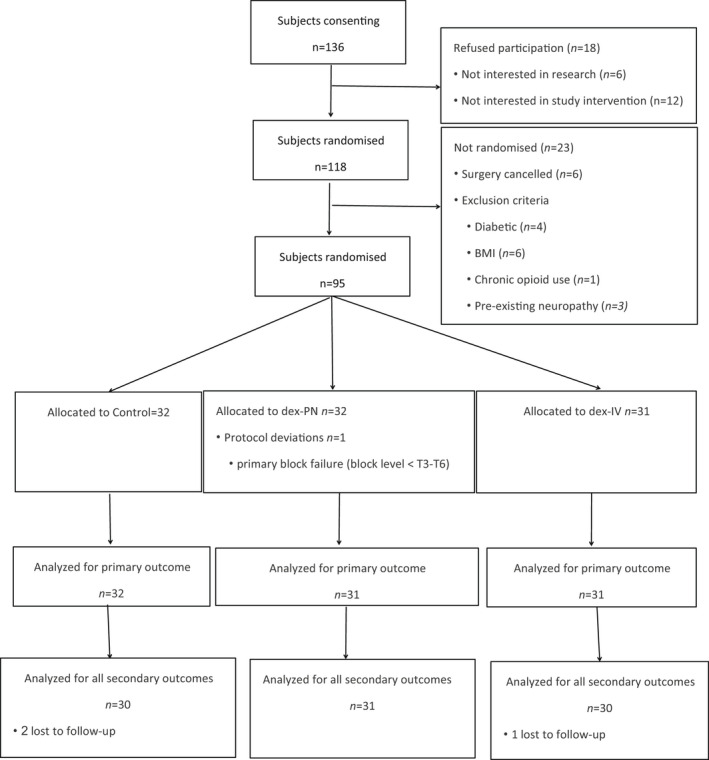
CONSORT flow diagram of screened, enrolled, randomized, and analyzed participants. BMI, body mass index; CONSORT, Consolidated Standards of Reporting Trials.
Patients providing informed consent who met all eligibility criteria were randomized (1:1:1) into the following three groups: normal saline (control), PN‐Dex (Dexamethasone sodium phosphate injection, Shiyao Medicine Co. Ltd. China), or i.v.‐Dex.
PN‐Dex group
TPVB injection mixture: ropivacaine 0.5% (15 ml) + [dexamethasone 8 mg + 0.9% saline] (3 ml);
i.v. infusion: 0.9% saline (3 ml).
-
2
i.v.‐Dex group
TPVB injection mixture: ropivacaine 0.5% (15 ml) + 0.9% saline (3 ml);
i.v. infusion: [dexamethasone 8 mg + 0.9% saline] (3 ml).
-
3
Control group
TPVB injection mixture: ropivacaine 0.5% (15 ml) + 0.9% saline (3 ml);
i.v. infusion: 0.9% saline (3 ml).
Anesthesia, monitoring, and surgery
After establishing i.v. access, standard monitors (electrocardiogram, invasive arterial pressure, and continuous oxygen saturation) were applied. Patients were placed in a standard lateral position to apply TPVB before induction of anesthesia. An assistant who neither participated in the surgery nor was involved in the study was responsible for drug preparation. TPVB was performed using an ultrasound guided parasagittal out‐plane approach, followed by i.v. Dex or saline. A real‐time ultrasound machine (SonoSite Edge II) with an rC60xi transducer (2–5 MHz) draped with a sterile cover (3 M Tegaderm) was used to guide a 22G, 80‐mm needle (stimuplex D; B. Braun Melsungen AG). Images of the transverse process, the superior costotransverse ligament, and the highlighted pleura were obtained via an ultrasonic probe (Figure 2). Then, different TPVB injection mixtures were administered at the paravertebral spaces between T5 and T6. Ultrasonography confirmed that a weak echogenic shade was located outside the pleura and that the pleura shifted downward due to the mixtures (Figure 3). The sensory block level was tested after 20‐min, and those with primary block failure were excluded (block level < T3–T6).
FIGURE 2.
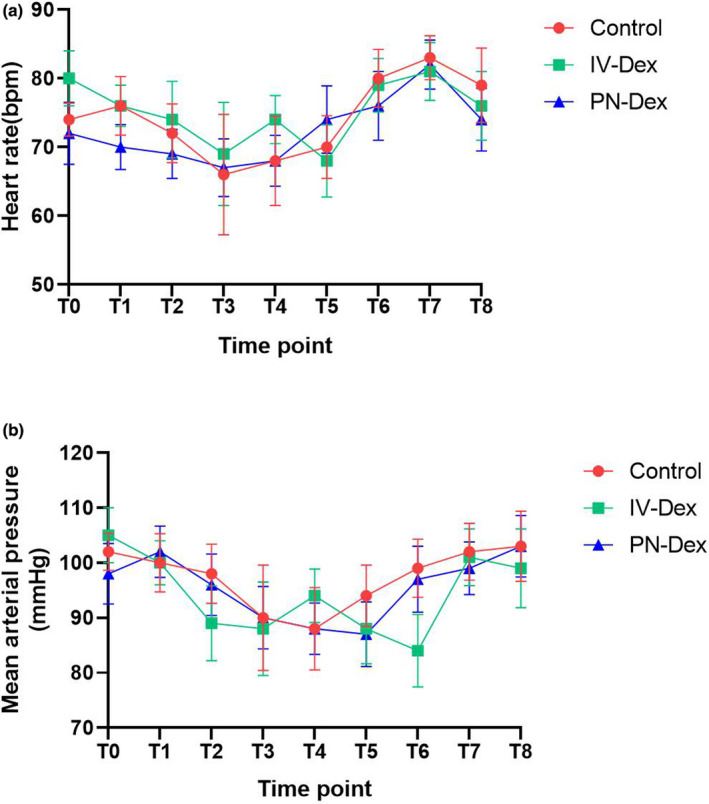
Observed mean (SD) heart rate (a) and mean arterial pressure (b) during perioperative period. T0, baseline; T1, 5 min after TPVB; T2, 5 min after induction; T3, 10 min after skin incision; T4, at the end of surgery; T5, at transfer to the PACU; T6, upon awakening; T7, upon extubation; T8, with transfer from the PACU. Dex, dexamethasone; PN, perineural; PACU, postanesthesia care unit.
FIGURE 3.
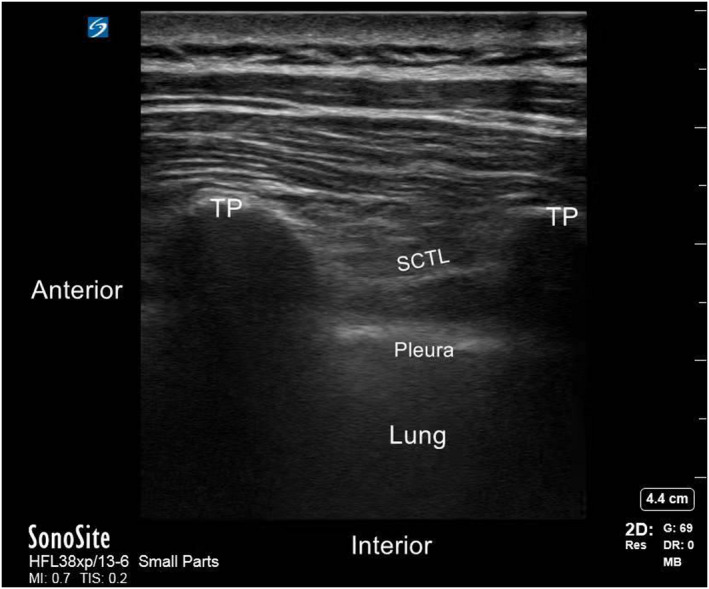
Schematic diagram of ultrasound guided thoracic paravertebral nerves. STCL, superior costotransverse ligament; TP, transverse process.
General anesthesia was induced using a standardized technique that included midazolam 0.05 mg/kg, etomidate 0.2 mg/kg, sufentanil 0.6 μg/kg, cisatracurium besilate 0.3 mg/kg to facilitate double‐lumen endobronchial intubation. Propofol and remifentanil were continuously infused to maintain anesthesia, and cisatracurium besilate was administered as needed. We maintained a bispectral index score of 40–60 during the whole operation by regulating the concentration of propofol. The operations were performed by the same experienced surgeon to eliminate possible confounders.
When the patients were transferred to the postanesthesia care unit, vital signs (heart rate, invasive blood pressure, respiratory rate, and SpO2) were monitored and recorded every 5 min. The bronchial tube was removed after the patients were fully awake and the tidal capacity was restored sufficiently. The patient controlled intravenous analgesia (PCIA) was linked postoperatively, but it was not operative in standby mode (sufentanil 2 μg/kg + palonosetron 7 μg/kg + 0.9% saline = 100 ml; 2 ml/h as basal infusion, control single dose 0.5 ml, lock‐on time 15 min). After the analgesia pump was started, an extra rescue dose of 5 mg oxycodone was administered for analgesia when the VAS was greater than or equal to four at rest.
Outcome measures
The primary end point was the duration of analgesic effect, defined as the time from a fixed level of sensory block to the first time that analgesics should be given (the time point when the patients pressed the button to start the analgesia pump).
The secondary end points included the following:
Postoperative VAS scores at 2, 4, 8, 12, 24, and 48 h after surgery at rest and when coughing;
The total of bolus times; remedial analgesic consumption;
The incidence of chronic postsurgical pain (CPSP) 3 months after surgery, which was diagnosed according to the International Pain Society (IASP) (International Classification of Disease‐11th revision [ICD]‐11) criteria 16 : (1) secondary to surgery; (2) pain persists for more than 3 months; (3) other causes, including pain caused by malignant tumor and chronic infection, are excluded; and (4) the pain nature changes. If patients were diagnosed with CPSP, the neuropathic pain (NP) scale (DN4 scale) was used, consisting of 10 questions in four different aspects (pain characteristics, related symptoms, hyperalgesia, and brush‐like response). The total score was 10, and patients whose scores were more than four were diagnosed with NP.
Block‐related complications (respiratory depression; and block‐related nerve palsy at 48 h after surgery), postoperative blood glucose, other complications (postoperative nausea and vomiting [PONV], and urinary retention).
Statistical analysis
The primary outcome was the duration of the sensory block. From our experience using ropivacaine 0.5% in TPVB, we anticipated a mean analgesia duration of 1020 min with an SD of 200 min in the PN‐Dex group, and a difference of 180 minutes between the groups represented clinical significance. We estimated that a sample size of 27 patients (power = 90%, a = 0.05) per group was appropriate. To account for losses to follow‐up, 30 patients per group were recruited.
The continuous variables were presented as the means ± SD or median (25th to 75th percentiles), and categorical data were presented as numbers and percentages. Normality was assessed using the Kolmogorov–Smirnov test. Continuous variables were assessed using one‐way analysis of variance and Tukey’s post hoc tests. Categorical variables were compared using Fisher’s exact test or the χ2 test for the expected low counts, for comparisons between the two groups. Time to first reported pain was evaluated by constructing Kaplan–Meier plots and using the log rank test with Bonferroni correction for multiple comparisons (n = 3). All data were processed using SPSS software (version 18.0, SPSS). A two‐sided p value <0.05 was considered statistically significant.
RESULTS
Patient characteristics
The flow diagram of the Consolidated Standards of Reporting Trial (CONSORT) showed the number of subjects that received the allocated interventions (Figure 1). Of the 136 candidate patients, 118 provided written informed consent to participate in the study from December 2019 to August 2021. Twenty‐three of the 118 patients who gave consent were not randomized due to exclusion criteria, whereas 95 subjects who met the inclusion criteria were randomized, with 32 allocated to the control group, 31 to the PN‐Dex group, and 31 to the i.v.‐Dex group. There were no significant differences among the three groups in basic information or operative data except that the consumption of remifentanil was significantly lower in both the PN‐Dex and i.v.‐Dex groups than in the control group, as shown in Tables 1 and 2. Figure 4 showed no differences in blood pressure or heart rate among all three groups.
TABLE 1.
Demographic and baseline characteristics
| Control (n = 30) | PN‐Dex (n = 31) | IV‐Dex (n = 30) | p Value | |||
|---|---|---|---|---|---|---|
| p a | p b | p c | ||||
| Male/Female | 13/17 | 14/17 | 16/14 | 0.89 | 0.44 | 0.52 |
| Age, year | 51.8 ± 3.7 | 51.9 ± 3.8 | 52.3 ± 4.1 | 0.99 | 0.87 | 0.91 |
| Body mass index, kg/m2 | 24.3 ± 2.2 | 24.5 ± 2.2 | 24.7 ± 2.1 | 0.95 | 0.82 | 0.95 |
| Comorbidities | ||||||
| Stroke | 0 | 1 | 0 | >0.99 | >0.99 | >0.99 |
| Hypertension | 3 | 5 | 2 | 0.71 | >0.99 | 0.42 |
| Coronary artery disease | 2 | 3 | 4 | >0.99 | 0.67 | 0.71 |
| Diabetes mellitus | 0 | 1 | 2 | >0.99 | 0.49 | 0.61 |
| Asthma/COPD | 0 | 1 | 1 | >0.99 | >0.99 | >0.99 |
| ASA class | 0.78 | 0.79 | 0.61 | |||
| I | 10 | 8 | 11 | |||
| II | 14 | 17 | 15 | |||
| III | 6 | 6 | 4 | |||
| Heart rate | 73.2 ± 2.6 | 73.3 ± 5.1 | 75.4 ± 3.8 | 0.99 | 0.09 | 0.10 |
| Mean arterial pressure (mmHg) | 91.4 ± 3.4 | 90.6 ± 5.2 | 92.6 ± 5.2 | 0.78 | 0.58 | 0.22 |
Note: Data represent mean ± SD. p a, PN‐Dex group vs. control group; p b, i.v.‐Dex group vs. control group; p c, PN‐Dex group vs. i.v.‐Dex group.
Abbreviations: ASA, American Society of Anesthesiology; COPD, chronic obstructive pulmonary disease; Dex, dexamethasone; PN, perineural.
TABLE 2.
Intra‐operative data and characteristics of recovery
| Control (n = 30) | PN‐Dex (n = 31) | i.v.‐Dex (n = 30) | p Value | |||
|---|---|---|---|---|---|---|
| p a | p b | p c | ||||
| Duration of anesthesia, min | 153.6 ± 43.1 | 147.5 ± 35.8 | 156.2 ± 30.3 | 0.79 | 0.96 | 0.63 |
| Intra‐operative medication | ||||||
| Midazolam, mg | 3.8 ± 0.4 | 3.7 ± 0.6 | 3.6 ± 0.2 | 0.64 | 0.18 | 0.64 |
| Propofol, mg | 1013.8 ± 99.2 | 1049.6 ± 101.3 | 1021.6 ± 98.6 | 0.42 | 0.99 | 0.62 |
| Cisatracurium, mg | 37.8 ± 3.4 | 38.7 ± 2.9 | 37.2 ± 3.1 | 0.50 | 0.74 | 0.15 |
| Sufentanil, μg | 56.4 ± 6.8 | 55.5 ± 7.6 | 56.8 ± 4.7 | 0.85 | 0.97 | 0.72 |
| Remifentanil, mg | 2.5 ± 0.3 | 1.9 ± 0.3* | 1.9 ± 0.4 # | <0.05 | <0.05 | >0.99 |
| Fluid balance | ||||||
| Total fluid infusion, ml | 1461.6 ± 244.4 | 1421.3 ± 230.2 | 1502 ± 333.1 | 0.83 | 0.83 | 0.48 |
| Estimated blood loss, ml | 154.7 ± 18.2 | 149.5 ± 11.1 | 154.4 ± 16.3 | 0.39 | 0.99 | 0.43 |
| Urine volume, ml | 352.2 ± 51.3 | 343.4 ± 52.6 | 365.4 ± 48.2 | 0.78 | 0.57 | 0.21 |
| Duration of surgery, min | 133.4 ± 37.6 | 129.2 ± 33.7 | 132.3 ± 36.3 | 0.89 | 0.99 | 0.94 |
Note: Data represent mean ± SD. p a, PN‐Dex group vs. control group; p b, i.v.‐Dex group vs. control group; p c, PN‐Dex group vs. i.v.‐Dex group.
Abbreviations: Dex, dexamethasone; PN, perineural.
The comparison between the PN‐Dex group and control group is statistically significant (p < 0.05).
The comparison between i.v.‐Dex group and control group is statistically significant (p < 0.05).
FIGURE 4.
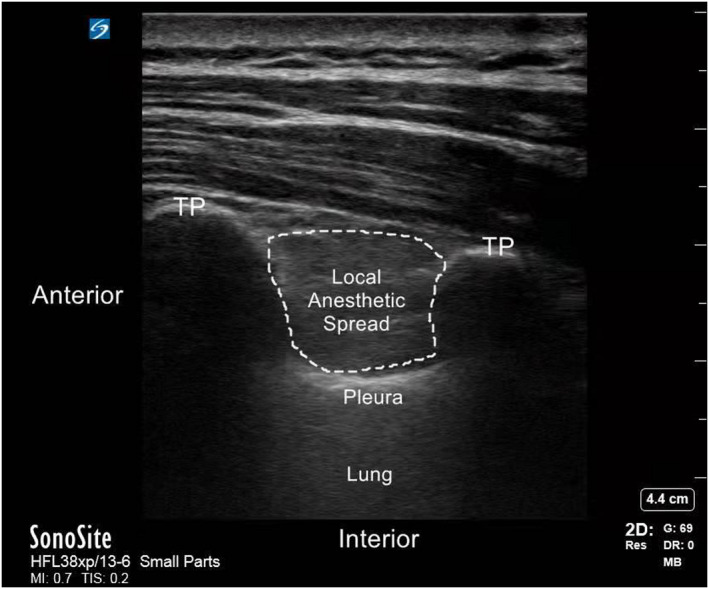
Schematic diagram of ultrasound guided thoracic paravertebral block. TP, transverse process.
Primary outcome
Both PN and i.v. Dex showed superior analgesic effects compared with the control group. In the PN‐Dex group, the duration of analgesia was longer than those in both the control and i.v.‐Dex groups, and the duration in the i.v.‐Dex group was longer than that in the control group. The median (95% confidence interval [CI]) difference in duration of analgesia for the PN‐Dex group was 5.2 h (2.6–10.4 h) compared with that of the control group, and 2.2 h (1.3–3.8 h) compared with that of the i.v.‐Dex group. The median difference between the i.v.‐Dex and control groups was 4.3 h (2.2–8.1 h). See Figure 5.
FIGURE 5.
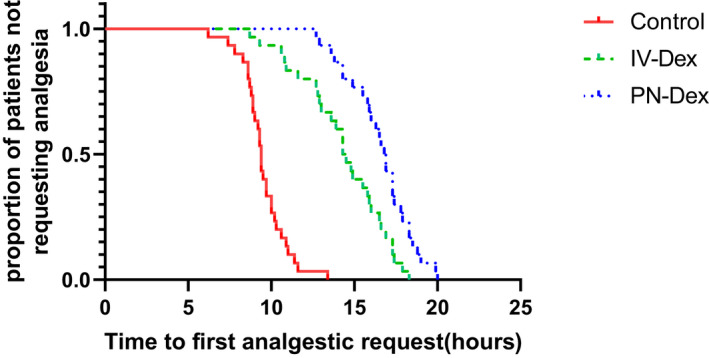
Kaplan–Meier survival plot for the time to first analgesia request. p < 0.001 (long‐rank test). The median (95% confidence interval [CI]) difference in analgesia duration for perineural dexamethasone compared with saline was 5.2 h (2.6–10.4 h), and 2.2 h (1.3–3.8 h) compared with the i.v.‐Dex group. The median difference between i.v.‐Dex and saline was 4.3 (2.2–8.1). Dex, dexamethasone; PN, perineural.
Secondary outcomes
The time course of the postoperative VAS score at rest and during coughing is shown in Figure 6; the VAS scores in the PN‐Dex and i.v.‐Dex groups were significantly lower compared with those in the control group both at rest and during coughing from 2 to 12 h postoperatively, and the VAS scores in the PN‐Dex group were lower than those in the i.v. group. Table 3 shows the postoperative analgesia consumption among the three groups. We found that both PN and i.v. Dex decreased the numbers of pressions for PCIA, the total amount of opioids and rescue analgesia compared with those of the control group, but there was no statistical significance between these results in the PN‐Dex and i.v.‐Dex groups. The intensities of PONV in the PN‐Dex and i.v.‐Dex groups were lower than those in the control group (Table 4), and the mean postoperative blood glucose in both the PN‐Dex and i.v.‐Dex groups was higher than that in the control group but levels were within normal ranges (Table 5). No patients experienced persistent nerve palsy or subjective symptoms of LA toxicity, and other postoperative adverse reactions did not differ significantly among the three groups (Table 4). For the incidence of chronic pain (Table 6), we found Dex (both in PN and i.v. administration) could decrease the incidence of CPSP and NP at 3 months after surgery, but without other clinically significant difference between i.v.‐Dex and PN‐Dex groups.
FIGURE 6.
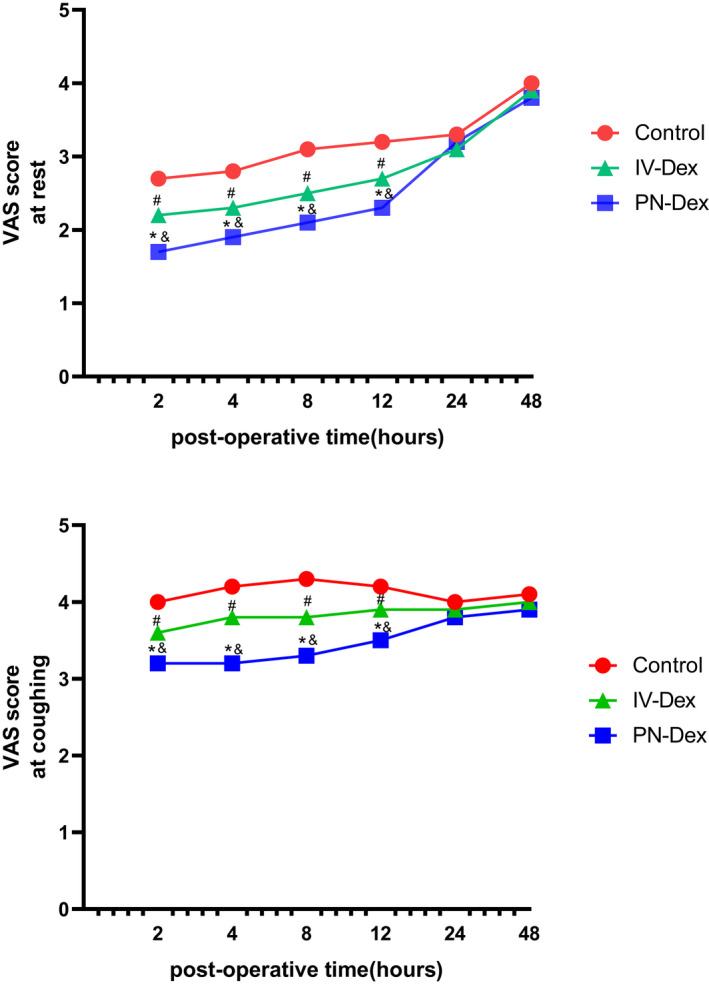
The VAS scores during 48 h after surgery at rest and coughing. Data are presented as mean ± SD. * p, the comparison between PN‐Dex group and control group was statistically significant (p < 0.05); # p, the comparison between the i.v.‐Dex group and the control group was statistically significant (p < 0.05); & p, the comparison between the PN‐Dex group and the i.v.‐Dex group was statistically significant (p < 0.05). Dex, dexamethasone; PN, perineural; VAS, visual analogue scale.
TABLE 3.
Postoperative analgesia consumption in three groups (48 h after surgery)
| Control (n = 30) | PN‐Dex (n = 31) | i.v.‐Dex (n = 30) | p Value | |||
|---|---|---|---|---|---|---|
| p a | p b | p c | ||||
| Sum of pressing numbers | 31.2 ± 2.2 | 21.9 ± 3.1* | 22.3 ± 3.6 # | <0.01 | <0.01 | 0.27 |
| Total amount of sufentanil (μg) | 91.2 ± 6.8 | 68.4 ± 8.9* | 65.3 ± 7.5 # | <0.01 | <0.01 | 0.13 |
| Numbers of rescue analgesia (%) | 8 (26.7) | 1 (3.2)* | 2 (6.7) | 0.03 | 0.08 | 0.61 |
Note: p a, PN‐Dex group vs. control group; p b, i.v.‐Dex group vs. control group; p c, PN‐Dex group vs. i.v.‐Dex group.
Abbreviations: Dex, dexamethasone; PN, perineural.
The comparison between the PN‐Dex group and control group was statistically significant (p < 0.05).
The comparison between i.v.‐Dex group and control group is statistically significant (p < 0.05).
TABLE 4.
Comparison of postoperative adverse reactions among three groups
| Control (n = 30) | PN‐Dex (n = 31) | i.v.‐Dex (n = 30) | p value | |||
|---|---|---|---|---|---|---|
| p a | p b | p c | ||||
| PONV (%) | 10 (33.3) | 2 (6.5)* | 1 (3.3) # | 0.01 | <0.01 | 0.99 |
| Respiratory depression (%) | 1 (3.3) | 0 (0) | 2 (6.7) | 0.49 | >0.99 | 0.24 |
| Urinary retention (%) | 0 (0) | 1 (3.2) | 2 (6.7) | >0.99 | 0.49 | 0.61 |
| Persistent nerve palsy (%) | 0 (0) | 0 (0) | 0 (0) | >0.99 | >0.99 | >0.99 |
Note: p a, PN‐Dex group vs. control group; p b, i.v.‐Dex group vs. control group; p c, PN‐Dex group vs. i.v.‐Dex group.
Abbreviations: Dex, dexamethasone; PN, perineural; PONV, postoperative nausea and vomiting.
The comparison between PN‐Dex group and control group was statistically significant (p < 0.05).
The comparison between the i.v.‐Dex group and the control group was statistically significant. (p < 0.05).
TABLE 5.
Comparison of blood glucose values among three groups (24 h after surgery)
| Control (n = 30) | PN‐Dex (n = 31) | i.v.‐Dex (n = 30) | p Value | |||
|---|---|---|---|---|---|---|
| p a | p b | p c | ||||
| Baseline | 5.3 ± 0.7 | 5.0 ± 0.9 | 4.9 ± 0.6 | 0.26 | 0.10 | 0.86 |
| Postoperative | 5.6 ± 0.6 | 6.3 ± 0.5* | 6.1 ± 0.7 # | <0.01 | <0.01 | 0.40 |
Note: Data represent mean ± SD, mmol/L. p a, PN‐Dex group vs. control group; p b, i.v.‐Dex group vs. control group; p c, PN‐Dex group vs. i.v.‐Dex group.
Abbreviations: Dex, dexamethasone; PN, perineural.
The comparison between the PN‐Dex group and the control group was statistically significant (p < 0.05).
The comparison between the i.v.‐Dex group and the control group was statistically significant (p < 0.05).
TABLE 6.
Incidence of postoperative chronic pain in three groups (3 months after surgery)
| Control (n = 30) | PN‐Dex (n = 31) | i.v.‐Dex (n = 30) | p value | |||
|---|---|---|---|---|---|---|
| p a | p b | p c | ||||
| CPSP (%) | 10 (33.3) | 3 (9.7)* | 2 (6.7) # | 0.03 | 0.02 | 0.99 |
| NP (%) | 9 (30) | 2 (6.5)* | 1 (3.3) # | 0.02 | 0.01 | 0.99 |
Note: p a, PN‐Dex group vs. control group; p b, i.v.‐Dex group vs. control group; p c, PN‐Dex group vs. i.v.‐Dex group.
Abbreviations: CPSP, chronic postsurgical pain; NP, neuropathic pain; Dex, dexamethasone; PN, perineural.
The comparison between the PN‐Dex group and the control group was statistically significant (p < 0.05).
The comparison between the i.v.‐Dex group and the control group was statistically significant (p < 0.05).
DISCUSSION
This double‐blinded randomized study compared the effect of different administration routes of Dex on block duration and analgesic effects in the context of TPVB in patients undergoing Ivor‐Lewis esophagectomy. To our knowledge, this is the first study to compare the analgesic duration between the two routes of administration in TPVB in patients undergoing thoracic surgery. We observed a reduction in perioperative analgesic drug consumption and a relatively long duration of analgesia in both the PN and i.v. Dex groups compared with those receiving ropivacaine alone. Significantly, PN Dex administration significantly improved the duration of analgesia and enhanced the analgesic effect compared to that of the i.v.‐Dex group. These results extend the knowledge of the superiority of Dex for the management of perioperative pain in the setting of Ivor‐Lewis esophagectomy.
Another important result of our study was the decreased incidence of chronic pain associated with PN or i.v. Dex compared with that of the control group. Our results are similar to the findings of Yu Mao et al. 7 who compared only PN Dex and saline and showed a longer block duration and incidence of CPSP with the use of Dex in TPVB. However, there was no significant difference relative to a large CI between PN and i.v. Dex on the incidence of CPSP. Because our follow‐up for chronic pain was relatively simple (just at 3 months after surgery), these conclusions need to be further explored.
In general, the safety of Dex as a PN adjuvant is promising. 17 Even though several studies have investigated the potential neuronal toxicity of Dex and other adjuvants, these data are limited to animal observations. 17 , 18 None of our study patients presented neurotoxicity or any other complaints related to Dex. Adverse events associated with the single‐dose use of Dex were rare. 19 A recent review 20 that examined the adverse side effects of short‐term treatment with Dex during surgery confirmed that Dex leads to a mild increase in blood sugar, but probably does not increase the risk of infection or the time it takes for surgical wounds to heal after surgery, which was similar to our results. The mean postoperative blood glucose in both the PN‐Dex and i.v.‐Dex groups was higher than that in the control group but levels were within normal ranges. Whether the increase in blood sugar results in any healing of surgical wounds has yet to be established.
The reason Dex reduces pain is not known. The decrease in pain intensity and the prolonged analgesia attained with the use of PN Dex may be the result of a systemic or local action, or both. 21 The PN Dex route might act locally on glucocorticosteroid receptors to induce vasoconstriction and have a direct effect on nerve cells to reduce neural discharge, 22 , 23 which might explain the advantage of PN Dex administration. In addition, the systemic effect of Dex in reducing the inflammatory response caused by surgical tissue injury cannot be ignored.
Intravenous Dex has been proven to be an effective adjunct in different regimens for decreasing postoperative pain and opioid consumption, as well as preventing PONV. 12 , 24 , 25 Our study confirmed that both i.v. and PN Dex administration could prolong the analgesic duration of TPVB and decrease the perioperative opioid consumption and VAS scores postoperatively. Moreover, a significant reduction in PONV in the i.v. and PN Dex groups was observed compared with the control group, which further confirmed the effectiveness of Dex in preventing PONV. Additionally, the dose of Dex (8 mg) administered in our research was consistent with the effective dose recommended by De Oliveira et al. 26 In addition, PN Dex administration was more effective than i.v.‐Dex with respect to the analgesic duration and VAS scores during the postoperative period. We found that there are no data regarding PN Dex compared to i.v. Dex for TPVB, which is mostly focused on interscalene brachial plexus blocks. A recent systematic review and meta‐analysis 27 confirmed the superiority of PN Dex compared with i.v. Dex for brachial plexus block. Several studies suggested that i.v. Dex provided superior analgesia, 17 , 28 , 29 and yet other studies found that there were no statistically significant differences between groups to conclude equivalence. 14 , 30
There are possible explanations for the controversial topic between our results and other studies identifying these differences. First, the concentration of Dex and block volumes among these studies are different. Previous studies reported superiority of the perineural route when using a low PN Dex dose (<8 mg). 17 In contrast, those studies using a high PN Dex dose (>8 mg) have found similar block durations for the two routes when added to long‐acting LAs. 26 , 27 Thus, we cannot exclude the possibility that a high Dex dose (>8 mg) could favor i.v. administration and thereby have parity with the PN route. Therefore, combined with our preliminary results, we chose a higher Dex dose (8 mg) to determine if PN administration has a superior analgesic effect over the i.v. route. Second, different nerve block types also influence the optimal administration of Dex. As the anatomy and tissue density are different, the Dex concentration in direct proximity to the plexus may be different, although we chose the same dose. It is reasonable to draw a different conclusion between sciatic nerve blockade 14 outcomes and our results.
Our study still had some limitations. First, our study is a single‐center study with a relatively small sample size, which implies that these conclusions need to be confirmed in a larger and multicentric study in the future. Second, we did not assess the incidence of CPSP in long‐term follow‐up in patients undergoing Ivor‐Lewis esophagectomy. We assessed the data only at 3 months after surgery. Further studies are needed to determine the equivalence of PN and i.v. routes in the incidence of CPSP. Third, the Dex dose chosen in this study was 8 mg, but the optimal dose of Dex for PN administration needs to be further explored.
In summary, the study investigated whether PN administration of Dex (8 mg) significantly improved the effect of analgesia and enhanced the analgesic effect as compared with that in the i.v.‐Dex group in TPVB in Ivor‐Lewis esophagectomy. However, there were no clinically significant differences between the two groups in the incidence of chronic pain.
AUTHOR CONTRIBUTIONS
Y.Z., L.Q., and L.W. wrote the manuscript. L.W. and Y.C. designed the research. Y.Z. and L.Q. performed the research. K.W. and W.D. analyzed the data. Y.C. and K.W. contributed new reagents/analytical tools.
CONFLICT OF INTEREST
The authors declared no competing interests for this work.
ACKNOWLEDGEMENTS
The authors thank Xin Wang and Yangzi Zhu, XuZhou Central Hospital, XuZhou, China, for providing technical support.
Zhang Y, Qiao L, Ding W, Wang K, Chen Y, Wang L. Comparison of the effects of perineural or intravenous dexamethasone on thoracic paravertebral block in Ivor‐Lewis esophagectomy: A double‐blind randomized trial. Clin Transl Sci. 2022;15:1926‐1936. doi: 10.1111/cts.13304
Yan Zhang and Lu Qiao contributed equally to this work.
Funding information
National Natural Science Foundation of China (grant number 81870857).
Contributor Information
Yuqiong Chen, Email: cosmoscyq@163.com.
Liwei Wang, Email: doctorlww@sina.com.
REFERENCES
- 1. Merritt RE, Kneuertz PJ, D'Souza DM, et al. Total laparoscopic and thoracoscopic Ivor Lewis esophagectomy after neoadjuvant chemoradiation with minimal overall and anastomotic complications. J Cardiothorac Surg. 2019;14(1):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brown MJ, Kor DJ, Allen MS, et al. Dual‐epidural catheter technique and perioperative outcomes after Ivor‐Lewis esophagectomy. Reg Anesth Pain Med. 2013;38(1):3‐8. [DOI] [PubMed] [Google Scholar]
- 3. Wang J, Yin Y, Zhu Y, et al. Thoracic epidural anaesthesia and analgesia ameliorates surgery‐induced stress response and postoperative pain in patients undergoing radical oesophagectomy. J Int Med Res. 2019;47(12):6160‐6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Booka E, Nakano Y, Mihara K, et al. The impact of epidural catheter insertion level on pain control after esophagectomy for esophageal cancer. Esophagus. 2020;17(2):175‐182. [DOI] [PubMed] [Google Scholar]
- 5. Li W, Li Y, Huang Q, et al. Short and long‐term outcomes of epidural or intravenous analgesia after esophagectomy: a propensity‐matched cohort study. PLoS One. 2016;11(4):e154380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saeki H, Ishimura H, Higashi H, et al. Postoperative management using intensive patient‐controlled epidural analgesia and early rehabilitation after an esophagectomy. Surg Today. 2009;39(6):476‐480. [DOI] [PubMed] [Google Scholar]
- 7. Mao Y, Zuo Y, Mei B, et al. Efficacy of perineural dexamethasone with ropivacaine in thoracic paravertebral block for postoperative analgesia in elective thoracotomy: a randomized, double‐blind, placebo‐controlled trial. J Pain Res. 2018;11:1811‐1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sabesan VJ, Shahriar R, Petersen‐Fitts GR, et al. A prospective randomized controlled trial to identify the optimal postoperative pain management in shoulder arthroplasty: liposomal bupivacaine versus continuous interscalene catheter. J Shoulder Elbow Surg. 2017;26(10):1810‐1817. [DOI] [PubMed] [Google Scholar]
- 9. Morita S, Oizumi N, Suenaga N, Yoshioka C, Yamane S, Tanaka Y. Dexamethasone added to levobupivacaine prolongs the duration of interscalene brachial plexus block and decreases rebound pain after arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2020;29(9):1751‐1757. [DOI] [PubMed] [Google Scholar]
- 10. Zhang P, Liu S, Zhu J, Rao Z, Liu C. Dexamethasone and dexmedetomidine as adjuvants to local anesthetic mixture in intercostal nerve block for thoracoscopic pneumonectomy: a prospective randomized study. Reg Anesth Pain Med. 2019;44:917‐922. [DOI] [PubMed] [Google Scholar]
- 11. Aliste J, Leurcharusmee P, Engsusophon P, et al. A randomized comparison between intravenous and perineural dexamethasone for ultrasound‐guided axillary block. Can J Anaesth. 2017;64(1):29‐36. [DOI] [PubMed] [Google Scholar]
- 12. Sehmbi H, Brull R, Ceballos KR, et al. Perineural and intravenous dexamethasone and dexmedetomidine: network meta‐analysis of adjunctive effects on supraclavicular brachial plexus block. Anaesthesia. 2021;76(7):974‐990. [DOI] [PubMed] [Google Scholar]
- 13. Desmet M, Braems H, Reynvoet M, et al. I.V. and perineural dexamethasone are equivalent in increasing the analgesic duration of a single‐shot interscalene block with ropivacaine for shoulder surgery: a prospective, randomized, placebo‐controlled study. Br J Anaesth. 2013;111(3):445‐452. [DOI] [PubMed] [Google Scholar]
- 14. Rahangdale R, Kendall MC, Mccarthy RJ, et al. The effects of perineural versus intravenous dexamethasone on sciatic nerve blockade outcomes: a randomized, double‐blind, placebo‐controlled study. Anesth Analg. 2014;118(5):1113‐1119. [DOI] [PubMed] [Google Scholar]
- 15. Leurcharusmee P, Aliste J, van Zundert TCRV, et al. A multicenter randomized comparison between intravenous and perineural dexamethasone for ultrasound‐guided infraclavicular block. Reg Anesth Pain Med. 2016;41(3):328‐333. [DOI] [PubMed] [Google Scholar]
- 16. Schug SA, Lavand'Homme P, Barke A, et al. The IASP classification of chronic pain for ICD‐11: chronic postsurgical or posttraumatic pain. Pain. 2019;160(1):45‐52. [DOI] [PubMed] [Google Scholar]
- 17. Sakae TM, Marchioro P, Schuelter‐Trevisol F, Trevisol DJ. Dexamethasone as a ropivacaine adjuvant for ultrasound‐guided interscalene brachial plexus block: a randomized, double‐blinded clinical trial. J Clin Anesth. 2017;38:133‐136. [DOI] [PubMed] [Google Scholar]
- 18. Kumar S, Palaria U, Sinha AK, et al. Comparative evaluation of ropivacaine and ropivacaine with dexamethasone in supraclavicular brachial plexus block for postoperative analgesia. Anesth Essays Res. 2014;8(2):202‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Waldron NH, Jones CA, Gan TJ, Allen TK, Habib AS. Impact of perioperative dexamethasone on postoperative analgesia and side‐effects: systematic review and meta‐analysis. Br J Anaesth. 2013;110(2):191‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Polderman JA, Farhang‐Razi V, Van Dieren S, et al. Adverse side effects of dexamethasone in surgical patients. Cochrane Database Syst Rev. 2018;11:CD011940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pehora C, Pearson AME, Kaushal A, Crawford MW, Johnston B, Cochrane Anaesthesia Group . Dexamethasone as an adjuvant to peripheral nerve block. Cochrane Database Syst Rev. 2017;11:CD011770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cummings KR, Napierkowski DE, Parra‐Sanchez I, et al. Effect of dexamethasone on the duration of interscalene nerve blocks with ropivacaine or bupivacaine. Br J Anaesth. 2011;107(3):446‐453. [DOI] [PubMed] [Google Scholar]
- 23. Wang PH, Tsai CL, Lee JS, Wu KC, Cheng KI, Jou IM. Effects of topical corticosteroids on the sciatic nerve: an experimental study to adduce the safety in treating carpal tunnel syndrome. J Hand Surg Eur. 2011;36(3):236‐243. [DOI] [PubMed] [Google Scholar]
- 24. Desmet M, Vanneste B, Reynvoet M, et al. A randomised controlled trial of intravenous dexamethasone combined with interscalene brachial plexus blockade for shoulder surgery. Anaesthesia. 2015;70(10):1180‐1185. [DOI] [PubMed] [Google Scholar]
- 25. Kang RA, Jeong JS, Yoo JC, et al. Improvement in postoperative pain control by combined use of intravenous dexamethasone with intravenous dexmedetomidine after interscalene brachial plexus block for arthroscopic shoulder surgery: a randomised controlled trial. Eur J Anaesthesiol. 2019;36(5):360‐368. [DOI] [PubMed] [Google Scholar]
- 26. De Oliveira GJ, Almeida MD, Benzon HT, McCarthy RJ. Perioperative single dose systemic dexamethasone for postoperative pain: a meta‐analysis of randomized controlled trials. Anesthesiology. 2011;115(3):575‐588. [DOI] [PubMed] [Google Scholar]
- 27. Heesen M, Klimek M, Imberger G, Hoeks SE, Rossaint R, Straube S. Co‐administration of dexamethasone with peripheral nerve block: intravenous vs perineural application: systematic review, meta‐analysis, meta‐regression and trial‐sequential analysis. Br J Anaesth. 2018;120(2):212‐227. [DOI] [PubMed] [Google Scholar]
- 28. Kahn RL, Cheng J, Gadulov Y, Fields KG, YaDeau JT, Gulotta LV. Perineural low‐dose dexamethasone prolongs interscalene block analgesia with bupivacaine compared with systemic dexamethasone: a randomized trial. Reg Anesth Pain Med. 2018;43(6):572‐579. [DOI] [PubMed] [Google Scholar]
- 29. Chun EH, Kim YJ, Woo JH. Which is your choice for prolonging the analgesic duration of single‐shot interscalene brachial blocks for arthroscopic shoulder surgery? Intravenous dexamethasone 5 mg vs. perineural dexamethasone 5 mg randomized, controlled, clinical trial. Medicine (Baltimore). 2016;95(23):e3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mchardy PG, Singer O, Awad IT, et al. Comparison of the effects of perineural or intravenous dexamethasone on low volume interscalene brachial plexus block: a randomised equivalence trial. Br J Anaesth. 2020;124(1):84‐91. [DOI] [PubMed] [Google Scholar]


