Abstract
The IMPROVE study (NCT02408315) compared the efficacy and safety of vaginal and buccal administration of misoprostol for full‐term, uncomplicated labor induction. This report compares the pharmacokinetics of misoprostol between vaginal and buccal routes. Women greater than or equal to 14 years of age undergoing induction of labor greater than or equal to 37 weeks gestation without significant complications were randomized to vaginal or buccal misoprostol 25 μg followed by 50 μg doses every 4 h. Misoprostol acid concentrations were determined using liquid chromatography‐tandem mass spectrometry for the first 8 h in a subgroup of participants. A population pharmacokinetic model was developed using NONMEM. Plasma concentrations (n = 469) from 47 women were fit to a one‐compartment nonlinear clearance model. The absorption rate constant (ka) was dependent on both route and dose of administration: buccal 25 μg 0.724 (95% confidence interval, 0.54–0.92) h−1; 50 μg 0.531 (0.37–0.63) h−1; vaginal 25 μg 0.507 (0. 2–1. 4) h−1; and 50 μg 0.246 (0.103–0.453) h−1. Relative bioavailability for vaginal compared to buccal route was 2.4 (1.63–4.77). There was no effect of body mass index or age on apparent clearance 705 (431–1099) L/h or apparent volume of distribution 632 (343–1008) L. The area under the concentration–time curve to 4 h following the first 25 μg dose of misoprostol was 16.5 (15.4–17.5) pg h/ml for buccal and 34.3 (32.5–36.1) pg h/ml for vaginal administration. The rate of buccal absorption was two times faster than that of vaginal, whereas bioavailability of vaginal administration was 2.4 times higher than that of buccal. Decreased time to delivery observed with vaginal dosing may be due to higher exposure to misoprostol acid compared to buccal.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Vaginal misoprostol administration for full‐term labor induction has a shorter time to delivery compared to buccal. Pharmacokinetic (PK) studies in first trimester pregnancies show higher plasma concentrations of misoprostol acid (MPA) following vaginal versus buccal administration of misoprostol.
WHAT QUESTION DID THIS STUDY ADDRESS?
This study compares PKs of MPA following vaginal and buccal administration for women undergoing full‐term labor induction in at term.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Vaginal misoprostol administration results in higher bioavailability, slower rate of absorption, and produces higher plasma concentrations of MPA compared to buccal administration when administered for full‐term induction of labor.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
This population PK model explains differences in clinical outcomes observed in women treated with vaginal versus buccal misoprostol for induction of labor at term. This can help inform clinician choice of route of administration for misoprostol for labor induction.
INTRODUCTION
The rate of labor induction has significantly increased in the United States, from 16.9% in 1996 to 24.5% in 2016. 1 Labor induction at term (>37 weeks gestation) may be recommended for many reasons. Cervical ripening and facilitation of uterine contraction are necessary for normal parturition. Misoprostol is a synthetic prostaglandin E1 analog (PGE1) that can be administered orally, buccally, sublingually, rectally, or intravaginally, and is widely used in clinical practice for cervical ripening and induction of uterine contractions. 2 Misoprostol is manufactured as 100‐ and 200‐μg unscored tablets. The American College of Obstetricians and Gynecologists (ACOG) recommends breaking the 100‐μg tablet to provide 25 μg doses every 3–6 h, stating that 50 μg doses every 6 h might be appropriate in some situations. 2 The World Health Organization gives a strong recommendation for oral misoprostol administration 25 μg every 2 h, and weak recommendation for vaginal misoprostol administration 25 μg every 6 h. 3
Multiple studies have demonstrated the safety and efficacy of misoprostol for labor induction by various routes. 4 , 5 , 6 After administration, misoprostol is rapidly de‐esterified into its active metabolite, misoprostol acid (MPA), which is observed at measurable concentrations in plasma. 7 A number of studies have assessed pharmacokinetics (PKs) of misoprostol in non‐pregnant women and during the first trimester of pregnancy. 8 , 9 , 10 , 11 , 12 , 13 , 14 These studies found significant differences in the area under the curve (AUC) and peak plasma concentrations (Cmax) between routes of administration. Oral and sublingual administration have faster absorption profiles and higher Cmax than vaginal and buccal routes. Vaginal absorption is more prolonged, with lower Cmax. Pregnancy leads to numerous physiologic changes that impact the PKs of drugs, including increased body size and total body water, changes in protein binding, increased renal clearance, and alterations in drug metabolizing enzymes. 15 , 16 Importantly, changes to the vaginal mucosa and epithelium, including alterations in pH, thickness, and blood flow, may lead to changes in vaginal drug absorption. 17 Despite the potential effects of parturition on PKs, minimal information is available regarding the PKs of misoprostol in pregnant women at term. One study describes the PKs of MPA following rectal or oral administration of 600 μg misoprostol during the third stage of labor. 11 Recently, Amini et al. reported PKs of two formulations of misoprostol following oral and sublingual administration at term. 18 To our knowledge, no study has compared the PKs of vaginal versus buccal administration of misoprostol for full‐term labor induction.
Historically, vaginal administration of misoprostol has been most common in the United States. However, there has been a recent practice shift toward buccal administration. The IMPROVE trial was a triple‐masked randomized placebo‐controlled trial that compared the efficacy and safety of vaginal versus buccal misoprostol in women undergoing labor induction at term. 19 The study was designed to test noninferiority of buccal administration compared to vaginal. Although noninferiority of buccal administration was not demonstrated, it did appear that vaginal administration was superior based on a higher rate of vaginal deliveries, faster time to delivery, and lower rate of cesarean sections for fetal distress. A secondary aim of the IMPROVE study, and the objective of this report, was to assess the PK characteristics of vaginal and buccal misoprostol in women at term.
METHODS
Study participants
The IMPROVE trial recruited 319 pregnant women (300 available for analysis) to compare safety and efficacy of buccal versus vaginal misoprostol for labor induction at term; 50 (25 per arm) participants were recruited into this PK substudy. The trial was conducted under a US Food and Drug Administration Investigational New Drug application (IND #122727), was approved by the Indiana University institutional review board, and was registered on clinicaltrials.gov (NCT 02408315). The participants were recruited and the study was carried out at Eskenazi Health and Indiana University Health Methodist Hospitals in Indianapolis, IN. All women provided written informed consent prior to participation in the study. A detailed description of the study has been previously reported. 19 Women who consented to the PK portion of the study were randomized separately from those who declined participation in the PK component.
Women were eligible for enrollment in the study if they were at least 14 years of age and undergoing either a medically indicated induction of labor at a gestational age beyond 370/7 weeks or an elective induction of labor after 390/7 weeks with a singleton pregnancy in the cephalic presentation and a modified Bishop Score of less than or equal to six. Women were excluded if they had a known allergy to misoprostol, known prior uterine scarring, untreated cervical infection, prior induction/cervical ripening methods during this pregnancy, any contraindication to labor induction or misoprostol therapy, planned cesarean section due to maternal or fetal condition, known major fetal congenital anomaly, or other evidence of fetal compromise (category 2 or 3 fetal tracing) before initiating induction.
Drug administration and blood sampling
Misoprostol 100 μg tablets were purchased from Novel Laboratories (Somerset, NJ). Placebo tablets that looked identical to controls were obtained from the University of Iowa Pharmaceuticals. Investigational pharmacists at participating sites split the tablets to provide 25 or 50 μg doses of misoprostol. Misoprostol or placebo tablets were then packaged in identical foil packets labeled “vaginal” or “buccal.” Other than the investigational pharmacists, who did not have direct subject contact, no investigators, providers, or patients had knowledge of the randomization assignment.
Misoprostol was administered vaginally (vaginal group) or buccally (buccal group) and placebo administered via the opposite route at an initial dose of 25 μg. Subsequent doses of 50 μg misoprostol were administered every 4 h, if clinically indicated. Study participation and drug administration continued until one of the following occurred: (1) there was adequate response and cervical ripening was no longer needed; (2) there were signs of tachysystole, nonreassuring fetal heart tracing, or other adverse event that led the provider to discontinue misoprostol; or (3) 24 h of study drug had been administered (maximum of 7 doses). For the PK study, serial blood samples were collected into EDTA‐treated tubes prior to and at ~0.25, 0.5, 1, 2, 3, and 4 h following administration of the first two doses of misoprostol. Samples were processed to plasma and stored at −80°C until analysis.
Misoprostol acid analysis
The MPA was measured by the Indiana University Simon Comprehensive Cancer Center Clinical Pharmacology Analytic Core (CPAC) Laboratory. MPA was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX). Methyl tertiary butyl ether (high‐performance liquid chromatography [HPLC] grade), methanol (Optima liquid chromatography mass spectrometry (LC‐MS] grade), acetonitrile (Optima LC‐MS grade), ammonium acetate, disodium phosphate, and phosphoric acid were purchased from Fisher Scientific (Fairlawn, NJ). Temazepam, sodium hydroxide, and citric acid monohydrate were purchased from Sigma Aldrich Chemical Co. (St. Louis, MO). Deionized water was purified using a Barnstead Nanopure Infinity ultrapure water system (Boston, MA). EDTA‐treated human plasma for standard curve and quality control samples was purchased from BioIVT (Westbury, NY).
Internal standard, temazepam (20 μl of 0.01 ng/μl), and 0.5 ml of 0.1 M citric acid buffer (pH 3.0) were added to 1.0 ml plasma. Samples were extracted with methyl tertiary butyl ether and the supernatant transferred to clean tubes and evaporated to dryness. Samples were reconstituted with 50 μl of methanol and a 10 μl aliquot was injected to the HPLC‐tandem mass spectrometry (HPLC‐MS/MS) system. Standards (0.3–300 pg/ml) and quality controls (1, 10, and 200 pg/ml) were processed identically. Limit of quantification was 0.3 pg/ml and inter‐ and intra‐day coefficients of variation were <15%.
MPA and temazepam quantification was carried out on an ExionLC coupled to a Sciex 6500+ QTRAP (Framingham, MA) HPLC‐MS/MS system. Chromatographic separation was achieved by a gradient mobile phase with acetonitrile: 5 mM ammonium acetate (mobile phase A: 20:80; v/v, and mobile phase B: 80:20; v/v) using a Phenomenex Kinetex C18 150 × 4.6 mm 2.6 μm column (Phenomenex, Torrance, CA). Flow rate was constant at 400 μl/min. At time zero, the mobile phase was 100% mobile phase A, by 2 min the mobile phase was 100% mobile phase B and held until 7 min. At 7.1 min, the gradient returned to 100% mobile phase A and held until 10 min for column equilibration. The mass spectrometer (MS) utilized an electrospray ionization probe in both positive and negative mode. MPA detection occurred in negative mode using the MS3 scan mode, the MS/MS/MS analysis was 367.2/249.2/151.2. Temazepam detection in positive mode used the multiple reaction monitoring scan mode at 301.0/255.0.
Pharmacokinetic analysis
Plasma PKs of MPA were characterized using a population PK model. NONMEM version 7.5 (ICON Development Solutions, Hanover, MD), 20 PsN 21 with Pirana version 3.0.0 (Pirana Software and Consulting, San Francisco, CA) 22 we utilized for model development. R version 3.5.3 23 was used for data cleaning, management, and graphical and statistical analyses.
First‐order conditional estimation with interaction method and one‐ and two‐compartment models were tested utilizing ADVAN2 and ADVAN4 subroutines, respectively. Nonlinear clearance mechanisms were evaluated using ADVAN6 subroutine. Interindividual and interoccasion variabilities were assumed to be log‐normally distributed. Residual variability was modeled by a proportional error model. Model development was guided by decrease of the objective function value (OFV) and diagnostic plots. After the base model was established, body mass index (BMI), race, gestational age, and maternal age were assessed as covariates. The relationships between model parameters and covariates were evaluated by plotting covariates versus empirical Bayes estimates of model parameters. Goodness‐of‐fit was evaluated using diagnostic plots, including observation versus individual prediction, observation versus population prediction (PRED), conditional weighted residual (CWRES) versus PRED, and CWRES versus time. A bootstrap resampling technique (n = 200) was used to determine the precision of the final model parameter estimates. The predictive performance of the model was evaluated with visual predictive check (VPC; n = 200) comparing the 5th, 50th, and 95th percentiles of predicted concentrations with observed concentrations of MPA. Area under the concentration–time profile (AUC) of MPA from 0 to 4 h following a 25 μg dose of misoprostol were determined from individual predicted (IPRED) plasma concentration–time profiles using noncompartmental methods.
RESULTS
Fifty women were initially enrolled in the PK substudy. Plasma observations from three study participants were excluded: two women in the vaginal misoprostol group expulsed the tablet shortly after it was placed; one subject was removed from the study shortly after placement of the first dose due to provider request. Demographics of the remaining 47 women are detailed in Table 1. There were no significant differences in age, BMI, gestational age, or race between the vaginal and buccal misoprostol groups. Concentrations below the lower limit of quantification after the initial dose were also excluded (n = 3). A total of 469 plasma observations from 47 subjects were analyzed.
TABLE 1.
Demographic characteristics of the study population
| Buccal misoprostol, median (95% CI) | Vaginal misoprostol, median (95% CI) | |
|---|---|---|
| N | 24 | 23 |
| Age, years | 26 (23.2–28.7) | 24 (21.6–26.4) |
| BMI, kg/m2 | 35 (31.6–38.3) | 36 (32.3–39.8) |
| Gestational age, weeks | 39 (38.5–39.6) | 39.8 (39.3–40.3) |
| RACE | ||
| African American/Black | 8 (33%) | 7 (30%) |
| White | 11 (46%) | 12 (52%) |
| Other | 5 (21%) | 4 (17%) |
Abbreviations: BMI, body mass index; CI, confidence interval.
Observed MPA concentration versus time plots demonstrated that absorption and bioavailability differed by route of administration (Figure 1). Thus, we included a relative bioavailability term (Fv/b) for the vaginal route relative to buccal (Fb, assumed to be one), and separate absorption rate constants (ka) for each route from the beginning of the model development. Based on OFV and goodness‐of‐fit diagnostic plots, a one‐compartment population PK model with nonlinear clearance was selected as a structural model. Interindividual variability (IIV) was estimated for apparent clearance (CL/Fb), apparent volume of distribution (V/Fb), and ka, and interoccasion variability (IOV) was estimated for Fv/b. Final model parameters are provided in Table 2. Introducing nonlinear clearance to the model did not significantly improve OFV, but it did improve individual fits. Addition of separate ka for each dose (25 and 50 μg) and route of administration, and incorporation of IOV on Fv/b significantly decreased OFV. No correlations were identified among BMI or age and empirical Bayes estimates for V/Fb, CL/Fb, or ka (Figure S1). Diagnostic plots demonstrate a satisfactory model fit (Figure 2a,b) based on both IPRED and population PRED versus observed concentrations, although with notable underprediction at the peak concentrations. Conditional weighted residuals were normally distributed around zero when plotted against predicted concentrations and time (Figure 2c,d).
FIGURE 1.
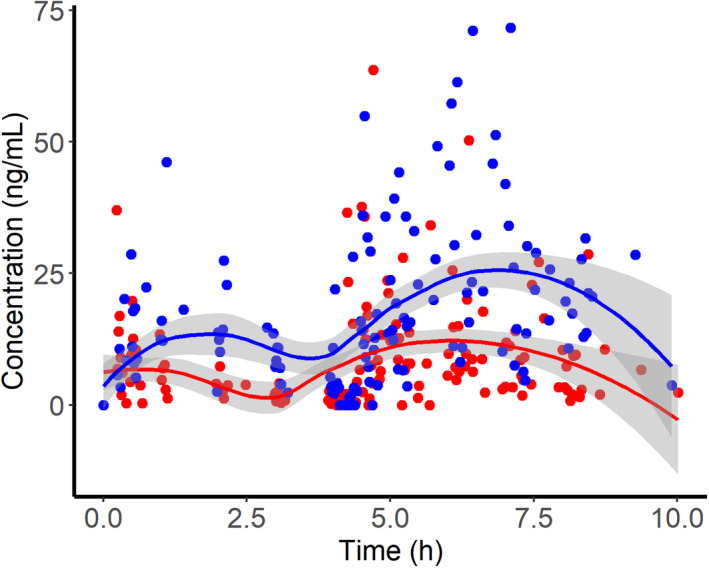
Observed misoprostol acid plasma concentration versus time profiles following vaginal (blue) or buccal (red) administration of misoprostol (25 μg followed by 50 μg at 4 h). Lines and shaded areas indicate the median and 95% confidence interval of a Loess smooth function.
TABLE 2.
Population parameter estimates
| Theta | Estimate | SE | RSE (%) | Bootstrapping median (95% CI) |
|---|---|---|---|---|
| CL/Fb, L/h | 730 | 164 | 22.5 | 705 (431–1099) |
| V/Fb, L | 610 | 204 | 33.4 | 632 (343–1008) |
| Fv/b | 2.3 | 0.479 | 20.8 | 2.4 (1.63–4.77) |
| ka, 1/h (buccal, 25 μg) | 0.709 | 0.111 | 15.7 | 0.724 (0.54–0.92) |
| ka, 1/h (buccal, 50 μg) | 0.537 | 0.0868 | 16.2 | 0.531(0.37–0.63) |
| ka, 1/h (vaginal, 25 μg) | 0.464 | 0.167 | 36 | 0.507 (0.2–1) |
| ka, 1/h (vaginal, 50 μg) | 0.24 | 0.0694 | 28.9 | 0.246 (0.103–0.453) |
| Vmax/Fb, pg/ml | 5.45 | 0.695 | 12.8 | 5.64 (3.141–10.453) |
| Km, pg | 2.5 | 1.03 | 41.2 | 2.864 (0.73–10.41) |
| Omega | Estimate | SE | RSE (%) | Etabar | p Value | Shrinkage (%) |
|---|---|---|---|---|---|---|
| CL, L/h | 0.602 | 0.203 | 33.7 | 0.01 (0.094) | 0.91 | 15.6 |
| V, L | 1.55 | 0.365 | 23.5 | 0.066 (0.151) | 0.66 | 16 |
| k a, 1/h | 0.0599 | 0.0636 | 106.2 | 0.022 (0.027) | 0.31 | 38.3 |
| IOV | 0.0848 | 0.0511 | 60.3 | −0.043 (0.027) | 0.11 | 39.4 |
| Sigma | Estimate | SE | RSE (%) | Shrinkage (%) |
|---|---|---|---|---|
| PROP | 0.262 | 0.0269 | 10.3 | 9 |
Abbreviations: CI, confidence interval; CL, clearance; CL/Fb, estimated for apparent clearance; IOV, interoccasion variability; ka, absorption rate constant; Km, kinetic metabolite; PROP, proportional error; RSE, relative standard error; V, volume; V/Fb, apparent volume of distribution; Vmax/Fb, apparent maximum velocity of drug clearance following buccal administration.
FIGURE 2.
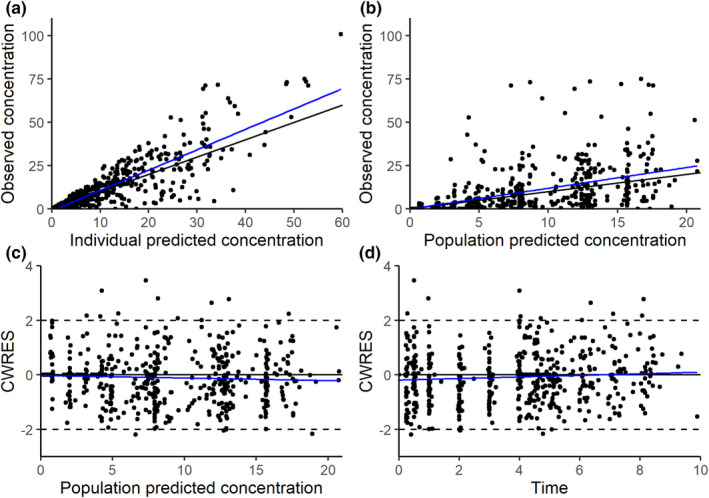
Goodness‐of‐fit plots of the final parametric model: observed versus individual predictions (a), observed versus population prediction (b), conditional weighted residuals (CWRES) versus population predicted concentrations (c), or time after dose (d). Black lines indicate the line of unity a and b or zero c and d, and blue lines indicate linear trends.
A VPC stratified by route of administration was used to compare model predictions with observed plasma concentration–time profiles of MPA (Figure S2). Due to high IIV and relatively small sample size, we observed model misspecification in the absorption profiles. Comparing the distribution of the observed AUC0–4h values to the simulated data was a more appropriate method in this case, and demonstrated that the model was able to replicate the AUC (Figure 3). Bootstrap analysis was performed and median and 95th percentile prediction intervals compared with the final parameter estimates (Table 2). Median parameters estimates were similar to those estimated from the final model. Consistent with high relative standard error (RSE), we noted large confidence intervals (CIs) for CL/Fb, V/Fb, ka,v, and K m.
FIGURE 3.
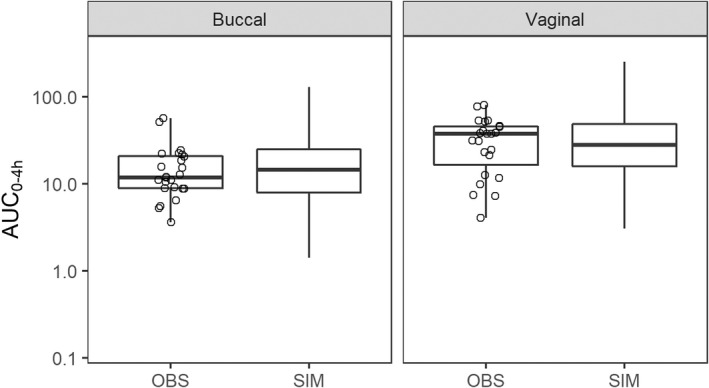
Predictive check plots of misoprostol acid plasma concentrations following buccal and vaginal dosing of 25 μg of misoprostol dose. AUC0–4h, area under the curve from 0 to 4 h; OBS indicate observed data, and SIM indicate simulated data.
Whereas CL/Fb (730 ± 164 L/h) and volume (610 ± 204 L) did not differ with route of administration, vaginal bioavailability of MPA compared to buccal was 2.3 ± 0.479. The rate of buccal absorption was 1.5 times faster for a 25 μg dose and 2.2 times faster for a 50 μg dose than that of vaginal dosing (Table 2). Consistent with differences in bioavailability, the median AUC0–4h following the first 25 μg dose of misoprostol was 16.5 (95% CI, 15.4–17.5) pg h/ml for buccal and 34.3 (95% CI, 32.5–36.1) pg h/ml for vaginal administration.
DISCUSSION
Misoprostol is extensively used in obstetric practice for induction of full‐term labor, treatment of postpartum hemorrhage, and incomplete, missed, and elective abortions in the first trimester. We observed marked differences between the plasma concentration–time profiles of MPA following vaginal and buccal dosing, resulting in higher bioavailability and AUC following vaginal dosing. Studies in first trimester pregnant women and healthy volunteers have compared MPA PK following various routes of misoprostol administration using higher doses (400–800 μg). PKs of misoprostol administered for full‐term labor induction have been challenging to study due to the low (25–50 μg) doses used for this indication. Recent advances in analytical technology now make it possible to detect low concentrations of plasma MPA, and to study the PKs of misoprostol at low doses used for labor induction at term. To our knowledge, this is the first study to compare PK measures of buccal versus vaginal misoprostol for labor induction at term using doses recommended in clinical practice.
The IMPROVE trial found decreased time to delivery and lower rate of cesarean delivery for fetal nonreassurance in women receiving vaginally administered misoprostol compared to buccally administered misoprostol. This difference appears to be related to differences in the PK profile of MPA following vaginal vs. buccal administration. Misoprostol is a highly lipophilic (logP = 3.6 24 ) acid. Differences in buccal and vaginal absorption may be attributed to differences in pH, tissue lipophilicity, blood flow, and volume of fluid (i.e., vaginal secretions or saliva) between the vagina buccal cavities. As patients were instructed to swallow the buccally administered tablet 30 min after administration, it is likely that a portion of the dose was administered orally, and subject to first pass metabolism. Whereas there is a possibility for the vaginal tablet to be expulsed prior to administration of the next tablet, it typically remains in the vagina for a longer period of time, with the residual tablet observed upon placement of the next dose (i.e., after 4 h). Although the MPA concentration of the buccal route achieved peak concentration in a shorter period of time than the vaginal route, the relative bioavailability following vaginal administration was 2.4 times that of buccal. This is consistent with studies of misoprostol in the first trimester of pregnancy. 13 Although absorption through the buccal route is more rapid, the extent of absorption through the vaginal route is higher, which could explain the favorable clinical results of vaginal misoprostol in the IMPROVE trial. In addition to the systemic absorption, vaginally administered misoprostol may also produce local actions which could contribute to the more advantageous results of the vaginal route.
Previous PK studies of MPA used 400–800 μg doses of misoprostol in non‐pregnant women or pregnant women seeking elective termination of pregnancy. 8 , 9 , 10 , 11 , 12 , 13 , 14 The majority of studies report AUC0–4h of MPA following 400 μg vaginal misoprostol in the first trimester of pregnancy to be 330–480 pg h/ml. 9 , 12 , 25 , 26 Meckstroth et al. found significantly higher exposure (925–1126 pg h/ml) following 400 μg vaginal dosing compared to other studies, which they attribute to cervical cleaning prior to placement of a uterine pressure catheter. 13 Zieman et al. did not find significant differences between first trimester pregnant and non‐pregnant women in the PKs of oral and vaginal misoprostol dosed at 400 μg. 14 However, physiologic changes associated with advanced pregnancy, such as increased plasma volume, cardiac output, and changes in drug metabolizing enzymes, likely alter the PKs in women with term gestations. The apparent clearance of MPA observed in our study (730 L/h) is almost twice greater than that observed following 800 μg buccal misoprostol administration in non‐pregnant women (386 L/h). 8 Dose‐corrected AUC0–4h following vaginal administration was over two‐fold higher in our study compared to studies in first‐trimester women. 9 , 12 , 25 , 26 The CL/Fb and V/Fb of MPA in our study were consistent with the PKs observed in a recent study comparing bioavailability of two formulations of misoprostol following oral and sublingual administration in full‐term women. 18 Thus, it appears that clearance of MPA in the third trimester of pregnancy is increased compared to non‐pregnant and first‐trimester pregnant women.
PK samples were collected following the first two doses of misoprostol. We noted high IIV and IOV in the MPA concentrations. This study was designed to closely mirror clinical practice within our institutions. Misoprostol tablets used in the study were 100 μg, the lowest dose formulation available. Investigational pharmacies at each hospital prepared the study dose by halving or quartering the tablets, as is routinely done in clinical practice. Whereas pieces of the tablets were relatively similar in size, it is possible that they contained different amounts of drug due to minor variations in size or non‐homogeneous distribution of misoprostol within the tablet.
Administration by buccal and vaginal routes may lead to additional sources of variability. Although study participants were instructed to keep the buccal dose between the mucous membranes of the cheek and gums for 30 min without disturbing it and then swallow the residual material, it is possible that some swallowed a larger portion of the tablets than others. Vaginal conditions rapidly change during labor, which could have an impact on vaginal absorption of misoprostol. Vaginal pH has been found to positively correlate with time to effect for misoprostol induction of missed abortions between 14 and 26 weeks. 27 The induction to abortion interval was twice as long in women with vaginal pH greater than or equal to five compared to those with vaginal pH less than five. Additionally, pre‐wetting misoprostol has been found to enhance dissolution rate and absorption. 13 , 26 , 28 Physicians in the IMPROVE trial did not receive instructions regarding moistening the tablet; some physicians may have moistened the tablet while others inserted a dry tablet. Similarly, the quantity of vaginal fluid and timing of the rupture of membranes could impact tablet dissolution rates. Tang et al. 12 found that peak plasma concentrations of misoprostol acid were decreased after multiple doses in women <12 weeks gestation undergoing termination, likely secondary to vaginal bleeding. Ruptured membranes additionally may affect absorption following vaginal administration. These factors could significantly impact bioavailability and k a, contributing to the high variability observed. In spite of these limitations, this study is one of the first to describe PKs of misoprostol in third‐trimester pregnant women at doses routinely used for induction of labor.
CONCLUSION
Bioavailability of misoprostol’s active metabolite, MPA, is higher following vaginal dosing than buccal dosing in pregnant women at term receiving similar dosing regimens of misoprostol for induction of labor. However, reduced rate of absorption (k a) results in lower Cmax and a more prolonged absorption phase for vaginal dosing. The increased bioavailability of misoprostol acid following vaginal administration of misoprostol may explain the faster time to delivery in the vaginally dosed group as compared to those who received misoprostol buccally that was observed in the IMPROVE trial.
AUTHOR CONTRIBUTIONS
Y.V., L.L.S., D.M.H., K.F., A.R.M., M.H., and S.K.Q. wrote the manuscript. D.H., K.F., R.C.P., and S.K.Q. designed the research. D.H., K.F., B.Y., G.H., and S.K.Q. performed the research. Y.V., A.R.M., D.G., M.H., L.L.S., and S.K.Q. analyzed the data.
CONFLICT OF INTEREST
The authors declare no competing interests for this work.
Supporting information
Figures S1‐S2
ACKNOWLEDGEMENTS
The authors thank the research staff, clinicians, and patients who participated in this study.
Vorontsova Y, Haas DM, Flannery K, et al. Pharmacokinetics of vaginal versus buccal misoprostol for labor induction at term. Clin Transl Sci. 2022;15:1937‐1945. doi: 10.1111/cts.13306
Funding informationY.V. and R.C.P. were supported by National Institute of General Medical Sciences (NIGMS), National Institutes of Health (NIH) grant T32GM008425. S.K.Q. was supported in part by 5K23HD071134 and P30 HD106451 from the Eunice Kennedy Shriver national Instituted of Child Health and Human Development (NICHD), NIH. The Indiana University School of Medicine Clinical Pharmacology Analytical Core is supported by the IU Simon Comprehensive Cancer Center Support Grant from the National Cancer Institute (NCI), NIH P30 CA082709. Additional funding provided by the Department of Obstetrics and Gynecology, Indiana University and the Indiana CTSI Disease and Therapeutic Response Modeling Program.
REFERENCES
- 1. Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. Births: final data for 2016. Natl Vital Stat Rep. 2018;67:1‐55. [PubMed] [Google Scholar]
- 2. ACOG . Practice bulletin no. 107: induction of labor. Obstet Gynecol. 2009;114:386‐397. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization & Department of Reproductive Health and Research . WHO Recommendations for Induction of Labour; 2011. Accessed January 20, 2022. https://apps.who.int/iris/bitstream/handle/10665/44531/9789241501156_eng.pdf?sequence=1 [Google Scholar]
- 4. Alfirevic Z, Aflaifel N, Weeks A. Oral misoprostol for induction of labour. Cochrane Database Syst Rev. 2014;2014:CD001338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hofmeyr GJ, Gülmezoglu AM, Pileggi C. Vaginal misoprostol for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2010;2010:CD000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Muzonzini G, Hofmeyr GJ. Buccal or sublingual misoprostol for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2004;2004:CD004221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schoenhard G, Oppermann J, Kohn FE. Metabolism and pharmacokinetic studies of misoprostol. Dig Dis Sci. 1985;30:126S‐128S. [DOI] [PubMed] [Google Scholar]
- 8. Frye LJ, Byrne ME, Winikoff B. A crossover spharmacokinetic study of misoprostol by the oral, sublingual and buccal routes. Eur J Contracept Reprod Health Care. 2016;21:265‐268. [DOI] [PubMed] [Google Scholar]
- 9. Aronsson A, Fiala C, Stephansson O, et al. Pharmacokinetic profiles up to 12 h after administration of vaginal, sublingual and slow‐release oral misoprostol. Hum Reprod. 2007;22:1912‐1918. [DOI] [PubMed] [Google Scholar]
- 10. Schaff EA, DiCenzo R, Fielding SL. Comparison of misoprostol plasma concentrations following buccal and sublingual administration. Contraception. 2005;71:22‐25. [DOI] [PubMed] [Google Scholar]
- 11. Khan RU, El‐Refaey H. Pharmacokinetics and adverse‐effect profile of rectally administered misoprostol in the third stage of labor. Obstet Gynecol. 2003;101:968‐974. [DOI] [PubMed] [Google Scholar]
- 12. Tang OS, Schweer H, Lee SW, Ho PC. Pharmacokinetics of repeated doses of misoprostol. Hum Reprod. 2009;24:1862‐1869. [DOI] [PubMed] [Google Scholar]
- 13. Meckstroth KR, Whitaker AK, Bertisch S, Goldberg AB, Darney PD. Misoprostol administered by epithelial routes: drug absorption and uterine response. Obstet Gynecol. 2006;108:582‐590. [DOI] [PubMed] [Google Scholar]
- 14. Zieman M, Fong SK, Benowitz NL, Banskter D, Darney PD. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. 1997;90:88‐92. [DOI] [PubMed] [Google Scholar]
- 15. Abduljalil K, Furness P, Johnson TN, Rostami‐Hodjegan A, Soltani H. Anatomical, physiological and metabolic changes with gestational age during normal pregnancy: a database for parameters required in physiologically based pharmacokinetic modelling. Clin Pharmacokinet. 2012;51:365‐396. [DOI] [PubMed] [Google Scholar]
- 16. Dallmann A, Ince I, Meyer M, Willmann S, Eissing T, Hempel G. Gestation‐specific changes in the anatomy and physiology of healthy pregnant women: an extended repository of model parameters for physiologically based pharmacokinetic modeling in pregnancy. Clin Pharmacokinet. 2017;56:1303‐1330. [DOI] [PubMed] [Google Scholar]
- 17. das Neves J, Notario‐Pérez F, Sarmento B. Women‐specific routes of administration for drugs: a critical overview. Adv Drug Deliv Rev. 2021;176:113865. [DOI] [PubMed] [Google Scholar]
- 18. Amini M, Reis M, Wide‐Swensson D. A relative bioavailability study of two misoprostol formulations following a single oral or sublingual administration. Front Pharmacol. 2020;11:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haas DM, Daggy Joanne, Flannery Kathleen M et al. A comparison of vaginal versus buccal misoprostol for cervical ripening in women for labor induction at term (the IMPROVE trial): a triple‐masked randomized controlled trial. Am J Obstet Gynecol 2019;221:259.e1‐259.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beal B, Sheiner L. NONMEM User's Guide, Part I. University of California at San Francisco; 1992. [Google Scholar]
- 21. Lindbom L, Ribbing J, Jonsson EN. Perl‐speaks‐NONMEM (PsN)—a Perl module for NONMEM related programming. Comput Methods Programs Biomed. 2004;75:85‐94. [DOI] [PubMed] [Google Scholar]
- 22. Keizer RJ, Karlsson MO, Hooker A. Modeling and simulation workbench for NONMEM: tutorial on Pirana, PsN, and Xpose. CPT Pharmacometrics Syst Pharmacol. 2013;2:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2018. https://www.R‐projectorg/. [Google Scholar]
- 24. National Center for Biotechnology Information . PubChem Compound Summary for CID 5282381, Misoprostol . April 10, 2022.
- 25. Khan RU, El‐Refaey H, Sharma S, Sooranna D, Stafford M. Oral, rectal, and vaginal pharmacokinetics of misoprostol. Obstet Gynecol. 2004;103:866‐870. [DOI] [PubMed] [Google Scholar]
- 26. Tang OS, Schweer H, Seyberth HW, Lee SW, Ho PC. Pharmacokinetics of different routes of administration of misoprostol. Hum Reprod. 2002;17:332‐336. [DOI] [PubMed] [Google Scholar]
- 27. Abd‐El‐Maeboud KH, Ghazy AA, Nadeem AA, Al‐Sharaky A, Khalil AE. Effect of vaginal pH on the efficacy of vaginal misoprostol for induction of midtrimester abortion. J Obstet Gynaecol Res. 2008;34:78‐84. [DOI] [PubMed] [Google Scholar]
- 28. Lee VC, Yung SSF, Li RHW, et al. A randomized comparison of pharmacokinetics of a single vaginal dose of dry misoprostol or misoprostol moistened with normal saline or with acetic acid. Hum Reprod. 2011;26:2981‐2987. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1‐S2


