Abstract
Background
Catheter ablation combined with left atrial appendage closure is effective in treating atrial fibrillation. However, the effectiveness of this combined treatment compared with catheter ablation alone is still controversial.
Methods
We searched studies in databases, including The Cochrane Library, PubMed, EMBASE, and Web of Science, that compared catheter ablation combined with left atrial appendage closure and catheter ablation alone in the treatment of atrial fibrillation. These studies reported at least one of the following outcomes: the freedom from atrial arrhythmia rate, the procedure time, the fluoroscopy time, perioperative complications, thromboembolic events, and bleeding events during follow-up. The risk ratio and standard mean difference with 95% CI were analyzed by the random-effects model.
Results
Five studies involving 699 people were included in our meta-analysis. We found no significant difference in the freedom from atrial arrhythmia rate (risk ratio = 0.93, 95% CI = 0.83-1.04, I2 = 0%, P = .21) between the 2 groups. Catheter ablation combined with left atrial appendage closure showed significantly longer procedure and fluoroscopy times than catheter ablation alone (standard mean difference = 1.26, 95% CI = 0.85-1.67, P < .00001 and standard mean difference = 1.19, 95% CI = 0.53-1.85, P = .0004, respectively). With regard to safety outcomes, no significant differences were observed in perioperative complications (RR = 1.62, 95% CI = 0.99-2.63, I2 = 0%, P = .05), thromboembolic events (RR = 0.67, 95% CI = 0.15-3.11, I2 = 0%, P = .61), or bleeding events (RR = 0.67, 95% CI = 0.11-3.88, P = .65) between the 2 groups during follow-up.
Conclusion
The freedom from atrial arrhythmia rate and safety outcomes of catheter ablation combined with left atrial appendage closure are similar to those of catheter ablation alone. Catheter ablation combined with left atrial appendage closure appears to have longer procedure and fluoroscopy times than catheter ablation alone.
Keywords: , Atrial fibrillation, catheter ablation, left atrial appendage closure, meta-analysis
HIGHLIGHTS
This is the first meta-analysis to compare the efficacy and safety of catheter ablation (CA) combined with left atrial appendage closure (LAAC) and CA alone.
The freedom from atrial arrhythmia rate and safety outcomes of CA combined with LAAC are similar to those of CA alone.
Catheter ablation combined with LAAC appears to have longer procedure and fluoroscopy times than CA alone.
Introduction
Atrial fibrillation (AF) is the most common arrhythmia. Approximately, 33 million people worldwide are affected by AF, and its rate is gradually increasing.1 Atrial fibrillation also leads to a huge economic burden.2 Catheter ablation (CA), which is an important method for treating AF, can improve symptoms and quality of life.3,4 In the 2020 European Society of Cardiology (ESC) Guidelines for the diagnosis and management of AF developed in collaboration with the European Association of Cardio-Thoracic Surgery, CA is also recommended as class I for patients with paroxysmal AF or persistent AF with or without major risk factors for AF recurrence after failure or intolerance of class I or III antiarrhythmic drug.5
However, there is still recurrence or thromboembolic events (TEs) in patients with AF after CA.6 Thromboembolic complications are the main cause of death and disability in AF.7 Although anticoagulant therapy reduces the incidence of thromboembolism,8 it also has its limitations. First, the proportion of anticoagulant therapy is insufficient.9 Second, many patients cannot adhere to long-term medication.10 Third, there may be drug interactions that affect the anticoagulant effect of antivitamin K anticoagulants. Additionally, the bleeding risk of anticoagulant therapy cannot be ignored.11
The left atrial appendage (LAA) plays an important role in thrombosis in patients with AF. A recent study of 1420 patients with nonvalvular AF or flutter showed that all patients with atrial thrombus had thrombus in the LAA.12 The combination of CA and LAA closure (LAAC) in the treatment of AF is expected to achieve rhythm control and reduce the risk of thrombus formation in the LAA. The recommendation of the current guideline in terms of anticoagulation is based on CHA2DS2-VASc score irrespective of AF ablative procedure outcome, and that patients who had concomitant LAAC can avoid taking long-term anticoagulation.5 A meta-analysis also showed the efficacy and safety of this combined strategy.13 However, whether the efficacy and safety of the CA combined with LAAC strategy is different from that of CA alone is still unknown. A recent study showed that the 1-year freedom from AF rate was lower with the combined strategy than that of CA alone.14 However, other studies reached different conclusions.15,16 Therefore, we aimed to conduct this systematic review and meta-analysis to evaluate the efficacy and safety of CA combined with LAAC and CA alone in the treatment of AF.
Methods
Our systematic review and meta-analysis were performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting checklist. The protocol for this meta-analysis is available in International prospective register of systematic reviews (PROSPERO) (CRD42021236262).
Literature Search
We searched The Cochrane Library, PubMed, EMBASE, and Web of Science for relevant literature up to January 16, 2021. We searched keywords, including atrial fibrillation, atrial fibrillat*, auricular fibrillat*, atrium fibrillat*, AF, ablat*, isolat*, “left atrial appendage,” LAA, occlu*, and clos* without language restriction. The specific search strategy is shown in the supplementary material. Two researchers (A.W. and J.J.) conducted the literature search independently. Differences in opinions were determined by a third reviewer (Z.X.). Approval from the Institutional Review Board was not required because this study was a systematic review and meta-analysis.
Study Selection
Inclusion criteria included the following: (1) randomized, controlled trials (RCTs) or observational studies; (2) CA combined with LAAC versus CA alone in the treatment of AF; and (3) at least one of the following outcomes was reported: efficacy outcomes included the freedom from atrial arrhythmia rate, procedure time, fluoroscopy time, and safety outcomes included perioperative complications, TEs, and bleeding events during follow-up. Exclusion criteria included the following: (1) a surgical operation; (2) studies including LAA isolation; and (3) conference abstract.
Data Extraction and Quality Assessment
Two reviewers independently extracted the following information from the studies that were selected for inclusion: authors, year of publication, country, sample size, age distribution, sex proportion, proportion of paroxysmal AF, left atrial diameter, congestive heart failure”/hypertension/age 75 years or older/diabetes mellitus/stroke/vascular disease/age 65 2 74 years/sex category (female) (CHA2DS2-VASc) score, uncontrolled hypertension/abnormal renal and/or hepatic function/stroke/bleeding history or predisposition/labile INR/elderly/drugs or excessive alcohol drinking (HAS-BLED) score, occlusion device, mean follow-up times, and study design. Efficacy and safety outcomes as described above were recorded. When the article only mentioned the recurrence of AF, we classified it as a recurrence of atrial arrhythmia. If stroke, transient ischemic attack, peripheral embolism, and myocardial infarction were mentioned in the included studies, we classified them as TEs. If the article only mentioned severe bleeding events, we classified them as bleeding events. Any disagreements between the 2 reviewers were determined by a third reviewer. The quality of RCTs was evaluated by Cochrane Collaboration’s risk of bias tools, and observational studies were evaluated by the Newcastle–Ottawa Scale.
Statistical Analysis
Data were analyzed by Review Manager (RevMan) (version 5.3 for Windows; Cochrane Collaboration, Oxford, UK). Risk ratios (RRs) with 95% CIs were used for dichotomous variables. We analyzed continuous data using the standard mean difference (SMD) with the corresponding 95% CI. Significant heterogeneity was determined by an I 2 greater than 50%.17 The random-effects model was chosen for analysis. P < .05 was considered to be statistically significant. Subgroup analysis was conducted depending on the type of AF, ablation energy, type of study, and patients with a high risk of stroke. We did not detect any publication bias owing to the small number of documents included in this meta-analysis.
Results
Study Selection
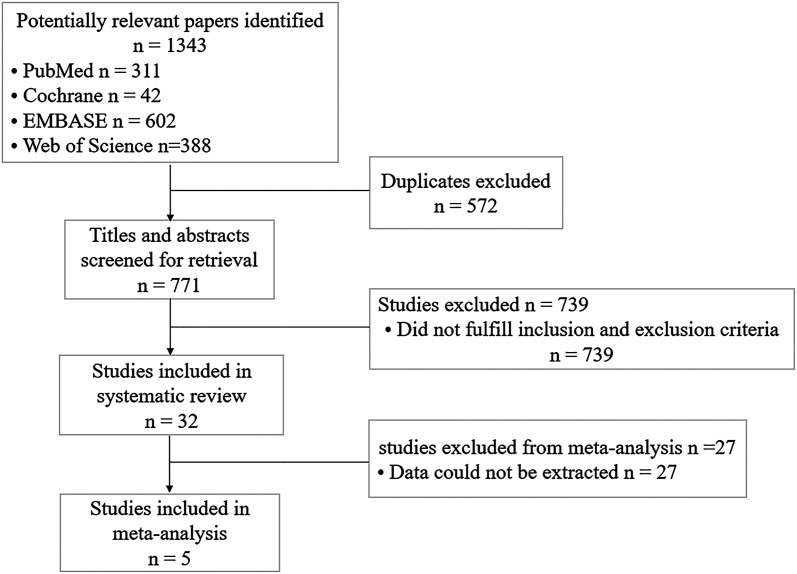
We identified 1343 articles by the search strategy. The screening process is shown in Figure 1. After eliminating repetitive or irrelevant literature by reading the title and/or abstract, 32 articles were further screened. Finally, a total of 5 studies14-16,18,19 (1 RCT and 4 observational studies) that met the inclusion criteria were included in the meta-analysis.
Figure 1.
Flowchart of literature selection.
Study Characteristics
Baseline characteristics of all included studies are shown in Table 1. A total of 699 patients were included in this meta-analysis, including 240 patients in the CA combined with LAAC group and 459 patients in the CA alone group. The mean time of follow-up ranged from 10.1 to 24 months. The research locations included China, Italy, and Russia. Two studies used radiofrequency energy,15,16 2 used radiofrequency or cryotherapy,18,19 and 1 used cryoballoon ablation.14 With regard to the closure device, 2 studies only used the Watchman,15,16 1 study used the Watchman and Amplatzer Cardiac Plug,18 and 1 study used the Watchman, LeFort, and Lacbes devices.14 Another study used 4 types of closure devices, including the Watchman, Lambre, Lagger, and Amplatzer Cardiac Plug.19 The average CHA2DS2-VASc score and HAS-BLED score ranged from 2.2 to 4.3 and 2.0 to 3.7, respectively. The risk of bias assessment of the RCT is shown in Figure 2. The observational studies were scored between 5 and 7 according to the Newcastle–Ottawa Scale score (Table 1).
Table 1.
Baseline Characteristics of Studies
| Trial (Year) | Country | Ablation Energy | Group | Sample Size (n) | Age (Years) | Female (%) | PAF (%) | LAD (mm) | CHA2DS2-VASc Score | HAS-BLED Score | Occlusion Device | Mean Follow-Up (Months) | Design | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mo (2020) | China | Radiofrequency† | CA + LAAC | 76 | 69.9 ± 7.9 | 48.7 | 48.7 | 42.7 ± 5.7 | 3.6 ± 1.3 | 3.3 ± 1.1 | Watchman | 24 | Observed | 7 |
| CA alone | 76 | 69.5 ± 7.8 | 52.6 | 50.0 | 41.7 ± 4.9 | 3.4 ± 1.4 | 2.6 ± 0.9 | 24 | ||||||
| Pelissero (2017) | Italy | Radiofrequency or cryoballoon | CA + LAAC | 21 | 66.86 ± 10.35 | 33.3 | 19.0 | NA | 2.8 ± 1.22 | 3.2 ± 0.83 | Watchman/ACP | 14.93 ± 10.05 | Observed | 7 |
| CA alone | 21 | 68.42 ± 10.61 | 28.6 | 14.3 | NA | 2.01 ± 0.93 | 3.1 ± 0.95 | 14.93 ± 10.05 | ||||||
| Ren (2020) | China | Cryoballoon | CA + LAAC | 42 | 70 ± 7.6 | 38.1 | 100 | 45.6 ± 5.8 | 3.8 ± 2.1 | 3.7 ± 1.2 | Watchman/Lefort/Lacbes | 20 ± 9 | Observed | 5 |
| CA alone | 262 | 66.3 ± 9.5 | 45.8 | 100 | 40.4 ± 5.6 | 2.8 ± 1.9 | 2.7 ± 1.2 | 22 ± 11 | ||||||
| Romanov (2015) | Russia | Radiofrequency | CA + LAAC | 45 | 60 ± 5 | 37.8 | 53.3 | 49 ± 6 | 2.2 ± 0.6 | 3.5 ± 0.8 | Watchman | 24 | RCT | - |
| CA alone | 44 | 60 ± 6 | 40.9 | 56.8 | 48 ± 7 | 2.3 ± 0.7 | 3.4 ± 0.8 | 24 | ||||||
| Zhu (2020) | China | Radiofrequency or cryoballoon | CA + LAAC | 56 | 65.2 ± 6.6 | 41.1 | 42.9 | 45.6 ± 6.3 | 4.3 ± 1.8 | 2.0 ± 1.3 | Watchman/Lambre/Lagger/ACP | 12.3 | Observed | 6 |
| CA alone | 56 | 64.8 ± 8.5 | 39.3 | 53.6 | 44.5 ± 6.2 | 4.1 ± 1.7 | 1.8 ± 1.1 | 10.1 |
ACP, Amplatzer Cardiac Plug; CA, catheter ablation; LAAC, left atrial appendage closure; LAD, left atrial diameter; LVEF, left ventricular ejection fraction; NA, not available; NOS, Newcastle–Ottawa Scale; PAF, paroxysmal atrial fibrillation; RCT, randomized controlled trial.
†Due to CARTO or Ensite 3D electroanatomic mapping system was used for mapping and ablation in the article, we speculate that the energy should be radiofrequency.
Figure 2.
Risk of bias of included randomized controlled trial in the meta-analysis.
Efficacy Outcomes
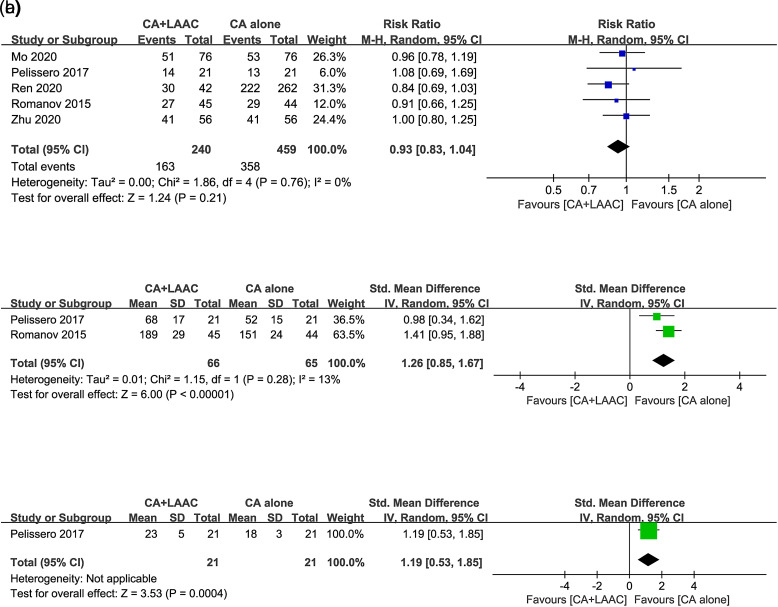
Our meta-analysis showed that for the main efficacy outcome, 163 (67.9%) patients in the CA combined with the LAAC group and 358 (78.0%) patients in CA alone group had freedom from atrial arrhythmia. There was no significant difference in the freedom from atrial arrhythmia rate between the 2 groups (RR = 0.93, 95% CI = 0.83-1.04, I 2 = 0%, P = .21, Figure 3A). Two15,18 of the included studies mentioned the procedure time. Patients in the CA combined with LAAC group showed a significantly longer procedure time than those in the CA alone group (SMD = 1.26, 95% CI = 0.85-1.67, P < .00001, Figure 3B). Only one study mentioned the fluoroscopy time.18 Similar to the procedure time, the CA combined with LAAC group had a longer fluoroscopy time than the CA alone group (SMD = 1.19, 95% CI = 0.53-1.85, P = .0004, Figure 3C).
Figure 3.
Forest plots of efficacy outcomes for CA + LAAC versus CA alone. (A) Freedom from atrial arrhythmia rate; (B) procedure time; (C) fluoroscopy time. CA, catheter ablation; LAAC, left atrial appendage closure.
Safety Outcomes
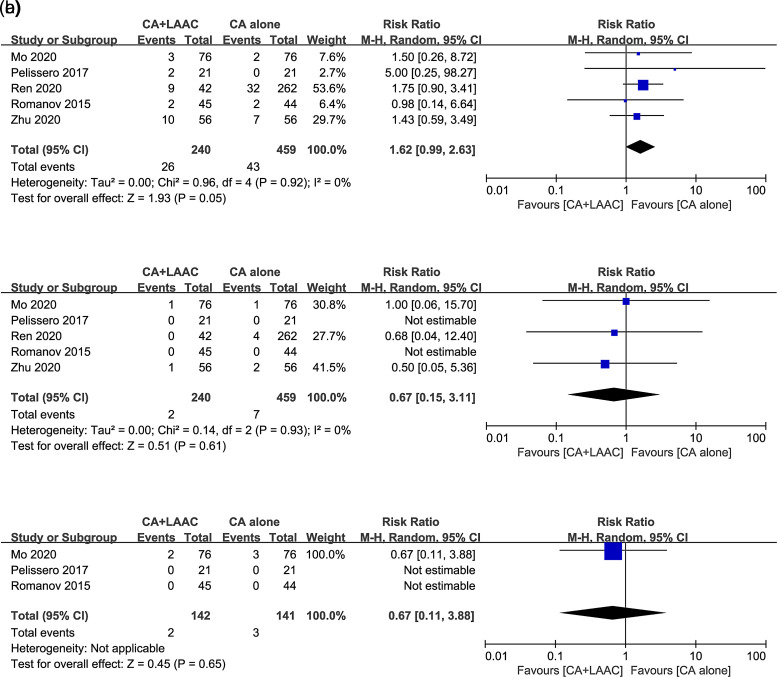
With regard to safety outcomes, all included studies reported perioperative complications. The incidence of perioperative complications was 10.8% (26/240) in the CA combined with LAAC group and 9.4% (43/459) in the CA alone group. No significant difference in the rate of perioperative complications was observed between the 2 groups (RR = 1.62, 95% CI = 0.99-2.63, I 2 = 0%, P = .05, Figure 4A). After an average follow-up ranging from 10.1 to 24 months, 2 patients in the CA combined with LAAC group and 7 patients in the CA alone group suffered from TEs. There was no significant difference in the rate of TEs between the 2 groups during the follow-up period (RR = 0.67, 95% CI = 0.15-3.11, I 2 = 0%, P = .61, Figure 4B). Three studies15,16,18 mentioned the safety outcome of bleeding events during follow-up, and there was no significant difference in the rate of these events between the 2 groups (RR = 0.67, 95% CI = 0.11-3.88, P = .65, Figure 4C).
Figure 4.
Forest plots of safety outcomes for CA + LAAC versus CA alone. (A) perioperative complications; (B) thromboembolic events during follow-up; (C) bleeding events during follow-up. CA, catheter ablation; LAAC, left atrial appendage closure.
Subgroup Analysis
We performed subgroup analysis by the type of AF, ablation energy, and type of study. There was no significant difference in the freedom from atrial arrhythmia rate, TEs, and bleeding events during follow-up (Supplemental Tables 1-3). However, subgroup analysis by the type of study showed that there was a significant difference in the rate of perioperative complications between the CA combined with LAAC group and the CA alone group in the observational study (RR = 1.67, 95% CI = 1.01-2.76, P = .04, Supplemental Table 3). This finding might be due to the inclusion of Ren et al’s study in the observational studies, which did not match with the basic characteristics of the included population (e.g., age, left atrial diameter, HAS-BLED score, CHA2DS2-VASc score, etc.), or because that the sample size of the 2 groups was different (42 vs. 262). These factors may have led to bias that affected the results.
Supplement Table 2.
Subgroup Analysis for Binary Variable Outcomes According to Ablation Energy
| Outcome | Trials | Participants | Risk Ratio (95% CI) | P |
|---|---|---|---|---|
| Freedom from atrial arrhythmia rate | ||||
| Radiofrequency | 2 | 241 | 0.95 (0.79, 1.13) | .54 |
| Cryoballoon | 1 | 304 | 0.84 (0.69, 1.03) | .09 |
| Radiofrequency or cryoballoon | 2 | 154 | 1.01 (0.83, 1.24) | .89 |
| Test for subgroup differences χ² = 1.70, df = 2, P = .43, I² = 0% | ||||
| Perioperative complications | ||||
| Radiofrequency | 2 | 241 | 1.25 (0.32, 4.79) | .75 |
| Cryoballoon | 1 | 304 | 1.96 (0.86, 4.47) | .11 |
| Radiofrequency or cryoballoon | 2 | 154 | 1.74 (0.64, 4.68) | .28 |
| Test for subgroup differences χ² = 0.32, df = 2, P = .85, I² = 0% | ||||
| thromboembolic events during follow-up | ||||
| Radiofrequency | 2 | 241 | 1.23 (0.34, 4.51) | .75 |
| Cryoballoon | 1 | 304 | 1.75 (0.90, 3.41) | .10 |
| Radiofrequency or cryoballoon | 2 | 154 | 1.58 (0.67, 3.72) | .29 |
| Test for subgroup differences χ² = 0.23, df = 2, P = .89, I² = 0% | ||||
| Bleeding events during follow-up | ||||
| Radiofrequency | 2 | 241 | 0.67 (0.11, 3.88) | .65 |
| Cryoballoon | - | - | - | - |
| Radiofrequency or cryoballoon | 1 | 41 | - | - |
| Test for subgroup differences - | ||||
In the subgroup analysis by the type of AF, ablation energy, and study type, the procedure time and fluoroscopy time showed significant differences between the groups (Supplemental Tables 4-6), which were similar to the results without subgroup analysis (Figure 3B, C).
Supplement Table 5.
Subgroup Analysis for Continuous Variable Outcomes According to Ablation Energy
| Outcome | Trials | Participants | Standard Mean Difference (95% CI) | P |
|---|---|---|---|---|
| Procedure time (minutes) | ||||
| Radiofrequency | 1 | 89 | 1.41(0.95, 1.88) | <.00001 |
| Cryoballoon | - | - | - | |
| Radiofrequency or cryoballoon | 1 | 42 | 0.98(0.34, 1.62) | .003 |
| Test for subgroup differences χ² = 1.15, df = 1, P = .28, I² = 12.9% | ||||
| Fluoroscopy time (minutes) | ||||
| Radiofrequency | - | - | - | - |
| Cryoballoon | - | - | - | |
| Radiofrequency or cryoballoon | 1 | 42 | 1.19 (0.53, 1.85) | .0004 |
| Test for subgroup differences - | ||||
Two studies (Romanov et al’s and Zhu et al’s studies)15,19 included patients with a high risk of stroke. In the subgroup analysis of patients with a high risk of stroke, no significant differences were observed in the freedom from atrial arrhythmia rate, perioperative complications, or TEs during follow-up between the groups (Supplement Table 7). During the follow-up, no severe bleeding events occurred in Romanov et al’s study, and data of bleeding events were not reported in Zhu et al’s study.
Supplement Table 6.
Subgroup Analysis for Continuous Variable Outcomes According to the Type of Study
| Outcome | Trials | Participants | Standard Mean Difference (95% CI) | P |
|---|---|---|---|---|
| Procedure time (minutes) | ||||
| RCT | 1 | 89 | 1.41 (0.95, 1.88) | <.00001 |
| Observational studies | 1 | 42 | 0.98 (0.34, 1.62) | .003 |
| Test for subgroup differences χ² = 1.15, df = 1, P = .28, I² = 12.9% | ||||
| Fluoroscopy time (minutes) | ||||
| RCT | - | - | - | - |
| Observational studies | 1 | 42 | 1.19 (0.53, 1.85) | .0004 |
| Test for subgroup differences - | ||||
RCT, randomized controlled trial.
Discussion
This meta-analysis showed similar freedom from atrial arrhythmia rate, perioperative complications, TEs, and bleeding events during follow-up in the CA combined with LAAC group compared with the CA alone group. Catheter ablation combined with LAAC appeared to have longer procedure and fluoroscopy times. To the best of our knowledge, this is the first meta-analysis to compare the efficacy and safety of CA combined with LAAC and CA alone.
A previous meta-analysis showed that the recurrence rate of AF was 32.89% with the CA combined with LAAC strategy,20 which is similar to our rate (32.08%). The authors of this previous meta-analysis speculated that the potential effect of long-term residual leakage on hemodynamics may affect the maintenance of sinus rhythm.20 However, some of the excitatory foci of AF (including paroxysmal and persistent AF) originate from the LAA.21 Exclusion of the LAA can reduce the burden of AF.22 However, previous studies showed no difference in the AF burden between CA combined with LAAC and CA alone.15,18 A meta-analysis showed that LAA isolation reduced the recurrence of AF.23 Our study showed no difference in the recurrence rate of AF between CA combined with LAAC and CA alone. This finding may be related to the fact that LAAC did not completely isolate LAA electrical activity. On the basis of combined strategy and LAA isolation, freedom from AF at 12 months was significantly higher than that of CA alone (95% vs 63%, P = .036).24 However, the study had a small sample and was a non-RCT. Therefore, more studies are required to evaluate the efficacy and safety of the above-mentioned strategies.
Our study showed that the procedure and fluoroscopy times of CA combined with LAAC were longer than those of CA alone, which may be related to the process of different procedures. The operation of CA combined with LAAC involves the addition of LAAC to CA, leading to prolongation of the procedure and fluoroscopy times. These factors are also related to the type of AF (e.g., persistent AF may require more ablation), ablation energy, experience of the operator, and other factors.
With regard to perioperative complications, because the combined strategy and CA alone use the same vascular approach, the main difference between the 2 methods is the process of LAAC. In early research of PROTECT AF, perioperative complications of LAAC reached 8.4%.25 With the development of new products, optimization of the operation process, and improvement of experience, this rate has dropped to 2.7%26 and even 1.44%.27 In theory, ablation of the posterior wall of the left atrium and the use of transesophageal echocardiography may increase the incidence of esophageal injury, which is a perioperative complication.28 However, we did not find any studies that reported the occurrence of esophageal injury or even atrial esophageal fistula.29-33 Postoperative gastroscopy may be able to detect and further evaluate the degree of esophageal injury. Our meta-analysis did not show a difference in perioperative complications between CA combined with LAAC and CA alone, which may be attributed to the low complication rate of LAAC itself.
The risk of stroke in patients with nonvalvular AF is significantly higher than that in patients without.34 Anticoagulant therapy is an important measure for preventing thrombosis. However, some patients have a high risk of stroke and also have contraindications to anticoagulation therapy. In the 2019 American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) and 2020 ESC guidelines for the management of AF, LAAC is recommended for such a group of patients as class IIb.5,35 Catheter ablation for AF has been recommended by multiple guidelines.5,36 Various studies have examined the strategy of CA combined with LAAC.29,37-39 A 2-year follow-up study from 11 centers showed that the composite endpoint ratio of transient ischemic attack, ischemic stroke, or systemic thromboembolism was 1.09/100 patient-years with CA combined with LAAC.40 A meta-analysis that included 18 studies (2 of them were RCTs) to evaluate the efficacy and safety of CA combined with LAAC showed that the incidence of stroke/transient ischemic attack was 0.01,13 compared with 0.008 in our study. Another meta-analysis of 12 RCTs showed no significant difference in TEs between the CA group and the non-ablation group (2.25% vs. 2.88%, P = .28).41 Our meta-analysis showed that the incidence of TEs in the CA alone group was 1.53%. The difference in rate between our study and the previous study may be due to the different numbers and types of literature that met the inclusion criteria. Moreover, our study showed that there was no difference in the incidence of TEs between the CA combined with LAAC and CA alone groups. Even in patients with the removal of the LAA and maintenance of sinus rhythm, atrial scarring caused by ablation injury or progression of the disease remains a risk of stroke.42 On one hand, thrombus formation in non-contractile left atrial space or appendage stump due to incomplete resection.42 On the other hand, we speculate that the LAA may be the “preferred site” for atrial thrombus formation. When the LAA is occluded, other atrial sites (e.g., the left atrial cavity and right atrial appendage) still have a risk of thrombus formation.
With regard to safety outcomes, the occurrence of bleeding events is related to anticoagulation or antiplatelet strategy. In the CA combined with LAAC group, the final strategy after the operation is frequently long-term, single antiplatelet therapy (e.g., aspirin). In the CA alone group, the strategy with a high CHA2DS2-VASc score tends to be continuation of oral anticoagulants (OACs). Studies have compared aspirin with OACs, warfarin, and novel OACs for the risk of bleeding. In a multinational, population-based cohort study, no difference was found in the risk of gastrointestinal bleeding between aspirin and rivaroxaban overall and low-dose rivaroxaban (≤15 mg/day).43 A meta-analysis showed that rivaroxaban 10 mg once daily or 5 mg twice daily and apixaban 5 mg twice daily were comparable to aspirin for intracranial hemorrhage.44 A meta-analysis that included 29 studies showed a similar risk of bleeding between warfarin and novel OACs.45 Our study did not show any difference in bleeding events between the CA combined with LAAC and CA alone groups.
The purpose of performing LAAC is mainly expected to benefit patients who are at a high risk of stroke and bleeding. In our subgroup analysis of patients with a high risk of stroke, patients with CA combined with LAAC and those with CA alone had similar rates of freedom from atrial arrhythmia, perioperative complications, and TEs during follow-up. In the analysis of bleeding events during the follow-up period, one study did not show any severe bleeding events, and the other study did not report data on bleeding events. However, because this subgroup analysis only included 2 studies and the follow-up time was not long enough, we were not able to accurately assess the role of LAAC in this part of the population.
Study Limitations
Our study has the following limitations. (1) The number of articles in this meta-analysis and the sample size of each study were small. (2) Of the 5 studies included, 4 of them were observational studies and only 1 was an RCT. (3) Different CA techniques and occlusion devices used in each study may have led to bias, such as residual peri-device leakage after different occlusion devices. (4) The follow-up time of the included studies was insufficient. (5) The conclusion of fluoroscopy time was based on data from a single-center observational study. (6) Subgroup analysis of patients with a high risk of stroke only included 2 studies. All of the above-mentioned factors may have affected the evaluation of the outcome events. A larger sample size and multicenter RCTs are required to evaluate the combined strategy of CA with LAAC and CA alone.
Conclusion
Our meta-analysis shows no significant difference in freedom from atrial arrhythmia rate, perioperative complications, TEs, and bleeding events during follow-up in patients who have CA combined with LAAC compared with those who have CA alone. Catheter ablation combined with LAAC tends to have a longer procedure and fluoroscopy time than CA alone. More RCTs are required for further study.
Supplement Table 1.
Subgroup Analysis for Binary Variable Outcomes According to the Type of AF
| Outcome | Trials | Participants | Risk Ratio (95% CI) | P |
|---|---|---|---|---|
| Freedom from atrial arrhythmia rate | ||||
| Paroxysmal AF | 1 | 304 | 0.84 (0.69, 1.03) | .09 |
| Paroxysmal/persistent/long-standing persistent AF | 4 | 395 | 0.98 (0.85, 1.12) | .72 |
| Test for subgroup differences χ² = 1.44, df = 1, P = .23, I² = 30.5% | ||||
| Perioperative complications | ||||
| Paroxysmal AF | 1 | 304 | 1.75 (0.90, 3.41) | .10 |
| Paroxysmal/persistent/long-standing persistent AF | 4 | 395 | 1.47 (0.72, 3.00) | .29 |
| Test for subgroup differences χ² = 0.13, df = 1, P = .72, I² = 0% | ||||
| Thromboembolic events during follow-up | ||||
| Paroxysmal AF | 1 | 304 | 0.68 (0.04, 12.40) | .79 |
| Paroxysmal/persistent/long-standing persistent AF | 4 | 395 | 0.67 (0.11, 4.05) | .66 |
| Test for subgroup differences χ² = 0.00, df = 1, P = .99, I² = 0% | ||||
| Bleeding events during follow-up | ||||
| Paroxysmal AF | - | - | - | - |
| Paroxysmal/persistent/long-standing persistent AF | 3 | 283 | 0.67 (0.11, 3.88) | .65 |
| Test for subgroup differences - | ||||
AF, atrial fibrillation.
Supplement Table 3.
Subgroup Analysis for Binary Variable Outcomes According to the Type of Study
| Outcome | Trials | Participants | Risk Ratio (95% CI) | P |
|---|---|---|---|---|
| Freedom from atrial arrhythmia rate | ||||
| RCT | 1 | 89 | 0.91 (0.66, 1.25) | .56 |
| Observational studies | 4 | 610 | 0.94 (0.83, 1.05) | .27 |
| Test for subgroup differences χ² = 0.02, df = 1, P = .88, I² = 0% | ||||
| Perioperative complications | ||||
| RCT | 1 | 89 | 0.98 (0.14, 6.64) | .98 |
| Observational studies | 4 | 610 | 1.67 (1.01, 2.76) | .04 |
| Test for subgroup differences χ² = 0.28, df = 1, P = .60, I² = 0% | ||||
| Thromboembolic events during follow-up | ||||
| RCT | 1 | 89 | - | - |
| Observational studies | 4 | 610 | 0.67 (0.15, 3.11) | .61 |
| Test for subgroup differences - | ||||
| Bleeding events during follow-up | ||||
| RCT | 1 | 89 | - | - |
| Observational studies | 2 | 194 | 0.67 (0.11, 3.88) | .65 |
| Test for subgroup differences - | ||||
RCT, randomized controlled trial.
Supplement Table 4.
Subgroup Analysis for Continuous Variable Outcomes According to the Type of AF
| Outcome | Trials |
Participants |
Standard Mean Difference (95% CI) |
P |
|---|---|---|---|---|
| Procedure time (minutes) | ||||
| Paroxysmal AF | - | - | - | |
| Paroxysmal/persistent/long-standing persistent AF | 2 | 131 | 1.26 (0.85, 1.67) | <.00001 |
| Test for subgroup differences - | ||||
| Fluoroscopy time (minutes) | ||||
| Paroxysmal AF | - | - | - | |
| Paroxysmal/persistent/long-standing persistent AF | 1 | 21 | 1.19 (0.53, 1.85) | .0004 |
| Test for subgroup differences - | ||||
AF, atrial fibrillation.
Supplement Table 7.
Subgroup Analysis of Patients with High Risk of Stroke
| Outcome | Trials | Participants | Risk Ratio (95% CI) | P |
|---|---|---|---|---|
| Freedom from atrial arrhythmia rate | 2 | 201 | 0.97 (0.81, 1.16) | .74 |
| Perioperative complications | 2 | 201 | 1.34 (0.59, 3.00) | .48 |
| Thromboembolic events during follow-up | 2 | 201 | 0.50 (0.05, 5.36) | .57 |
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – A.W., G.Z.; Design – A.W., G.Z.; Supervision – G.Z.; Fundings – G.Z.; Materials – None; Data collection &/or processing – A.W., Z.X, J.J.; Analysis &/or interpretation – A.W., Z.X.; Literature search – A.W., J.J., Z.X.; Writing – A.W., G.Z.; Critical review – A.W., J.J., Z.X., G.Z.
Declaration of Interests: The authors declare no conflict of interest.
Funding: This work was supported by the National Natural Science Foundation of China [No. 82060068].
References
- 1. Chung MK, Refaat M, Shen WK.et al. Atrial fibrillation: JACC council perspectives. J Am Coll Cardiol. 2020;75(14):1689 1713. 10.1016/j.jacc.2020.02.025) [DOI] [PubMed] [Google Scholar]
- 2. Coyne KS, Paramore C, Grandy S, Mercader M, Reynolds M, Zimetbaum P. Assessing the direct costs of treating nonvalvular atrial fibrillation in the United States. Value Health. 2006;9(5):348 356. 10.1111/j.1524-4733.2006.00124.x) [DOI] [PubMed] [Google Scholar]
- 3. Blomström-Lundqvist C, Gizurarson S, Schwieler J.et al. Effect of catheter ablation vs antiarrhythmic medication on quality of life in patients with atrial fibrillation: the CAPTAF randomized clinical trial. JAMA. 2019;321(11):1059 1068. 10.1001/jama.2019.0335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mark DB, Anstrom KJ, Sheng S.et al. Effect of catheter ablation vs medical therapy on quality of life among patients With atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321(13):1275 1285. 10.1001/jama.2019.0692) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hindricks G, Potpara T, Dagres N.et al. ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2020;42:373 498. [DOI] [PubMed] [Google Scholar]
- 6. Mao YJ, Wang H, Chen JX, Huang PF. Meta-analysis of medical management versus catheter ablation for atrial fibrillation. Rev Cardiovasc Med. 2020;21(3):419 432. 10.31083/j.rcm.2020.03.60) [DOI] [PubMed] [Google Scholar]
- 7. Chinese Society of Cardiology of Chinese Medical Association, Editorial Board of Chinese Journal of Cardiology. 2019 Chinese Society of Cardiology (CSC) expert consensus statement on left atrial appendage closure in the prevention of stroke in patients with atrial fibrillation. Zhonghua Xin Xue Guan Bing Za Zhi. 2019;47(12):937 955. 10.3760/cma.j.issn.0253-3758.2019.12.002) [DOI] [PubMed] [Google Scholar]
- 8. Pallazola VA, Kapoor RK, Kapoor K, McEvoy JW, Blumenthal RS, Gluckman TJ. Anticoagulation risk assessment for patients with non-valvular atrial fibrillation and venous thromboembolism: a clinical review. Vasc Med. 2019;24(2):141 152. 10.1177/1358863X18819816) [DOI] [PubMed] [Google Scholar]
- 9. Gebreyohannes EA, Salter S, Chalmers L, Bereznicki L, Lee K. Non-adherence to thromboprophylaxis guidelines in atrial fibrillation: a narrative review of the extent of and factors in guideline non-adherence. Am J Cardiovasc Drugs. 2021;21(4):419 433. 10.1007/s40256-020-00457-3) [DOI] [PubMed] [Google Scholar]
- 10. Gumbinger C, Holstein T, Stock C, Rizos T, Horstmann S, Veltkamp R. Reasons underlying non-adherence to and discontinuation of anticoagulation in secondary stroke prevention among patients with atrial fibrillation. Eur Neurol. 2015;73(3-4):184 191. 10.1159/000371574) [DOI] [PubMed] [Google Scholar]
- 11. Granger CB, Alexander JH, McMurray JJ.et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981 992. 10.1056/NEJMoa1107039) [DOI] [PubMed] [Google Scholar]
- 12. Cresti A, García-Fernández MA, Sievert H.et al. Prevalence of extra-appendage thrombosis in non-valvular atrial fibrillation and atrial flutter in patients undergoing cardioversion: a large transoesophageal echo study. EuroIntervention. 2019;15(3):e225 e230. 10.4244/EIJ-D-19-00128) [DOI] [PubMed] [Google Scholar]
- 13. Jiang Y, Li F, Li D.et al. Efficacy and safety of catheter ablation combined with left atrial appendage occlusion for nonvalvular atrial fibrillation: a systematic review and meta-analysis. Pacing Clin Electrophysiol. 2020;43(1):123 132. 10.1111/pace.13845) [DOI] [PubMed] [Google Scholar]
- 14. Ren Z, Zhang J, Zhu M.et al. Cryoablation combined with left atrial appendage closure: a safe and effective procedure for paroxysmal atrial fibrillation patients. Cardiol Res Pract. 2020;2020:6573296. 10.1155/2020/6573296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Romanov A, Pokushalov E, Artemenko S.et al. Does left atrial appendage closure improve the success of pulmonary vein isolation? Results of a randomized clinical trial. J Interv Card Electrophysiol. 2015;44(1):9 16. 10.1007/s10840-015-0030-4) [DOI] [PubMed] [Google Scholar]
- 16. Mo BF, Sun J, Zhang PP.et al. Combined therapy of catheter ablation and left atrial appendage closure for patients with atrial fibrillation: a case-control study. J Interv Cardiol. 2020;2020:8615410. 10.1155/2020/8615410) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higgins JPT, López-López JA, Becker BJ.et al. Synthesising quantitative evidence in systematic reviews of complex health interventions. BMJ Glob Health. 2019;4(Suppl 1):e000858. 10.1136/bmjgh-2018-000858) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pelissero E, Giuggia M, Todaro MC, Trapani G, Giordano B, Senatore G. [Combined left atrial appendage percutaneous closure and atrial fibrillation ablation: a single center experience]. G Ital Cardiol (Rome). 2017;18(12):11S 17S. 10.1714/2835.28627) [DOI] [PubMed] [Google Scholar]
- 19. Zhu S, Zheng M, Yan R.et al. [Success rate of one-stop procedure for atrial fibrillation ablation and its impact on cardiac function: a propensity-matched study]. Nan Fang Yi Ke Da Xue Xue Bao. 2020;40(10):1415 1421. 10.12122/j.issn.1673-4254.2020.10.06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Han Z, Wu X, Chen Z.et al. Residual flow may increase the risk of adverse events in patients received combined catheter ablation and transcatheter left atrial appendage closure for nonvalvular atrial fibrillation: a meta-analysis. BMC Cardiovasc Disord. 2019;19(1):138. 10.1186/s12872-019-1123-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Di Biase L, Burkhardt JD, Mohanty P.et al. Left atrial appendage: an underrecognized trigger site of atrial fibrillation. Circulation. 2010;122(2):109 118. 10.1161/CIRCULATIONAHA.109.928903) [DOI] [PubMed] [Google Scholar]
- 22. Afzal MR, Kanmanthareddy A, Earnest M.et al. Impact of left atrial appendage exclusion using an epicardial ligation system (LARIAT) on atrial fibrillation burden in patients with cardiac implantable electronic devices. Heart Rhythm. 2015;12(1):52 59. 10.1016/j.hrthm.2014.09.053) [DOI] [PubMed] [Google Scholar]
- 23. Friedman DJ, Black-Maier EW, Barnett AS.et al. Left atrial appendage electrical isolation for treatment of recurrent atrial fibrillation: a meta-analysis. JACC Clin Electrophysiol. 2018;4(1):112 120. 10.1016/j.jacep.2017.07.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Panikker S, Jarman JW, Virmani R.et al. Left atrial appendage electrical isolation and concomitant device occlusion to treat persistent atrial fibrillation: a first-in-human safety, feasibility, and efficacy study. Circ Arrhythm Electrophysiol. 2016;9(7):e003710. 10.1161/CIRCEP.115.003710) [DOI] [PubMed] [Google Scholar]
- 25. Holmes DR, Reddy VY, Turi ZG.et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374(9689):534 542. 10.1016/S0140-6736(09)61343-X) [DOI] [PubMed] [Google Scholar]
- 26. Boersma LV, Schmidt B, Betts TR.et al. Implant success and safety of left atrial appendage closure with the WATCHMAN device: peri-procedural outcomes from the EWOLUTION registry. Eur Heart J. 2016;37(31):2465 2474. 10.1093/eurheartj/ehv730) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reddy VY, Gibson DN, Kar S.et al. Post-approval U.S. experience with left atrial appendage closure for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2017;69(3):253 261. 10.1016/j.jacc.2016.10.010) [DOI] [PubMed] [Google Scholar]
- 28. Hu H, Cui K, Jiang J, Fu H, Zeng R. Safety and efficacy analysis of one-stop intervention for treating nonvalvular atrial fibrillation. Pacing Clin Electrophysiol. 2018;41(1):28 34. 10.1111/pace.13250) [DOI] [PubMed] [Google Scholar]
- 29. Liu FZ, Lin WD, Liao HT.et al. Mid-term outcomes of concomitant left atrial appendage closure and catheter ablation for non-valvular atrial fibrillation: a multicenter registry. Heart Vessels. 2019;34(5):860 867. 10.1007/s00380-018-1312-4) [DOI] [PubMed] [Google Scholar]
- 30. Wintgens L, Romanov A, Phillips K.et al. Combined atrial fibrillation ablation and left atrial appendage closure: long-term follow-up from a large multicentre registry. Europace. 2018;20(11):1783 1789. 10.1093/europace/euy025) [DOI] [PubMed] [Google Scholar]
- 31. Phillips KP, Pokushalov E, Romanov A.et al. Combining watchman left atrial appendage closure and catheter ablation for atrial fibrillation: multicentre registry results of feasibility and safety during implant and 30 days follow-up. Europace. 2018;20(6):949 955. 10.1093/europace/eux183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Feng XF, Zhang PP, Sun J, Wang QS, Li YG. Feasibility and safety of left atrial appendage closure using the LAmbre device in patients with nonvalvular atrial fibrillation with or without prior catheter ablation. Int Heart J. 2019;60(1):63 70. 10.1536/ihj.18-070) [DOI] [PubMed] [Google Scholar]
- 33. Du X, Chu H, Ye P.et al. Combination of left atrial appendage closure and catheter ablation in a single procedure for patients with atrial fibrillation: multicenter experience. J Formos Med Assoc. 2019;118(5):891 897. 10.1016/j.jfma.2018.10.006) [DOI] [PubMed] [Google Scholar]
- 34. Lin HJ, Wolf PA, Kelly-Hayes M.et al. Stroke severity in atrial fibrillation. The Framingham study. Stroke. 1996;27(10):1760 1764. 10.1161/01.str.27.10.1760) [DOI] [PubMed] [Google Scholar]
- 35. January CT, Wann LS, Calkins H.et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients With atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74(1):104 132. 10.1016/j.jacc.2019.01.011) [DOI] [PubMed] [Google Scholar]
- 36. Andrade JG, Aguilar M, Atzema C.et al. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society Comprehensive Guidelines for the management of atrial fibrillation. Can J Cardiol. 2020;36(12):1847 1948. 10.1016/j.cjca.2020.09.001) [DOI] [PubMed] [Google Scholar]
- 37. Calvo N, Salterain N, Arguedas H.et al. Combined catheter ablation and left atrial appendage closure as a hybrid procedure for the treatment of atrial fibrillation. Europace. 2015;17(10):1533 1540. 10.1093/europace/euv070) [DOI] [PubMed] [Google Scholar]
- 38. Walker DT, Phillips KP. Left atrial catheter ablation subsequent to Watchman® left atrial appendage device implantation: a single centre experience. Europace. 2015;17(9):1402 1406. 10.1093/europace/euv037) [DOI] [PubMed] [Google Scholar]
- 39. Fassini G, Conti S, Moltrasio M.et al. Concomitant cryoballoon ablation and percutaneous closure of left atrial appendage in patients with atrial fibrillation. Europace. 2016;18(11):1705 1710. 10.1093/europace/euw007) [DOI] [PubMed] [Google Scholar]
- 40. Phillips KP, Romanov A, Artemenko S.et al. Combining left atrial appendage closure and catheter ablation for atrial fibrillation: 2-year outcomes from a multinational registry. Europace. 2020;22(2):225 231. 10.1093/europace/euz286) [DOI] [PubMed] [Google Scholar]
- 41. Liu M, Wang Y, Li J.et al. Opposite effect of ablation on early/late-phase thromboembolic incidence in patients with atrial fibrillation: a meta-analysis on more than 100 000 individuals. Clin Cardiol. 2020;43(6):594 605. 10.1002/clc.23354) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Buber J, Luria D, Sternik L.et al. Left atrial contractile function following a successful modified Maze procedure at surgery and the risk for subsequent thromboembolic stroke. J Am Coll Cardiol. 2011;58(15):1614 1621. 10.1016/j.jacc.2011.05.051) [DOI] [PubMed] [Google Scholar]
- 43. Fanning L, Wong ICK, Li X.et al. Gastrointestinal bleeding risk with Rivaroxaban vs aspirin in atrial fibrillation: a multinational study. Pharmacoepidemiol Drug Saf. 2020;29(12):1550 1561. 10.1002/pds.5130) [DOI] [PubMed] [Google Scholar]
- 44. Huang WY, Singer DE, Wu YL.et al. Association of intracranial hemorrhage risk With non-vitamin K antagonist oral anticoagulant use vs aspirin use: a systematic review and meta-analysis. JAMA Neurol. 2018;75(12):1511 1518. 10.1001/jamaneurol.2018.2215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vallakati A, Sharma A, Madmani M.et al. Efficacy and safety of novel oral anticoagulants for atrial fibrillation ablation: an updated meta-analysis. Cardiol Ther. 2016;5(1):85 100. 10.1007/s40119-016-0061-7) [DOI] [PMC free article] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a