Abstract
Introduction: Vitamin D deficiency is a worldwide public health concern, which can lead to severe diseases, such as rickets in children and osteomalacia in adults. Most studies have compared equimolar unit-to-unit doses of vitamin D2 and D3.
Objectives: The current study aimed to answer the research question: “How effective is vitamin D2 (600,000 U/1.5 ml) compared to vitamin D3 (300,000 U/1 ml) parenteral supplementation for raising serum vitamin D levels in adult patients treated in a primary health care setting?”
Setting: Primary Health Care Corporation (PHCC) runs 28 health centers distributed throughout the State of Qatar and its capital city, Doha. Qatar is on the east coast of the Arabic peninsula, with very hot and sunny summers and a desert climate.
Study design: This was a retrospective observational cohort study.
Method: A total of 15,716 participants were recruited following ethical approval. They were identified by electronic medical records (EMR) describing the clinical encounters of individuals aged 18 to 60-years-old who attended a health center operated by the PHCC during the 3.5-year study period from January 1, 2017 to June 30, 2020. The PHCC EMR system uses SNOMED codes (a systematically organized computer-processable collection of medical terms providing codes, names, synonyms, and definitions implemented for clinical documentation and reporting). Four study groups were created depending on the type of vitamin D injection and the oral form of replacement therapy. The analysis scheme used the serum vitamin D level within the preceding 4 weeks (pretreatment), followed by administration of the treatment dose. The post-treatment serum testing value should have been available within a maximum of 12 weeks. The Statistical Package for Social Sciences (IBMSPSS; IBM Corp., Armonk, NY, USA) version 23 software was used for the statistical analysis.
Results: Four treatment options were compared, including a vitamin D2 injection, a vitamin D3 injection, combined use of a vitamin D2 injection + a D2 tablet, and combined use of a vitamin D3 injection + a D2 tablet. All four treatment groups were associated with a statistically significant increase in serum vitamin D within a maximum of 12 weeks of follow-up. The vitamin D2 injection alone was associated with the lowest increase in serum concentration by a mean of 3.2 ng/ml. In contrast, the vitamin D3 injection alone or with a D2 tablet increased serum vitamin D by 6.1 and 5.6 ng/ml, respectively. Using the combination of a vitamin D2 injection and a tablet only added a marginal increase of 2.3 ng/ml in serum vitamin D on top of the 3.2 ng/ml increase attained after administering the D2 injection alone.
Conclusion: Utilizing vitamin D3 in an injectable form is the best choice to restore severe vitamin D deficiency. Furthermore, it was superior to the injectable form of vitamin D2, even though vitamin D2 has double the molar units.
Keywords: primary; health; care,; vitamin; D; deficiency,; vitamin; D2; (ergocalciferol),; and; vitamin; D3; (cholecalciferol)
Introduction
Vitamin D is a fat-soluble vitamin consisting of vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). The primary source of vitamin D2 is plants, and D2 can be manufactured synthetically, whereas vitamin D3 is synthesized in the human skin from 7-dehydrocholesterol after exposure to the sun. Both forms of vitamin D are inactive until processed by enzymatic hydroxylation 1 . Vitamin D is involved in the function of vital organs, such as the kidneys, intestinal mucosa, and bones to regulate calcium and phosphate metabolism. In addition, severely low levels can lead to rickets in children and osteomalacia in adults 2 .
Vitamin D is also essential in calcium and phosphorous homeostasis and is associated with parathyroid hormone. A vitamin D deficiency is thought to be associated with osteoporotic and stress-related fractures 3 . Some studies have linked vitamin D deficiency to colon cancer, arthritis, diabetes mellitus, and cardiovascular diseases 4,5,6,7 . Vitamin D deficiency is considered a significant healthcare concern worldwide in all age groups. It is more of a problem for residents in countries located at high latitudes where ultraviolet radiation is insufficient. In addition, residents of developed countries suffer despite the fortification of vitamin D in their food for many years 8 . A recent study in Qatar showed that the prevalence of severe vitamin D deficiency (serum level < 10 ng/ml) among adults attending the Primary Health Care Corporation (PHCC) health centers and aged 18–65 years was 14%. Using less stringent criteria for defining vitamin D deficiency (serum level < 20 ng/mL) increases the prevalence rate to 71.4% 9 .
Many factors are involved in the etiology of vitamin D deficiency, including lack of sun exposure and insufficient consumption of foods rich in vitamin D 10 . Other factors associated with low serum vitamin D levels are age, gender, clothing style, darker skin, socioeconomic status, and body mass index 9 . Moreover, the lifestyle in the Arabian Gulf area is another major factor in vitamin D deficiency because many people travel in their cars rather than walk, run, or cycle because of convenience and the hot climate 6,11,12 .
The preferred marker of vitamin D status is 25(OH)D because it is the principal circulating form of vitamin D in the blood with a half-life of 2–3 weeks 13 . Recent evidence suggests that inter-laboratory variability in 25(OH)D assays could provide an unclear interpretation of low vitamin D serum levels 14 .
The ideal serum 25(OH)D concentration is controversial. The majority of classifications consider that a severe vitamin D deficiency is defined as < 12 ng/ml (30 nmol/L) 25(OH)D. Moderate deficiency is defined as 12–20 ng/ml (30–50 nmol/L) 25(OH)D. Mild deficiency is defined as 25(OH)D >20 ng/ml (50 nmol/L) 15 . PHCC in Qatar considers a severe deficiency to be < 10 ng/ml and a moderate deficiency as < 20 ng/ml.
Recent clinical practice recommendations consider that vitamin D2 and vitamin D3 have the same equivalence in the therapeutic field 2 . However, numerous recent clinical trials have evaluated the ability of equimolar dosing regimens of vitamin D2 vs. vitamin D3 by their capability to increase and sustain the serum total 25(OH)D level 16 . Although most studies have found that orally-administered vitamin D3 increases total serum 25D more vigorously than D2 17,18,19,20 , others have found them to have the same efficacy 21,22 . In contrast, studies that show greater efficacy for vitamin D3 may be limited by small sample size 23 . Furthermore, most studies compared the equal molar quantity of either vitamin D2 or vitamin D3 to elevate serum 25(OH)D.
The current study aimed to answer the research question: “How effective is parenteral supplementation of vitamin D2 (600,000 U/1.5 ml) compared to vitamin D3 (300,000 U/1 ml) on serum vitamin D levels in adult patients treated in a primary healthcare setting?”
Methods
Study settings: The PHCC is a publicly funded primary healthcare provider in Qatar. It provides healthcare services to a large part of the country's population and runs 27 health centers distributed throughout the country 24 . PHCC operates an EMR system called CERNER, which was introduced in July 2016. Qatari citizens and ex-pats who live with their families in Qatar can access and utilize healthcare services provided by the PHCC after registering and paying a nominal annual fee.
Study design: This was a retrospective observational cohort study.
Study population: The data analyzed covers the EMR describing the clinical encounters of individuals aged 18–60-years-old who presented at a health center operated by the PHCC during the 3.5-year study period from January 1, 2017 to June 30, 2020. The inclusion criterion for study participants was having a prescription for at least one dose of a vitamin D2 (600,000 units) or D3 (300,000 units) injection during the study period.
Study variables: The following variables were extracted from the EMR for the targeted study population:
-
•
All serum vitamin D measurements and their dates.
-
•
All prescribed vitamin D2 (600,000 units) or vitamin D3 (300,000 units) injections and tablets with the dates administered.
-
•
Sociodemographic variables, including age at first serum vitamin D measurement (before starting vitamin D replacement therapy), gender, and nationality.
-
•
SNOMED codes for comorbidities including diabetes mellitus, dyslipidemia, asthma, chronic obstructive pulmonary disease, hypertension, cerebrovascular disorders, and chronic kidney disease 25 .
Data Collection: The PHCC EMR system uses SNOMED codes (a systematically organized collection of medical terms providing codes, names, synonyms, and definitions used in clinical documentation and reporting). SNOMED International is a not-for-profit organization that owns and maintains this medical coding system. These codes are quality-controlled and reviewed by the Business Health Intelligence (BHI) department of PHCC. The BHI department is responsible for translating SNOMED codes into ICD-10 codes (International Classification of Disease, Tenth Revision) and continuously updates the coding manual at monthly intervals with new codes used in the organizational database 26 . The BHI department provided a complete list of variables for the study population using custom-made filters.
Data cleaning: A total of 21,269 adults had their first vitamin D injection (D2 or D3) during the study period. Among them, 785 had their first serum vitamin D test after being prescribed the treatment with no available laboratory test before the prescription. These individuals were excluded from the analysis. Another 4,768 individuals had a recorded value for serum vitamin D older than 4 weeks before the first vitamin D injection. This group of study participants was also excluded from the analysis.
The database was restructured to allow a retrospective cohort study analysis using the study date range, with each participant's interaction with the health care system. The aim was to create a paired data design in the form of a pretreatment and post-treatment comparison of serum vitamin D measurements. Four study groups were created depending on the type of vitamin D injection and the oral form of replacement therapy that they may have received as an add on. The primary unit of analysis was the treatment intervention. To qualify for inclusion in the database, a participant should be preceded by a serum vitamin D test measurement within the previous 4 weeks (pretreatment), followed by administration of the treatment. The post-treatment serum testing value should be available within a maximum of 12 weeks.
Data analysis: IBMSPSS version 23 software (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. A P value < 0.05 was considered significant. The paired t-test was used to assess the statistical significance of mean change in a quantitative normally distributed variable (serum vitamin D) after treatment. Cohen's d was used to assess the magnitude of the effect size of each treatment option compared to the vitamin D2 injection alone. A multiple linear regression model was employed to measure the net and independent effect of the treatment options on the magnitude of change in serum vitamin D attributed to the treatment after controlling for possible confounding effects of age, gender, and comorbidities.
Vitamin D deficiency status was measured at two pre-identified cut-off values. The severe deficiency status was defined as a serum vitamin D concentration < 10 ng/ml. Less stringent criteria for defining deficiency status was the < 20 ng/ml cut-off value. The effect of the four treatment options in overcoming the deficiency status after treatment was measured by the paired odds ratio 27 .
Quality control measures: An extensive review of the literature was undertaken during the preparative phase of the study. The authors are responsible for data collection in collaboration with the BHI department.
Ethical considerations: The study presented minimal risk of harm to the participants. The original research proposal was an anonymized data request. This research proposal was approved by the Research Sub-Committee of the PHCC (PHCC/DCR/2020/01/006). This study was conducted according to generally accepted ethical principles.
Results
As shown in Table 1 and Figure 1, all four treatment groups were associated with a statistically significant increase in serum vitamin D within a maximum of 12 weeks of follow-up. A vitamin D2 injection alone was associated with the smallest increase in serum concentration by a mean of 3.2 ng/ml. In contrast, the vitamin D3 injection alone or with a D2 tablet conferred an extra 6.1 and 5.6 ng/ml of serum vitamin D, respectively, on top of the changes introduced by the D2 injection alone. This additional effect of the vitamin D3 injection compared to the D2 injection was classified as a strong effect (Cohen's d >0.6). Using a combination of a vitamin D2 injection and a tablet only conferred a marginal increase of 2.3 ng/ml in serum vitamin D on top of the 3.2 ng/ml increase attainable with an injection alone.
Table 1.
Mean change in serum vitamin D after four types of replacement therapies.
| Serum vitamin D ng/ml-Changes (after the first dose) | Difference in mean compared to the reference | Effect size (Cohen's d) compared to the reference | P value for difference in mean compared to the reference | |||||
|
| ||||||||
| Range | Mean | SD | SE | N | category | category | category | |
|
| ||||||||
| Type of vitamin D prescribed | ||||||||
|
| ||||||||
| vitamin D2 injection | (-16 to 50) | 3.2 | 0.51 | 0.33 | 411 | Reference | ||
|
| ||||||||
| vitamin D3 injection | (-17 to 48) | 9.3 | 0.69 | 0.49 | 454 | 6.1 | 0.69 | < 0.001 |
|
| ||||||||
| vitamin D2 injection + D2 tablet | (-18 to 47) | 5.5 | 0.32 | 0.18 | 1585 | 2.3 | 0.32 | < 0.001 |
|
| ||||||||
| vitamin D3 injection + D2 tablet | (-18 to 47) | 8.8 | 0.94 | 0.27 | 997 | 5.6 | 0.94 | < 0.001 |
|
| ||||||||
P (ANOVA) < 0.001
P (Paired t-test) for each of the four treatments < 0.001
Figure 1.
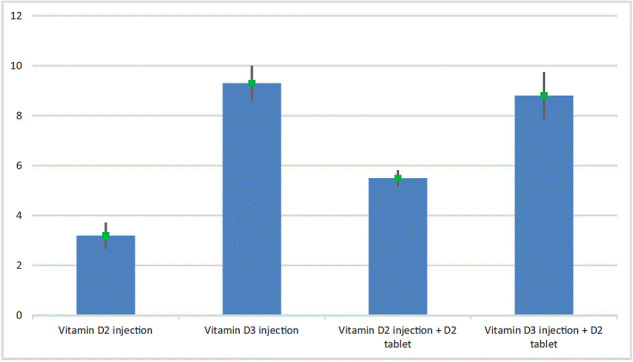
Mean change in serum vitamin D (ng/ml) after treatment.
The effect of age, gender, and six comorbidities on the magnitude of change in serum vitamin D in response to replacement therapy was evaluated in Table 2. Almost all of the explanatory variables had a statistically significant but weak effect (Cohen's d < 0.3) on the treatment response measured by the change in serum vitamin D. In addition, age and chronic kidney disease had no significant effect on the magnitude of the response. Male patients had a better response to replacement therapy than females. Comorbidities were associated with a smaller magnitude of response in the serum vitamin D level to replacement therapy.
Table 2.
Mean change in serum vitamin D after treatment with selected explanatory variables
| Serum vitamin D ng/ml-Changes (after the first dose) | Effect size (Cohen's d) compared to the reference | Difference in mean compared to the reference | P value for difference in mean compared to the reference | |||||
|
| ||||||||
| Range | Mean | SD | SE | N | category | category | category | |
|
| ||||||||
| Gender | ||||||||
|
| ||||||||
| Female | ( − 17 to 50) | 6.5 | 7.9 | 0.16 | 2501 | Reference | ||
|
| ||||||||
| Male | ( − 18 to 48) | 7.2 | 9.4 | 0.31 | 946 | 0.08 | 0.7 | 0.0497 |
|
| ||||||||
| Age group (years) | ||||||||
|
| ||||||||
| < 30 | ( − 10 to 47) | 7 | 7.7 | 0.23 | 1119 | Reference | ||
|
| ||||||||
| 30–39 | ( − 18 to 50) | 6.6 | 8.3 | 0.24 | 1224 | − 0.05 | − 0.4 | 1[NS] |
|
| ||||||||
| 40–49 | ( − 18 to 48) | 6.5 | 8.8 | 0.32 | 754 | − 0.06 | − 0.5 | 1[NS] |
|
| ||||||||
| 50–60 | ( − 15 to 45) | 6.4 | 9.7 | 0.52 | 350 | − 0.06 | − 0.6 | 1[NS] |
|
| ||||||||
| History of Diabetes Mellitus | ||||||||
|
| ||||||||
| Negative | ( − 18 to 50) | 6.9 | 8.4 | 0.16 | 2798 | Reference | ||
|
| ||||||||
| Positive | ( − 15 to 48) | 5.6 | 8.1 | 0.32 | 649 | − 0.16 | − 1.3 | < 0.001 |
|
| ||||||||
| History of Hypertension | ||||||||
|
| ||||||||
| Negative | ( − 18 to 50) | 6.8 | 8.4 | 0.15 | 3021 | Reference | ||
|
| ||||||||
| Positive | ( − 15 to 39) | 5.5 | 7.8 | 0.38 | 426 | − 0.16 | − 1.3 | 0.001 |
|
| ||||||||
| History of Dyslipidemia | ||||||||
|
| ||||||||
| Negative | ( − 18 to 50) | 7 | 8.4 | 0.16 | 2727 | Reference | ||
|
| ||||||||
| Positive | ( − 15 to 48) | 5.5 | 8.2 | 0.3 | 720 | − 0.18 | − 1.5 | < 0.001 |
|
| ||||||||
| History of Cerebrovascular Disorder | ||||||||
|
| ||||||||
| Negative | ( − 18 to 50) | 6.7 | 8.4 | 0.14 | 3394 | Reference | ||
|
| ||||||||
| Positive | ( − 9 to 36) | 4.8 | 7.9 | 1.09 | 53 | − 0.23 | − 1.9 | 0.09[NS] |
|
| ||||||||
| History of Chronic Kidney Disease | ||||||||
|
| ||||||||
| Negative | ( − 18 to 50) | 6.7 | 8.3 | 0.14 | 3422 | Reference | ||
|
| ||||||||
| Positive | ( − 8 to 31) | 6.8 | 9.4 | 1.88 | 25 | 0.01 | 0.1 | 0.97[NS] |
|
| ||||||||
| History of Asthma / Chronic Obstructive Pulmonary Disease | ||||||||
|
| ||||||||
| Negative | ( − 18 to 50) | 6.8 | 8.4 | 0.15 | 3037 | Reference | ||
|
| ||||||||
| Positive | ( − 15 to 48) | 5.6 | 8.1 | 0.4 | 410 | − 0.14 | − 1.2 | 0.004 |
|
| ||||||||
A multiple linear regression model was used to assess the net and independent effects of the explanatory variables on changes in serum vitamin D after treatment. A conclusion similar to the bivariate model considered in Table 2 was reached here. The vitamin D3 injection alone or with a D2 tablet was associated with the highest treatment response compared to a vitamin D2 injection alone after adjusting for age, gender, and comorbidities. The model was statistically significant, as illustrated in Table 3.
Table 3.
Multiple linear regression model and the changes in serum vitamin D after treatment as the dependent (outcome) variable and the treatment type, gender, age, and history of selected chronic conditions as the explanatory variables.
| Unstandardized regression Coefficient (B) | ||||
|
| ||||
| estimate | 95% confidence interval | P | Standardized Coefficients | |
|
| ||||
| (Constant) | 3.5 | (2.6 to 4.5) | < 0.001 | |
|
| ||||
| vitamin D3 (Cholecalciferol) injection compared to vitamin D2 injection | 6.2 | (5.2 to 7.3) | < 0.001 | 0.25 |
|
| ||||
| Combined (Cholecalciferol) injection [D3] + D2 tablet) compared to vitamin D2 injection | 5.7 | (4.8 to 6.6) | < 0.001 | 0.31 |
|
| ||||
| Combined (Ergocalciferol) injection [D2] + D2 tablet) compared to vitamin D2 injection | 2.4 | (1.5 to 3.2) | < 0.001 | 0.14 |
|
| ||||
| Male gender compared to female | 1.3 | (0.6 to 1.9) | < 0.001 | 0.07 |
|
| ||||
| History of Diabetes Mellitus | − 1.3 | ( − 2.1 to − 0.5) | < 0.001 | − 0.06 |
|
| ||||
| History of Dyslipidemia | − 1.5 | ( − 2.2 to -0.7) | < 0.001 | − 0.07 |
|
| ||||
| History of Asthma / Chronic Obstructive Pulmonary Disease | − 0.9 | ( − 1.7 to − 0.1) | 0.036 | − 0.03 |
|
| ||||
| History of Hypertension | − 0.7 | ( − 1.6 to 0.2) | 0.13[NS] | − 0.03 |
|
| ||||
| History of Cerebrovascular Disorder | − 1.2 | ( − 3.4 to 1.1) | 0.31[NS] | − 0.02 |
|
| ||||
| History of Chronic Kidney Disease | 1.7 | ( − 1.5 to 4.9) | 0.29[NS] | 0.02 |
|
| ||||
| Age group (years) | − 0.01 | ( − 0.3 to 0.3) | 0.98[NS] | − 0.001 |
|
| ||||
P (model) < 0.001
Determination coefficient (R2) = 0.076
The effectiveness of the four types of treatments in reducing the prevalence of vitamin D deficiency at the two preset cut-off values was assessed in Tables 4 to 15. A serum vitamin D concentration of < 10 ng/ml was identified as a severe vitamin D deficiency, while < 20 ng/ml was identified as a vitamin D deficiency. In addition, the magnitude of the response to treatment was measured by the paired odds ratio. This ratio measures the probability of achieving the treatment target (overcoming deficiency status after treatment) compared to developing a new deficiency status after treatment (treatment failure). As a result, the beneficial treatment effect was higher and more pronounced when the target for the four treatment types was to correct a severe deficiency as opposed to a deficiency status. In addition, a vitamin D3 injection, whether alone or combined with a D2 tablet, provided the most substantial treatment effect.
Table 4.
Relative frequency (prevalence) of two definitions of serum vitamin D deficiency before and after treatment with a vitamin D2 injection.
| vitamin D2 injection (Total examined | Before treatment | 95% Confidence | After treatment | 95% Confidence | ||
|
| ||||||
| N = 411) | N | % | Interval | N | % | Interval |
|
| ||||||
| Severe vitamin D deficiency (serum conc < 10 ng/ml) | 124 | 30.2 | (25.9 to 34.7) | 41 | 10 | (7.4 to 13.2) |
|
| ||||||
| vitamin D deficiency (serum conc < 20 ng/ml) | 364 | 88.6 | (85.2 to 91.4) | 326 | 79.3 | (75.2 to 83) |
|
| ||||||
Table 15.
Risk (paired odds ratio) of responding to vitamin D3 injection + D2 tablet replacement therapy considering severe serum vitamin D deficiency as the outcome compared to pretreatment deficiency status.
| vitamin D3 injection | vitamin D deficiency (serum conc < 20 ng/ml)-After treatment (within 12 weeks) | Paired | 95% Confidence Interval | |||
|
| ||||||
| + D2 tablet | Negative | Positive | Total | OR | OR | P |
|
| ||||||
| vitamin D deficiency (serum conc < 20 ng/ml)-Baseline (before treatment) | ||||||
|
| ||||||
| Negative | 103 | 22 | 125 | 21 | (14 to 32) | < 0.001 |
|
| ||||||
| Positive | 452 | 420 | 872 | |||
|
| ||||||
| Total | 555 | 442 | 997 | |||
|
| ||||||
Discussion
Clinicians must often reconcile the encouraging results of well-designed studies that have evaluated treatment effectiveness against the apparent replication in everyday medical practice on ambulatory patients in a primary care setting. Therefore, this retrospective cohort study was designed to compare the effectiveness of two forms of vitamin D parenteral supplementation available in the primary healthcare setting, such as vitamin D2 (600,000 U/1.5 ml) and vitamin D3 (300,000 U/1 ml), on improving serum vitamin D levels in adult patients. In addition, the treatment effect of parenteral supplementation in the current study was adjusted for the confounding effect of oral supplementation forms readily available in the health center. Four treatment options were compared. These included a vitamin D2 injection, a vitamin D3 injection, combined use of a vitamin D2 injection + a D2 tablet, and a vitamin D3 injection + a D2 tablet. The results showed that both vitamin D treatments (injectable forms of vitamin D2 and vitamin D3) increased serum vitamin D levels. This finding is consistent with many studies indicating that both types of vitamin D replacement therapy (D2 and D3) increase serum 25(OH)D levels using various vitamin D concentrations and dosing schedules 28,29,30,31,32,33 . In contrast, few studies failed to determine a significant effect of both vitamin D2 and vitamin D3 treatment in their participants. These studies used a small sample size ( < 100 participants in both studies and low doses of vitamin D2 and D3 (1,000 IU daily) 22,21 .
A small sample-sized study compared the treatment response of 50 participants to a bolus dose of 300,000 IU IM (injectable) vitamin D2 (ergocalciferol) to that of 19 participants who received 300,000 IU oral vitamin D3 (cholecalciferol). The authors concluded that both preparations were practical, well-tolerated, and safe. Vitamin D3 had greater potency than equimolar vitamin D2 34 . To evaluate this recommendation, the participants in our study received a double concentration of vitamin D2 compared to D3 (600,000 IU D2 compared to 300,000 IU D3). Nevertheless, vitamin D3 replacement therapy was superior to that of D2 in achieving higher serum vitamin D levels, despite the double concentration of vitamin D2.
The current study demonstrated that a vitamin D3 injection alone or combined with a D2 tablet achieved the best treatment response compared to a vitamin D2 injection or a vitamin D2 injection + a D2 tablet in the primary care setting. This finding agrees with a systematic review and meta-analysis concluding that vitamin D3 replacement is more effective than vitamin D2 35 However, that meta-analysis was the subject of controversy and considerable criticism because of the small number of studies included and the inadequate sample sizes of the participants in each of the studies.
Another observation made by the current study was the large difference in treatment effect between the vitamin D3 injection and the vitamin D2 injection. The former is twice as effective as D2 when used alone and three times more effective when combined with an oral tablet. A similar conclusion was reached in another study comparing 50,000 IU oral administration of vitamin D2 with D3, which showed an 87% increase in D3 potency compared to D2 36 . Another study revealed that vitamin D2 replacement therapy is almost one-third the potency of vitamin D3 37 .
The current study showed that combining the less potent form of vitamin D in parenteral replacement therapy (vitamin D2) with oral tablets resulted in a doubling of the potency of the injection alone. This result could be explained by the time needed to reach the steady-state concentration. The half-life of these medications ranges from 5 weeks to 5 months 38 , and the vitamin D binding protein affinity of the injection and tablet, as well as the oily nature of injectable therapy, are other reasons. In addition, combining parenteral vitamin D3 with a D2 tablet was more effective than the isolated use of the parenteral preparation for managing a severe vitamin D deficiency. However, this added advantage of vitamin D2 tablets failed to show when the absolute mean change in serum vitamin D was examined. This may reflect the varying focus of the managing physician or the individual (whenever possible) in achieving higher serum levels Vs simply achieving the target of overcoming the deficiency status.
Including a large number of individuals with comorbidities in this study enabled an in-depth analysis of the treatment effect. In general, having comorbidities (diabetes mellitus, hypertension, dyslipidemia, cerebrovascular disorder, asthma, or chronic obstructive pulmonary disease) was associated with a significant but weak effect (Cohen's d < 0.3) on the treatment response based on changes in serum vitamin D for both treatments. This may relate to the multiple medications that these patients may be taking to control their conditions. The only exception was chronic kidney disease, which had no statistically significant effect on the magnitude of the response.
The current study demonstrated that the age of the patients was not associated with a significant difference in vitamin D levels post-treatment for any type of regimen. Moreover, male participants had a significantly better response than females. This difference could be related to many factors, such as the dietary habits of the participants or the amount of sun exposure, which is influenced by the exposed body surface. In general, social customs in the Middle East prohibit females from openly exposing most parts of their body.
Another question that was addressed by the current study was “is it better to target moderate or severe vitamin D deficiency with replacement therapy?” Targeting severe deficiency was associated with a much better treatment response compared to treating a moderate deficiency. This outcome was evident when using vitamin D3 alone or with a D2 tablet and the same was applied to using the vitamin D2 injection alone or with a vitamin D2 tablet. This outcome has financial implications, considering the cost of medication used to restore normal levels of vitamin D and the cost of hospitalization due to a vitamin D deficiency. It has been suggested that of the 30 leading causes of death in the United States in 2010, 19 were linked to low vitamin D status, including various forms of cardiovascular disease, various cancers, diabetes mellitus, Alzheimer's disease, falls, and fractures in the elderly 39 . If the population of the United States was to increase their vitamin D status to 40 ng/mL, we could expect to see a potential reduction of as much as 336,000 deaths each year (out of 2.1 million deaths attributed to the diseases of concern), so raising 25(OH)D concentrations would be a cost-effective route to reduce the burden of disease and increase life expectancy in the United States 40 .
In summary, using a vitamin D3 injectable form to correct a severe vitamin D deficiency is an appropriate choice to restore acceptable levels and has superiority over a vitamin D2 injectable form. The double molar units used to achieve the greater impact of vitamin D2 were insufficient to achieve a comparable improvement level to that with vitamin D3. The synergistic effect of a vitamin D2 tablet with a vitamin D2 injection is notable and could help to achieve a better treatment response.
Limitations
The main limitation of this study arises from the fact that it was based on the patients’ electronic health records. This resulted in the inability to adjust for the confounding effect of vitamin D intake from food sources, sun exposure, smoking history, alcohol intake, and physical exercise status of the participants 13 . Another possible source of bias was the variable follow-up period, ranging from 1 to 12 weeks. In addition, the long-term outcomes of the intervention were not evaluated.
The number of injections received during the 3-month follow-up period would affect the resulting serum vitamin D level after treatment. However, this bias would have a small effect when comparing the two treatments (since it is a non-differential bias) as it affected both the same. In addition, the magnitude of the response was not the primary objective of this study, but rather the difference incurred by treatment choices in a primary health care setting.
Conclusion
Vitamin D2 and vitamin D3 increased serum vitamin D levels, but doubling the vitamin D2 dose failed to match the better treatment response of vitamin D3. Relying on a vitamin D3 injectable form would be a preferable choice for treating the severe form of vitamin D deficiency in a primary care setting.
The vitamin D3 injectable form had a more favorable effect in terms of treating vitamin D deficiency and could be a more cost-effective option to achieve the targeted goal of treatment.
It would be interesting to undertake further investigations to compare the effectiveness of vitamin D2 and D3 in a randomized controlled trial (intervention compared to a control group).
Acknowledgments
We gratefully acknowledge Dr. Ahmed Sameer Alnuaimi for his continuous guidance and assistance. Many thanks to the team at Al Wajbah Health Center, including Dr. Meshal Al Mesaifri (Health Center Manager), Dr. Alia Al Ruwaili (Deputy Health Center Manager), Dr. Muhammad Arsalan Zamir (Physician Lead), and the Pharmacy team.
Special thanks also to the Business Intelligence Department at the PHCC for their support in data extraction.
Table 5.
Risk (paired odds ratio) of responding to vitamin D2 injection replacement therapy considering a severe serum vitamin D deficiency as the outcome compared to pretreatment deficiency status.
| Sever vitamin D deficiency (serum conc < 10 ng/ml)-After treatment (within 12 weeks) | Paired | 95% Confidence Interval | ||||
|
| ||||||
| vitamin D2 injection | Negative | Positive | Total | OR | OR | P |
|
| ||||||
| Severe vitamin D deficiency (serum conc < 10 ng/ml)-Baseline (before treatment) | ||||||
|
| ||||||
| Negative | 280 | 7 | 287 | 13 | (6 to 28) | < 0.001 |
|
| ||||||
| Positive | 90 | 34 | 124 | |||
|
| ||||||
| Total | 370 | 41 | 411 | |||
|
| ||||||
Table 6.
Risk (paired odds ratio) of responding to vitamin D2 injection replacement therapy considering vitamin D deficiency as the outcome compared to pretreatment deficiency status.
| vitamin D deficiency (serum conc < 20 ng/ml)-After treatment (within 12 weeks) | Paired | 95% Confidence Interval | ||||
|
| ||||||
| vitamin D2 injection | Negative | Positive | Total | OR | OR | P |
|
| ||||||
| vitamin D deficiency (serum conc < 20 ng/ml)-Baseline (before treatment) | ||||||
|
| ||||||
| Negative | 25 | 22 | 47 | 3 | (2 to 5) | < 0.001 |
|
| ||||||
| Positive | 60 | 304 | 364 | |||
|
| ||||||
| Total | 85 | 326 | 411 | |||
|
| ||||||
Table 7.
Relative frequency (prevalence) of two definitions of serum vitamin D deficiency before and after treatment with vitamin D3 injection.
| vitamin D3 injection (Total | Before treatment | 95% Confidence | After treatment | 95% Confidence | ||
|
| ||||||
| examined N = 454) | N | % | Interval | N | % | Interval |
|
| ||||||
| Severe vitamin D deficiency (serum conc < 10 ng/ml) | 119 | 26.2 | (22.3 to 30.4) | 17 | 3.7 | (2.3 to 5.8) |
|
| ||||||
| vitamin D deficiency (serum conc < 20 ng/ml) | 378 | 83.3 | (79.6 to 86.5) | 187 | 41.2 | (36.7 to 45.8) |
|
| ||||||
Table 8.
Risk (paired odds ratio) of responding to vitamin D3 injection replacement therapy considering severe serum vitamin D deficiency as the outcome compared to pretreatment deficiency status.
| Sever vitamin D deficiency (serum conc < 10 ng/ml)-After treatment (within 12 weeks) | Paired | 95% Confidence Interval | ||||
|
| ||||||
| vitamin D3 injection | Negative | Positive | Total | OR | OR | P |
|
| ||||||
| Severe vitamin D deficiency (serum conc < 10 ng/ml)-Baseline (before treatment) | ||||||
|
| ||||||
| Negative | 331 | 4 | 335 | 27 | (10 to 73) | < 0.001 |
|
| ||||||
| Positive | 106 | 13 | 119 | |||
|
| ||||||
| Total | 437 | 17 | 454 | |||
|
| ||||||
Table 9.
Risk (paired odds ratio) of responding to vitamin D3 injection replacement therapy considering vitamin D deficiency as the outcome compared to pretreatment deficiency status.
| vitamin D deficiency (serum conc < 20 ng/ml)-After treatment (within 12 weeks) | Paired | 95% Confidence Interval | ||||
|
| ||||||
| vitamin D3 injection | Negative | Positive | Total | OR | Or | P |
|
| ||||||
| vitamin D deficiency (serum conc < 20 ng/ml)-Baseline (before treatment) | ||||||
|
| ||||||
| Negative | 57 | 19 | 76 | 11 | (7 to 18) | < 0.001 |
|
| ||||||
| Positive | 210 | 168 | 378 | |||
|
| ||||||
| Total | 267 | 187 | 454 | |||
|
| ||||||
Table 10.
Relative frequency (prevalence) of two definitions of serum vitamin D deficiency before and after treatment with a vitamin D2 injection + a D2 tablet.
| vitamin D2 injection + D2 tablet | Before treatment | 95% Confidence | After treatment | 95% Confidence | ||
|
| ||||||
| (Total examined N = 1585) | N | % | Interval | N | % | Interval |
|
| ||||||
| Severe vitamin D deficiency (serum conc < 10 ng/ml) | 622 | 39.2 | (36.9 to 41.7) | 72 | 4.5 | (3.6 to 5.7) |
|
| ||||||
| vitamin D deficiency (serum conc < 20 ng/ml) | 1462 | 92.2 | (90.8 to 93.5) | 1172 | 73.9 | (71.7 to 76.1) |
|
| ||||||
Table 11.
Risk (paired odds ratio) of responding to vitamin D2 injection + D2 tablet replacement therapy considering severe serum vitamin D deficiency as the outcome compared to pretreatment deficiency status.
| vitamin D2 injection + D2 | Sever vitamin D deficiency (serum conc < 10 ng/ml)-After treatment (within 12 weeks) | Paired | 95% Confidence Interval | |||
|
| ||||||
| tablet | Negative | Positive | Total | OR | OR | P |
|
| ||||||
| Severe vitamin D deficiency (serum conc < 10 ng/ml)-Baseline (before treatment) | ||||||
|
| ||||||
| Negative | 941 | 22 | 963 | 26 | (17 to 40) | < 0.001 |
|
| ||||||
| Positive | 572 | 50 | 622 | |||
|
| ||||||
| Total | 1513 | 72 | 1585 | |||
|
| ||||||
Table 12.
Risk (paired odds ratio) of responding to vitamin D2 injection + D2 tablet replacement therapy considering severe serum vitamin D deficiency as the outcome compared to pretreatment deficiency status.
| vitamin D deficiency (serum conc < 20 ng/ml)-After treatment (within 12 weeks) | Paired | 95% Confidence Interval | ||||
|
| ||||||
| vitamin D2 injection + D2 tablet | Negative | Positive | Total | OR | OR | P |
|
| ||||||
| vitamin D deficiency (serum conc < 20 ng/ml)-Baseline (before treatment) | ||||||
|
| ||||||
| Negative | 76 | 47 | 123 | 7 | (5 to 10) | < 0.001 |
|
| ||||||
| Positive | 337 | 1125 | 1462 | |||
|
| ||||||
| Total | 413 | 1172 | 1585 | |||
|
| ||||||
Table 13.
Relative frequency (prevalence) of two definitions of serum vitamin D deficiency before and after treatment with a vitamin D3 injection + a D2 tablet.
| vitamin D3 injection + D2 tablet | Before treatment | 95% Confidence | After treatment | 95% Confidence | ||
|
| ||||||
| (Total examined N = 997) | N | % | Interval | N | % | Interval |
|
| ||||||
| Severe vitamin D deficiency (serum conc < 10 ng/ml) | 314 | 31.5 | (28.7 to 34.4) | 14 | 1.4 | (0.8 to 2.3) |
|
| ||||||
| vitamin D deficiency (serum conc < 20 ng/ml) | 872 | 87.5 | (85.3 to 89.4) | 442 | 44.3 | (41.3 to 47.4) |
|
| ||||||
Table 14.
Risk (paired odds ratio) of responding to vitamin D3 injection + D2 tablet replacement therapy considering severe serum vitamin D deficiency as the outcome compared to pretreatment deficiency status.
| vitamin D3 injection | Sever vitamin D deficiency (serum conc < 10 ng/ml)-After treatment (within 12 weeks) | Paired | 95% Confidence Interval | |||
|
| ||||||
| + D2 tablet | Negative | Positive | Total | OR | OR | P |
|
| ||||||
| Severe vitamin D deficiency (serum conc < 10 ng/ml)-Baseline (before treatment) | ||||||
|
| ||||||
| Negative | 679 | 4 | 683 | 76 | (28 to 204) | < 0.001 |
|
| ||||||
| Positive | 304 | 10 | 314 | |||
|
| ||||||
| Total | 983 | 14 | 997 | |||
|
| ||||||
References
- 1. Heaney RP, Weaver CM. Overview of vitamin D. Diet Ref Intakes Calcium Vitam D 2003;32:75–134.
- 2. Blanco A, Blanco G. Medical biochemistry. J Med Biochem 2017;185–188 p.
- 3. Hamilton B. Vitamin D and human skeletal muscle. Scand J Med Sci Sports 2010;20(2):182–90. [DOI] [PMC free article] [PubMed]
- 4. Mozos I, Marginean O. Links between vitamin D deficiency and cardiovascular diseases. BioMed Res Int 2015;2015:109275. [DOI] [PMC free article] [PubMed]
- 5. Tsoucalas G, Sgantzos M. Mediterranean. Journal 2017;28(4):223–6. [DOI] [PMC free article] [PubMed]
- 6. Sadiya A, Ahmed SM, Skaria S, Abusnana S. Vitamin D status and its relationship with metabolic markers in persons with obesity and type 2 diabetes in the UAE: A cross-sectional study. J Diabetes Res 2014;2014:869307. [DOI] [PMC free article] [PubMed]
- 7. Pani MA, Knapp M, Donner H, Braun J, Baur MP, Usadel KH, Badenhoop K. Vitamin D receptor allele combinations influence genetic susceptibility to type 1 diabetes in Germans. Diabetes 2000;49(3):504–7. [DOI] [PubMed]
- 8. Craik C. Is vitamin D deficiency a major global public health problem. Bone 2008;23(1):1–7.
- 9. Zainel AAL, Qotba H, Al Nuaimi A, Syed M. Vitamin D status among adults (18–65 years old) attending primary healthcare centres in Qatar: A cross-sectional analysis of the Electronic Medical Records for the year 2017. BMJ Open 2019;9(8):e029334. [DOI] [PMC free article] [PubMed]
- 10. Al-Saleh Y, Al-Daghri NM, Khan N, Alfawaz H, Al-Othman AM, Alokail MS, Chrousos GP. Vitamin D status in Saudi school children based on knowledge. BMC Pediatr 2015;15(1):53. [DOI] [PMC free article] [PubMed]
- 11. Hussain AN, Alkhenizan AH, El Shaker M, Raef H, Gabr A. Increasing trends and significance of hypovitaminosis D: A population-based study in the Kingdom of Saudi Arabia. Arch Osteoporos 2014;9(1):190. [DOI] [PubMed]
- 12. Gannagé-Yared MH, Chemali R, Yaacoub N, Halaby G. Hypovitaminosis D in a sunny country: Relation to lifestyle and bone markers. J Bone Miner Res 2000;15(9):1856–62. [DOI] [PubMed]
- 13. Malaeb D, Hallit S, Salameh P. Assessment of vitamin D levels, awareness among Lebanese pharmacy students, and impact of pharmacist counseling. J Epidemiol Glob Health 2017;7(1):55–62. [DOI] [PMC free article] [PubMed]
- 14. Binkley N, Krueger D, Cowgill CS, Plum L, Lake E, Hansen KE, et al. Assay variation confounds the diagnosis of hypovitaminosis D: A call for standardization. J Clin Endocrinol Metab 2004;89(7):3152–7. [DOI] [PubMed]
- 15. Giustina A, Adler RA, Binkley N, Bouillon R, Ebeling PR, Lazaretti-Castro M, et al. Controversies in vitamin D: Summary statement from an international conference. J Clin Endocrinol Metab 2019;104(2):234–40. [DOI] [PubMed]
- 16. Shieh A, Chun RF, Ma C, Witzel S, Meyer B, Rafison B, et al. Effects of high-dose vitamin D2 versus D3 on total and free 25-hydroxyvitamin D and markers of calcium balance. J Clin Endocrinol Metab 2016;101(8):3070–8. [DOI] [PMC free article] [PubMed]
- 17. Cipriani C, Romagnoli E, Pepe J, Russo S, Carlucci L, Piemonte S, et al. Long-term bioavailability after a single oral or intramuscular administration of 600,000 IU of ergocalciferol or cholecalciferol: Implications for treatment and prophylaxis. J Clin Endocrinol Metab 2013;98(7):2709–15. [DOI] [PubMed]
- 18. Romagnoli E, Mascia ML, Cipriani C, Fassino V, Mazzei F, D'Erasmo E, et al. Short and long-term variations in serum calciotropic hormones after a single very large dose of ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) in the elderly. J Clin Endocrinol Metab 2008;93(8):3015–20. [DOI] [PubMed]
- 19. Trang HM, Cole DEC, Rubin LA, Pierratos A, Siu S, Vieth R. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr 1998;68(4):854–8. [DOI] [PubMed]
- 20. Lehmann U, Hirche F, Stangl GI, Hinz K, Westphal S, Dierkes J. Bioavailability of vitamin D(2) and D(3) in healthy volunteers, a randomized placebo-controlled trial. J Clin Endocrinol Metab 2013;98(11):4339–45. [DOI] [PubMed]
- 21. Holick MF, Biancuzzo RM, Chen TC, Klein EK, Young A, Bibuld D, et al. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab 2008;93(3):677–81. [DOI] [PMC free article] [PubMed]
- 22. Biancuzzo RM, Young A, Bibuld D, Cai MH, Winter MR, Klein EK, et al. Fortification of orange juice with vitamin D(2) or vitamin D(3) is as effective as an oral supplement in maintaining vitamin D status in adults. Am J Clin Nutr 2010;91(6):1621–6. [DOI] [PMC free article] [PubMed]
- 23. Tripkovic L, Lambert H, Hart K, Smith CP, Bucca G, Penson S, et al. Reinhold Vieth and SL-N. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: A systematic review and meta-analysis. Rev Iberoam 2008;74(224):685–701. Available from: http://www.academia.edu/download/31819832/Birkenmaier__Revista_Iberoamericana_2008.pdf. [DOI] [PMC free article] [PubMed]
- 24. Al-Kuwari MG, Al-Abdulla SA, Abdulla MY, Bakri AH, Mohammed AM, Kandy MC, et al. Epidemiological health assessment in primary healthcare in the State of Qatar- 2019. Qatar Med J 2021;2021(3):1–12. [DOI] [PMC free article] [PubMed]
- 25. Bowman SE. Coordination of SNOMED-CT and ICD-10: Getting the most out of electronic health record systems. Coord SNOMED-CT ICD-10 Get Most out Electron Heal Rec Syst Am Heal Inf Manag Assoc. 2005.
- 26. Isaradech N, Khumrin P. Auto-mapping clinical documents to ICD-10 using SNOMED-CT. In: AMIA Jt Summits Transl Sci Proc. American Medical Informatics Association 2021;2021:296–304. [PMC free article] [PubMed]
- 27. Kleinbaum DG, Sullivan KM, Barker ND. A pocket guide to epidemiology. Springer Science+Business Media. 2007.
- 28. Hammami MM, Yusuf A. Differential effects of vitamin D2 and D3 supplements on 25-hydroxyvitamin D level are dose, sex, and time dependent: A randomized controlled trial. BMC Endocr Disord 2017;17(1):12. [DOI] [PMC free article] [PubMed]
- 29. Aterrado S, Ono G, Kanehira-Mar S, Meier J, Swislocki A. Evaluating vitamin D repletion regimens and effects in veteran patients. Ann Pharmacother 2015;49(9):969–77. [DOI] [PubMed]
- 30. Biancuzzo RM, Clarke N, Reitz RE, Travison TG, Holick MF. Serum concentrations of 1, 25-dihydroxyvitamin D2 and 1, 25-dihydroxyvitamin D3 in response to vitamin D2 and vitamin D3 supplementation. J Clin Endocrinol Metab 2013;98(3):973–9. [DOI] [PMC free article] [PubMed]
- 31. Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr 2003;77(1):204–10. [DOI] [PubMed]
- 32. Whiting SJ, Bonjour JP, Payen FD, Rousseau B. Moderate amounts of vitamin D3 in supplements are effective in raising serum 25-hydroxyvitamin D from low baseline levels in adults: A systematic review. Nutrients 2015;7(4):2311–23. [DOI] [PMC free article] [PubMed]
- 33. Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest 2006;116(8):2062–72. [DOI] [PMC free article] [PubMed]
- 34. Leventis P, Kiely PDW. The tolerability and biochemical effects of high-dose bolus vitamin D2 and D3 supplementation in patients with vitamin D insufficiency. Scand J Rheumatol 2009;38(2):149–53. [DOI] [PubMed]
- 35. Laura T, Helen L, Kathryn H, Colin P, Smith, Giselda B, et al. Elina, Hyppo nen. Jacqueline, Berry. Reinhold, Vieth and SL-N. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. [Internet]. American Society for Nutrition; 2012. Available from: http://epubs.surrey.ac.uk/725769/1/ajcn9561357.pdf. [DOI] [PMC free article] [PubMed]
- 36. Heaney RP, Recker RR, Grote J, Horst RL, Armas LAG. Vitamin D(3) is more potent than vitamin D(2) in humans. J Clin Endocrinol Metab 2011;96(3):E447–52. [DOI] [PubMed]
- 37. Armas LAG, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab 2004;89(11):5387–91. [DOI] [PubMed]
- 38. Cashman KD, Seamans KM, Lucey AJ, Stöcklin E, Weber P, Kiely M, Hill TR. Relative effectiveness of oral 25-hydroxyvitamin D3 and vitamin D3 in raising wintertime serum 25-hydroxyvitamin D in older adults. Am J Clin Nutr 2012;95(6):1350–6. [DOI] [PubMed]
- 39. Baggerly CA, Cuomo RE, French CB, Garland CF, Gorham ED, Grant WB, et al. Sunlight and vitamin D: Necessary for public health. J Am Coll Nutr 2015;34(4):359–65. [DOI] [PMC free article] [PubMed]
- 40. Grant WB. An estimate of the global reduction in mortality rates through doubling vitamin D levels. Eur J Clin Nutr 2011;65(9):1016–26. [DOI] [PubMed]


