Abstract
Objective
To characterize return to ovulation after injecting Sayana Press (104 mg/0.65 mL medroxyprogesterone acetate [MPA] in the Uniject device) every 4 months for 1 year of treatment.
Study design
We followed a subset of women for return to ovulation in a trial that demonstrated Sayana Press remains highly effective when the subcutaneous reinjection interval is extended from 3 to 4 months. We measured serum progesterone in weeks 38 to 42 and 46 to 50 after a final (third) injection and used a concentration ≥4.7 ng/mL as a surrogate for ovulation. We also performed pharmacokinetic and pharmacodynamic modeling to predict differences in MPA accumulation and return to ovulation had - contrary to fact - injections been given every 3 months.
Results
Ten of 19 women (53%; 95% confidence interval: 29–76) ovulated within 50 weeks of their last injection. We predicted that typical 12-month trough MPA concentrations are 34% lower (0.46 vs 0.69 ng/mL) and the median time from last dose to ovulation is 1.1 months shorter (13.1 vs 14.2 months) when injections are given every four months for 1 year.
Conclusion
Extending the Sayana Press reinjection interval from 3 to 4 months leads to less drug accumulation, without a noticeable loss in efficacy. Although the Sayana Press patient leaflet specifies that over 80% of women desiring pregnancy will conceive within a year of stopping the method (independent of treatment duration), our empirical and modeling results indicate women should anticipate waiting a year or more for fertility to return after repeat dosing, with a somewhat shorter delay were the reinjection interval extended to four months.
Implications
Providers should counsel women regarding the distinct possibility that return to fertility will take a year or longer following repeat use of Sayana Press. Extending the dosing interval from 3 to 4 months would result in approximately a 1-month shorter delay, without any appreciable reduction in contraceptive efficacy.
Keywords: Medroxyprogesterone acetate, Depo-subQ provera, Pharmacokinetics, Pharmacodynamics
1. Introduction
Depo-subQ Provera 104 (104 mg medroxyprogesterone acetate [MPA] in 0.65 mL suspension) is a safe and highly efficacious contraceptive method, with a pregnancy rate less than 0.25 per 100 women-years [1,2]. Depo-subQ Provera 104 is supplied in a prefilled glass syringe and labelled for subcutaneous (SC) injection every 3 months (13 weeks ±1 week) in the abdomen or anterior thigh [1]. The same formulation in a Uniject delivery system, Sayana Press, is approved in many African, Asian, and European countries. Sayana Press facilitates access to the method beyond clinic-based settings due to its ease of administration, including self-injection [3]. The two products may not be strictly equivalent due to differences in how drug is expelled from the devices, but they exhibited similar pharmacokinetics (PK) and pharmacodynamics (PD) in a comparative trial that supported regulatory approval of Sayana Press in the United Kingdom [4]. Here we refer to both products as Depo-SC unless reference to a specific delivery device is warranted.
We previously hypothesized that Depo-SC would remain highly effective if the reinjection interval were extended from 3 to 4 months, based on PK and PD studies of the method [5]. One motivation for extending the reinjection interval is the potential to reduce exposure-related side effects, including delayed return to fertility. Prescribing information for Depo-SC in the United States (U.S.) notes that 12 of 15 women (80%) who received multiple doses (clarified elsewhere as three injections) ovulated within 12 months of their last dose, but only one of 21 women (5%) who stopped using the method to become pregnant did so within 12 months [1,6]. The U.S. patient product information is similarly cautious, indicating that it may take a year or longer after a last injection to become pregnant [7]. In contrast, the United Kingdom patient leaflet for Depo-SC in Uniject states the effect of the last injection wears off in most women after 6 months, regardless of how long it has been used, and that over 80% of women who want to get pregnant will do so within a year [8]. The latter presumably refers to the ability to conceive, but even that may be optimistic: we previously observed 0 of 15 women ovulating within 7.5 months of a single injection and only 4 of 9 (44.4%) ovulating within 12 months of a second injection in phase 1 studies of Depo-SC [9,10,11].
Here we report on return to ovulation among a subset of women who participated in a phase 3 trial of Depo-SC administered with the Uniject device every four months for 1 year of treatment [5]. We contrast our findings with historical data and use modeling to predict differences in MPA accumulation and return to ovulation had – contrary to fact – injections been given every 3 months.
2. Materials and methods
We previously reported results of an efficacy trial conducted between September 2017 and April 2020 in which 750 women in Brazil, Chile, and the Dominican Republic received three injections of Depo-SC with the Uniject device at 4-month intervals (Clinical Trials Registration Number NCT03154125) [5]. The primary analysis was restricted to 710 participants randomized 1:1 to receive injections in the abdomen or thigh. Eighty of these women (40 per injection site, constituting a PK Cohort) agreed to sparse MPA sampling at baseline, months 2 and 3; prior to reinjections at months 4 and 8; and at their final month 12 visit. Noting that Depo-SC in Uniject may be injected off-label in the upper arm, an additional 40 participants were randomized to that site for comparative PK analyses [12]. The FHI 360 Protection of Human Subjects Committee and ethics boards applicable to each research site approved the study. No pregnancies occurred during treatment, resulting in a Pearl Index of 0.00 per 100 women-years (95% confidence interval [95% CI]: 0.00, 0.59). Geometric mean trough MPA concentrations four months after each dose were highest among women receiving injections in the abdomen and lowest in the upper arm. Here we focus on the abdomen and thigh group data, since those are the injection sites specified in the product label.
A subset of 20 participants (the Ovulation Cohort) from Brazil and the Dominican Republic who were treated every four months per protocol, received their injections in the abdomen or thigh, and did not plan to use hormonal contraception after the study were monitored for return to ovulation. Participants in the Ovulation Cohort (none of whom were in the PK Cohort) contributed five weekly serum progesterone measurements between weeks 38 and 42 (month 10) after their last SC injection; if a single progesterone concentration ≥4.7 ng/mL was observed then ovulation was assumed to have occurred (the same threshold used in the Depo-SC development program [13,14]). If ovulation was not detected, then the procedure was repeated during weeks 46 to 50 (approximately 1 year) after last injection. In our primary analysis, return to ovulation was summarized among all women with evaluable data using exact 95% CIs for binomial proportions. We also collected specimens for MPA, levonorgestrel (LNG), and etonogestrel (ENG) testing 38 and 46 weeks after last injection and excluded anovulatory participants with detectable LNG or ENG (indicative of hormonal contraception use in violation of the protocol) from a post hoc per protocol analysis. Progesterone levels were measured at local laboratories using chemiluminescence immunoassays (Roche Elecsys 2010 and Siemens Advia Centaur) with sensitivity ≤0.05 ng/mL and inter-assay coefficient of variation <10%. Exogenous hormone levels were determined with validated high-performance liquid chromatography tandem mass spectrometry assays at PPD Development (Richmond, VA). The lower limit of quantification for MPA was 0.02 ng/mL, the inter-assay coefficient of variation was <11%, the inter-assay accuracy was −0.4 to 4.0%, and the average intra-assay coefficient of variation was <8%.
The observed return to ovulation results were augmented with PK/PD modeling. Because only sparse MPA data were available for analysis, it was not possible to characterize complete pharmacokinetic profiles. However, we could model trough MPA concentrations and drug levels after treatment ended by assuming that a terminal, log-linear phase of absorption was achieved by the third month after each injection. Our mixed-effects model included parameters for the typical MPA concentration attributable to an injection four months (122 days) earlier (μ; equivalent to the 4-month concentration in a single dose study); the typical absorption rate in the terminal phase (λ; inversely proportional to apparent half-life); fixed effects of injection site on μ; fixed effects of body weight on μ and λ; and correlated random subject effects for μ and λ. We incorporated an external estimate of the distribution of threshold MPA levels when ovulation returns into a joint PK/PD model to predict the cumulative probability of ovulation after treatment ends. The latter presumed each woman has her own intrinsic threshold value, with approximately 10% of women ovulating at MPA levels exceeding 0.1 ng/mL and a median threshold of 0.07 ng/mL [11]. However, we also performed a sensitivity analysis that conservatively assumed 10% of women ovulate at MPA levels exceeding 0.2 ng/mL; a fixed level cited as sufficient to ensure a contraceptive effect in some previous studies [13,14].
We estimated pharmacokinetic model parameters and MPA levels over time for a typical woman (analogous to median levels in our model framework) using maximum likelihood, and predicted return to ovulation for a population of women with similar weight distribution as the Ovulation Cohort using Monte Carlo simulations. The same methods were used to predict MPA levels and return to ovulation had - contrary to fact - participants been dosed every 3 months for up to 1 year of treatment. We assessed the external validity of the model by comparing predictions to results of previous Depo-SC studies. All analyses were performed using SAS/STAT software (SAS Institute, Cary, NC). Details of data handling rules, model assumptions, and sensitivity analyses are provided in the Supplemental Appendix.
3. Results
3.1. Participant demographics and disposition
Participants in the Ovulation Cohort had a median age at enrolment of 29 years (range: 18–35); 35% identified as white and 65% as black or biracial; and the median weight at the final (treatment month 8) reinjection visit was 66 kg (range: 48–105). Nine of 20 women (45%) received their injections in the abdomen and 11 (55%) in the thigh. One participant with progesterone concentrations ≤0.5 ng/mL at weeks 38 through 41 discontinued prior to completing her first PD assessment period at week 42 and was excluded from the return to ovulation analysis. The remaining 19 Ovulation Cohort participants completed their prescribed post-treatment follow-up (Table 1). Among 80 participants in the PK Cohort, eight (10%) were excluded from our modeling: five had MPA concentrations >0.10 ng/mL in their baseline specimens, one may have received more than a single dose at baseline due to partial needle blockage on the first injection attempt, and two discontinued before their month 3 visit. The remaining 72 PK Cohort participants were somewhat more likely to identify as white (49%) and had a higher median weight at their final injection visit (71 kg) than in the Ovulation Cohort, although the differences were not statistically significant (Table 1).
Table 1.
Demographics and disposition data for the cohorts of participants who contributed to pharmacokinetics (PK) and return to ovulation analysesa The parent trial that assessed efficacy of Depo-SC when the injection interval is extended to 4 months was conducted between 2017 and 2020 [5].
| PK cohort (N = 72) | Ovulation cohort (N = 20) | p-valueb | |
|---|---|---|---|
| Age (y) | |||
| Baseline | 26 (18–35) | 29 (18–35) | 0.45 |
| Race | |||
| White | 35 (48.6%) | 7 (35.0%) | 0.32 |
| Biracial or Black | 37 (51.4%) | 13 (65.0%) | |
| Weight (kg) | |||
| Baseline | 68 (45–167) | 64 (44–111) | 0.30 |
| Month 8 (last injection) | 71 (47–152) | 66 (48–105) | 0.50 |
| Injection site | |||
| Abdomen | 36 (50.0%) | 9 (45.0%) | 0.80 |
| Upper thigh | 36 (50.0%) | 11 (55.0%) | |
| Investigational clinic | |||
| Brazil | 24 (33.3%) | 16 (80.0%) | <0.01 |
| Chile | 24 (33.3%) | 0 (0.0%) | |
| Dominican Republic | 24 (33.3%) | 4 (20.0%) | |
| Visits contributedc | |||
| Mo 3 | 67 (93.0%) | NA | NA |
| Mo 4 | 66 (91.7%) | 20 (100%) | |
| Mo 8 | 64 (88.9%) | 20 (100%) | |
| Mo 12 | 59 (81.9%) | 20 (100%) | |
| Post-treatment | NA | 19 (95%) | |
Data presented are median (range) or n (%).
p-values are based on Kruskal Wallace tests for continuous variables and Fisher's Exact tests for categorical data.
Participation in the PK Cohort (contribution of MPA specimens at months 3, 4, 8, and 12) was determined prior to study enrollment, while completion of month 4, 8, and 12 visit procedures per protocol was a requirement for participation in the return to Ovulation Cohort.
We did not obtain MPA specimens at the end of the 1-year treatment period (18 weeks after last injection) in the Ovulation Cohort. However, their MPA levels 38 weeks after last injection (range: <0.02–0.31 ng/mL) were generally well below those at the end of treatment in the PK Cohort (range: 0.22–0.88), providing qualitative assurance that additional injections were not covertly taking place in the return to ovulation assessment period. Likewise, there was no evidence of ENG use in violation of the protocol. However, two participants in the Ovulation Cohort had detectable LNG in their specimens 46 weeks after last injection: one with a relatively high LNG level (0.46 ng/mL) nonetheless ovulated 3 weeks later and one with a low level (0.07 ng/mL) never had ovulation detected.
3.2. Return to ovulation: Empirical results
Four of 19 evaluable participants (21%; 95% CI: 6–46) in the Ovulation Cohort had a progesterone concentration ≥4.7 ng/mL within 42 weeks of last injection, including two of eight (25%) and two of 11 (18%) in the abdomen and thigh groups, respectively (two other participants with progesterone concentrations of 4.3 and 4.4 ng/mL at week 42 exceeded the protocol-defined 4.7 ng/mL threshold when they returned for their second PD assessment period). Week 38 MPA levels were <0.02, 0.05, 0.07, and 0.11 ng/mL in the four participants who ovulated, and they were above 0.1 ng/mL in 13 of 15 participants who did not have ovulation detected by week 42.
By 50 weeks after last injection, 10 of 19 participants (53%; 95% CI: 29–76) had ovulated, including four of eight (50%) and six of 11 (54%) in the abdomen and thigh groups, respectively (Table 2). Week 46 MPA levels were 0.06, 0.06, 0.06, 0.09, 0.10, and 0.12 ng/mL in the six women who ovulated between 46 and 50 weeks after their last injection, and they were above 0.1 ng/mL in seven of nine participants who never had ovulation detected. Among participants who never ovulated, none had a progesterone concentration ≥3.0 ng/mL (a lower threshold used as a surrogate for ovulation by some authors; [15]). In an exploratory per protocol analysis that excluded the participant with LNG detected in her week 46 specimen who did not ovulate, the probability of ovulation within 50 weeks of last injection was 56% (95% CI: 31–78).
Table 2.
Observed probability of ovulation within 12 months of a third injection of Depo-SC at 4-month intervals in a trial conducted between 2017 and 2020 (top row), within 12 months of a third injection at 3-month intervals in prescribing information for Depo-SC (middle row), and within 12 months of a second injection at 3-month intervals in a trial conducted between 2015 and 2018 (bottom row)
| Regimen | Injection device [ref] | N | Ovulated | Probability (95% CI) |
|---|---|---|---|---|
| 3 injections in the abdomen or thigh at 4-mo intervals | Uniject [5] | 19 | 10 | 0.53 (0.29–0.76)a,b |
| 3 injections in the abdomen or thigh at 3-mo intervals | Glass syringe [1,6] | 15 | 12 | 0.80 (0.52, 0.96)b |
| 2 injections in the abdomen at 3-mo intervals | Glass syringe [10,11] | 9 | 4 | 0.44 (0.14, 0.79) |
The estimate was 0.56 (95% CI: 0.31–0.78) when excluding one subject with LNG detected who never ovulated.
p-value = 0.15 for post hoc Fisher's Exact Test of no difference between the 3- and 4-month regimens.
3.3. Return to ovulation: Pharmacokinetic and Pharmacodynamic modeling results
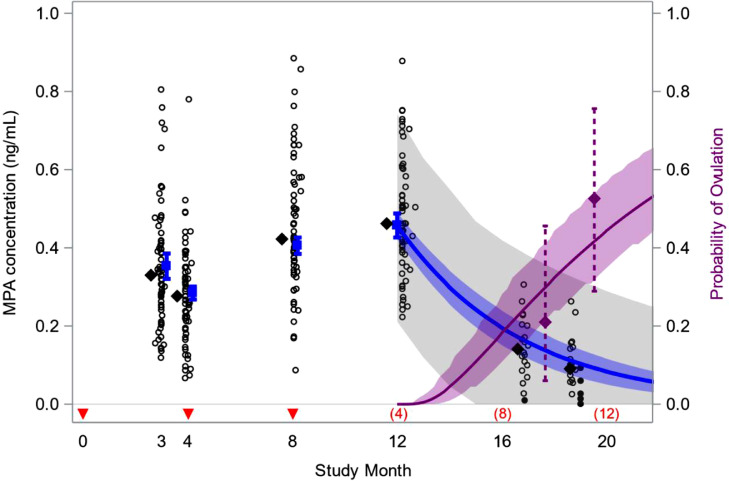
The predicted 4-month trough MPA concentration for a typical 66 kg woman (the median weight at time of last injection in the Ovulation Cohort) was higher for injections in the abdomen (0.30 ng/mL; 95% CI: 0.28–0.33) than the thigh (0.26 ng/mL; 95% CI: 0.24–0.29) (p = 0.01), but the apparent half-life (99 days; 95% CI: 79–119) was similar for the two injection sites (p = 0.42). Increasing body weight was associated with both a lower 4-month trough (p < 0.01) and a longer apparent half-life (p = 0.01) (Supplemental Table S1). Based on these results, we simulated the distribution of MPA levels in a hypothetical population of women following the same dosing regimen as evaluated in the study, including three injections in the abdomen or thigh at 4-month intervals (50% in each injection site) and with a weight distribution similar to the Ovulation Cohort. The predicted median trough MPA concentrations 4, 8, and 12 months after treatment initiation were 0.28 ng/mL (95% CI: 0.26–0.30), 0.40 ng/mL (0.38–0.43), and 0.46 ng/mL (0.42–0.49), respectively, and the median MPA level 12-months after the final (third) injection was 0.08 ng/mL (0.05–0.12) (Table 3). The probability of ovulation within 12 months of the final injection was 0.44 (0.35–0.57) and the median time from last injection to ovulation was 13.1 months (10.9–14.9) (Fig. 1).
Table 3.
Predicted median MPA concentrations (ng/mL) and 95% CIs for a population of women with median weight of 66 kg who inject Depo-SC every three or every four months in the abdomen or thigh for 1 year of treatment. Observed values are from the trial of the 4-month regimen conducted between 2017 and 2020 [5].
| 4-Mo regimen |
3-Mo regimen | Prediction ratio (4- vs 3-mo) | ||
|---|---|---|---|---|
| Timepoint | Observed | Predicted | Predicted | |
| First dose trough | 0.28 | 0.28 (0.26, 0.30) | 0.35 (0.32, 0.39) | 0.80 (0.77, 0.84) |
| Second dose trough | 0.42 | 0.40 (0.38, 0.43) | 0.54 (0.50, 0.58) | 0.75 (0.71, 0.79) |
| 12-mo trougha | 0.46 | 0.46 (0.42, 0.49) | 0.69 (0.65, 0.73) | 0.66 (0.64, 0.68) |
| Steady state trougha | NA | 0.49 (0.44, 0.55) | 0.75 (0.69, 0.81) | 0.66 (0.64, 0.68) |
| 12-mo after last doseb | 0.08c | 0.08 (0.05, 0.12) | 0.10 (0.06, 0.14) | 0.82 (0.80, 0.83) |
12-month troughs are MPA level at the end of the 1-year treatment period. Asymptotic, steady state troughs were estimated based on the accumulation ratio, 1/[1-exp(-λ∙τ)], where λ is the terminal absorption rate and τ is the dosing interval.
12-months after last dose corresponds to 8 and 9 months after the end of a 1-year treatment period for the four- and 3-month regimens, respectively. Since an extra month has passed for the 3-month regimen, the ratio of MPA levels 12-months after last dose (0.82) is not as extreme as the ratio at treatment month 12 (0.66).
46 weeks after last dose. This value conservatively assumes that 5 of 20 subjects who ovulated or discontinued prior to week 46 would have had the lowest MPA levels, had they been observed.
Fig. 1.
Observed and predicted medroxyprogesterone acetate (MPA) concentrations and the probability of ovulation following subcutaneous injection of Depo-SC. Data are from 92 women who received injections in the abdomen or thigh at months 0, 4, and 8, including 20 followed for return to ovulation after the 12-month treatment period, in a study conducted between 2017 and 2020 [5]. Injection times are denoted by red arrows and months since last injection are in parentheses above the x-axis. Empirical results include cumulative proportions ovulating 10 and 12 months after last injection (purple diamonds, 95% CIs); observed MPA levels (open circles); and medians of observed levels (black diamonds). Filled circles at study month 19 are empirical Bayes estimates of MPA levels among women who discontinued or ovulated in the tenth month after their last injection. Model predictions are for a population of women with a median weight of 66 kg, and include median MPA levels (blue, with 95% CIs); a 90% prediction interval for individual MPA levels (gray band); and the cumulative probability of ovulation at the end of treatment (solid purple, 95% CIs). The model predicts 44% of women ovulate within 12 months of their final injection, and a median time to ovulation of 13.1 months since last injection.
A number of counterfactual dosing regimens were modeled to gain further insight the PK and PD of Depo-SC. Assuming women receive four injections of Depo-SC at 3-month intervals per the current product label led to a median trough MPA concentration 12 months after treatment initiation of 0.69 ng/mL (0.65–0.73), a probability of ovulation within 12 months of the final injection of 0.40 (0.31–0.53), and a median time to ovulation of 14.2 months (11.6–16.1). By comparison, the trough MPA level at the end of 12 months of treatment was 34% lower (95% CI: 32–36), and the median time from last injection to ovulation 1.1 months shorter, with the 4-month reinjection interval. Since predicted MPA levels were within 10% of steady state by month 12, differences between the 3- and 4-month dosing regimens did not meaningfully increase with longer treatment durations (Table 3). In contrast, the difference in median time to ovulation was only 0.4 months when considering a hypothetical scenario in which treatment stops after receiving only two doses: 12.4 (10.6–14.0) and 12.8 (10.9–14.4) for the 4- and the 3-month reinjection interval, respectively.
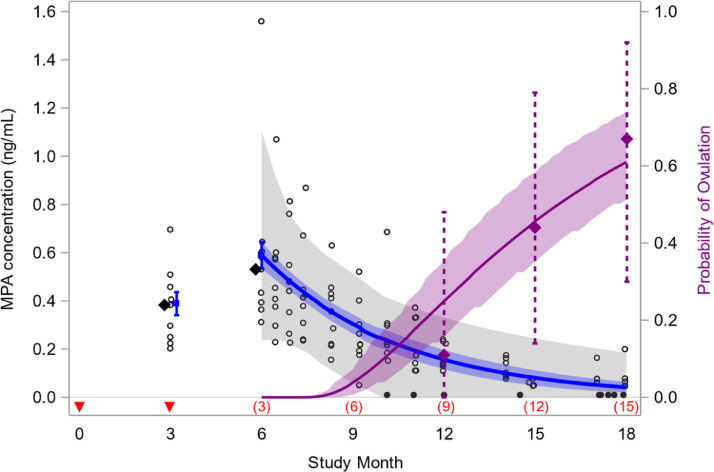
We assessed the external validity of our model by simulating data assuming two doses are injected in the abdomen at 3-month intervals and contrasting the results with what we previously observed in a phase 1 study of that regimen [10]. The predicted 3- and 6-month median trough MPA levels (0.39 ng/mL and 0.59 ng/mL, respectively) were similar to the median levels observed in the trial (0.38 ng/mL and 0.53 ng/mL), and the predicted probability of ovulation within 12 months of a second dose (0.46; 95% CI: 0.35–0.58) was similar to the study value (0.44; 95% CI: 0.14–0.79) (Fig. 2). We also contrasted arithmetic mean trough MPA levels predicted by our counterfactual model with results of a large post-marketing study of the labelled 3-month regimen, and our findings (0.64 and 0.80 ng/mL at months 6 and 12, respectively) were similar to the published values (0.67 and 0.79 ng/mL) [16].
Fig. 2.
External validity of model used to predict medroxyprogesterone acetate (MPA) concentrations and the probability of ovulation following subcutaneous injection of Depo-SC. Model fit to sparse MPA data in a study of Depo-SC injected every 4 months was used to predict MPA levels and the cumulative probability of ovulation in a hypothetical population of women who receive two injections at 3-month intervals [5]. Injection times are denoted by red arrows and months since last injection in parentheses above the x-axis. Model predictions include median MPA levels (blue, 95% CIs); a 90% prediction interval for individual MPA levels (gray band); and the cumulative probability of ovulation after treatment (solid purple, 95% CIs). Predictions are compared to results of a phase 1 trial of this 2-dose regimen, including cumulative proportions ovulating by 12, 15, and 18 months after last injection (purple diamonds, 95% CIs); observed MPA levels (open circles); medians of 3- and 6-month trough levels (black diamonds); and MPA levels below the limit of quantification (filled circles). The model predicts that 46% of women ovulate within 12 months of their final injection, versus 44% observed in the trial [10,11].
4. Discussion
We previously demonstrated that Depo-SC remains highly effective when the reinjection interval is extended from 3 to 4 months [5]. This simple change in regimen could reduce service delivery costs and exposure-related side effects, including delayed return to ovulation. We characterized the length of this delay by following a subset of participants after their last (third) injection, and 10 of 19 (53%) ovulated within 12 months. We predicted a somewhat smaller probability, 44%, based on a model that assumed approximately 10% of women ovulate at MPA levels exceeding 0.1 ng/mL [11]. The predicted probability was still only 57% in a sensitivity analysis that conservatively assumed 10% of women ovulate at MPA levels exceeding 0.20 ng/mL, indicating that our model was reasonably robust to the threshold assumption (see Supplemental Appendix for details).
Our empirical estimate of 53% ovulating within 12 months of a third injection is noticeably smaller than the 80% (12 of 15 women) reported in U.S. prescribing information for the 3-month reinjection interval. The sample sizes of both studies were small, and the difference is not statistically significant (Fisher's Exact test p = 0.15). Nonetheless the result is surprising given that we unambiguously reduced MPA accumulation by extending the reinjection interval 1 month. We previously observed only 4 of 9 women (44%) ovulating within 12 months of a second injection at 3-month intervals, which closely aligns with our current finding [11]. Our finding is also consistent with the low rate of return to fertility described in U.S. prescribing information for Depo-SC, according to which only one of 21 women (5%) who stopped using the method to become pregnant did so within a year of their last injection [1].
A major strength of our study was the availability of PK data to support the empirical findings. The maximum observed MPA concentration among women who ovulated in the subsequent 4-week pharmacodynamic assessment period was 0.12 ng/mL, supporting our assumption that the risk of ovulation is low when MPA levels exceed 0.10 ng/mL. Likewise, the median MPA level 46 weeks after a third injection (0.08 ng/mL) was above the median level required to suppress ovulation (0.07 ng/mL) reported elsewhere, indicating our empirical estimate of 53% ovulating within 12 months is not spurious [11]. The MPA data also allowed us to model drug accumulation and return to ovulation had – contrary to fact – injections been given every 3 months for one year of treatment. Based on this analysis, the typical 12-month trough MPA level is 34% lower, and the median time from last injection to ovulation 1.1 months shorter, with the 4-month regimen. Although our analysis was focused on women using Depo-SC for exactly 1 year, the median time from last injection to ovulation was predicted to exceed 12 months even if treatment stopped after only two doses were received (well before steady state drug levels are achieved). These results emphasize how PK/PD modeling, in conjunction with limited clinical outcome data, could improve product labels and counseling messages for modified dosing regimens. By providing more precise estimates of clinical outcome rates, modeling may also enable more innovative and economic drug development strategies.
Our model inferences rely on a typical apparent half-life estimate of 99 days, the validity of which assumes that a terminal, log-linear phase of absorption occurs by the third month after each injection. We obtained a similar half-life estimate (92 days) when assuming a terminal phase was not achieved until month four, and it was 103 days in a supportive population PK analysis of single-dose data from two phase 1 studies of Depo-SC in glass syringe (Supplemental Fig. S2) [9,10]. A median half-life of 77 days was previously reported in a single-dose PK study in Asian women, but we also predicted a shorter value (86 days) when accounting for the lower average body weight (55 kg) in that study [13]. And although not the focus here, we obtained a half-life estimate of 72 days when injections are given in the upper arm (Supplemental Table S2). All of these values are substantially larger than the mean of 43 days in the study which informed the prescribing information for Depo-SC [1,14]. Whether the differences are due to delivery device, trial design (single- vs multi-dose), estimation method (parametric vs non-parametric), or study population is not clear. Regardless, a half-life of 43 days is insufficient to explain the accumulation of MPA observed in our study of the 4-month regimen or reported in a previous post-marketing study of the 3-month regimen [16].
There are several limitations to our analysis. First, our empirical estimator of the probability of ovulation within 12 months of a third injection was slightly biased because we stopped testing at week 50, not week 52. In addition, we could have missed events if women ovulated in the 4-week gap between assessment periods without experiencing a progesterone concentration ≥4.7 ng/mL in the subsequent month. However, the transitory nature of such events would raise doubts as to whether functional ovulation had truly returned. There was also low precision associated with our empirical estimate of return to ovulation (95% CI: 29–76%), although we were still able to rule out probabilities greater than 80%. Second, all of the women in our study were from Latin America, which may limit generalizability of results. Although clinically significant associations with race or ethnicity have not been reported for Depo-SC, most studies which have looked at this question have been modest in size [11,14], and a robust assessment of relevant pharmacogenomics is a valuable area for future research. Similarly, our supportive PK/PD model assumed that the distribution of MPA levels required to suppress ovulation is not a function of weight, which has not been validated for Class II+ obesity [11]. Finally, sparse MPA sampling meant we could not fully distinguish within- from between-subject random effects in our pharmacokinetic model (see Supplemental Appendix for discussion). We predicted MPA levels that were similar to those observed in independent multi-dose studies of Depo-SC, however, suggesting we were not substantially over- or under-estimating typical drug accumulation. Nonetheless we cannot preclude biases, and caution against extrapolating beyond the range of data described here.
Extending the Depo-SC reinjection interval from 3 to 4 months reduces cumulative MPA exposure by 25% while maintaining excellent contraceptive effectiveness. The 4-month dosing interval is associated with substantially less drug accumulation and a shorter delay between time of last injection and return to ovulation. The delay remains considerable, however, and women initiating Depo-SC should be counseled regarding the distinct possibility that return to fertility may take a year or longer after repeat dosing. In the case of Sayana Press (Depo-SC in the Uniject delivery system) this may require a change to the patient leaflet, which currently states that over 80% of women desiring pregnancy will do so within a year of stopping method use, regardless of how long it has been used.
Data sharing
De-identified, individual participant data analyzed here have been submitted to the Development Data Library (DDL), USAID's publicly available repository for Agency-funded data-on-demand.
Acknowledgments
We are grateful to the study participants and research staff at PROFAMILIA (Dominican Republic), Universidade Estadual de Campinas (Brazil), and Instituto Chileno de Medicina Reproductiva (Chile) for contributing the study data used in our analyses.
Footnotes
Conflicts of interest: We declare no conflicts of interest.
Funding: This work is made possible by the generous support of the American people through the U.S. Agency for International Development (USAID), provided to FHI 360 through Cooperative Agreement AID-OAA-A-15–00045, and grant 2006–04825 from the Children's Investment Fund Foundation (CIFF). The funders provided technical review, but were not involved in the analysis, interpretation, or writing of this report. The contents are the responsibility of FHI 360, and do not necessarily reflect the views of USAID, the United States Government, or CIFF. Nor does any mention of trade names, commercial products, or organizations imply endorsement by FHI 360, USAID, the United States Government, or CIFF.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.conx.2022.100080.
Appendix. Supplementary materials
References
- 1.Pharmacia and Upjohn, Division of Pfizer Inc.; New York, NY: 2020. DEPO-subQ provera [U.S. physician prescribing information]https://www.pfizer.com/products/product-detail/depo_subq_provera_104 (accessed on February 26, 2022) [Google Scholar]
- 2.Jain J., Jakimiuk A.J., Bode F.R., Ross D., Kaunitz A.M. Contraceptive efficacy and safety of DMPA-SC. Contraception. 2004;70:269–275. doi: 10.1016/j.contraception.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 3.Burke H., Chen M., Buluzi M., Fuchs R., Wevill S., Venkatasubramanian L., et al. Effect of self-administration versus provider-administered injection of subcutaneous depot medroxyprogesterone acetate on continuation rates in Malawi: a randomized controlled trial. Lancet Glob Health. 2018;6:e568–e578. doi: 10.1016/S2214-109X(18)30061-5. [DOI] [PubMed] [Google Scholar]
- 4.Medicines and healthcare products regulatory agency (MHRA), public assessment report, mutual recognition procedure, Sayana Press 104 mg/0.65 mL Suspension for Injection (last updated August 2015). https://products.mhra.gov.uk (accessed on February 13, 2022).
- 5.Deese J., Brache V., Bahamondes L., Salinas A., Jorge A., Veiga N., et al. Contraceptive effectiveness, pharmacokinetics, and safety of Sayana Press when injected every four months. eClinicalMedicine. 2022;44 doi: 10.1016/j.eclinm.2022.101273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.U.S. Food and Drug Administration . 2005. Drug approval package. depo-subq provera 104 (Medroxyprogesterone acetate) injectable suspension. Application no.: 021584. Center for drug evaluation and research. Medical review(s) (accessed on March 21, 2022) [Google Scholar]; https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021584s000_depo-subQTOC.cfm
- 7.Pharmacia and Upjohn, Division of Pfizer Inc; New York, NY: 2020. DEPO-subQ provera [U.S. patient product information]https://www.pfizer.com/products/product-detail/depo_subq_provera_104 Revised December. (accessed on February 26, 2022) [Google Scholar]
- 8.2022. Medicines and healthcare products regulatory agency (MHRA), patient leaflet: information for the user, SAYANA PRESS 104 mg/0.65 mL Suspension for Injection.https://www.medicines.org.uk/emc/product/3148/smpc at. (accessed on March 8, 2022) [Google Scholar]
- 9.Halpern V., Fuchs R., Brache V., Bahamondes L., Miranda M., Lendvay A., et al. Suppression of ovulation and pharmacokinetics following subcutaneous administration of various doses of Depo-Provera®: a randomized trial. Contraception X. 2021;3 doi: 10.1016/j.conx.2021.100070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halpern V., Brache V., Taylor D., Lendvay A., Cochón L., Jensen J., et al. Clinical trial to evaluate pharmacokinetics and pharmacodynamics of medroxyprogesterone acetate after subcutaneous administration ofDepo-Provera. Fertil Steril. 2021;115:1035–1043. doi: 10.1016/j.fertnstert.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor D., Halpern V., Brache V., Bahamondes L., Jensen J., Dorflinger L. Ovulation suppression following subcutaneous administration of depot medroxyprogesterone acetate. Contraception X. 2022;4 doi: 10.1016/j.conx.2022.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sayana Press: A Guide for Trainers of Providers; 2016. Program for Appropriate Technology in Health. (accessed on February 13, 2022) [Google Scholar]; https://toolkits.knowledgesuccess.org/sites/default/files/sayana_press_a_guide_for_trainers_of_providers_.pdf
- 13.Toh Y.C., Jain J., Rahimy M.H., Bode F.R., Ross D. Suppression of ovulation by a new subcutaneous depot medroxyprogesterone acetate (104 mg/0.65 mL) contraceptive formulation in Asian women. Clin Ther. 2004;26:1845–1854. doi: 10.1016/j.clinthera.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 14.Jain J., Dutton C., Nicosia A., Wajszczuk C., Bode F.R., Mishell D.R. Pharmacokinetics, ovulation suppression and return to ovulation following a lower dose subcutaneous formulation of Depo-Provera. Contraception. 2004;70:11–18. doi: 10.1016/j.contraception.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Westoff C.L., Torgal A.H., Mayeda E.R., Stanczyk F.Z., Lerner J.P., Benn E.K.T., et al. Ovarian suppression in normal-weight and obese women during oral contraceptive use: a randomized controlled trial. Obstet Gynecol. 2010;116:275–283. doi: 10.1097/AOG.0b013e3181e79440. [DOI] [PubMed] [Google Scholar]
- 16.Kaunitz A.M., Darney P.D., Ross D., Wolter K.D., Speroff L. Subcutaneous DMPA vs. intramuscular DMPA: a 2-year randomized study of contraceptive efficacy and bone mineral density. Contraception. 2009;80:7–17. doi: 10.1016/j.contraception.2009.02.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.