Abstract
Anesthetics penetrate the blood-brain-barrier (BBB) and - as confirmed preclinically – transiently disrupt it. An analogous consequence in humans has remained unproven. In mice, we previously reported that upon BBB dysfunction, the brain acts as ‘immunoprecipitator’ of autoantibodies against N-methyl-D-aspartate-receptor subunit-NR1 (NMDAR1-AB). We thus hypothesized that during human anesthesia, pre-existing NMDAR1-AB will specifically bind to brain. Screening of N = 270 subjects undergoing general anesthesia during cardiac surgery for serum NMDAR1-AB revealed N = 25 NMDAR1-AB seropositives. Only N = 14 remained positive post-surgery. No changes in albumin, thyroglobulin or CRP were associated with reduction of serum NMDAR1-AB. Thus, upon anesthesia, BBB opening likely occurs also in humans.
Keywords: Blood-brain-barrier opening, BBB breakdown, Seroprevalence, Serum proteins, Immunoglobulin class, Cardiac surgery, Heart disease
Highlights
-
•
Whether the blood brain barrier opens on general anesthesia in humans is unclear.
-
•
Serum NMDAR1-AB titers drop upon anesthesia during cardiac surgery.
-
•
Drop of serum NMDAR1-AB after anesthesia indicates ‘immunoprecipitation’ by brain.
-
•
Immunoprecipitation needs brain access of NMDAR1-AB, indicating barrier opening.
-
•
Neither hemodilution nor inflammation explain this loss of NMDAR1-AB from serum.
1. Introduction
Autoantibodies against the N-methyl-D-aspartate-receptor subunit NR1 (NMDAR1-AB) are to date the most frequently detected circulating autoantibodies directed against brain antigens. Originally described in the context of the so-called NMDAR encephalitis, they were first claimed to be disease-specific (Dalmau et al., 2008), until NMDAR1-AB of all immunoglobulin classes were discovered to be equally seroprevalent across health and many different diseases (Hammer et al., 2014; Dahm et al., 2014; Castillo-Gomez et al., 2017). Circulating NMDAR1-AB are functional, leading to NMDAR internalization, and their seroprevalence increases with age (Hammer et al., 2014; Castillo-Gomez et al., 2017; Zerche et al., 2015). Upon acute access to the brain, they can exert various different NMDAR antagonistic effects, which may best be described as ketamine-like (Ehrenreich, 2018). Access to the brain, in turn, is predominantly dependent on the functionality of the blood-brain-barrier (BBB). Under circumstances of BBB impairment and thus increased permeability, the brain virtually acts as immunoprecipitator, efficiently extracting circulating NMDAR1-AB from blood through their specific binding to brain tissue (Zerche et al., 2015; Castillo-Gomez et al., 2016).
Whereas studies in animals, including rodents and cats, indicate that during general anesthesia, the BBB temporarily opens, providing a defined time window for normally non-passing molecules to reach the brain during surgery, a comparable process in humans is still a matter of dispute (Acharya et al., 2015; Spieth et al., 2021; Tétrault et al., 2008; Yang et al., 2017; Kiviniemi et al., 2017; Sweeney et al., 2018; Diamond et al., 2009; Merino et al., 2013). Worth mentioning, also in the rodent literature, there is still a bit of controversy regarding the BBB effect of anesthetics. Some reports, building on models that cause BBB opening by themselves, e.g. experimental ischemia, hyperosmolar mannitol or carbogen, claim that anesthetics do not affect or even decrease BBB permeability (Chi et al., 1998, 2017; Poon et al., 2020). These observations, however, clearly differ from the present work in humans investigating the effect of general anesthesia without evidence of pre-existing or additionally induced BBB disruption.
As it would be of major interest to know whether a transient loss of BBB integrity takes place during human anesthesia, we exploited our knowledge of the brain acting in conditions of compromised BBB as immunoprecipitator of NMDAR1-AB (Zerche et al., 2015; Castillo-Gomez et al., 2016), to undertake the present study. We hypothesized that a substantial amount of individuals undergoing heart surgery would carry circulating NMDAR1-AB before the intervention, and that after anesthesia, their NMDAR1-AB in blood would be reduced or eliminated.
2. Methods
2.1. Patients
A total of N = 270 subjects, undergoing 3–4 h of general anesthesia during cardiac surgery or percutaneous intervention for valve replacement, coronary bypass or a combination thereof at Hannover Medical School were included into the study between August 2018 and March 2019. Anesthesia was induced with etomidate or thiopental, sufentanil and rocuronium or atracurium as muscle-relaxant. Maintenance of anesthesia was accomplished with high-dose sufentanil and sevoflurane. During cardiopulmonary bypass, anesthesia was maintained with propofol instead of sevoflurane. Patients with percutaneous valve replacements received a mild sedation with low-dose sufentanil and dimenhydrinate. Patient data were collected in accordance with ethical guidelines (Ethics Committee of Hannover Medical School, Approval No. 7876 BO S 2018) and the Declaration of Helsinki. All participants gave written informed consent.
2.2. Clinical and laboratory data
Blood samples for NMDAR1-AB and serum protein determination including determinants of inflammation, were taken the day before and after surgery. Albumin, thyroglobulin and C-reactive protein (CRP) in serum were measured using clinical routine techniques, i.e. albumin and CRP by immunoturbidimetric assays and thyroglobulin by an electrochemiluminescence immunoassay (ECLIA) on a cobas 8000 modular analyzer platform (Roche Diagnostics, Mannheim, Germany).
2.3. NMDAR1-AB determination
An established commercial assay and standard procedure for clinical diagnosis, based on NMDAR1-transfected HEK293 cells, was employed to detect NMDAR1-AB in plasma, using secondary antibodies against human IgA, IgG or IgM, and accurately following the manufacturer's instructions (Euroimmun, Lübeck, Germany). All conditions (exploring NMDAR1-AB of the IgG, IgA, IgM class in all samples) were evaluated independently by two investigators (JBHW, MH) and all discrepant or questionable results (<10%) double-checked by a third, experienced rater (HE). Samples were processed in random order, with raters being unaware of the time point pre-OP versus post-OP (blinded). A dilution of 1:10 was selected as cutoff to positivity (Dahm et al., 2014). All positive samples had their titers evaluated.
2.4. Statistical analyses
Group differences in categorical variables were assessed using Chi-squared test. Pearson's chi-squared p-values are displayed. In case of N < 5 in one cell, Fisher's exact test was used. Ordinal scaled pre- and post-OP titer ranks were compared using the Wilcoxon-test. Group differences in continuous variables were assessed using Mann-Whitney U test for unpaired and Wilcoxon-test for paired samples. At normal distribution of continuous variables, T-tests were performed. Values of p < 0.05 were considered significant. Data in text and figures are displayed as mean values ± standard deviation. SPSS 25.0 (IBM-Deutschland GmbH, Munich, Germany) or Prism 9 (GraphPad Software, San Diego, California, USA) were used for statistical analyses.
3. Results and discussion
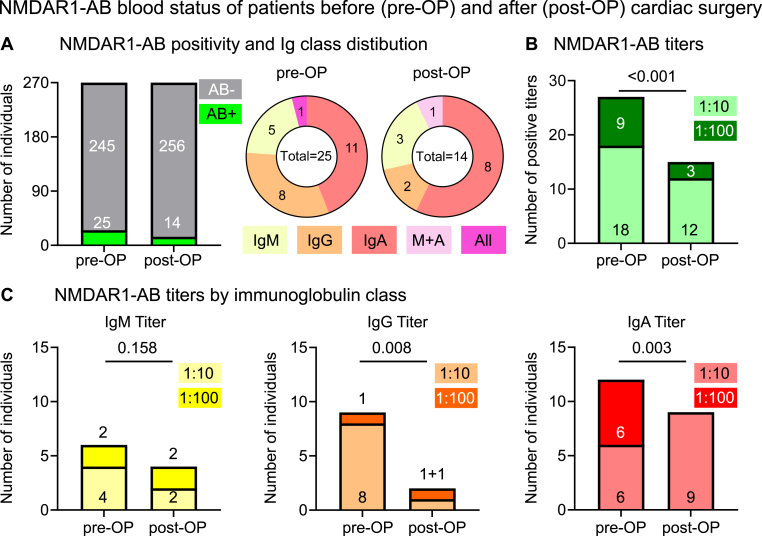
Of the 270 subjects (71 ± 12 years; 62% males), undergoing 3–4 h of general anesthesia during cardiac surgery for valve replacement, coronary bypass or a combination thereof, a total of 25 (9.3%) were NMDAR1-AB positive before general anesthesia (68 ± 10 years; 64% males), and only 14 of those (i.e. 56%; 67 ± 12 years; 64.3% males) remained positive post-surgery (Fig. 1A). In fact, from the mean age of our 270 included patients (>70 years), we would have anticipated well over 10% positive blood samples, as established in meanwhile data on thousands of subjects (Hammer et al., 2014; Dahm et al., 2014; Zerche et al., 2015; Castillo-Gomez et al., 2016), but the unexpectedly low seroprevalence may be related to the still small number of individuals we screened. Similarly surprising was the high percentage of IgG carriers, relative to IgM and IgA (Hammer et al., 2014; Dahm et al., 2014; Zerche et al., 2015; Castillo-Gomez et al., 2016), but again, we are admittedly dealing with ‘statistics of small numbers' and thus by chance findings. Nevertheless, the observation of the here identified positive individuals before versus after surgery helped to provide proof-of-concept for our study hypothesis.
Fig. 1.
NMDAR1-AB blood status of patients before and after cardiac surgery (A) Overview of NMDAR1-AB positivity and Ig class distribution before and after cardiac surgery (pre-OP and post-OP). (B) Overview of all NMDAR1-AB titers pre-OP and post-OP. Note that carriers of more than one NMDAR1-AB Ig class count two or three times (C) Presentation of NMDAR1-AB titers by immunoglobulin class. N = 270 paired samples, Wilcoxon signed rank test with method of Pratt for ties, Prism 9.2.
Importantly, not only did we find >40% of carriers completely losing their NMDAR1-AB status, but NMDAR1-AB titers dropped overall (Fig. 1B; please note that carriers of more than one NMDAR1-AB Ig class count here twice or three times). Looking at immunoglobulin classes separately, titers of IgG and IgA were found clearly reduced, whereas those of IgM, as the largest immunoglobulin molecule, only tended to decrease (Fig. 1C).
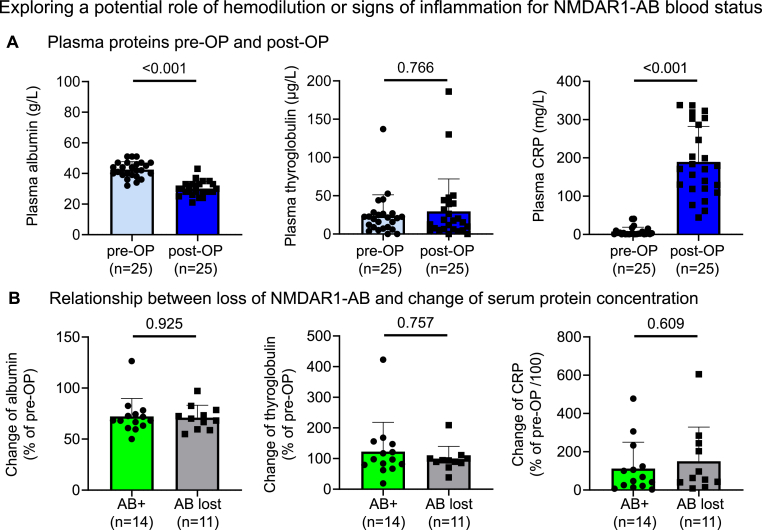
In principle, the reduction or disappearance of NMDAR1-AB from the circulation could reflect a general decrease in serum proteins relative to pre-OP levels, possibly connected to hemodilution during and after surgery. Indeed, we found the expected overall reduction in serum albumin. However, no differences, related to persistence or loss of NMDAR1-AB, were detectable regarding degree of albumin decrease from pre-OP to post-OP (Fig. 2A and B). Human serum albumin (HSA) substitution during/after surgery was similar in individuals who kept (N = 14) versus lost (N = 11) NMDAR1-AB (57.1% versus 54.5%, p > 0.999; mean HSA substitution 33.0 g versus 21.4 g, p = 0.729).
Fig. 2.
Exploring a potential role of hemodilution or signs of inflammation for NMDAR1-AB blood status
(A) Serum proteins pre-OP and post-OP: Absolute values of albumin, thyroglobulin and CRP. N = 25 paired samples, Wilcoxon signed rank test with method of Pratt for ties, Prism 9.2 (B) Relationship between loss of NMDAR1-AB and change of serum protein concentration: Data are expressed in % of pre-OP values and compared among individuals that kept versus lost NMDAR1-AB during general anesthesia. Mean ± SD presented. N = 25 unpaired samples, Mann-Whitney test, Prism 9.2.
Nevertheless, we decided to determine another, even larger plasma protein, thyroglobulin, which is not subject to any targeted perioperative substitution, even though contained at low amount in blood transfusions. Transfusions, however, did not significantly differ between subjects who kept (N = 14) versus lost (N = 11) NMDAR1-AB (red blood cells: 92.9% with 5.07 ± 4.2 units versus 54.5% with 2.46 ± 2.8 units, p = 0.202; fresh frozen plasma, FFP: 50% with 1.71 ± 2.7 units versus 27.3% with 0.45 ± 1.3 units, p = 0.291; platelet transfusions: 64.3% with 1.57 ± 1.4 units versus 27.3% with 0.53 ± 1.2 units, p = 0.053). Thus, with thyroglobulin levels, we could widely exclude a potential hemodilution effect to explain our findings on NMDAR1-AB (Fig. 2A and B).
A considerable increase in the inflammation marker CRP was noted upon surgery in all 25 original NMDAR1-AB carriers. Again, however, the change in CRP from pre-OP to post-OP was comparable in subjects who kept versus lost their NMDAR1-AB (Fig. 2A and B). Nevertheless, inflammation during cardiac surgery is soundly established and could very well have contributed to the leakiness of the BBB upon anesthesia, but is unlikely the sole cause (Okamura et al., 2010; Scott et al., 2014; Huang et al., 2021; Gao et al., 2021).
Together, these data indirectly indicate that upon general anesthesia, at least during heart surgery with its known inflammatory component, substantial BBB opening may also occur in humans. This temporary BBB insufficiency supports the transfer of molecules into the brain that are normally prevented from entering, like circulating NMDAR1-AB. These bind specifically to brain tissue, which acts as an immunoprecipitator (Zerche et al., 2015; Castillo-Gomez et al., 2016). As a consequence, we measure post-OP a substantial overall reduction of NMDAR1-AB from the circulation. Of course, binding of NMDAR1-AB to other organs and tissues expressing NMDAR cannot be entirely excluded, but would most likely happen before as much as during and after surgery, due to absence of a barrier comparable to the BBB. We have to be aware that NMDAR1-AB (like other compounds, transferring to the brain during surgery) can ultimately have phenotypical consequences both upon acute and chronic binding to NMDAR (Zerche et al., 2015; Deutsch et al., 2021). For solidly analysing these consequences of NMDAR1-AB during/after anesthesia, however, much larger numbers of subjects will be needed.
Author contributions
Concept, design: HE, HW, and KW.
Drafting manuscript: HE and KW.
Drafting display items: JBHW, JT, CJ, together with HE and KW.
Data acquisition: JT, CJ, JBHW, SS, MH, LH, MMG, CF, AS, HL, RL, HW.
All authors read and approved the final version of the manuscript.
Declaration of competing interest
The authors declare no competing financial or other interests.
Acknowledgements
This work was funded by the Else Kröner-Fresenius-Stiftung within the structured doctorate program KlinStrucMed of Hannover Medical School as well as by the Max Planck Society and the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) TRR 274/1 2020 - 408885537.
References
- Acharya N.K., Goldwaser E.L., Forsberg M.M., Godsey G.A., Johnson C.A., Sarkar A., DeMarshall C., Kosciuk M.C., Dash J.M., Hale C.P., et al. Sevoflurane and Isoflurane induce structural changes in brain vascular endothelial cells and increase blood−brain barrier permeability: possible link to postoperative delirium and cognitive decline. Brain Res. 2015;1620:29–41. doi: 10.1016/j.brainres.2015.04.054. [DOI] [PubMed] [Google Scholar]
- Castillo-Gomez E., Kastner A., Steiner J., Schneider A., Hettling B., Poggi G., Ostehr K., Uhr M., Asif A.R., Matzke M., et al. The brain as immunoprecipitator of serum autoantibodies against N-Methyl-D-aspartate receptor subunit NR1. Ann. Neurol. 2016;79(1):144–151. doi: 10.1002/ana.24545. [DOI] [PubMed] [Google Scholar]
- Castillo-Gomez E., Oliveira B., Tapken D., Bertrand S., Klein-Schmidt C., Pan H., Zafeiriou P., Steiner J., Jurek B., Trippe R., et al. All naturally occurring autoantibodies against the NMDA receptor subunit NR1 have pathogenic potential irrespective of epitope and immunoglobulin class. Mol. Psychiatr. 2017;22(12):1776–1784. doi: 10.1038/mp.2016.125. [DOI] [PubMed] [Google Scholar]
- Chi O.Z., Chun T.W., Liu X., Weiss H.R. The effects of pentobarbital on blood-brain barrier disruption caused by intracarotid injection of hyperosmolar mannitol in rats. Anesth. Analg. 1998;86(6):1230–1235. doi: 10.1097/00000539-199806000-00018. [DOI] [PubMed] [Google Scholar]
- Chi O.Z., Mellender S.J., Kiss G.K., Liu X., Weiss H.R. Blood-brain barrier disruption was less under isoflurane than pentobarbital anesthesia via a PI3K/Akt pathway in early cerebral ischemia. Brain Res. Bull. 2017;131:1–6. doi: 10.1016/j.brainresbull.2017.02.007. [DOI] [PubMed] [Google Scholar]
- Dahm L., Ott C., Steiner J., Stepniak B., Teegen B., Saschenbrecker S., Hammer C., Borowski K., Begemann M., Lemke S., et al. Seroprevalence of autoantibodies against brain antigens in health and disease. Ann. Neurol. 2014;76(1):82–94. doi: 10.1002/ana.24189. [DOI] [PubMed] [Google Scholar]
- Dalmau J., Gleichman A.J., Hughes E.G., Rossi J.E., Peng X., Lai M., Dessain S.K., Rosenfeld M.R., Balice-Gordon R., Lynch D.R. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7(12):1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch N.R., Worthmann H., Steixner-Kumar A.A., Schuppner R., Grosse G.M., Pan H., Gabriel M.M., Hasse I., van Gemmeren T., Lichtinghagen R., et al. Autoantibodies against the NMDAR subunit NR1 are associated with neuropsychiatric outcome after ischemic stroke. Brain Behav. Immun. 2021;96:73–79. doi: 10.1016/j.bbi.2021.05.011. [DOI] [PubMed] [Google Scholar]
- Diamond B., Huerta P.T., Mina-Osorio P., Kowal C., Volpe B.T. Losing your nerves? Maybe it's the antibodies. Nat. Rev. Immunol. 2009;9(6):449–456. doi: 10.1038/nri2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich H. Autoantibodies against N-methyl-d-aspartate receptor 1 in health and disease. Curr. Opin. Neurol. 2018;31(3):306–312. doi: 10.1097/WCO.0000000000000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q., Hernandes M.S., Encephalopathy Sepsis-Associated, Barrier Blood-Brain. Dysfunction. Inflammation. 2021;44(6):2143–2150. doi: 10.1007/s10753-021-01501-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer C., Stepniak B., Schneider A., Papiol S., Tantra M., Begemann M., Siren A.L., Pardo L.A., Sperling S., Mohd Jofrry S., et al. Neuropsychiatric disease relevance of circulating anti-NMDA receptor autoantibodies depends on blood-brain barrier integrity. Mol. Psychiatr. 2014;19(10):1143–1149. doi: 10.1038/mp.2013.110. [DOI] [PubMed] [Google Scholar]
- Huang X., Hussain B., Chang J. Peripheral inflammation and blood–brain barrier disruption: effects and mechanisms. CNS Neurosci. Ther. 2021;27(1):36–47. doi: 10.1111/cns.13569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviniemi V., Korhonen V., Kortelainen J., Rytky S., Keinänen T., Tuovinen T., Isokangas M., Sonkajärvi E., Siniluoto T., Nikkinen J., et al. Real-time monitoring of human blood-brain barrier disruption. PLoS One. 2017;12(3):1–16. doi: 10.1371/journal.pone.0174072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino J.G., Latour L.L., Tso A., Lee K.Y., Kang D.W., Davis L.A., Lazar R.M., Horvath K.A., Corso P.J., Warach S. Blood-brain barrier disruption after cardiac surgery. Am. J. Neuroradiol. 2013;34(3):518–523. doi: 10.3174/ajnr.A3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura T., Ishibashi N., Zurakowski D., Jonas R.A. Cardiopulmonary bypass increases permeability of the blood-cerebrospinal fluid barrier. Ann. Thorac. Surg. 2010;89(1):187–194. doi: 10.1016/j.athoracsur.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon K.-S., Pan Y.-L., Liao K.H., Wang H.-L., Chen K.-B., Chen H.-Y., Liu Y.-C., Lai T.W. Isoflurane attenuates carbogen-induced blood–brain barrier disruption independent of body temperature in mice and rats. Neuroreport. 2020;31(2):118–124. doi: 10.1097/WNR.0000000000001390. [DOI] [PubMed] [Google Scholar]
- Scott D.A., Evered L.A., Silbert B.S. Cardiac surgery, the brain, and inflammation. J. Extra Corpor. Technol. 2014;46(1):15–22. [PMC free article] [PubMed] [Google Scholar]
- Spieth L., Berghoff S.A., Stumpf S.K., Winchenbach J., Michaelis T., Watanabe T., Gerndt N., Düking T., Hofer S., Ruhwedel T., et al. Anesthesia triggers drug delivery to experimental glioma in mice by hijacking caveolar transport. Neuro-Oncology Advances. 2021;3(1):1–13. doi: 10.1093/noajnl/vdab140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney M.D., Zhao Z., Montagne A., Nelson A.R., Zlokovic B.V. Blood-brain barrier: from physiology to disease and back. Physiol. Rev. 2018;99(1):21–78. doi: 10.1152/physrev.00050.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tétrault S., Chever O., Sik A., Amzica F. Opening of the blood–brain barrier during isoflurane anaesthesia. Eur. J. Neurosci. 2008;28(7):1330–1341. doi: 10.1111/j.1460-9568.2008.06443.x. [DOI] [PubMed] [Google Scholar]
- Yang S., Gu C., Mandeville E.T., Dong Y., Esposito E., Zhang Y., Yang G., Shen Y., Fu X., Lo E.H., et al. Anesthesia and surgery impair blood–brain barrier and cognitive function in mice. Front. Immunol. 2017;8:1–17. doi: 10.3389/fimmu.2017.00902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerche M., Weissenborn K., Ott C., Dere E., Asif A.R., Worthmann H., Hassouna I., Rentzsch K., Tryc A.B., Dahm L., et al. Preexisting serum autoantibodies against the NMDAR subunit NR1 modulate evolution of lesion size in acute ischemic stroke. Stroke. 2015;46(5):1180–1186. doi: 10.1161/STROKEAHA.114.008323. [DOI] [PubMed] [Google Scholar]