Abstract
Contaminated chicken meat is a major source of human Campylobacteriosis and rates of infection remain high, despite efforts to limit the colonisation of broiler (meat) chicken flocks on farms. Using conventional testing methods of culture or qPCR, Campylobacter is typically detected amongst broiler flocks from 3 wk of age, leading to the assumption that infection is introduced horizontally into chicken rearing houses at this time. In this study, we use parallel sequencing of a fragment of the Campylobacter outer membrane protein, encoded by the porA gene, to test for presence of Campylobacter DNA amongst fresh fecal samples collected from broiler flocks aged 23 to 28 d. Campylobacter DNA was detected in all of the 290 samples tested using the porA target, and in 48% of samples using 16S bacterial profiling, irrespective of whether or not Campylobacter could be detected using conventional qPCR thresholds. A single porAf2 variant was predominant among flocks that would be determined to be Campylobacter ‘positive’ by conventional means, but a diverse pattern was seen among flocks that were Campylobacter ‘negative’. The ability to routinely detect low levels of Campylobacter amongst broiler flocks at a much earlier age than would conventionally be identified requires a re-examination of how and when biosecurity measures are best applied for live birds. In addition, it may be useful to investigate why single Campylobacter variants proliferate in some broiler flocks and not others.
Key words: broiler, campylobacter, parallel sequencing, multistrain
INTRODUCTION
Raw or uncooked poultry meat has been identified as the main route by which humans become infected with Campylobacter, one of the major causes of gastroenteritis (Sheppard et al., 2009). In the European Union, over 70% of broiler (meat) chicken flocks were found to be Campylobacter positive at the time of slaughter (European Food Safety Authority, 2010). The EU prohibits disinfection of chicken carcases by chlorine which means that contaminated chicken can easily make its way onto supermarket shelves. Public Health England (now the UK Health Security Agency), for example, found Campylobacter in 73% of supermarket chicken and on 7% of the outer packaging (Jorgensen et al., 2019).
Using standard culture or qPCR methods, Campylobacter are not usually detected in chicken flocks until the birds are at least 2 to 3 wk of age (Newell and Fearnley, 2003; Awad, et al., 2018; Ijaz, et al., 2018). We, henceforth, refer to Campylobacter status based upon these methods as ‘Campylobacter culture/qPCR positive/negative’ for clarity. The widespread use of such methods as the main way of detecting the presence of Campylobacter gave rise to the assumption that because the bacteria could not be detected until this age, they were not present in the birds (Evans and Sayers, 2000; Rushton et al., 2009; Awad et al., 2018). It therefore seemed logical that the key to controlling Campylobacter was to prevent infection from outside sources by stricter biosecurity. However, the combination of continuing Campylobacter infection despite the introduction of tighter biosecurity measures (Anonymous, 2017) and sensitive genetic techniques for detecting the bacteria has challenged the idea of initially pristine flocks becoming later contaminated from outside due to a breach in biosecurity (Cox et al., 2012; Colles et al., 2021). Using a deep sequencing approach, Colles et al. (2021) detected Campylobacter DNA in faecal samples from all the broiler flocks they tested when birds were less than 8 days old (Colles et al., 2021). Campylobacter DNA was detected amongst a total of 87.5% of 16 broiler flocks from the UK, Switzerland and France by 16S bacterial profiling assay and among 100% of 34 flocks using the porAf2 assay. The amount of Campylobacter DNA identified in these young flocks was very small (typically less than 0.01% of the microbiome) and it was also notably diverse, showing wide variation at the porA locus. By contrast, flocks that later (at 28–46 days old) tested positive for Campylobacter using culture or PCR assay had an average 100-fold increase in Campylobacter DNA (perhaps associated with ‘super-shedder’ individuals), but predominantly of one porA genotype. Furthermore, >28-day-old flocks that tested negative using culture or qPCR tests retained the juvenile pattern of very small quantities of Campylobacter DNA of high genetic diversity.
These findings suggest new strategies for the control of Campylobacter. If Campylobacter are universally present in chicken flocks by the time the birds are a week old (and possibly earlier), but not all flocks later develop infections severe enough to be detected as ‘positive’ by culture or qPCR, then it might pay to investigate why some flocks retain the early pattern of low levels of diverse Campylobacter and others develop high levels of a single strain. As higher levels of Campylobacter are likely to be most dangerous to humans through increased risk of transmission, understanding what triggers the increase could be the first step in reducing the risk of human infection.
In this paper, we provide further supporting evidence that at 28 d of age, flocks testing positive for Campylobacter via culture or qPCR tests are shedding large quantities of mainly single strains of Campylobacter, although precisely which strain they shed varies from flock to flock. At the same time, flocks testing negative by the same conventional tests are also shedding Campylobacter as detectable by deep sequencing but only in minute quantities and of diverse strains. We also show that different testing methodology for Campylobacter can give different results as to whether a flock is classified as positive or negative and argue that the resulting uncertainty over the Campylobacter status of a flock may be one reason why the search for effective control measures has proved so difficult.
MATERIALS AND METHODS
Ethical Approval
The study was approved by the Institutional Review Board for the approval of animal experiments of the LANAT office of the Canton of Bern (BE97/16) and met all cantonal and federal regulations for the ethical treatment of animals on 30-09-2016. The procedure was declared to be severity level 0.
Sample Collection
Fresh fecal samples were collected from 20 flocks from 3 different farms for qPCR and porA analysis; 8 from Farm 1, 7 from Farm 2 and 5 from Farm 3. Details of housing and management were described by Gebhardt-Henrich et al. (2021). Samples were collected between November 2017 and October 2018, with 5 flocks sampled in Winter months (December, January, February), 4 flocks sampled in Spring months (March, April, May), 4 flocks sampled in Summer months (June, July) and 7 flocks sampled in Autumn (September, October, November). Up to 16 samples were collected per flock, when the birds were aged between 23 and 28 d of age (Table S1, supplementary data). Samples were stored at −20°C in RNAlater before shipping on dry ice for DNA extraction. In addition, end-point samples from each flock were routinely tested for Campylobacter at the abattoir by a commercial laboratory, using pooled fecal swabs, neck skin, and meat samples and following the ISO 10272:2017 protocol.
DNA Extraction
Cecal content samples were homogenized in a MagNALyzer (Roche, Basel, Switzerland). Following homogenization, the DNA was extracted using a QIAamp DNA Stool Mini Kit according to the manufacturer's instructions (Qiagen, Hilden, Germany). The DNA concentration was determined spectrophotometrically and DNA samples diluted to 5 ng/mL.
qPCR
DNA extracts from samples were tested individually using the method published previously (Colles et al., 2021). Presence of C. jejuni DNA was detected using primers/probe for the mapA gene and the presence of C. coli DNA detected using primers/probe for the ceuE gene (Best et al., 2003). Positive results were recorded for Ct values of 16 to 35, corresponding to copy number >100, based upon plasmid controls. All results, including detection of lower copy number, with higher Ct values are given in Table S1, supplementary data.
16S Bacterial Profile Sequencing
The V3/V4 region of 16S rRNA genes were amplified using the method described previously (Kubasova et al., 2019). Briefly, the following primers were used, with MIDs representing different 5, 6, 7, or 9 base pair sequences to allow multiplexing of samples; forward primer 5´- TCGTCGGCAGCGTCAGATGTGTATAAGAGACAG-MID-GT-CCTACGGGNGGCWGCAG-3´ and reverse primer 5´-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAG-MID-GT GACTACHVGGGTATCTAATCC-3´. The KAPA HiFi Hot Start Ready Mix kit (Kapa Biosystems, Woburn, MA) was used for PCR amplification, and the resulting PCR products were purified using AMPure beads. The PCR products were indexed using the Nextera XT Index Kit following the manufacturer's instructions (Illumina, San Diego, CA) and the concentration of the differently indexed samples determined using a KAPA Library Quantification Complete kit (Kapa Biosystems). Sequencing was performed using the MiSeq Reagent Kit v3 (600 cycle), with 20 pM phiX DNA added to the pooled indexed PCR products to give a final concentration of 5% (v/v). Quality trimming of the raw reads was performed using TrimmomaticPE v0.32 with sliding window 4 bp and quality read score equal or higher than 20 (Bolger et al., 2014). Minimal read length was at least 150 bp. The fastq files generated after quality trimming were uploaded into QIIME software (Caporaso et al., 2010). Forward and reverse sequences were joined and in the next step, chimeric sequences were predicted and excluded by the slayer algorithm. The resulting sequences were then classified by RDP Seqmatch with an OTU (operational taxonomic units) discrimination level set to 97%.
Campylobacter porA Parallel Sequencing
A short fragment of the short variable region of the porA gene (“porAf2”) was amplified in triplicate 25-µL reactions, using the method published previously (Colles et al., 2019). Primers were designed to match short sections of porA nucleotide sequence data without polymorphisms, covering both C. jejuni and C. coli. Briefly, the MOMP B 5’- CCA CAA TTA TGG TTA GCT TA -3’ and MOMP 2R 5′-TGA GAA GTT AAG TTT TGG AGA G-3’ primers were used, with the MOMP 2R primer tagged with a 7 nucleotide barcode specific for each reaction, enabling the reactions to be multiplexed within the same sequencing library. The PCR mastermix was made according to the manufacturer's recommendations, using high fidelity Phusion Hot Start Flex DNA polymerase enzyme and 5X Phusion HF buffer (M0535, New England Biolabs, Hitchen, UK). Library preparation was performed using the NEB ultra DNA library preparation kit for Illmina (E7370) and Indexing primers (E7335S and E7500S; New England Biolabs, Hitchen, UK), following protocols described previously (Colles et al., 2019). PCR products were loaded with 10% phiX onto the Illumina MiSeq platform, following analysis by TapeStation (Agilent Genomics, Santa Clara, US) and qPCR (E7630, New England Biolabs, Hitchen, UK) to confirm template size and concentration. The 600-cycle MiSeq Reagent Kit v3 (Illumina, Cambridge UK, MS-102-3003) was used, giving paired 300 nucleotide reads. Raw sequencing reads were processed using the DADA2 pipeline (Callahan et al., 2016) and then the phyloseq package in R was used to produce a table of OTUs (McMurdie and Holmes, 2013), as published previously. Custom python scripts were used to demultiplex reads according to their barcode, and cutadapt v1.15 (Martin, 2011) was used to trim any remaining primer sequence ahead of the DADA2 pipeline. OTU's were assigned both porAf2 nucleotide allele and its translated MOMPf2 peptide allele using the PubMLST database (https://pubmlst.org/organisms/campylobacter-jejunicoli/). The assigned alleles and sequence information are publicly available on the database by searching ‘Typing’-‘Downloads’-‘Loci not in schemes’.
Cross-Contamination Control
The parallel sequencing for Campylobacter porA was performed in a separate institute to the bacterial 16S profiling, but using aliquots of the same DNA extractions. PCR reactions were performed in separate PCR cabinets for mastermix preparation and template addition, within a designated clean room. Equipment was cleaned before use and between batches of samples using DNAZap (AM98902, ThermoFisher Scientific, UK), 70% ethanol and UV light for a minimum of 15 min. Fresh aliquots of reagents and newly opened plastic ware were used for each set of PCR reactions, and non-template controls with molecular water used in place of sample were included for every batch. Sequencing reactions were prepared in a separate room to that of the PCR reactions.
Data Analyses
Data were transformed to an even sampling depth, giving proportional frequency of each porAf2 type, using the phyloseq package in R (McMurdie and Holmes, 2013). Excel and Tableau 2019.4 software were used to produce color matched bar charts. The Simpson's and Shannon's diversity indices were performed on raw and interpolated/extrapolated data calculated the iNEXT (iNterpolation/EXTrapolation) R package to ensure standardized comparison (Hsieh et al., 2016). For Simpson's diversity index, a 1-D value of 1.0 indicated that all members of a population could be distinguished from each other, and a 1-D value of 0 indicated that all members of a population were identical (Hunter, 1990). Shannon's diversity index was included as it is considered to give more weight to rare species (pofAf2 variants) (Shannon, 1948). An H value of 0 indicated that all species were the same. H increases with increasing number of species. Spearman's rank correlation coefficient measuring the relationship between qPCR value and % Campylobacter 16S DNA was calculated using R. Bray-Curtis dissimilarity indices were calculated using the Phyloseq package in R (McMurdie and Holmes, 2013) for the porAf2 populations identified in each flock, and compared by flock, ‘florid/non-florid’ status (predominance or otherwise of a single porAf2 variant), and parent flock. The results were plotted using ggplot2 (Wickham, 2016) as non-metric multidimensional scaling (NMDS) ordination plots. The evolutionary distances between the porAf2 variants were calculated using the Neighbor joining and p distance methods (Saitou and Nei, 1987), using the MEGA-X software (Kumar et al., 2018).
RESULTS
Detection of Campylobacter DNA by qPCR, Parallel Sequencing of the 16S Ribosomal RNA and porAf2 Gene Fragment Targets
Using Ct value thresholds of 16 to 35, corresponding to copy number >100, > 90% of samples from 3 broiler flocks (flocks 3, 14, and 19) were determined to be positive for Campylobacter, using the mapA and ceuE targets. Two other flocks recorded Ct values of 31–34, corresponding to ∼1,000–30,000 copy number for 4/16 (25%) samples (flock 7) and 6/16 (37.5%) samples (flock 11). Samples were collected from these flocks in June (farm 1), May and September 2018 (farm 2) and February and December 2018 (farm 3). If the Ct threshold was removed, and less stringent accuracy therefore adopted, Campylobacter DNA was detected among at least one sample from all of the 20 flocks tested at 23 to 28 days of age by qPCR. Of these, 14 (70%) flocks were positive for both C. jejuni and C. coli, 4 (20%) flocks were positive for C. jejuni only and 2 (10%) flocks were positive for C. coli only. Campylobacter DNA was detected among 145 of the 290 (50%) samples tested individually by qPCR. Of these, both C. jejuni and C. coli were detected among 39 (26.9%) of 145 samples, C. jejuni only was detected among 72 (49.6%) of 145 samples and C. coli only was detected among 35 (24.1%) of 145 samples (Table S1, supplementary data).
Variants of Campylobacter 16S rDNA were recovered from at least one sample from 17 of the 20 (85%) flocks, and 142 of the 291 (48.8%) samples tested. Amongst the positive samples, the proportion of Campylobacter DNA identified amongst the bacterial 16S variant sequences ranged from <0.01 to 79.03% per sample (Table S1, supplementary data). The number of ‘positive’ samples/birds ranged from one bird within a flock, to all 16 birds tested from a flock. Presence/absence of Campylobacter DNA matched by 16S bacterial variant profile and low stringency qPCR in 176 (60.7%) of the 290 samples. Campylobacter DNA was detected in 43 (14.8%) of 290 samples by low stringency qPCR but not by 16S bacterial profile, and in 72 (24.8%) of 290 samples by 16S bacterial variant profile but not by qPCR. The different methods of C. jejuni and C. coli quantification by qPCR and the percent Campylobacter DNA detected by 16S rDNA profile were correlated (when comparing 16S rDNA with qPCR results for C. jejuni alone (Spearman's rank correlation coefficient ρ = 0.47, P < 0.01), and qPCR results for C. jejuni and C. coli combined (ρ = 0.38, P < 0.01)) among the samples tested.
PorAf2 variants were detected from all of the samples from all of the flocks tested in the study, with an average sequencing depth of 2,000 (range 31–220,000) per sample. The fragment is specific for C. jejuni and C. coli; no-cross reaction to other bacterial species was detected by BLAST searching the NCBI nucleotide database, or amongst whole genome sequence from a further 40 Campylobacter species, Helicobacter pullorum or H. pylori, tested using the multispecies Ribosomal MLST database (Jolley et al., 2012).
Diversity of porAf2 Variants
A total of 245 porAf2 nucleotide variants were recovered from the samples and included in the study (Figure 1). Of these, 149/245 (60.8%) porAf2 variants were newly described in this study compared to previous. The number of different porAf2 variants recovered from an individual sample ranged from 1 to 79, with an average of 21.1. The porAf2 variant types 1–9 were most commonly isolated in the study, accounting for 70.5% of the total sequences recovered (Figure 2). The 245 porAf2 variants translated to 170 peptide variants, with the 79 porAf2 variants amongst an individual bird translating to 65 different peptide sequences. Point mutations and deletions were spread along the porAf2 fragment sequenced, with the most disparate alleles varying by ∼80% sequence homology.
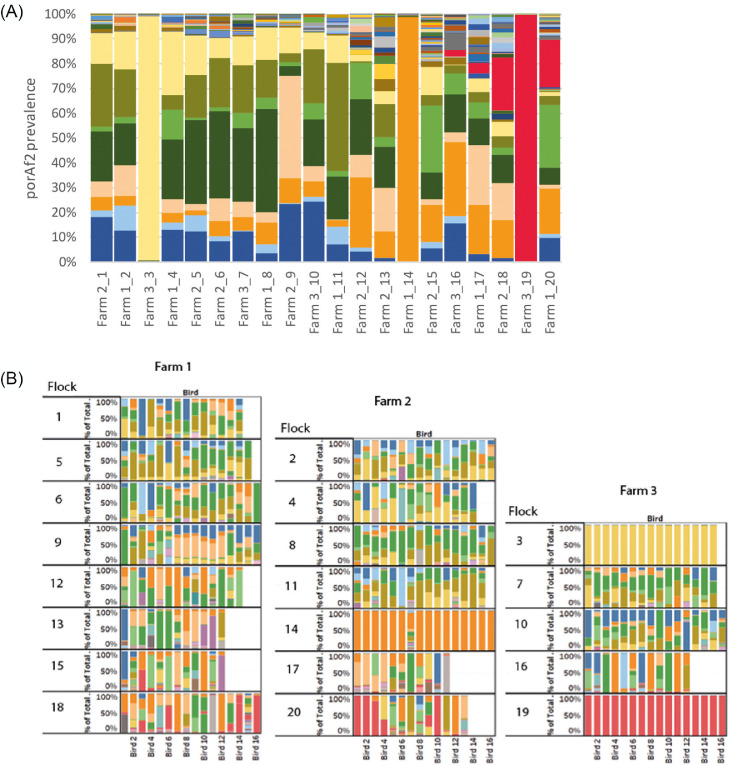
Figure 1.
The Campylobacter porAf2 nucleotide variants shown by (A) Farm_flock id, and (B) individual sample, with each color representing a different variant.
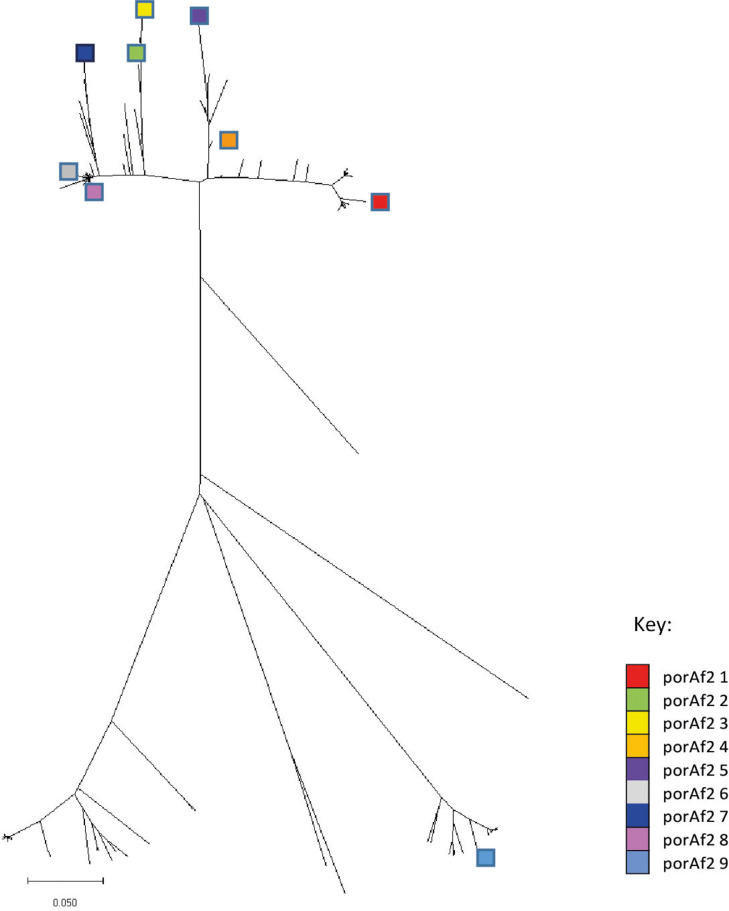
Figure 2.
A neighbor joining tree showing the genetic distance between the 245 porAf2 variants identified amongst the broiler flocks. The most commonly isolated porAf2 variants 1–9 are highlighted in color.
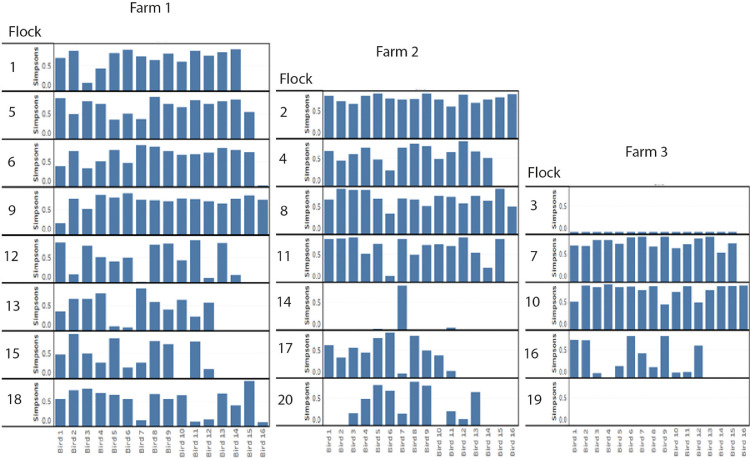
In 3 flocks (flocks 3, 14, and 19), a single porAf2 variant was predominant amongst all of the samples tested (Figures 1 and 2). These 3 flocks also had the highest levels of Campylobacter detected by qPCR, percentage of Campylobacter amongst 16S bacterial variant sequence and number of birds testing positive within a flock. Campylobacter 16S DNA was not detected in flock 7 that had 4 positive samples by qPCR, and at very low quantities <1% in flock 11 that had 6 positive samples by qPCR. The porAf2 variants were more evenly spread among samples for each of the remaining samples and flocks, excluding those from flocks 3, 14, and 19. The Simpson's and Shannon's diversity indices were close to zero for all except one bird/sample in flocks 3, 14, and 19 (Figure 3). For these 3 flocks, the Simpson's diversity ranged from <0.01 to 0.04 (0.81 for the outlying sample), with an average of 0.03, and the Shannon's diversity ranged from 0.03 to 0.15 (2.28 for the outlying sample), with an average of 0.12. For the remaining flocks, the Simpson's diversity ranged from 0 to 0.91, with an average of 0.59, and the Shannon's diversity ranged from 0 to 2.57, with an average of 1.22.
Figure 3.
Measures of Simpson's diversity 1-D for Campylobacter porAf2 variants identified amongst each of the flocks. A value of 1 indicates that all variants in a flock were different, and 0 indicates that all variants within a flock were identical.
The porAf2 variant that became dominant in flocks 3, 14, and 19 was different in each of the flocks (Figures 1 and 2). These were porAf2 variants 3, 8, and 736. The porAf2 variant that became dominant in one of these flocks was present in the other 2 flocks at low frequency on 2 of the three occasions.
Differences in Campylobacter porAf2 Variant Population Structure Between Flocks, Farms, and Parent Flocks
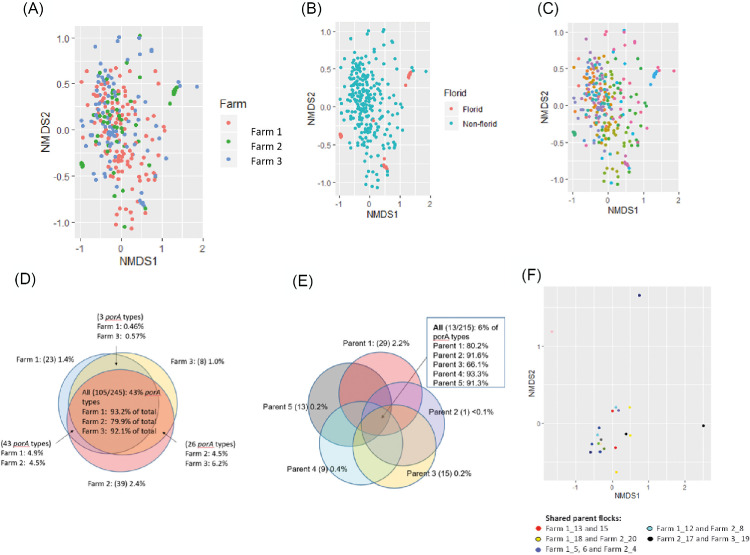
With the exceptions of flocks 3, 14, and 19, the population structure was not distinguishable between individual flocks or farms. Flocks 3, 14, and 19 can been seen clustered to the extremes of the ordination plots, reflecting the dominance of different porAf2 variants, but even then, they are not distinct from others (Figures 4A–4C). Of the 245 porAf2 variants identified amongst the study, 105 (42.9%) were detected on all three farms (Figure 4D). In total, they accounted for 93.2% of porAf2 variants identified from samples from Farm 1, 92.1% of porAf2 variants identified from Farm 3, and 79.9% of porAf2 variants identified from Farm 2. Individually, the farms had between 8 and 39 unique porAf2 variants, accounting for between 1 and 2.4% of the porAf2 variants identified from each farm in total. A subset of broiler flocks were derived from matching parent flocks, and the porAf2 variants were compared between parent-matched flocks for evidence of vertical transfer, either directly, or indirectly via fecally contaminated transport crates for example. In fact, between 1 (<0.1%) and 29 (2.2%) porAf2 variants were unique to each group of broiler flocks with matched parents (Figure 4E) and parent-matched flocks could not be distinguished from each other using Bray-Curtis dissimilarity indices (Figure 4F).
Figure 4.
Non-metric multidimensional scaling (NMDS) ordination plots showing Bray-Curtis dissimilarity measures between the porAf2 populations identified (A) farm, (B) ‘florid’ vs. ‘non-florid’ status (C) flock (each different color representing a different flock). Venn diagrams showing the overlap of porAf2 variants identified by farm (D) and parent flock for a subset of 11 flocks (E). NMDS plot showing Bray-Curtis dissimilarity measures between the porAf2 population detected among flocks derived from matched parent flocks (F).
DISCUSSION
Results from this study show that Campylobacter DNA could be detected among fecal samples from 85 to 100% of the boiler flocks tested using nucleotide sequence based methods, depending on which gene target (porAf2 or 16S) was used. The results were irrespective of whether or not the flocks may be called ‘Campylobacter positive’ by routine culture or qPCR testing methods. Rather, the pattern of overgrowth of a predominant porAf2 type coincided with flocks that would usually be determined Campylobacter qPCR/culture positive using Ct thresholds, whilst a pattern of high porAf2 diversity at low prevalence was apparent amongst Campylobacter qPCR/culture negative flocks. The findings support the strong correlation between predominant porA type among culture/qPCR positive birds/flocks and diverse porA types among culture/qPCR negative birds/flocks shown in our previous work (Colles et al., 2021). Two of the flocks had a small proportion of samples that were positive by conventional qPCR, but Campylobacter 16S nucleotide sequence was detected at very low frequency, if at all, with the porAf2 pattern remaining diverse. Both of these flocks later tested positive for Campylobacter by standard culture methods in the abattoir, and may have been in early stages of transition when we tested them in this study. More work is needed to understand how a flock transitions from negative to positive for Campylobacter by conventional testing means, and could be critical for the control of Campylobacter infection in poultry flocks.
That Campylobacter DNA was not equally detected for samples tested by both 16S and porA is not surprising given they are different targets, with porA specifically amplifying Campylobacter DNA, and therefore, giving a greater sensitivity of detection. In contrast, the 16S bacterial profile, among which Campylobacter in our studies represented <0.01% of species recovered from the broiler chicken fecal samples (Colles et al., 2021) gives a high chance that more prevalent species will be detected ahead of Campylobacter, and may give greater variation between samples. Unlike our previous studies, however, Campylobacter DNA was detected at a prevalence >1% of the bacterial profile in 14.8% (43/290) of samples in this study, and at 51 to 79% of the bacterial profile in 1.7% (5/290) of samples collected from the Swiss broiler flocks. Other studies report Campylobacter prevalence in excess of 10% for experimentally infected chickens (Han et al., 2016; Rychlik, 2020). It is possible that some birds within the flocks were unusual in some way, for example with gut dysbiosis brought about by co-infection with another pathogen. Farm 3, with two Campylobacter qPCR/culture positive flocks from which the 5 samples with greatest Campylobacter prevalence were identified, was suspected to be having some problems with coccidial infections over the course of this study.
The porAf2 variants 1–9 accounted for 70.5% of the total sequences recovered, and it was not possible to distinguish the porAf2 populations isolated from broiler flocks by either farm or parent flock. Of the 245 porAf2 variants identified among the study, 43% were identified from flocks on all 3 farms, with only 1 to 2.4% (8–39 porAf2 variants) of the total, unique to each of the farms. These results imply that a large proportion of the porAf2 variants are well-adapted for persistence in the poultry industry and co-exist amongst broiler flocks from different farms over a number of months. On a broader context, MLST-based studies also demonstrate a number of Campylobacter types show host association with chicken sources (Sheppard et al., 2010). Deep sequencing using the porAf2 target gives a useful indication of Campylobacter diversity within a sample, but further development of the method, including additional gene targets is required for more refined strain typing. It is not currently possible to distinguish between C. jejuni and C. coli species using the porAf2 fragment, though the presence of both species among samples was detected by qPCR and it is likely the 80% homology between the most disparate nucleotide sequences reflects the different species also.
It was notable that nucleotide changes detected in the porAf2 led to a new amino acid sequence (MOMPf2) in 70% of cases, with up to 79 porAf2 nucleotide/65 MOMPf2 variants detected from an individual sample. This finding fits with the short variable region of the outer membrane protein being under host immune selection (Cody et al., 2009), and the pattern of high diversity may reflect evasion of the host immune response (Bloomfield et al., 2021). It was unpredictable which porAf2 variant would become predominant in the 3 Campylobacter qPCR/culture positive flocks. Two of the porAf2 variants colonizing the Campylobacter qPCR/culture positive flocks were commonly observed among all flocks, implying they could have greater fitness, but the third was not. The stochastic nature of Campylobacter infection by different strain types has been shown to relate to the susceptibility of individual birds infected by a Campylobacter type by chance, more than the Campylobacter strain type itself, using Bayesian modeling approaches (Rawson et al., 2020).
In conclusion, we demonstrate that Campylobacter testing methodology may need to be reviewed, depending on the circumstances in which it is needed. While it is still useful to identify those flocks that are most heavily contaminated by Campylobacter at slaughter and pose greatest risk to human health by conventional means that are timely and cost-effective, identifying routes of transmission among a relatively rare gut inhabitant is much more challenging. The ability to routinely detect low levels of Campylobacter amongst broiler flocks at a much earlier age than would conventionally be identified requires a re-examination of how and when biosecurity measures are best applied. In addition, other interventions, such as good animal welfare and management to maintain optimal gut health may help to prevent the overgrowth of a single Campylobacter type, characteristic of problematic flocks that test Campylobacter positive by conventional means.
Acknowledgments
ACKNOWLEDGMENTS
This work was supported by the Biotechnology and Biological Science Research Council (grant numbers BB/N023803/1 and BB/K004468/1) including part of the Animal Health and Welfare ERA-net call. ALS was also supported by the UK Department for Environment, Food and Rural Affairs, (grant number OD0221). The collection of feces was funded by the Federal Office of Food Safety and Veterinary Affaires (No. 2.16.03). We also acknowledge the support of the Bell AG, Zell, Switzerland, and the five farmers who provided access to their barns and logistic support.
DISCLOSURES
The authors declare no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2022.102048.
Appendix. Supplementary materials
REFERENCES
- Anonymous. 2017. Campylobacter data 2008-2017. https://www.gov.uk/government/publications/campylobacter-infection-annual-data/campylobacter-data-2008-to-2017. Accessed Mar. 2022.
- Awad W.A., Hess C., Hess M. Re-thinking the chicken-Campylobacter jejuni interaction: a review. Avian Pathol. 2018;47:352–363. doi: 10.1080/03079457.2018.1475724. [DOI] [PubMed] [Google Scholar]
- Best E.L., Powell E.J., Swift C., Grant K.A., Frost J.A. Applicability of a rapid duplex real-time PCR assay for speciation of Campylobacter jejuni and Campylobacter coli directly from culture plates. FEMS Microbiol. Lett. 2003;229:237–241. doi: 10.1016/S0378-1097(03)00845-0. [DOI] [PubMed] [Google Scholar]
- Bloomfield S.J., Midwinter A.C., Biggs P.J., French N.P., Marshall J.C., Hayman D.T.S., Carter P.E., Mather A.E., Fayaz A., Thornley C., Kelly D.J., Benschop J. Genomic adaptations of Campylobacter jejuni to long-term human colonization. Gut Pathog. 2021;13:72. doi: 10.1186/s13099-021-00469-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan B.J., McMurdie P.J., Rosen M.J., Han A.W., Johnson A.J., Holmes S.P. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Pena A.G., Goodrich J.K., Gordon J.I., Huttley G.A., Kelley S.T., Knights D., Koenig J.E., Ley R.E., Lozupone C.A., McDonald D., Muegge B.D., Pirrung M., Reeder J., Sevinsky J.R., Turnbaugh P.J., Walters W.A., Widmann J., Yatsunenko T., Zaneveld J., Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody A.J., Maiden M.J.C., Dingle K.E. Genetic diversity and stability of the porA allele as a genetic marker in human Campylobacter infection. Microbiology. 2009;155:4145–4154. doi: 10.1099/mic.0.031047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colles F.M., Hedges S.J., Dixon R., Preston S.G., Thornhill P., Barfod K.K., Gebhardt-Henrich S.G., Creach P., Maiden M.C.J., Dawkins M.S., Smith A.L. Parallel sequencing reveals Campylobacter spp in commercial meat chickens less than 8 days old. Appl. Environ. Microbiol. 2021;87:e01060–21. doi: 10.1128/AEM.01060-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colles F.M., Preston S.G., Barfod K.K., Flammer P.G., Maiden M.C.J., Smith A.L. Parallel sequencing of porA reveals a complex pattern of Campylobacter genotypes that differs between broiler and broiler breeder chickens. Sci. Rep. 2019;9:6204. doi: 10.1038/s41598-019-42207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox N.A., Richardson L.J., Maurer J.J., Berrang M.E., Fedorka-Cray P.J., Buhr R.J., Byrd J.A., Lee M.D., Hofacre C.L., O'Kane P.M., Lammerding A.M., Clark A.G., Thayer S.G., Doyle M.P. Evidence for horizontal and vertical transmission in Campylobacter passage from hen to her progeny. J. Food Prot. 2012;75:1896–1902. doi: 10.4315/0362-028.JFP-11-322. [DOI] [PubMed] [Google Scholar]
- European Food Safety Authority Analysis of the baseline survey on the prevalence of Campylobacter in broiler batches and of Campylobacter and Salmonella on broiler carcasses in the EU, 2008. EFSA J. 2010;8:1503–1602. [Google Scholar]
- Evans S.J., Sayers A.R. A longitudinal study of Campylobacter infection of broiler flocks in Great Britain. Prev. Vet. Med. 2000;46:209–223. doi: 10.1016/s0167-5877(00)00143-4. [DOI] [PubMed] [Google Scholar]
- Gebhardt-Henrich S.G., Stratmann A., Dawkins M.S. Groups and individuals: optical flow patterns of broiler chicken flocks are correlated with the behavior of individual birds. Animals (Basel) 2021;11:568. doi: 10.3390/ani11020568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z., Willer T., Pielsticker C., Gerzova L., Rychlik I., Rautenschlein S. Differences in host breed and diet influence colonization by Campylobacter jejuni and induction of local immune responses in chicken. Gut Pathog. 2016;8:56. doi: 10.1186/s13099-016-0133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh T.C., M K.H., Chao A. iNEXT: an R package for rarefaction and extrapolation of species diversity (Hill numbers) Methods Ecol. Evol. 2016;7:1451–1456. [Google Scholar]
- Hunter P.R. Reproducibility and indices of discriminatory power of microbial typing methods. J. Clin. Microbiol. 1990;28:1903–1905. doi: 10.1128/jcm.28.9.1903-1905.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijaz U.Z., Sivaloganathan L., McKenna A., Richmond A., Kelly C., Linton M., Stratakos A.C., Lavery U., Elmi A., Wren B.W., Dorrell N., Corcionivoschi N., Gundogdu O. Comprehensive longitudinal microbiome analysis of the chicken cecum reveals a shift from competitive to environmental drivers and a window of opportunity for Campylobacter. Front. Microbiol. 2018;9:2452. doi: 10.3389/fmicb.2018.02452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolley K.A., Bliss C.M., Bennett J.S., Bratcher H.B., Brehony C., Colles F.M., Wimalarathna H., Harrison O.B., Sheppard S.K., Cody A.J., Maiden M.C.J. Ribosomal multilocus sequence typing: universal characterization of bacteria from domain to strain. Microbiology. 2012;158:1005–1015. doi: 10.1099/mic.0.055459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen, F., A. Charlett, C. Swift, N. Corcionivoschi, and N. C. Elviss. 2019. A microbiological survey of Campylobacter contamination in fresh whole UK- produced chilled chicken at retail sale. Accessed July 2022. https://www.food.gov.uk/sites/default/files/media/document/antimicrobial-resistance-in-campylobacter-jejuni-and-campylobacter-coli-from-retail-chilled-chicken-in-the-uk-year-4-2017-18.pdf.
- Kubasova T., Kollarcikova M., Crhanova M., Karasova D., Cejkova D., Sebkova A., Matiasovicova J., Faldynova M., Pokorna A., Cizek A., Rychlik I. Contact with adult hen affects development of caecal microbiota in newly hatched chicks. PLoS One. 2019;14 doi: 10.1371/journal.pone.0212446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, M. 2011. Cutadapt removes adapter sequences from high-throughput sequencing reads. Accessed July 2022. https://journal.embnet.org/index.php/embnetjournal/article/view/200.
- McMurdie P.J., Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8:e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell D.G., Fearnley C. Sources of Campylobacter colonization in broiler chickens. Appl. Environ. Microbiol. 2003;69:4343–4351. doi: 10.1128/AEM.69.8.4343-4351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson T., Paton R.S., Colles F.M., Maiden M.C.J., Dawkins M.S., Bonsall M.B. A mathematical modeling approach to uncover factors influencing the spread of Campylobacter in a flock of broiler-breeder chickens. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.576646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton S.P., Humphrey T.J., Shirley M.D., Bull S., Jorgensen F. Campylobacter in housed broiler chickens: a longitudinal study of risk factors. Epidemiol. Infect. 2009;137:1099–1110. doi: 10.1017/S095026880800188X. [DOI] [PubMed] [Google Scholar]
- Rychlik I. Composition and function of chicken gut microbiota. Animals (Basel) 2020;10:103. doi: 10.3390/ani10010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Shannon C.E. A mathematical theory of communication. AT&T Tech. J. 1948;27:379–423. [Google Scholar]
- Sheppard S.K., Colles F., Richardson J., Cody A.J., Elson R., Lawson A., Brick G., Meldrum R., Little C.L., Owen R.J., Maiden M.C., McCarthy N.D. Host association of Campylobacter genotypes transcends geographic variation. Appl. Environ. Microbiol. 2010;76:5269–5277. doi: 10.1128/AEM.00124-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard S.K., Dallas J.F., Strachan N.J., MacRae M., McCarthy N.D., Wilson D.J., Gormley F.J., Falush D., Ogden I.D., Maiden M.C., Forbes K.J. Campylobacter genotyping to determine the source of human infection. Clin. Infect. Dis. 2009;48:1072–1078. doi: 10.1086/597402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H. Springer-Verlag; New York, NY: 2016. ggplot2: Elegant Graphics for Data Analysis. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.