ROS1
gene rearrangements occur in 1% to 2% of NSCLC. Acquired “on-target” mutations within the ROS1 kinase domain are a known resistance mechanism to the first-line ROS1 inhibitor crizotinib. Here, we report the first case of a patient with an acquired ROS1 G2101A resistance mutation after first-line crizotinib, who responded to lorlatinib. The response was dramatic but short in duration.
Keywords: Non–small cell lung cancer, ROS1 fusion, ctDNA, Lorlatinib, Case report
Introduction
ROS1 gene fusions are known oncogenic drivers in NSCLC, accounting for 1% to 2% of cases. Crizotinib and entrectinib are approved by the Food and Drug Administration and European Medicines Agency for first-line treatment of ROS1-rearranged NSCLC. Nevertheless, secondary resistance to these remains a challenge. Lorlatinib is a brain-penetrant, adenosine triphosphate-competitive, small molecule inhibitor of ALK and ROS1, which has efficacy in patients with both ALK and ROS1 kinase resistance mutations, such as the solvent-front G1202R mutation and its ROS1 analog G2032R.1 Genomic predictors of lorlatinib durable response post crizotinib or post entrectinib are important in drug decision-making.
Case Presentation
A 75-year-old female never smoker presented with dyspnea. Results of initial computed tomography (CT) pulmonary angiogram with subsequent 18F-fluorodeoxyglucose positron emission tomography (PET)-CT and brain magnetic resonance imaging (MRI) revealed a right lower lobe lung primary with widespread metastases involving supraclavicular and bilateral mediastinal nodes, pleura (with effusion), lungs, liver, adrenal gland, multiple bone, and multiple brain sites. Result of pleural aspirate confirmed metastatic ROS1-positive, TTF1-positive adenocarcinoma by immunohistochemistry. Circulating tumor DNA (ctDNA) next-generation sequencing (NGS) (Guardant360 CDx, Guardant Health, Redwood City, CA) confirmed a dominant CD74-ROS1 fusion at 2.0% variant allele frequency (VAF), alongside additional driver variants, including CDKN2A E120∗, TP53 H193Y, TP53 A159D, and KRAS K117N all at lower VAFs.
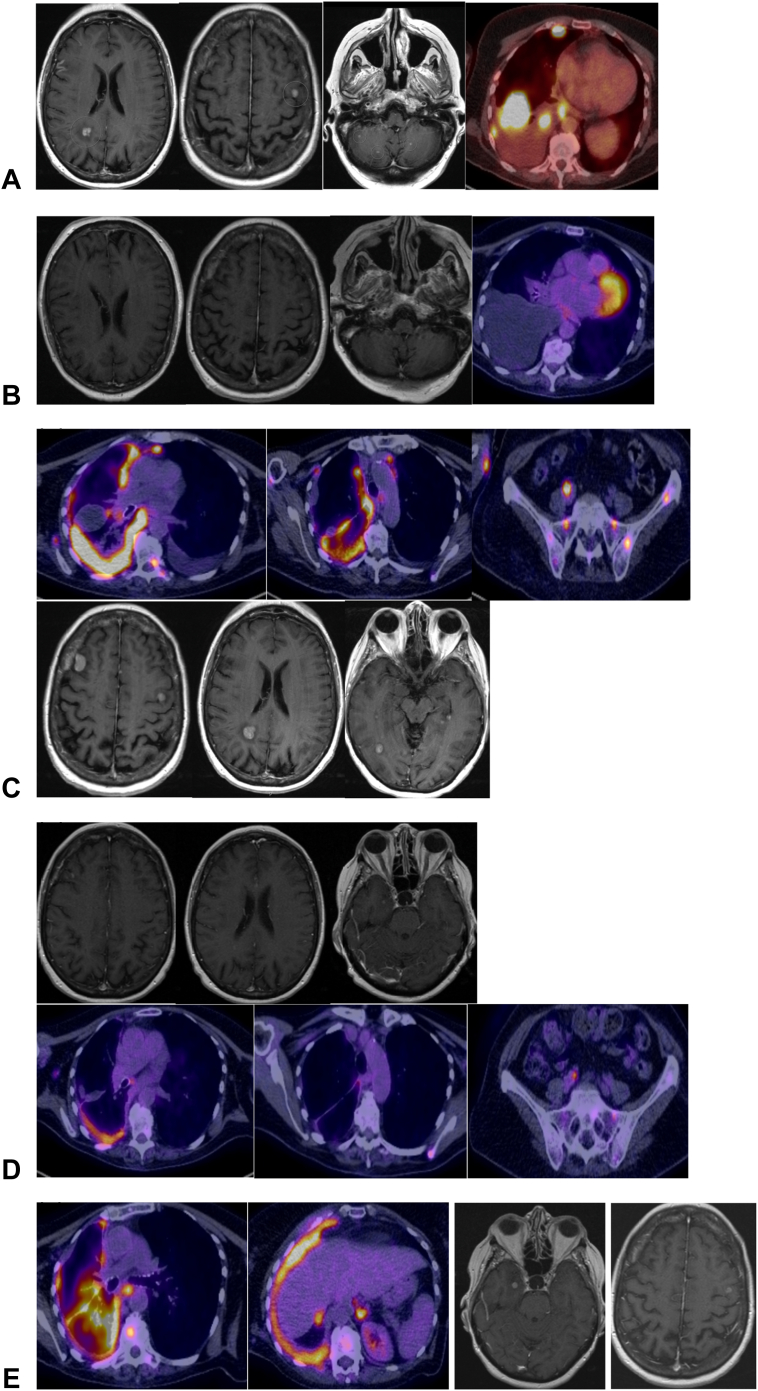
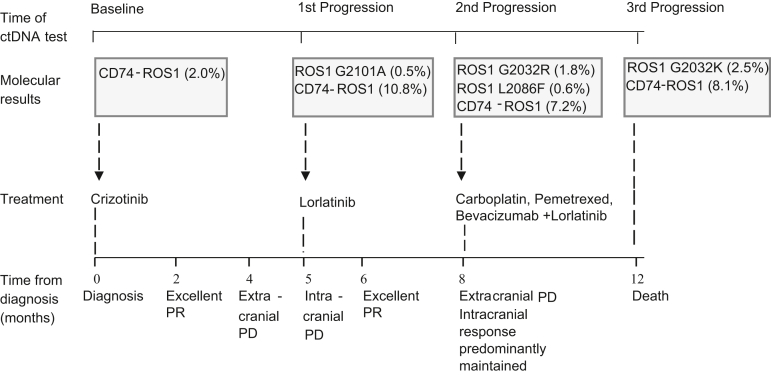
The patient commenced crizotinib 250 mg twice daily with denosumab because entrectinib was unavailable. Result of response imaging with PET-CT and MRI brain after 2 months revealed a rapid intracranial and extracranial partial response (Fig. 1).
Figure 1.
Radiology images on therapy. (A) CT, FDG PET-CT, and MRI brain images at diagnosis. (B) Response imaging after two months on crizotinib, revealing a good response. (C) Imaging at progression on crizotinib, coinciding with emergence of a ROS1 G2101A variant. (D) Imaging revealing a good extracranial and intracranial response after 1 month of lorlatinib treatment. (E) Imaging revealing extracranial progression and intracranial oligoprogression at two sites, after 2 months of lorlatinib treatment. NGS result at this point revealed loss of G2101A and emergence of ROS1 G2032R and L2086F. CT, computed tomography; FDG PET-CT, 18F-fluorodeoxyglucose positron emission tomography-computed tomography; MRI, magnetic resonance imaging.
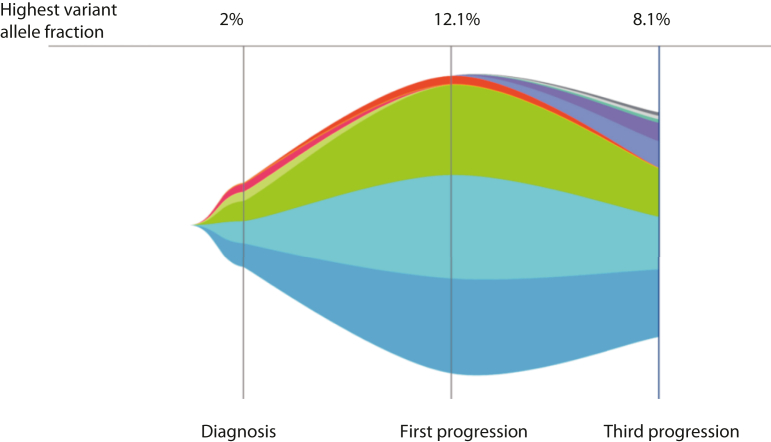
Nevertheless, imaging after 3 additional months identified extracranial and intracranial progression (at pleural, nodal, adrenal, skeletal, and brain sites, Fig. 1). At this point, repeat ctDNA NGS for therapy selection identified progressive dominance of the truncal CD74-ROS1 driver (VAF10.8%) alongside the CDKN2A E120∗ and TP53 H193Y variants. In addition, an acquired ROS1 G2101A mutation was identified (VAF 0.5%; Fig. 2). After discussion with the patient and family, she commenced lorlatinib (100 mg once daily). Her response evaluation PET-CT and brain MRI scan 4 weeks after commencement revealed an excellent intracranial and extracranial partial response (Fig. 1) underpinning excellent clinical benefit.
Figure 2.
Guardant360 tumor response map. Tumor response map illustrating the dynamic changes in variant allele fractions of (% cfDNA) observed somatic variants at each sample submission. Amplifications are not plotted. cfDNA, cell-free DNA.
After 2 months, dyspnea deteriorated and PET-CT with brain MRI confirmed widespread extracranial progression with predominantly maintained intracranial response. Result of repeat ctDNA NGS revealed reduction in the CD74-ROS1 fusion (VAF 7.2%), CDKN2A E120∗, and TP53 H193Y drivers, loss of the ROS1 G2101A target, and emergence of the lorlatinib compound ROS1 G2032R (VAF 1.8%) and L2086F (VAF 0.6%) resistance mutations (Fig. 3). The patient commenced third-line carboplatin-pemetrexed-bevacizumab combination therapy with continued lorlatinib for ongoing intracranial control, and in parallel, she received stereotactic radiosurgery to two intracranial sites owing to oligoprogressive metastases with edema. Despite an initial response after two cycles of systemic therapy associated with clinical benefit, result of PET-CT following cycle 4 revealed disease progression. Result of ctDNA NGS testing revealed progression of the CD74-ROS1 driver (VAF 8.1%) with minor reduction of the CDKN2A E120∗ (VAF 5.3%) and TP53 H193Y (VAF 6.0%) drivers, loss of ROS1 G2032R, gain of ROS1 G2032K, and gain in other variants, including NRAS G12D. She unfortunately continued to deteriorate and was admitted with dyspnea, acute kidney injury, a raised troponin level, and multiple watershed embolic infarcts in both cerebral hemispheres on MRI imaging results. Progressive clinical and neurological deterioration occurred thereafter, shortly followed by the patient’s death.
Figure 3.
A timeline of the patient’s clinical, treatment, and molecular profile. At the first progression on crizotinib treatment, there is emergence of a ROS1 G2101A variant. Lorlatinib is started at this point with excellent response. At the second progression, there is loss of G2101A and emergence of ROS1 G2032R and L2086F. At the final progression, while still on lorlatinib, there is emergence of a ROS1 G2032K variant. Variant allele frequencies are provided in parentheses. PD, progressive disease; PR, partial response.
Discussion
Crizotinib and entrectinib are first-line treatments for ROS1-rearranged NSCLC. Nevertheless, acquired resistance remains a major challenge in its management, especially the development of solvent-front and gatekeeper mutations with no other ROS1 kinase inhibitors currently licensed. ctDNA provides a useful minimally invasive tool for the temporal detection of variants that can identify on- or off-target resistance mechanisms and potentially guide treatment. Here, we reveal that the acquisition of the on-target G2101A ROS1 crizotinib-resistance mutation is associated with lorlatinib response: a novel clinical finding. G2101A has previously been identified as crizotinib resistant in preclinical assays,2 an analog of ALK G1269A, itself previously found to result in responses of short duration to next-generation ALK inhibitors3 and retains preclinical sensitivity to foretinib,2 although no preclinical or clinical data have previously been reported for its sensitivity to lorlatinib. G2101A sits away from the ROS1 L2026 gatekeeper residue1 and outside the G2302 solvent-front region, and its precise mechanism of crizotinib resistance and lorlatinib sensitivity remains uncertain. It remains unclear whether G2101A results in lorlatinib response of brief duration as observed in this case or whether the brief lorlatinib sensitivity observed here was underpinned by the volume of her disease and diversity of other drivers (CDKN2A and TP53). Moreover, this case highlights that G2101A resistance may be mediated by on-target compound G2032R-L2086F ROS1 mutations, previously associated with lorlatinib resistance,4 as well as with conferring resistance to taletrectinib (DS6051b) and sensitivity to cabozantinib.5 L2086F, being analogous to ALK L1256F, which confers crizotinib and lorlatinib resistance in an ALK-positive NSCLC preclinical model through steric interference.4 Finally, this case demonstrates the utility of ctDNA NGS at diagnosis and each progression point allowing identification of extracranial acquired ROS1-kinase inhibitor resistance mechanisms and optimal drug decision-making.
Conclusion
This is the first report of a ROS1 G2101A mutation associated with acquired crizotinib resistance and lorlatinib sensitivity with immediate response. We further identify lorlatinib resistance through G2101A loss and compound G2032R-L2086F gain, adding additional evidence for lorlatinib sensitivity to ROS1 NSCLCs with specific acquired crizotinib-resistance mutations.
CRediT Authorship Contribution Statement
Sanjay Popat: Conceptualization ideas, Writing manuscript, review and editing.
Coordinated patient care.
Parvin Begum: Writing manuscript, review and editing, Creating figures.
Wanyuan Cui: Writing manuscript, review and editing.
Acknowledgments
The authors acknowledge the patient for providing informed consent for her case to be reported. Drs. Popat and Cui acknowledge the National Health Service funding to the National Institute for Health and Care Research Biomedical Research Centre at the Royal Marsden Hospital/Institute of Cancer Research.
Footnotes
Disclosure: Dr. Cui declares receiving grant funding from the Breast Cancer Trials and honoraria from Pfizer, United States, Merck, United States, Janssen, and AstraZeneca, United Kingdom outside of the submitted work. Prof. Popat is a consultant to Amgen, AstraZeneca, Bayer, Beigene, Blueprint, Bristol-Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo, Guardant Health, Janssen, Lilly, Merck KGaA, Novartis, Roche, and Takeda. Dr. Begum declares no conflict of interest.
Cite this article as: Begum P, Cui W, Popat S. Crizotinib-resistant ROS1 G2101A mutation associated with sensitivity to lorlatinib in ROS1-rearranged NSCLC: case report. JTO Clin Res Rep. XXXX;X:XXXXXX.
References
- 1.Zou H.Y., Li O., Engstrom L.D., et al. PF-06463922 is a potent and selective next-generation ROS1/ALK inhibitor capable of blocking crizotinib-resistant ROS1 mutations. Proc Natl Acad Sci. 2015;112:3493–3498. doi: 10.1073/pnas.1420785112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song A., Kim T.M., Kim D., et al. Molecular changes associated with acquired resistance to crizotinib in ROS1-rearranged non–small cell lung cancer. Clin Cancer Res. 2015;21:2379–2387. doi: 10.1158/1078-0432.CCR-14-1350. [DOI] [PubMed] [Google Scholar]
- 3.Michels S., Scheel A.H., Wündisch T., et al. ALKG1269A mutation as a potential mechanism of acquired resistance to crizotinib in an ALK-rearranged inflammatory myofibroblastic tumor. npj Precision Onc. 2017;1:4. doi: 10.1038/s41698-017-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin J.J., Choudhury N.J., Yoda S., et al. Spectrum of mechanisms of resistance to crizotinib and lorlatinib in ROS1 fusion-positive lung cancer. Clin Cancer Res. 2021;27:2899–2909. doi: 10.1158/1078-0432.CCR-21-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papadopoulos K.P., Borazanci E., Shaw A.T., et al. U.S. phase I first-in-human study of taletrectinib (DS-6051b/AB-106), a ROS1/TRK inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2020;26:4785–4794. doi: 10.1158/1078-0432.CCR-20-1630. [DOI] [PubMed] [Google Scholar]