Abstract
Mosquitoes are vectors for a number of infectious diseases. Only females feed on blood to provision for their embryos and in doing so transmit pathogens to the associated vertebrate hosts. Therefore, sex is an important phenotype in the context of genetic control programs, both for sex separation in the rearing facilities to avoid releasing the biting females and for ways to distort the sex ratio towards the non-biting males. We review recent progress in the fundamental knowledge of sex determination and sex chromosomes in mosquitoes and discuss new methods to achieve sex separation and sex ratio distortion to help control mosquito-borne infectious diseases. We conclude by suggesting a few critical areas for future research.
Keywords: Sex determination, Homomorphic sex chromosomes, Gene-drive, Meiotic drive, Sterile insect technique, X-shredder
The importance of sex in the genetic control of mosquito-borne infectious disease
Mosquitoes transmit various pathogens that cause a number of infectious diseases such as malaria, dengue, Zika and yellow fever. Despite recent advances in vaccine development [1, 2] current prevention depends mainly on effective vector control, which is hindered by increasing insecticide-resistance. Novel genetic control measures are being actively explored that either suppress the target mosquito populations or render them refractory to the causative pathogens. An example of population suppression is the release of sterile male mosquitoes (Sterile Insect Technique, SIT, see Glossary) to reduce the vector population density. In contrast, population modification strategies involve the release of insects carrying an effector gene(s) that confers resistance to the targeted pathogen to prevent its transmission. This strategy is based on the ability to increase the frequency of the introduced effector genes in the target population through a gene-drive mechanism (e.g., [3]). These genetic methods are species-specific and only need to target the small number of species that transmit important human pathogens. For example, in many parts of the world Aedes aegypti is the primary vector of a number of arboviruses such as dengue, chikungunya, yellow fever and Zika. Thus, effective genetic control targeting this single species could help reduce or prevent multiple arboviral diseases. Regardless of the vector species, only females feed on blood to provision for their embryos and in doing so transmit pathogens to their vertebrate hosts. Therefore, sex is an important phenotype in the context of genetic control programs, both for sex separation in the rearing facilities to avoid releasing biting females and for ways to distort the sex ratio towards non-biting males to help control mosquito-borne infectious diseases. We previously reviewed this topic in the context of sex-determination and gene-drive [4]. Several recent reviews have discussed new developments and challenges to controlling mosquito-borne infectious diseases using gene-drive and other genetic strategies (e.g., [3, 5–8]). Here we review recent advances in mosquito sex chromosome genomics, sex-determination pathways, and a diverse array of methods to achieve sex separation and sex ratio distortion. We conclude by suggesting a few critical areas for future research.
Genomic characterization of the homomorphic and heteromorphic sex chromosomes in mosquitoes
The mosquito family (Culicidae) include more than 3500 described species that are grouped into two main subfamilies consisting of many diverse genera [9]. The Anophelinae subfamily includes the Anopheles genus to which many malaria vectors including Anopheles gambiae belong. The Culicinae subfamily includes other medically important genera such as Aedes and Culex. For example, Ae. aegypti is an important arboviral vector as described earlier, while Culex quinquefasciatus is a key vector of West Nile and other encephalitis viruses as well as lymphatic filariasis worms. All mosquitoes studied so far have three pairs of chromosomes. Anopheles mosquitoes contain two pairs of autosomes and one pair of well-differentiated sex chromosomes, X and Y, with males being the heterogametic sex. Aedes and Culex mosquitoes have a pair of homomorphic sex-determining chromosomes (Figure 1A) that are cytologically indistinguishable [10, 11]. In males, which are also the heterogametic sex, one of the pair has a male-determining locus (the M locus) while the other has an m locus (Figure 1A). A dominant male-determining factor (M factor), which resides either on the Y chromosome in Anophelinae mosquitoes, or within the M locus in Culicinae mosquitoes (reviewed in [12]), is the master switch for sex determination.
Figure 1. Sex chromosomes in Aedes aegypti and Anopheles gambiae.
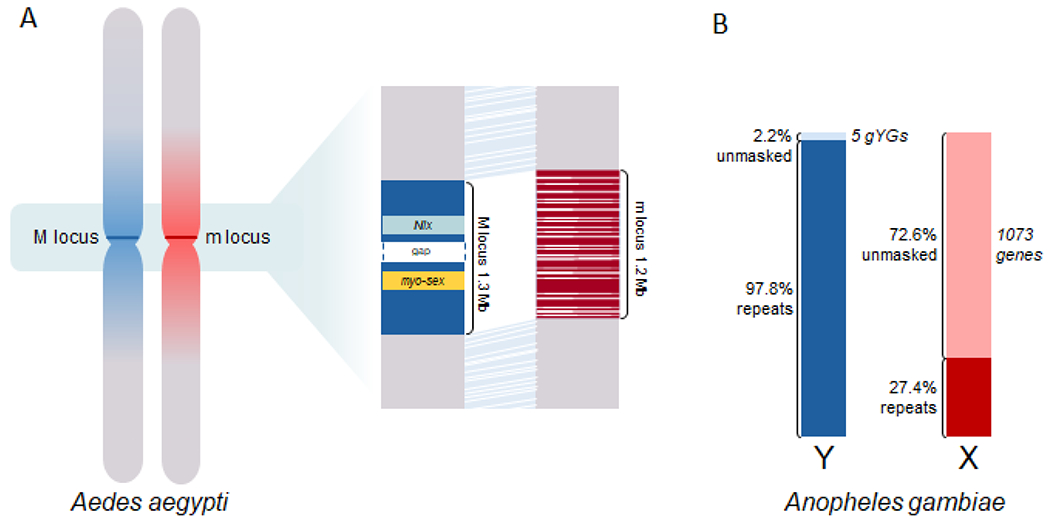
A) Sex-determining chromosomes in Ae. aegypti are homomorphic and karyotypically indistinguishable. A small 1.2-1.3 Mb region of divergence represents the M (blue line) or m (red line) locus near the centromere [22]. At the molecular level, however, a much broader 100Mb region showed sequence differentiation between the M-bearing and m-bearing chromosomes surrounding the M/m locus (e.g., [22]), indicated by the blue gradient and red gradient, respectively. The M locus harbors male-specific genes including the male-determining factor Nix and the myo-sex gene encoding a myosin heavy-chain protein necessary for male flight. White bars/lines within the M/m loci indicate gaps of various sizes. B) An. gambiae sex chromosomes are well differentiated X and Y chromosomes. As the Y chromosome has not been assembled at the chromosomal level, we compared the repeat and gene content of the non-recombining region of the Y (NRY, [18]) with those of the 24.39 Mb X chromosome assembly (vectorbase.org). The NRY is largely repetitive and only 2.2% of it is not masked by RepeatMasker (www.repeatmasker.org [97]), including five experimentally verified genes (An. gambiae Y genes or gYGs) such as the male determining factor gYG2/Yob [17–19]. Approximately 72.6% of the assembled X chromosome is comprised of single or low copy sequences that are not masked by RepeatMasker (www.repeatmasker.org) and it contains 1073 protein-coding genes.
The Culicinae sex-determining loci as well as the Anopheles Y chromosomes were largely missing in previous genome assemblies (e.g., [13–15]), due to the repetitive nature of these genomic regions. Using next-generation sequencing and a Chromosome Quotient (CQ) method, several Y chromosome genes were uncovered including the early embryonic signals Guy1 and gYG2, in An. stephensi and An. gambiae, respectively [16, 17]. gYG2 is the only experimentally verified Y-linked gene that is found in all species of the An. gambiae complex [18] and it is identical to the An. gambiae M factor Yob [19]. Using PacBio sequencing and CQ, the sequence and structure of the non-recombining region of the Y (NRY) of An. gambiae was further determined [18]. Only ~2% of the NRY is made of low copy number sequences including a handful of genes such as gYG2 while the vast majority of the NRY consists of tandem arrays of highly amplified repeats (Figure 1B). Up to 86% of the repetitive NRY varied as the subspecies within the An. gambiae complex diverged [18]. These genomic insights were obtained by analyzing fragments of the NRY without a Y chromosome assembly. More extensive collections of Y chromosome contigs are reported in recent genome assemblies using PacBio or Oxford Nanopore sequencing [20, 21], resulting in the identification of seven male-linked gene candidates in An. stephensi [20].
Recent advances in sequencing and assembly methods also revealed for the first time the genomic characteristics of the Ae. aegypti M/m loci [22]. By aligning a male Ae. aegypti PacBio-HiC assembly (AaegL5, [22]) with a previous Sanger-based assembly [14] scaffolded using female HiC reads (AaegL4, [23]), a region of clear divergence was identified that represents the M and m locus in AaegL5 and AaegL4, respectively (Figure 1A). The male-specificity of the 1.3 Mb M locus, which contains the previously characterized M factor Nix [24], another male-specific gene myo-sex [25] and 25 non-coding RNA genes, was confirmed by CQ analysis and in situ hybridization [22]. Because haplotype switching between homologous chromosomes caused by potential assembly artefacts could mix M- or m-haplotypes in regions of sufficient similarity in the male assembly, the precise borders of the M and m loci are not resolved. A 163 kb gap exists in the M locus as indicated by Bionano optical mapping [22]. The 1.2 Mb m locus contains 44 gaps of known and unknown sizes. Moreover, the M/m locus is embedded in an ~100 Mb region of suppressed recombination near the centromere [22, 23, 26]. Extensive sequence variations or genetic differentiation were also found between the M-chromosome and the m-chromosome in an ~100 Mb region surrounding the M/m locus among multiple strains and populations [22, 27]. This may reflect the nascent differentiation between the two homomorphic sex chromosomes beyond the sex locus.
Recent development in mosquito sex determination
Insects have evolved highly diverse primary signals for sex determination. However, through a cascade of events (Figure 2), these primary signals are eventually transduced as sex-specific isoforms of doublesex (dsx) and fruitless (fru), two conserved transcription factors that program sexual differentiation [12, 28]. As mentioned earlier, a dominant M factor serves as the primary signal that triggers male development in mosquitoes, regardless of the karyotypes of sex chromosomes. The first described mosquito M factor, the RNA-binding protein Nix, was discovered in Ae. aegypti using the CQ method [24] prior to characterization of the M locus [22]. Nix is both required and sufficient to initiate male development as somatic Nix-knockout resulted in partial feminization in males and ectopically expressing Nix in females resulted in partial masculinization [24]. At the molecular level, Nix knockout feminized the sex-specific splicing of doublesex (dsx) and fruitless (fru). In Drosophila melanogaster and many other insects, the sex-specific splicing of dsx and fru is modulated by a protein complex that includes a fast-evolving transformer (TRA) and a conserved transformer 2 (TRA2), where TRA is the sex-specific protein in the complex. However, TRA does not appear to exist in mosquitoes and the role of TRA2 in sex-determination remains unresolved. Nix was also found in the Asian tiger mosquito Aedes albopictus [24, 29–31] and it also regulates dsx splicing in this species [32]. There is some evidence that the Ae. albopictus Nix indirectly regulates dsx splicing through an unknown intermediary splicing factor (Figure 2, [33]). Stable germline transformation and expression of a Nix transgene from its native promoter successfully converted genetic female Ae. aegypti into fertile males [34]. These converted males are flightless due to the lack of myo-sex, a second gene in the M locus that encodes a myosin heavy-chain protein required specifically for flight in males. Thus, the converted males cannot mate on their own as flight is required for mating. However, when assisted by providing them with cold-anesthetized virgin females these converted m/m males fathered transgenic m/m males and wildtype m/m females at a 1:1 ratio, suggesting complete masculinization of the reproductive system in the absence of the native M locus. Stable germline transformation of the Ae. albopictus Nix converted genetic females into fertile and flying males [35], indicating that the M-linked myo-sex is not required and an autosomal paralog(s) is sufficient to sustain male flight in this species.
Figure 2. Simplified models of the sex determination pathway in male (A) and female (B) Aedes mosquitoes.
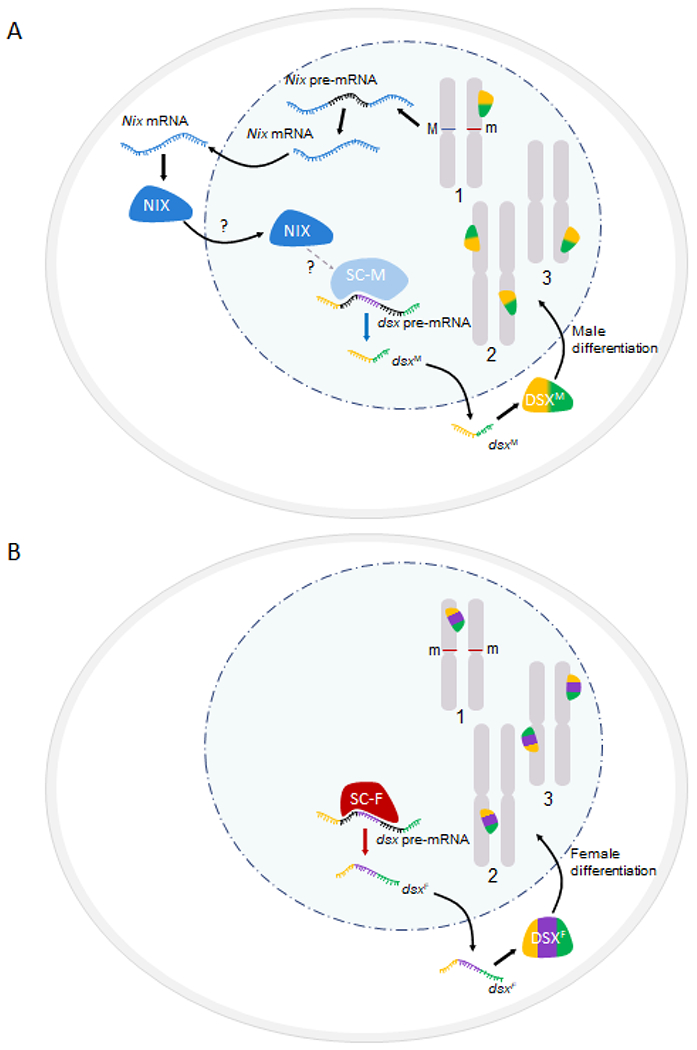
Events leading to the sex-specific splicing of the dsx pre-mRNA are depicted based on information from Ae. aegypti and Ae. albopictus. Details on possible activation or repression of certain splice sites can be found elsewhere [12, 33, 98]. A). Nix, which is located in the M locus of the M/m males, is transcribed, processed, and translated in the early embryo. The NIX protein has predicted RNA recognition motifs and is distantly related to a splicing factor TRA2 [24]. Although we cannot rule out a role in regulating translation or mRNA stability, NIX is hypothesized to function as a splicing factor that somehow modulates an uncharacterized splicing complex in males (SC-M), ultimately affecting dsx splicing. It appears that the effect of NIX on dsx splicing is indirect [33]. The dashed arrow and question mark indicate the uncertainty about the number of steps and the mechanism involved in this modulation. The resulting male DSXM protein isoform participates in programming male differentiation. B) Females (m/m) do not have the M locus and do not express Nix. An uncharacterized splicing complex in the female (SC-F) is responsible for the female-specific splicing of dsx pre-mRNA to produce the dsxF mRNA, which is translated in the cytoplasm to make the female DSXF protein isoform. The DSXF protein is a transcription factor that programs female differentiation through the regulation of gene expression in the nucleus.
Guy1 encodes a 56 amino acid protein that is the primary Y-linked signal in An. stephensi, and as its expression is at the onset of the maternal-to-zygotic transition, requiring no other Y chromosome genes [16, 36]. Instead of sex conversion, stable germline transformation of Guy1 resulted in female-specific lethality in An. stephensi [36]. This female-specific lethality was explained by evidence that Guy1 also controls X chromosome dosage compensation by upregulating X-linked genes at a chromosomal scale [37]. Increased X chromosome gene expression is needed to compensate for the single X chromosome in XY males [38]. Upregulation of the X-linked genes in the Guy1 transgenic females, which already have two X chromosomes, cause abnormally high X expression and could lead to female-specific lethality.
Yob, the M factor in An. gambiae [19] also encodes a 56 amino acid protein but it is not clear whether or not it is orthologous to Guy1. Yob modulates dsx splicing and confers female-specific lethality when ectopically expressed either transiently or as a stable transgene [19, 39]. In addition, an autosomal gene, femaleless, was recently found to regulate sex-determination by modulating dsx splicing and control X chromosome dosage compensation [40]. Knockdown of femaleless confers female-specific lethality in An. gambiae and An. stephensi [40], providing a link between sex-determination and dosage compensation. We note that sex conversion was described in organisms where X-chromosome dosage compensation is presumably not needed (e.g., Aedes mosquitoes and medfly: [24, 34, 35, 41]). In contrast, sex-specific lethality is observed when the upstream regulators of sex-determination are perturbed early in development in organisms that require X-chromosome dosage compensation (e.g., [36, 37, 39, 40, 42, 43].
Sterile insect technique (SIT) and the need for sex separation methods
In the following sections, we will discuss various methods to accomplish sex separation and sex ratio distortion with an emphasis on recent progress in mosquitoes (Table 1). We begin by discussing sex separation in the context of the classic genetic control method, sterile insect technique (SIT) [44]. SIT requires the mass rearing, sterilization and release of males into a designated area with the goal of reducing the fertility of the corresponding wild population. As females usually mate only once in their lifetime, mating with a sterile male will render the female sterile. Though not absolutely required for all pests, SIT programs stand to benefit substantially from a scalable sex-sorting technology, as factory-reared females do not contribute to the goals of the program, while their presence en masse requires resources in terms of diet and space within the rearing facility. In particular for mosquito SIT programmes, the release of females could also contribute to pathogen transmission and nuisance via biting. In addition, the release of females can decrease the efficacy of those strategies as they would be readily available to mate with the sterile males possibly preventing them to look for wild-type ones.
Table 1.
Sex separation and sex distortion methods in mosquitoes and other dipteran insects
| Mechanism/Strategy | Results | Species | Reference |
|---|---|---|---|
| Sexual dimorphism and other morphological markers | |||
|
| |||
| Pupal sieve and adult real-time visual sorter | Sex separation | Ae. aegypti | [49] |
| Pupal size sorter and eye color image analysis | Sex separation | Ae. aegypti | [51] |
|
| |||
| Manipulation of sex-determination factors | |||
|
| |||
| Expression of transgenic Guy1 | Female lethality | An. stephensi | [36] |
| Expression of transgenic Yob | Female lethality | An. gambiae | [39] |
| Expression of transgenic Nix | Female to male conversion | Ae. aegypti | [34] |
| Expression of transgenic Nix | Female to male conversion | Ae. albopictus | [35] |
| RNAi of dsxF via bacterial feeding | Female lethality | Ae. aegypti | [72] |
| RNAi of dsxF via bacterial feeding | Female lethality | An. gambiae | [75] |
| CRISPR/Cas9 homing gene drive affecting dsxF splicing | Sterile and intersex females | An. gambiae | [76] |
|
| |||
| Other means to cause female lethality or male bias | |||
|
| |||
| RNAi of female essential genes via Yeast feeding | Female lethality | Ae. aegypti | [55–57] |
| CRISPR/Cas9 targeting male fertility and female viability | Female lethal/intersex; sterile male | Drosophila | [58] |
| CRISPR/Cas9 targeting male fertility and female viability | Flightless female; sterile male | Ae. aegypti | [59] |
| Female-specific splicing of a tet-responsive tTa feedback loop | Conditional female lethal | Drosophila | [64] |
| Y-linked Cas9 targeting of essential genes | Female lethality | Drosophila | [70] |
| Transgenic RNAi knockdown of a male germline-specific Tra2 | Biased transmission of M-sperm | Ae. aegypti | [73] |
|
| |||
| X-shredder or X-poisoning during spermatogenesis | |||
|
| |||
| I-Ppol shredding of X-linked rDNA | Biased transmission of Y-sperm | An. gambiae | [81, 82] |
| Introgression of the I-Ppol X-shredder to a sibling species | Biased transmission of Y-sperm | An. arabiensis | [90] |
| CRISPR/Cas9 shredding of X-linked rDNA | Biased transmission of Y-sperm | An. gambiae | [83] |
| CRISPR/Cas9 X-poisoning of RpS5a/RpS6/Mucin14A | Female lethality | Drosophila | [85] |
| CRISPR/Cas9 X-shredding of X-specific kmers | Biased transmission of Y-sperm | C. capitata | [86] |
| I-Ppol X-shredder coupled with a gene drive targeting dsx | Combined effects of an X-shredder and a drive targeting dsxF splicing | An. gambiae | [78] |
Many SIT pilot studies have used methods for sex separation based on the sexual dimorphism of the pupal stages [45, 46], as the females are significantly larger than males in some species. Low female contamination rates of 0.02-0.10% were achieved using the Fay Morlan glass plate separator in pilot field trials that combined SIT with Wolbachia-mediated incompatible insect technique (IIT) to suppress Ae. aegypti [46]. Although mechanical separation methods can provide low female contamination, they are time consuming, increasing the cost associated with the techniques [47]. Moreover, they are not effective for all vector mosquitoes as pupal size overlaps substantially between sexes in Anopheles mosquitoes [48]. To overcome these obstacles, a combination of automation and machine learning was able to achieve essentially perfect sex separation as part of an IIT trial for Ae. aegypti [49]. While effective, this approach may be cost prohibitive in many places where Ae. aegypti control is most needed. The lack of strong sexual dimorphism in An. gambiae pupae was overcome by engineering a male specific promoter derived from the β2-tubulin gene to drive the expression of a green fluorescent protein (GFP) in the testes of transgenic mosquitoes [50]. Larvae of this transgenic line can then be separated into EGFP+ (male) and EGFP− (female) groups using a large particle flow sorter (COPAS). Although no female contamination was observed in this system, the cost of flow sorting systems and its relatively low throughput are substantial barriers for its usage. Most recently, a sex-linked spontaneous red-eye mutation is being explored for sex separation by eye color [51] and the determination that cardinal is the causal gene for this mutant [52] provides an opportunity to expand its use in other mosquito species.
Methods to selectively eliminate or incapacitate females
An alternative to separating males away from females is to apply methods that might selectively kill females. The earlier this lethality takes place the more cost effective it would be for the corresponding male release program. Simply offering a blood meal containing an insecticide like malathion or ivermectin to mixed populations exploits the specific blood feeding behavior of adult females. Although this method was designed to kill females only, males were affected as well (through contact with females or excretions) and overall separation efficiencies were low [53, 54]. Promising strategies of female killing have been pursued based on larval consumption of the yeast Saccharomyces cerevisiae engineered to deliver interfering RNA to specifically kill females. RNAi mediated silencing of gamma-glutamyl transpeptidase (GGT), methylthioribulose 1-phosphate dehydratase (MtnB) and lncRNA genes resulted in significant female death and no impact on male survival or fitness [55–57]. This system is advantageous as heat-killed yeast retain their ability to induce effective gene silencing, making packaging, shipping and storage of this diet straightforward and extremely cost-effective. However, improvements in this system are needed as single gene silencing did not completely eliminate the females; combined engineered yeast targeting multiple genes could potentially increase female mortality.
Other methods of sex separation have made use of genetic and transgenic technologies. Precision-guided SIT (pgSIT) [58, 59] and temperature-inducible pgSIT ([60]) utilize CRISPR/Cas9 to simultaneously sterilize males for population suppression and kill or incapacitate females for sex sorting. Initially developed in Drosophila [58], pgSIT was adapted to Ae. aegypti [59], where a cross between Cas9-expressing and guide RNA-expressing strains resulted in flightless females and sterile males by targeting myo-fem gene essential for female flight [61] and the β-tubulin gene essential for spermatogenesis [62]. In other work, the insertion of sex-specific introns into drug resistance genes led to the ability to select for either males or females based on the application of the corresponding drug, as upon splicing, the resistance genes are restored. This system has 100% accuracy in selection although the effect of antibiotic treatment on fitness for the non-selected sex should be further evaluated [63]. Similarly, a conditional female-lethality system was developed based on the differential splicing of a female sex differentiation factor to restore a gene. In this case the intron from the tra gene of D. melanogaster was used to disrupt the coding DNA sequence of the tetracycline transactivator (tTa) driven by several copies of the Tet operator (TetO) upstream of HsP70 promoter. Only females are able to splice this construct, restoring tTa that will bind to the TetO which in turn expresses more tTa, killing only the females [64]. This conditional female-lethality system was coupled with an engineered genetic incompatibility method based on creating mating incompatibility in a bidirectional manner (male to female, female to male) [65–67]. Both systems based on sex specific-splicing were developed for the proof-of-concept in the model organism Drosophila, thus it will be interesting to see these technologies adapted into vector species. These studies build on prior work from the past decade where the Tet-Off system was used to engineer female-specific flightless phenotypes in mosquitoes. Using an indirect flight muscle promoter derived by the Actin-4 gene to drive tTA, transgenic mosquitoes displayed a flightless phenotype that could be used to sort males from females and applied to RIDL (release of insects carrying a dominant lethal) systems [68, 69].
The ease and precision of CRISPR/Cas9-based methods to edit genomes has enabled the insertion of genes into the Y chromosome, which are more difficult to target since they are repeat-rich, heterochromatic, and have less highly conserved regions [18]. A novel method termed sex-linked CRISPR selection (SELECT) targeted an essential gene in Drosophila, DNA Polymerase gamma subunit 2 (PolG2), with a X- or Y-linked source of Cas9. Crosses between individuals expressing the gRNA against PolG2 and individuals expressing Cas9 selectively killed males or females, depending on whether the Cas9 source was X- or Y-linked enabling efficient sex selection [70]. The concept of using sex-linkage to achieve sex-specific killing was initially demonstrated by radiation induced translocation of a dieldrin-resistant gene to the Y chromosome, killing the females upon larval treatment allowing for male selection (reviewed in [71]). However, insecticide resistance is not considered a good selectable marker for field releases even when the vast majority of the males are sterile, due to the potential to introduce resistance genes into wild populations.
Sex distortion through the manipulation of factors involved in sex-determination
As described above, there are several techniques available for sex-separation in mosquitoes, ranging from simple mechanical separation to more elaborate methods involving complex transgene design. All have some limitations in terms of accuracy, cost, and applicability to new species, and the usage of each strategy will have to be evaluated in case-by-case scenario. Synthetic sex distorters are attractive new strategies that take advantage of recent advances in our knowledge of the sex determination pathway in mosquitoes. The An. stephensi male determining factor Guy1 was the first among mosquitoes to be used in this type of approach. Transgenic mosquitoes expressing Guy1 under either its native promoter or an early zygotic bZip1 promoter rendered 100% male transformants [36]. As noted above, rather than the dominant conversion of females to males, the sex bias observed was the result of complete female lethality in the presence of Guy1 [36]. Similarly, ectopic expression of Yob, the M factor in the An. gambiae, under the control of the vasa2 promoter also produced a significant male bias, also through female-lethality [39]. While in this case male bias was not 100%, it is possible that switching the vasa2 promoter for the endogenous Yob promoter could yield male-only transgenic progeny. In contrast, transgenic Ae. aegypti ectopically expressing Nix (male determining factor) under the control of its native promoter resulted in complete male bias arising from female to male conversion [34]. Successful conversion, rather than female lethality, may have been due to the fact that dosage compensation does not occur in Ae. aegypti. While either female lethality or sex conversion result in extreme male bias, all of these experiments have relied on the constitutive expression of the respective sex factor. As a consequence, maintenance of these strains requires continuous out-crossing, and in the case of Ae. aegypti, continuous sex separation since the transgene would only be inherited by half of the progeny. To translate these breakthroughs into products useful for population suppression programs, control of sex determination gene expression needs to be placed under repressible/activatable expression systems. This will enable the production of homozygous transgenic lines that substantially increase efficiency and allow large-scale production of male-only populations for genetic vector control programs. Alternatively, the fact that all transformants are males can be used for sex separation by the transformation marker [34, 35].
As an alternative to the dominant expression of male-determining factors, RNAi-based knockdown of transcripts involved in female development could also result in extreme male bias. Using the bacteria E. coli as an RNAi delivery method to silence the female isoform of doublesex (dsx) during the larval stages resulted in ~97 % sex bias towards males in the mosquito Ae. aegypti [72]. Sex ratio distortion was observed in transgenic mosquitoes expressing RNAi against one of the three tra2 genes in Ae. aegypti [73], while RNAi of the other two tra2 genes suppressed ovarian development in Ae. albopictus [74]. Feeding An. gambiae larvae with E. coli that produce dsRNA against dsx resulted in ~72% males [75]. While these studies involved transient silencing, transgenic expression of dsRNA would be expected to result in more scalable silencing. Such expression cassettes could also be made inducible/repressible to facilitate strain maintenance and mass rearing.
Homing-based gene-drive approaches have been developed targeting the autosomal sex-determination gene dsx [76]. In these experiments, CRISPR/Cas9 disruption of the intron-exon boundary critical only for the dsxF isoform led to intersex females that were unable to bloodfeed and died shortly after emergence. When introduced into small cage populations [76] or larger contained environments [77], successful homing-based gene-drive spread the engineered transgene to near fixation, with the resulting sex distortion causing elimination of egg production and crash of the colony. This process was accelerated by combining the autosomal dsx-gene-drive transgene with the I-PpoI X-shredding system as described below [78], providing a powerful tool that may be capable of suppressing malaria mosquito populations at regional scales.
Sex distortion through selective disruption of the X chromosome
The process of sex determination in mosquitoes leads to an approximate 1:1 sex ratio, however natural sex distorters known as meiotic drives have been found in the Aedes and Culex mosquitoes (Figure 3A, [12]). By definition, any change in the mechanics of the normal meiotic process where a heterozygote produces a bias in the production or transmission of the types of gametes outside of the expected 1:1 ratio is considered a meiotic drive. Sex distortion systems producing a preponderance of males can also be useful in population suppression programs in which male-only releases are required, avoiding the need for costly sex separation techniques. However, while a number of genes have been implicated as being differentially regulated in the presence or absence of meiotic drivers [79], the molecular basis of naturally occurring meiotic drives in these organisms remains poorly understood, and more research needs to be done in order to manipulate and develop systems useful for vector control.
Figure 3. Meiotic sex ratio distortion in Aedes aegypti (A) and Anopheles gambiae (B).
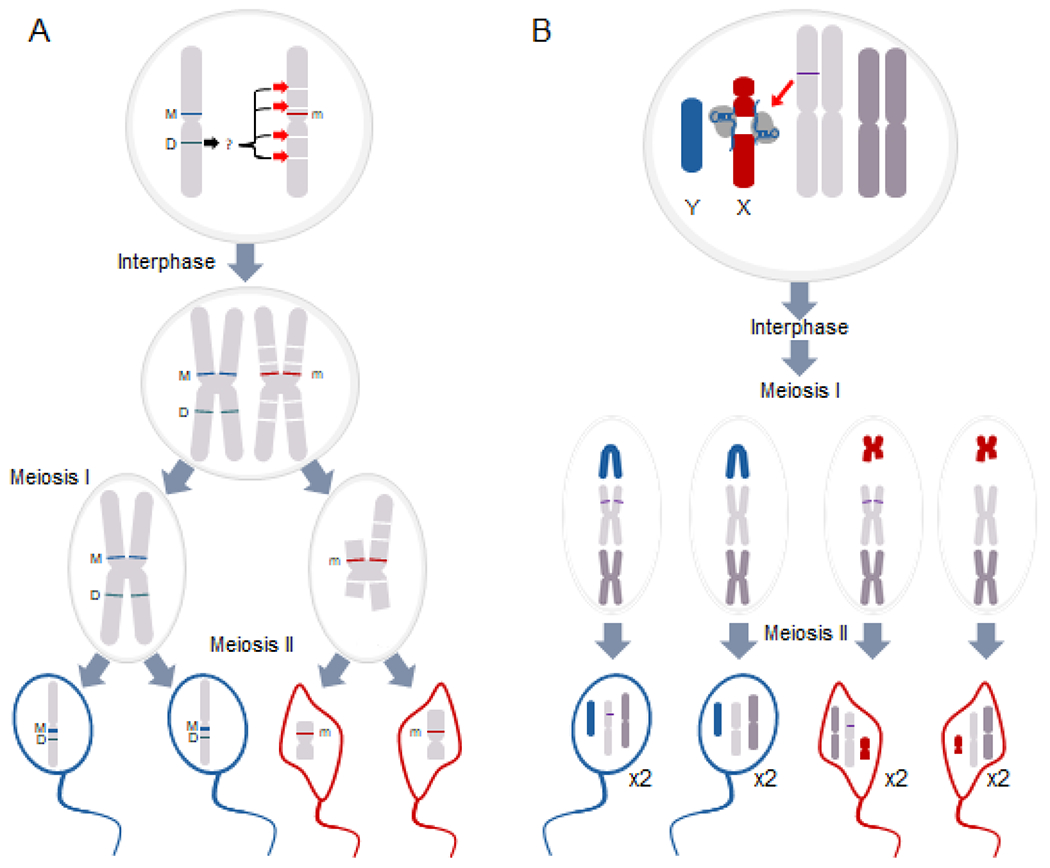
A) The naturally occurring meiotic drive distorts sex ratio in Aedes aegypti. It is comprised of a male-linked distorter (D) allele that targets drive sensitive alleles on the m chromosome for breakage during spermatogenesis [99]. Although the mechanism remains unknown, targeting of the sensitive alleles results in m chromosome breakage and disrupts the formation of m chromosome-bearing sperm, favoring transmission of the M-bearing (and D-bearing) sperm and causing male-bias in the progeny. B) An engineered X-shredder in An. gambiae (purple line on one of the autosomes) uses either an I-PpoI homing endonuclease or the RNA-guided Cas9 endonuclease (depicted as Cas9) to target an rDNA gene cluster located exclusively on the X chromosome. This reduces transmission of the X-bearing sperm and results in a sex ratio bias in the following generation. Since the shredder allele is not on the sex chromosome, the inheritance of the shredder allele follows Mendelian segregation and is not sex biased.
The differentiation of sex chromosomes into heteromorphic forms opens up a critical vulnerability during meiotic development. For XY systems like those in malaria mosquitoes, selective destruction of X-bearing gametes has the potential to yield male-only progeny (Figure 3B). The development of such X-shredding systems thus relies only on the discovery of X-specific sequences and the means to generate double-stranded DNA breaks at these targets. Fortuitously, in several malaria vectors including An. gambiae the ribosomal DNA repeats (rDNA) are localized to the X chromosome, providing a critical set of targets. As the homing-endonuclease I-PpoI targets a 15 bp sequence conserved in eukaryotic rDNA [80] transgenic expression of this nuclease in An. gambiae testis was evaluated for the ability to achieve sex distortion [81]. Surprisingly, I-PpoI expressing males were found to be completely sterile, due to the unexpected deposition of I-PpoI enzyme carried by transgenic sperm into the fertilized egg, where the maternal X chromosome was also targeted [81]. Structure-guided mutagenesis of the I-PpoI enzyme produced a version with a substantially shorter half-life, and the transgenic expression of this modified nuclease resulted in robust sex distortion, with ~95% of progeny being male [82]. Similar results were obtained by using CRISPR/Cas9 as the nuclease [83].
The identification of new target sites beyond the rDNA repeats has been facilitated by specialized bioinformatics pipelines [84], making this approach potentially generalizable to other vectors. While targeting X-specific repeats in Drosophila resulting in only mild sex distortion [85], similar experiments in the medfly, Ceratitis capitata, approached ~80% male bias [86]. At the same time, nuclease targeting of a unique haploinsufficient X-linked gene was able to achieve ~95% male bias [85], suggesting that simple DSBs alone are insufficient to destabilize the X chromosome. Indeed, sex distortion increased when end-joining repair was inhibited [85], suggesting existing metabolic processes can cope with even a few hundred interspersed DSBs, and that the resulting inactivation of critical genes (rDNA or otherwise) is a substantial contributing factor.
The extreme male bias generated by I-PpoI-based X-shredders has been shown to cause the collapse of small cage populations reared as discrete generations [82], and strongly suppress populations reared in indoor enclosures consisting of stable overlapping generations [87, 88]. As the source transgene in these experiments is integrated autosomally, this approach is inherently self-limiting, with the transgene predicted to become rare shortly after releases cease, and disappear completely within several years [88]. Attempts to generate Y-linked X-shredders, which would be self-sustaining rather than self-limiting have not been successful, as the Y-chromosome in An. gambiae is transcriptionally inactivated during meiosis, preventing expression from the Y-linked transgene [89].
As malaria transmission is sustained in sub-Saharan Africa by multiple vectors within the An. gambiae species complex, the suppression of a single species through a powerful sex distortion approach such as I-PpoI may be insufficient to impact transmission. To address this, Bernardini et al (2019) [90] successfully introgressed the integrated I-PpoI transgene from An. gambiae into Anopheles arabiensis through F1 hybrid transgenic females that were backcrossed with the parental An. arabiensis strain. Male bias consistently exceeded 95% over the course of 40 generations, confirming that sex distortion approaches could be used to control multiple vectors of malaria. Even in cases where rDNA repeats are present on both X and Y chromosomes, sex distortion can still be observed provided the corresponding area of targets is larger on the X [91].
Concluding remarks
The past several years has witnessed significant progress in the fundamental knowledge of mosquito sex chromosomes and sex determination and in the methods to achieve sex separation and sex ratio distortion to control mosquito-borne infectious diseases. We suggest a few critical areas for future research (see Outstanding Questions). As described earlier, the Y chromosomes of the vast majority of the Anopheles mosquitoes remain essentially uncharacterized, which hinders our understanding of male mosquito biology and the development of sex-related genetic manipulations. New sequencing and scaffolding methods are significantly better at assembling repeat-rich regions of the genome (reviewed in [92]) and thus provide a great opportunity to both improve the X chromosome assembly in its heterochromatic regions and to actually produce a Y chromosome assembly for evolutionary divergent Anopheles mosquitoes. Although the M and m loci of the homomorphic sex chromosomes have been assembled in Ae. aegypti, a representative of the Culicinae subfamily, gaps and uncertainties remain both within and at the borders of the sex loci [22]. Haploid genome assemblies are so far the most accurate reflection of the diploid genome [93], which will help close the gaps and resolve the uncertainties. In addition, haplotype-resolved assemblies will reveal the molecular basis underlying the high levels of differentiation between the ~100 Mb M- and m-linked regions of the homomorphic sex chromosomes (e.g., [22]). These results will have direct impact on identifying m-chromosome-specific sequences for sex ratio distortion through m-shredding, and increase knock-in efficiency in M-linked sequences by specifically targeting the M-linked haplotypes [94]. Therefore, beyond revealing the pattern of sex-linked sequence differentiation, haplotype-resolved assemblies will enable new ways to study allele-specific expression, long-range allelic interaction, genetic recombination, and epigenetic inheritance (e.g., [95]). As accurate genomic characterizations of both the homomorphic and heteromorphic sex chromosomes accumulate across the mosquito family, significant insights will be gained on the evolutionary dynamics of these two types of sex chromosomes in this important taxon.
Outstanding questions.
What are the complete content and fine structure of the mosquito Y chromosomes and male-determining loci, and how have they been shaped through evolution across mosquito species?
What other essential factors are required to establish complete female to male conversion to produce fully competitive males?
Can improvements in conditional expression systems afford simple and effective control of sex ratio distortion methods?
What are the molecular and biochemical mechanisms that transduce the sex determining signal from the key initiating factors to dsx and fru splicing?
The timing of female-specific lethality should be considered in the context of specific applications. Separation or female killing approaches that act at the pupal or adult stages result in the needless rearing of female larvae at substantial space and cost. New approaches that result in early larval-, or better, early embryonic-lethality would be preferred to avoid these resource-intensive investments into material that will be ultimately discarded. On the other hand, female killing approaches that act at the late larval, pupal or adult stages will be beneficial when density-dependent competition is important in field applications. As an alternative to sex separation or female lethality, sex conversion approaches also potentially avoid the problem of rearing material not needed for releases. Moreover, the release of homozygous males carrying a sex-conversion unit is, according to models, more effective than SIT and the female-killing methods in suppressing mosquito populations [96]. However, this requires that pseudomales (converted females) possess fitness characteristics on par with their genetic male siblings. This has not been the case so far, as shown by expression of the sex conversion factor Nix in Ae. aegypti (flightless, [34]) and Ae. albopictus (decreased mating competitiveness, [35]). Thus, future work should establish additional components needed to generate fully converted, fully fit pseudo-males.
Progress in genetic systems that result in X-shredding, female lethality or male sterility in mosquitoes has been strong. However, most of these systems have been expressed constitutively, making their maintenance in the factory setting impractical as they require continuous outcrossing and sorting. While some recent work in D. melanogaster has taken advantage of heat-inducible systems [64], and the main RIDL approach is tet-repressible, more work is needed to combine conditional expression methods with these promising genetic approaches. Ideally, female lethal factors or male-programming genes would be repressed in a homozygous strain to allow simple maintenance of the parental strain, with a release from repression (removal of repressor) allowing the large-scale production of sterile or x-shredding males.
We are only beginning to investigate the molecular and biochemical mechanisms that underlie the complex sex-determination pathway in mosquitoes that transduce the primary and dominant male-determining signals to effect sex-specific splicing of dsx and fru. In this regard, integrating new biochemical methods that facilitate the characterization of protein-protein and protein-RNA interactions with current genetic methods will be fruitful (e.g.,[43]). In addition, a better understanding of the sex-determination pathway and its connection to dosage compensation in Anopheles mosquitoes [37, 40] may point to ways to de-couple sex-determination and dosage compensation to accomplish sex conversion instead of sex-specific lethality. Progress in the characterization of sex chromosomes in mosquitoes uncovered genes in the M locus and Y chromosome in addition to the M factor. One such gene myo-sex is required for male flight in Ae. aegypti. However, the function of all other M-locus genes and Y chromosome genes remain unknown. It will be interesting to see if they play roles in male fertility and male competitiveness.
Highlights.
Primary and downstream sex determination signaling molecules have been identified in critical mosquito vectors
3rd generation sequencing approaches are enabling the assembly and analysis of repetitive sex chromosome regions
Transgenic, CRISPR/Cas9 and RNAi approaches are enabling new methods to achieve sex separation and sex ratio distortion to help control mosquito-borne infectious diseases.
Acknowledgement
We thank the three reviewers for helpful comments. This work was supported by grants AI157491, AI154871, AI123338 and AI121284 from the National Institutes of Health, by the Virginia Experimental Station, Texas A&M Agrilife Research (Insect Vectored Diseases Grant Program), and by the USDA National Institute of Food and Agriculture, Hatch project 1018401. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the USDA.
Glossary
- Aedes aegypti
primary vector of arboviruses such as dengue virus, yellow fever virus, Zika virus and chikungunya virus, highly adapted to urban environments.
- Aedes albopictus
vector of arboviruses such as dengue virus, Zika virus, and chikungunya virus, commonly found in peri-urban and suburban environments.
- Anopheles gambiae
most important vector of human malaria parasites in the Afrotropical region.
- CRISPR/Cas9
site-specific genetic technology used to generate double-stranded breaks in genomic DNA; repair of these breaks can result in the insertion or deletion of DNA sequences.
- Fay Morlan glass plate separator
device consisting of two glass plates with an adjustable wedge shape space between them used to separate the developmental stages and sexes of mosquitoes based on size.
- Homozygous
presence of two identical alleles at corresponding loci.
- I-PpoI
intron-encoded endonuclease from the slime mold Physarum polycephalum.
- Incompatible insect technique (IIT)
insect pest control method involving a mass release of Wolbachia-infected males with the goal of producing no offspring after successful mating with wild-type females.
- Large particle flow sorter (COPAS)
Flow cytometry equipment that sorts and dispenses viable multicellular organisms based on size and fluorescent parameters.
- Precision-guided SIT (pgSIT)
genetic system that utilizes CRISPR/Cas9 technology to generate sterilized male mosquitoes for use in the sterile insect technique.
- RNAi mediated silencing
a method for silencing gene expression based on the sequence-specific degradation of double-stranded RNA and the subsequent targeting of corresponding messenger RNA.
- Sex determination
process that designates the sex of an organism.
- Sterile insect technique (SIT)
insect pest control method involving a mass release of sterile males with the goal of producing no offspring after successful mating with wild-type females.
- Synthetic sex distorters
engineered genetic system that favors the production of one sex, altering normal sex ratios.
- Tet-Off system
transgenic conditional expression system that is dependent on the antibiotic tetracycline or tetracycline-analogs to inhibit gene expression.
- X-shredders
system to distort sex ratio via the incapacitation of X-bearing sperm resulting in a male bias.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare no competing interests.
References
- 1.Paz-Bailey G et al. (2021) Dengue Vaccine: Recommendations of the Advisory Committee on Immunization Practices, United States, 2021. MMWR Recomm Rep 70 (6), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Datoo MS et al. (2021) Efficacy of a low-dose candidate malaria vaccine, R21 in adjuvant Matrix-M, with seasonal administration to children in Burkina Faso: a randomised controlled trial. Lancet 397 (10287), 1809–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nolan T (2021) Control of malaria-transmitting mosquitoes using gene drives. Philos Trans R Soc Lond B Biol Sci 376 (1818), 20190803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adelman ZN and Tu Z (2016) Control of Mosquito-Borne Infectious Diseases: Sex and Gene Drive. Trends Parasitol 32 (3), 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lutrat C et al. (2019) Sex Sorting for Pest Control: It’s Raining Men! Trends Parasitol 35 (8), 649–662. [DOI] [PubMed] [Google Scholar]
- 6.Devos Y et al. (2021) Gene Drive-Modified Organisms: Developing Practical Risk Assessment Guidance. Trends Biotechnol 39 (9), 853–856. [DOI] [PubMed] [Google Scholar]
- 7.Wang GH et al. (2021) Combating mosquito-borne diseases using genetic control technologies. Nat Commun 12 (1), 4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rasic G et al. (2021) Monitoring Needs for Gene Drive Mosquito Projects: Lessons From Vector Control Field Trials and Invasive Species. Front Genet 12, 780327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harbach RE (2007) The Culicidae (Diptera): a review of taxonomy, classification and phylogeny. Zootaxa 1668 (1). [Google Scholar]
- 10.Motara MA and Rai KS (1977) Chromossomal differentiation in two species of Aedes and their hybrids revealed by Giemsa C-banding. Chromossoma (Berlin) 64, 125–132. [Google Scholar]
- 11.Motara MA and Rai K (1978) Giemsa C-banding patterns in Aedes (Stegomyia) mosquitoes. Chromosoma 70, 51–58. [Google Scholar]
- 12.Biedler JK and Tu Z (2016) Chapter Two - Sex Determination in Mosquitoes. In Advances in Insect Physiology (Raikhel AS ed), pp. 37–66, Academic Press. [Google Scholar]
- 13.Holt RA et al. (2002) The genome sequence of the malaria mosquito Anopheles gambiae. Science 298 (5591), 129–49. [DOI] [PubMed] [Google Scholar]
- 14.Nene V et al. (2007) Genome sequence of Aedes aegypti, a major arbovirus vector. Science 316 (5832), 1718–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arensburger P et al. (2010) Sequencing of Culex quinquefasciatus establishes a platform for mosquito comparative genomics. Science 330 (6000), 86–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Criscione F et al. (2013) A unique Y gene in the Asian malaria mosquito Anopheles stephensi encodes a small lysine-rich protein and is transcribed at the onset of embryonic development. Insect Mol Biol 22 (4), 433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall AB et al. (2013) Six novel Y chromosome genes in Anopheles mosquitoes discovered by independently sequencing males and females. BMC Genomics 14, 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall AB et al. (2016) Radical remodeling of the Y chromosome in a recent radiation of malaria mosquitoes. Proceedings of the National Academy of Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krzywinska E et al. (2016) A maleness gene in the malaria mosquito Anopheles gambiae. Science 353 (6294), 67–9. [DOI] [PubMed] [Google Scholar]
- 20.Chakraborty M et al. (2021) Hidden genomic features of an invasive malaria vector, Anopheles stephensi, revealed by a chromosome-level genome assembly. BMC Biol 19 (1), 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zamyatin A et al. (2021) Chromosome-level genome assemblies of the malaria vectors Anopheles coluzzii and Anopheles arabiensis. Gigascience 10 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthews BJ et al. (2018) Improved reference genome of Aedes aegypti informs arbovirus vector control. Nature 563 (7732), 501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dudchenko O et al. (2017) De novo assembly of the Aedes aegypti genome using Hi-C yields chromosome-length scaffolds. Science 356 (6333), 92–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall AB et al. (2015) SEX DETERMINATION. A male-determining factor in the mosquito Aedes aegypti. Science 348 (6240), 1268–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall AB et al. (2014) Insights into the preservation of the homomorphic sex-determining chromosome of Aedes aegypti from the discovery of a male-biased gene tightly linked to the M-locus. Genome Biol Evol 6 (1), 179–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juneja P et al. (2014) Assembly of the genome of the disease vector Aedes aegypti onto a genetic linkage map allows mapping of genes affecting disease transmission. PLoS Negl Trop Dis 8 (1), e2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fontaine A et al. (2017) Extensive Genetic Differentiation between Homomorphic Sex Chromosomes in the Mosquito Vector, Aedes aegypti. Genome Biol Evol 9 (9), 2322–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hopkins B and Kopp A (2021) Evolution of sexual development and sexual dimorphism in insects. Current Opinion in Genetics & Development 69, 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomulski LM et al. (2018) The Nix locus on the male-specific homologue of chromosome 1 in Aedes albopictus is a strong candidate for a male-determining factor. Parasites & Vectors 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller JR et al. (2018) Analysis of the Aedes albopictus C6/36 genome provides insight into cell line utility for viral propagation. Gigascience 7 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palatini U et al. (2020) Improved reference genome of the arboviral vector Aedes albopictus. Genome Biology 21 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu PW et al. (2020) Nix is a male-determining factor in the Asian tiger mosquito Aedes albopictus. Insect Biochemistry and Molecular Biology 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin B et al. (2021) Alternative splicing patterns of doublesex reveal a missing link between Nix and doublesex in the sex determination cascade of Aedes albopictus. Insect Sci 28 (6), 1601–1620. [DOI] [PubMed] [Google Scholar]
- 34.Aryan A et al. (2020) Nix alone is sufficient to convert female Aedes aegypti into fertile males and myo-sex is needed for male flight. Proc Natl Acad Sci U S A 117 (30), 17702–17709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lutrat C et al. (2022) Transgenic expression of Nix converts genetic females into males and allows automated sex sorting in Aedes albopictus. Communications Biology 5 (1), 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Criscione F et al. (2016) GUY1 confers complete female lethality and is a strong candidate for a male-determining factor in Anopheles stephensi. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qi Y et al. (2019) Guy1, a Y-linked embryonic signal, regulates dosage compensation in Anopheles stephensi by increasing X gene expression. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang XF et al. (2015) Complete Dosage Compensation in Anopheles stephensi and the Evolution of Sex-Biased Genes in Mosquitoes. Genome Biology and Evolution 7 (7), 1914–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krzywinska E and Krzywinski J (2018) Effects of stable ectopic expression of the primary sex determination gene Yob in the mosquito Anopheles gambiae. Parasit Vectors 11 (Suppl 2), 648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krzywinska E et al. (2021) femaleless Controls Sex Determination and Dosage Compensation Pathways in Females of Anopheles Mosquitoes. Curr Biol 31 (5), 1084–1091 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meccariello A et al. (2019) Maleness-on-the-Y (MoY) orchestrates male sex determination in major agricultural fruit fly pests. Science 365 (6460), 1457–1460. [DOI] [PubMed] [Google Scholar]
- 42.Cline TW (1978) Two closely linked mutations in Drosophila melanogaster that are lethal to opposite sexes and interact with daughterless. Genetics 90 (4), 683–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhuo JC et al. (2021) A feminizing switch in a hemimetabolous insect. Sci Adv 7 (48), eabf9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knipling EF (1955) Possibilities of insect control or eradication through the use of sexually sterile males. J Econ Entomol 48, 459–469. [Google Scholar]
- 45.Bellini R et al. (2021) Field Competitiveness of Aedes albopictus (Diptera: Culicidae) Irradiated Males in Pilot Sterile Insect Technique Trials in Northern Italy. J Med Entomol 58 (2), 807–813. [DOI] [PubMed] [Google Scholar]
- 46.Kittayapong P et al. (2018) Combined sterile insect technique and incompatible insect technique: sex separation and quality of sterile Aedes aegypti male mosquitoes released in a pilot population suppression trial in Thailand. Parasit Vectors 11 (Suppl 2), 657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Araujo HRC et al. (2018) Sex determination and Aedes population control. Parasit Vectors 11 (Suppl 2), 644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dame DA et al. (1974) Release of chemosterilized males for the control of Anopheles albimanus in El Salvador. II. Methods of rearing, sterilization, and distribution. Am J Trop Med Hyg 23 (2), 282–7. [DOI] [PubMed] [Google Scholar]
- 49.Crawford JE et al. (2020) Efficient production of male Wolbachia-infected Aedes aegypti mosquitoes enables large-scale suppression of wild populations. Nat Biotechnol 38 (4), 482–492. [DOI] [PubMed] [Google Scholar]
- 50.Catteruccia F et al. (2005) An Anopheles transgenic sexing strain for vector control. Nat Biotechnol 23 (11), 1414–7. [DOI] [PubMed] [Google Scholar]
- 51.Koskinioti P et al. (2021) Genetic sexing strains for the population suppression of the mosquito vector Aedes aegypti. Philos Trans R Soc Lond B Biol Sci 376 (1818), 20190808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen C et al. (2021) Marker-assisted mapping enables effective forward genetic analysis in the arboviral vector Aedes aegypti, a species with vast recombination deserts. bioRxiv, 2021.04.29.442065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lowe RE et al. (1981) Separation of sexes of adult Anopheles albimanus by feeding of insecticide-laden blood. Mosquito News 41 (4), 634–638. [Google Scholar]
- 54.Yamada H et al. (2013) Eliminating female Anopheles arabiensis by spiking blood meals with toxicants as a sex separation method in the context of the sterile insect technique. Parasit Vectors 6, 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mysore K et al. (2021) A functional requirement for sex-determination M/m locus region IncRNA genes in Aedes aegypti female larvae. Sci Rep 11 (1), 10657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mysore K et al. (2022) A Conserved Female-Specific Requirement for the GGT Gene in Mosquito Larvae Facilitates RNAi-Mediated Sex Separation in Multiple Species of Disease Vector Mosquitoes. Pathogens 11, 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mysore K et al. (2021) A conserved female-specific larval requirement for MtnB function facilitates sex separation in multiple species of disease vector mosquitoes. Parasit Vectors 14 (1), 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kandul NP et al. (2019) Transforming insect population control with precision guided sterile males with demonstration in flies. Nat Commun 10 (1), 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li M et al. (2021) Suppressing mosquito populations with precision guided sterile males. Nat Commun 12 (1), 5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kandul NP et al. (2021) Temperature-Inducible Precision Guided Sterile Insect Technique. bioRxiv, 2021.06.14.448312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O’Leary S and Adelman ZN (2020) CRISPR/Cas9 knockout of female-biased genes AeAct-4 or myo-fem in Ae. aegypti results in a flightless phenotype in female, but not male mosquitoes. PLOS Neglected Tropical Diseases 14 (12), e0008971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen J et al. (2021) Suppression of female fertility in Aedes aegypti with a CRISPR-targeted male-sterile mutation. Proc Natl Acad Sci U S A 118 (22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kandul NP et al. (2020) A drug-inducible sex-separation technique for insects. Nat Commun 11 (1), 2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Upadhyay A et al. (2022) Genetically engineered insects with sex-selection and genetic incompatibility enable population suppression. Elife 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Waters AJ et al. (2018) Rationally-engineered reproductive barriers using CRISPR & CRISPRa: an evaluation of the synthetic species concept in Drosophila melanogaster. Sci Rep 8 (1), 13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buchman A et al. (2021) Engineered reproductively isolated species drive reversible population replacement. Nat Commun 12 (1), 3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maselko M et al. (2020) Engineering multiple species-like genetic incompatibilities in insects. Nat Commun 11 (1), 4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fu G et al. (2010) Female-specific flightless phenotype for mosquito control. Proc Natl Acad Sci U S A 107 (10), 4550–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marinotti O et al. (2013) Development of a population suppression strain of the human malaria vector mosquito, Anopheles stephensi. Malar J 12, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gamez S et al. (2021) Exploiting a Y chromosome-linked Cas9 for sex selection and gene drive. Nat Commun 12 (1), 7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bernardini F et al. (2018) Molecular tools and genetic markers for the generation of transgenic sexing strains in Anopheline mosquitoes. Parasites & vectors 11 (Suppl 2), 660–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Whyard S et al. (2015) Silencing the buzz: a new approach to population suppression of mosquitoes by feeding larvae double-stranded RNAs. Parasit Vectors 8, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoang KP et al. (2016) Mechanisms of sex determination and transmission ratio distortion in Aedes aegypti. Parasit Vectors 9, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li X et al. (2019) Two of the three Transformer-2 genes are required for ovarian development in Aedes albopictus. Insect Biochem Mol Biol 109, 92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taracena ML et al. (2019) Downregulation of female doublesex expression by oral-mediated RNA interference reduces number and fitness of Anopheles gambiae adult females. Parasit Vectors 12 (1), 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kyrou K et al. (2018) A CRISPR-Cas9 gene drive targeting doublesex causes complete population suppression in caged Anopheles gambiae mosquitoes. Nat Biotechnol 36 (11), 1062–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hammond A et al. (2021) Gene-drive suppression of mosquito populations in large cages as a bridge between lab and field. Nature Communications 12 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Simoni A et al. (2020) A male-biased sex-distorter gene drive for the human malaria vector Anopheles gambiae. Nat Biotechnol 38 (9), 1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shin D et al. (2019) Genome-Wide Transcriptome Profiling Reveals Genes Associated with Meiotic Drive System of Aedes aegypti. Insects 10 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nomura N et al. (2008) Recognition of a common rDNA target site in archaea and eukarya by analogous LAGLIDADG and His-Cys box homing endonucleases. Nucleic Acids Res 36 (22), 6988–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Windbichler N et al. (2008) Targeting the X Chromosome during Spermatogenesis Induces Y Chromosome Transmission Ratio Distortion and Early Dominant Embryo Lethality in Anopheles gambiae. Plos Genetics 4 (12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Galizi R et al. (2014) A synthetic sex ratio distortion system for the control of the human malaria mosquito. Nat Commun 5, 3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Galizi R et al. (2016) A CRISPR-Cas9 sex-ratio distortion system for genetic control. Sci Rep 6, 31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Papathanos PA and Windbichler N (2018) Redkmer: An Assembly-Free Pipeline for the Identification of Abundant and Specific X-Chromosome Target Sequences for X-Shredding by CRISPR Endonucleases. Crispr Journal 1 (1), 88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fasulo B et al. (2020) A fly model establishes distinct mechanisms for synthetic CRISPR/Cas9 sex distorters. PLoS Genet 16 (3), e1008647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Meccariello A et al. (2021) Engineered sex ratio distortion by X-shredding in the global agricultural pest Ceratitis capitata. BMC Biol 19 (1), 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Facchinelli L et al. (2019) Large-cage assessment of a transgenic sex-ratio distortion strain on populations of an African malaria vector. Parasites & Vectors 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pollegioni P et al. (2020) Detecting the population dynamics of an autosomal sex ratio distorter transgene in malariavector mosquitoes. Journal of Applied Ecology 57 (10), 2086–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alcalay Y et al. (2021) The Potential for a Released Autosomal X-Shredder Becoming a Driving-Y Chromosome and Invasively Suppressing Wild Populations of Malaria Mosquitoes. Front Bioeng Biotechnol 9, 752253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bernardini F et al. (2019) Introgression of a synthetic sex ratio distortion system from Anopheles gambiae into Anopheles arabiensis. Sci Rep 9 (1), 5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Haghighat-Khah RE et al. (2020) Cellular mechanisms regulating synthetic sex ratio distortion in the Anopheles gambiae germline. Pathogens and Global Health 114 (7), 370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Compton A et al. (2020) Recent advances and future perspectives in vector-omics. Curr Opin Insect Sci 40, 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cheng HY et al. (2021) Haplotype-resolved de novo assembly using phased assembly graphs with hifiasm. Nature Methods 18 (2), 170-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ang JX et al. (2022) Considerations for homology-based DNA repair in mosquitoes: Impact of sequence heterology and donor template source. PLoS Genet 18 (2), e1010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Garg S et al. (2021) Chromosome-scale, haplotype-resolved assembly of human genomes. Nature Biotechnology 39 (3), 309–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schliekelman P et al. (2005) Pest control by genetic manipulation of sex ratio. Journal of economic entomology 98, 18–34. [DOI] [PubMed] [Google Scholar]
- 97.Smit AFA et al. (2013-2015) RepeatMasker Open-4.0. http://www.repeatmasker.org, (accessed).
- 98.Salvemini M et al. (2011) Genomic organization and splicing evolution of the doublesex gene, a Drosophila regulator of sexual differentiation, in the dengue and yellow fever mosquito Aedes aegypti. Bmc Evolutionary Biology 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Newton ME et al. (1976) A cytogenetic analysis of meiotic drive in the mosquito, Aedes aegypti (L.). Genetica 46, 297–318. [Google Scholar]


