Abstract
The radionuclides 225Ac3+ and 213Bi3+ possess favorable physical properties for targeted alpha therapy (TAT), a therapeutic approach that leverages α radiation to treat cancers. A chelator that effectively binds and retains these radionuclides is required for this application. The development of ligands that can be used for this purpose, however, is challenging because the large ionic radii and charge-diffuse nature of these metal ions give rise to weaker metal-ligand interactions. In this study, we evaluated two 18-membered macrocyclic chelators, macrodipa and py-macrodipa, for their ability to complex 225Ac3+ and 213Bi3+. Their coordination chemistry with Ac3+ was probed computationally and with Bi3+ experimentally via NMR spectroscopy and X-ray crystallography. Furthermore, radiolabeling studies were conducted, revealing the efficient incorporation of both 225Ac3+ and 213Bi3+ by py-macrodipa that matches or surpasses the well-known chelators macropa and DOTA. Incubation in human serum at 37 °C showed that ~90% of the 225Ac3+–py-macrodipa complex dissociates after 1 d. The Bi3+–py-macrodipa complex possesses remarkable kinetic inertness in an EDTA transchelation challenge study, surpassing that of Bi3+–macropa. This work establishes py-macrodipa as a valuable candidate for 213Bi3+ TAT, providing further motivation for its implementation within new radiopharmaceutical agents.
Graphical Abstract
Targeted alpha therapy (TAT) is a promising therapeutic strategy that leverages α-particle-emitting radionuclides to annihilate tumor cells. Compared to conventional internal radiotherapy using β-particle emitters, the implementation of significantly more massive α particles, which deposit their energy over much shorter distances, provides key advantages. The short range of α radiation can yield enhanced selectivity for targeted cancer cells, while minimizing damage to surrounding healthy cells. Moreover, the very large linear energy transfer (LET) of α particles is significantly more effective in causing lethal DNA double strand breaks that kill cancer cells in a more efficacious manner compared to the lower-LET β particles.1-6
To date, over eight radionuclides have been identified as potential candidates for use in TAT based on their decay properties and production routes.7 Among these nuclides, 225Ac3+ and 213Bi3+ have received considerable attention that has manifested in clinical studies.8-10 225Ac (t1/2 = 9.9 d) emits four α particles through its decay chain, a property that confers it with high cytotoxic potency. Its 9.9-day half-life is also well matched with the in vivo circulation timescales of macromolecular targeting vectors like antibodies.11,12 213Bi (t1/2 = 45.6 min), a daughter of 225Ac3+, emits one α particle through its decay chain and can be conveniently obtained from 225Ac/213Bi generators.13 Its shorter half-life can be optimally matched to small-molecule targeting vectors, rendering it useful for different systems than those used for 225Ac3+.14,15
To convert these promising radionuclides into useful radiotherapeutic agents, a chelator that efficiently binds and stably retains them is required.16,17 The development of chelators for large metal ions like Ac3+ and Bi3+, however, is challenging, partly because their low charge density weakens electrostatic interactions with ligand donor atoms.
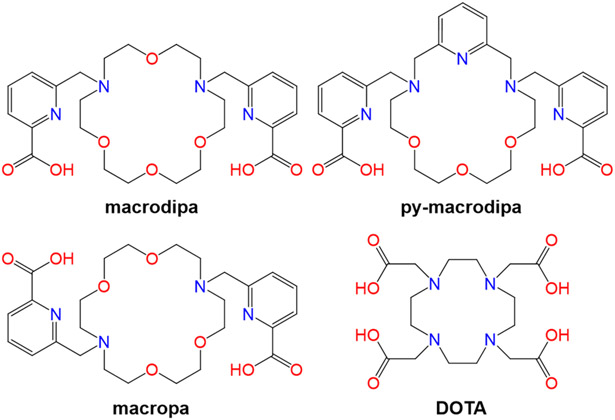
We recently reported a new ligand called macrodipa18 and its second-generation analogue py-macrodipa19 (Chart 1). These “macrodipa-type” chelators feature a unique “dual size selectivity”, characterized by their good affinities for both the large and small rare-earth metal ions (Ln3+). This unusual selectivity profile arises from a significant conformational toggle that occurs when they form complexes with Ln3+ ions of different sizes. Large Ln3+ form 10-coordinate, nearly C2-symmetric complexes (Conformation A), whereas an 8-coordinate, asymmetric complex arises for small Ln3+ (Conformation B).18,19 We have further demonstrated that this property makes py-macrodipa a valuable candidate for nuclear medicine applications with both 135La3+ and 44Sc3+, Ln3+ radiometal ions with the largest and smallest ionic radii within this series.19
Chart 1.
Structures of Chelators Discussed in This Work.
Based on this successful application of macrodipa and py-macrodipa for the Ln3+ ions, we sought to evaluate these ligands with biomedically relevant ions beyond the Ln3+ series, namely Ac3+ and Bi3+. The potentials of both chelators for TAT applications using their radioisotopes 225Ac3+ and 213Bi3+ were determined and benchmarked to those of the well-known chelators macropa and DOTA (Chart 1), which have established precedence for nuclear medicine applications with these radiometals.20-23
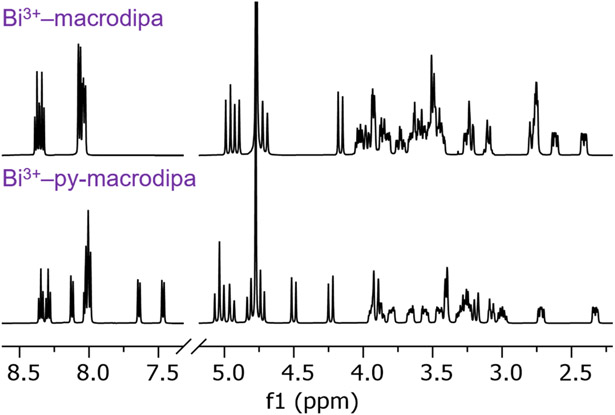
We assessed the coordination chemistry of these ligands with stable Bi3+. The 1H and 13C{1H} NMR spectra of their Bi3+ complexes (Bi3+–macrodipa and Bi3+–py-macrodipa) were acquired in D2O (Figures 1 and S1-S4). These spectra reveal the presence of a single, well-resolved species that lacks symmetry for both complexes. Thus, Bi3+–macrodipa and Bi3+–py-macrodipa most likely attain the asymmetric Conformation B, which is the preferred binding mode of these ligands for small Ln3+ (Figure S5-S6).
Figure 1.
1H NMR spectra of Bi3+–macrodipa and Bi3+–py-macrodipa (500 MHz, D2O, pD 5, 25 °C).
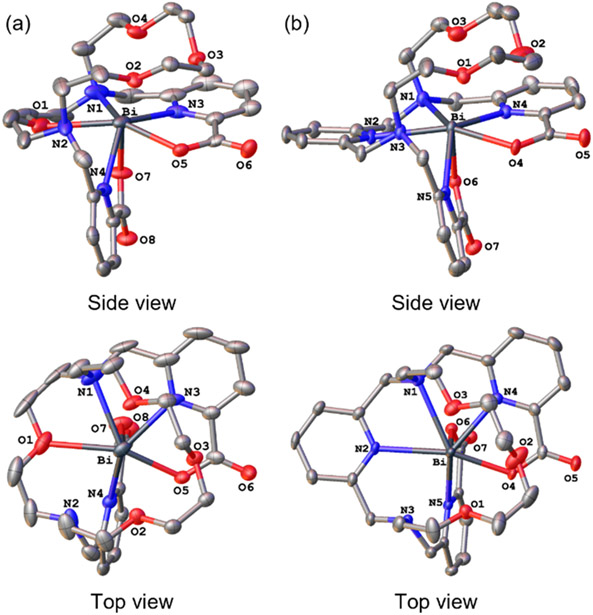
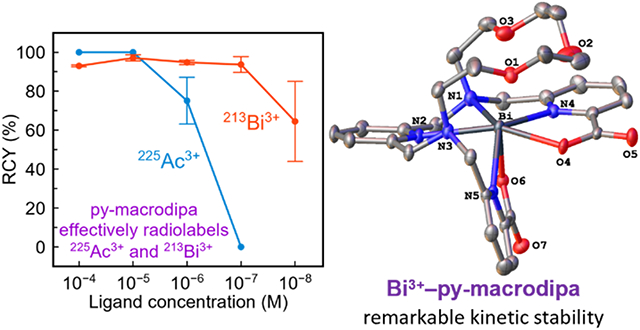
As further validation, we characterized Bi3+–macrodipa and Bi3+–py-macrodipa by X-ray crystallography (Figure 2). The crystal structures of these complexes confirm that they attain the asymmetric Conformation B, consistent with our observations from NMR spectroscopy. Like their NMR spectra, these Bi3+ structures are comparable to those of the small Ln3+ analogues, Lu3+–macrodipa and Sc3+–py-macrodipa, with respect to the orientation of the picolinate donors and the lack of full engagement of all six macrocycle donor atoms.18,19 A key difference between these Ln3+ and Bi3+ structures, however, is the absence of a coordinated water molecule in the latter. This void is most likely a consequence of the stereochemical activity24,25 of the Bi3+ 6s2 lone pair. These observations that Bi3+–macrodipa and Bi3+–py-macrodipa attain the asymmetric Conformation B rather than the symmetric Conformation A is somewhat surprising based on the similar ionic radii of Bi3+ and La3+,26,27 a representative large Ln3+. This result suggests that the stereochemical activity of the 6s2 lone pair plays a pronounced role in mediating the preferred conformations of these Bi3+ complexes.
Figure 2.
Crystal structures of (a) [Bi(macrodipa)]+ and (b) [Bi(py-macrodipa)]+. Thermal ellipsoids are drawn at the 50% probability level. Solvent and counterions are omitted for clarity.
Experimental characterization of Ac3+ complexes is challenging due to the high radioactivity and extremely limited availability of its longest-lived isotope 227Ac (t1/2 = 21.8 y).28 Thus instead, we probed the structures of Ac3+–macrodipa and Ac3+–py-macrodipa computationally using density functional theory (DFT) with Gaussian 16.29 The hybrid TPSSh functional,30 which has been validated for studying Ac3+ chemistry,31,32 was adopted. A large-core relativistic effective core potential (LCRECP) and the associated basis set was assigned to the Ac3+ center,33-35 whereas the 6-31G(d,p) basis set36,37 was applied to all other lighter atoms. Aqueous solvation effects were accounted for with the SMD solvation model.38
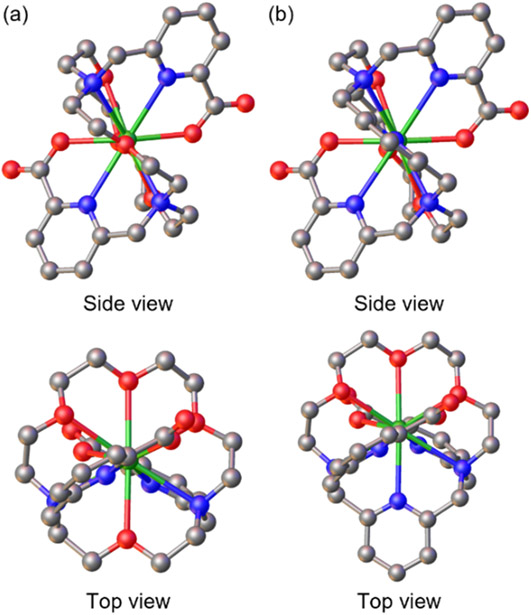
Because the ionic radii and coordination chemistry of Ac3+ and La3+ are similar,28 we optimized Ac3+–macrodipa and Ac3+–py-macrodipa starting from the geometries of the corresponding La3+ complexes, which attain the symmetric Conformation A.18,19 Within these structures (Figure 3), the Ac–O interatomic distances are 2.45–2.48 Å for negatively charged O and 2.70–2.79 Å for neutral O, whereas the Ac–N interactions range from 2.76–2.92 Å. These calculated distances are in expectation with experimentally measured Ac–O and Ac–N interatomic distances.39-43 Additionally, we optimized both complexes in Conformation B. Consistent with our expectations, Conformation B is energetically unfavored for both complexes (Table S2).
Figure 3.
DFT-optimized structures of (a) [Ac(macrodipa)]+ and (b) [Ac(py-macrodipa)]+. Hydrogen atoms are omitted for clarity. Green: Ac, grey: C, blue: N, red: O.
Having established the coordination chemistry of these ligands, we next carried out radiolabeling studies to evaluate their potential value for 225Ac3+ and 213Bi3+ TAT in comparison to the state-of-the-art chelators macropa and DOTA. These radionuclides were produced and purified according to previously-described protocols.44-46
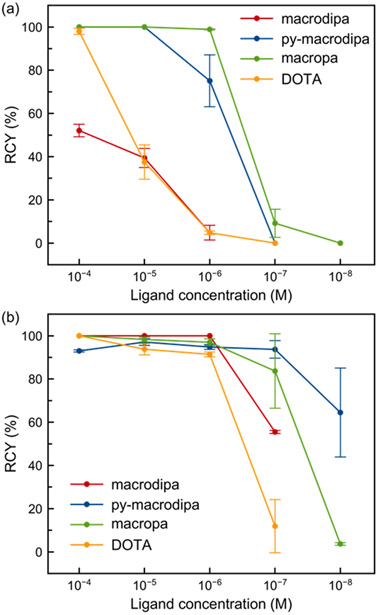
Different concentrations of macrodipa, py-macrodipa, macropa, and DOTA were combined with pH 5.5–6 buffered solutions containing either 20–40 or 30–300 kBq of 225Ac3+ and 213Bi3+ at ambient or elevated temperature, and the radiochemical yields (RCYs) were determined by radio-TLC. The concentration-dependent RCYs for these four chelators are summarized in Figure 4. For both radionuclides, py-macrodipa is able to achieve significantly higher RCYs than its analogue macrodipa and the conventional chelator DOTA, which also required high temperatures for radiolabeling. RCYs of approximately 75% and 65% are obtained when using low py-macrodipa concentrations of 10−6 M and 10−8 M for 225Ac3+ and 213Bi3+, respectively. With respect to 225Ac3+ chelation, py-macrodipa was slightly less effective than macropa, but was better at radiolabeling 213Bi3+. We also performed 225Ac3+ radiolabeling with macrodipa and py-macrodipa at pH 7 (Table S3). Under this condition, both chelators were able to access greater RCYs, but still failed to surpass macropa. Overall, these studies show that py-macrodipa effectively radiolabels both 225Ac3+ and 213Bi3+ under mild conditions.
Figure 4.
Radiochemical yields at different ligand concentrations. (a) RCYs of 225Ac3+ radiolabeling (25 °C for py-macrodipa, macropa, 40 °C for macrodipa, and 80 °C for DOTA; pH 5.5–6; 60 min reaction time). (b) RCYs of 213Bi3+ labeling (25 °C for macrodipa, py-macrodipa, macropa and 95 °C for DOTA; pH 5.5–6; 6–8 min reaction time). Error bars represent the standard deviations. The 213Bi3+ data with macropa and DOTA was taken from Ref 21.
We next assessed the kinetic inertness of 225Ac3+–py-macrodipa by incubating it in human serum at 37 °C (Table S5). These studies show that 225Ac3+–py-macrodipa is fairly labile, as ~90% of the complex dissociated after 1 d. By contrast, 225Ac3+–macropa remained 98% intact in human serum after 5 d. This excellent kinetic inertness is consistent to a previously reported serum challenge on 225Ac3+–macropa.20 Hence, despite the efficient radiolabeling properties of py-macrodipa, it is not an optimal candidate for TAT applications with 225Ac3+.
Because 213Bi3+ decays quickly (t1/2 = 45.6 min), probing the 213Bi3+ complex kinetic inertness by this serum challenge assay is impractical. Instead, we performed a transchelation challenge assay19-21,47-49 on the macrodipa, py-macrodipa, and macropa complexes with stable Bi3+. The transchelation reactions of these Bi3+ complexes were monitored by UV–Vis spectroscopy in the presence of a 10-fold excess EDTA, a ligand with high affinity for Bi3+,50,51 at pH 5.0 and 25 °C. Under this condition, the Bi3+ ion is transchelated by EDTA, following pseudo-first-order kinetics. The resulting half-lives (t1/2) for this transchelation process, a comparative measure of complex kinetic inertness, are shown in Table 1. Bi3+–macrodipa is kinetically labile to this transchelation challenge. The kinetic inertness of Bi3+–py-macrodipa is remarkably enhanced, as reflected by a t1/2 of 13 d. Moreover, its inertness is greater than that of Bi3+–macropa, indicating that py-macrodipa is a promising candidate for TAT applications with 213Bi3+.
Table 1.
Half-lives of Bi3+ Complexes when Challenged with 10 Equivalents of EDTA.a
| t 1/2 | |
|---|---|
| Bi3+–macrodipa | 9.2 ± 0.1 min |
| Bi3+–py-macrodipa | 13.2 ± 1.2 d |
| Bi3+–macropa | 2.2 ± 0.2 d |
[BiL] = 100 μM, pH 5.0, 25 °C.
In summary, we evaluated the viability of macrodipa and py-macrodipa as chelators for 225Ac3+ and 213Bi3+. Their coordination chemistry with Ac3+ and Bi3+ were characterized computationally and experimentally, respectively. Our radiolabeling studies revealed that py-macrodipa is highly effective at radiolabeling both radiometals, outperfoming both macrodipa and DOTA. Although the lability of Ac3+–py-macrodipa precludes its use with 225Ac3+ in nuclear medicine, the efficient formation and high stability of Bi3+–py-macrodipa, which surpasses Bi3+–macropa, suggests that this ligand is a valuable candidate for 213Bi3+ chelation. These results highlight that py-macrodipa joins other promising candidates for 213Bi3+ chelation that have arisen in recent years.21,52-60 Ongoing work is directed towards the synthesis of a bifunctional analogue of py-macrodipa to apply this chelator in TAT, as well as the development of “macrodipa-type” chelators with enhanced Ac3+ complex stabilities.
Supplementary Material
SYNOPSIS.
The α-emitting radionuclides 225Ac3+ and 213Bi3+ are promising candidates for targeted alpha therapy (TAT), a form of nuclear medicine that harnesses α radiation to kill cancer cells. Here, we investigate the chelation of these radiometals with the ligands macrodipa and py-macrodipa to assess their suitability for TAT. In particular, py-macrodipa is demonstrated to be a promising candidate for 213Bi3+ chelation, surpassing the current state-of-the art chelators macropa and DOTA.
ACKNOWLEDGMENT
This research was supported by the National Institutes of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Numbers R21EB027282 and R01EB029259, as well as the Research Corporation for Science Advancement through a Cottrell Research Scholar Award to J.J.W. This research made use of the NMR Facility at Cornell University, which was supported, in part, by the U.S. National Science Foundation under award number CHE-1531632. TRIUMF receives funding via a contribution agreement with the Natural Research Council of Canada. The authors acknowledge the TRIUMF actinium-production team for their work to produce and isolate 225Ac from the 500 MeV Isotope Production Facility. V.B. was funded by a Natural Sciences and Engineering Research Council (NSERC) Canada Graduate Scholarship – Masters (CGS-M).
Footnotes
The authors declare no competing financial interest.
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/xxxxxxx.
Experimental procedures and supplementary data (PDF)
Crystallographic data for Bi3+–macrodipa and Bi3+–py-macrodipa (CIF)
Geometry outputs for DFT-optimized structures (ZIP)
Accession Codes
CCDC 2124116–2124117 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: + 44 1223 336033.
REFERENCES
- (1).Brechbiel MW Targeted α-Therapy: Past, Present, Future? Dalton Trans. 2007, 4918–4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Kim Y-S; Brechbiel MW An Overview of Targeted Alpha Therapy. Tumor Biol. 2012, 33, 573–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Seidl C Radioimmunotherapy with α-Particle-Emitting Radionuclides. Immunotherapy 2014, 6, 431–458. [DOI] [PubMed] [Google Scholar]
- (4).Guerra Liberal FDC; O’Sullivan JM; McMahon SJ; Prise KM Targeted Alpha Therapy: Current Clinical Applications. Cancer Biother. Radiopharm 2020, 35, 404–417. [DOI] [PubMed] [Google Scholar]
- (5).Radchenko V; Morgenstern A; Jalilian AR; Ramogida CF; Cutler C; Duchemin C; Hoehr C; Haddad F; Bruchertseifer F; Gausemel H; Yang H; Osso JA; Washiyama K; Czerwinski K; Leufgen K; Pruszyński M; Valzdorf O; Causey P; Schaffer P; Perron R; Maxim S; Wilbur DS; Stora T; Li Y Production and Supply of α-Particle–Emitting Radionuclides for Targeted α-Therapy. J. Nucl. Med 2021, 62, 1495–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Yang H; Wilson JJ; Orvig C; Li Y; Wilbur DS; Ramogida C; Radchenko V; Schaffer P Harnessing Alpha-Emitting Radionuclides for Therapy: Radiolabeling Method Review. J. Nucl. Med 2021, doi: jnumed.121.262687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Eychenne R; Chérel M; Haddad F; Guérard F; Gestin J-F Overview of the Most Promising Radionuclides for Targeted Alpha Therapy: The “Hopeful Eight”. Pharmaceutics 2021, 13, 906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Morgenstern A; Apostolidis C; Kratochwil C; Sathekge M; Krolicki L; Bruchertseifer F An Overview of Targeted Alpha Therapy with 225Actinium and 213Bismuth. Curr. Radiopharm 2018, 11, 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Bruchertseifer F; Kellerbauer A; Malmbeck R; Morgenstern A Targeted Alpha Therapy with Bismuth-213 and Actinium-225: Meeting Future Demand. J. Labelled Compd. Radiopharm 2019, 62, 794–802. [DOI] [PubMed] [Google Scholar]
- (10).Morgenstern A; Apostolidis C; Bruchertseifer F Supply and Clinical Application of Actinium-225 and Bismuth-213. Semin. Nucl. Med 2020, 50, 119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Geerlings MW; Kaspersen FM; Apostolidis C; van der Hout R The Feasibility of 225Ac as a Source of α-Particles in Radioimmunotherapy. Nucl. Med. Commun 1993, 14, 121–125. [DOI] [PubMed] [Google Scholar]
- (12).Thiele NA; Wilson JJ Actinium-225 for Targeted α Therapy: Coordination Chemistry and Current Chelation Approaches. Cancer Biother. Radiopharm 2018, 33, 336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Morgenstern A; Bruchertseifer F; Apostolidis C Bismuth-213 and Actinium-225 – Generator Performance and Evolving Therapeutic Applications of Two Generator-Derived Alpha-Emitting Radioisotopes. Curr. Radiopharm 2012, 5, 221–227. [DOI] [PubMed] [Google Scholar]
- (14).Hassfjell S; Brechbiel MW The Development of the α-Particle Emitting Radionuclides 212Bi and 213Bi, and Their Decay Chain Related Radionuclides, for Therapeutic Applications. Chem. Rev 2001, 101, 2019–2036. [DOI] [PubMed] [Google Scholar]
- (15).Ahenkorah S; Cassells I; Deroose CM; Cardinaels T; Burgoyne AR; Bormans G; Ooms M; Cleeren F Bismuth-213 for Targeted Radionuclide Therapy: From Atom to Bedside. Pharmaceutics 2021, 13, 599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Price EW; Orvig C Matching Chelators to Radiometals for Radiopharmaceuticals. Chem. Soc. Rev 2014, 43, 260–290. [DOI] [PubMed] [Google Scholar]
- (17).Radiopharmaceutical Chemistry; Lewis JS, Windhorst AD, Zeglis BM, Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2019. [Google Scholar]
- (18).Hu A; MacMillan SN; Wilson JJ Macrocyclic Ligands with an Unprecedented Size-Selectivity Pattern for the Lanthanide Ions. J. Am. Chem. Soc 2020, 142, 13500–13506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Hu A; Aluicio-Sarduy E; Brown V; MacMillan SN; Becker KV; Barnhart TE; Radchenko V; Ramogida CF; Engle JW; Wilson JJ Py-Macrodipa: A Janus Chelator Capable of Binding Medicinally Relevant Rare-Earth Radiometals of Disparate Sizes. J. Am. Chem. Soc 2021, 143, 10429–10440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Thiele NA; Brown V; Kelly JM; Amor-Coarasa A; Jermilova U; MacMillan SN; Nikolopoulou A; Ponnala S; Ramogida CF; Robertson AKH; Rodríguez-Rodríguez C; Schaffer P; Williams C Jr.; Babich JW; Radchenko V; Wilson JJ An Eighteen-Membered Macrocyclic Ligand for Actinium-225 Targeted Alpha Therapy. Angew. Chem., Int. Ed 2017, 56, 14712–14717. [DOI] [PubMed] [Google Scholar]
- (21).Fiszbein DJ; Brown V; Thiele NA; Woods JJ; Wharton L; MacMillan SN; Radchenko V; Ramogida CF; Wilson JJ Tuning the Kinetic Inertness of Bi3+ Complexes: The Impact of Donor Atoms on Diaza-18-Crown-6 Ligands as Chelators for 213Bi Targeted Alpha Therapy. Inorg. Chem 2021, 60, 9199–9211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).McDevitt MR; Ma D; Simon J; Frank RK; Scheinberg DA Design and Synthesis of 225Ac Radioimmunopharmaceuticals. Appl. Radiat. Isot 2002, 57, 841–847. [DOI] [PubMed] [Google Scholar]
- (23).Norenberg JP; Krenning BJ; Konings IRHM; Kusewitt DF; Nayak TK; Anderson TL; de Jong M; Garmestani K; Brechbiel MW; Kvols LK 213Bi-[DOTA0, Tyr3]Octreotide Peptide Receptor Radionuclide Therapy of Pancreatic Tumors in a Preclinical Animal Model. Clin. Cancer Res 2006, 12, 897–903. [DOI] [PubMed] [Google Scholar]
- (24).Shimoni-Livny L; Glusker JP; Bock CW Lone Pair Functionality in Divalent Lead Compounds. Inorg. Chem 1998, 37, 1853–1867. [Google Scholar]
- (25).Pujales-Paradela R; Rodríguez-Rodríguez A; Gayoso-Padula A; Brandariz I; Valencia L; Esteban-Gómez D; Platas-Iglesias C On the Consequences of the Stereochemical Activity of the Bi(iii) 6s2 Lone Pair in Cyclen-Based Complexes. The [Bi(DO3A)] Case. Dalton Trans. 2018, 47, 13830–13842. [DOI] [PubMed] [Google Scholar]
- (26).Shannon RD Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr., Sect. A: Found. Adv 1976, 32, 751–767. [Google Scholar]
- (27).Näslund J; Persson I; Sandström M Solvation of the Bismuth(III) Ion by Water, Dimethyl Sulfoxide, N,N′-Dimethylpropyleneurea, and N,N-Dimethylthioformamide. An EXAFS, Large-Angle X-ray Scattering, and Crystallographic Structural Study. Inorg. Chem 2000, 39, 4012–4021. [DOI] [PubMed] [Google Scholar]
- (28).Deblonde GJ-P; Zavarin M; Kersting AB The Coordination Properties and Ionic Radius of Actinium: A 120-Year-Old Enigma. Coord. Chem. Rev 2021, 446, 214130. [Google Scholar]
- (29).Frisch MJ; Trucks GW; Schlegel HB; Scuseria GE; Robb MA; Cheeseman JR; Scalmani G; Barone V; Petersson GA; Nakatsuji H; Li X; Caricato M; Marenich AV; Bloino J; Janesko BG; Gomperts R; Mennucci B; Hratchian HP; Ortiz JV; Izmaylov AF; Sonnenberg JL; Williams-Young D; Ding F; Lipparini F; Egidi F; Goings J; Peng B; Petrone A; Henderson T; Ranasinghe D; Zakrzewski VG; Gao J; Rega N; Zheng G; Liang W; Hada M; Ehara M; Toyota K; Fukuda R; Hasegawa J; Ishida M; Nakajima T; Honda Y; Kitao O; Nakai H; Vreven T; Throssell K; Montgomery JA Jr.; Peralta JE; Ogliaro F; Bearpark MJ; Heyd JJ; Brothers EN; Kudin KN; Staroverov VN; Keith TA; Kobayashi R; Normand J; Raghavachari K; Rendell AP; Burant JC; Iyengar SS; Tomasi J; Cossi M; Millam JM; Klene M; Adamo C; Cammi R; Ochterski JW; Martin RL; Morokuma K; Farkas O; Foresman JB; Fox DJ Gaussian 16, Revision C. 01; Gaussian, Inc.: Wallingford, CT, 2016. [Google Scholar]
- (30).Tao J; Perdew JP; Staroverov VN; Scuseria GE Climbing the Density Functional Ladder: Nonempirical Meta–Generalized Gradient Approximation Designed for Molecules and Solids. Phys. Rev. Lett 2003, 91, 146401. [DOI] [PubMed] [Google Scholar]
- (31).Kovács A Theoretical Study of Actinide Complexes with Macropa. ACS Omega 2020, 5, 26431–26440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Kovács A Theoretical Study of Actinide(III)-DOTA Complexes. ACS Omega 2021, 6, 13321–13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Küchle W; Dolg M; Stoll H; Preuss H Energy-Adjusted Pseudopotentials for the Actinides. Parameter Sets and Test Calculations for Thorium and Thorium Monoxide. J. Chem. Phys 1994, 100, 7535–7542. [Google Scholar]
- (34).Cao X; Dolg M; Stoll H Valence Basis Sets for Relativistic Energy-Consistent Small-Core Actinide Pseudopotentials. J. Chem. Phys 2003, 118, 487–496. [Google Scholar]
- (35).Cao X; Dolg M Segmented Contraction Scheme for Small-Core Actinide Pseudopotential Basis Sets. J. Mol. Struct.: THEOCHEM 2004, 673, 203–209. [Google Scholar]
- (36).Hehre WJ; Ditchfield R; Pople JA Self-Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian-Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys 1972, 56, 2257–2261. [Google Scholar]
- (37).Hariharan PC; Pople JA The Influence of Polarization Functions on Molecular Orbital Hydrogenation Energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar]
- (38).Marenich AV; Cramer CJ; Truhlar DG Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [DOI] [PubMed] [Google Scholar]
- (39).Ferrier MG; Batista ER; Berg JM; Birnbaum ER; Cross JN; Engle JW; La Pierre HS; Kozimor SA; Lezama Pacheco JS; Stein BW; Stieber SCE; Wilson JJ Spectroscopic and Computational Investigation of Actinium Coordination Chemistry. Nat. Commun 2016, 7, 12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Ferrier MG; Stein BW; Batista ER; Berg JM; Birnbaum ER; Engle JW; John KD; Kozimor SA; Lezama Pacheco JS; Redman LN Synthesis and Characterization of the Actinium Aquo Ion. ACS Cent. Sci. 2017, 3, 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Ferrier MG; Stein BW; Bone SE; Cary SK; Ditter AS; Kozimor SA; Lezama Pacheco JS; Mocko V; Seidler GT The Coordination Chemistry of CmIII, AmIII, and AcIII in Nitrate Solutions: an Actinide L3-Edge EXAFS Study. Chem. Sci 2018, 9, 7078–7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Stein BW; Morgenstern A; Batista ER; Birnbaum ER; Bone SE; Cary SK; Ferrier MG; John KD; Lezama Pacheco J; Kozimor SA; Mocko V; Scott BL; Yang P Advancing Chelation Chemistry for Actinium and Other +3 f-Elements, Am, Cm, and La. J. Am. Chem. Soc 2019, 141, 19404–19414. [DOI] [PubMed] [Google Scholar]
- (43).Jones ZR; Livshits MY; White FD; Dalodière E; Ferrier MG; Lilley LM; Knope KE; Kozimor SA; Mocko V; Scott BL; Stein BW; Wacker JN; Woen DH Advancing Understanding of Actinide(iii) (Ac, Am, Cm) Aqueous Complexation Chemistry. Chem. Sci 2021, 12, 5638–5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Robertson AKH; McNeil BL; Yang H; Gendron D; Perron R; Radchenko V; Zeisler S; Causey P; Schaffer P 232Th-Spallation-Produced 225Ac with Reduced 227Ac Content. Inorg. Chem 2020, 59, 12156–12165. [DOI] [PubMed] [Google Scholar]
- (45).Ma D; McDevitt MR; Finn RD; Scheinberg DA Breakthrough of 225Ac and Its Radionuclide Daughters from an 225Ac/213Bi Generator: Development of New Methods, Quantitative Characterization, and Implications for Clinical Use. Appl. Radiat. Isot 2001, 55, 667–678. [DOI] [PubMed] [Google Scholar]
- (46).McDevitt MR; Finn RD; Sgouros G; Ma D; Scheinberg DA An 225Ac/213Bi Generator System for Therapeutic Clinical Applications: Construction and Operation. Appl. Radiat. Isot 1999, 50, 895–904. [DOI] [PubMed] [Google Scholar]
- (47).Thiele NA; Woods JJ; Wilson JJ Implementing f-Block Metal Ions in Medicine: Tuning the Size Selectivity of Expanded Macrocycles. Inorg. Chem 2019, 58, 10483–10500. [DOI] [PubMed] [Google Scholar]
- (48).Aluicio-Sarduy E; Thiele NA; Martin KE; Vaughn BA; Devaraj J; Olson AP; Barnhart TE; Wilson JJ; Boros E; Engle JW Establishing Radiolanthanum Chemistry for Targeted Nuclear Medicine Applications. Chem. - Eur. J 2020, 26, 1238–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Hu A; Keresztes I; MacMillan SN; Yang Y; Ding E; Zipfel WR; DiStasio RA Jr.; Babich JW; Wilson JJ Oxyaapa: A Picolinate-Based Ligand with Five Oxygen Donors that Strongly Chelates Lanthanides. Inorg. Chem 2020, 59, 5116–5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Martell AE; Smith RM Critical Stability Constants; Plenum Press: New York, 1974; Vol. 1. [Google Scholar]
- (51).Ramaiah NA; Tewari GD; Trivedi SR; Katiyar SS Spectrophotometric Titration of Bismuth with EDTA. Talanta 1968, 15, 352–356. [DOI] [PubMed] [Google Scholar]
- (52).Lima LMP; Beyler M; Oukhatar F; Le Saec P; Faivre-Chauvet A; Platas-Iglesias C; Delgado R; Tripier R H2Me-do2pa: An Attractive Chelator with Fast, Stable and Inert natBi3+ and 213Bi3+ Complexation for Potential α-Radioimmunotherapy Applications. Chem. Commun 2014, 50, 12371–12374. [DOI] [PubMed] [Google Scholar]
- (53).Lima LMP; Beyler M; Delgado R; Platas-Iglesias C; Tripier R Investigating the Complexation of the Pb2+/Bi3+ Pair with Dipicolinate Cyclen Ligands. Inorg. Chem 2015, 54, 7045–7057. [DOI] [PubMed] [Google Scholar]
- (54).Wilson JJ; Ferrier M; Radchenko V; Maassen JR; Engle JW; Batista ER; Martin RL; Nortier FM; Fassbender ME; John KD; Birnbaum ER Evaluation of Nitrogen-Rich Macrocyclic Ligands for the Chelation of Therapeutic Bismuth Radioisotopes. Nucl. Med. Biol 2015, 42, 428–438. [DOI] [PubMed] [Google Scholar]
- (55).Egorova BV; Matazova EV; Mitrofanov AA; Aleshin GY; Trigub AL; Zubenko AD; Fedorova OA; Fedorov YV; Kalmykov SN Novel Pyridine-Containing Azacrownethers for the Chelation of Therapeutic Bismuth Radioisotopes: Complexation Study, Radiolabeling, Serum Stability and Biodistribution. Nucl. Med. Biol 2018, 60, 1–10. [DOI] [PubMed] [Google Scholar]
- (56).Šimeček J; Hermann P; Seidl C; Bruchertseifer F; Morgenstern A; Wester H-J; Notni J Efficient Formation of Inert Bi-213 Chelates by Tetraphosphorus Acid Analogues of DOTA: Towards Improved Alpha-Therapeutics. EJNMMI Res. 2018, 8, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Matazova EV; Egorova BV; Konopkina EA; Aleshin GY; Zubenko AD; Mitrofanov AA; Karpov KV; Fedorova OA; Fedorov YV; Kalmykov SN Benzoazacrown Compound: A Highly Effective Chelator for Therapeutic Bismuth Radioisotopes. MedChemComm 2019, 10, 1641–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Bruchertseifer F; Comba P; Martin B; Morgenstern A; Notni J; Starke M; Wadepohl H First-Generation Bispidine Chelators for 213BiIII Radiopharmaceutical Applications. ChemMedChem 2020, 15, 1591–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Lange JL; Davey PRWJ; Ma MT; White JM; Morgenstern A; Bruchertseifer F; Blower PJ; Paterson BM An Octadentate Bis(semicarbazone) Macrocycle: A Potential Chelator for Lead and Bismuth Radiopharmaceuticals. Dalton Trans. 2020, 49, 14962–14974. [DOI] [PubMed] [Google Scholar]
- (60).Horváth D; Travagin F; Guidolin N; Buonsanti F; Tircsó G; Tóth I; Bruchertseifer F; Morgenstern A; Notni J; Giovenzana GB; Baranyai Z Towards 213Bi Alpha-Therapeutics and beyond: Unravelling the Foundations of Efficient BiIII Complexation by DOTP. Inorg. Chem. Front 2021, 8, 3893–3904. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.