Summary
Background
We assessed the efficacy of a receptor-binding domain (RBD)-based protein subunit COVID-19 vaccine.
Methods
A randomised Phase-1/2 trial followed by a Phase-2 trial were conducted to assess the safety and immunogenicity of the COVID-19 vaccine Corbevax and select to an optimum formulation. Healthy adults (n=460) without COVID-19 vaccination or SARS-CoV-2 infection in the Phase-1/2 study were randomly divided into four vaccine formulation groups.
Findings
A low incidence of adverse events was reported post-vaccination. All formulations showed similar profiles of humoral and cellular immune responses that were associated with the content of CpG1018 adjuvant in the vaccine. In the Phase-2 study, 750 µg of CpG1018 showed significant improvement (> 4-fold increase from baseline) in immune responses, including the titres of anti-RBD IgG and neutralising antibody (nAb), and cellular immune responses, while maintaining the safety profile. Antibodies persisted consistently for 12 months after the second dose of vaccine.
Interpretations
Corbevax (two-dose schedule with 28 days of interval between doses) was well tolerated with no observed safety concerns. Previous observations from efficacy studies by Moderna and AstraZeneca and the correlation between nAb titres post-vaccination and a human convalescent serum panel showed that Corbevax induced significantly high nAb titres. These studies were prospectively registered with the Clinical Trial Registry of India (CTRI/2021/06/034014 and CTRI/2020/11/029032).
Funding
Bill & Melinda Gates Foundation, BIRAC-Division of Department of Biotechnology, Govt of India, and the Coalition for Epidemic Preparedness Innovations funded this study.
Keywords: Covid-19, Vaccine, Receptor-binding domain, SARS-CoV-2, Spike protein, Protein subunit
Research in context.
Evidence before this study
A search on PubMed on the 30th June 2022 with the search terms ‘COVID-19’, ‘vaccine’, ‘protein subunit’, with no language restrictions applied, and filtering for clinical trial, generated only a few results: the SCB-2019 vaccine, comprising S-Trimer protein formulated with either AS03 or CpG/alum adjuvants and tested in Phase-1, 2, and 3 studies; a recombinant tandem-repeat dimeric RBD-based vaccine containing full-length SARS-CoV-2 spike protein with alum (ZF2001); a Sanofi vaccine (CoV2 preS dTM) tested in Phase-1 and 2 studies; and a recombinant subunit vaccine, AKS-452, tested in a Phase-1 study, comprising of a Fc fusion protein of the SARS-CoV-2 viral spike protein RBD antigen and human IgG1 Fc emulsified in the water-in-oil adjuvant. Many studies based on protein subunit vaccines are in the clinical trial stage and only limited data are available on RBD-based vaccine candidates.
Added value of this study
The present study is based on using a soluble monomeric RBD protein as an antigen. To our knowledge, this is the first vaccine for COVID-19, which is adjuvanted with alum and CpG1018. This Phase-1/2 study focused on identifying the optimal formulation of the Corbevax vaccine, that is, relative concentrations of the three key components: RBD antigen, alum, and CpG1018 adjuvants, to meet key benchmarks of product safety, reactogenicity, and immunogenicity. Testing included robust humoral responses (binding and nAb titres) and cellular immune responses. The studies were successful, and an optimal formulation (RBD antigen - 25 µg, alum – 750 µg, and CpG1018 – 750 µg) was identified from the Phase-2 study, which was then advanced into pivotal Phase-3 studies in adults as well as paediatric populations. These studies are currently ongoing.
Implications of all the available evidence
These findings suggest that the RBD-based protein subunit Corbevax vaccine using a two-dose schedule is safe, well tolerated, and highly immunogenic. The Phase-3 trial will provide information on the safety and immunogenicity of this vaccine in a larger adult and paediatric population.
Alt-text: Unlabelled box
Introduction
Coronavirus disease (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 Multiple vaccines have either been developed or are under development to prevent infection and reduce disease severity. Most vaccines utilise the spike protein or entire inactivated virus as the antigen. Protein subunit vaccines consist of either whole protein or specific protein regions from the pathogen containing key B- and T-cell epitopes combined with an adjuvant.2 Subunit proteins are often poorly immunogenic by themselves and adjuvants are added to enhance their ability to induce robust and long-lasting immune responses. Animal experiments suggest that vaccine-primed T helper type 2 (Th2) or T helper type 17 (Th17) responses are associated with immunopathology. Therefore, caution should be exercised when selecting an appropriate adjuvant. CpG motifs are Toll Like Receptor-9 (TLR-9) agonists that generate potent T helper type 1 (Th1)-biased responses and can mitigate Th2 or Th17-induced immunopathology.3 In pre-clinical studies, the subunit vaccine from the spike region of SARS-CoV-2, when mixed with CPG1018 and Al3+, was highly effective at inducing antibodies that neutralised pseudovirus (PSV) and wild-type live virus with no vaccine-related adverse effects.4 Biological E developed a receptor-binding domain (RBD)-based subunit vaccine with aluminium hydroxide and CpG1018 as adjuvants. Baylor College of Medicine/Texas Children's Hospital produced and licenced the recombinant Pichia Pastoris strain expressing RBD protein, consisting of residues 331–549 of the spike protein of SARS-CoV-2 Wuhan-Hu1 (GenBank Accession Number: QHD43416.1) to Biological E,5, 6, 7, 8, 9 and Dynavax Inc. supplied the CpG1018 adjuvant. The RBD of spike protein can induce an excellent immune response in terms of neutralising antibody (nAb)titres against SARS-COV-2 virus.10, 11, 12, 13 After satisfactory results from pre-clinical and developmental studies, clinical studies were planned to finalise vaccine formulation for late-stage clinical studies.
This manuscript describes clinical studies used to evaluate the optimal formulation of the vaccine (Corbevax). The Phase-1/2 study assessed the key role played by the RBD and adjuvant CpG1018. The subsequent Phase-2 study confirmed the safety and immunogenicity of the optimum formulation. In addition, the persistence of immune response at six and 12 months after the second dose is also presented in the Phase-1/2 study.
Methods
Study population and study design
In total, 1497 subjects were screened for both studies, and 460 subjects (n=360 and 100 in Phase 1/2 and Phase-2 studies, respectively) were vaccinated with different formulations of the Corbevax vaccine (Table 1).
Table 1.
Composition of the different formulations of Corbevax™.
| Phase-1/2 study |
Phase-2 study | ||||
|---|---|---|---|---|---|
| Component details | A | B | C | D | E |
| RBD antigen of SARS-CoV-2 (Covid-19) | 50 µg | 25 µg | 25 µg | 15 µg | 25 µg |
| Aluminium Hydroxide gel as Al+++ | 750 µg | 750 µg | 500 µg | 750 µg | 750 µg |
| CpG 1018 | 500 µg | 500 µg | 250 µg | 500 µg | 750 µg |
| Buffer (Tris and NaCl in water for injection) | q·s to 0·5 mL | q·s to 0·5 mL | q·s to 0·5 mL | q·s to 0·5 mL | q·s to 0·5 mL |
RBD: receptor binding domain; NaCl: sodium chloride; µg: micrograms; mL: milli liters.
Study design
The Phase-1/2 and Phase-2 studies were conducted in five and seven centres, respectively, across India. Studies were prospectively registered as multicentre, open-label, and randomised (Phase-2 part of Phase-1/2) and aimed to assess the optimal vaccine formulation for safety, tolerability, reactogenicity, and immunogenicity in real-time polymerase chain reaction (RT-PCR) for COVID-19 and anti-SARS-CoV-2 antibody seronegative subjects. Phase-1/2 and 2 trials were not placebo-controlled, as they were primarily designed to select the optimum formulation of the candidate vaccine. The results of these studies are currently being tested in a placebo-controlled Phase-3 clinical trial.
In the Phase-1/2 study, healthy volunteers were randomly assigned into four groups to receive a 0.5 mL dose of four different Corbevax formulations, designated as A, B, C, and D, as intramuscular injections into the deltoid muscle in a two-dose schedule with a 28-day interval between the doses (Figure 1a). All subjects were followed up for one year after the second dose. The optimum Corbevax formulation consisting of RBD antigen (25 µg) + aluminium hydroxide (750 µg) + CpG 1018 (750 µg) in a 0.5 mL volume (single dose for a human subject), was evaluated in the Phase-2 study (formulation-E, Table 1). All participants are being followed up for 12 months after the second dose of vaccine.
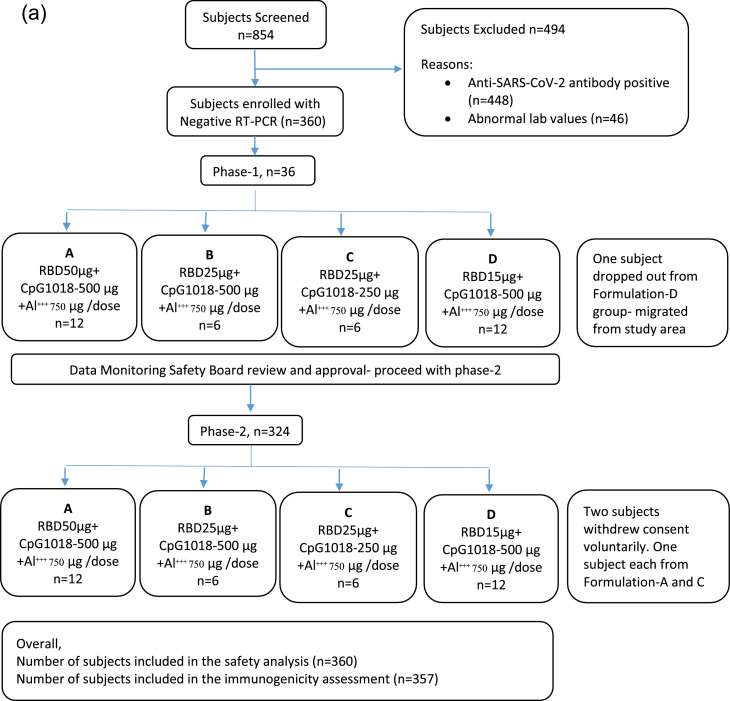
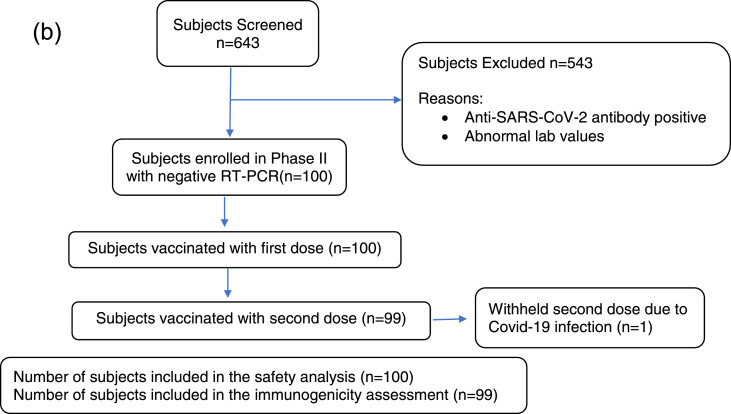
Figure 1.
(a) Subject disposition in Phase-1/2 study (consort diagram). A total of 854 subjects were screened, and 360 subjects were randomly randomised to four different formulation groups (n=90 in each group). All subjects were of Indian origin; 305 (84.72%) were male the mean ±SD age was 34.4±8.26 years and BMI (kg/m2) was 24.8±3.03. All subjects received the first dose, and 358 subjects received the second dose of the COVID-19 vaccine of Biological E. Three participants voluntarily withdrew from the study. A total of 357 (99.17%) and 360 (100%) patients were included in the immunogenicity and safety analyses, respectively.
N, number; RT-PCR, reverse transcription polymerase chain reaction; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
(b) Subject disposition in Phase-2 part of Phase-2/3 study. A total of 643 subjects were screened, and 100 were enrolled in the study. All 100 subjects were of Indian origin and received the first dose, and 99 subjects received the second dose of COVID-19 vaccine of Biological E. Out of the 100 subjects, 86 (86.00%) were male, and the mean ±SD age was 33.2 ±8.41 years. In one subject, the second dose of the vaccine was withheld owing to COVID-19 infection. A total of 99 (99%) and 100 (100%) patients were included in the immunogenicity and safety analyses, respectively.
n, number; RT-PCR, reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Healthy adult volunteers of either sex, between 18 to 65 years of age at the time of the first vaccination, were included in these studies. In addition, participants who were virologically seronegative for SARS-CoV-2 infection by RT-PCR (Dr.Dangs lab) and an anti-SARS-CoV-2 antibody (Liaison-SARS-CoV-2 ELISA kit14) were included in the study. Subjects with antibody concentrations below 12 units/mL were designated seronegative and selected for the trial (DiaSorin). Health status assessed during the screening period was based on medical history, clinical laboratory findings, vital signs, and physical examination. Participants who had a history of vaccination against COVID-19, were seropositive for IgG antibodies against SARS-CoV-2, or were exposed to COVID-19 patients were excluded from the study (detailed eligibility criteria are described in the protocol in the supplement section).
Ethics
The Ethics Committee at each study site approved the protocol (details of the Ethics Committee are available in the supplement.) The study was conducted in accordance with the principles of the Declaration of Helsinki, the International Conference on Harmonisation, and local regulatory guidelines. Written informed consent was obtained from all healthy volunteers prior to enrolment.
Safety assessments
Each subject was under direct observation for any immediate local and systemic adverse reactions, up to 120 min post-vaccination. All subjects were provided with a subject diary and trained to observe and capture adverse symptoms post-vaccination for the next seven days.
The number and percentage of subjects with adverse events (AEs) and serious adverse events (SAEs) were presented by system organ class (SOC) and preferred term (PT). The percentage of subjects with at least one local AE (solicited and unsolicited), one systemic AE (solicited and unsolicited), and with any AE during the solicited follow-up period was tabulated with an exact 95% confidence interval (CI). The same calculations were performed for symptoms rated as grade 3 or higher. Systemic and local tolerability, recorded in subject diaries, were summarised in a frequency table with percentages based on the number of observed values. SAEs, related AEs, AEs leading to death or withdrawal, solicited AEs, and medically attended adverse events (MAAEs) were separately summarised.
Immunological assays
Anti-SARS-CoV-2 antibody concentrations were measured using a DiaSorin kit.14 Anti-RBD antibody and IgG subclass responses were measured using a validated enzyme-linked immunosorbent assay (ELISA). In addition, nAbs against SARS-COV-2 were measured using a microneutralisation assay (MNA). PSV neutralisation assay (PNA) and cellular immune responses analysis were conducted by measuring cytokine secretion using TrueCulture tubes (Q2 solutions, USA) coated with SARS-COV-2 peptides.
Humoral immune responses were evaluated using the following methods:
-
1.
Anti-RBD antibody response: Anti-RBD IgG concentrations in serum samples were measured pre-vaccination (Day 0), post first dose (Day 28), and at multiple time points post second dose (Days 42, 56, and 208) using a validated ELISA at Dr.Dangs Lab, New Delhi, India. CR-3022 (Lake Pharma Inc., CA, USA), a monoclonal antibody against the RBD protein, was used to generate a standard curve comprising optical density (OD) values in ELISA and antibody concentrations. An ELISA concentration equivalent to 1 ng/mL of CR-3022 binding concentration was assigned as the concentration of one anti-RBD ELISA Unit/mL. The anti-RBD IgG concentration was reported as EU/mL. The National Institute for Biological Standards and Control, UK plasma reference standard 20/130, was used as a positive control (range, 8151–15137 EU/mL).15 Geometric means were calculated at specific time-points, fold rise in anti-RBD concentrations for all time points post-vaccination was calculated in relation to the pre-vaccination concentrations, and then fold rise in geometric mean (GMFR) was calculated.
-
2.Anti-RBD IgG-subclass response: The titres of anti-RBD IgG1 and IgG4 subclasses were measured in serum samples pre-vaccination (Day 0), post-first dose (Day 28), and at multiple timepoints post second dose (Days 42 and 56) using a validated ELISA executed at Dr.Dangs Lab. A pool of serum was prepared with very low anti-RBD IgG concentrations from pre-vaccination samples. The threshold ELISA OD for titre assignment was calculated as following:
(I)
Each serum sample was tested as a series of two-fold dilutions (starting from 50-fold), and the dilution that resulted in an OD higher than the threshold OD was assigned as the titre of IgG1 or IgG4. Geometric means were calculated at specific time points, and GMFR in anti-RBD IgG1 and IgG4 titres for all time points postvaccination were calculated with respect to the pre-vaccination titres.
-
3.
SARS-COV-2 virus neutralisation: SARS-COV-2 nAb-titres were measured using two different methods.16 MNA was employed using wild-type SARS-COV-2 strain (Victoria isolate 01/2020). A PNA was employed using Vesicular stomatitis virus expressing SARS-COV-2 spike protein and luciferase reporter gene. MNA and PNA were conducted at the Translational Health Science and Technology Institute (THSTI), Faridabad, India and Nexelis, Quebec, Canada, respectively; both are Coalition for Epidemic Preparedness Innovation (CEPI-network) labs. nAb was tested as previously described.16 Both laboratories have established conversion factors to enable conversion of the neutralisation titre (NT50) values to World Health Organization (WHO) International Standard (NIBSC-20/136) and report the NT50 values in International Units/mL.17 MNA and PNA values were divided by 4·064 and 1·875, respectively, to obtain the titres in IU/mL when required for comparison. Geometric mean titres were calculated at scheduled time points, and fold increases from the pre-vaccination values were calculated along with GMFR. For serum samples that did not demonstrate a minimum of 50% neutralisation of the virus at 10-fold dilution [the lower limit of quantitation (LLOQ) of the assay], titres were assigned as LLOQ/2. For key geometric mean titre (GMT)/ geometric mean concentration (GMC) values, 95% CI were calculated. All serum samples were assessed for nAb titres using the PNA method. Subjects with PNA titres below and above the LLOQ were considered seronegative and seropositive, respectively. Seroconversion rates for anti-RBD IgG concentration and nAb titres were calculated as follows: for seronegative subjects, a ≥ 4-fold rise in IgG or nAb titres and for seropositive subjects, a ≥ 2-fold rise in IgG or nAb titres were seroconverted.
Cellular immune responses were assessed in a subset of subjects using terms of cytokine secretion in TrueCulture tubes.18 Whole blood samples were incubated in TrueCulture tubes coated with SARS-COV-2 peptides (Myriad Inc., TX, USA). Peripheral blood mononuclear cells in the blood are stimulated by peptides that resemble the vaccine antigen to produce cytokines. The culture supernatants were collected, and the levels of interferon-gamma (IFN-γ) and interleukin 4 (IL-4) were measured using standard ELISA kits (Becton Dickinson, Franklin Lakes, New Jersey, USA) at Dr.Dangs Lab. Cytokine contents were reported in pg/mL, and averages were calculated for each cohort.
Serum samples were tested for breakthrough infections after vaccination to detect IgG antibodies against the nucleocapsid (N) protein of SARS-CoV-2 using an automated, two-step chemiluminescent microparticle immunoassay (06R9020, Abbott Alinity CMIA ELISA kit; Abbott Diagnostics) following the manufacturer's instructions. Intensity of the chemiluminescent reaction was measured in relative light units, which were calculated as an index value S/C (specimen absorbance/calibrator absorbance). An index ≥ 1·4 was considered seropositive for anti-N.
Statistics
Sample size
The sample size used in Phase-1 of the Phase-1/2 study was determined based on the WHO guidelines on clinical evaluation of vaccines,19 as this is the first human study to assess the safety, tolerability, and reactogenicity of each drug dose. The sample size used in Phase-2 of the Phase-1/2 study was calculated based on the difference in seroconversion rates between higher and lower dose groups using “SAS Proc” to detect a treatment difference of −20%, −20%, and −30%, for formulations B, C and D respectively, for each of the comparisons with the highest dose group (formulation A). Multiplicity adjustment was applied for each comparison with an overall significance level of 0·05. Therefore, the total sample size needed for Phase-1 to be seamlessly followed by the Phase-2 study was 320 subjects. With the addition of not less than a 10% dropout rate, 360 subjects were enrolled. The sample size of the Phase-2 study was based on human trials using a similar protein subunit-based vaccine,20 and was calculated for a minimum power of 90% to assess superiority against the background seroconversion rate of 15% in the target population. The estimated seroconversion rate in the cohort and the population background seroconversion rate were assumed to be 71% and 15%, respectively, and the superiority margin was 60% based on human trials with similar vaccines along with a significance level of 2.5% (one-sided test for superiority) with not less than 90% power. Based on these assumptions, a total sample size of 1268 was determined (n=100 in Phase-2 and 1168 in Phase-3), assuming a 10% dropout rate.
Randomisation and masking
In this study, the Phase-2 part of Phase-1/2 employed randomisation. First, equal randomisation of subjects into different formulation groups was performed using Interactive Web Response System containing randomisation numbers and intended allocation. The randomisation numbers were assigned as follows: EA001 (E-enrolment; A-site code; 001-number of the enrolled subjects), and this number continued in the same serial order for all subjects.
Statistical analyses
All data are presented using descriptive statistics. Demographic and primary safety analyses were based on the total vaccinated population. The full analysis set (FAS) included participants who provided informed consent. Intention-to-treat (ITT) analysis set included all participants from FAS who received vaccines in this study. Per-protocol analysis set included all subjects from ITT set. All subjects who received at least one dose of the study vaccine were included in the safety analysis. Statistical analyses were conducted using SAS 9·4 or higher (SAS Institute, Cary, NC, USA).
Immunogenicity data (IgG titres, IFN-γ levels, and nAb titres) were assessed in the protocol population. Serum anti-SARS-CoV-2 IgG titres were determined using validated ELISA and nAb assay against live and/or pseudo-type SARS-CoV-2 virus once at baseline, on days 28, 42, and 56 and at six and 12 months after the second dose. Seroconversion was defined as the appearance of antibodies (titre ≥ 4-fold rise) in sera of subjects who were seronegative before vaccination. A significant vaccine response rate was defined as an increase in antibody concentration ≥ 4-fold post-vaccination in a seronegative subject. Seronegative was defined as a person with no detectable levels of antibodies against the vaccine-specific antigen. GMT was calculated at baseline, and on days 28, 42, and 56 by taking the anti-log of the mean of log-concentration transformations. Antibody titres below the LLOQ were assigned an arbitrary value of half the LLOQ cut-off for GMT calculation. In addition, GMFR in anti-SARS-CoV IgG antibody titres and nAb titres on Day 28 post second dose, from baseline, along with their corresponding two-sided 95% CIs, was presented.
Role of the funding source
The selection of a laboratory for immunogenicity analysis was based on the recommendations of CEPI. Funding sources were not involved in the study, data analysis/interpretation, or writing of the manuscript.
Results
Study population
The subjects’ demographics and baseline characteristics are described in Table 2, and their disposition is shown in Figure 1a and b. The Phase-1/2 study was conducted between November 2020 and March 2021. The Phase-2 study was conducted between June to December in 2021.
Table 2.
Demographics and baseline characteristics of participants in phase-1/2 study and phase-2 of phase-2/3 study.
| Parameter/Statistics/Category | Phase-1/2 study |
Phase-2 Study | ||||
|---|---|---|---|---|---|---|
| A (N=90) | B (N=90) | C (N=90) | D (N=90) | Overall (N=360) | E (n=100) | |
| Age (Year) Mean ±SD | 35·0±8·7 | 35·0±9·1 | 32·7±8·1 | 34·7±6·7 | 34·4±8·2 | 33·2 ±8·41 |
| Gender, N1 (%) | ||||||
| Male | 74 (82·22%) | 75 (83·33%) | 74 (82·22%) | 82 (91·11%) | 305 (84·72%) | 86 (86·00%) |
| Female | 16 (17·78%) | 15 (16·67%) | 16 (17·78%) | 8 (8·89%) | 55 (15·28%) | 14 (14·00%) |
| Height (Cms) Mean ±SD | 166·8±8·9 | 166·9±7·8 | 165·7±8·4 | 165·8±6·8 | 166·3±8·0 | 167·7 ±7·23 |
| Weight (Kgs) Mean ±SD | 69·8±10·0 | 68·8±11·6 | 67·5±11·2 | 68·9±9·7 | 68·8±10·7 | 65·7 ±10·90 |
| BMI (Kg/m2) Mean ±SD | 25·1±2·6 | 24·6±3·2 | 24·5±3·2 | 25·1±3·1 | 24·8±3·0 | 23.30±3.2 |
| Ethinicty: Asian | 90 (100.00%) | 90 (100.00%) | 90 (100.00%) | 90 (100.00%) | 360 (100.00%) | 100(100.00%) |
| Centres | No of participants recruited (N) | |||||
| GTB Hospital, Delhi | 1 | 10 | 11 | 3 | 25 | 3 |
| AIIMS, Patna | 45 | 27 | 29 | 17 | 118 | 12 |
| KGH, Vizag | 6 | 7 | 4 | 1 | 18 | - |
| STH, Hyderabad | 34 | 43 | 43 | 67 | 187 | 31 |
| MASH, Jaipur | 4 | 3 | 3 | 2 | 12 | 10 |
| ESIC, Faridabad | - | - | - | - | - | 23 |
| Prakhar Hospital, Patna | - | - | - | - | - | 17 |
| AIG Hospital, Hyderabad | - | - | - | - | - | 4 |
Note: Percentages were calculated using column header group count as denominator.The Age were calculated using following formula: Age (at vaccination) =round of ((Vaccination Date-Birth Date)/365·25); BMI= Weight/(Height (in mts)2); N1: Subject Count, N: Sample Size.
Safety findings
In the Phase-1/2 study, AEs were reported in 42 subjects (11.67%) with a range of 8·89–15·56%. The lowest and highest numbers of subjects with AEs were reported in B and D formulation groups, respectively (Table 3a). In the Phase-2 study, 27 (27·00%) subjects reported AEs post-vaccination. No AEs were reported in any subject within 120 min post-vaccination. None of the AEs was serious or of Grade-3 severity. Details of solicited local and systemic AEs and unsolicited AEs are listed in Table 3a (Phase-1/2) and Table 3c (Phase-2). Most AEs were related to the study vaccine. MAAEs were reported in 10 (2·78%) and six (6%) subjects in Phase-1/2 and Phase-2 studies, respectively, which were not serious (Table 3b, c).
Table 3a.
Adverse events and reactions in the Phase-1/2 study.
| N1 (%) [95% CI] n | A (N=90) |
B (N=90) |
C (N=90) |
D (N=90) |
Overall (N= 360) |
|---|---|---|---|---|---|
| Overall Adverse Events | |||||
| Any AE | 11 (12·22%) [6·26, 20·82] 11 |
9 (10·00%) [4·68, 18·14] 9 |
8 (8·89%) [3·92, 16·77] 10 |
14 (15·56%) [8·77, 24·72] 16 |
42 (11·67%) [8·54, 15·44] 46 |
| Any Local AE | 7 (7·78%) [3·18, 15·37] 7 |
3 (3·33%) [0·69, 9·43] 3 |
3 (3·33%) [0·69, 9·43] 4 |
12 (13·33%) [7·08, 22·13] 12 |
25 (6·94%) [4·54, 10·08] 26 |
| Solicited Local Adverse Events | |||||
| Injection site erythema | 1 (1·11%) [0·03, 6·04] 1 |
0 (0·00%) [NE] 0 | 0 (0·00%) [NE] 0 | 0 (0·00%) [NE] 0 | 1 (0·28%) [0·01, 1·54] 1 |
| Injection site pain | 6 (6·67%) [2·49, 13·95] 6 |
3 (3·33%) [0·69, 9·43] 3 |
2 (2·22%) [0·27, 7·80] 3 |
10 (11·11%) [5·46, 19·49] 10 |
21 (5·83%) [3·65, 8·78] 22 |
| Injection site swelling | 0 (0.00%) [0.00, 4.02] 0 | 0 (0.00%) [0.00, 4.02] 0 | 1 (1·11%) [0·03, 6·04] 1 |
2 (2·22%) [0·27, 7·80] 2 |
3 (0·83%) [0·17, 2·42] 3 |
| Any Systemic AEs | 4 (4·44%) [1·22, 10·99] 4 |
5 (5·56%) [1·83, 12·49] 5 |
5 (5·56%) [1·83, 12·49] 6 |
4 (4·44%) [1·22, 10·99] 4 |
18 (5·00%) [2·99, 7·79] 19 |
| Solicited Systemic Adverse Events | |||||
| Chills | 0 (0.00%) [0.00, 4.02] 0 | 0 (0.00%) [0.00, 4.02] 0 | 1 (1·11%) [0·03, 6·04] 1 |
0 (0.00%) [0.00, 4.02] 0 | 1 (0·28%) [0·01, 1·54] 1 |
| Pyrexia | 3 (3·33%) [0·69, 9·43] 3 |
2 (2·22%) [0·27, 7·80] 2 |
2 (2·22%) [0·27, 7·80] 2 |
1 (1·11%) [0·03, 6·04] 1 |
8 (2·22%) [0·96, 4·33] 8 |
| Myalgia | 0 (0.00%) [0.00, 4.02] 0 | 1 (1·11%) [0·03, 6·04] 1 |
1 (1·11%) [0·03, 6·04] 1 |
0 (0.00%) [0.00, 4.02] 0 | 2 (0·56%) [0·07, 1·99] 2 |
| Headache | 1 (1·11%) [0·03, 6·04] 1 |
2 (2·22%) [0·27, 7·80] 2 |
0 (0.00%) [0.00, 4.02] 0 | 3 (3·33%) [0·69, 9·43] 3 |
6 (1·67%) [0·61, 3·59] 6 |
| Urticaria | 0 (0.00%) [0.00, 4.02] 0 | 0 (0.00%) [0.00, 4.02] 0 | 2 (2·22%) [0·27, 7·80] 2 |
0 (0.00%) [0.00, 4.02] 0 | 2 (0·56%) [0·07, 1·99] 2 |
| Any Unsolicited Systemic AE | |||||
| Dyspepsia | 0 (0.00%) [0.00, 4.02] 0 | 1 (1·11%) [0·03, 6·04] 1 |
0 (0.00%) [0.00, 4.02] 0 | 0 (0.00%) [0.00, 4.02] 0 | 1 (0·28%) [0·01, 1·54] 1 |
AE: adverse event; NE: non estimable; CI: confidence interval.
Table 3c.
Adverse events and reactions in the phase-2 study.
| N1 (%) [95% CI] n | Corbevax (N= 100) |
|---|---|
| Overall adverse events | |
| Any Adverse events | 27 (27·0) [0·19, 0·37] 105 |
| Solicited adverse event | 20 (20·0) [0·13, 0·29] 52 |
| Unsolicited adverse events | 10 (10·0) [0·05, 0·18] 17 |
| Medically Attended Adverse Events | 6 (6·0) [0·02, 0·13] 12 |
| Adverse Events Following Immunization | 24 (24·0) [0·16, 0·34] 47 |
| Local Adverse events | |
| Injection site pain | 14 (14·0) [0·08, 0·22] 39 |
| Injection site erythema | 2 (2·0) [0·00, 0·07] 5 |
| Injection site swelling | 1 (1·0) [0·00, 0·05] 2 |
| Systemic Adverse events | |
| Pyrexia | 9 (9·0) [0·04, 0·16] 15 |
| Fatigue | 4 (4·0) [0·01, 0·10] 9 |
| Pain | 2 (2·0) [0·00, 0·07] 2 |
| Gastrointestinal disorders | 4 (4·0) [0·01, 0·10] 6 |
| Musculoskeletal and connective tissue disorders | 4 (4·0) [0·01, 0·10] 10 |
| Nervous system disorders | 4 (4·0) [0·01, 0·10] 13 |
| Infections and infestations | 3 (3·0) [0·01, 0·09] 3 |
| Reproductive system and breast disorders | 1 (1·0) [0·00, 0·05] 1 |
| Respiratory, thoracic and mediastinal disorders | 1 (1·0) [0·00, 0·05] 3 |
N1: Subject count in specified category; N= Total number of subjects; n= Event Count; CI: confidence interval.
Table 3b.
Medically attended AEs by system organ class and preferred term.
| Phase-1/2 | |||||
|---|---|---|---|---|---|
| SOC/PTN1 (%) [95% CI] n | Treatment Groups |
OVERALL (N=360) |
|||
| A (N=90) |
B (N=90) |
C (N=90) |
D (N=90) |
||
| Gastrointestinal disorders | 0 (0.00%) [0.00, 4.02] 0 | 1 (1·11%) [0·03, 6·04] 1 |
0 (0.00%) [0.00, 4.02] 0 | 0 (0.00%) [0.00, 4.02] 0 | 1 (0·28%) [0·01, 1·54] 1 |
| Dyspepsia | 0 (0.00%) [0.00, 4.02] 0 | 1 (1·11%) [0·03, 6·04] 1 |
0 (0.00%) [0.00, 4.02] 0 | 0 (0.00%) [0.00, 4.02] 0 | 1 (0·28%) [0·01, 1·54] 1 |
| General disorders and administration site conditions | 1 (1·11%) [0·03, 6·04] 1 |
1 (1·11%) [0·03, 6·04] 1 |
1 (1·11%) [0·03, 6·04] 1 |
1 (1·11%) [0·03, 6·04] 1 |
4 (1·11%) [0·30, 2·82] 4 |
| Pyrexia | 1 (1·11%) [0·03, 6·04] 1 |
1 (1·11%) [0·03, 6·04] 1 |
1 (1·11%) [0·03, 6·04] 1 |
1 (1·11%) [0·03, 6·04] 1 |
4 (1·11%) [0·30, 2·82] 4 |
| Nervous system disorders | 1 (1·11%) [0·03, 6·04] 1 |
0 (0.00%) [0.00, 4.02] 0 | 0 (0.00%) [0.00, 4.02] 0 | 2 (2·22%) [0·27, 7·80] 2 |
3 (0·83%) [0·17, 2·42] 3 |
| Headache | 1 (1·11%) [0·03, 6·04] 1 |
0 (0.00%) [0.00, 4.02] 0 | 0 (0.00%) [0.00, 4.02] 0 | 2 (2·22%) [0·27, 7·80] 2 |
3 (0·83%) [0·17, 2·42] 3 |
| Skin and subcutaneous tissue disorders | 0 (0.00%) [0.00, 4.02] 0 | 0 (0.00%) [0.00, 4.02] 0 | 2 (2·22%) [0·27, 7·80] 2 |
0 (0.00%) [0.00, 4.02] 0 | 2 (0·56%) [0·07, 1·99] 2 |
| Urticaria | 0 (0.00%) [0.00, 4.02] 0 | 0 (0.00%) [0.00, 4.02] 0 | 2 (2·22%) [0·27, 7·80] 2 |
0 (0.00%) [0.00, 4.02] 0 | 2 (0·56%) [0·07, 1·99] 2 |
| Phase-2 | |||||
|---|---|---|---|---|---|
| E (N=100) N1(%), 95% CI, n | |||||
| Subjects with medically attended adverse events | 6 (6·0) [0·02, 0·13] 12 | ||||
| Infections and infestations | 3 (3·0) [0·01, 0·09] 3 | ||||
| COVID-19 | 1 (1·0) [0·00, 0·05] 1 | ||||
| Nasopharyngitis | 1 (1·0) [0·00, 0·05] 1 | ||||
| Pharyngitis | 1 (1·0) [0·00, 0·05] 1 | ||||
| General disorders and administration site conditions | 2 (2·0) [0·00, 0·07] 4 | ||||
| Pain | 2 (2·0) [0·00, 0·07] 2 | ||||
| Pyrexia | 2 (2·0) [0·00, 0·07] 2 | ||||
| Musculoskeletal and connective tissue disorders | 1 (1·0) [0·00, 0·05] 1 | ||||
| Pain in extremity | 1 (1·0) [0·00, 0·05] 1 | ||||
| Nervous system disorders | 1 (1·0) [0·00, 0·05] 1 | ||||
| Lethargy | 1 (1·0) [0·00, 0·05] 1 | ||||
Note: Percentages were calculated using column header count as denominator.
N1: Subject count in specified category; N= Total number of subjects; n= Event Count (One subject may be counted more than once),NE: Not Estimable; 95% CI was based on percentage calculated by Clopper–Pearson Method.
No abnormal laboratory values, vital signs, or physical examinations were reported to be clinically significant. No SAEs were reported during the study.
Immunogenicity findings
The Phase-1/2 study
Anti-RBD IgG concentrations moderately increased after the first dose, significantly after the second dose, and plateaued between days 42 and 56 (Figure 2a). Seroconversion was highest (90%) for B formulation (Table 4a). IgG1 titres significantly increased in all four groups, with the highest GMT (2940 on Day 56) and GMFR (39·7) observed for B formulation. The Day 0 titres were very low for both isotypes in all groups.
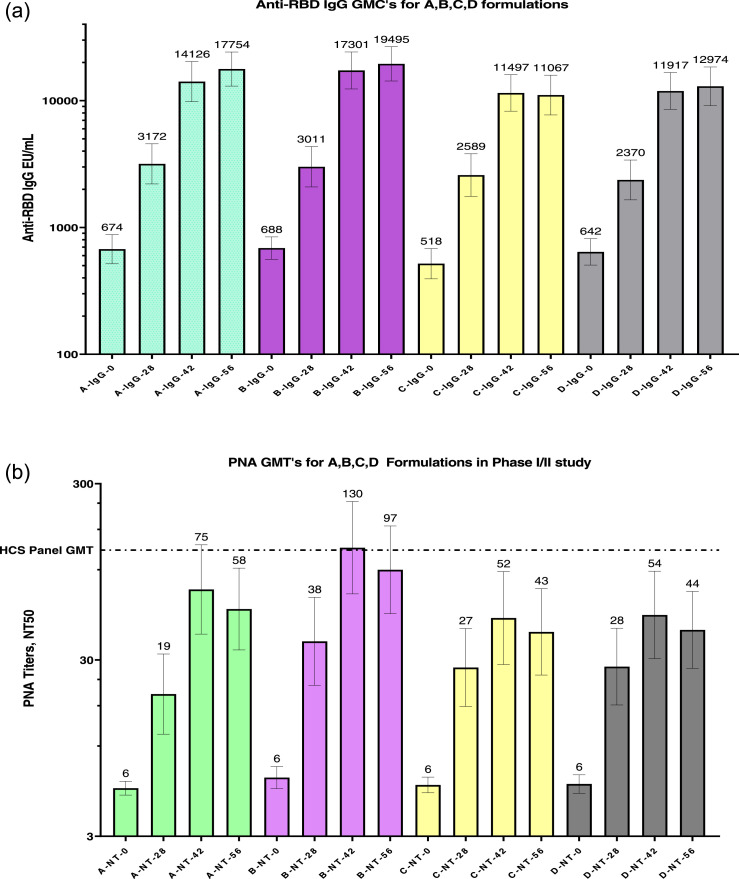
Figure 2.
(a) GMCs of anti-RBD IgG concentrations for all four formulation groups on Day 0 (pre-vaccination), Day 28 (post-first dose), and Days 42 and 56 (post second dose). GMCs (top of each column) with a 95% confidence interval (two-sided bars) are included in the figure.
RBD, receptor-binding domain; GMC, geometric mean concentration; IgG, immunoglobulin G.
(b) nAb titres determined by the PNA method for all four formulation groups on Day 0 (pre-vaccination), Day 28 (post-first dose), and Days 42 and 56 (post second dose). GMT was determined from 273 serum samples (human convalescent serum panel; HCS) collected from COVID-19 patients (determined by RT-PCR) with a range of disease severity. GMCTs (top of each column) with 95% confidence intervals (two-sided bars) are shown in the figure.
nAb: neutralising antibody titres; PNA: pseudovirus neutralisation assay;; GMT: geometric mean titre.
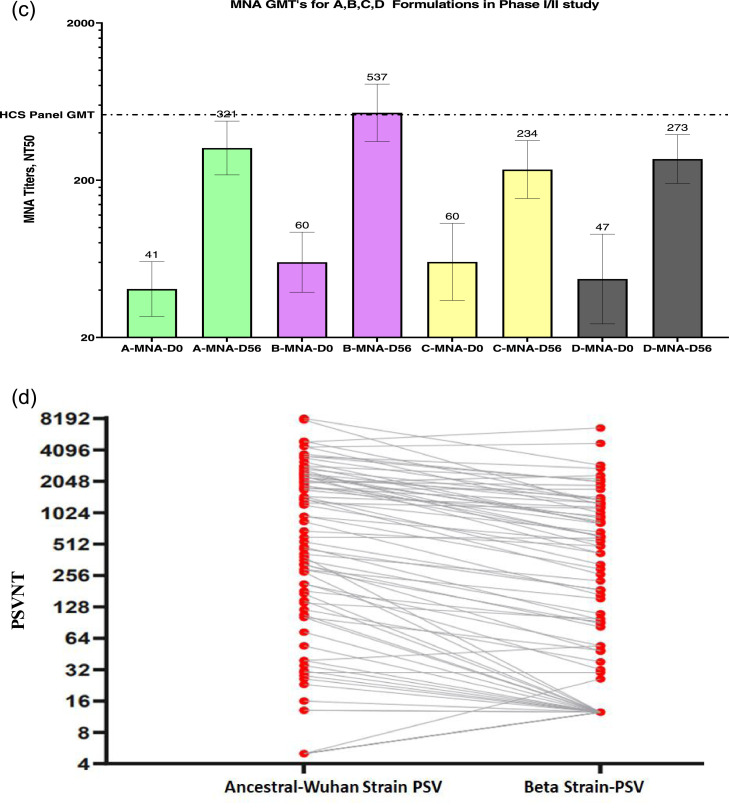
(c) nAb titres determined by the MNA method for all four formulation groups on Day 0 (pre-vaccination) and Day 56 (post second dose). The GMT for human convalescent serum panel was determined using serum samples from 32 subjects. The GMTs (top of each column) with 95% confidence intervals (two-sided bars) are included in the figure.
nAb, neutralising antibody; MNA, microneutralisation assay; GMT, geometric mean titre.
(d) Direct comparison of nAb titres by the PNA method against PSV mimicking the ancestral Wuhan and beta strains of SARS-COV-2 for a subset of subjects from all four formulation groups on Day 56 (post second dose). A direct comparison of neutralising antibodies was performed in a randomly selected subset of subjects from all four formulation groups on Day 56 (post second dose). The geometric mean reduction in nAb titres of the ancestral Wuhan to those of the beta strain was 2.25-fold.
PSV: pseudovirus neutralization assay; GMT: geometric mean titre, PvSNT: pseudovirus neutralisation titres.
Table 4a.
Anti-RBD IgG concentration Geometric Mean Fold Rise from Day0 (pre-vaccination) to Day28 (post first-dose) and to Day42 & 56 (post second-dose) for all four formulation cohorts.
| Formulation | Day28 GMFR | Day42 GMFR | Day56 GMFR | Percentage of Seroconversion |
|---|---|---|---|---|
| A | 4·71 | 24·97 | 24·90 | 83% |
| B | 4·74 | 25·15 | 28·35 | 90% |
| C | 4·58 | 22·03 | 21·21 | 79% |
| D | 3·59 | 18·46 | 19·48 | 79% |
GMFR: Geometric mean fold rise; RBD: Receptor binding domain; IgG: Immunoglobulin; Note: Percentage seroconversion observed for Day56 time-point sera samples based on ≥4-fold rise in anti-RBD-IgG concentration).
A minor increase was observed in IgG4 titres of the four groups on Day 56 (Table 4b). Nexelis tested 273 plasma samples collected from convalescent patients detected COVID-19 positive by RT-PCR. The range of disease severity in the collected plasma samples was asymptomatic to that of hospitalised patients. The GMT calculated using the PNA method for these convalescent serum panels was 126 and is shown in Figure 2b for days 0, 28, 42, and 56. The observed nAb titres were similar to the anti-RBD IgG concentrations, with the highest NT50 titres (130) on Day 42 induced by formulation B. Figure 2c represents the GMTs for the nAb titres measured using the MNA method for the four groups on days 0 and 56, showing a similar increase in the overall titre. However, GMFR and percentage seroconversion were not calculated, as the Day 0 titres were measured for only a subset of randomly selected subjects by the MNA method. UK Health Security Agency (UKHSA)21 tested 32 convalescent plasma samples collected from RT-PCR-positive COVID-19 subjects with severe disease. The GMT obtained via the MNA method was 522. Formulation B induced a higher NT50 (60 at Day 0 vs 537 at Day 56) than did the other formulations (Figure 2c). nAb titres were measured on Day 56 using the PNA method, where Vascular Stomatitis Virus (VSV) PSV expressed spike protein from the beta strain of SARS-COV-2. Serum samples were obtained from a subset of randomly selected subjects (81 of 358) in all four groups. A comparison of the PNA titres measured against the ancestral (Wuhan, GMT, 362) and the beta (GMT, 161) strains is shown in Figure 2d. The ratio was calculated for each pair of PNA titres, and the overall fold reduction of geometric means in PNA titres from the ancestral to the beta strain was 2·25. A significantly high IFN-γ response was induced by B formulation (31·4 pg/mL) on Day 56 compared to that by formulations A, C, and D (Table 4c).
Table 4b.
Anti-RBD-IgG1 and IgG4 GMTs for all four formulation cohorts at Day0 (pre-vaccination), and Day56 (post second-dose).
| Formulation | Subjects | IgG1 Titer |
IgG4 Titer |
D56-G1/G4 Ratio GMR | ||||
|---|---|---|---|---|---|---|---|---|
| D0 GMT | D56 GMT | GMFR | D0 GMT | D56 GMT | GMFR | |||
| A | 89 | 74 | 2696 | 36·25 | 31 | 57 | 1·84 | 48·7 |
| B | 90 | 74 | 2940 | 39·7 | 31 | 76 | 2·48 | 37·1 |
| C | 89 | 65 | 1884 | 28·92 | 27 | 50 | 1·81 | 38·0 |
| D | 89 | 89 | 2219 | 24·94 | 32 | 57 | 1·79 | 38·4 |
IgG: Immunoglobulin G; RBD: Receptor binding protein; GMT: Geometric mean titer; D0: Day0, D56: Day56.
Note: GMFR for each formulation cohort is calculated from the Geometric Mean for the Fold Rise in titer at Day56 vs Day0 time-point for each cohort; Geometric Mean Ratio was calculated for IgG1 to IgG4 titers at Day56 for all four cohorts.
Table 4c.
Average cytokine concentration at Day0 (pre-vaccination) and Day56 (post second-dose).
| Formulation | Average Interferon-gamma concentration (pg/mL) |
Average IL-4 concentration (pg/mL) |
||||||
|---|---|---|---|---|---|---|---|---|
| D-0 Null | D-0 Active | D-56 Null | D-56 Active | D-0 Null | D-0 Active | D-56 Null | D-56 Active | |
| A | 2·24 | 7·95 | 1·75 | 14·01 | 1·45 | 1·63 | 1·31 | 1·36 |
| B | 2·04 | 3·73 | 1·91 | 31·42 | 3·77 | 3·72 | 2·73 | 3·19 |
| C | 1·99 | 5·59 | 2·08 | 22·03 | 1·06 | 1·69 | 1·02 | 1·60 |
| D | 2·59 | 3·02 | 1·87 | 23·03 | 1·07 | 1·23 | 0·89 | 1·22 |
D-0: Day0; D-56; Day56; IL-4: Interleukin 4; pg/mL: picogram per milliliter.
Note: Cytokine measured in the supernatants of whole-blood samples incubated in tubes coated with SARS-COV-2 peptides (Active) and without coating (Null) for a subset of subjects from all four cohorts.
Phase-2 study
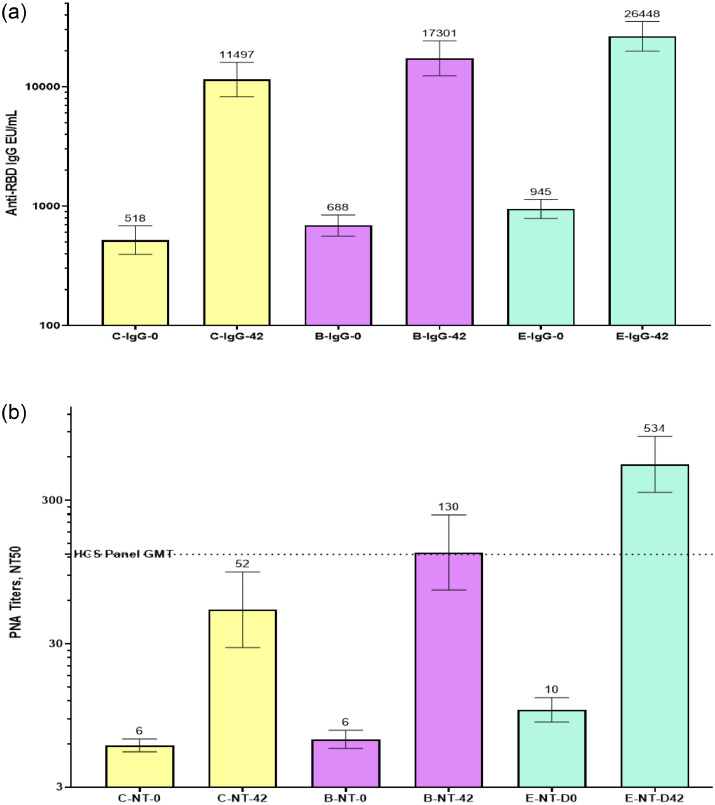
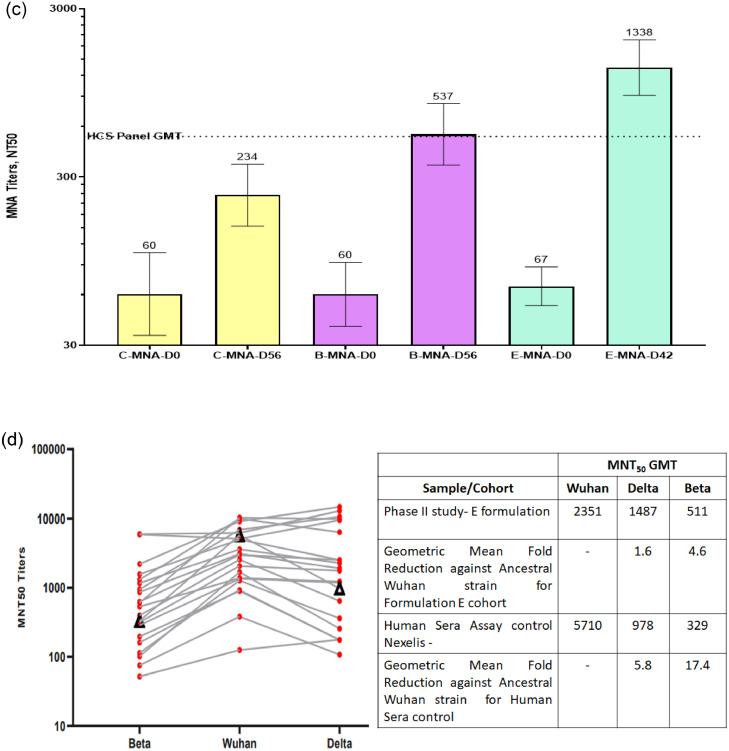
The subjects in the Phase-2 study received the modified vaccine formulation containing a relatively high CpG1018 adjuvant (Formulation E, Table 1) to increase the robustness and magnitude of the immune response. Immunogenicity parameters were measured in 100 subjects based on anti-SARS-COV-2 IgG seronegative status. The primary aim of this immunogenicity analysis was to assess the increase in immune response owing to changes in vaccine formulation; therefore, data are presented for the C and B groups from Phase-1/2, along with the E cohort from the Phase-2 trial. Formulation E induced a stronger anti-RBD IgG response (GMC, E-26448 vs. B-17301 and C-11497) than did formulations C and B, whereas the seroconversion rate was comparable for all three formulations (E, 89%; B, 88%; and C, 82%) on Day 42 (Figure 3a and Table 5a). Anti-RBD IgG1/IgG4 ratio was significantly higher in the Formulation E (75·4) group than in the Formulation C (38·0) or B (37·1) groups on Day 42 (Table 5b). The GMTs of nAb measured via the PNA method are shown in Figure 3b for days 0 and 42 with strong NT50 induced by Formulation E (GMT, 534) compared to that by B (GMT, 130) and C (GMT, 52). Similarly, Formulation E induced very high nAb GMTs measured via the MNA method on days 42 or 56 (E-1338 vs C-234 vs B-537) (Figure 3c). The delta and beta strains of SARS-COV-2 were isolated from Indian subjects by THSTI and were used to assess nAb-titres on Day 42 serum samples from a subset of 20 subjects (Figure 3d) using the MNA method. The results showed a minimal reduction in nAb titres against these two variants of concern (VOCs) indicating a significant potential of the vaccine under study to prevent infections. Most other vaccines have shown a significant drop (5-to 15-fold) in nAb titres against these two VOCs. Significantly high IFN-γ responses were induced by E formulation (99·82 pg/mL) on Day 56 compared to that by C (22·03 pg/mL) and B (31·42 pg/mL) (Table 5c).
Figure 3.
(a) Comparison of GMCs of anti-RBD IgG concentrations for formulations C and B in the Phase-1/2 study vs formulation E in the Phase- 2 study. The GMCs on Day 0 (pre-vaccination) and Day 42 (post-two-dose vaccination) are shown in the figure. Formulation E induced a stronger anti-RBD IgG response than did formulations C and B on Day 42 compared to that on Day 0. The GMCs are shown at the top of the respective columns, and the 95% confidence intervals are shown as two-sided bars.
RBD, receptor-binding domain; GMC, geometric mean concentration; IgG, immunoglobulin G.
(b) Comparison of nAb titres determined by the PNA method against PSV mimicking the ancestral Wuhan strain of SARS-COV-2 for formulations C and B in the Phase- 1/2 study vs formulation E in the Phase- 2 study. The GMTs on Day 0 (pre-vaccination) and Day 42 (post-two-dose vaccination) are shown in the figure. Formulation E induced high NT50 compared to that by formulations C and B tested using the PNA method. GMTs are shown at the top of the respective columns and the 95% confidence interval is shown as two-sided bars.
PNA, pseudovirus neutralisation assay; PSV, pseudovirus; HCS, high-content screening; GMT, geometric mean titre; NT, neutralising titre.
(c) Comparison of nAb titres determined by the MNA method against the ancestral Wuhan strain of SARS-COV-2 for formulations C and B in the Phase- 1/2 study vs formulation E in the Phase- 2 study. GMTs on Day 0 (pre-vaccination) and Days 56 or 42 (post-two-dose vaccination) are shown in the figure. Formulation E induced a very high NT50 compared to that by formulations C and B tested using the MNA method. GMTs are shown at the top of the respective columns and the 95% confidence interval is shown as two-sided bars.
nAb, neutralising antibody; MNA, micro neutralisation assay; HCS, high-content screening; GMT, geometric mean titre.
(d) Comparison of nAb titres determined by the PNA method against PSV mimicking the ancestral Wuhan strain of SARS-COV-2 for formulations C and B in the Phase-1/2 study vs formulation E in the Phase-2 study. The GMTs of MNA were measured for the beta and delta strains and compared with those for the ancestral Wuhan strain on serum samples of Day 42 in a subset of 20 subjects.
MNT, microneutralisation test; GMT, geometric mean titre; PNA, pseudovirus neutralisation assay.
Table 5a.
Anti-RBD IgG concentration Geometric Mean Fold Rise from Day0 (pre-vaccination) to Day42 (post second-dose) for all formulation C&B cohorts from Phase-1/2 and Formulation-E cohort from Phase II study.
| Formulation | Day42 GMFR | % Seroconversion at Day42 |
|---|---|---|
| C | 22·03 | 82% |
| B | 25·15 | 88% |
| E | 27·99 | 89% |
GMFR: Geometric mean fold rise; IgG: Immunoglobulin G.
Note: Percentage seroconversion observed for Day42 time-point sera samples based on ≥4-fold rise in anti-RBD-IgG concentration is also reported.
Table 5b.
Comparison of anti-RBD-IgG1 and IgG4·GMTs for all formulation C&B cohorts from phase-1/2 study and formulation E from phase-2 study.
| Formulation | N | IgG1 Titer |
IgG4 Titer |
IgG1/IgG4 RatioGMR post vaccination | ||||
|---|---|---|---|---|---|---|---|---|
| D-0 GMT | D-56 or D-42 | GMFR | D-0 GMT | D-56 or D-42 | GMFR | |||
| GMT | GMT | |||||||
| C | 89 | 65 | 1884 (D56) | 28·92 | 27 | 50 (D56) | 1·81 | 38·0 |
| B | 90 | 74 | 2940 (D56) | 39·7 | 31 | 76 (D56) | 2·48 | 37·1 |
| E | 98 | 126 | 7167 (D42) | 56·89 | 41 | 95 (D42) | 2·32 | 75·4 |
N:number; D-0: Day0; D-42: Day42; D-56: Day56; GMT: Geometric mean titer; GMFR: Geometric mean fold rise; IgG: Immunoglobulin G; GMR: geometric mean ratio.
Note: GMFR for each formulation cohort is calculated from the Geometric Mean for the Fold Rise in titer at Day56 or Day42 vs Day0 time-point for each cohort· Geometric Mean Ratio was calculated for IgG1 to IgG4 titers post vaccination for the three cohorts.
Table 5c.
Average cytokine concentration at day0 (pre-vaccination) and day56 or day42 (post second-dose).
| Formulation | Average Interferon-gamma concentration (pg/mL) |
Average IL-4 concentration (pg/mL) |
||||||
|---|---|---|---|---|---|---|---|---|
| D-0 Null | D-0 Active | D-56 Null | D-56 Active | D-0 Null | D-0 Active | D-56 Null | D-56 Active | |
| C | 1·99 | 5·59 | 2·08 | 22·03 | 1·06 | 1·69 | 1·02 | 1·60 |
| B | 2·04 | 3·73 | 1·91 | 31·42 | 3·77 | 3·72 | 2·73 | 3·19 |
| E | 7·03 | 26·24 | 2·63 | 99·82 | 5·19 | 4·26 | 8·10 | 10·96 |
D-0: Day0; D-56: Day56; IL-4: Interleukin 4; pg/mL: picograms per milliliter.
Note: The cytokine concentrations are measured in the supernatants of whole-blood samples incubated in tubes coated with SARS-COV-2 peptides (Active) and without coating (Null) for a subset of subjects from Formulation C&B cohorts from Phase-1/2 study and Formulation-E cohort from Phase-2 study.
Persistence of immune response in study subjects of Phase-1/2
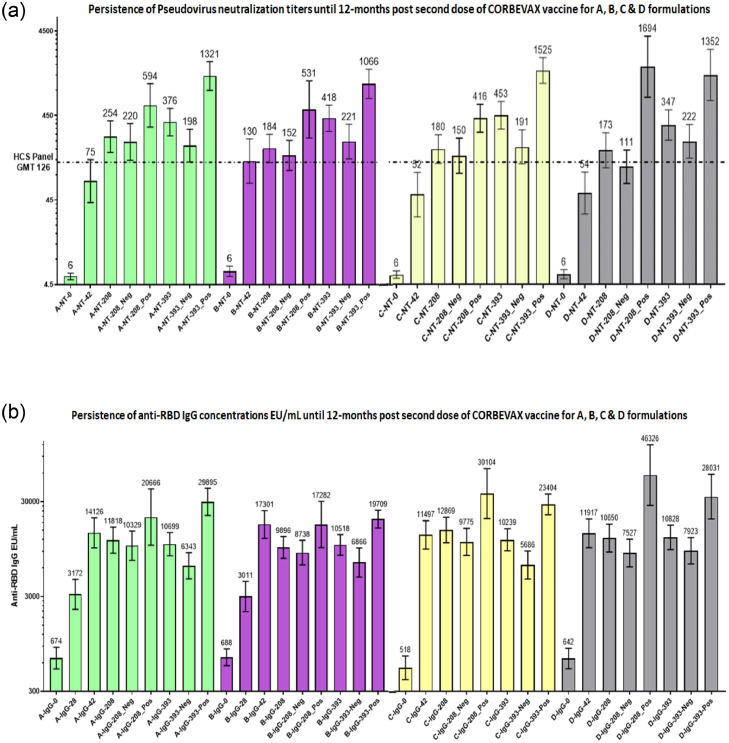
The persistence of immune response was assessed at six and 12 months after the second dose of vaccination. All serum samples were assessed for anti-RBD IgG concentration and nAb titres using the PNA method. Sero-surveillance studies have shown that a significant percentage of the population demonstrates asymptomatic infections, particularly during large waves. To determine the potential impact of asymptomatic infections on the overall immune response, serum samples were tested for antibodies raised against the N protein using a commercial kit (Abbott) with a threshold for anti-N IgG S/C ratio > 1·4 indicative of seropositive status. Serum samples of 6 and 12 months after the second vaccine dose were assessed for anti-N IgG concentration, and the serostatus of the subjects was assigned. nAb titres against PSV were measured at 6 and 12 months after the second vaccine dose. These data were further divided into anti-N seronegative and anti-N seropositive groups. nAb titres in anti-N seronegative and seropositive subjects on days 208 (six months) and 393 (one year) along with the titres at different time-points (Day 0, 28, 42,56, 208, and 393) are presented in Figure 4a and b. Approximately, 78% and 65% of the study subjects remained anti-N seronegative at six and 12 months, respectively. nAb titres persisted even six and 12 months after the second dose in vaccinated anti-N seronegative subjects, indicating that nAbs were vaccine-induced and not influenced by breakthrough infections. As expected, higher nAb titres were detected in anti-N positive subjects owing to asymptomatic infections. nAb titres in anti-N negative subjects at six- and 12-months post Corbevax vaccination are indicative of very high effectiveness of this vaccine when compared with the established high content screening (HCS) panel threshold (GMT of HCS panel, 126) or Correlate of Protection observed in other trials assessing vaccine efficacy.
Figure 4.
(a) Persistence of immune response in terms of nAb titres by PNA for formulations A, B, C, and D. Summary of the GMTs of nAb titres determined using the PNA method for all four groups on Day 0, after two weeks (D 42), six months (D 208), and 12 months (D 393) post second dose of vaccination is presented. Neg and Pos designations are for subjects who were seronegative and seropositive, respectively, as per ELISA results for nucleocapsid conducted using samples of six and 12 months post second dose of vaccine.
GMT, geometric mean titre; PNA, pseudovirus neutralisation assay; PSVNT, pseudovirus neutralisation titres; HCS, high-content screening; nAb, neutralising antibody.
(b) Persistence of anti-RBD IgG immune response. The GMCs of anti-RBD IgG concentration in all four groups on Day 0, Day 42, Day 208 (six months), and Day 393 (12 months). Minimal changes in the GMCs of anti-RBD IgG were observed at six and 12 months compared to those at two and four weeks after the second dose of vaccine. Neg and Pos represent subjects who were seronegative and seropositive as per ELISA results for nucleocapsid conducted using samples after six and 12 months post second dose of vaccine.
RBD, receptor-binding domain; GMC, geometric mean concentration; IgG, immunoglobulin G.
Discussion
The studies presented here were prospective, open-label, Phase-1/2 (randomised) and Phase-2 studies to assess the safety, tolerability, reactogenicity, and immunogenicity of four vaccine formulations (Phase-1/2) and optimal formulations (Phase-2) that contained the same antigen, i.e., the RBD protein, an essential target for vaccine development.22
The Corbevax vaccine, with a two-dose schedule and 28-day interval between doses, was safe and well-tolerated in all formulation groups. The percentage of subjects with reported AEs was comparable among all formulations (A, B, C, D, and E) tested in Phase-1/2 and Phase-2 studies. Most AEs were of mild intensity. No Grade-3 or serious AEs were reported. To date, very few MAAEs have been reported. During the long-term follow-up of subjects, other than two cases of mild COVID-19, no additional AEs were reported after 10–12 and five months for the Phase-1/2 and Phase-2 studies, respectively. Therefore, the optimised Corbevax formulation E was considered safe with minimal reactogenicity and will advance into pivotal Phase-3 studies.
In the Phase-1/2 study, both humoral and cellular immune responses were analysed to determine the impact of various compositions on the overall immune response. All four groups had similar GMCs on Day 0, which increased moderately by Day 28, representing a low immune response after the first dose. The GMCs increased substantially on Day 42 and plateaued by Day 56, showing a significant and stable immune response after the second vaccination dose. Formulation B induced the highest immune response among all four formulations.
Antibodies induced by vaccines neutralise disease-causing agents. All four formulations in this study induced comparable nAb titres that were significant after the second dose. Formulation B demonstrated the highest GMTs in the PNA and MNA methods and GMFR post-vaccination. The GMTs of nAb corresponding to Formulation B, 132 (PNA method) and 533 (MNA method), were higher than the established GMTs of the HCS panels of 126 (PNA method) and 522 (MNA method).
The Th1/Th2 balance in COVID-19 has been linked to disease outcome. An appropriate Th1 immune response is associated with disease clearance, whereas a Th2 response is associated with poor prognosis.23,24 in this study, all four formulations showed significant and consistently high ratio of IgG titres on Day 56, indicative of an immune response skewed towards Th1. This may be owing to the adjuvant CpG1018 in the formulation. CpG1018 is known to skew the immune response towards Th1, as opposed to alum-only adjuvants that tend to skew the immune response towards Th2.
The key cytokines monitored were IFN-γ (Th1-biased) and IL-4 (Th2–biased). On Day 0, cytokine concentrations were low or undetectable. A significant increase was observed in active IFN-γ concentration on Day 56 across all cohorts, with the highest by B-formulation. However, a slight increase was observed in IL-4 concentration on Day 56. This significant increase in Th1-biased immune response induced by the Corbevax vaccine further strengthens the role of Th1 responses in fighting COVID-19 infection.23,24
Significant increases in the magnitude of immune responses were observed by B formulation compared to that by C formulation, presumably owing to the CpG1018 content (250 and 500 µg per dose in C and B formulations, respectively). To further increase the magnitude and consistency of immune response, we increased the CpG1018 content in E-formulation to 750 µg per dose; the Phase-2 study confirmed expected improvement in immune response parameters while maintaining an excellent safety profile. Significant improvements anti-RBD IgG1 and nAb titres measured by the PNA and MNA methods were observed for E formulation compared to that for B formulation. We also tested the ability of Corbevax to protect from VOC. During the Phase-1/2 study, the dominant SARS-CoV-2 VOC in circulation in India was the beta strain. Nexelis laboratory created a PSV that expressed the spike protein of the beta strain of SARS-COV-2 with all known mutations. The same PSV strain was used in the PNA method to assess nAb titres against beta-PSV, which were measured and compared with observed nAb titres against ancestral D614G-PSV strain. The Corbevax formulation demonstrated excellent cross-neutralisation potential (2·25-fold reduction in nAb titres against the beta strain compared to that of the ancestral strain), which is significantly higher than that of other vaccines (e.g., mRNA and adenovector vaccines), which show 5–10-fold reduction in nAb titres.25,26
In the Phase-2 study, a subset of subject serum samples was tested against the wild-type beta and delta strains of SARS-COV-2, which showed only 1·6- and 4.6-fold reduction in nAb GMTs from the ancestral strain to the delta and beta strains, respectively. More importantly, detectable nAb titres were found in all serum samples against both beta and delta strains. Furthermore, the corresponding convalescent serum control (obtained during the initial wave, i.e., infection from the ancestral strain) showed 5·8-fold and 17·4-fold reduction in nAb titres against the delta and beta strains, respectively. These results suggest that Corbevax confers the most consistent cross-protection against the two most relevant VOCs.
The longevity of immune response is an essential attribute of any candidate vaccine, which is routinely assessed during long-term monitoring. The subjects showed excellent persistence of the humoral immune response over a significant duration of 12 months post-vaccination. This attribute of Corbevax is significantly superior to that of other vaccines that have demonstrated a 70–90% drop in the titres of binding antibody and nAb for the same duration.27, 28, 29 During the 12-month monitoring period after two doses of Corbevax in the Phase-1/2 study, only two subjects (one each from C and D formulation groups) reported mild symptomatic COVID19 infection. This corresponds to a COVID19 incidence rate of approximately seven cases per 1000 person per year, indicating a high vaccine efficacy. nAb titres in serum samples after two doses of vaccine correlated with protection (CoP) against symptomatic COVID19 infection, which was reported in Phase-3 efficacy studies of Spikevax30 (Moderna Inc.) and Vaxzveria31 (AstraZeneca Inc.). Both studies reported the CoP information in terms of nAb titres (IU/mL) using calibration factors to convert nAb titres to the WHO International Standard. Their CoP evaluation studies suggest that nAb GMT > 100 IU/mL post two-dose vaccination correspond to a significant vaccine efficacy > 90% higher than that by placebo control. The nAb GMTs on Day 42 (14 days after the second dose, similar to that in case of Spikevax and Vaxzveria) in the Phase-2 study using Corbevax were 285 and 329 IU/mL based using the PNA and MNA methods, which are indicative of a vaccine effectiveness > 90%. The ratio of nAb GMTs post-vaccination to the HCS panel nAb GMT correlates with vaccine efficacy independent of its nature.32 These ratios after the Corbevax vaccination in the Phase-2 trial were 4·2 and 2·6 based on the PNA and MNA methods, respectively, which also indicate > 90% vaccine efficacy.
This was an open-label and not a double-blind study. The study population did not include paediatric and elderly (> 65 years) age groups. nAb titres against the Omicron strain were not tested because this variant did not start circulating in India during the study. A limited number of subjects were included in Phase-1/2 and Phase-2 studies. Vaccine performance will be further tested in a larger cohort with a broader age range (5–80 years) in an ongoing Phase-3 study.
To evaluate the persistence of an immune response generated by vaccination, serum samples were tested to determine anti-N IgG levels. Subjects were designated seropositive or seronegative based on the recommended cut-off for seropositivity. However, the serostatus indicated by anti-N IgG testing is a point assessment and is also subject to waning of the immune response over time. Therefore, it may not be fully representative of viral exposure/asymptomatic infections during long-term monitoring.
The Corbevax vaccine is safe and well-tolerated in healthy adult volunteers (18–55 years) of Indian origin with no AEs of clinical concern, shows > 90% effectiveness, and provides protection against symptomatic COVID-19 infection. The excellent maintenance of antibody binding and nAb titres over a 6-month duration after two doses of all four vaccine formulations indicates that a high level of protection from symptomatic infection will be sustained for an extended duration. This is in contrast to the significant waning of immune responses and effectiveness observed for most other COVID-19 vaccines. Based on the safety profile, significant and robust humoral and cellular immune responses, and desired Th1 skewed immune response post Corbevax vaccination, pivotal Phase-3 clinical trials have been initiated using the selected optimal formulation of the vaccine.
Contributors
ST and VP conceptualised the study and edited the manuscript for intellectual content. ST, SG, VY, RM, and KT curated, accessed, and verified the data, and helped in interim report generation. VP, MK, SKM, GM, and NG performed immunogenicity experiments. GM and NG contributed to the performance and analysis of neutralising antibody assays. CS and VRA were the key contributors to the study. ST was responsible for the overall supervision of the project. All authors contributed to data interpretation, review, and editing of this manuscript. All authors have read and approved the final version of the manuscript.
Data sharing statement
Study data presented in the manuscript can be made available upon request and addressed to the corresponding author Dr. Subhash Thuluva at his email: subhash.thuluva@biologicale.com.
Declaration of interests
ST, VP, KT, SG, VY, RM, MK, and SKM are employees of Biological E Limited, and do not have any stock options or incentives.
All the other participating authors declare no conflicts of interest.
Acknowledgements
We are thankful to all study participants, principal investigators, and study staff at all clinical sites. We are thankful to Dr. Leena Chatterjee, Dr Arjun Dang, Mr. Dinesh Kuma, and Mr. Shakeeb Mohammad, Dr.Dangs Lab, New Delhi, India for study sample coordination (receipt, accessioning, aliquoting, labelling, storage, and dispatch) and performing ELISA assays (anti-RBD and cytokines). We thank THSTI, India for conducting neutralising antibody titre testing. We are thankful to Anbalagan Anantharaj, Kamal Pargai, Parveen Kumar, Alok Tripathi, Neha Garg, and Shamsher Singh from the Bioassay Lab, and the microneutralisation assay group from THSTI, India. Finally, we appreciate the help rendered by the team at Nexelis in conducting the neutralising antibody titre testing against pseudovirus expressing SARS-COV-2 spike protein. Dr. Maria Bottazzi and Dr. Peter Hotez, and their scientific team at the Centre for Vaccine Development at Baylor College of Medicine/Texas Children's Hospital created and produced the recombinant Pichia pastoris strain expressing the RBD protein. Dynavax Inc. supplied CpG1018. The clinical assay development team led by Dr. Arun Kumar at CEPI, India helped with neutralising antibody titre assays by supplying reagents and establishing assay consistency across multiple laboratories. Writing support for this manuscript was provided by Syneos Health. The authors would like to thank Dr. Suneetha Pothakamuri, Mr. Kamal Thammireddy, Mr. Kalyan Kumar P, Mr. Raju Esanakarra, and Mr. Naga Ganesh B for their valuable support in reviewing and finalising the manuscript. The authors also thank Mr. Srinivas Kosaraju and Mr. Varma Bhupathiraju for their regulatory support and guidance. Development of this vaccine candidate would not have been possible without the efforts of the manufacturing, quality control, quality assurance, and regulatory teams from Biological E. The authors would like to thank the Scientific Advisory Board (SAB) and the Management of Biological E Limited for their support and valuable guidance. All authors wish to express their appreciation and gratitude to all the frontline healthcare workers. We would also like to thank the members of the data safety monitoring board (DSMB) for the safety monitoring of the study data. The study was funded by grants from the Bill & Melinda Gates Foundation, BIRAC, a division of the Department of Biotechnology, Government of India, and by the Coalition for Epidemic Preparedness Innovations.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.104217.
Appendix. Supplementary materials
References
- 1.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flanagan KL, MacIntyre CR, McIntyre PB, Nelson MR. SARS-CoV-2 vaccines: where are we now? J Allergy Clin Immunol Pract. 2021;9:3535–3543. doi: 10.1016/j.jaip.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang Z, Zhu H, Wang X, et al. Adjuvants for coronavirus vaccines. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.589833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuo TY, Lin MY, Coffman RL, et al. Development of CpG-adjuvanted stable prefusion SARS-CoV-2 spike antigen as a subunit vaccine against COVID-19. Sci Rep. 2020;10:20085. doi: 10.1038/s41598-020-77077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen WH, Wei J, Kundu RT, et al. Genetic modification to design a stable yeast-expressed recombinant SARS-CoV-2 receptor binding domain as a COVID-19 vaccine candidate. Biochim Biophys Acta Gen Subj. 2021;1865 doi: 10.1016/j.bbagen.2021.129893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pollet J, Chen WH, Versteeg L, et al. SARSCoV-2 RBD219-N1C1: a yeast-expressed SARS-CoV-2 recombinant receptor-binding domain candidate vaccine stimulates virus neutralizing antibodies and T-cell immunity in mice. Hum Vaccin Immunother. 2021;17:2356–2366. doi: 10.1080/21645515.2021.1901545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee J, Liu Z, Chen WH, et al. Process development and scale-up optimization of the SARS-CoV-2 receptor binding domain–based vaccine candidate, RBD219-N1C1. Appl Microbiol Biotechnol. 2021;105:4153–4165. doi: 10.1007/s00253-021-11281-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen WH, Tao X, Agrawal AS, et al. Yeast-expressed SARS-CoV recombinant receptor-binding domain (RBD219-N1) formulated with aluminum hydroxide induces protective immunity and reduces immune enhancement. Vaccine. 2020;38:7533–7541. doi: 10.1016/j.vaccine.2020.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen WH, Pollet J, Strych U, et al. Yeast-expressed recombinant SARS-CoV-2 receptor binding domain RBD203-N1 as a COVID-19 protein vaccine candidate. Protein Expr Purif. 2022;190 doi: 10.1016/j.pep.2021.106003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dispinseri S, Secchi M, Pirillo MF, et al. Neutralizing antibody responses to SARS-CoV-2 in symptomatic COVID-19 is persistent and critical for survival. Nat Commun. 2021;12:2670. doi: 10.1038/s41467-021-22958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varnaitė R, García M, Glans H, et al. Expansion of SARS-CoV-2-Specific antibody-secreting cells and generation of neutralizing antibodies in hospitalized COVID-19 patients. J Immunol. 2020;205:2437–2446. doi: 10.4049/jimmunol.2000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robbiani DF, Gaebler C, Muecksch F, et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584:437–442. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang J, Wang W, Chen Z, et al. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature. 2020;586:572–577. doi: 10.1038/s41586-020-2599-8. [DOI] [PubMed] [Google Scholar]
- 14.LIAISON® SARS-CoV-2 S1/S2 IgG. The fully automated serology test for the detection of SARS-CoV-2 IgG antibodies, Saluggia; Italy.
- 15.Data Sheet NIBSC. Research Reagent for Anti-SARS-CoV-2 Ab NIBSC Code 20/130. Medicines and Healthcare products Regulatory Agency, Hertfordshire, United Kingdom.
- 16.Bewley KR, Coombes NS, Gagnon L, et al. Quantification of SARS-CoV-2 neutralizing antibody by wild-type plaque reduction neutralization, microneutralization and pseudotyped virus neutralization assays. Nat Protoc. 2021;16:3114–3140. doi: 10.1038/s41596-021-00536-y. [DOI] [PubMed] [Google Scholar]
- 17.Mattiuzzo G, Bentley EM, Hassall M, et al. Establishment of the WHO international standard and reference panel for anti-SARS-CoV-2 antibody. World Health Organization, Geneva. 2020 WHO/BS/2020.2403; 2020, p. 60. [Google Scholar]
- 18.Petrovsky N, Harrison LC. Cytokine-based human whole blood assay for the detection of antigen-reactive T cells. J Immunol Methods. 1995;186:37–46. doi: 10.1016/0022-1759(95)00127-v. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization; Geneva: 2015. Guidelines on Clinical Evaluation of Vaccines: Regulatory Expectations; pp. 1–91. [Google Scholar]
- 20.Yang S, Li Y, Dai L, et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomised, double-blind, placebo-controlled, Phase 1 and 2 trials. Lancet Infect Dis. 2021;21:1107–1119. doi: 10.1016/S1473-3099(21)00127-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadoff J, Le Gars M, Shukarev G, et al. Interim Results of a Phase 1–2a Trial of Ad26.COV2.S Covid-19 Vaccine. N Engl J Med. 2021;384:1824–1835. doi: 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Min L, Sun Q. Antibodies and vaccines target RBD of SARS-CoV-2. Front Mol Biosci. 2021;8 doi: 10.3389/fmolb.2021.671633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neidleman J, Luo X, Frouard J, et al. SARS-CoV-2-specific T cells exhibit phenotypic features of helper function, lack of terminal differentiation, and high proliferation potential. Cell Rep Med. 2020;1 doi: 10.1016/j.xcrm.2020.100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roncati L, Nasillo V, Lusenti B, Riva G. Signals of Th2 immune response from COVID-19 patients requiring intensive care. Ann Hematol. 2020;99:1419–1420. doi: 10.1007/s00277-020-04066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Planas D, Veyer D, Baidaliuk A, et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596:276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 26.Davis C, Logan N, Tyson G, et al. Reduced neutralisation of the Delta (B.1.617.2) SARS-CoV-2 variant of concern following vaccination. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flaxman A, Marchevsky NG, Jenkin D, et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: a substudy of two randomised controlled trials (COV001 and COV002) Lancet. 2021;398:981–990. doi: 10.1016/S0140-6736(21)01699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doria-Rose N, Suthar MS, Makowski M, et al. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for Covid-19. N Engl J Med. 2021;384:2259–2261. doi: 10.1056/NEJMc2103916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Falsey AR, Frenck RW, Walsh EE, et al. SARS-CoV-2 neutralization with BNT162b2 vaccine Dose 3. N Engl J Med. 2021;385:1627–1629. doi: 10.1056/NEJMc2113468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilbert PB, Montefiori DC, McDermott AB, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375:43–50. doi: 10.1126/science.abm3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng S, Phillips DJ, White T, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27:2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.