Abstract
Ablative surgery of the mandibular condyle poses a unique reconstructive challenge for many reasons. The condyle and it's relationship to the TMJ is a unique, complex, functional and aesthetically relevant piece of human anatomy. Resection may be required for both malignant and benign pathologies; each posing a differing set of surgical variables. Particularly in neoplastic processes, there must remain a certain degree of peri-operative flexibility with regards to the extent of the resection, and forethought to the requirement for post-operative radiotherapy; both of which further complicate choice of reconstructive option and surgical or prosthetic planning. The cases involved can often concern paediatric patients, and an additional aspect to be considered is that of growth potential.
In this piece, we will discuss the indications for ablation and the techniques involved. We will elaborate on the reconstructive challenges specific to reconstructing the condyle in post-ablative cases. We will then describe and analyse the established reconstructive techniques; aiming to provide a balanced view on the advantages and disadvantages. Our focus will include autologous options such as vascularised and non-vascularised free tissue transfer, and the non-autologous options of custom and stock implants. We will also touch on distraction osteogenesis and ramus osteotomies. Lastly we will look to the future and consider possible innovative techniques which may become available to the surgeon.
Keywords: TMJ, Temporo-mandibular joint, Reconstruction, Condyle, Post-ablation, Ablative surgery, Resection
Graphical abstract
1. Introduction
Ablative resection of the mandibular condyle poses a challenging reconstructive problem and there remains a lack of evidence base, and significant controversy, regarding the optimal reconstructive option to restore form and function.1, 2, 3 The mandibular condyle has unique anatomy in view of morphological recreation, but also biomechanically due to it’s involvement in the temporo-mandibular joint (TMJ.) The relationship makes the condyle integral to function in mastication and speech, and vital to maintain vertical height for a stable occlusion and for facilitating facial symmetry.2,3 Pathology that requires ablative surgery or resection of the condyle includes malignant processes (e.g. oral squamous cell carcinoma), benign tumours (e.g. ameloblastoma), osteonecrosis, osteomyelitis or other rarer pathologies such as vascular lesions.3 The ablative techniques required are varied and may range from simple condylar head excision to hemi-mandibulectomy with associated soft tissue; which poses a unique set of variables in planning the reconstruction.3,4 In addition, aspects such as post-operative radiotherapy and donor site morbidity must be considered. The latter plays a significant part in the decision regarding autologous vs non-autologous reconstruction3; this distinction is particularly pertinent in the treatment of paediatric patients and the consideration of the effect on, and the potential for, growth.5,6 In this review the authors will discuss the established techniques for reconstruction of the condyle in post-ablation surgery but assume the reader has some pre-existing knowledge of the specific techniques. The paper will analyse the advantages and disadvantages, explore the challenges and look to the future in an attempt to inform the reader in view of deciding upon the ideal reconstructive option.
2. Pathology of the condyle requiring ablation (see Table 1)
Table 1.
A table summarising examples of pathologies involving the condyle that may require ablative surgery.
| Benign Pathology | Malignant Pathology |
|---|---|
| Tumours | Primary |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Other | Secondary |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pathologies of the mandibular condyle warranting ablation are not commonplace but can affect adults and children, and are often complex in their resection and subsequent reconstruction. Benign odontogenic tumours such as ameloblastomas or myxomas, can extend to the condyle requiring subcondylar resection as a minimum. Benign tumours affecting the condylar head itself include chondroma or osteoma, as well as giant cell lesions and rarities such as chondroblastomas.7 Other benign pathology includes fibro-osseous lesions (e.g. fibrous dysplasia) vascular lesions (e.g. haemangioma, arterio-venous malformations) neural tumours (e.g. neurofibroma) and rarer conditions like vilonodular synovitis.7
Malignant pathology requiring ablation of the condyle in adults can be carcinoma, either local invasion from surrounding tissues e.g. oral squamous cell carcinoma, skin (e.g SCC, BCC, melanoma) or parotid, or from metastatic spread (e.g. lung, thyroid, breast, prostate or renal.) Lymphoma or multiple myeloma can present with bony lesions but these rarely require surgical treatment.3,7 Less commonly primary malignancy of the bone such as osteosarcoma, chondrosarcoma, synovial fibrosarcoma can occur and would require radical wide local excision.8 Sarcomatous malignancies are more commonly seen in paediatric patients and locally invasive non-osseous neoplasms such as rhabdomyosarcoma can result in requirement for bony resection.5,6,8 Osteomyelitis (usually odontogenic origin) can, when extensive, involve the condyle and warrant significant debridement or ostectomy resulting in loss of the condyle.3 Osteoradionecrosis can affect the ramus and condyle and although generally conservatively managed could rarely require resection.9
3. Ablative techniques
The ablative technique and degree/extent of resection is usually based predominantly on the pathology. Malignancies may require wide local excision involving not just a standard condylectomy, but segmental resection or hemi-mandibulectomy.1,4 More extensive tumours may require concomitant excision of the glenoid fossa and temporal or zygomatic bone, or local soft tissue. There will also to some extent be variations in the ability to preserve the disc, some form of capsule and the muscular attachments.3
4. Reconstructive challenges in post-ablation cases
In comparison to joint replacement surgery for degenerative or inflammatory aetiologies, ablation cases have unique aspects that can make the reconstruction challenging. The resection margin is not always predictable and even if planned, may need to be adapted peri-operatively.1,3 The necessity for intra-operative flexibility requires the surgeon to often adapt their reconstruction on table. Computer assisted design and manufacture (CAD-CAM) and virtual surgical planning (VSP) with consideration of alterations to the osteotomies can mitigate this somewhat, but it is costly, takes time, cannot always flexibly compensate and is not always completely predictable.10, 11, 12 Although this is not such an issue in benign cases as margin modification is not so common, it is not always appropriate for malignant cases. In cancer resection, the potential for requiring post-operative radiotherapy plays a large part in the reconstructive decision. A non-vascularised reconstruction or prosthesis is at significant risk of necrosis or becoming exposed during the oncological treatment.3 However, the use of autologous, whether vascularised or not, poses the issue of donor site morbidity as well as not being immune to effects of radiotherapy.
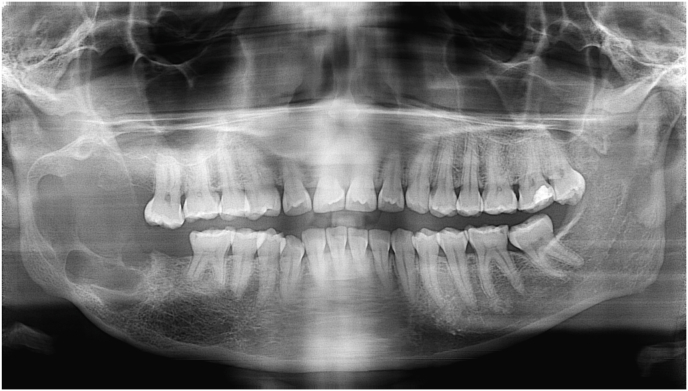
When the lesion is not isolated to the condyle (e.g. extensive ameloblastoma,) the ablation is not restricted to the condyle alone and requires different reconstructive solutions. A hemi-mandibulectomy poses a significantly more challenging reconstructive problem whether autologous or non-autologous materials are used, with the addition of forward planning for dental rehabilitation (See Fig. 1.).
Fig. 1.
An OPG showing an extensive ameloblastoma of the right hemi-mandible. When considering the post-resection defect, one can appreciate the associated reconstructive challenges.
Paediatric cases pose an additional problem of the effect of growth of the child, but also the reconstruction having growth potential too.5,6 However some clinicians who support Moss's theory of functional growth have utilised alloplastic prostheses in paediatric patients with some degree of functional growth; this is an ongoing area of controversy and academic discussion.2,6
5. Techniques
The last 20–30 years has seen a significant shift in thinking in TMJ reconstruction regarding the use of non-autologous materials, particularly as development in material science and innovation in VSP and CAD/CAM has increased.2,13, 14, 15, 16, 17, 18 However, in the majority of ablative cases for malignancy and paediatric cases, autologous reconstruction provides tissue that is vascularised, biocompatible, resistant to the effects of radiotherapy and may have the potential for growth.1, 2, 3, 4,6,19
5.1. Autologous reconstruction
5.1.1. Vascularised free tissue transfer (see Table 2)
Table 2.
A table summarising the advantages and disadvantages of some common autologous reconstructive options.
| Reconstruction | Advantages | Disadvantages |
|---|---|---|
| Non-Vascularised |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
| Vascularised |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In cases of hemi-mandibulectomy for resection of a malignant process including all or part of the condyle, the fibula free flap is now the autogenous reconstructive mainstay.3,19 It involves the raising of a length of the fibula from the leg with a pedicle of the fibular/peroneal artery. The flap is disconnected and transferred to the jaw where it is anastomosed to suitable vessels in the neck and inset to create a neo-mandible.1,3 The fibula free flap provides a long length (up to 26 cm) of tubular vascularised bone that is very amenable to virtual surgical planning. 3D printed cutting guides can be used to plan multiple segmental osteotomies to recreate the mandibular contour, and the graft may accordingly be ‘double-barrelled’ to provide greater bone height.5,10,19 It is raised with a reliable and long pedicle, and from a donor site with low morbidity.12,18 The free flap bio-integrates, has a good soft tissue relationship, can be harvested with a skin paddle and will better resist the post-operative radiotherapy. However, the downside of using an osteotomised segment from a long bone, is that it cannot be shaped to accurately recreate the condylar contour, has no cartilaginous cap and thus results in sup-optimal joint mechanics. To some extent, the issue of limited recreation of joint anatomy with the fibula can be overcome by combining the free tissue graft with an alloplastic implant. In the long term, there is a risk of ankylosis and recurrent dislocation.3,19 In addition, although the donor site morbidity is low, complications include an unsightly scar, paraesthesia, foot drop or ischaemia of the distal limb.3,6,12,19
Alternative free flap options include iliac crest, scapula or composite radial; each with their own advantages and disadvantages depending on the reconstructive requirements.1,3,12 The deep circumflex iliac artery/iliac crest free flap, raised as an osseous flap or with myo-cutaneous components provides very good quality and thickness of vascularised bone which has favourable natural morphology to the mandible. However, it cannot provide as much length of bone as the fibula, is raised with a relatively short pedicle and the post-operative donor site complications can result in significant morbidity. Many consider it superior for short segmental defects in which osseo-integrated dental implants are planned.1,3,12 Scapula flaps have a reasonable pedicle length and very versatile soft tissue. However the thickness of the bone is often limited for dental rehabilitation, does not have segmental blood supply and requires a change in the patient's intra-operative position for raising. Composite radial flaps have a long pedicle and a useful skin paddle, however the bone is of low volume and quality. It has been used in reconstructing the posterior mandible and condyle but is rarely the first choice.1.3.12 These flaps can be the preferred choice for segmental mandibular reconstruction or for restoring the posterior ramus, their limited use in reconstruction of the condyle is preferred in cases where the condylar head remains largely intact and their predominate role is for maintaining vertical ramus height.1,3,12 Other less common free tissue osseous flaps for mandibular reconstruction such as the rib, femoral medial epicondyle and second metatarsal phalangeal flaps exist and have been used for condylar reconstruction but have limited published outcomes.3
Many connotations and modifications exist regarding how the condylar head is recreated. If the resection margin is through the distal condylar neck then the condyle may be preserved in situ with disc and capsule intact, and the fibular can be plated to this.4,12,19 If the condyle is unaffected, but the osteotomy is more proximal or the surgery requires joint disarticulation, the condylar head can be removed and transplanted back as a free tissue graft.4,12,19 If the ablative surgery requires total condylectomy then an excess of bone can be planned and shaped to create a neo-condyle; this can be inset with or without the retained disc and capsule.20 Small alloplastic condylar components can also be connected proximally; usually integrated to the plate and can be combined with glenoid resurfacing or reconstruction. Hemi-arthroplasty reconstruction of this sort have however been shown to be prone to glenoid fossa erosion.3
5.1.2. Non-vascularised autologous reconstruction (see Table 2)
One the most commonly used non-vascularised autogenous reconstructive options for reconstruction of the condyle/TMJ is the free costochondral graft.3 The contralateral fifth, sixth or seventh rib is the most commonly used and has obvious benefits of appropriate size, adaptability, a cartilaginous cap, and low donor site morbidity. In the growing patient, it also has the potential for growth.6 However this can be hard to predict and result in late asymmetry or facial deformity. The unpredictable bone biology can result in late complications of resorption or formation of heterotrophic bone.6 In adult patients, it has the significant potential to ankylose.3 Although generally donor site morbidity is low, there is the potential for inadvertent pneumothorax. An alternative less common donor sites is the sternoclavicular joint, but can result in significant morbidity and has limited long term outcome data.3 Alternative non-cartilaginous techniques include iliac crest or calvarium.3 An innovative technique that uses autogenous mandibular bone is the use of the ipsilateral coronoid.22 The bone has been shown to resist the typical resorption of iliac crest or rib, and has the obvious benefit of no distant donor site. It's limitations lie in the small size of defect it can be used for (i.e. condylar head and neck only), and it's minimal published cases. The techniques described above are much more commonly utilised in the paediatric population due to the transfer of a potential growth centre and the possible inclusion of a cartilaginous cap. In adults previous data suggests a higher likelihood of revision, and better outcomes with alloplastic TMJ replacement.2,13,14
5.1.3. Distraction osteogenesis
Although not used as commonly in ablative cases, there are a few suitable scenarios where distraction osteogenesis can provide benefits such as the use of native bone, neo-genesis of the soft tissue envelope, no donor site morbidity and ability for subsequent bony reconstruction to grow with the patient.3,6 The major issue is determining the correct vector for the transport segment, to recreate what is complex bony anatomy. Recent advances in computer assisted virtual planning and multi-directional/multi-vector distractors have the potential to improve outcomes and make distraction osteogenesis a more favourable option. However pin site infection and scarring is not an uncommon complication, as well as device failure, non-union, bony resorption and subsequent malocclusion. Furthermore, there are potential long term limitations due to muscle contracture and host bone biochemistry resulting in resorption, particularly at the neo-condylar head.3,6
5.1.4. Ramus osteotomies
Restoring posterior height and reconstructing the condyle can be achieved by designing osteotomies to either slide bone superiorly, to maintain height and provide bone for either the neo-condyle, or for plating the condylar head – either as a free graft or maintaining capsular blood supply.2,3,21 Examples include sub-sigmoid vertical ramus osteotomy and closing wedge osteotomies have been described to alter the angle of bone to form a neo-condylar neck.21
5.2. Non-autologous reconstruction
Although historically, autogenous reconstruction has been preferred due to sub-optimal outcomes and failure rate in alloplastic reconstruction, there has been a significant shift over the last few decades as advances in material science and VSP/CADCAM has facilitated excellent long term results with alloplastic TMJ replacement in adults.3,13, 14, 15, 16,18 In comparison to autogenous options, benefits include a lack of donor site morbidity, in biological response e.g. growth or resorption of the reconstruction, reduced intra-operative time, immediate function and surgical consistency/predictability. It is now generally considered the first choice in the reconstruction of benign ablative defects involving the condyle in adults.3,13, 14, 15, 16, 17, 18
Over the last 100 years a multitude of materials, shapes, fixation and biomechanics have been used in attempts to create an optimal reconstructive option. The currently most accepted prosthesis is a titanium condylar component with an ultra-high molecular weight polyethylene glenoid fossa component.3,13, 14, 15, 16, 17, 18 The predominant prosthetic decision surrounds the use of stock implants vs custom implants (see Table 3). Custom implants have the obvious advantage of being bespoke to the patients anatomy and can be carefully designed using CAD/VSP software to create cutting guides and custom implants. However the obvious downsides are cost and time. Although stock implants are only produced in a series of sizes, angulations and designs, this does not preclude them from being utilised alongside VSP/CADCAM process.11,12 In particular, the evaluation with regards to potential interferences is key in ensuring stability in fixation. This also facilitates the use of custom cutting guides planned based on the stock dimensions and anatomical planning for ideal placement of the prostheses based on alveolar nerve position and defect.12
Table 3.
A table summarising some of the advantages and disadvantages of stock vs custom TMJ implants.
| Stock Prosthesis | Custom Prosthesis | |
|---|---|---|
| Advantages |
|
|
| Disadvantages |
|
|
In cases of severe craniofacial deformity, or cases with significant biomechanical discrepancy, stock implants are not as suitable. In benign ablative surgery, custom implants are often the reconstructive option of choice in many cases over stock implants. Custom prostheses ensure optimal primary fixation in the context of marked angulation or shape of defect.16,17 Nonetheless, in the setting of ablative surgery a custom implant has it’s disadvantages such as in cases where there is greater than planned resection, poor bone quality, altered shape of fixation site, or intra-operative features that indicate a requirement for post-operative radiotherapy.3,16,17 A specific aspect of the decision-making process in custom reconstruction is to utilise a single or 2-stage approach. The single stage approach requires pre-operative CT scanning and either data upload or model creation. Virtual surgical planning is undertaken with planned resection/osteotomies and subsequent implant manufacture. The ablative surgery takes place and the implant is placed during the same surgery. The two-stage process involves the ablative surgery being undertaken, MMF placement and the patient is re-scanned post-operatively. During the initial surgery a pre-fabricated or intra-operatively formed spacer may be placed. The custom implant is then planned, designed and manufactured based on the defect. Obvious advantages are that the occlusion and defect are set and consistent. The disadvantage is clearly a second surgical procedure but also the long term MMF during device manufacture.3,16,17 Custom implants can now be manufactured entirely via computer with virtual surgical planning and designed without any physical models. This provides the technological advantages of manipulation of the virtual anatomy and visualisation of the nerve and bone thickness for screw position. Furthermore, it allows unlimited degree of modification of shape and dimensions of the prosthesis and also, by overlapping pre- and post-operative imaging, assess any degree of torqueing or rotation of the contralateral joint. Lastly, for very complex cases cutting guides and drill templates can be manufactured, or the computer software data can be imported in CT-guided surgical software to ensure correct osteotomy and implant positioning intra-operatively.
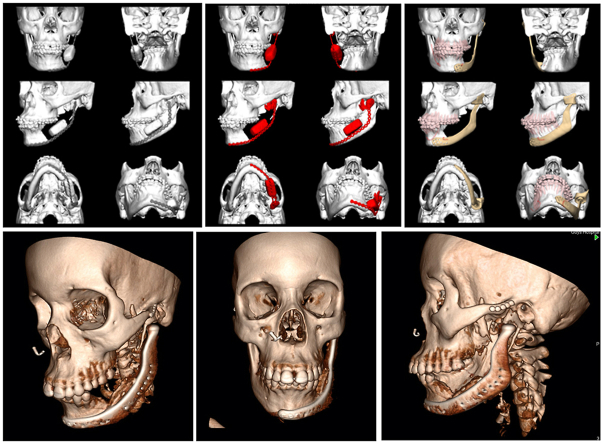
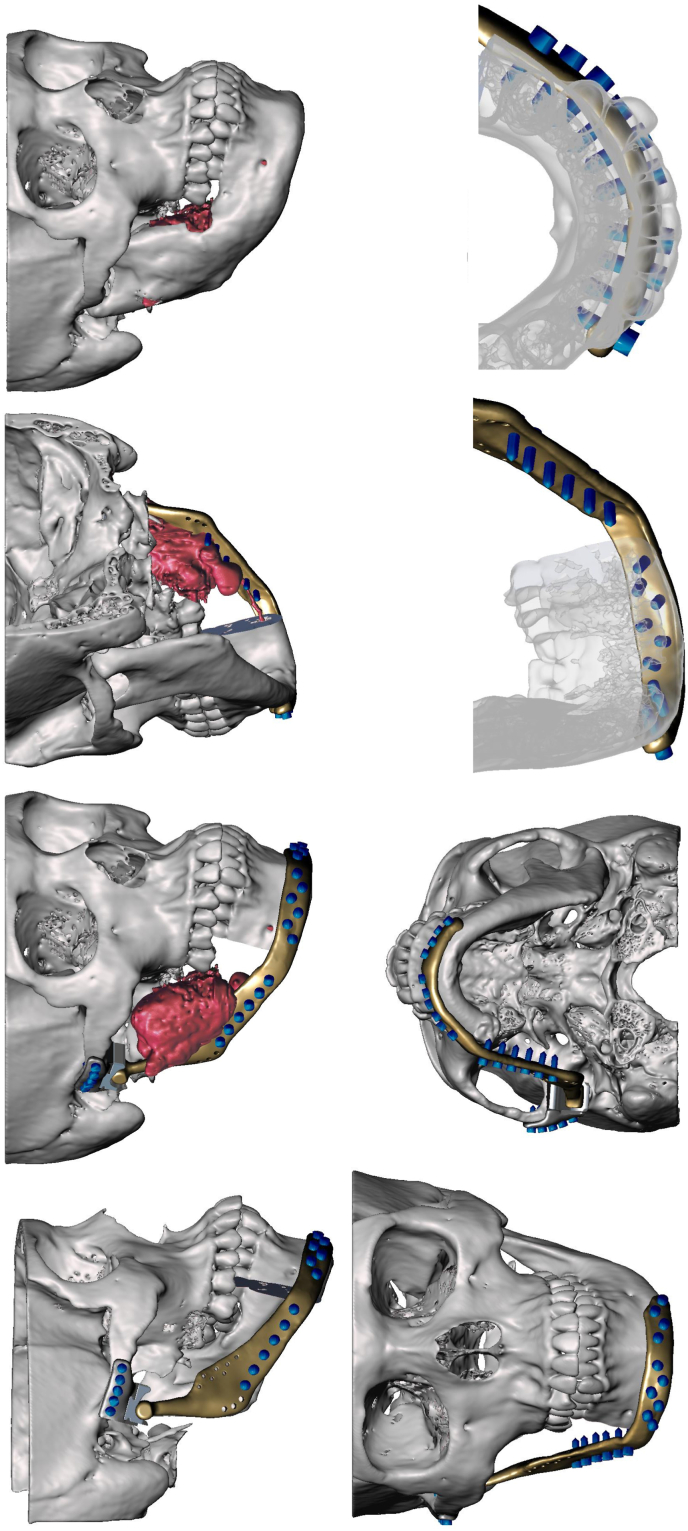
A relatively less common concept that can be utilised in cases where there is significant loss of native mandible after ablation such as hemi-mandibulectomy is an extended custom made TMJ replacement.23 In a large proportion of cases, the pathology will be malignant and thus require post-operative radiotherapy. This would in all likelihood determine a vascularised osseous free flap such as a fibula as the first line choice. However in extensive benign cases such as ameloblastoma, myxoma or fibro-osseous lesions, it may be necessary to resect significantly more than the condyle. In these cases an option is what has been termed an extended TMJ implant, eTJR or TMJe (see Fig. 2, Fig. 3).23 In these cases the custom planning incorporates the possibility of a titanium condylar component that can reach to the midline or even the whole mandible. At time of writing, there is not any large scale trials or long-term evidence but the largest cases series as yet published by our group, includes some ablative cases which have undergone extended TMJ prosthesis reconstruction with favourable outcomes. One important aspect to bear in mind in these cases is vertical stabilisation to prevent “condylar sag” resulting in a condyle seated outside of the glenoid fossa. The authors mitigate this by using a technique described by Westermark, whereby an additional hole is incorporated into the proximal ramus prosthesis to facilitate it's suspension from the zygomatic arch with a polydioxanone suture. Furthermore, consideration of the soft tissue envelope; specifically conscientious repair of the pterygo-masseteric sling, contributes to the support of the hard tissue reconstruction, implant coverage and function.
Fig. 2.
A series of images of the virtual surgical planning of the case in Fig. 1. Note the planned defect involved the mandibular condyle and was planned to be reconstructed with a custom extended TMJ replacement.
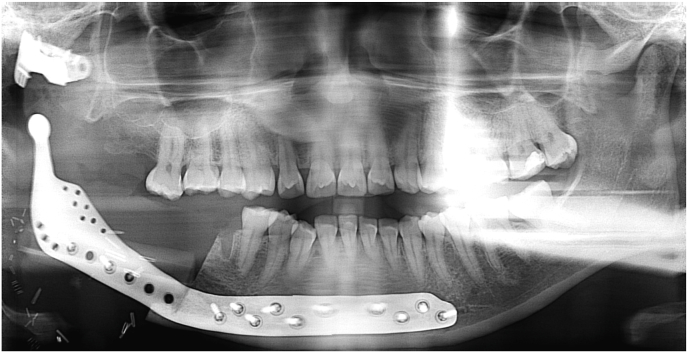
Fig. 3.
An OPG showing the post-operative imaging of the case from Fig. 1, Fig. 2. Note the right hemi-mandibular defect reconstructed with a custom extended TMJ replacement.
5.3. Future advances
There is the significant potential for relevant and exciting advances in reconstruction of the condyle in the fields of prosthetics, biomaterials and tissue engineering. Currently, only Stryker TMJ concepts and Zimmer Biomet have long term outcome data for alloplastic TMJ protheses.24 However, there are currently over 25 new systems being developed worldwide. The majority are custom designs, use ultra-high-molecular-weight polyethylene for the glenoid component and 3D printed (selective laser melting or direct metal laser sintering) titanium alloys for the condylar component.24 This is a promising area in which the hope is that different designs, material composition and manufacturing techniques will both provide surgeons and patients with greater choice, but also drive quality and innovation. The authors also look forward to larger and longer term outcome datasets using established implants to form a more established evidence base and inform surgical and bio-engineering decision-making.
Research into bone substitute materials and modern regenerative medicine holds huge potential for reconstruction of the mandible. Although the vast majority remains in research stages and is unlikely to be in mainstream practice for many years, there are significant steps being made to further the goal of more optimal reconstructive options. Bio-ceramics as bone substitutes are established in practice, and in particular nano-hydroxyapatite, which can be 3D printed into a porous biomimetic scaffold. This can be bound with active molecules such as bone morphogenetic protein 2 (BMP-2.)25 Individually, there is limited clinical applicability due to biological performance in vivo, but these structures can be hybridised with other biological compounds to create composite constructs with multi-faceted benefits.25 An area of interest has been printable organic and synthetic hydrogels such as collagen, chitosan or polyethylene glycol which can be used as a filling suspension for the scaffold or as a functional biological membrane. An alternative is an autologous substance like platelet-rich fibrin which has both the structural characteristics as well as containing growth factors.25 As a suspension there is work being done on creating composite gels containing stem cells (e.g. bone mesenchymal stem cells) or autologous bone marrow that can be introduced into the internal aspect of the scaffolds.25
The research ideal is a hybrid structure designed on a surgical condylar defect that could, via stem cell culture, bioprinting and biological maturation, form an autologous pluripotent bioactive tissue analogue for implantation and full mechanical and immunological integration.
6. Conclusion
Reconstruction of the mandibular condyle after ablative surgery is challenging and a paucity of high quality evidence makes the decision making process a complex one for the surgeon.
The choice of reconstructive technique can be driven by the pathology, the requirement for post-operative oncological treatment, the age and growth potential of the patient, and the complexity and extent of the defect, as well as surgeon-related expertise. There are effective and suitable options currently available such as vascularised osseous free flaps, non-vascularised autologous bone grafts and alloplastic prostheses. It is clear that this is an area that is in need of large scale intelligently designed studies and the future appears to hold exciting prospects in the fields of computer aided design, 3D printing, biomimetic structures and tissue engineering.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
Nil.
References
- 1.Batstone M.D. Reconstruction of major defects of the jaws. Aust Dent J. 2018;63(Suppl 1):S108–S113. doi: 10.1111/adj.12596.PMID:29574815. Mar. [DOI] [PubMed] [Google Scholar]
- 2.Sidebottom A.J. Alloplastic or autogenous reconstruction of the TMJ. J Oral Biol Craniofac Res. 2013;3(3):135–139. doi: 10.1016/j.jobcr.2013.07.003. Sep-Dec. Epub 2013 Sep 6. PMID: 25737902; PMCID: PMC3942016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vega L.G., González-García R., Louis P.J. Reconstruction of acquired temporomandibular joint defects. Oral Maxillofac Surg Clin. 2013;25(2):251–269. doi: 10.1016/j.coms.2013.02.008. May. PMID: 23642672. [DOI] [PubMed] [Google Scholar]
- 4.Wang L., Liu K., Shao Z., Shang Z.J. Management of the condyle following the resection of tumours of the mandible. Int J Oral Maxillofac Surg. 2017;46(10):1252–1256. doi: 10.1016/j.ijom.2017.04.029. Oct. Epub 2017 Jul 5. PMID: 28688540. [DOI] [PubMed] [Google Scholar]
- 5.Abramowicz S., Goudy S.L., Mitchell C.E., et al. A protocol for resection and immediate reconstruction of pediatric mandibles using microvascular free fibula flaps. J Oral Maxillofac Surg. 2020;26(20):S0278–S2391. doi: 10.1016/j.joms.2020.08.020. Aug. 31085-5, Epub ahead of print. PMID: 32950472. [DOI] [PubMed] [Google Scholar]
- 6.Resnick C.M. Temporomandibular joint reconstruction in the growing child. Oral Maxillofac Surg Clin. 2018;30(1):109–121. doi: 10.1016/j.coms.2017.08.006. Feb. PMID: 29153233. [DOI] [PubMed] [Google Scholar]
- 7.Berkovitz B.K.B., Holland G.R., Moxham B.J. vol. 15. 4e. Edinburgh, Mosby/Elsevier; 2017. p. 293. (Oral Anatomy, Histology and Embryology). 13:p.237. [Google Scholar]
- 8.Petrovic I., Ahmed Z.U., Hay A., et al. Sarcomas of the mandible. J Surg Oncol. 2019;120(2):109–116. doi: 10.1002/jso.25477. Aug. Epub 2019 Apr 16. PMID: 30993699; PMCID: PMC6635012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haroun K., Coblens O.M. Reconstruction of the mandible for osteoradionecrosis. Curr Opin Otolaryngol Head Neck Surg. 2019;27(5):401–406. doi: 10.1097/MOO.0000000000000571. Oct. PMID: 31389851. [DOI] [PubMed] [Google Scholar]
- 10.Tarsitano A., Battaglia S., Ramieri V., et al. Short-term outcomes of mandibular reconstruction in oncological patients using a CAD/CAM prosthesis including a condyle supporting a fibular free flap. J Cranio-Maxillo-Fac Surg. 2017;45(2):330–337. doi: 10.1016/j.jcms.2016.12.006. Feb. Epub 2016 Dec 16. PMID: 28052811. [DOI] [PubMed] [Google Scholar]
- 11.Mascha F., Winter K., Pietzka S., Heufelder M., Schramm A., Wilde F. Accuracy of computer-assisted mandibular reconstructions using patient-specific implants in combination with CAD/CAM fabricated transfer keys. J Cranio-Maxillo-Fac Surg. 2017;45(11):1884–1897. doi: 10.1016/j.jcms.2017.08.028. Nov. Epub 2017 Sep 5. PMID: 28965991. [DOI] [PubMed] [Google Scholar]
- 12.Chang E.I., Boukovalas S., Liu J., Largo R.D., Hanasono M.M., Garvey P.B. Reconstruction of posterior mandibulectomy defects in the modern era of virtual planning and three-dimensional modelling. Plast Reconstr Surg. 2019;144(3):453e–462e. doi: 10.1097/PRS.0000000000005954. Sep. PMID: 31461040. [DOI] [PubMed] [Google Scholar]
- 13.Mercuri L.G. Costochondral graft versus total alloplastic joint for temporomandibular joint reconstruction. Oral Maxillofac Surg Clin. 2018;30(3):335–342. doi: 10.1016/j.coms.2018.05.003. Aug. Epub 2018 Jul 5. PMID: 30008343. [DOI] [PubMed] [Google Scholar]
- 14.Tang W., Long J., Feng F., Guo L., Gao C., Tian W. Condyle replacement after tumor resection: comparison of individual prefabricated titanium implants and costochondral grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(2):147–152. doi: 10.1016/j.tripleo.2009.01.028.Epub.2009.Apr.22. Aug. PMID: 19386520. [DOI] [PubMed] [Google Scholar]
- 15.Johnson N.R., Roberts M.J., Doi S.A., Batstone M.D. Total temporomandibular joint replacement prostheses: a systematic review and bias-adjusted meta-analysis. Int J Oral Maxillofac Surg. 2017;46(1):86–92. doi: 10.1016/j.ijom.2016.08.022. Jan. Epub 2016 Sep 17. PMID: 27644588. [DOI] [PubMed] [Google Scholar]
- 16.Siegmund B.J., Winter K., Meyer-Marcotty P., Rustemeyer J. Reconstruction of the temporomandibular joint: a comparison between prefabricated and customized alloplastic prosthetic total joint systems. Int J Oral Maxillofac Surg. 2019;48(8):1066–1071. doi: 10.1016/j.ijom.2019.02.002. Aug. Epub 2019 Feb 15. PMID: 30777713. [DOI] [PubMed] [Google Scholar]
- 17.De Meurechy N.K.G., Zaror C.E., Mommaerts M.Y. Total temporomandibular joint replacement: stick to stock or optimization by customization? Craniomaxillofacial Trauma Reconstr. 2020;13(1):59–70. doi: 10.1177/1943387520904874.Epub.2020.Feb.27. Mar. PMID: 32642034; PMCID: PMC7311846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zou L., He D., Ellis E. A comparison of clinical follow-up of different total temporomandibular joint replacement prostheses: a systematic review and meta-analysis. J Oral Maxillofac Surg. 2018;76(2):294–303. doi: 10.1016/j.joms.2017.08.022. Feb. Epub 2017 Aug 24. PMID: 28919368. [DOI] [PubMed] [Google Scholar]
- 19.Brown J.S., Lowe D., Kanatas A., Schache A. Mandibular reconstruction with vascularised bone flaps: a systematic review over 25 years. Br J Oral Maxillofac Surg. 2017;55(2):113–126. doi: 10.1016/j.bjoms.2016.12.010. Feb. Epub 2017 Jan 5. PMID: 28065645. [DOI] [PubMed] [Google Scholar]
- 20.Yu Y., Zhang W.B., Liu X.J., Guo C.B., Yu G.Y., Peng X. Regeneration of the neocondyle after free fibular flap reconstruction of the mandibular condyle. J Oral Maxillofac Surg. 2020;78(3):479–487. doi: 10.1016/j.joms.2019.11.009. Mar. Epub 2019 Nov 20. PMID: 31838093. [DOI] [PubMed] [Google Scholar]
- 21.Markowitz N.R., Allan P.G., Duffy M.T. Reconstruction of the mandibular condyle using ramus osteotomies: a preliminary report. J Oral Maxillofac Surg. 1989;47(4):367–377. doi: 10.1016/0278-2391(89)90338-8. Apr. PMID: 2647938. [DOI] [PubMed] [Google Scholar]
- 22.Heffez L.B. The inverted coronoid-ramus graft for condylar reconstruction. J Oral Maxillofac Surg. 2019;77(6):1315. doi: 10.1016/j.joms.2019.02.035. Jun. e1-1315.e19, Epub 2019 Mar 2. PMID: 30926545. [DOI] [PubMed] [Google Scholar]
- 23.Elledge R., Mercuri L.G., Speculand B. Extended total temporomandibular joint replacements: a classification system. Br J Oral Maxillofac Surg. 2018;56(7):578–581. doi: 10.1016/j.bjoms.2018.06.002. Sep. Epub 2018 Jun 27. PMID: 29958720. [DOI] [PubMed] [Google Scholar]
- 24.Elledge R., Mercuri L.G., Attard A., Green J., Speculand B. Review of emerging temporomandibular joint total joint replacement systems. Br J Oral Maxillofac Surg. 2019;57(8):722–728. doi: 10.1016/j.bjoms.2019.08.009. Oct. Epub 2019 Aug 25. PMID: 31455594. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Q., Wu W., Qian C., et al. Advanced biomaterials for repairing and reconstruction of mandibular defects. Mater Sci Eng C Mater Biol Appl. 2019;103 doi: 10.1016/j.msec.2019.109858. Oct. Epub 2019 Jun 5. PMID: 31349473. [DOI] [PubMed] [Google Scholar]