Abstract
Endoscopic ultrasound guided liver biopsy (EUS-LB) has emerged as a minimally-invasive alternative to the traditional (percutaneous or transjugular) liver biopsy techniques for the diagnosis of liver parenchymal diseases. Po-tentially, EUS-LB combines the advantages of percutaneous and transjugular liver biopsy in addressing focused sampling in addition to measuring portal pressure. Additionally, EUS-LB facilitates access to both the lobes of the liver which is not considered with the traditional percutaneous liver biopsy. Multiple studies have compared EUS-LB with conventional liver biopsy and reported comparable diagnostic yield, increased acquisition of complete portal tracts, and longer specimen length as compared to the traditional approaches. EUS-LB is associated with lesser post-procedural pain and shorter recovery time, while providing lower risk of complications when compared to traditional liver biopsy. Innovations in needle types, needle sizes and suction techniques have aimed at further optimizing the EUS-LB technique. This review article updates current literature with focus on the variations in the technique and equipment used for EUS-LB, and compares EUS-LB with traditional methods of liver biopsy.
Keywords: Endoscopic ultrasound guided liver biopsy, Liver biopsy, Percutaneous liver biopsy, Transjugular liver biopsy, Liver parenchymal disease, Portal pressure gradient
Core Tip: Endoscopic ultrasound guided liver biopsy (EUS-LB) has emerged as a minimally invasive alternative to the traditional (percutaneous or transjugular) liver biopsy techniques for the diagnosis of liver parenchymal diseases. EUS-LB facilitates access to both the lobes of the liver and allows measurement of portal pressure. EUS-LB has comparable diagnostic accuracy, increased yield of complete portal tracts, and longer specimen length compared to the traditional approaches. Innovations in needle technology and variations in suction have further optimized the EUS-LB technique. This review article updates current literature comparing EUS-LB to traditional liver biopsy and advances in this field.
INTRODUCTION
Liver biopsy is helpful in diagnosis of parenchymal pathologies such as alcoholic liver disease, autoimmune hepatitis, viral hepatitis, metabolic liver diseases (non-alcoholic fatty liver disease, alfa-1 anti-trypsin deficiency, Wilson disease, hemochromatosis, Gaucher’s disease, etc.), drug-induced liver injury and infiltrative liver disease (i.e., malignancy, abscess, sarcoidosis, etc.). Tissue examination also allows for diagnosis of rare overlapping liver diseases. The liver biopsy has traditionally been obtained via two routes: percutaneous liver biopsy (PC-LB) and transjugular liver biopsy (TJ-LB). In recent years, endoscopic technique and hardware advancement have led to the rise of endoscopic ultrasound-guided liver biopsy (EUS-LB). There is changing epidemiology of liver disease with increased global incidence of non-alcoholic fatty liver disease over the last two decades; it has reached to an estimated global prevalence of 25%[1]. This increased prevalence has led to advancements in lesser or non-invasive diagnostic tests such as ultrasound-elastography and MRI-proton density fat fraction[2]. Although these tests provide greater diagnostic accuracy compared to traditional peripheral blood laboratory tests, liver biopsy remains the gold standard for diagnosing focal lesions and parenchymal liver disease[3]. In this review, we will compare EUS-LB with traditional liver biopsy and highlight its, advantages and disadvantages in context of changing epidemiology of liver disease. Further, we will summarize the latest advancements on EUS-LB, focusing on technique, needle types/size, and suction type.
METHODS OF LIVER BIOPSY
The first mode of acquisition of liver tissue was PC-LB, as it provides the most direct route to access the liver. Percutaneous needle aspiration biopsies have been performed since the late 19th century and popularized in the 1930s[4]. Initial PC-LB techniques used percussion to guide needle placement; however, modern PC-LB is done under ultrasound- or fluoroscopic-image guidance[5]. If hepatomegaly is present, a subcostal route for PC-LB is preferred; however, a transthoracic approach is employed in the absence of hepatomegaly. In the early days of PC-LB, interventionists preferred 14 gauge (G) or 16 G needles to provide large, intact tissue samples. In recent years, spring-loaded 18 G and 20 G needles have been common[6]. PC-LB is usually done while the patient is awake with local anesthesia, but conscious sedation is often used. Common complications of PC-LB include pain (74%), minor bleeding (30%), and infection at the biopsy site[7,8]. As the liver capsule is punctured to obtain biopsy, intra-peritoneal hemorrhage is a severe, albeit rare complication[7]. Other less-common complications include pneumothorax and hemothorax if the transthoracic approach is employed (0.1%-0.9%)[7-9]. Contraindications to PC-LB include significant ascites, large body-habitus, and severe coagulopathy[5]. As it has been a long-standing method of biopsy, PC-LB is widely available. Furthermore, given its short procedure time and the fact that general anesthesia is not required, PC-LB provides a cost-effective approach to diagnosing liver diseases. Drawbacks to PC-LB include access to only the right hepatic lobe and a limited view of surrounding anatomy. Further, if real-time imaging is not used for guidance, there is an increased risk of serious complications[10].
More recently, TJ-LB has been developed to obtain liver samples in patients with contraindications to PC-LB. In this technique, an interventional radiologist cannulates the internal jugular vein and accesses one of the hepatic vein under fluoroscopic guidance. Biopsy of the right hepatic lobe is preferred because of its size and the acute angulation of the veins with the inferior vena cava[11]. This procedure can be performed in patients with high body mass index, and significant ascites. Additionally, as the liver capsule is not punctured, it is the preferred method in those with profound coagulopathies[11]. This technique provides the added benefit of assessing the hepatic venous pressure gradient (HVPG), which is an indirect measure of the absence, presence and degree of portal hypertension. HVPG measurement will be compared to EUS-guided measurement of portal pressure in the discussion of future research and practice below. Complications from TJ-LB include pain, hematoma, hemobilia, arterial aneurysm, and major hemorrhage[11]. In addition to subverting contraindications posed by the PC-LB, advantages of TJ-LB include lack of capsular puncture and the ability to obtain a portal pressure gradient measurement[7,10,11]. One of the limitation of TJ-LB is inability to obtain tissue from a focal lesion, due to limited view of surrounding structures[10].
Endoscopic ultrasound-guided fine-needle aspiration was first done in 1993 and EUS-LB was first described by Mathew in 2007[12,13]. EUS-LB poses several advantages over the traditional LB techniques. Firstly, EUS-LB allows access to both lobes of the liver (except in patients with Roux-en-Y or gastric bypass anatomy)[10]. Secondly, like TJ-LB, EUS-LB enables a practitioner to obtain liver tissue regardless of body habitus or ascites. The most advantageous aspects of EUS-LB, when compared to PC-LB and TJ-LB, lie in its real-time multi-dimensional evaluative abilities. Those undergoing workup for liver disease often require endoscopic evaluation – the advent of EUS-LB posed a solution to facilitate patients to undergo multiple endoscopic procedures in the same session. With EUS, a liver biopsy can be performed along with simultaneous evaluation for varices (esophageal and gastric) and portal pressure measurement. Further, the use of EUS in close proximity to the liver allows for better visualization of liver lesions for targeted liver biopsies[10]. In fact, multiple studies have shown a diagnostic accuracy between 85%-90% for solid liver masses using EUS-guided FNB[14-16]. Use of ultrasound-guided technique also allows practitioners to avoid critical structures during biopsy procurement. The drawbacks to EUS-LB mirror some of the drawbacks found in PC-LB and TJ-LB. Although the number of EUS-trained practitioners have grown rapidly over the past ten years in the United States, this procedure is less widely accessible when compared to PC-LB[10]. Further, unlike TJ-LB, EUS-LB does require capsular puncture and is not readily performed in patients with severe coagulopathy. Additionally, EUS-LB does require moderate or deep sedation, as with TJ-LB.
METHODS OF LIVER BIOPSY - COMPARISONS
Recovery times and complications
With the advent of multiple modalities for obtaining liver biopsies, recent studies have comparatively evaluated these approaches. The comparison between each of the three liver biopsy methods is summarized in Table 1. With regards to length of recovery, EUS-LB patients (n = 30) experienced a shorter post-procedural monitoring time (3 h) when compared to those (n = 60) undergoing PC-LB (4.2 h, P = 0.004)[17]. A recent study done in 2021 showed the mean recovery time for those undergoing EUS-LB was 90 min vs 141.3 min (P = 0.004) for TJ-LB[18]. The most common minor complication in each of the three modes of liver biopsy is pain. In a 2020 study, EUS-LB had significantly less pain scores when compared to PC-LB (0/10 for EUS-LB vs 3.5 for PC-LB, P = 0.0009)[17]. When comparing pain in EUS-LB and TJ-LB patients, one 2019 study found a significantly higher incidence of post-procedural pain in TJ-LB patients compared to EUS-LB patients, 8% vs 0% (P < 0.0001), respectively[19]. Adverse events with regard to liver biopsy include, but are not limited to, severe hemorrhage, hemobilia, abdominal wall injury, and intraperitoneal injury. Although no studies have directly compared adverse event rates between the three liver biopsy modalities, adverse event rates appear to be similar across each modality – EUS-LB: 1%-2.3%, PC-LB: 0.9%-3.1%, TJ-LB: 0.56%-6.5%[18-22].
Table 1.
Comparison of liver biopsy methods1
|
|
Percutaneous liver biopsy
|
Transjugular liver biopsy
|
Endoscopic ultrasound-guided liver biopsy
|
| Most common form of anesthesia | Local | Moderate sedation | Moderate sedation, deep sedation |
| Imaging | Fluoroscopy, ultrasound | Fluoroscopy | Ultrasound |
| Capsular puncture | Yes | No | Yes |
| Ability to evaluate focal hepatic lesions | No | No | Yes |
| Liver lobe access | Right | Right | Right, left |
| Contraindications | INR > 1.5, body habitus, significant ascites | Venous thrombosis, cholangitis | INR > 1.5 |
| Post-procedural pain | +++ | + | + |
| Post-procedural minor bleeding | + | + | + |
| Adverse event rate | + | + | + |
| Post-procedural recovery time | +++ | ++ | + |
| Specimen length | + | + | +++ |
| Total complete portal tracts | + | ++ | +++ |
| Procedure cost | + | ++ | +++ |
| Institutional availability | +++ | ++ | + |
Specimen adequacy
Regarding histological adequacy of liver biopsy samples, the American Association for the Study of Liver Diseases recommends a tissue length of between 2-3 cm with greater-than-or-equal-to 11 complete portal tracts (CPT)[23]. Systematic reviews have shown a total specimen length for PC-LB to be 17.7mm (± 5.5 mm), TJ-LB: 13.5 mm (± 4.5 mm), and EUS-LB 36.9 mm (± 6.2 mm)[20,24]. CPT numbers in these same studies revealed an aggregate CPT for PC-LB of 7.7 (± 3.4), TJ-LB: 6.8 (± 2.3), and EUS-LB: 9.0 (± 3.1)[20,24]. A recent 2021 study compared each liver biopsy technique head-to-head and found that EUS-LB (n = 53) had a significantly greater mean aggregate length (22.95 mm) compared to PC-LB (n = 20) (14.5 mm) (P = 0.03) and TJ-LB (n = 20) (14.6 mm) (P = 0.02)[18]. Further, both EUS-LB and TJ-LB provided a greater number of CPTs when compared to PC-LB: EUS-LB (19.36), TJ-LB (20.2), PC-LB (9.1) (EUS-LB vs PC-LB: P < 0.0001, EUS-LB vs TJ-LB P = 1)[18].
TECHNIQUE OF EUS-GUIDED LIVER BIOPSY
Since the inception of EUS-LB in 2007 with a Tru-Cut core biopsy needle (QuickCore, Cook Medical, Winston Salem, NC, United States), multiple studies have aimed at optimizing EUS-LB technique[13]. These changes coincided with procedural upgrades and improvements in needle technology. Incorporating these advancements into EUS-LB comes with technical (skills and training) and logical challenges (cost and availability). However, the essential technique of EUS-LB remains unchanged.
General technique
Patients undergoing EUS-LB are either moderately sedated with short-acting benzodiazepines and opiates or deeply sedated with propofol, per the availability of dedicated anesthetists. The patients are prone positioned and a linear array echo-endoscope is inserted for endo-sonography. Once the area of interest is identified, the stylet is removed, and a needle primed (with heparin or saline) or unprimed (air) is inserted[6,10]. Per endoscopist and center preference, the needle should be attached either to a wet suction (saline or heparin) or a dry suction (air). Before needle puncture, color Doppler should be used to ensure there are no vascular structures in the trajectory of the needle. Suction should be applied via a syringe once adequate liver parenchymal penetration is achieved (depending on the needle selection). Figure 1 shows the echoendoscopic image of a needle passing through into the left hepatic lobe. In 1 pass, actuations or fanning back-and-forth motions can be done with continued suction to allow for improved tissue acquisition[25]. Multiple such passes can be done overall to increase tissue acquisition. After each pass, the needle should be removed from the echo-endoscope and the tissue should be stored in formalin solution directly for preservation[6,10]. This gross tissue can be analyzed for adequacy and should guide the decision if more passes are required. Following the procedure, patients can be discharged after 1-2 h observation in the recovery unit.
Figure 1.
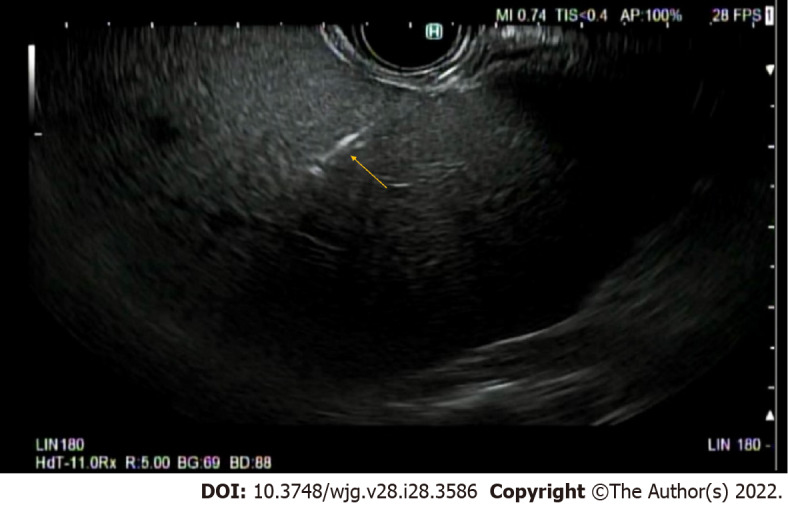
Needle for endoscopic ultrasound guided liver biopsy accessing left lobe of the liver. Orange arrow denotes needle. Image obtained by Krishna SG at the Ohio State University Wexner Medical Center Division of Gastroenterology, Hepatology, and Nutrition.
Accessing liver parenchyma requires identification of endoscopic gastric landmarks. The left lobe of liver adjoins the gastroesophageal junction can be accessed through the gastroesophageal junction in the proximal stomach. The right lobe of the liver can be accessed through the duodenal bulb[26]. Among patients with altered anatomy, EUS-LB is limited. For Roux-en-Y gastric bypass patients, only left liver lobe biopsy is feasible through the transgastric approach[10].
Suction
Suction can be applied in a wet or dry fashion. In dry suction, an empty 10 cc or 20 cc syringe is applied to maintain suction after passing the needle into the liver parenchyma. In wet suction, the needle lumen is primed with fluid, causing negative pressure at the needle tip. As outlined above, wet suction utilizes either saline or heparin. A 2018 study compared wet heparin suction technique with dry suction technique. The study showed significantly increased aggregate specimen length (wet: 49.2 mm, dry: 23.9 mm, P = 0.003) and number of CPTs (7.0 vs 4.0, P = 0.01) for wet suction compared to dry suction techniques[27]. With wet suction, the sample is suspended in a column of fluid—leading to reduced shearing forces as it is not in contact with the needle wall. This likely leads to increased CPTs and intact specimen length for wet suction technique. To our knowledge, no studies directly comparing wet suction with saline vs heparin have been performed.
In contrast, specimens can be obtained without applying suction via stylet, in a “slow-pull” technique. A 2020 meta-analysis showed that FNB with a slow-pull technique provided similar total specimen length (44.3 mm vs 53.9 mm, P = 0.40) when compared to suction application; however, the slow-pull technique provided improved CPT than suction (30 vs 14.6, P < 0.001)[28]. Authors hypothesize that this stems from reduced fragmentation of the tissue specimen as it is subjected to an environment of less negative pressure with the slow-pull technique.
Needle pass/Actuation
Needle pass refers to the number of times a needle is introduced into the liver parenchyma through puncture of the liver capsule, while actuation refers to the number of back-and-forth motions are made in a specified needle pass. Through our literature review, it appears the 1-pass 1-actuation technique is the most common mode of EUS-LB acquisition[25,29]. There are few studies that have been done comparing needle pass and needle actuation with regards to pain, adverse events, and specimen quality. A 2021 study showed that EUS-LB using a 1-pass, 3-actuation technique (n = 40) provided longer liver cores with more CPTs than a 1-to-1 technique (n = 40), with an equivalent safety profile: CPT (24.5 vs 17.25, P < 0.008), aggregate specimen length (12.85 cm vs 6.89 cm P < 0.001)[25].
Needle selection: Size
When considering needle selection, a EUS-LB practitioner must take needle size and type into account. With regards to needle size, multiple studies have been done to determine the optimal needle size for EUS-LB. In the early days of EUS-LB, large 14G – 16 G spring-loaded cutting needles were used; however, these needles had varied diagnostic yields ranging from 29%-100%[30,31]. As time progressed, researchers found that smaller-gauge needles provided better results than their 14-16 G counterparts. In a 2017 study, 18 G percutaneous, 19 G FNA, 19G FNB, and 22 G FNB needles were compared on human cadaveric tissue to test for tissue adequacy. The study showed that a 19 G needle provided a significantly greater number of CPTs when compared to its 22 G and 18 G counterparts (6.2 vs 3.5 vs 3.2, respectively, P < 0.001 for both comparisons)[32]. In a similar study in 2019 on fresh bovine liver, 19 G and 20 G fork-tip needles yielded similar CPTs and total specimen length; however, both the 19 G and 20 G fork-tip needles significantly outperformed their 22 G fork-tip counterpart in terms of CPTs and specimen length[33].
More recent in vivo studies have confirmed the superiority of 19 G needles when compared to 22 G needles. A recent (2021) study showed that 19 G Franseen tip needle performed better than a 22 G Franseen tip needle in terms of specimen adequacy (19 G: 81.5%, 22 G: 66.7%, P < 0.01)[34]. The researchers posit that the 22 G samples had greater fragmentation, leading to reduced intact specimen length and CPT, reducing histological adequacy. The advantage of 19 G compared to 22 G needle was also shown in core needles, where 19 G core needles had a greater CPT (8.8 vs 3, P < 0.0001), longer core length (2.5 cm vs 1.1 cm, P < 0.0001), and a higher rate of pathological diagnostic samples (85% vs 10%, P < 0.0001) when compared to 22 G core needles[35].
Needle selection: Tip and design
As noted earlier, large spring-loaded cutting needles were initially used to perform EUS-LB. As EUS-LB grew, practitioners noted that fine-needle biopsy (FNB) needles may provide better samples in the endoscopic setting. In 2015, Dewitt et al[36] showed that a 19 G FNB histology needle performed better than a 19 G spring-loaded cutting needle and provided longer specimens (19.4 mm vs 4.3 mm, P = 0.0001), greater CPTs (10.4 vs 1.3, P = 0.0004), and a higher percent of diagnostic histology (85% vs 57%, P = 0.006)[36].
Holding needle gauge constant, Franseen needle types have shown to be superior than their counterparts in recent studies. A 2019 study showed that a 19 G Franseen needle provided lengthier specimens when compared to a 19 G FNA needle for in vivo EUS-LB (2.09 cm vs 1.47 cm, P < 0.001), more CPTs (42.6 vs 18.1, P < 0.001), and similar pain scores[37]. In the late portion of the last decade, EUS-LB has been most commonly performed with either Franseen or Fork-Tip biopsy needles. Multiple studies have compared the effectiveness with regards to specimen quality and adverse events between these two needles. A 2020 study showed a similar adverse event and abdominal pain profile between the use of 19 G Franseen and fork-tip needles[29]. The Franseen needle, however, had a significantly greater total specimen length when compared to the fork-tip needle (3.1 cm vs 2.7 cm, P = 0.01) and greater total CPTs (24.0 vs 19.55, P < 0.01)[29]. The Franseen tip has also shown equal-to-superior diagnostic yield when compared to fork-tip, with one study showing nonsignificant-difference in histological adequacy rates but an increased number of CPTs (14.4 vs 9.5, P < 0.001) and another study showing a higher diagnostic yield of 97.2% for Franseen tip compared to 79.4% for fork-tip (P < 0.001)[38,39].
FUTURE RESEARCH AND PRACTICE
Portal pressure gradient
In trained hands it has become possible to obtain accurate measures of portal pressure gradients by various approaches[36]. The measure of the portal pressure gradient is primarily done through the transjugular approach. Although described as the gold standard, the hepatic venous pressure gradient (HVPG) is actually an indirect measure of portal pressure calculated by subtracting the free hepatic venous pressure from the wedge hepatic venous pressure[40]. In contrast, EUS-guided measurement of portal pressure is a direct measurement of sinusoidal pressure rather than an estimate, and may prove to be more accurate[40]. To obtain EUS-guided measurement of portal pressure, endoscopists first puncture the hepatic vein via a transgastric transhepatic approach, with the needle hooked up to a digital manometer via non-compressible tubing[41]. Once obtaining hepatic vein pressure averages, endoscopists then turn their attention to the portal vein, which is accessed in a transgastric transhepatic approach usually at the umbilical portion of the portal vein. Once the portal vein pressure is obtained, the portal pressure gradient is calculated by subtracting the mean portal vein pressure from the mean hepatic vein pressure[41]. These readings are usually obtained with a small-gauge (25 G) FNA needle[40,41].
Since the HVPG can be obtained without performing a liver biopsy, it is a very safe procedure when performed alone, mainly imparting the risk associated with right internal jugular access[9,11]. Therefore, it is essential to ensure that EUS-guided measurement of portal pressure is performed with specific indications to ensure safety. For example, patients with splenomegaly may benefit from EUS-LB and EUS-guided measurement of portal pressure to confirm whether cirrhosis and portal hypertension are the underlying causes of splenomegaly. However, if a patient has esophageal or gastric varices, it can be safely assumed that the portal pressure is 10 mmHg or higher and the patient has clinically significant portal hypertension[40]. That information should be used to determine whether EUS-guided measurement of portal pressure is warranted at the time of the procedure.
Concomitant EUS-LB with other endoscopic procedures
EUS-LB provides an exciting new method for liver tissue acquisition. A novel application is for special patient populations such as liver transplant recipients (LTRs). Laboratory abnormalities in liver transplant patients often requires an extensive workup including imaging, liver biopsy, and ERCP. Combining EUS-LB with ERCP can potentially provide a “one-stop-shop” for evaluation in this patient population. This will hopefully reduce the time, cost, and healthcare resources needed to accurately diagnose and treat post-transplant liver function abnormalities. Our group at Ohio State University, has recently submitted a case series of 12 consecutive LTRs with abnormal liver function tests (Han S, Jalil S, Groce JR, Krishna SG, Lara L, Lee P, Limkemann A, Papachristou GI, Mumtaz K. Feasibility of Single-Session EUS-guided Liver Biopsy and ERCP in Liver Transplant Recipients with Abnormal Liver Function Tests), who underwent concomitant EUS-LB and ERCP. In this case series, tissue adequacy was obtained in 100% of patients with the most common diagnoses including anastomotic stricture (75%) and T-cell mediated rejection (66.7%). Seven (58.3%) patients had dual diagnoses of T-cell mediated rejection and anastomotic stricture. There were no 30 d adverse events. Authors concluded that single-session EUS-guided LB and ERCP for the evaluation of elevated liver function tests in LTRs appears to be safe and feasible, but larger studies are needed to verify these findings.
Similarly, a recent study published by Hajifathalian et al[42] on concomitant EUS-LB and portal pressure gradient measurement reported 24 (100%) and 23 (96%) patients had successful portosystemic gradient (PSG) measurement and EUS-liver biopsy, respectively. Analysis revealed a significant association between both PSG and liver stiffness measured on transient elastography (P = 0.01) and FIB-4 score (P = 0.02). There was no significant correlation between the fibrosis stage on histology and measured PSG (P = 0.56)[42].
Lastly, EUS-LB has been discussed anecdotally as a more effective option in pediatric population, as it may reduce patient anxiety when compared to PC-LB or TJ-LB – although, to our knowledge, formal studies in pediatric populations are yet to be done.
CONCLUSION
Overall, EUS-LB provides unique advantages when compared to its counterparts: ability to evaluate the GI tract and pancreato-biliary system, while obtaining access to both lobes of the liver. It also has proven to produce more histologically/pathologically complete samples than PC-LB and TJ-LB. EUS-LB appears to be a safe, diagnostic modality for obtaining liver tissue. Concomitant EUS-LB, EUS portal pressure gradient measurement and other endoscopic procedure (simple endoscopy and ERCP) is another emerging area in the field of endo-hepatology.
Footnotes
Conflict-of-interest statement: All authors report no relevant conflicts of interest for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: January 16, 2022
First decision: March 8, 2022
Article in press: June 23, 2022
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Alali AA, Kuwait; El-Karaksy H, Egypt; Treeprasertsuk S, Thailand; Zhu YY, China S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
Contributor Information
Shiva Rangwani, Division of Gastroenterology, Hepatology, and Nutrition,Department of Internal Medicine, Ohio State University Wexner Medical Center, Columbus, OH 43210, United States.
Devarshi R Ardeshna, Division of Gastroenterology, Hepatology, and Nutrition,Department of Internal Medicine, Ohio State University Wexner Medical Center, Columbus, OH 43210, United States.
Khalid Mumtaz, Division of Gastroenterology, Hepatology, and Nutrition,Department of Internal Medicine, Ohio State University Wexner Medical Center, Columbus, OH 43210, United States.
Sean G Kelly, Division of Gastroenterology, Hepatology, and Nutrition,Department of Internal Medicine, Ohio State University Wexner Medical Center, Columbus, OH 43210, United States.
Samuel Y Han, Division of Gastroenterology, Hepatology, and Nutrition,Department of Internal Medicine, Ohio State University Wexner Medical Center, Columbus, OH 43210, United States.
Somashekar G Krishna, Division of Gastroenterology, Hepatology, and Nutrition,Department of Internal Medicine, Ohio State University Wexner Medical Center, Columbus, OH 43210, United States. somashekar.krishna@osumc.edu.
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Piazzolla VA, Mangia A. Noninvasive Diagnosis of NAFLD and NASH. Cells. 2020;9 doi: 10.3390/cells9041005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castera L, Friedrich-Rust M, Loomba R. Noninvasive Assessment of Liver Disease in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;156:1264–1281.e4. doi: 10.1053/j.gastro.2018.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLoughlin MJ, Ho CS, Tao LC. Percutaneous needle aspiration biopsy. Can Med Assoc J. 1978;119:1324–1328. [PMC free article] [PubMed] [Google Scholar]
- 5.Gilmore IT, Burroughs A, Murray-Lyon IM, Williams R, Jenkins D, Hopkins A. Indications, methods, and outcomes of percutaneous liver biopsy in England and Wales: an audit by the British Society of Gastroenterology and the Royal College of Physicians of London. Gut. 1995;36:437–441. doi: 10.1136/gut.36.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diehl DL. Endoscopic Ultrasound-guided Liver Biopsy. Gastrointest Endosc Clin N Am. 2019;29:173–186. doi: 10.1016/j.giec.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Shah AR, Al-Hanayneh M, Chowdhry M, Bilal M, Singh S. Endoscopic ultrasound guided liver biopsy for parenchymal liver disease. World J Hepatol. 2019;11:335–343. doi: 10.4254/wjh.v11.i4.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chi H, Hansen BE, Tang WY, Schouten JN, Sprengers D, Taimr P, Janssen HL, de Knegt RJ. Multiple biopsy passes and the risk of complications of percutaneous liver biopsy. Eur J Gastroenterol Hepatol. 2017;29:36–41. doi: 10.1097/MEG.0000000000000731. [DOI] [PubMed] [Google Scholar]
- 9.Lavian JD, Thornton LM, Zybulewski A, Kim E, Nowakowski SF, Ranade M, Patel RS, Lookstein RA, Fischman A, Bishay V. Safety of percutaneous versus transjugular liver biopsy: A propensity score matched analysis. Eur J Radiol. 2020;133:109399. doi: 10.1016/j.ejrad.2020.109399. [DOI] [PubMed] [Google Scholar]
- 10.Madhok IK, Parsa N, Nieto JM. Endoscopic Ultrasound-Guided Liver Biopsy. Clin Liver Dis. 2022;26:127–138. doi: 10.1016/j.cld.2021.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Kalambokis G, Manousou P, Vibhakorn S, Marelli L, Cholongitas E, Senzolo M, Patch D, Burroughs AK. Transjugular liver biopsy--indications, adequacy, quality of specimens, and complications--a systematic review. J Hepatol. 2007;47:284–294. doi: 10.1016/j.jhep.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Gress FG. The Early History of Interventional Endoscopic Ultrasound. Gastrointest Endosc Clin N Am. 2017;27:547–550. doi: 10.1016/j.giec.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 13.Mathew A. EUS-guided routine liver biopsy in selected patients. Am J Gastroenterol. 2007;102:2354–2355. doi: 10.1111/j.1572-0241.2007.01353_7.x. [DOI] [PubMed] [Google Scholar]
- 14.Chon HK, Yang HC, Choi KH, Kim TH. Endoscopic Ultrasound-Guided Liver Biopsy Using a Core Needle for Hepatic Solid Mass. Clin Endosc. 2019;52:340–346. doi: 10.5946/ce.2018.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee YN, Moon JH, Kim HK, Choi HJ, Choi MH, Kim DC, Lee TH, Cha SW, Kim SG, Kim YS. Usefulness of endoscopic ultrasound-guided sampling using core biopsy needle as a percutaneous biopsy rescue for diagnosis of solid liver mass: Combined histological-cytological analysis. J Gastroenterol Hepatol. 2015;30:1161–1166. doi: 10.1111/jgh.12922. [DOI] [PubMed] [Google Scholar]
- 16.Kongkam P, Nalinthassanai N, Prueksapanich P, Sanpavat A, Cañones AR, Luangsukrerk T, Angsuwatcharakon P, Ridtitid W, Kullavanijaya P, Treeprasertsuk S, Rerknimitr R. A comparison of the antegrade core trap and reverse bevel needles for EUS-guided fine-needle biopsy sampling of liver mass: a prospective randomized cross over study. HPB (Oxford) 2022;24:797–805. doi: 10.1016/j.hpb.2021.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Ali AH, Panchal S, Rao DS, Gan Y, Al-Juboori A, Samiullah S, Ibdah JA, Hammoud GM. The efficacy and safety of endoscopic ultrasound-guided liver biopsy versus percutaneous liver biopsy in patients with chronic liver disease: a retrospective single-center study. J Ultrasound. 2020;23:157–167. doi: 10.1007/s40477-020-00436-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel HK, Therapondos G, Galliano G, Romero R, Evans J, Cohen A, Mubarak MF, Shah JN, Chafic AHE. Endoscopic Ultrasound Guided Liver Biopsy Using Newer 19G FNB Needles Compared to Percutaneous and Trans-jugular Liver Biopsy: A Tertiary Center Experience. Tech Innov Gastrointest Endosc. 2022;24:127–135. [Google Scholar]
- 19.Shuja A, Alkhasawneh A, Fialho A, Shukri A, Harris C, Smotherman C, Malespin M, de Melo SW Jr. Comparison of EUS-guided versus percutaneous and transjugular approaches for the performance of liver biopsies. Dig Liver Dis. 2019;51:826–830. doi: 10.1016/j.dld.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Dhar J, Samanta J. Role of endoscopic ultrasound in the field of hepatology: Recent advances and future trends. World J Hepatol. 2021;13:1459–1483. doi: 10.4254/wjh.v13.i11.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Facciorusso A, Crinò SF, Ramai D, Fabbri C, Mangiavillano B, Lisotti A, Muscatiello N, Cotsoglou C, Fusaroli P. Diagnostic yield of endoscopic ultrasound-guided liver biopsy in comparison to percutaneous liver biopsy: a systematic review and meta-analysis. Expert Rev Gastroenterol Hepatol. 2022;16:51–57. doi: 10.1080/17474124.2022.2020645. [DOI] [PubMed] [Google Scholar]
- 22.Johnson KD, Laoveeravat P, Yee EU, Perisetti A, Thandassery RB, Tharian B. Endoscopic ultrasound guided liver biopsy: Recent evidence. World J Gastrointest Endosc. 2020;12:83–97. doi: 10.4253/wjge.v12.i3.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD American Association for the Study of Liver Diseases. Liver biopsy. Hepatology. 2009;49:1017–1044. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 24.Cholongitas E, Senzolo M, Standish R, Marelli L, Quaglia A, Patch D, Dhillon AP, Burroughs AK. A systematic review of the quality of liver biopsy specimens. Am J Clin Pathol. 2006;125:710–721. doi: 10.1309/W3XC-NT4H-KFBN-2G0B. [DOI] [PubMed] [Google Scholar]
- 25.Ching-Companioni RA, Johal AS, Confer BD, Forster E, Khara HS, Diehl DL. Single-pass 1-needle actuation versus single-pass 3-needle actuation technique for EUS-guided liver biopsy sampling: a randomized prospective trial (with video) Gastrointest Endosc. 2021;94:551–558. doi: 10.1016/j.gie.2021.03.023. [DOI] [PubMed] [Google Scholar]
- 26.Bhatia V, Hijioka S, Hara K, Mizuno N, Imaoka H, Yamao K. Endoscopic ultrasound description of liver segmentation and anatomy. Dig Endosc. 2014;26:482–490. doi: 10.1111/den.12216. [DOI] [PubMed] [Google Scholar]
- 27.Mok SRS, Diehl DL, Johal AS, Khara HS, Confer BD, Mudireddy PR, Kirchner HL, Chen ZE. A prospective pilot comparison of wet and dry heparinized suction for EUS-guided liver biopsy (with videos) Gastrointest Endosc. 2018;88:919–925. doi: 10.1016/j.gie.2018.07.036. [DOI] [PubMed] [Google Scholar]
- 28.Baran B, Kale S, Patil P, Kannadath B, Ramireddy S, Badillo R, DaVee RT, Thosani N. Endoscopic ultrasound-guided parenchymal liver biopsy: a systematic review and meta-analysis. Surg Endosc. 2021;35:5546–5557. doi: 10.1007/s00464-020-08053-x. [DOI] [PubMed] [Google Scholar]
- 29.Nieto J, Khaleel H, Challita Y, Jimenez M, Baron TH, Walters L, Hathaway K, Patel K, Lankarani A, Herman M, Holloman D, Saab S. EUS-guided fine-needle core liver biopsy sampling using a novel 19-gauge needle with modified 1-pass, 1 actuation wet suction technique. Gastrointest Endosc. 2018;87:469–475. doi: 10.1016/j.gie.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 30.Dewitt J, McGreevy K, Cummings O, Sherman S, Leblanc JK, McHenry L, Al-Haddad M, Chalasani N. Initial experience with EUS-guided Tru-cut biopsy of benign liver disease. Gastrointest Endosc. 2009;69:535–542. doi: 10.1016/j.gie.2008.09.056. [DOI] [PubMed] [Google Scholar]
- 31.Gleeson FC, Clayton AC, Zhang L, Clain JE, Gores GJ, Rajan E, Smyrk TC, Topazian MD, Wang KK, Wiersema MJ, Levy MJ. Adequacy of endoscopic ultrasound core needle biopsy specimen of nonmalignant hepatic parenchymal disease. Clin Gastroenterol Hepatol. 2008;6:1437–1440. doi: 10.1016/j.cgh.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 32.Schulman AR, Thompson CC, Odze R, Chan WW, Ryou M. Optimizing EUS-guided liver biopsy sampling: comprehensive assessment of needle types and tissue acquisition techniques. Gastrointest Endosc. 2017;85:419–426. doi: 10.1016/j.gie.2016.07.065. [DOI] [PubMed] [Google Scholar]
- 33.Eskandari A, Koo P, Bang H, Gui D, Urayama S. Comparison of Endoscopic Ultrasound Biopsy Needles for Endoscopic Ultrasound-Guided Liver Biopsy. Clin Endosc. 2019;52:347–352. doi: 10.5946/ce.2019.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel HK, Saxena R, Rush N, Patel SK, Dasari CS, Mneimneh W, Quickery A, Rahal MA, Temnykh L, DeWitt J, Al-Haddad M. A Comparative Study of 22G versus 19G Needles for EUS-Guided Biopsies for Parenchymal Liver Disease: Are Thinner Needles Better? Dig Dis Sci. 2021;66:238–246. doi: 10.1007/s10620-020-06165-x. [DOI] [PubMed] [Google Scholar]
- 35.Shah RM, Schmidt J, John E, Rastegari S, Acharya P, Kedia P. Superior Specimen and Diagnostic Accuracy with Endoscopic Ultrasound-Guided Liver Biopsies Using 19-Gauge versus 22-Gauge Core Needles. Clin Endosc. 2021;54:739–744. doi: 10.5946/ce.2020.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeWitt J, Cho CM, Lin J, Al-Haddad M, Canto MI, Salamone A, Hruban RH, Messallam AA, Khashab MA. Comparison of EUS-guided tissue acquisition using two different 19-gauge core biopsy needles: a multicenter, prospective, randomized, and blinded study. Endosc Int Open. 2015;3:E471–E478. doi: 10.1055/s-0034-1392222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ching-Companioni RA, Diehl DL, Johal AS, Confer BD, Khara HS. 19 G aspiration needle versus 19 G core biopsy needle for endoscopic ultrasound-guided liver biopsy: a prospective randomized trial. Endoscopy. 2019;51:1059–1065. doi: 10.1055/a-0956-6922. [DOI] [PubMed] [Google Scholar]
- 38.Aggarwal SN, Magdaleno T, Klocksieben F, MacFarlan JE, Goonewardene S, Zator Z, Shah S, Shah HN. A prospective, head-to-head comparison of 2 EUS-guided liver biopsy needles in vivo. Gastrointest Endosc. 2021;93:1133–1138. doi: 10.1016/j.gie.2020.09.050. [DOI] [PubMed] [Google Scholar]
- 39.Hashimoto R, Lee DP, Samarasena JB, Chandan VS, Guo W, Lee JG, Chang KJ. Comparison of Two Specialized Histology Needles for Endoscopic Ultrasound (EUS)-Guided Liver Biopsy: A Pilot Study. Dig Dis Sci. 2021;66:1700–1706. doi: 10.1007/s10620-020-06391-3. [DOI] [PubMed] [Google Scholar]
- 40.Rudnick SR, Conway JD, Russo MW. Current state of endohepatology: Diagnosis and treatment of portal hypertension and its complications with endoscopic ultrasound. World J Hepatol. 2021;13:887–895. doi: 10.4254/wjh.v13.i8.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samarasena JB, Chang KJ. Endoscopic Ultrasound-Guided Interventions for the Measurement and Treatment of Portal Hypertension. Gastrointest Endosc Clin N Am. 2019;29:311–320. doi: 10.1016/j.giec.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Hajifathalian K, Westerveld D, Kaplan A, Dawod E, Herr A, Ianelli M, Saggese A, Kumar S, Fortune BE, Sharaiha RZ. Simultaneous EUS-guided portosystemic pressure measurement and liver biopsy sampling correlate with clinically meaningful outcomes. Gastrointest Endosc. 2022;95:703–710. doi: 10.1016/j.gie.2021.11.037. [DOI] [PubMed] [Google Scholar]


