Abstract
Background and Objectives
To recruit and characterize a national cohort of individuals who have a genetic variant (LRRK2 G2019S) that increases risk of Parkinson disease (PD), assess participant satisfaction with a decentralized, remote research model, and evaluate interest in future clinical trials.
Methods
In partnership with 23andMe, Inc., a personal genetics company, LRRK2 G2019S carriers with and without PD were recruited to participate in an ongoing 36-month decentralized, remote natural history study. We examined concordance between self-reported and clinician-determined PD diagnosis. We applied the Movement Disorder Society Prodromal Parkinson's Disease Criteria and asked investigators to identify concern for parkinsonism to distinguish participants with probable prodromal PD. We compared baseline characteristics of LRRK2 G2019S carriers with PD, with prodromal PD, and without PD.
Results
Over 15 months, we enrolled 277 LRRK2 G2019S carriers from 34 states. At baseline, 60 had self-reported PD (mean [SD] age 67.8 years [8.4], 98% White, 52% female, 80% Ashkenazi Jewish, and 67% with a family history of PD), and 217 did not (mean [SD] age 53.7 years [15.1], 95% White, 59% female, 73% Ashkenazi Jewish, and 57% with a family history of PD). Agreement between self-reported and clinician-determined PD status was excellent (κ = 0.94, 95% confidence interval 0.89–0.99). Twenty-four participants had prodromal PD; 9 met criteria for probable prodromal PD and investigators identified concern for parkinsonism in 20 cases. Compared with those without prodromal PD, participants with prodromal PD were older (63.9 years [9.0] vs 51.9 years [15.1], p < 0.001), had higher modified Movement Disorders Society-Unified Parkinson's Disease Rating Scale motor scores (5.7 [4.3] vs 0.8 [2.1], p < 0.001), and had higher Scale for Outcomes in PD for Autonomic Symptoms scores (11.5 [6.2] vs 6.9 [5.7], p = 0.002). Two-thirds of participants enrolled were new to research, 97% were satisfied with the overall study, and 94% of those without PD would participate in future preventive clinical trials.
Discussion
An entirely remote national cohort of LRRK2 G2019S carriers was recruited from a single site. This study will prospectively characterize a large LRRK2 G2019S cohort, refine a new model of clinical research, and engage new research participants willing to participate in future therapeutic trials.
Gene-directed therapies have the potential to delay or prevent the onset of neurologic disorders.1,2 However, clinical trials of such gene-directed therapies require the identification of genetically at-risk individuals and recruitment of primarily asymptomatic persons willing to participate in such trials.3
Two advances may accelerate therapeutic development for genetically defined conditions. The first is direct-to-consumer genetic testing. Despite its limitations,4 such testing has been purchased by more than 26 million people,5 vastly expanding the number of individuals who know their risk for different diseases. The second advance is “decentralized” studies in which research assessments are moved away from clinical sites into participant homes.6,7 Remote assessments remove geographic barriers to participation, reduce recruitment time and cost, and reduce the burden of participation.8 The coronavirus disease 2019 (COVID-19) pandemic has accelerated the adoption of such models, which limit the spread of infectious diseases and are less susceptible to study disruption.9,10
Although promising, linking direct-to-consumer genetic testing with decentralized research studies has had few applications to date.11 Consequently, we launched a national, decentralized, natural history study of individuals who underwent direct-to-consumer genetic testing and carry a LRRK2 Gly2019Ser (G2019S) variant. The LRRK2 G2019S variant, which is the only LRRK2 variant included in 23andMe's Parkinson's disease report, is the most prevalent cause of autosomal dominant Parkinson disease (PD) in the United States and exhibits reduced penetrance.12 We sought to assess the extent to which we could characterize participants remotely and to determine the value of the study in creating a cohort ready for gene-based clinical trials.
Methods
Study Design
Virtual Assessment of LRRK2 carriers to Optimize Research of PD is a decentralized, remote natural history study of LRRK2 G2019S variant carriers with and without self-reported PD.13 Participants, all of whom are LRRK2 G2019S carriers, complete annual video visits for 36 months via a HIPAA-compliant web-based video platform from Zoom (San Jose, CA) and participant-reported outcomes via REDCap (Vanderbilt University). Study recruitment took place from June 2019 to August 2020 and was conducted from a single site at the University of Rochester. This study is ongoing with data collection anticipated to conclude in September 2023. The study design and methods have been previously reported in detail13; key elements are provided in this manuscript, which describes baseline results.
Standard Protocol Approvals, Registrations, and Patient Consents
The study was reviewed and approved by the University of Rochester's Research Subjects Review Board (STUDY00003703). All participants provided consent to participate in the study via a process that included reviewing an electronic consent form, being given an opportunity to ask questions and producing an electronic signature.
Participants
Adult individuals who pursued genetic testing through 23andMe, chose to be informed of their LRRK2 G2019S carrier status, and consented to be contacted by 23andMe about research opportunities were eligible. Access to an internet-enabled device and United States residence were required for participation. Consistent with NIH policy mandating the reporting of sex, race, and ethnicity, participants were asked to self-identify their sex, race (American Indian or Alaska Native, Asian, Black of African American, Native Hawaiian or Other Pacific Islander, White), and ethnicity (Hispanic/Latino or not Hispanic/Latino). Given the high prevalence of LRRK2 variants among those who are Ashkenazi Jewish and the fact that no genetic information was received directly from 23andMe, participants were also asked whether they are Ashkenazi Jewish and were asked to provide percentage Ashkenazi Jewish according to their 23andMe report, if known. This information was provided after providing consent to participate.
Outcome Measures
In advance of the annual video visits, participants are emailed a link to complete several participant-reported outcome measures that cover sleep, mood, motor, and autonomic symptoms.13 During the annual video visits, assessments of motor function, independence, and cognition are completed.13 At baseline, 1 of 4 trained investigators administers a modified version of the Movement Disorders Society-Unified Parkinson's Disease Rating Scale (MDS-UPDRS) motor assessment14 that excludes assessments of postural instability and rigidity, which cannot be performed via video. The feasibility and reliability of such remote assessments for PD has been previously demonstrated in other studies.15 Investigators, who are blinded to self-reported PD status, also assess all participants for features of PD to determine whether the disease is clinically present. This includes application of the Movement Disorder Society Clinical Diagnostic Criteria for Parkinson's disease,16 the UK Parkinson's Disease Society Brain Bank Criteria,17 and the NIH Diagnostic Criteria for Parkinson's disease.18 Items that cannot be adequately assessed remotely (e.g., rigidity, postural instability, hyperreflexia, and graphesthesia) are excluded. In addition, participants receive the University of Pennsylvania Smell Identification Test (UPSIT) via mail and are asked to complete and return the test prior to their baseline video visit. After the visit, participants are emailed a link to complete a survey on satisfaction and research interest.
Determining Prodromal PD and Concern for Parkinsonism
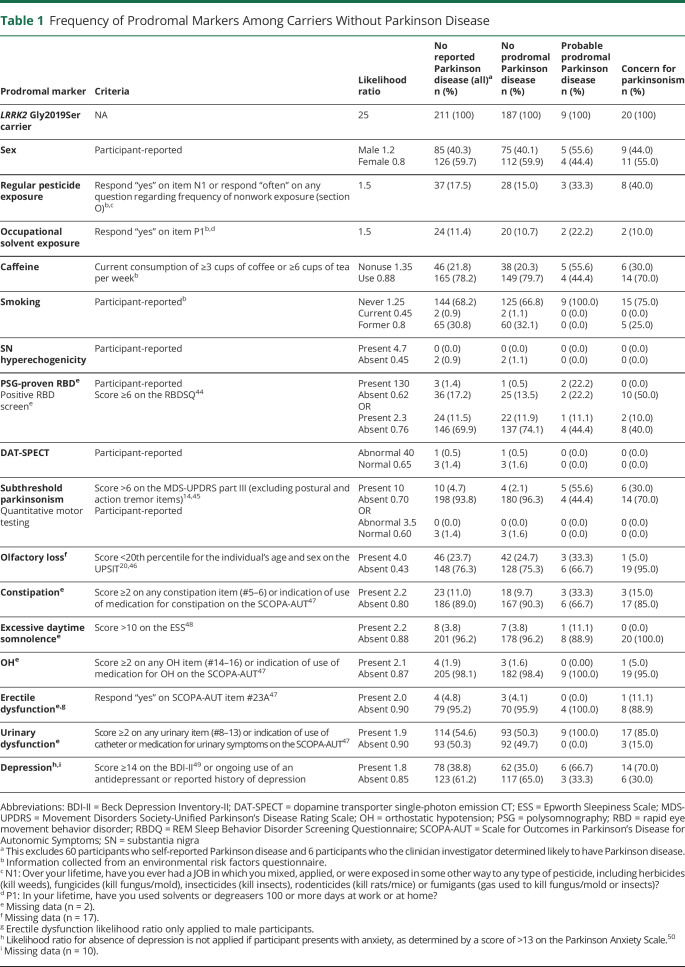
We used the 2015 Movement Disorder Society Prodromal Parkinson's Disease Criteria to determine the probability of prodromal PD in participants without self-reported PD at baseline.19 These criteria assign a likelihood ratio (LR) to a range of markers, all of which are captured in this study (Table 1). Pesticide exposure, occupational solvent exposure, caffeine use, and smoking status were captured on an environmental risk factors questionnaire completed by participants after providing consent to participate and prior to completing any research visits. During the baseline visit, participants were asked specifically about diagnostic testing, including dopamine transporter single-photon emission CT imaging, transcranial ultrasound, quantitative motor testing, and polysomnography. We did not attempt to obtain or verify participant-reported testing results. The cutoffs used to determine the presence of other clinical features are provided in Table 1. We multiplied LRs for each item to determine the overall LR; missing information was excluded from the equation. Participants with an overall LR that exceeded the specified age-based minimum LR required to meet the recommended 80% probability threshold19,20 were classified as having probable prodromal PD. We did not assess the probability of prodromal PD for participants younger than 50 years because no estimated prior probability is provided for these younger age groups. In addition, clinicians identified cases of concern for parkinsonism in individuals without self-reported PD at baseline. In these cases, examination was consistent with parkinsonism but findings were insufficient to support the clinical presence of PD according to expert determination. For the purposes of analyses, both participants who met the 2015 Movement Disorder Society Prodromal Parkinson's Disease Criteria and those where clinicians identified concern for parkinsonism were categorized as prodromal PD.
Table 1.
Frequency of Prodromal Markers Among Carriers Without Parkinson Disease
Safety
Safety assessments for this observational study are limited to the collection of reportable events either spontaneously offered by the participant (e.g., hospitalization) or observed by a study team member (e.g., fall). Participants who have a score ≥14 or endorse suicidal thoughts on the Beck Depression Inventory-II (BDI-II) inventory undergo a focused safety assessment by an investigator during their video visit. A detailed description of safety oversight has been reported previously.13
Return of Results
Participants were asked at the start of the study whether they would like their neurologist and/or primary care provider to receive an individualized research summary after each visit. Participants may also receive their own results if requested. A detailed description of the process for the return of individual research results has been reported previously.13
Bias
Traditionally, LRRK2 carriers with manifest PD are identified by screening individuals with PD who present to academic research centers, and LRRK2 nonmanifest carriers are identified by screening relatives of these individuals. This process results in a biased identification of LRRK2 carriers. In our model, LRRK2 carriers may pursue 23andMe genetic testing for reasons other than finding out their LRRK2 carrier status and testing is not dependent on the genetic status of relatives, so the bias expected with family-based methods and clinic-based recruitment is reduced. However, the resulting population reflects those who pursue direct-to-consumer genetic testing and is not representative of the general population.21
Statistical Analysis
We planned to enroll at least 250 LRRK2 G2019S carriers without and 50 carriers with self-reported PD. A detailed justification of the sample size has been reported previously.13 Predictors of enrollment in the study for all participants invited from 23andMe were identified using logistic regression with enrollment (yes or no) as the outcome variable and age, sex, genetic ancestry (i.e., Ashkenazi Jewish, European, Latino, or other), education level, marital status, PD status/duration, and family history of PD as independent variables. Genetic ancestry is used rather than self-reported ancestry because the latter is more likely to have missing data and yet still highly correlates with genetic ancestry. Given low numbers, to maintain anonymity, African American and Asian were not pulled out as separate categories under genetic ancestry.
Concordance between baseline self-reported PD status and expert clinician-determined PD, as well as PD determined according to each of the different diagnostic criteria (Movement Disorder Society Clinical Diagnostic Criteria for Parkinson's disease,16 the UK Parkinson's Disease Society Brain Bank Criteria,17 and the NIH Diagnostic Criteria for Parkinson's disease18), was determined using Cohen κ with 95% confidence intervals (CIs). To determine whether our study cohorts differed from those of published reports of other established LRRK2 cohorts22-27 regarding baseline demographic characteristics, t tests were used for continuous characteristics and χ2 or Fisher exact tests were used for categorical characteristics.
Those without self-reported PD and without clinician-determined PD were further categorized as having prodromal PD, defined as either meeting the 2015 Movement Disorder Society Prodromal Parkinson's Disease Criteria or by clinician determination of concern for parkinsonism. Baseline demographic and clinical characteristics were compared among those without prodromal PD, with prodromal PD, and with clinician-determined PD using analysis of variance for continuous characteristics or χ2 tests for categorical characteristics. Pairwise group comparisons were performed using the Tukey-Kramer test for analysis of variance and Bonferroni adjustment for χ2 tests to control the type I error probability.
The percentage of participants reporting at baseline being satisfied or very satisfied with the study and the percentage of participants being willing to participate in other virtual research studies were used to assess the acceptance of virtual study designs. In addition, interest in future PD research participation was reported as the percentage willing to participate in observational and interventional studies. Analyses were performed using SAS v9.4. p Values <0.05 were considered statistically significant. Missing data were minimal; therefore, results are based on actual responses with no imputation of missing values.
Data Availability
Anonymized data not published in the article may be shared on request from qualified researchers.
Results
Recruitment
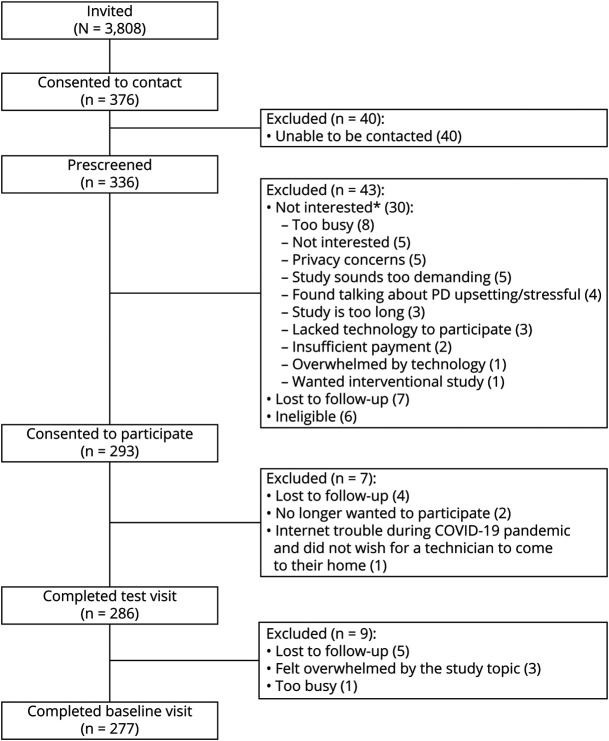
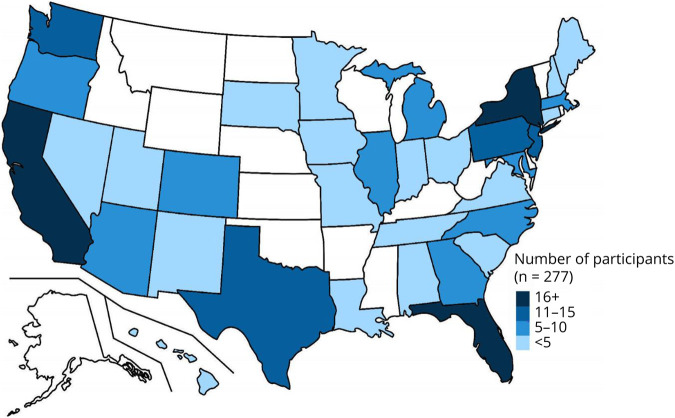
23andMe emailed 3,808 individuals inviting them to participate in the study, and 4,199 (38.9%) of all emails were opened. Of those invited, 376 participants (9.8%) consented to be contacted by a research coordinator at the University of Rochester to confirm eligibility, 336 (8.8%) contacts were successfully completed, and 277 (7.3%) LRRK2 G2019S carriers from 34 states enrolled in the study from June 2019 to August 2020 (Figures 1 and 2). A total of 217 individuals without self-reported PD (mean [SD] age 53.7 years [15.1], 95% White, 59% female, 73% Ashkenazi Jewish, and 57% with a family history of PD) and 60 individuals with self-reported PD (mean [SD] age 67.8 years [8.4], 98% White, 52% female, 80% Ashkenazi Jewish, and 67% with a family history of PD) enrolled in the study. Forty-three percent of enrolled participants without self-reported PD were 60 years or older compared with 85% of enrolled participants with self-reported PD (p < 0.001). Independent predictors of enrollment in the study were greater number of years of education (odds ratio [95% CI] = 1.16 [1.09–1.24], p < 0.001), being separated vs married (2.8 [1.2–6.7], p = 0.02), early PD (<5 years since diagnosis) vs no PD (3.5 [1.6–7.8], p = 0.002), and having a family history of PD (1.7 [1.2–2.3], p = 0.002).
Figure 1. Flowchart of Study Participants.
*Participants were instructed to choose all that applied. COVID-19 = coronavirus disease 2019; PD = Parkinson disease.
Figure 2. Location of Study Participants.
Assessment of PD
At baseline, the clinician assessment of PD matched closely with the participant's self-report of their PD status (κ = 0.94 95% CI 0.89–0.99). For 6 individuals, the investigator's expert assessment did not match the participant's self-report; all were suspected new PD diagnoses. In all cases where the individual self-reported PD, the investigator concurred. Of those with self-reported PD, 22 (37%) met Movement Disorder Society Clinical Diagnostic Criteria, 24 (40%) met UK Parkinson's Disease Society Brain Bank Criteria, and 49 (82%) met NIH Diagnostic Criteria (eTable 1, links.lww.com/NXG/A531). Across the entire cohort, agreement between self-reported diagnosis and application of the diagnostic criteria was moderate (κ = 0.45, 95% CI 0.31–0.58) for the Movement Disorder Society Clinical Diagnostic Criteria, moderate (κ = 0.49, 95% CI 0.36–0.62) for the UK Parkinson's Disease Society Brain Bank Criteria, and good (κ = 0.79, 95% CI 0.71–0.88) for the NIH Diagnostic Criteria. Of those without self-reported or clinician-identified PD (n = 211), 9 (4%) met the threshold for probable prodromal PD, investigators identified 20 (10%) with concern for parkinsonism, and 5 participants met both conditions. The frequency of each prodromal marker is presented in Table 1.
Study Cohort
The baseline characteristics of the study cohort are presented in Table 2. Median (interquartile range [IQR]) time for completion of the participant-reported outcome measures, and the baseline video visit was 7 (4–13) days. On average, individuals without PD or prodromal PD were younger than those with prodromal PD (those who either met the threshold for probable prodromal PD according to MDS criteria or had concern for parkinsonism) and those with clinician-determined PD. Participants with self-reported PD (n = 60) had a mean (SD) age at diagnosis of 61.1 (9.2) years. Aside from participation in the 23andMe Research Program required for participation in this study, over 75% of those without self-reported PD did not report participation in a prior PD or LRRK2 research study.
Table 2.
Baseline Characteristics of the Study Cohort
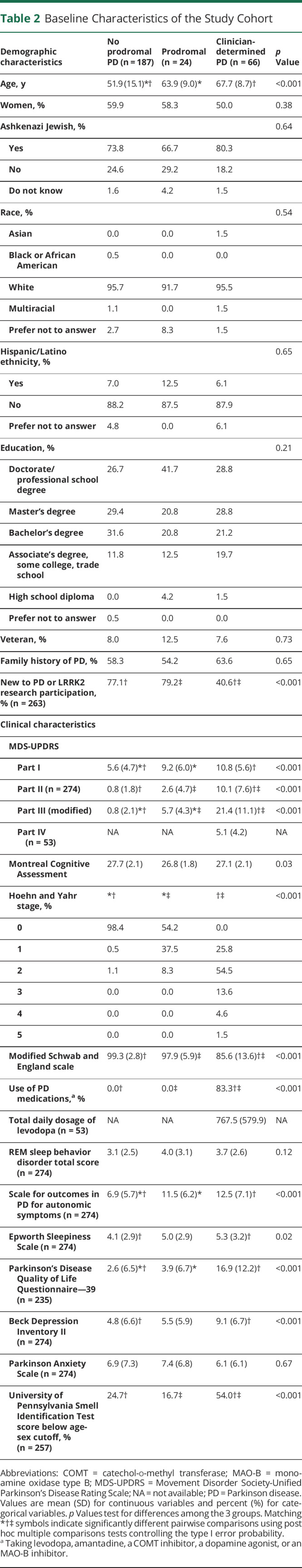
Compared with participants without prodromal PD, those with prodromal PD had higher autonomic scores (Scale for Outcomes in Parkinson's Disease for Autonomic Symptoms mean [SD] score 11.5 [6.2] vs 6.9 [5.7], p = 0.002) and higher motor scores (modified MDS-UPDRS part III mean [SD] score 5.7 [4.3] vs 0.8 [2.1], p < 0.001). There were no significant differences in depressive symptom scores, sleep symptom scores, cognitive scores, or rates of hyposmia between these 2 groups. Overall, those with concern for parkinsonism who did not meet the threshold for probable prodromal PD (n = 15) had higher motor scores and a lower rate of hyposmia than those who met the threshold for probable prodromal PD but did not have concern for parkinsonism (n = 4) (data not shown). Compared with participants without PD or prodromal PD, those with clinician-determined PD had higher depressive symptom scores (BDI-II mean [SD] score 9.1 [6.7] vs 4.8 [6.6], p < 0.001), more daytime sleepiness (Epworth Sleepiness Scale mean [SD] score 5.3 [3.2] vs 4.1 [2.9], p = 0.02), and were more likely to have hyposmia (UPSIT score below age-sex cutoff 54.0% vs 24.7%, p < 0.001).
Comparison With Traditional LRRK2 Cohorts
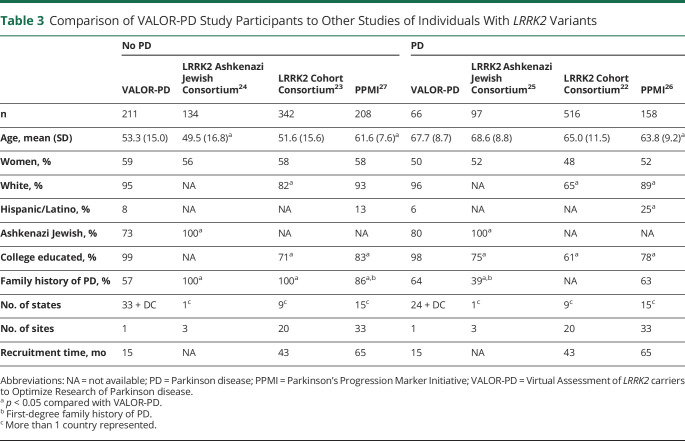
In comparison with traditional, in-person LRRK2 cohorts without PD, we recruited a comparable number of individuals from a single location over a shorter period of time. As shown in Table 3, in comparison with traditional LRRK2 cohorts, our participants were more likely to identify as White, more likely to have a college education, and less likely to be Ashkenazi Jewish. In addition, our participants without PD were less likely to have a family history of PD.
Table 3.
Comparison of VALOR-PD Study Participants to Other Studies of Individuals With LRRK2 Variants
Safety
One fall occurred during the baseline remote examinations, and no injuries occurred. No breaches of privacy or confidentiality were reported. No adverse clinical outcomes related to the study occurred.
Participant Feedback
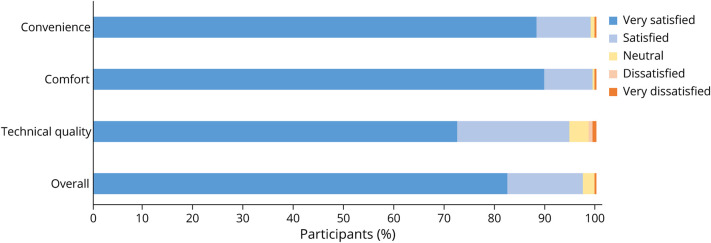
Of the 262 participants who completed a postbaseline visit survey, 97% reported that they were “satisfied” or “very satisfied” with the study overall. The satisfaction ratings for the technical quality, convenience, and comfort of the video visits were similarly high (Figure 3). About one-third would not have participated in this study if it required in-person study visits. In addition, over 99% of participants indicated willingness to participate in another observational study with video visits, and 94% of those without self-reported PD were willing to participate in a PD prevention trial (Figure 4). Median (IQR) time for completion of all baseline activities, including the participant-reported outcome measures, the video visit, and the postvisit survey, was 14 (8–21) days.
Figure 3. Participant Satisfaction With Video Visits.
Figure 4. Percent Willing to Participate in PD Clinical Trials.
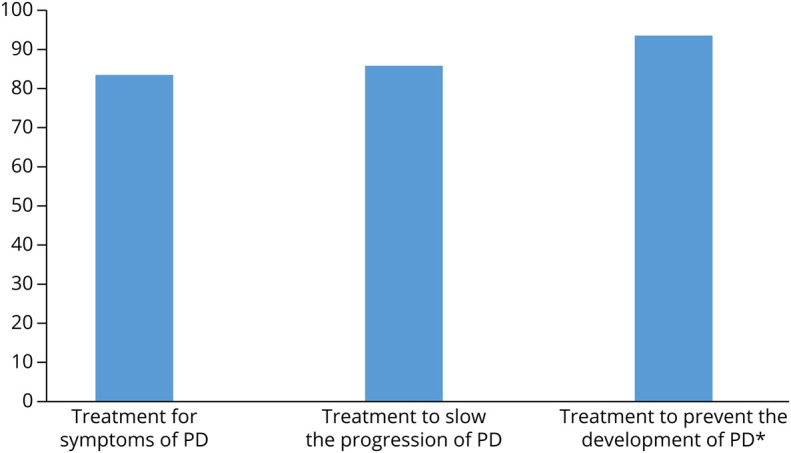
*Responses include only individuals without self-reported PD, those with self-reported PD were not asked. PD = Parkinson disease.
Discussion
Despite the COVID-19 pandemic, a large, national cohort of individuals with and at genetic risk for developing PD enrolled in an entirely remote observational study in just over a year. The scope of clinical assessments conducted was similar to that in traditional clinical studies, and the model was well received by participants who expressed high levels of satisfaction and interest in future clinical trials.
Precision medicine clinical trials aimed at individuals who carry genetic variants that put them at high risk of developing a neurologic disorder will likely need to screen and recruit large numbers of individuals.3 We have demonstrated that the combination of direct-to-consumer genetic testing and a remote, decentralized study design can identify and characterize a large number of at-risk carriers. Genetic testing is not yet standard practice in the care of individuals with PD. Traditional recruitment methods would require a large number of sites and widespread testing to identify a sufficient number of individuals at genetic risk for PD. For example, the Rostock International PD (ROPAD) study offers comprehensive genetic testing to individuals with PD and individuals without PD at higher likelihood of having a LRRK2 variant.28 ROPAD has thus far enrolled 67 individuals without PD at higher likelihood of having a LRRK2 variant and, among 1,288 individuals with PD, identified 23 LRRK2 Gly2019Ser carriers. It is clear that we will need to use multiple different methods of identification to recruit sufficient numbers of individuals for precision medicine trials.
As shown in Table 3, this cohort differs from traditionally established LRRK2 cohorts by race (a greater proportion are White) and education (more are college educated).22-27 This cohort, though, is also more geographically dispersed across the United States, less likely to be Ashkenazi Jewish, less likely to have a family history of PD (at least among those without PD, which expands the sampling frame), and includes many participants who are new to research, including more than 75% of those who do not have PD. This latter population may be especially important for trials aimed at delaying or preventing onset of the disease. In addition, recruitment was faster than has occurred for similar cohorts, covered a larger geographical area within the United States at likely lower cost, and unlike most other research studies,29 was not delayed or otherwise affected by the COVID-19 pandemic.
This study also demonstrated that a wide range of clinical assessments, including patient-reported outcomes, which may be better administered in an individual's natural setting than in an artificial clinical one, can be conducted remotely. Consistent with prior studies,11,30 when assessed remotely, 100% of individuals with self-reported PD were judged to have PD by the study investigators and 97% of those who did not report PD were judged not to have the disease. Agreement between self-reported PD status and modified formal diagnostic criteria was moderate to good. As suggested by Myers et al., these lower levels of agreement may reflect the inability to assess the core criterion of rigidity and to adequately assess the core criterion of rest tremor during remote assessment.30 Diagnostic criteria that are validated for remote application are needed.
We also demonstrated the ability to apply the Movement Disorder Society Prodromal Parkinson's Disease Criteria in LRRK2 carriers remotely, identifying 9 individuals who met the criteria for probable prodromal PD. We anticipate that long-term follow-up of this cohort will identify additional cases and add to the literature regarding the sensitivity and specificity of these criteria for identifying LRRK2 carriers who will ultimately develop PD.20
Among carriers without PD, compared with those without prodromal PD, those with prodromal PD (according to prodromal criteria or presence of concern for parkinsonism, n = 24) are older, have higher motor scores, and have higher autonomic symptom scores. Similarly, Mirelman et al.20 found that, compared with those without prodromal PD, those with prodromal PD (n = 20) were older, had higher motor scores, and more often reported constipation and erectile dysfunction. Prior studies have also demonstrated higher motor scores23,27 and more autonomic symptoms24,27 in nonmanifest LRRK2 G2019S carriers compared with noncarriers. We did not detect differences in cognitive scores, sleepiness, depressive symptoms, anxiety symptoms, REM sleep behavior disorder symptoms, or hyposmia between those with and without prodromal PD. Mirelman et al.20 found that those with prodromal PD more frequently reported sleepiness, had lower cognitive scores, and had higher frequency of hyposmia than those without prodromal PD. Multiple prior studies have not shown higher rates of sleepiness or REM sleep behavior disorder among nonmanifest LRRK2 G2019S carriers compared with noncarriers.23,27,31,32 While Simuni et al. found lower cognitive scores and higher rates of hyposmia among nonmanifest LRRK2 G2019S carriers compared with noncarriers, other studies did not.23,24,33 Given the incomplete penetrance of LRRK2 variants, the ability to accurately identify individuals with prodromal disease will be of high value in preparation for clinical trials.
Participants who chose to enroll in this longitudinal study expressed high levels of study satisfaction as they have in previous, remote cross-sectional studies in several neurodegenerative conditions.11,34-36 Participants also expressed high levels of interest in participating in future clinical trials, whether the treatment was aimed at prevention or symptomatic benefit. Whether this translates to actual participation remains to be seen.
Despite these benefits, the study has some significant limitations. The first is that recruitment relied on individuals who chose to pursue direct-to-consumer genetic testing. While increasingly common, such individuals are not representative of the general population.21 Moreover, while 23andMe invited all potentially eligible LRRK2 G2019S carriers, only a small minority (7.3%) of those contacted chose to enroll in the study. Only 217 individuals without self-reported PD were enrolled (falling short of the target of 250 individuals without self-reported PD) and 43% were older than 60 years (falling short of the target 50%). A family history of PD, early PD, and greater number of years of education predicted enrollment in the study. The resulting cohort was 95% White and 99% had at least some college education. The study was thus able to reduce geographical but not social barriers to research participation.37 The digital divide38 that is common in clinical applications of video visits may also apply to research studies.39
The second is that the characterization of this cohort is incomplete. The clinical examination performed remotely is inferior to an in-person examination and can be further compromised by poor connectivity. Certain features (e.g., rigidity, balance, eye movements) are difficult to assess remotely.40 This may be of particular concern when asking clinicians to identify concern for parkinsonism. In addition, while numerous clinical assessments were conducted, biological assessments, including those that could be collected remotely (e.g., urine) and those that would require in-person visits (e.g., lumbar puncture), imaging, and digital measures, were lacking. This cohort may be well positioned to participate in studies using digital sensors to develop deep clinical phenotypes41 and identify novel digital endpoints for future research studies. These new measures may be especially important in a population that is largely asymptomatic and in which biomarkers are lacking. The third is that this study lacks a control group of noncarriers for comparison. This may be of particular relevance because investigators are aware that all participants are at high risk for developing PD, which may introduce bias to over call subtle signs. The fourth is that we did not use objective criteria or cutoffs for the determination of concern for parkinsonism, relying instead on investigator judgment, which could limit reproducibility. Finally, this study was limited to carriers of a specific variant linked to a single disease. Whether this research model is generalizable to other variants (e.g., GBA)42 and to other conditions (e.g., Alzheimer disease, amyotrophic lateral sclerosis, autism) is yet to be determined. However, the use of video visits, which have now been used to provide care to a wide range of patients with neurologic 43 and other medical conditions, is likely well suited to a broad range of conditions.
Notwithstanding these limitations, this study demonstrates the feasibility and value of a new model of research. This model, largely based on clinical applications of video visits, will likely become more common as individuals, researchers, and sponsors become increasingly familiar and comfortable with the underlying technology. Its value will also rise as gene-based therapies for a wide range of conditions move from the laboratory to the clinic.2 Future studies, and likely clinical trials, could include a mixture of remote and in-person assessments, allowing for a more complete characterization of the study cohort while minimizing participant burden.
This study has demonstrated that remote recruitment of a geographically diverse cohort from a single site is feasible. Moreover, among this sample of study participants who obtained direct-to-consumer genetic testing, most like and even prefer this model to traditional in-person research visits and are interested in participating in future clinical trials. Longitudinal assessment will allow prospective characterization of this large LRRK2 G2019S cohort and address important gaps in knowledge, such as the identification of protective factors and risk factors for PD. The study will also inform the design of future clinical trials for this population and serve as a model for other genetically defined cohorts. As a new class of gene-based therapies arise for neurologic conditions, novel models of clinical research will be required. This study, which broadens recruitment, lowers barriers to participation, and may reduce complexity and cost, provides a glimpse of what those models may look like.
Acknowledgment
The authors thank the research participants and employees of 23andMe for making this work possible. The following members of the 23andMe Research Team contributed to this study: Stella Aslibekyan, Adam Auton, Elizabeth Babalola, Robert K. Bell, Jessica Bielenberg, Katarzyna Bryc, Emily Bullis, Gabriel Cuellar Partida, Devika Dhamija, Sayantan Das, Sarah L. Elson, Teresa Filshtein, Kipper Fletez-Brant, Pierre Fontanillas, Will Freyman, Pooja M. Gandhi, Karl Heilbron, Barry Hicks, David A. Hinds, Ethan M. Jewett, Yunxuan Jiang, Katelyn Kukar, Keng-Han Lin, Maya Lowe, Jey C. McCreight, Matthew H. McIntyre, Steven J. Micheletti, Meghan E. Moreno, Joanna L. Mountain, Priyanka Nandakumar, Elizabeth S. Noblin, Jared O'Connell, Aaron A. Petrakovitz, G. David Poznik, Morgan Schumacher, Anjali J. Shastri, Janie F. Shelton, Jingchunzi Shi, Suyash Shringarpure, Vinh Tran, Joyce Y. Tung, Xin Wang, Wei Wang, Catherine H. Weldon, Peter Wilton, Alejandro Hernandez, Corinna Wong, Christophe Toukam Tchakouté.
Glossary
- BDI-II
Beck Depression Inventory-II
- CI
confidence interval
- COVID-19
coronavirus disease 2019
- IQR
interquartile range
- LR
likelihood ratio
- MDS-UPDRS
Movement Disorders Society-Unified Parkinson's Disease Rating Scale
- PD
Parkinson disease
- ROPAD
Rostock International PD
- UPSIT
University of Pennsylvania Smell Identification Test
Appendix. Authors
Contributor Information
Stella Jensen-Roberts, Email: stella.jensenroberts@chet.rochester.edu.
Taylor L. Myers, Email: taylorlmyers@gmail.com.
Peggy Auinger, Email: peggy.auinger@chet.rochester.edu.
Paul Cannon, Email: pcannon@23andme.com.
Helen M. Rowbotham, Email: hrowbotham@23andme.com.
Daniella Coker, Email: daniellac@23andme.com.
Eli Chanoff, Email: elic@23andme.com.
Julia Soto, Email: julia.soto@chet.rochester.edu.
Meghan Pawlik, Email: meghan.pawlik@chet.rochester.edu.
Katherine Amodeo, Email: katherine.amodeo@wmchealth.org.
Saloni Sharma, Email: saloni.sharma@chet.rochester.edu.
Blanca Valdovinos, Email: blanca_valdovinos@urmc.rochester.edu.
Renee Wilson, Email: renee.wilson@chet.rochester.edu.
Aayush Sarkar, Email: aayush.sarkar@gmail.com.
Michael P. McDermott, Email: michael_mcdermott@urmc.rochester.edu.
Roy N. Alcalay, Email: royalcalay@gmail.com.
Kevin Biglan, Email: biglan_kevin@lilly.com.
Daniel Kinel, Email: dkinel@hselaw.com.
Caroline Tanner, Email: caroline.tanner@ucsf.edu.
Reni Winter-Evans, Email: lrrk2mutant@gmail.com.
Erika F. Augustine, Email: augustinee@kennedykrieger.org.
Robert G. Holloway, Email: robert_holloway@urmc.rochester.edu.
E. Ray Dorsey, Email: ray.dorsey@chet.rochester.edu.
Study Funding
Research reported in this publication was supported by the NINDS of the NIH under Award Number P50NS108676.
Disclosure
S. Jensen-Roberts, T.L. Myers, and P. Auinger report no disclosures relevant to the manuscript. P. Cannon is employed by and holds stock or stock options in 23andMe, Inc. H. Rowbotham is employed by and holds stock or stock options in 23andMe, Inc. D. Coker is employed by and holds stock or stock options in 23andMe, Inc. E. Chanoff is employed by and holds stock or stock options in 23andMe, Inc. J. Soto and M. Pawlik report no disclosures relevant to the manuscript. K. Amodeo has served as investigator for trials funded by Biogen, Aptinyx, NINDS, Roche, Acadia, and EIP Pharma and her movement disorders fellowship was funded by the Edmond J Safra MJFF. S. Sharma, B. Valdovinos, R. Wilson, A. Sarkar, and M.P. McDermott report no disclosures relevant to the manuscript. R.N. Alcalay received consultation fees from Genzyme/Sanofi, Avrobio, Jenssen, Caraway, Merck, GSK, Ono Therapeutics. K. Biglan is employed by Eli Lilly and Company. D. Kinel reports no disclosures relevant to the manuscript. C.M. Tanner reports grants from Gateway LLC, Roche/Genentech, Biogen Idec, consulting personal fees from Adamas Therapeutics, CNS Ratings, Lundbeck, Cadent, and Kyowa Kirin, all outside the submitted work. R. Winter-Evans reports no disclosures relevant to the manuscript. E.F. Augustine has a consulting agreement with PTC Therapeutics. R.G. Holloway reports no disclosures relevant to the manuscript. E.R. Dorsey has received honoraria for speaking at American Neurological Association, Excellus BlueCross BlueShield, International Parkinson's and Movement Disorders Society, National Multiple Sclerosis Society, Northwestern University, Stanford University, Texas Neurological Society, and Weill Cornell, received compensation for consulting services from Abbott, Abbvie, Acadia, Acorda, Alzheimer's Drug Discovery Foundation, Ascension Health Alliance, Biogen, BluePrint Orphan, Clintrex, Curasen Therapeutics, DeciBio, Denali Therapeutics, Eli Lilly, Grand Rounds, Huntington Study Group, medical-legal services, Medical Communications Media, Medopad, Medrhythms, Michael J. Fox Foundation, MJH Holding LLC, NACCME, Olson Research Group, Origent Data Sciences, Otsuka, Pear Therapeutic, Praxis, Prilenia, Roche, Sanofi, Spark, Springer Healthcare, Sunovion Pharma, Sutter Bay Hospitals, Theravance, University of California Irvine, and WebMD, research support from Acadia Pharmaceuticals, Biogen, Biosensics, Burroughs Wellcome Fund, CuraSen, Greater Rochester Health Foundation, Huntington Study Group, Michael J. Fox Foundation, NIH, Patient-Centered Outcomes Research Institute, Pfizer, PhotoPharmics, Safra Foundation, and Wave Life Sciences, editorial services for Karger Publications; and ownership interests with Grand Rounds (second opinion service). R.B. Schneider has received research funding from NIH, Michael J. Fox Foundation for Parkinson's Research, Biohaven Pharmaceuticals, Acadia Pharmaceuticals, and CHDI Foundation, consulting work with Escape Bio and the Parkinson's Foundation. Go to Neurology.org/NG for full disclosures.
References
- 1.Deverman BE, Ravina BM, Bankiewicz KS, Paul SM, Sah DWY. Gene therapy for neurological disorders: progress and prospects. Nat Rev Drug Discov. 2018;17(10):641-659. [DOI] [PubMed] [Google Scholar]
- 2.Sun J, Roy S. Gene-based therapies for neurodegenerative diseases. Nat Neurosci. 2021;24(3):297-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tolosa E, Vila M, Klein C, Rascol O. LRRK2 in Parkinson disease: challenges of clinical trials. Nat Rev Neurol. 2020;16(2):97-107. [DOI] [PubMed] [Google Scholar]
- 4.Horton R, Crawford G, Freeman L, Fenwick A, Wright CF, Lucassen A. Direct-to-consumer genetic testing. BMJ. 2019;367:l5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Regalado A. More than 26 million people have taken an at-home ancestry test [online]. Accessed December 22, 2021. technologyreview.com/s/612880/more-than-26-million-people-have-taken-an-at-home-ancestry-test/.
- 6.Dorsey ER, Kluger B, Lipset CH. The new normal in clinical trials: decentralized studies. Ann Neurol. 2020;88(5):863-866. [DOI] [PubMed] [Google Scholar]
- 7.Smolensky L, Amondikar N, Crawford K, et al. Fox Insight collects online, longitudinal patient-reported outcomes and genetic data on Parkinson's disease. Sci Data. 2020;7(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Initiative CTT. Decentralized clinical trials [online]. Accessed August 13, 2020. ctti-clinicaltrials.org/projects/decentralized-clinical-trials.
- 9.Broderick JP, Elm JJ, Janis LS, et al. National Institutes of Health StrokeNet during the time of COVID-19 and beyond. Stroke. 2020;51(8):2580-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doherty GJ, Goksu M, de Paula BHR. Rethinking cancer clinical trials for COVID-19 and beyond. Nat Cancer. 2020:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorsey ER, Darwin KC, Mohammed S, et al. Virtual research visits and direct-to-consumer genetic testing in Parkinson's disease. Digit Health. 2015;1:2055207615592998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Healy DG, Falchi M, O'Sullivan SS, et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson's disease: a case-control study. Lancet Neurol. 2008;7(7):583-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider RB, Myers TL, Rowbotham HM, et al. A virtual cohort study of individuals at genetic risk for Parkinson's disease: study protocol and design. J Parkinsons Dis. 2020;10(3):1195-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goetz CG, Fahn S, Martinez-Martin P, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): process, format, and clinimetric testing plan. Mov Disord. 2007;22(1):41-47. [DOI] [PubMed] [Google Scholar]
- 15.Tarolli CG, Andrzejewski K, Zimmerman GA, et al. Feasibility, reliability, and value of remote video-based trial visits in Parkinson's disease. J Parkinsons Dis. 2020;10(4):1779-1786. [DOI] [PubMed] [Google Scholar]
- 16.Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord. 2015;30(12):1591-1601. [DOI] [PubMed] [Google Scholar]
- 17.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56(1):33-39. [DOI] [PubMed] [Google Scholar]
- 19.Berg D, Postuma RB, Adler CH, et al. MDS research criteria for prodromal Parkinson's disease. Mov Disord. 2015;30(12):1600-1611. [DOI] [PubMed] [Google Scholar]
- 20.Mirelman A, Saunders-Pullman R, Alcalay RN, et al. Application of the Movement Disorder Society prodromal criteria in healthy G2019S-LRRK2 carriers. Mov Disord. 2018;33(6):966-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Majumder MA, Guerrini CJ, McGuire AL. Direct-to-consumer genetic testing: value and risk. Annu Rev Med. 2021;72:151-166. [DOI] [PubMed] [Google Scholar]
- 22.Marras C, Alcalay RN, Caspell-Garcia C, et al. Motor and nonmotor heterogeneity of LRRK2-related and idiopathic Parkinson's disease. Mov Disord. 2016;31(8):1192-1202. [DOI] [PubMed] [Google Scholar]
- 23.Pont-Sunyer C, Tolosa E, Caspell-Garcia C, et al. The prodromal phase of leucine-rich repeat kinase 2-associated Parkinson disease: clinical and imaging Studies. Mov Disord. 2017;32(5):726-738. [DOI] [PubMed] [Google Scholar]
- 24.Mirelman A, Alcalay RN, Saunders-Pullman R, et al. Nonmotor symptoms in healthy Ashkenazi Jewish carriers of the G2019S mutation in the LRRK2 gene. Mov Disord. 2015;30(7):981-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alcalay RN, Mirelman A, Saunders-Pullman R, et al. Parkinson disease phenotype in Ashkenazi Jews with and without LRRK2 G2019S mutations. Mov Disord. 2013;28(14):1966-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simuni T, Brumm MC, Uribe L, et al. Clinical and dopamine transporter imaging characteristics of leucine rich repeat kinase 2 (LRRK2) and glucosylceramidase beta (GBA) Parkinson's disease participants in the Parkinson's progression markers initiative: a cross-sectional study. Mov Disord. 2020;35(5):833-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simuni T, Uribe L, Cho HR, et al. Clinical and dopamine transporter imaging characteristics of non-manifest LRRK2 and GBA mutation carriers in the Parkinson's Progression Markers Initiative (PPMI): a cross-sectional study. Lancet Neurol. 2020;19(1):71-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skrahina V, Gaber H, Vollstedt EJ, et al. The Rostock International Parkinson's Disease (ROPAD) study: protocol and initial findings. Mov Disord. 2021;36(4):1005-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xue JZ, Smietana K, Poda P, Webster K, Yang G, Agrawal G. Clinical trial recovery from COVID-19 disruption. Nat Rev Drug Discov. 2020;19(10):662-663. [DOI] [PubMed] [Google Scholar]
- 30.Myers TL, Tarolli CG, Adams JL, et al. Video-based Parkinson's disease assessments in a nationwide cohort of Fox Insight participants. Clin Park Relat Disord. 2021;4:100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saunders-Pullman R, Alcalay RN, Mirelman A, et al. REM sleep behavior disorder, as assessed by questionnaire, in G2019S LRRK2 mutation PD and carriers. Mov Disord. 2015;30(13):1834-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pont-Sunyer C, Iranzo A, Gaig C, et al. Sleep disorders in parkinsonian and nonparkinsonian LRRK2 mutation carriers. PLoS One. 2015;10(7):e0132368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saunders-Pullman R, Mirelman A, Wang C, et al. Olfactory identification in LRRK2 G2019S mutation carriers: a relevant marker? Ann Clin Transl Neurol. 2014;1(9):670-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dorsey ER, Wagner JD, Bull MT, et al. Feasibility of virtual research visits in fox trial finder. J Parkinsons Dis. 2015;5(3):505-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bull MT, Darwin K, Venkataraman V, et al. A pilot study of virtual visits in Huntington disease. J Huntingtons Dis. 2014;3(2):189-195. [DOI] [PubMed] [Google Scholar]
- 36.Tarolli CG, Zimmerman GA, Goldenthal S, et al. Video research visits for atypical parkinsonian syndromes among Fox Trial Finder participants. Neurol Clin Pract. 2020;10(1):7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider MG, Swearingen CJ, Shulman LM, Ye J, Baumgarten M, Tilley BC. Minority enrollment in Parkinson's disease clinical trials. Parkinsonism Relat Disord. 2009;15(4):258-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norris P. Digital Divide: Civic Engagement, Information Poverty, and the Internet Worldwide. Cambridge University Press, 2001. [Google Scholar]
- 39.Beck CA, Beran DB, Biglan KM, et al. National randomized controlled trial of virtual house calls for Parkinson disease. Neurology. 2017;89(11):1152-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dorsey ER, Bloem BR, Okun MS. A new day: the role of telemedicine in reshaping care for persons with movement disorders. Mov Disord. 2020;35(11):1897-1902. [DOI] [PubMed] [Google Scholar]
- 41.Dorsey ER, Omberg L, Waddell E, et al. Deep phenotyping of Parkinson's disease. J Parkinsons Dis. 2020;10(3):855-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gan-Or Z, Liong C, Alcalay RN. GBA-associated Parkinson's disease and other synucleinopathies. Curr Neurol Neurosci Rep. 2018;18(8):44. [DOI] [PubMed] [Google Scholar]
- 43.Hatcher-Martin JM, Adams JL, Anderson ER, et al. Telemedicine in neurology: Telemedicine Work Group of the American Academy of Neurology update. Neurology. 2020;94(1):30-38. [DOI] [PubMed] [Google Scholar]
- 44.Stiasny-Kolster K, Mayer G, Schafer S, Moller JC, Heinzel-Gutenbrunner M, Oertel WH. The REM sleep behavior disorder screening questionnaire—a new diagnostic instrument. Mov Disord. 2007;22(16):2386-2393. [DOI] [PubMed] [Google Scholar]
- 45.Dorsey ER, Deuel LM, Voss TS, et al. Increasing access to specialty care: a pilot, randomized controlled trial of telemedicine for Parkinson's disease. Mov Disord. 2010;25(11):1652-1659. [DOI] [PubMed] [Google Scholar]
- 46.Doty RL, Bromley SM, Stern MB. Olfactory testing as an aid in the diagnosis of Parkinson's disease: development of optimal discrimination criteria. Neurodegeneration. 1995;4(1):93-97. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez-Blazquez C, Forjaz MJ, Frades-Payo B, de Pedro-Cuesta J, Martinez-Martin P, Longitudinal Parkinson's Disease Patient Study ELdPcEdPG. Independent validation of the scales for outcomes in Parkinson's disease-autonomic (SCOPA-AUT). Eur J Neurol. 2010;17(2):194-201. [DOI] [PubMed] [Google Scholar]
- 48.Spira AP, Beaudreau SA, Stone KL, et al. Reliability and validity of the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Scale in older men. J Gerontol A Biol Sci Med Sci. 2012;67(4):433-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leentjens AF, Verhey FR, Luijckx GJ, Troost J. The validity of the Beck Depression Inventory as a screening and diagnostic instrument for depression in patients with Parkinson's disease. Mov Disord. 2000;15(6):1221-1224. [DOI] [PubMed] [Google Scholar]
- 50.Leentjens AF, Dujardin K, Pontone GM, Starkstein SE, Weintraub D, Martinez-Martin P. The Parkinson Anxiety Scale (PAS): development and validation of a new anxiety scale. Mov Disord. 2014;29(8):1035-1043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published in the article may be shared on request from qualified researchers.