Abstract
Background and Objectives
Chronic pain (CP) and cognitive decline (CD) are highly comorbid and debilitating among older adults. We iteratively developed Active Brains–Fitbit (AB-F), a group mind–body activity program aided by a Fitbit that is feasible and associated with improvements in physical, cognitive, and emotional functioning when delivered in-person to older adults with CP and CD. We adapted our intervention and methodology for remote delivery to bypass barriers to participation. Here we report on a feasibility randomized controlled trial of the virtual AB-F versus a Health Enhancement Program (HEP) educational control followed by qualitative exit interviews.
Research Design and Methods
Older adults (aged ≥60) with CP and CD (2 cohorts) completed 8 weeks of AB-F (n = 8) or HEP (n = 11). Study procedures were fully remote via live video. Quantitative analyses explored feasibility and acceptability markers and within-group improvements in outcomes. Qualitative analyses were primarily deductive using the Framework Method.
Results
AB-F met a priori set feasibility benchmarks, similar to our in-person pilot. Participation in AB-F was associated with preliminary signals of improvement in multimodal physical function, emotional function (anxiety), cognitive function, pain intensity, and coping (e.g., pain self-efficacy, catastrophizing). Participation in HEP was associated with smaller or negligible improvements. Exit interviews confirmed feasibility and satisfaction with our completely remote interventions and methodology.
Discussion and Implications
Results provide evidence for the feasibility of our completely remote study and for initial markers of improvement after AB-F. The results will inform a fully powered remote efficacy trial.
Clinical Trial Registration
Keywords: Chronic illness, Chronic pain, Cognitive decline, Mind–body, Physical activity
Chronic musculoskeletal pain (CP) and cognitive decline (CD) are challenging to treat, often co-occur, and worsen each other over time. An estimated 60%–75% of older adults (65 or older) report at least some persistent pain (Molton & Terrill, 2014), and rates increase with age (Tsang et al., 2008). The prevalence of mild cognitive impairment (CD that precedes dementia confirmed by objective testing) ranges from 10% to 25% in older adults and up to 50% report subjective CD (Si et al., 2020). Older adults with CP are 2 times more likely to also report CD (Whitlock et al., 2017). Recent research shows that the CP–CD relationship is bidirectional among older adults. CP accelerates CD and increases the risk for dementia (Whitlock et al., 2017), while neurodegeneration associated with CD can exacerbate pain perceptions and disability (van Kooten et al., 2016). Older adults with comorbid CP and CD can become caught in a “disability spiral,” whereby impairments in cognitive, physical, emotional, and social functioning exacerbate one another and progressively worsen over time (Vlaeyen & Linton, 2012).
To the best of our knowledge, there are no evidence-based nonpharmacological treatments that comprehensively address this comorbidity in older adults. Mind–body interventions (Greenberg et al., 2020) and strategies to safely increase physical activity (e.g., walking) aided by digital monitoring devices (e.g., Fitbit) show promise in improving physical, emotional, and social functioning. However, these interventions have not been tailored to meet the unique needs of older adults with CP and CD.
To address these treatment gaps, we iteratively developed the Active Brains–Fitbit (AB-F; Mace, Doorley et al., 2021) program following the National Institutes of Health (NIH) Stage Model (Nielsen et al., 2018). Our multimodal mind–body activity program is grounded in the fear-avoidance model (Vlaeyen & Linton, 2012) and integrates relaxation response (Park et al., 2013) and mindfulness with pain-specific cognitive-behavioral and operant/physical restoration elements and uses technology (digital monitoring devices) to reinforce step count linked to activities of daily living. Program skills are conceptualized as working together to decrease nonadaptive reactions to pain (kinesiophobia, fear of movement due to pain; pain catastrophizing, magnifying the threat of pain), increase adaptive coping strategies (mindfulness, relaxation, gratitude, and self-compassion skills) to promote pain self-efficacy and acceptance, increase adaptive social functioning (engaging social support, decreasing social isolation), and increase memory compensatory strategies (Supplementary Figure 1). In a previous open pilot, AB-F was feasible when delivered in an in-person group format and associated with improvement in multimodal physical, cognitive, emotional function, and decreased pain intensity (Mace et al., 2020). Qualitative exit interviews revealed significant barriers to in-person attendance (e.g., costs, coordinating rides) and that technological devices (e.g., Fitbit, ActiGraph accelerometer) were well accepted by participants, highlighting the need to adapt AB-F for remote delivery.
Virtual interventions delivered via live videoconferencing software are effective in reducing these barriers (Mahmoud et al., 2019). Despite stereotypes, older adults can effectively use technology, including live video software, and, when introduced to these technologies, are often motivated to gain mastery (Ng, 2007). Our virtual adaptations to mind–body interventions for patients with neurofibromatosis (who frequently report cognitive challenges) and stroke highlight the potential for live video adaptations to AB-F.
To increase accessibility and incorporate previous participants’ feedback, we adapted the program and study procedures to be fully remote (Mace, Doorley et al., 2021). Here, we report on a feasibility, single-blind randomized controlled trial (RCT) of AB-F versus a time- and dose-matched Health Enhancement Program (HEP; Mahaffey et al., 2020) education control, both delivered via secure live video (NIH Stage 1B). We hypothesized that the virtual AB-F and fully remote study procedures would meet a priori feasibility, acceptability, credibility, expectancy, and satisfaction benchmarks comparable to our in-person open pilot. We also hypothesized that participation in AB-F would be associated with preliminary signals of improvement in multimodal physical, emotional, cognitive, and social outcomes, as well as proposed mechanistic program targets (e.g., coping, mindfulness, self-compassion, social functioning). Finally, we expected that qualitative exit interview findings would inform our subsequent fully powered remote efficacy trial (NIH Stage II).
Method
This single-blind feasibility RCT is consistent with the NIH Stage Model (1B) and builds on prior AB-F pilot studies. For additional methodological details, see our published study protocol (Mace, Doorley et al., 2021) and Supplementary Materials. Our Institutional Review Board (IRB) approved all study procedures (#2018P002152).
Participants
We recruited older adults from Massachusetts General Hospital with CP and CD using IRB-approved recruitment flyers distributed to physicians and posted on social media (Facebook) as well as an online recruitment platform for medical research within our hospital system (Partners Rally), which we used in previous iterations of our study (Mace et al., 2020; Mace, Gates et al., 2021; Mace, Greenberg et al., 2021). Our eligibility criteria were informed by intervention development studies in older adults with CP (Morone et al., 2009) and CD (Mace, Gates et al., 2021).
Inclusion criteria were as follows: (a) ≥age 60, (b) heterogeneous (i.e., multiple etiologies) chronic pain for ≥3 months (Bryce et al., 2012), (c) self-reported CD (Jessen et al., 2014), (d) ability to perform the 6-min walk test (6MWT), (e) no psychotropic or pain medications for the past 2 weeks or stable on these medications for the past 6 weeks and willing to maintain this dose, (f) cleared by a physician for study participation and no self-reported concerns about physical functioning on the Physical Activity Readiness Questionnaire (Adams, 1999), and (g) access to a smartphone with Bluetooth 4.0 capability and a tablet or computer.
Exclusion criteria were as follows: (a) medical illness expected to worsen in the next 6 months, (b) serious mental illness for which hospitalization may be likely in the next 6 months, (c) current substance abuse, (d) current suicidal ideation, (e) regular use of a digital activity monitoring device in the past 3 months, (f) intense exercise for more than 30 min per day, (g) mindfulness practice (e.g., meditation, yoga) for more than 45 min per week in the past 3 months, and (h) inability to walk without assistance. Additional rationale for our CP and CD criteria are reported in Supplementary Material. Table 1 presents demographics and clinical characteristics for the sample.
Table 1.
Demographics and Clinical Characteristics for the HEP (n = 11) and AB-F (n = 9)
| Characteristic | HEP M (SD, range or n [%]) | AB-F M (SD, range or n [%]) |
|---|---|---|
| Age | 70.8 (8.4, 60.0–87.1) | 68.9 (5.9, 61.2–79.3) |
| Gender | ||
| Male | 4 (36.36%) | 3 (33.33%) |
| Female | 7 (63.64%) | 6 (66.67%) |
| Ethnicity | ||
| Not Hispanic or Latino/Latina | 11 (100%) | 9 (100%) |
| Race | ||
| American Indian/Alaskan Native | 0 | 1 (11.11%) |
| White | 10 (90.91%) | 7 (77.78%) |
| More than one race | 1 (9.09%) | 1 (11.11%) |
| Marital status | ||
| Single, never married | 1 (9.09%) | 0 |
| Living with significant other | 6 (54.54%) | 3 (33.33%) |
| Married | 0 | 1 (11.11%) |
| Separated/divorced | 3 (27.27%) | 2 (22.22%) |
| Widowed | 1 (9.09%) | 3 (33.33%) |
| Education | ||
| Completed high school or GED (12 years) | 1 (9.09%) | 0 |
| Some college/associates degree (<16 years) | 3 (27.27%) | 3 (33.33%) |
| Completed college (16 years) | 2 (18.18%) | 3 (33.33%) |
| Graduate/professional degree (>16 years) | 5 (45.45%) | 3 (33.33%) |
| Employment | ||
| Employed full-time | 1 (9.09%) | 0 |
| Employed part-time | 1 (9.09%) | 1 (11.11%) |
| Retired | 6 (54.54%) | 4 (44.44%) |
| Unemployed | 1 (9.09%) | 1 (11.11%) |
| Other situation | 2 (18.18%) | 3 (33.33%) |
| Pain medications | ||
| Opioids | 4 (36.36%) | 2 (22.22%) |
| Other analgesics | 4 (36.36%) | 5 (55.56%) |
| None | 3 (27.27%) | 2 (22.22%) |
| Reported pain location | ||
| Upper | 4 (36.36%) | 3 (33.33%) |
| Lower | 0 | 2 (22.22%) |
| Multisite | 7 (63.64%) | 4 (44.44%) |
| Pain duration | ||
| ≤5 years | 2 (18.18%) | 4 (44.44%) |
| 6–10 years | 3 (27.27%) | 2 (22.22%) |
| ≥11 years | 6 (54.54%) | 3 (33.33%) |
| Cognitive status | ||
| Normal | 8 (72.73%) | 4 (44.44%) |
| Cognitive impairment | 3 (27.27%) | 5 (55.56%) |
Notes: HEP = Health Enhancement Program (control group); AB-F = Active Brains–Fitbit. Cognitive impairment identified with a Montreal Cognitive Assessment cutoff of 26. While only eight participants completed the AB-F intervention, demographic data were available for all nine participants who completed the baseline assessment.
Procedures
We adapted our previously published study procedures to facilitate completely remote study procedures and virtual delivery via Zoom (Mace, Doorley et al., 2021). To optimize feasibility, acceptability, and adherence, we followed guidelines for facilitating older adults’ use of technology, including harnessing social support (learning study technology in groups supported by multiple research staff, leveraging support from participants’ family members) and giving participants time to explore Zoom functions (Tsai et al., 2017).
We aimed to enroll and randomize up to 10 participants for each of the two rounds of this pilot RCT (N = 20) to evaluate the feasibility of delivering the programs in groups of five to six participants (Chadi et al., 2020) for the subsequent efficacy trial. After providing electronic informed consent, participants were randomized to either AB-F or the HEP using a block design (blocks of 12) to ensure equivalent group sizes. To maintain single-arm blinding, the study staff referred to the AB-F and HEP as AB1 and AB2, respectively.
After randomization, the research assistant emailed Zoom appointment instructions and mailed participants: (a) welcome letters from the principal investigator, (b) testing materials for the Montreal Cognitive Assessment (MoCA; Nasreddine et al., 2005), (c) a wGT3X-BT ActiGraph accelerometer (including wear-time log, instructional document, reminder card), and (d) prepaid return envelopes for the accelerometer. AB-F group received an additional sealed envelope with a Fitbit, charger, wall-plug, device instructions, and log-in information. AB-F participants were asked not to open that envelop until their Fitbit pairing session (1 week before Session 1).
For the baseline assessment, participants (a) wore the ActiGraph accelerometer for 1 week (during waking hours except while bathing), (b) completed the MoCA via Zoom with trained study staff, (c) self-administered the 6MWT using the Timed Walk app (outside using GPS), and (d) completed quantitative self-report measures via REDCap on a Zoom call with two research assistants for technical support. Participants were notified of their group assignment after baseline assessments were complete.
Participants completed 8 weeks of AB-F or HEP delivered by a study clinician via Zoom (Supplementary Table 1). We repeated all assessment procedures 1 week postintervention. After the postintervention assessment, participants returned their accelerometers using a prepaid envelope. Participants who attended at least one session agreed to an optional 30-min Zoom group exit interview (AB-F and HEP conducted separately) with the study clinicians. Participants earned $30 for each assessment, $10 for each intervention session, and homework (AB-F only), and $30 for the exit focus group ($170 total).
Measures
Feasibility measures
The primary outcomes were a priori feasibility acceptability, credibility, expectancy, and satisfaction benchmarks. We used intervention development guidelines (Leon et al., 2011) and our previous feasibility and pilot studies (Greenberg et al., 2020) to determine scoring criteria. See Supplementary Table 2 for details on feasibility measures.
Quantitative measures
We measured (a) Multimodal physical function: objectively measured step count with wGT3X-BT ActiGraph accelerometer (ActiGraph LLC, n.d.), performance-based via 6MWT using the Timed Walk smartphone application (Dario, 2020; Salvi et al., 2020), and patient-reported with PROMIS Physical Function (Stone et al., 2016), World Health Organization Disability Assessment Schedule (WHODAS; Ustün et al., 2010), Godin Leisure-Time Questionnaire (GLQ; Amireault & Godin, 2015); (b) Cognitive function: virtual MoCA (Nasreddine et al., 2005), Everyday Cognition Scale (eCog-12; Tomaszewski Farias et al., 2011); (c) Emotional function: PROMIS Anxiety (v1.08a) and Depression (v1.08b; Pilkonis et al., 2011); (d) Social function: PROMIS Emotional Support (4a; Hahn et al., 2014), UCLA Loneliness Scale (UCLA-8; Russell et al., 1978); (e) Pain intensity: Numerical Rating Scale (Farrar et al., 2001); (f) Pain-specific coping: Pain Catastrophizing Scale (Sullivan et al., 1995), Pain Self-Efficacy Questionnaire (Nicholas, 2007), and Chronic Pain Acceptance Questionnaire-8 (Fish et al., 2010); (g) General coping: Measure of Current Status—Part A (Carver, 2006), Cognitive and Affective Mindfulness Scale—Revised (Feldman et al., 2006), Gratitude Questionnaire (Mccullough et al., 2002), and Self-Compassion Scale—Short Form (Raes et al., 2011). See Supplementary Materials for details on quantitative measures.
Group Exit Interviews
We used group exit interviews to gather perceptions about virtual program delivery and technological adaptations to further optimize AB-F. We followed procedures for virtually conducting focus groups (Jacobs et al., 2021) and collecting valid qualitative data (Rubin & Rubin, 2005). Supplementary Materials contain the exit interview script.
Data Analysis
Quantitative analysis
This pilot RCT focused primarily on the feasibility and acceptability of our study procedures and virtual adaptations. However, consistent with the NIH Stage Model guidelines for pilot studies (Nielsen et al., 2018), our secondary focus was to explore preliminary signals of improvement on key study outcomes via quantitative analyses.
Our sample size met established standards for exploring feasibility and signals of improvement for a future, large-scale RCT (Leon et al., 2011). We used R (R Core Team, 2020) for quantitative analyses. Study staff blinded to group assignment conducted validity checks to assess data quality and missing data. First, we assessed a priori set feasibility benchmarks by calculating frequency and proportions for AB-F and HEP separately. Next, we conducted an exploratory analysis on descriptive statistics for each measure, within-group pre–post comparisons using paired t-tests, calculation of Cohen’s d effect sizes (0.20 = small; 0.50 = medium; 0.80 = large) to explore signals for improvement in both groups reported separately. We used nonparametric tests when appropriate.
Qualitative analysis
Qualitative analyses were primarily deductive (Braun & Clarke, 2006) using the Framework Method (Gale et al., 2013) in NVivo 12 (Richards, 2018). We allowed inductive flexibility to explore unexpected themes (e.g., unanticipated barriers to technology). We manually transcribed and de-identified the recordings and checked them for accuracy. We read all transcripts, collaborated on an initial thematic framework, and created the codebook. We conducted line-by-line independent coding of key participant statements on one practice transcript. We incorporated gaps identified by the independent raters (initially coded as “other” according to Gale et al., 2013), incorporated divergent viewpoints, and agreed on the final codebook for remaining transcripts (interrater reliability, Kappa = 0.90). The study investigators resolved all disagreements through consensus. We charted and visualized the qualitative data using a spreadsheet matrix, balancing data reduction with preserving the original context and sentiment (Gale et al., 2013).
Results
Participants
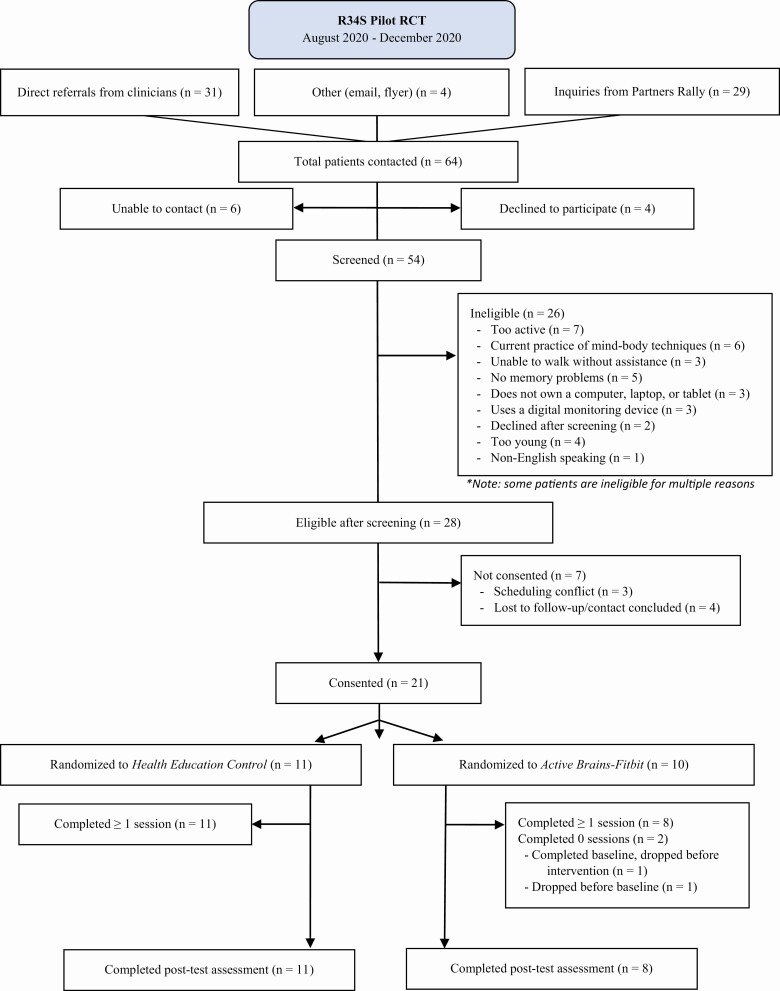
Figure 1 presents the participant flow. Of the 64 participants who expressed interest in the study, we screened 54 participants (84.4%, excellent feasibility of recruitment), 28 were eligible, and 21 were randomized to AB-F (n = 10) or HEP (n = 11). Two participants randomized to AB-F dropped out (one before baseline due to unwillingness to attend any proposed group times, one after baseline due to elective surgery). Thus, a total of eight participants in AB-F and 11 in HEP completed the program, assessments, and exit interviews (AB-F: n = 8; HEP: n = 11; completion rate = 90.5%; excellent program acceptability).
Figure 1.
Participant flow. RCT = randomized controlled trial.
Table 1 presents the sample demographics. Participants endorsed a range of pain conditions, durations, and medications. The majority of participants (HEP: n = 6, 60%; AB-F: n = 8, 73%) were sedentary at baseline (<5,000 steps; Tudor-Locke & Bassett, 2004). Participants reported moderate pain levels at rest (HEP: M = 4.4, SD = 2.6; AB-F: M = 5.9, SD = 2.5) and during activity (HEP: M = 5.7, SD = 2.6; AB-F: M = 6.2, SD = 1.8) comparative to norms (Nicholas et al., 2008). Average cognitive functioning scores on the MoCA approached clinically significant cognitive impairment (<26; HEP: M = 26.3, SD = 3.5; AB-F: M = 24.8, SD = 2.4). Self-reported CD symptoms on the eCog-12 (HEP: M = 1.9, SD = 0.6; AB-F: M = 2.3, SD = 1) were comparable to norms for mild cognitive impairment (Tomaszewski Farias et al., 2011).
Feasibility Markers
Table 2 reports feasibility and acceptability results by group. HEP and AB-F programs met the criteria for “excellent” on nearly all of the a priori set benchmarks. Participants had low initial expectations of improving during either program. One AB-F participant reported a fall at home but no adverse events were reported related to the study.
Table 2.
Feasibility Markers by Group
| Outcome | Health Education Control | Active Brains–Fitbit |
|---|---|---|
| Feasibility of recruitment | 54 participants out of 64 (84.4%) successfully contacted agreed to complete screening (excellent) | |
| Program acceptability | 11 out of 11 participants (100%) attended at least six out of eight sessions (excellent) | Eight out of 9 participants (88.9%) attended at least six out of eight sessions (excellent) |
| Credibility and expectancy | 11 out of 11 participants (100%) scored above the scale midpoint for credibility (excellent) Four out of 11 participants (36.4%) scored above the scale midpoint for expectancy (poor) |
Nine out of 9 participants (100%) scored above the scale midpoint for credibility (excellent) Five out of 9 participants (55.6%) scored above the scale midpoint for expectancy (poor) |
| Therapist adherence to the manual | 91.4% adherence (excellent) | 97.3% adherence (excellent) |
| Feasibility of quantitative measures | 99% of questionnaires were fully completed (excellent) | 85% of questionnaires were fully completed (excellent) |
| Adherence to homework | N/A | Eight out of 9 participants (88.9%) completed at least five of seven homework logs (excellent) |
| Adherence to ActiGraphs | 10 out of 11 participants at baseline and 10 out of 11 at post-test recorded ≥6 days of ActiGraph data (90.9% total; excellent) | Nine out of 9 participants at baseline and eight out of nine at post-test recorded ≥6 days of ActiGraph data (94.4%; excellent) |
| Adherence to Fitbit | N/A | Eight out of 9 (88.9%) wore the Fitbit for at least 5 out of 7 days for 6 out of the 8 weeks of the program (excellent) |
| Modified Patient Global Impression of Change | Six out of 11 participants (54.5%) reported overall improvement | Eight out of 9 participants (88.9%) reported overall improvement |
| Client satisfaction | Nine out of 11 (81.8%) of participants scored above the scale midpoint (excellent) | Eight out of 9 participants (88.9%) scored above the scale midpoint (excellent) |
| Program safety and adverse events | None | One participant reported falling in their home, unrelated to the study (excellent) |
Notes: N/A = not applicable. Calculations for the client satisfaction measure include missing data. For the health enhancement program, 1 participant out of 11 chose not to complete this measure and 1 participant scored below the midpoint. For Active Brains-Fitbit (AB-F), 1 participant out of 9 chose not to complete this measure and another participant only completed Item #2. We included this participant’s score, which was above the midpoint.
Quantitative Outcomes
Multimodal physical functioning
On average, AB-F participants exhibited greater improvements in physical functioning compared with HEP (Table 3, Supplementary Figure 2). Improvements in step count were clinically and statistically significant for AB-F participants, while step count significantly decreased for the HEP. Improvements on PROMIS physical functioning were moderate for AB-F participants and small for HEP participants. AB-F participants reported large improvements in physical activity intensity on GLQ compared to small-to-medium improvements for HEP participants. Participants in both groups had small-to-medium improvements in timed walking performance on the 6MWT, after removing an outlier (>2 SD below the mean change for AB-F). AB-F participants had small-to-medium improvements in disability on the WHODAS, which were smaller for HEP (Table 3). Overall, participants perceived their levels of independent engagement in activities of daily living as “much improved” or “very much improved” as a result of AB-F (75%) and HEP (18.2%).
Table 3.
Objective, Performance-Based, and Self-Report Physical, Pain, and Cognitive Functioning Outcomes
| Physical function assessments | Health Education Control | Active Brains–Fitbit | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline M (SD, range); n |
Post-test M (SD, range); n |
M difference (Post–Pre) | p | Cohen’s d | Baseline M (SD, range); n |
Post-test M (SD, range); n |
M difference (Post–Pre) | p | Cohen’s d | |
| ActiGraph average steps | 3,195.3 (1,975.7, 1,114.5–7, 518.12); n = 9 | 2,604.4 (1,912.8, 634.38–7, 377.12); n = 10 | −590.8 | .02 | 0.3 | 3,643.1 (1,679.9, 1,925.5–5, 734.12); n = 9 | 4,283.8 (1,905.7, 2,250.5–6, 925.12); n = 8 | 640.7 | .04 | 0.4 |
| 6MWT (m) | 415.6 (123.1, 207.3–601.99); n = 10 | 435.2 (101.6, 321.44–632.18); n = 11 | 31.0 | .22 | 0.3 | 407.6 (110.2, 192.47–559.51); n = 9 | 426.6 (147.6, 180.93–661.34); n = 8 | 33.1 | .19 | 0.3 |
| PROMIS Phys | 37.1 (6.2, 27.5–44.6); n = 11 | 38.2 (5.8, 25.8–45.4); n = 11 | 1.1 | .21 | 0.2 | 39.9 (5.5, 31.8–50.5); n = 9 | 43.4 (9.6, 30.5–60.1); n = 8 | 3.6 | .09 | 0.5 |
| WHODAS | 23.9 (17.2, 3.3–52.74); n = 11 | 22.2 (15, 3.12–45.8); n = 11 | −1.7 | .47 | 0.1 | 17.1 (14.3, 0–38.61); n = 10 | 16 (14.7, 1.04–39.34); n = 8 | −3.8 | .08 | 0.3 |
| GLQ (total) | 20 (20.9, 0–56); n = 11 | 30.8 (35.8, 0–113); n = 11 | 10.8 | .24 | 0.4 | 36 (34.9, 9–119); n = 9 | 74 (57.5, 9–180); n = 8 | 48.4 | .04 | 1.2 |
| Pain at rest | 4.4 (2.6, 0–10); n = 11 | 4.3 (1.8, 0–6); n = 11 | −0.2 | .74 | 0.1 | 5.9 (2.5. 1–9); n = 9 | 3.9 (2.1, 1–7); n = 8 | −1.6 | .12 | 0.8 |
| Pain with activity | 5.7 (2.6, 2–9); n = 11 | 5.7 (2.2, 3–9); n = 11 | 0 | 1 | 0 | 6.2 (1.8, 3–8); n = 9 | 4.2 (2.5, 1–8); n = 8 | −2.1 | .05 | 1 |
| CPAQ | 24.3 (10.7, 6–37); n = 11 | 27.1 (10.4, 7–46); n = 11 | 2.8 | .15 | 0.3 | 30.1 (8.7, 18–41); n = 8 | 31 (8.2, 23–45); n = 7 | 0.4 | .87 | 0 |
| MoCA | 26.3 (3.5, 19–30); n = 11 | 26.6 (3.7, 18–30); n = 11 | 0.4 | .6 | 0.1 | 24.8 (2.4, 21–28); n = 9 | 26.2 (1.7, 23–29); n = 8 | 1.6 | .05 | 0.8 |
| eCog-12 | 1.9 (0.6, 1–2.75); n = 11 | 1.8 (0.5, 1.17–2.5); n = 11 | −0.1 | .16 | 0.3 | 2.3 (1, 1–3.92); n = 8 | 2 (0.9, 1.08–4); n = 8 | −0.4 | .14 | 0.4 |
Notes: 6MWT = 6-min walk test; PROMIS Phys = PROMIS physical function; WHODAS = World Health Organization Disability Assessment Schedule; GLQ = Godin Leisure-Time Questionnaire; CPAQ = Chronic Pain Acceptance Questionnaire; MoCA = Montreal Cognitive Assessment; eCog-12 = Everyday Cognition Scale. “M Difference” = mean of the differences from the paired samples t-test.
Cognitive functioning and pain measures
AB-F participants exhibited large improvements in objective cognitive function on the MoCA while changes for HEP participants were negligible. Improvements in self-reported CD were small to medium for participants in both groups on eCog-12 (Table 3, Supplementary Figure 3). In the exit interview, participants in AB-F reported that their cognition was at least “minimally improved” as a result of AB-F (62.5%) or HEP (45.5%).
AB-F participants endorsed very large reductions in pain intensity with activity and large reductions in pain intensity at rest. Changes in pain intensity were negligible among HEP participants at rest and there was no change with activity (Table 3, Supplementary Figure 3). This aligns with the proportion of participants in AB-F (50%) who reported their pain was “much improved” or “very much improved” compared to participants in HEP (9%). Similarly, AB-F participants endorsed small-to-medium improvements in pain catastrophizing and pain self-efficacy, while the changes for HEP participants were negligible and small, respectively. Furthermore, 50% of AB-F participants perceived that their pain self-efficacy was either “much improved” or “very much improved” compared to 18.2% in the HEP. HEP participants reported small improvements in pain acceptance, which was negligible for the AB-F group. Participants in both groups did not endorse reductions in kinesiophobia.
Emotional functioning, social functioning, and general coping
AB-F participants endorsed medium reductions in anxiety on the PROMIS while changes in HEP participants were negligible. AB-F participants exhibited small-to-medium improvements in mindfulness and adaptive coping while these changes were smaller in HEP participants. For gratitude, HEP participants endorsed medium improvement, whereas gratitude in AB-F participants did not change. Overall, 63% of participants in HEP reported that their emotional functioning was at least “minimally improved” compared to 50% in AB-F participants. Changes in the remaining measures of emotional support, loneliness, depression, and self-compassion were negligible for participants in both groups (Table 4, Supplementary Figure 4).
Table 4.
Quantitative Self-Report Outcomes of Emotional and Social Functioning and Pain and General Coping
| Measures | Health Education Control | Active Brains–Fitbit | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline M (SD, range) |
Post-test M (SD, range) |
M difference (Post–Pre) | p | Cohen’s d | Baseline M (SD, range) |
Post-test M (SD, range) |
M difference (Post–Pre) | p | Cohen’s d | |
| PCS | 11.6 (9.4, 1–28); n = 10 | 11 (7.1, 2–24); n = 11 | −0.4 | .8 | 0 | 9.4 (6.8, 2–19); n = 9 | 7.1 (5.6, 1–18); n = 8 | −1.5 | .31 | 0.3 |
| PROMIS Dep | 55.6 (11.1, 37.1–73.8); n = 11 | 54.3 (11.7, 37.1–73.8); n = 11 | −1.4 | .54 | 0.1 | 51.6 (6.6, 43.1–61.7); n = 9 | 52.5 (8.6, 37.1–60.7); n = 8 | 0.4 | .81 | 0.1 |
| PROMIS Anx | 52.9 (10.1, 37.1–74.2); n = 11 | 53.7 (11, 37.1–68.9); n = 11 | 0.8 | .7 | 0.1 | 51 (10, 37.1–62.5); n = 9 | 47.9 (10.2, 37.1–60.5); n = 8 | −4.8 | .16 | 0.5 |
| PROMIS ES | 49.4 (9.6, 34.2–62); n = 11 | 48.8 (9.5, 34.2–62); n = 11 | −0.6 | .28 | 0.1 | 51.1 (11.2, 37.5–62); n = 9 | 52.1 (9.1, 41.1–62); n = 8 | 0.3 | .86 | 0 |
| UCLA-8 | 13.4 (4.4, 6–19); n = 10 | 13.3 (3.6, 8–19); n = 9 | −0.6 | .67 | 0.1 | 12.9 (3.3, 8–16); n = 9 | 12.6 (5.2, 3–18); n = 8 | −0.1 | .91 | 0 |
| CAMS-R | 33.8 (8, 18–44); n = 11 | 35.3 (6, 23–42); n = 11 | 1.4 | .46 | 0.2 | 31.9 (7.3, 25–45); n = 8 | 33.9 (6.1, 26–42); n = 8 | 2.4 | .4 | 0.4 |
| PSEQ | 35.5 (18.9, 9–58); n = 10 | 38 (13.5, 13–58); n = 11 | 2.5 | .46 | 0.2 | 38 (14.2, 23–60); n = 9 | 42.9 (14.5, 28–60); n = 7 | 9.0 | .1 | 0.7 |
| MOCS-A | 25.6 (10.1, 19–30); n = 11 | 28.3 (8.7, 11–42); n = 11 | 2.6 | .29 | 0.3 | 30.4 (12.4, 11–52); n = 9 | 36.1 (14.2, 16–52); n = 7 | 5.7 | .08 | 0.4 |
| GQ-6 | 32.5 (7.6, 19–41); n = 11 | 35.9 (5.1, 26–42); n = 11 | 3.4 | .12 | 0.5 | 36.2 (5.9, 27–42); n = 9 | 35.5 (7.1, 21–42); n = 8 | −0.7 | .67 | 0.1 |
| TKS | 37.6 (8.9, 17–47); n = 8 | 35.8 (7.4, 25–45); n = 9 | 0 | 1 | 0 | 31.9 (6.2, 21–40); n = 8 | 31.2 (13.5, 19–55); n = 6 | 1.0 | .8 | 0.1 |
| SCS-SF | 44.4 (8.5, 31–56); n = 11 | 43.6 (6.9, 33–56); n = 10 | −1.3 | .37 | 0.1 | 45.6 (7.9, 34–60); n = 8 | 43.2 (7.4, 35–56); n = 8 | −1.9 | .48 | 0.2 |
Notes: PCS = Pain Catastrophizing Scale; PROMIS Dep = PROMIS depression; PROMIS Anx = PROMIS anxiety; PROMIS ES = PROMIS emotional support; UCLA-8 = UCLA Loneliness Scale; CAMS-R = The Cognitive and Affective Mindfulness Scale; PSEQ = Pain Self-Efficacy Questionnaire; MOCS-A = The Measure of Current Status—Part A; GQ-6 = The Gratitude Questionnaire; TKS = Tampa Kinesiophobia Scale; SCS-SF = Self-Compassion Scale—Short Form. “M difference” = mean of the differences from the paired samples t-test.
Qualitative Exit Interviews
We present the themes that we identified through semistructured exit interviews in Supplementary Table 3. We describe the main findings for each theme below.
Theme 1: Technological adaptations
Participants reported positive experiences with technological adaptations including remote enrollment, secure Zoom platform, and online assessments. Participants described the remote enrollment as easy and user-friendly. The virtual program helped to overcome barriers (e.g., transportation, time) and increased access (e.g., “only way” they could participate). Participants said the virtual format can help increase group member diversity. For participants who were new to the Zoom platform, they appreciated research coordinator assistance. The 6MWT was acceptable through mobile application format. Participants had mixed reactions to the online questionnaire and cognitive assessments, with some expressing concerns about completing them too slowly while others believed the process was smooth.
Consistent with the pilot RCT, participants reported positive experiences with wearable technologies and remote training procedures. Using ActiGraph at baseline and postintervention was well-received by participants. Most participants noted it was “a nonissue,” although a few had difficulties remembering to wear it similar to our in-person pilot. They were grateful for the on-call research coordinator’s support (assistance and reminders). For AB-F, the Fitbit kept them engaged and on track with step goals. Participants explained that it was “very informative,” “easy to set up,” and kept them motivated with the live feedback.
Theme 2: Program experience
Participants had positive reactions to the program format, content, and execution: “easiest study I’ve done … effortless on my part.” They found the materials to be valuable and “user-friendly.” Participants said the Fitbit was essential for improvements and engagement. They enjoyed the “group aspect” and noted that “personal interactions” helped to enhance engagement and accountability. They endorsed a good balance between skill development, education, and social connections. Program reinforcements, such as reminders to engage in skills between sessions, were generally well-received. Participants identified several aspects of their program experience that were affected by coronavirus disease 2019 (COVID-19), including socializing, walking, and general health and safety.
Theme 3: Perceptions of improvements
Participants reported several benefits following program participation. They endorsed enhanced activity, gratitude, physical and social functioning, as well as decreases in pain. They also reported an improved understanding of the relationship between pain and activity. Participants noted a new appreciation for social interactions: “encouraged me to be more social. I started dating and it’s been 16 years.” Some participants felt their memory-related problems were stable and more prepared on how to manage them. One participant acknowledged that COVID-19 stress may have influenced the postintervention assessments: “I was really nervous about getting contaminated and I just know, for me, anxiety. A lot of reasons for my pain is from somatic.”
Discussion
Our entirely virtual RCT of AB-F demonstrated proof of concept for remotely teaching older adults mind–body and activity skills to address the CP and CD comorbidity. The remote trial met a priori feasibility benchmarks comparable to our in-person open pilot (Supplementary Table 4) and had higher ActiGraph adherence (94.4%) and patient-reported global improvements (88.9%). We demonstrated feasibility in recruitment and randomization and high retention (90.5%), similar to our open pilot study (91.7%) and higher than the previous mind–body interventions RCTs for older adults with CP (Morone & Greco, 2007) and CD (Eyre et al., 2017; Lam et al., 2014). Credibility, satisfaction, and attendance were high for both groups, suggesting strong acceptability among our target population. Participants were highly adherent to homework, accelerometer, and Fitbit.
Signals of within-group improvement were stronger for AB-F compared to HEP. AB-F exhibited clinically and statistically significant improvements in both objective (step count) and self-reported physical functioning while changes for HEP were smaller and even decreased for step count. AB-F had large to very large improvements in objective cognitive functioning and pain intensity while HEP had negligible improvements on both. Furthermore, AB-F endorsed small-to-medium improvements in pain catastrophizing and pain self-efficacy, while these changes were negligible and small, respectively, for HEP. These results build on positive findings for AB-F from our in-person open pilot (Mace, Gates et al., 2021) and provide preliminary support for our conceptual model of addressing fear-avoidance in AB-F (Supplementary Figure 1). The multimodal program skills target habitual negative affective reactions to pain and cognitive errors, focusing on acceptance, and learning to disengage from cognition and pain-related worries and sensations (e.g., during increased activity). Combining program skills with the digital monitoring device may bypass barriers to activity, maintain motivation, reinforce activity in real time, and avoid setbacks (e.g., doing too much too soon). Of note, baseline differences in these scores across groups (e.g., pain intensity, step count, cognitive functioning per the MoCA) may have influenced observed changes from baseline to postintervention (e.g., mean reversion) which we will address in our efficacy RCT (i.e., fully powered, adjust for baseline differences).
Emotional and social functioning and coping outcomes were more modest for both groups and favored AB-F. AB-F endorsed medium reductions in anxiety compared to negligible improvements for HEP. Both groups exhibited small-to-medium improvements in mindfulness and adaptive coping. Contrary to our hypothesis, HEP endorsed medium improvements in gratitude with no change for AB-F, and changes in kinesiophobia, pain acceptance, emotional support, loneliness, depression, and self-compassion were negligible for both groups. Notably, participants in our small sample reported relatively low levels of emotional distress (1 SD below our prior open pilot; Mace, Doorley et al., 2021) and greater self-compassion than norms for older adults (Bratt & Fagerström, 2020), which may have restricted the range for improvement on these measures. We will use participants’ individual accounts of improvement in emotional and social functioning from qualitative exit interviews (e.g., increased gratitude while walking, increased engagement in social/romantic relationships) to enhance emotional and social components of AB-F for the subsequent efficacy RCT.
Participants offered valuable suggestions to enhance the programs for the subsequent trial, including transitioning to smartphone-delivered homework assignments with automated reminders. Other suggestions included a more detailed self-report of daily pain during at-home practice (e.g., average and peak pain along with coping strategies) and beginning each AB-F session with a group mindfulness exercise. Using self-report questionnaires, such as the Mindfulness Adherence Questionnaire (Hassed et al., 2021), may facilitate the assessment of mindfulness practice in between sessions beyond the current homework log system. During the exit interviews, some participants reported that they have continued to use AB-F skills, such as meeting their Fitbit step count goal or self-compassion. We will assess the maintenance of intervention effects with 6-month follow-up assessments in the subsequent efficacy RCT.
Treatment expectancy in both groups was the only benchmark that fell below our previous in-person pilot. Because treatment expectations can influence outcomes, considerations for boosting baseline expectations are important to enhance efficacy in future trials. While low program expectations and high perceived credibility may be initially puzzling, credibility is more strongly related to perceptions gained through direct experience in an intervention (e.g., early experiences interacting with study staff). In contrast, expectations may be influenced by a wider range of factors, including patients’ own history (Schulte, 2008). During the exit interviews, some participants reported that COVID-19 stress affected multiple areas of life, which could have also dampened program expectations. We will administer a self-report of COVID-19 stress that captures these domains in the subsequent efficacy trial (Park et al., 2020). We will also aim to enhance expectations by refining our recruitment materials and communicating our successful technological support strategies. Participants identified several aspects of their program experience that were affected by COVID-19, including socializing, walking, and general health and safety.
Strengths of the study include (a) applying the NIH Stage Model to iteratively adapt methodology, (b) emphasizing feasibility benchmarks prior to efficacy testing in a full-scale multisite RCT, (c) collecting multimodal physical function data following Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) criteria (Gewandter et al., 2020), (d) exploring within-group analyses to estimate a signal of improvement (Leon et al., 2011), (e) technological adaptations to conduct the program virtually for patient safety during COVID-19 and preferences for remote interventions, and (f) our mixed-methods approach, which integrated qualitative data from exit interviews with feasibility markers and quantitative outcomes (Supplementary Figure 1).
There are also limitations: (a) Given the small sample size and heterogeneity of the patient population, interpretations of our quantitative results are limited to guiding the iterative development of AB-F for a subsequent fully powered RCT. (b) As with all trials, study compensation may have influenced patients’ willingness to participate, but we did not collect data to evaluate this. However, we believe that the total compensation offered was not enough to be coercive, and this level of compensation is similar to other mind–body, virtually delivered interventions by our group and approved by our IRB (Greenberg et al., 2020). (c) Our sample was comprised primarily of White, female, and urban-dwelling individuals residing in Massachusetts and does not represent all older adults with CP and CD. We plan to circumvent these limitations by collecting a larger, more diverse pool of participants for a subsequent efficacy RCT and collecting data on potential unmeasured confounds (e.g., COVID-19 distress, the influence of weather on physical activity, the influence of study compensation on study outcomes).
Multimodal and longitudinal assessment in future trials can help rule out confounds, including COVID-19 distress and weather. We plan to expand recruitment to geographically diverse regions and provide devices and data plans to older adults without access to promote more equitable, representative participation for the next trial.
This feasibility RCT involved technological adaptations to our AB-F program for older adults with CP and CD and comparison with a time- and attention-matched control (HEP). Mixed-methods data provided evidence of feasibility for our virtual interventions, stronger preliminary signals of improvement for AB-F compared to HEP, and valuable feedback regarding the feasibility and acceptability of technological adaptations. The next phase of intervention development will evaluate AB-F versus HEP in a full-scale, multisite clinical trial in a larger and more diverse sample. With remote technologies, there is potential to scale the accessibility of mind–body and activity skills to address CP and CD among older adults.
Supplementary Material
Contributor Information
James D Doorley, Integrated Brain Health Clinical and Research Program, Massachusetts General Hospital, Boston, USA; Harvard Medical School, Boston, Massachusetts, USA.
Ryan A Mace, Integrated Brain Health Clinical and Research Program, Massachusetts General Hospital, Boston, USA; Harvard Medical School, Boston, Massachusetts, USA.
Paula J Popok, Integrated Brain Health Clinical and Research Program, Massachusetts General Hospital, Boston, USA.
Victoria A Grunberg, Integrated Brain Health Clinical and Research Program, Massachusetts General Hospital, Boston, USA; Harvard Medical School, Boston, Massachusetts, USA.
Anya Ragnhildstveit, Integrated Brain Health Clinical and Research Program, Massachusetts General Hospital, Boston, USA.
Ana-Maria Vranceanu, Integrated Brain Health Clinical and Research Program, Massachusetts General Hospital, Boston, USA; Harvard Medical School, Boston, Massachusetts, USA.
Funding
This work was supported by a supplement from the National Institute on Aging (3R34AT009356-02S1) to an R34 grant funded by the National Institute of Complementary and Integrative Health (1R34AT009356-01A1).
Conflict of Interest
None declared.
References
- ActiGraph LLC . (n.d.). ActiGraph accelerometer (Version wGT3X-BT ActiGraph accelerometer) [Computer software]. https://actigraphcorp.com/actigraph-wgt3x-bt/
- Adams, R. (1999). Revised physical activity readiness questionnaire. Canadian Family Physician, 45, 992, 995, 1004–1005. [PMC free article] [PubMed] [Google Scholar]
- Amireault, S., & Godin, G. (2015). The Godin–Shephard leisure-time physical activity questionnaire: Validity evidence supporting its use for classifying healthy adults into active and insufficiently active categories. Perceptual and Motor Skills, 120(2), 604–622. doi: 10.2466/03.27.PMS.120v19x7 [DOI] [PubMed] [Google Scholar]
- Braun, V., & Clarke, V. (2006). Using thematic analysis in psychology. Qualitative Research in Psychology, 3(2), 77–101. [Google Scholar]
- Bratt, A., & Fagerström, C. (2020). Self-compassion in old age: Confirmatory factor analysis of the 6-factor model and the internal consistency of the self-compassion scale-short form. Aging & Mental Health, 24(4), 642–648. doi: 10.1080/13607863.2019.1569588 [DOI] [PubMed] [Google Scholar]
- Bryce, T. N., Biering-Sørensen, F., Finnerup, N. B., Cardenas, D. D., Defrin, R., Lundeberg, T., Norrbrink, C., Richards, J. S., Siddall, P., Stripling, T., Treede, R.-D., Waxman, S. G., Widerström-Noga, E., Yezierski, R. P., & Dijkers, M. (2012). International spinal cord injury pain classification: Part I. Background and description. Spinal Cord, 50(6), 413–417. doi: 10.1038/sc.2011.156 [DOI] [PubMed] [Google Scholar]
- Carver, C. S. (2006). Measure of Current Status (MOCS) [Data set]. Whitaker Institute. doi: 10.13072/midss.541 [DOI] [Google Scholar]
- Chadi, N., Weisbaum, E., Vo, D. X., & Ahola Kohut, S. (2020). Mindfulness-based interventions for adolescents: Time to consider telehealth. Journal of Alternative and Complementary Medicine (New York, N.Y.), 26(3), 172–175. doi: 10.1089/acm.2019.0302 [DOI] [PubMed] [Google Scholar]
- Dario, S. (2019). Timed walk (Version 0.1.2). Apple Store. https://apps.apple.com/us/app/timed-walk/id1515893887 [Google Scholar]
- Eyre, H. A., Siddarth, P., Acevedo, B., Van Dyk, K., Paholpak, P., Ercoli, L., St Cyr, N., Yang, H., Khalsa, D. S., & Lavretsky, H. (2017). A randomized controlled trial of Kundalini yoga in mild cognitive impairment. International Psychogeriatrics, 29(4), 557–567. doi: 10.1017/S1041610216002155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar, J. T., Young, J. P.Jr, LaMoreaux, L., Werth, J. L., & Poole, M. R. (2001). Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain, 94(2), 149–158. doi: 10.1016/S0304-3959(01)00349-9 [DOI] [PubMed] [Google Scholar]
- Feldman, G., Hayes, A., Kumar, S., Greeson, J., & Laurenceau, J.-P. (2006). Mindfulness and emotion regulation: The development and initial validation of the Cognitive and Affective Mindfulness Scale—Revised (CAMS-R). Journal of Psychopathology and Behavioral Assessment, 29(3), 177. doi: 10.1007/s10862-006-9035-8 [DOI] [Google Scholar]
- Fish, R. A., McGuire, B., Hogan, M., Morrison, T. G., & Stewart, I. (2010). Validation of the chronic pain acceptance questionnaire (CPAQ) in an Internet sample and development and preliminary validation of the CPAQ-8. Pain, 149(3), 435–443. doi: 10.1016/j.pain.2009.12.016 [DOI] [PubMed] [Google Scholar]
- Gale, N. K., Heath, G., Cameron, E., Rashid, S., & Redwood, S. (2013). Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Medical Research Methodology, 13, 117. doi: 10.1186/1471-2288-13-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewandter, J. S., Dworkin, R. H., Turk, D. C., Devine, E. G., Hewitt, D., Jensen, M. P., Katz, N. P., Kirkwood, A. A., Malamut, R., Markman, J. D., Vrijens, B., Burke, L., Campbell, J. N., Carr, D. B., Conaghan, P. G., Cowan, P., Doyle, M. K., Edwards, R. R., Evans, S. R., … Witter, J. (2020). Improving study conduct and data quality in clinical trials of chronic pain treatments: IMMPACT recommendations. The Journal of Pain, 21(9–10), 931–942. doi: 10.1016/j.jpain.2019.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg, J., Popok, P. J., Lin, A., Kulich, R. J., James, P., Macklin, E. A., Millstein, R. A., Edwards, R. R., & Vranceanu, A. M. (2020). A mind–body physical activity program for chronic pain with or without a digital monitoring device: Proof-of-concept feasibility randomized controlled trial. JMIR Formative Research, 4(6), e18703. doi: 10.2196/18703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn, E. A., DeWalt, D. A., Bode, R. K., Garcia, S. F., DeVellis, R. F., Correia, H., & Cella, D.; PROMIS Cooperative Group . (2014). New English and Spanish social health measures will facilitate evaluating health determinants. Health Psychology, 33(5), 490–499. doi: 10.1037/hea0000055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassed, C., Flighty, A., Chambers, R., Hosemans, D., Bailey, N., Connaughton, S., Lee, S., & Kazantzis, N. (2021). Advancing the assessment of mindfulness-based meditation practice: Psychometric evaluation of the Mindfulness Adherence Questionnaire. Cognitive Therapy and Research, 45(1), 190–204. doi: 10.1007/s10608-020-10150-z [DOI] [Google Scholar]
- Jacobs, C. A., Mace, R. A., Greenberg, J., Popok, P. J., Reichman, M., Lattermann, C., Burris, J. L., Macklin, E. A., & Vranceanu, A. M. (2021). Development of a mind–body program for obese knee osteoarthritis patients with comorbid depression. Contemporary Clinical Trials Communications, 21, 100720. doi: 10.1016/j.conctc.2021.100720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen, F., Amariglio, R. E., van Boxtel, M., Breteler, M., Ceccaldi, M., Chételat, G., Dubois, B., Dufouil, C., Ellis, K. A., van der Flier, W. M., Glodzik, L., van Harten, A. C., de Leon, M. J., McHugh, P., Mielke, M. M., Molinuevo, J. L., Mosconi, L., Osorio, R. S., Perrotin, A., … Wagner, M.; Subjective Cognitive Decline Initiative (SCD-I) Working Group . (2014). A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s & Dementia, 10(6), 844–852. doi: 10.1016/j.jalz.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam, L. C., Chan, W. M., Kwok, T. C., & Chiu, H. F. (2014). Effectiveness of Tai Chi in maintenance of cognitive and functional abilities in mild cognitive impairment: A randomised controlled trial. Hong Kong Medical Journal, 20(3 Suppl. 3), 20–23. [PubMed] [Google Scholar]
- Leon, A. C., Davis, L. L., & Kraemer, H. C. (2011). The role and interpretation of pilot studies in clinical research. Journal of Psychiatric Research, 45(5), 626–629. doi: 10.1016/j.jpsychires.2010.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace, R. A., Doorley, J. D., Popok, P. J., & Vranceanu, A. M. (2021). Live video adaptations to a mind–body activity program for chronic pain and cognitive decline: Protocol for the virtual active brains study. JMIR Research Protocols, 10(1), e25351. doi: 10.2196/25351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace, R. A., Gates, M. V., Bullard, B., Lester, E. G., Silverman, I. H., Quiroz, Y. T., & Vranceanu, A. M. (2021). Development of a novel mind–body activity and pain management program for older adults with cognitive decline. The Gerontologist, 61(3), 449–459. doi: 10.1093/geront/gnaa084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace, R. A., Gates, M. V., Popok, P. J., Kulich, R., Quiroz, Y. T., & Vranceanu, A. M. (2020). Feasibility trial of a mind-body activity pain management program for older adults with cognitive decline. The Gerontologist, gnaa179. Advance online publication. doi: 10.1093/geront/gnaa179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace, R. A., Greenberg, J., Stauder, M., Reynolds, G., & Vranceanu, A. M. (2021). My Healthy Brain: a multimodal lifestyle program to promote brain health. Aging & Mental Health, 0(0), 1–12. doi: 10.1080/13607863.2021.1904828 [DOI] [PubMed] [Google Scholar]
- Mahaffey, B. L., Mackin, D. M., Vranceanu, A. M., Lofaro, L., Bromet, E. J., Luft, B. J., & Gonzalez, A. (2020). The stony brook health enhancement program: The development of an active control condition for mind–body interventions. Journal of Health Psychology, 25(13–14), 2129–2140. doi: 10.1177/1359105318787024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud, H., Vogt, E. L., Sers, M., Fattal, O., & Ballout, S. (2019). Overcoming barriers to larger-scale adoption of telepsychiatry. Psychiatric Annals, 49(2), 82–88. doi: 10.3928/00485713-20181228-02 [DOI] [Google Scholar]
- Mccullough, M. E., Emmons, R. A., & Tsang, J. A. (2002). The grateful disposition: A conceptual and empirical topography. Journal of Personality and Social Psychology, 82(1), 112–127. doi: 10.1037//0022-3514.82.1.112 [DOI] [PubMed] [Google Scholar]
- Molton, I. R., & Terrill, A. L. (2014). Overview of persistent pain in older adults. American Psychologist, 69(2), 197–207. doi: 10.1037/a0035794 [DOI] [PubMed] [Google Scholar]
- Morone, N. E., & Greco, C. M. (2007). Mind–body interventions for chronic pain in older adults: A structured review. Pain Medicine (Malden, Mass.), 8(4), 359–375. doi: 10.1111/j.1526-4637.2007.00312.x [DOI] [PubMed] [Google Scholar]
- Morone, N. E., Rollman, B. L., Moore, C. G., Li, Q., & Weiner, D. K. (2009). A mind–body program for older adults with chronic low back pain: Results of a pilot study. Pain Medicine (Malden, Mass.), 10(8), 1395–1407. doi: 10.1111/j.1526-4637.2009.00746.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine, Z. S., Phillips, N. A., Bédirian, V., Charbonneau, S., Whitehead, V., Collin, I., Cummings, J. L., & Chertkow, H. (2005). The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695–699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- Ng, C. (2007). Motivation among older adults in learning computing technologies: A grounded model. Educational Gerontology, 34(1), 1–14. doi: 10.1080/03601270701763845 [DOI] [Google Scholar]
- Nicholas, M. K. (2007). The pain self-efficacy questionnaire: Taking pain into account. European Journal of Pain (London, England), 11(2), 153–163. doi: 10.1016/j.ejpain.2005.12.008 [DOI] [PubMed] [Google Scholar]
- Nicholas, M. K., Asghari, A., & Blyth, F. M. (2008). What do the numbers mean? Normative data in chronic pain measures. Pain, 134(1–2), 158–173. doi: 10.1016/j.pain.2007.04.007 [DOI] [PubMed] [Google Scholar]
- Nielsen, L., Riddle, M., King, J. W., Aklin, W. M., Chen, W., Clark, D., ... & Weber, W. (2018). The NIH Science of Behavior Change Program: Transforming the science through a focus on mechanisms of change. Behaviour Research and Therapy, 101, 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, C. L., Russell, B. S., Fendrich, M., Finkelstein-Fox, L., Hutchison, M., & Becker, J. (2020). Americans’ COVID-19 stress, coping, and adherence to CDC guidelines. Journal of General Internal Medicine, 35(8), 2296–2303. doi: 10.1007/s11606-020-05898-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, E. R., Traeger, L., Vranceanu, A. M., Scult, M., Lerner, J. A., Benson, H., Denninger, J., & Fricchione, G. L. (2013). The development of a patient-centered program based on the relaxation response: The Relaxation Response Resiliency Program (3RP). Psychosomatics, 54(2), 165–174. doi: 10.1016/j.psym.2012.09.001 [DOI] [PubMed] [Google Scholar]
- Pilkonis, P. A., Choi, S. W., Reise, S. P., Stover, A. M., Riley, W. T., & Cella, D.; PROMIS Cooperative Group . (2011). Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS®): Depression, anxiety, and anger. Assessment, 18(3), 263–283. doi: 10.1177/1073191111411667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2020). R: What is R?https://www.r-project.org/about.html
- Raes, F., Pommier, E., Neff, K. D., & Van Gucht, D. (2011). Construction and factorial validation of a short form of the Self-Compassion Scale. Clinical Psychology & Psychotherapy, 18(3), 250–255. doi: 10.1002/cpp.702 [DOI] [PubMed] [Google Scholar]
- Richards, L. (2018). Using NVIVO in qualitative research (PAP/CDR edition). SAGE Publications. [Google Scholar]
- Rubin, H. J., & Rubin, I. S. (2005). Qualitative interviewing: The art of hearing data (2nd ed.). SAGE Publications Ltd. doi: 10.4135/9781452226651 [DOI] [Google Scholar]
- Russell, D., Peplau, L. A., & Ferguson, M. L. (1978). Developing a measure of loneliness. Journal of Personality Assessment, 42(3), 290–294. doi: 10.1207/s15327752jpa4203_11 [DOI] [PubMed] [Google Scholar]
- Salvi, D., Poffley, E., Orchard, E., & Tarassenko, L. (2020). The mobile-based 6-minute walk test: Usability study and algorithm development and validation. JMIR MHealth and UHealth, 8(1), e13756. doi: 10.2196/13756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte, D. (2008). Patients’ outcome expectancies and their impression of suitability as predictors of treatment outcome. Psychotherapy Research, 18(4), 481–494. doi: 10.1080/10503300801932505 [DOI] [PubMed] [Google Scholar]
- Si, T., Xing, G., & Han, Y. (2020). Subjective cognitive decline and related cognitive deficits. Frontiers in Neurology, 11, 1–13. doi: 10.3389/fneur.2020.00247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone, A. A., Broderick, J. E., Junghaenel, D. U., Schneider, S., & Schwartz, J. E. (2016). PROMIS fatigue, pain intensity, pain interference, pain behavior, physical function, depression, anxiety, and anger scales demonstrate ecological validity. Journal of Clinical Epidemiology, 74, 194–206. doi: 10.1016/j.jclinepi.2015.08.029 [DOI] [PubMed] [Google Scholar]
- Sullivan, M. J. L., Bishop, S. R., & Pivik, J. (1995). The pain catastrophizing scale: Development and validation. Psychological Assessment, 7(4), 524–532. doi: 10.1037/1040-3590.7.4.524 [DOI] [Google Scholar]
- Tomaszewski Farias, S., Mungas, D., Harvey, D. J., Simmons, A., Reed, B. R., & Decarli, C. (2011). The measurement of everyday cognition: Development and validation of a short form of the Everyday Cognition scales. Alzheimer’s & Dementia, 7(6), 593–601. doi: 10.1016/j.jalz.2011.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai, H. S., Shillair, R., & Cotten, S. R. (2017). Social support and “playing around”: An examination of how older adults acquire digital literacy with tablet computers. Journal of Applied Gerontology, 36(1), 29–55. doi: 10.1177/0733464815609440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang, A., Von Korff, M., Lee, S., Alonso, J., Karam, E., Angermeyer, M. C., Borges, G. L., Bromet, E. J., Demytteneare, K., de Girolamo, G., de Graaf, R., Gureje, O., Lepine, J. P., Haro, J. M., Levinson, D., Oakley Browne, M. A., Posada-Villa, J., Seedat, S., & Watanabe, M. (2008). Common chronic pain conditions in developed and developing countries: Gender and age differences and comorbidity with depression-anxiety disorders. The Journal of Pain, 9(10), 883–891. doi: 10.1016/j.jpain.2008.05.005 [DOI] [PubMed] [Google Scholar]
- Tudor-Locke, C., & Bassett, D. R. (2004). How many steps/day are enough? Preliminary pedometer indices for public health. Sports Medicine, 34(1), 1–8. doi: 10.2165/00007256-200434010-00001 [DOI] [PubMed] [Google Scholar]
- Ustün, T. B., Chatterji, S., Kostanjsek, N., Rehm, J., Kennedy, C., Epping-Jordan, J., Saxena, S., von Korff, M., & Pull, C.; WHO/NIH Joint Project . (2010). Developing the World Health Organization disability assessment schedule 2.0. Bulletin of the World Health Organization, 88(11), 815–823. doi: 10.2471/BLT.09.067231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kooten, J., Binnekade, T. T., van der Wouden, J. C., Stek, M. L., Scherder, E. J., Husebø, B. S., Smalbrugge, M., & Hertogh, C. M. (2016). A review of pain prevalence in Alzheimer’s, vascular, frontotemporal and Lewy body dementias. Dementia and Geriatric Cognitive Disorders, 41(3–4), 220–232. doi: 10.1159/000444791 [DOI] [PubMed] [Google Scholar]
- Vlaeyen, J. W. S., & Linton, S. J. (2012). Fear-avoidance model of chronic musculoskeletal pain: 12 years on. Pain, 153(6), 1144–1147. doi: 10.1016/j.pain.2011.12.009 [DOI] [PubMed] [Google Scholar]
- Whitlock, E. L., Diaz-Ramirez, L. G., Glymour, M. M., Boscardin, W. J., Covinsky, K. E., & Smith, A. K. (2017). Association between persistent pain and memory decline and dementia in a longitudinal cohort of elders. JAMA Internal Medicine, 177(8), 1146–1153. doi: 10.1001/jamainternmed.2017.1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.