Abstract
The Yersinia pestis low-Ca2+ response stimulon is responsible for the environmentally regulated expression and secretion of antihost proteins (V antigen and Yops). We have previously shown that yscO encodes a secreted core component of the Yop secretion (Ysc) mechanism. In this study, we constructed and characterized in-frame deletions in the adjacent gene, yscP, in the yscN–yscU operon. The ΔP1 mutation, which removed amino acids 246 to 333 of YscP, had no effect on Yop expression or secretion, and the mutant protein, YscP1, was secreted, as was YscP in the parent. In contrast, the ΔP2 strain expressed and secreted less of each Yop than did the parent under the inductive conditions of 37°C and the absence of Ca2+, with an exception being YopE, which was only minimally affected by the mutation. The YscP2 protein, missing amino acids 57 to 324 of YscP, was expressed but not secreted by the ΔP2 mutant. The effect of the ΔP2 mutation was at the level of Yop secretion because YopM and V antigen still showed limited secretion when overproduced in trans. Excess YscP also affected secretion: overexpression of YscP in the parent, in either yscP mutant, or in an lcrG mutant effectively shut off secretion. However, co-overexpression of YscO and YscP had no effect on secretion, and YscP overexpression in an lcrE mutant had little effect on Yop secretion, suggesting that YscP acts, in conjunction with YscO, at the level of secretion control of LcrE at the bacterial surface. These findings place YscP among the growing family of mobile Ysc components that both affect secretion and themselves are secreted by the Ysc.
The pathogenic Yersinia species, Y. pestis, Y. enterocolitica, and Y. pseudotuberculosis, cause human diseases ranging from the systemic bubonic plague to a variety of gastroenteric symptoms collectively referred to as yersiniosis. Despite the differences in disease severity, these bacteria share several virulence properties that enable them to resist the nonspecific host immune response (10). Key among these is the ability to secrete a set of virulence proteins, V antigen and Yops, which function to incapacitate host cells and thus enable yersiniae to survive and multiply in lymphoid tissue (10).
V antigen has functions both to regulate the delivery of Yops and as an antihost protein (10, 31, 32, 34, 36, 48). Yops appear to fall in two categories: ones that have direct antihost function and ones whose function relates to the delivery of the antihost Yops (10). Upon cell contact, six Yops (YopE, YopH, YopJ, YopM, YopT, and YpkA) are directly transferred (targeted) into the eukaryotic cell at the point of contact (10). The intracellular activities of these Yops require their secretion and targeting. Secretion and targeting are dependent on a functional Yop secretion mechanism (Ysc); in addition, targeting requires YopB, YopD, and YopK (10).
The genes that encode V antigen and Yops, most of the proteins that regulate their expression, and the Ysc are located on a virulence plasmid (pCD1 in Y. pestis (10, 39) and have been referred to as the Yop virulon for their function (10) and as the low-Ca2+-response stimulon (LCRS) for their regulation in vitro (18, 52). The transcription of LCRS operons is thermally regulated by the transcriptional activator LcrF (10). The extent of activation is determined by environmental conditions. At 37°C, eukaryotic cell contact induces maximal expression of Yops (40). In vitro, the presence of millimolar concentrations of Ca2+ limits the activation of LCRS expression whereas the lack of Ca2+ mimics cell contact and results in strong expression of Yops (52). Under these conditions, there is high-level Yop expression and secretion accompanied by cessation of bacterial growth (referred to as growth restriction), collectively termed the low-Ca2+ response (LCR) (52). Growth restriction probably does not occur in vivo (17) but has been a useful marker of Yop induction (51).
In the absence of cell contact or in the presence of Ca2+ in vitro, LcrE (also called YopN) and TyeA at the bacterial surface and LcrG in the cytoplasm are thought to block the putative Ysc secretion pore at the outer and inner membranes, respectively. LcrE is thought to act at the surface as a Ca2+ sensor, although that activity has not been directly demonstrated (16, 57). TyeA interacts with LcrE and appears to function both at the level of secretion control and in the targeting of a subset of Yops (24). LcrG is located mostly in the cytosol, although a significant amount also is membrane associated; a small amount is secreted in the absence of Ca2+ (36, 49). The block of the secretion pore prevents the secretion of LcrQ, which, in conjunction with YopD, acts as a negative regulator of Yop and V-antigen expression (44, 55). Mutations in LcrE, TyeA, or LcrG result in strong Yop expression and secretion independent of Ca2+ levels or in the absence of host cell contact, presumably due to the secretion of LcrQ and the loss of control over Yop release (16, 24, 49). Upregulation of Yop expression is thought to occur when cell contact or the absence of Ca2+ relieves the block of LcrE and TyeA at the bacterial surface. Some LcrQ is then secreted, which results in the increased expression of V antigen and Yops. LcrG is thought to be maximally titrated away from the Ysc by directly interacting with V antigen when elevated levels of V antigen are produced upon LCR induction (36).
The Ysc mechanism is composed of 20 identified protein products of genes encoded by multiple operons (yscVlcrR, yscBCDEFGHIJKLM, yscNOPQRSTU, and yscW) (1–3, 6, 10, 14, 20, 29, 37, 39, 41, 42, 56). Mutants with mutations of essential Ysc components are unable to secrete Yops and thus are not induced for high-level Yop expression, presumably due to the inability to secrete LcrQ (14, 18, 40–42, 44). These mutants are characterized by Yop expression at the level seen in the presence of Ca2+ and by failure to undergo growth restriction regardless of Ca2+ levels (a growth phenotype referred to as Ca2+ independence). Ysc-related secretion mechanisms exist in several pathogenic gram-negative bacteria and are involved in the direct delivery of bacterial proteins into eukaryotic cells (23). Most of the proteins encoded by the Yersinia yscN–yscU operon share a high sequence similarity to their Spa counterparts in Salmonella typhimurium (where the locus is also called inv) and Shigella flexneri (6, 14, 23). However, the YscO and YscP proteins have little similarity to their Spa counterparts, which suggests that they have a Yersinia-specific function. We have previously characterized yscO (37). The present work focuses on yscP. We constructed two nonpolar deletions in yscP, and the resulting mutants were characterized for the expression and secretion of V antigen and Yops. Under LCR-inductive conditions, one mutation had no effect on either the growth of the strain or the expression and secretion of Yops. The second yscP mutation caused a decrease in the secretion and expression of some but not all Yops. YscP itself was found to belong to the LCRS and to be secreted by the Ysc. It appears to be a mobile component of the Ysc itself and may act in conjunction with YscO at the level of LcrE function.
MATERIALS AND METHODS
Bacteria, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study and their relevant properties are listed in Table 1, and some are shown in Fig. 1. Y. pestis KIM5-3001 (LCR+) was the parent strain used in these studies. It contains three naturally occurring Y. pestis plasmids: pCD1 (carrying the genes necessary for the LCR phenotype) (12, 18), pPCP1 (encoding the plasminogen activator [Pla] responsible for degradation of Yops) (50), and pMT1 (encoding the F1 capsular protein) (43). Y. pestis KIM8-3002, (LCR+, pPCP1−; parent Pla−), was used in experiments in which degradation of proteins by the surface protease Pla would have affected the analysis of results. Escherichia coli strains were typically grown in Luria-Bertani broth or on Luria-Bertani agar (11). Y. pestis strains were routinely grown in heart infusion broth (HIB) or on tryptose blood agar base plates (Difco Laboratories, Detroit, Mich.) at 26°C. For physiological studies, Y. pestis strains were grown in the defined liquid medium TMH (51), supplemented with 2.5 mM CaCl2 as indicated. The medium was inoculated to an optical density at 620 nm (A620) of ca. 0.1 from a culture that had been growing exponentially at 26°C with shaking at 200 rpm for about seven generations. Cultures were started at 26°C and then shifted to 37°C when the A620 reached ca. 0.2. Cells and secreted proteins were harvested 5 or 6 h after the temperature shift. All bacteria with antibiotic resistances were grown in the presence of the appropriate antibiotics, ampicillin and/or streptomycin, at 100 μg/ml.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or description | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | end-1 hsdR17 (rK− mK+) supE44 thi-1 recA1 gyrA (Nalr) relA1 Δ(lacIZYA-argF)U169 deoR [φ80dlacΔ(lacZ)M15] | GIBCO-BRL |
| XL1-Blue | end-1 hsdR17 (rK− mK+) supE44 thi-1 λ recA1 gyrA96 (Nalr) relA1 (Δlac)[F′ proAB+ lacIqZΔM15::Tn10 (Tcr)] | Stratagene |
| Y. pestisa | ||
| KIM5-3001 (parent) | Smr; pCD1 (LCR+), pPCP1(Pla+) pMT1 | 27 |
| KIM5-3001.5 | Smr; pCD1 lcrG (Δ39–53)b, pPCP1(Pla+), pMT1 | 49 |
| KIM5-3001.6 | Smr; pCD1 lcrE (Δ48–197), pPCP1(Pla+), pMT1 | 42 |
| KIM5-3001.17 (ΔP1) | Smr; pCD1 yscP(Δ246–333), pPCP1(Pla+), pMT1 | This study |
| KIM5-3001.18 (ΔP2) | Smr; pCD1 yscP(Δ57–324), pPCP1(Pla+), pMT1 | This study |
| KIM5-3401 | Smr, pCD1 lcrH::cat yopJ::Mu d1734 [Kmr Lac+] [LcrH−, YopB−, YopD−, YopJ−], pPCP1, pMT1 | 42 |
| KIM8-3002 (parent Pla−) | Smr; pCD1 (LCR+), pPCP1−(Pla−), pMT1 | 34 |
| KIM8-3002.1 | Smr, pCD1 yopB (Δ8–399), pPCP1−(Pla−), pMT1 | 13 |
| KIM8-3002.4 (ΔP2-Pla−) | Smr; pCD1 yscP(Δ57–324), pPCP1−(Pla−) pMT1 | This study |
| Plasmids | ||
| pBluescript II SK+ | Apr; cloning vector | Stratagene |
| pBluescript II SK− | Apr; cloning vector | Stratagene |
| pYP-F2 | Apr; BamHI F fragment cloned from pBGCD1 carrying yscN′OPQRS, with frameshift mutation in yscR, was cloned into pBluescript II SK− with the insert oriented with the lac promoter | 14 |
| pYscOP.2 | Apr; 2.38-kb Bpu1102I fragment of pYP-F2 carrying yscO and yscP was filled in with Klenow and cloned into XhoI digested/Klenow blunt-ended pBluescript II SK+ with the insert oriented with the lac promoter | 37 |
| pYscOP | Apr; same as pYscOP.2 with the insert oriented with the T7 promoter | 37 |
| pYscO.2 | Apr; SphI-KpnI (KpnI site in vector) digestion of pYscOP.2 followed by blunt ending with T4 polymerase and religation, resulting in elimination of yscP and carrying yscO | 37 |
| pYscP | Apr; 1.8-kb AvaI fragment of pYP-F1 (14) carrying yscP, filled in with Klenow and cloned into the EcoRV site of pBluescript II SK+ with the insert oriented with the T7 promoter | This study |
| pYscP.2 | Apr; same as pYscP but oriented with the lac promoter | This study |
| pYscP1 | Apr; BclI digest and religation of plasmid pYscP, resulting in deletion of yscP (Δ246–333) | This study |
| pYscP2 | Apr; pYscOP digested with AgeI, filled in with Klenow followed by digestion with StyI and blunt ending with mung bean nuclease; religation of plasmid resulted in deletion of yscP (Δ57–324) | This study |
| pHT-V | Apr; expression vector carrying lcrV; translationally fused to a leader encoding 23 residues, including 6 histidines | 15, 33 |
| pTRCM.2 | Apr; expression vector carrying yopM behind the trc promoter | 42 |
| pGEX-3X | Apr; GST fusion expression vector with the tac promoter | Pharmacia |
| pGST-YscP | Apr; BamHI (in vector site)-AgeI digest of pGST-YscO (37) filled in with Klenow followed by religation of plasmid (expresses fusion protein of GST and aa 328–455 of YscP) | This study |
| pUK4134 | Apr; suicide vector oriR6K oriT cos rpsL | 47 |
| pUKΔP1 | XhoI digest of pYscP1 filled in with Klenow followed by SmaI (vector site) digest; XhoI-SmaI fragment cloned into EcoRV site of pUK4134 | This study |
| pUKΔP2 | MluI digest of pYscP2 filled in with Klenow followed by digestion with EcoRV (vector site); MluI-EcoRV band cloned into EcoRV site of pUK4134 | This study |
All Y. pestis strains are Pgm− (54).
Numbers in parentheses are the amino acids deleted from the protein product.
AvrII sites introduced by site-directed mutagenesis (see Materials and Methods).
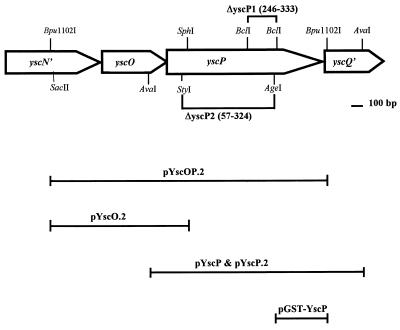
FIG. 1.
Physical and genetic map of the region of pCD1 that encompasses yscP. The coding regions for yscP, yscO, and parts of yscN and yscQ carried on pYscOP.2, as well as selected restriction sites, are shown. Regions included in selected clones are diagrammed below the map.
DNA methods.
Cloning, including the use of restriction endonucleases and T4 DNA ligase, was performed essentially as described previously (28). Plasmid DNA was isolated by an alkali lysis procedure (7), by the method of Kado and Liu (25), by cesium chloride gradients (28), or with Qiagen columns (Qiagen, Inc., Studio City, Calif.). Certain DNA fragments were isolated and purified from agarose gels by using a Qiaex DNA purification kit (Qiagen). Transformation of E. coli was done by a standard CaCl2 procedure (28). Electroporation of E. coli and Y. pestis was done as previously described (38). The PCR technique (30) was performed with 20 to 30 cycles of amplification. The denaturing, annealing, and extending conditions were 94, 55, and 72°C, respectively, for 30 s each with a 480 thermocycler (Perkin-Elmer Cetus, Norwalk, Conn.). Nucleotide primers synthesized by the Macromolecular Structure Analysis Facility (University of Kentucky, Lexington, Ky.) or Integrated DNA Technologies (Coralville, Iowa) were used for PCR.
Mutagenesis of yscP.
To study the effect of yscP on the LCR, we made two in-frame deletions in yscP. Plasmids pYscP1 and pYscP2 were constructed by restriction endonuclease digestions as described in Table 1. Restriction endonuclease fragments that contained the deleted sequence flanked by homologous DNA were subcloned into the EcoRV site of the suicide vector pUK4134, generating pUKΔP1 and pUKΔP2 (Table 1). Plasmids pUKΔP1 and pUKΔP2 were introduced into Y. pestis KIM5-3001 and pUKΔP2 was introduced into Y. pestis KIM5-3002 by electroporation, and recipient bacteria that had integrated the clone into pCD1 by homologous recombination were selected for their resistance to ampicillin as previously described (47). Following passage under nonselective conditions to allow a second crossover, clones which had resolved the cointegrate by excision of the vector sequences were selected by growth on streptomycin and screened for ampicillin sensitivity, and the presence of the correct deletion was confirmed by PCR and restriction endonuclease digestion. Y. pestis KIM5-3001.17 (ΔP1), KIM5-3001.18 (ΔP2), and KIM8-3000.4 (ΔP2 Pla−) contained the correct in-frame deletions (Table 1) and were used in this study.
T7 promoter-polymerase expression system.
The T7 promoter-polymerase expression system in E. coli was used to determine if the predicted coding sequence of yscP expressed a protein. E. coli XL1-Blue was transformed with pBluescript II SK+ (vector-only control) and plasmids carrying yscP and its derivatives. Plasmid pYscP carried yscP oriented for expression from the T7 promoter, and pYscP.2 carried yscP oriented with the lac promoter and against the T7 promoter, an orientation that would prevent transcription in the T7 system. The clone-specific and control (pBluescript II SK+) proteins were expressed by using bacteriophage mGP1-2 to supply T7 RNA polymerase as previously described (5). The proteins expressed by the T7 polymerase were labeled with Tran35S-label (35S-Met and 35S-Cys; ICN Pharmaceuticals). Equal numbers of cells as determined by measurement of the A620 of the labeled culture were pelleted in a microcentrifuge at 4°C and solubilized in 100 μl of electrophoresis sample buffer (37). Portions of each sample were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (12% [wt/vol] acrylamide) and visualized by autoradiography.
Virulence tests.
To assess the importance of yscP for virulence, we used a systemic plague model in intravenously infected mice. Bacterial inocula were prepared by growing the bacteria for 10 generations at 26°C in HIB containing streptomycin. The cells were sedimented by centrifugation at room temperature. They were washed once with room temperature (RT) phosphate-buffered saline (PBS) (135 mM NaCl, 2.68 mM KCl, 10 mM Na2HPO4, 1.76 mM KH2PO4 [pH 7.2]) and resuspended in RT PBS before use. Serial 10-fold dilutions of each strain (103 and/or 105 dose) were spread in triplicate onto tryptose blood agar for later determination of viable numbers (CFU). Groups of five female BALB/c mice, 6 to 8 weeks old and caged separately, were anesthetized with Metofane and injected intravenously retro-orbitally with 0.1 ml of bacterial suspensions containing 103 CFU of Y. pestis KIM5-3001 (parent) or 103 or 105 CFU of the yscP mutants Y. pestis KIM5-3001.17 (ΔP1) and KIM5-3001.18 (ΔP2). For the high-dose infection group of each mutant, an extra mouse was inoculated and euthanatized 24 h after infection for recovery from macerated spleen and verification by plasmid profile that the challenge strain was unaltered by growth in vivo. The other mice were observed until they succumbed to infection or for a maximum of 14 days (ΔP1) or 33 days (ΔP2).
Antibody preparation.
To determine the localization of YscP in bacterial fractions, antibody was raised in rabbits against fusion proteins of glutathione S-transferase (GST) and a portion of YscP (amino acids [aa] 328 to 455). Plasmid pGST-YscP (Table 1; Fig. 1), which encoded the fusion protein, was transformed into E. coli DH5α. The fusion protein, GST-YscP, was expressed as specified by the manufacturer for use of the pGEX-3X vector (Pharmacia-LKB, Piscataway, N.J.). After harvest, cell pellets were resuspended in PBS and lysed by two passages through a chilled French pressure cell at 20,000 lb/in2. Unlysed cells, large debris, and inclusion bodies were removed by centrifugation at 8,800 × g for 5 min at 4°C. The GST-YscP fusion protein was found almost exclusively in the 8,800 × g pellet, indicating that this protein was in inclusion bodies. The GST-YscP-containing inclusion bodies were resuspended in PBS containing 1% (vol/vol) Triton X-100 and then subjected to centrifugation at 8,800 × g for 5 min at 4°C. This treatment, which did not solubilize the inclusion bodies, would remove most of the contaminating soluble and membrane proteins from the inclusion body preparation and was performed a total of three times. GST-YscP fusion protein was then purified from the inclusion bodies by solubilization in electrophoresis sample buffer and SDS-PAGE. The protein was isolated from excised gel pieces by electroelution with a Centrilutor microelectroluter (Amicon), concentrated in a Centricon-10 concentrator (Amicon), and stored at −20°C until use. A sample of GST-YscP and known amounts of the similar-sized bovine serum albumin separated by SDS-PAGE were compared for their intensity of staining by Coomassie brilliant blue to estimate the concentration of GST-YscP in the sample.
Two female New Zealand White rabbits were injected subcutaneously at multiple sites along the back with a total of 0.1 mg of GST-YscP which had been eluted from a gel and emulsified 1:1 (vol/vol) in Freund’s complete adjuvant. At 4 and 8 weeks, the rabbits were boosted with 0.1 mg of the same fusion protein preparation mixed 1:1 in Freund’s incomplete adjuvant. Antiserum was collected and applied to a protein A-Sepharose column (Pharmacia-LKB) to purify the immunoglobulin G fraction.
Cell fractionation and immunoblot analysis.
Samples for analysis of protein content by immunoblotting were prepared from Y. pestis strains grown in TMH (with and without 2.5 mM Ca2+). Cell pellets and culture supernatants were separated by centrifugation at 4°C. Cell pellets were washed in ice-cold 100 mM Tris-HCl (pH 7.4)–1 mM EDTA. Whole-cell fractions were prepared by resuspending the pellet in 2× sample electrophoresis buffer. Cell extracts were made by disintegration of the Tris-EDTA-resuspended cell pellets in an ice-cold cell of a French pressure cell (20,000 lb/in2). Unlysed cells and cellular debris were removed by centrifugation at 8,800 × g for 5 min at 4°C. Total soluble proteins (cytoplasmic plus periplasmic) were separated from membranes of the cleared lysates by ultracentrifugation at 417,000 × g for 15 min at 4°C in a TLA 100.4 rotor (Beckman, Inc., Palo Alto, Calif.). Membranes were resuspended in ice-cold 100 mM Tris-HCl (pH 7.4)–1 mM EDTA. Soluble and membrane fractions were stored at −20°C. Before electrophoresis, the samples were thawed and diluted 1:1 with 2× electrophoresis sample buffer. Secreted proteins were precipitated with 5% (vol/vol) trichloroacetic acid (TCA) for 2 h to overnight on ice. After centrifugation (14,000 × g for 30 min at 4°C) to pellet the precipitated proteins, the pellet was neutralized with 1 M Tris-HCl (pH 8.0), resuspended in electrophoresis sample buffer, and stored at −20°C. All samples were boiled for 2 min before being loaded onto gels for electrophoresis. YscP was subject to degradation by Pla, even with immediate processing and storage at −20°C. Accordingly, samples to be analyzed for YscP were separated by SDS-PAGE on the day when the bacteria were grown. Gels were loaded such that each lane contained proteins corresponding to equal numbers of bacteria. Bacterial fractions were analyzed by denaturing SDS-PAGE (12 to 15% [wt/vol] acrylamide) (26) followed by transfer to Immobilon-P (Millipore Corp., Bedford, Mass.) with Towbin transfer buffer (53). Specific proteins were visualized on the membranes by using the polyclonal antibodies specific for the proteins (37) and a secondary antibody (goat anti-rabbit or goat anti-mouse [Sigma]) conjugated to either alkaline phosphatase or horseradish peroxidase.
Chemical cross-linking of proteins.
To determine if YscP was part of a protein complex or associated with other Yersinia proteins, proteins in whole cells of Y. pestis strains growing in TMH (lacking Ca2+) were cross-linked with either disuccinimidyl suberate (DSS; spacer arm, 11.4 Å) or 1,4-di-[3′-(2′-pyridyldithio)propionamido]butane (DPDPB; spacer arm, 16 Å) (Pierce) at 1 mM as specified by the manufacturer. The optimal concentration of each crosslinker was determined in separate experiments with various concentrations of DSS and DPDPB (0.5, 1, 2, and 5 mM, and 0.1, 1, and 2.5 mM, respectively). Each Y. pestis strain was grown in TMH (lacking Ca2+), and after ca. 5 h of growth, a portion representing 2 OD620 ml was transferred to a 2.5-ml prewarmed flask in a shaking water bath. For each strain tested, cultures were cross-linked by the addition of either DSS or DPDPB (final concentration, 1 mM). Additionally, a sample from each strain was mock treated with dimethyl sulfoxide at a concentration equal to that in samples containing cross-linker. After 30 min at 37°C, the DPDPB-cross-linked cultures were removed to ice. The DSS-cross-linked cultures were quenched by the addition of Tris-HCl (pH 8.0) (final concentration, 50 mM), allowed to react for 15 min, and then removed to ice. Each sample was lysed in a French pressure cell, and soluble proteins were separated from the cellular debris by low-speed centrifugation. TCA was added to the soluble fraction to a final concentration of 5% (vol/vol), and the proteins were allowed to precipitate overnight on ice. Proteins and cross-linked protein complexes were collected by centrifugation (543,300 × g for 10 min at 4°C) in a Beckman 100.4 TLC rotor. After being air dried, each protein pellet was neutralized, and the mock-treated and DSS-cross-linked proteins were resuspended in electrophoresis sample buffer lacking β-mercaptoethanol (BME) and boiled for 2 min. The proteins were separated by SDS-PAGE and subjected to immunoanalysis with anti-YscP. Duplicate samples were run for DPDPB, one in the sample buffer lacking BME and one with BME added to a final concentration of 10% to cleave any sulfhydryl cross-links. In other experiments, Y. pestis cultures grown as described above were fractionated into soluble proteins, membranes, and culture medium, and these were subjected to chemical cross-linking and SDS-PAGE.
Yop targeting studies.
To assess the effect of yscP on the targeting of Yops into eukaryotic cells, we used a tissue culture model of Yersinia infection to induce Yop targeting. We detected the amount of Yops released into the tissue culture medium, the amount targeted (that in the soluble fraction of eukaryotic cells), and the amount associated with the bacteria and the surface of eukaryotic cells (that in cellular debris of lysed eukaryotic cells with adherent bacteria) by immunoanalysis of SDS-PAGE-separated fractions.
HeLa and J774 murine macrophage-like cells were seeded in 3.5-cm-diameter tissue culture wells (∼2.5 × 105 cells/well) and grown to semiconfluence (5-8 × 105 cells/well) as previously described (46). The bacteria for these targeting studies were grown at 26°C in HIB, diluted into warm RPMI 1640 (Life Technologies, Grand Island, N.Y.), and immediately added at an infectious dose of 10 per eukaryotic cell. The cluster dishes containing infected eukaryotic cells were centrifuged in a Beckman TJ-6 centrifuge (200 × g for 5 min at room temperature) to facilitate bacterial contact with the eukaryotic cells and then incubated for 30 min at 37°C under a 5% CO2 atmosphere. Nonadherent bacteria were removed by washing twice with 2 ml of warm PBS. Then 2 ml of warm RPMI (with 5 μg of cytochalasin D per ml for J774 cells, to prevent phagocytosis of the nonencapsulated 26°C-grown yersiniae) was added to the appropriate wells, and the incubation was continued for 3.5 h (for a total of 4 h). After infection, one replicate well of HeLa and J774 cells per infecting strain was treated for 5 min at 37°C with trypsin (final concentration, 100 μg/ml) to differentiate between surface (trypsin-susceptible) and cytosol-localized (trypsin-nonsusceptible) proteins. After a maximum 5-min treatment, protease inhibitors (Pefabloc and leupeptin; Boehringer Mannheim Biochemicals, Indianapolis, Ind.) were added (final concentration, 100 μg/ml each) to stop the trypsin treatment. To each well that had not been treated with trypsin, 2 μg of each of the two protease inhibitors per ml was added. The contents of each well were harvested and fractionated as previously described (46). Briefly, the RPMI 1640 was removed from each well and placed in a separate capped 5-ml syringe. A 1-ml volume of RT PBS was used to gently wash the cells adhering to the wells, and this was added to the syringe. The combined supernatant and wash from each well was filtered into a tube on ice. Infected eukaryotic cells remaining in the wells after removal of the supernatant were gently washed a second time in RT PBS, and this wash was discarded. The cells were completely lysed by addition of 1 ml of ice-cold distilled water containing protease inhibitors (Pefabloc and leupeptin, each at a final concentration of 2 μg/ml) followed by incubation on ice for ∼30 min. Complete lysis of cells was achieved by scraping and vigorous pipetting of well contents. The soluble fraction (eukaryotic cytosolic contents including any targeted Yops) from each well was separated from the cellular debris (nonlysed eukaryotic cells, nonlysed large organelles, large pieces of membrane or cytoskeleton, and intact bacteria) by centrifugation (20,800 × g for 15 min at 4°C). The soluble and cell-free supernatant fractions were precipitated (10% [vol/vol] TCA) overnight on ice, neutralized in 1 M Tris-HCl (pH 8.0), and resolubilized in no more than 50 μl (total volume) of electrophoresis sample buffer. The debris (low-speed pellet) was washed in cold PBS and then solubilized in 50 μl of electrophoresis sample buffer. Proteins were analyzed by immunoblotting after separation by SDS-PAGE.
DNA sequence analysis.
The yscP nucleotide sequence of Y. pestis was previously submitted (accession no. L25667) (37). The DNA and predicted protein sequences of yscP were analyzed by using PCGene (IntelliGenetics, Inc., Mountain View, Calif.) and IntelliGenetics Suite (IntelliGenetics, Inc.) software. The deduced amino acid sequence was compared with available sequences in the GenBank database via the National Center for Biotechnology Information BLAST (4) mail server (blast@ncbi.nlm.nih.gov).
RESULTS
Analysis of yscP of Y. pestis KIM.
The focus of this study is yscP of Y. pestis, the third gene in the yscN–yscU operon. Computer analysis of yscP predicted a 455-residue protein having a molecular mass of 50.4 kDa and an isoelectric point (pI) of 5.23; the predicted sequence does not contain any hydrophobic domain, remarkable secondary structure, or protein motif. The corresponding Spa proteins in Salmonella (SpaN, also called InvJ) (9, 19) and Shigella (Spa32) (45) are predicted to be proteins of 36.4 and 32.9 kDa with pIs of 6.3 and 5.6, respectively. There was no ca. 32-kDa region of YscP that showed significant similarity to either Spa protein, and the predicted amino acid sequence of yscP is not significantly similar to any other protein outside the Yersinia spp.
T7 expression of yscP in E. coli.
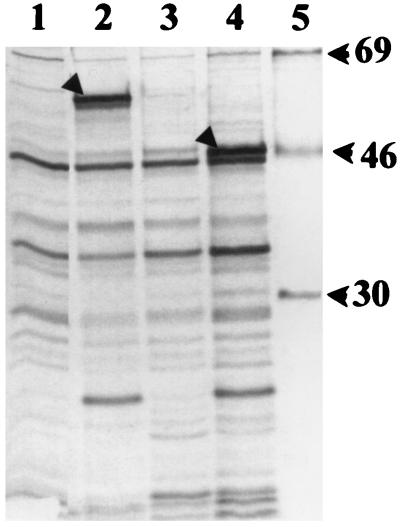
Products were expressed from the cloned yscP gene on plasmid pYscP and its derivatives (Fig. 1; Table 1) in E. coli by using a bacteriophage T7 promoter-polymerase system (Fig. 2). This system provides high-level transcription of cloned genes, while background expression is minimized due to inhibition of E. coli RNA polymerase by rifampin. pYscP expressed a unique ca. 60-kDa protein product, which was a higher molecular mass than expected for YscP (predicted 50.4 kDa) (Fig. 2, lane 2). Plasmids pYscP.2 (with yscP oriented opposite the T7 promoter) and pYscP1 did not express this protein, suggesting that the 60-kDa band was the product of yscP (lanes 3 and 4). pYscP1 expressed a unique ca. 48-kDa protein (lane 4) that was higher than the predicted molecular mass of 40.7 kDa.
FIG. 2.
T7 promoter/polymerase expression of cloned yscP in E. coli. Radiolabeled proteins were separated by SDS-PAGE (12% [wt/vol] polyacrylamide), and the gels were dried and autoradiographed. Lanes 1 to 4 were loaded with equivalent numbers of E. coli XLI-Blue cells carrying (left to right) pBluescript II SK+, pYscP, pYscP.2, and pYscP1. Lane 5 was loaded with 14C-labeled protein markers (sizes are given in kilodaltons to the right). Arrowheads in lanes 2 and 4 indicate putative full-length and mutant YscP products, respectively.
Identification of yscP in Y. pestis KIM.
In an effort to identify a role for YscP, it was of interest to determine its cellular location. We were also interested to see if YscP would be expressed in Y. pestis at the same molecular mass as in E. coli. Polyclonal antiserum directed against a GST fusion protein containing the C terminus of YscP (aa 328 to 455) was used to identify the yscP gene product in immunoanalysis of bacterial fractions of Y. pestis.
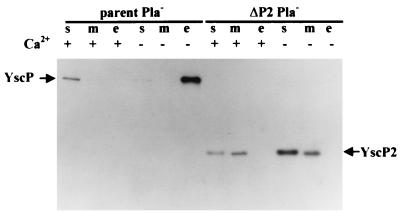
We found that in Y. pestis KIM 5-3001, YscP was subject to rapid degradation and was seen predominantly as a 26-kDa band, even if the bacterial fractions were prepared and electrophoresed on the same day (data not shown). At least some of the degradation spared the carboxyl terminus, because putative degradation products as small as ca. 7 kDa were detected by our antiserum against the C terminus of YscP. The degradation was suspected to be Yersinia specific and to be due to Pla, since no degradation products of YscP were observed in E. coli (Fig. 2). Thus, fractions of the Pla− Y. pestis strain KIM8-3002 (parent Pla−) were analyzed for the presence of YscP (Fig. 3). At 37°C in the presence of calcium, a single band corresponding to the putative YscP was detected in the soluble fraction of Y. pestis KIM8-3002 (parent Pla−) and in very small amounts in the membrane fraction. It migrated as a ca. 60-kDa protein, greater than the expected molecular mass (50.4 kDa) but similar to that seen in E. coli. These data suggest that Pla, which is known to degrade Yops (50), had been the major cause of YscP degradation in Pla+ Y. pestis. YscP1 also was found as degradation products in fractions from Pla+ Y. pestis KIM5-3001.17 (ΔP1) (data not shown). However, in whole-cell preparations, it was observed as a 48-kDa protein, the same mass as in E. coli (predicted, 40.7 kDa) (see Fig. 7). In Y. pestis KIM8-3002.4 (ΔP2 Pla−) grown in the presence of Ca2+, the putative YscP2 was detected in the soluble and membrane fractions as a 22-kDa species, the expected molecular mass (21.1 kDa) for this mutant protein (Fig. 3). In Pla+ Y. pestis, we also saw smaller putative YscP2 degradation products in the same bacterial fractions (data not shown).
FIG. 3.
Localization of YscP in Pla− Y. pestis. An immunoblot analysis of Y. pestis KIM8-3002 (parent Pla−) and Y. pestis KIM8-3002.4 (ΔP2 Pla−) is shown. Bacteria were grown in TMH with (+) and without (−) Ca2+. Proteins from the soluble (s), membrane (m), and culture medium (e) fractions were separated by SDS-PAGE (12% polyacrylamide gels) and transferred to Immobilon P. The proteins were detected with polyclonal antibody specific to a fusion protein of GST-YscP and a secondary antibody conjugated to horseradish peroxidase and were visualized by enhanced chemiluminescence.
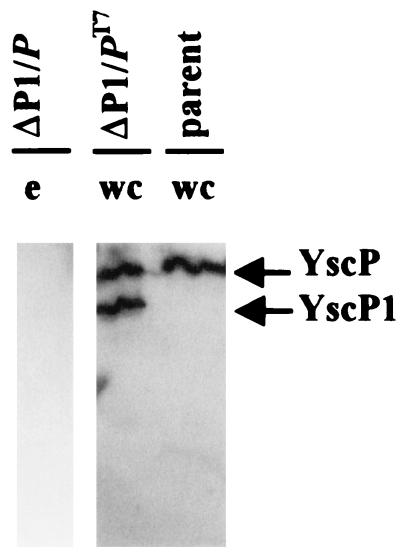
FIG. 7.
Evidence of an independent promoter for yscP. An immunoblot analysis of the Pla− Lcr+ parent strain of Y. pestis (KIM8-3002, parent) and the Pla+ ΔP1 Y. pestis KIM5-3001.17 carrying yscP in trans on pYscP.2 or pYscP (ΔP1/P and ΔP1/PT7, respectively) is shown. Bacteria were grown in TMH without Ca2+. Proteins from the whole-cell (wc) and culture medium (e) fractions were separated by SDS-PAGE and transferred to Immobilon P. They were detected by using polyclonal antibody specific to the fusion protein GST-YscP and a secondary antibody conjugated to horseradish peroxidase and were visualized by enhanced chemiluminescence.
The aberrant migration of YscP and YscP1 but not YscP2 might be due to aa 57 to 233, since this region is present in YscP and YscP1 but lacking in YscP2. Computer analysis did not reveal any posttranslational modification site in aa 57 to 233 that would account for these differences; therefore, the effect may be due to the protein conformation.
In the absence of calcium, both YscP and YscP2 were detected at higher levels (Fig. 3). This shows that yscP belongs to the LCRS stimulon, as previously shown for yscO (37) and yscR (14). YscP was present almost exclusively in the culture medium of the parent (parent Pla−), whereas YscP2 still was observed in the soluble and membrane fractions of Y. pestis KIM8-3002.4 (ΔP2 Pla−). This suggests that YscP is a secreted protein. Its lack of a signal sequence suggested the possibility that it was being secreted by the Ysc mechanism, and indeed, we had previously found that YscP was not secreted by a yscO mutant (37).
LCR phenotype of yscP Y. pestis.
Ca2+-independent growth, decreased Yop and V-antigen expression, and blocked secretion of Yops and V antigen have been previously described for yscO and yscR mutants when grown under LCR-inductive conditions (14, 37), and we anticipated that a yscP mutant would be similar. To determine the LCR phenotype of our yscP strains, we compared the growth and secretion phenotypes of the parent and mutant strains (ΔP1 and ΔP2) growing in TMH under both inductive and noninductive conditions for the LCR (see Fig. 4 for growth and Fig. 5 for secretion).
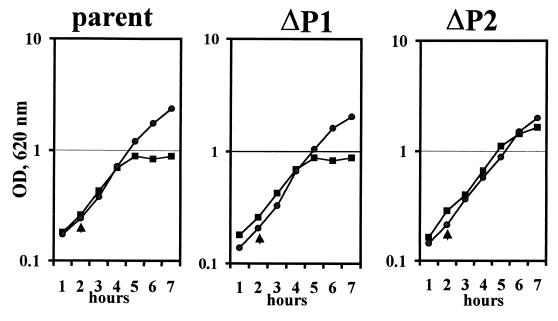
FIG. 4.
Growth phenotype of yscP Y. pestis. Y. pestis KIM5-3001 (parent), KIM5-3001.17 (ΔP1), and KIM5-3001.18 (ΔP2) were grown at 37°C in the presence or absence of Ca2+ in the defined medium TMH. The temperature was shifted from 26 to 37°C (temperature shifts are denoted by arrowheads). Symbols: circles, +Ca2+; squares, −Ca2+. OD, optical density.
FIG. 5.
Secretion of YopM by yscP Y. pestis. An immunoblot analysis of YopM secreted from Y. pestis KIM5-3001 (parent), KIM5-3001.17, (ΔP1), KIM5-3001.18 (ΔP2), and the mutants carrying yscP in trans on pYscP.2 or pYscP (/P and /PT7, respectively) is shown. Bacteria were grown in TMH at 37°C without Ca2+. Proteins from the culture medium were separated by SDS-PAGE (12% polyacrylamide gels), transferred to Immobilon P, and visualized with antibody specific to YopM and a secondary antibody conjugated to alkaline phosphatase.
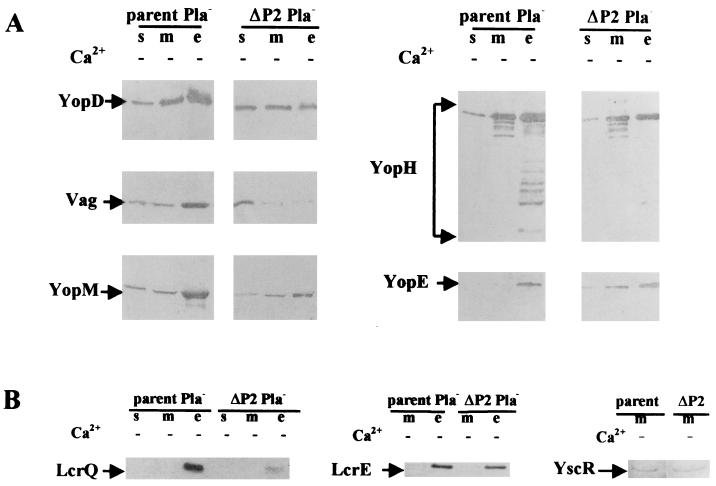
All strains exhibited full growth yield at 37°C in the presence of Ca2+ (Fig. 4). In the absence of Ca2+, the parent, Y. pestis KIM5-3001, showed growth restriction typical for media like TMH (17) following a temperature shift from 26 to 37°C. Y. pestis KIM5-3001.17 (ΔP1) grew in the same manner as the parent. Y. pestis KIM5-3001.18 (ΔP2) displayed a growth phenotype that ranged from a slight amount of growth restriction to Ca2+-independent growth (Fig. 4, ΔP2). Growth restriction has previously been used as a marker for LCR induction, and strains that display Ca2+-independent growth under inductive conditions generally also express less Yop and V antigen than does the parent and do not secrete these proteins. The ΔP1 mutant, in keeping with the growth pattern of the parent, expressed and secreted YopM (Fig. 5) YopH, and V antigen (data not shown) at a similar level. However, for the ΔP2 strain, the growth response was not a sensitive indicator of induction: although there was some decrease in the abundance of some secreted proteins, this mutant showed significant Yop expression and secretion (Fig. 6). Y. pestis KIM8-3002.4 (ΔP2 Pla−) expressed and secreted less V antigen, YopD, YopM, and YopH than did the parent (Fig. 6A). The negative regulators LcrE and LcrQ were detected only in the culture medium fraction for either the parent or the mutant, as expected under LCR-inductive conditions (Fig. 6B). The amount of LcrQ was smaller for the ΔP2 mutant than for the parent, whereas LcrE secretion by the mutant was slightly decreased. The significance of these differences is not obvious at present. Interestingly, YopE abundance was not affected in the ΔP2 mutant, and secretion of YopE occurred at near wild-type levels (Fig. 6A). This phenotype is believed to be due to the mutation in yscP alone and not to a polar effect on downstream genes, because YscR, the product of the second gene downstream of yscP in the same operon, was detected at similar levels in membranes of the parent and the ΔP2 mutant (Fig. 6B).
FIG. 6.
Expression and secretion of LCRS proteins in yscP Y. pestis. An immunoblot analysis of proteins expressed and secreted from Y. pestis KIM5-3001 (parent) and KIM5-3001.18 (ΔP2) or their Pla− counterparts. KIM8-3002 (parent Pla−) and KIM5-3002.4 (ΔP2 Pla−), respectively, is shown. Bacteria were grown in TMH at 37°C without Ca2+. Proteins from soluble (s), membrane (m), and culture medium (e) fractions were separated by SDS-PAGE (12% polyacrylamide gel) and transferred to Immobilon P. Primary antibodies were used that were specific to YopE, YopD, YopH, YopM, LcrE, LcrQ, YscR, and V antigen (Vag), and the secondary antibody was conjugated to alkaline phosphatase. Arrows denote the positions of the respective proteins.
No proteins were detected in the culture medium of the parent or either yscP mutant at 37°C in the presence of Ca2+ (data not shown; the antibodies used were to YopE, YopH, YopM, V antigen, and total secreted proteins). This showed that the mechanism for blocking secretion through the Ysc in response to Ca2+ was functional in the yscP mutants.
Complementation of the ΔP2 mutant.
To test whether the ΔP2 mutation could be complemented, we expressed YscP in trans from the lac promoter on pYscP.2 in the ΔP2 Y. pestis KIM5-3001.18. To our surprise, this caused Yop secretion to be almost completely blocked (Fig. 5, ΔP2/P). A similar secretion phenotype was also observed for the ΔP1 Y. pestis KIM5-3001.17 carrying pYscP.2 in trans (Fig. 5, ΔP1/P). These effects were not due to the vector, since introduction of vector only into the parent and both yscP-mutant Y. pestis strains did not affect the secretion phenotype of the respective strains (data not shown). Unlike pYscP.2, pYscP was not expected to have any effect on the phenotype of either mutant, since in this construct yscP is oriented against the lac promoter and with the T7 promoter, which is not functional in Yersinia. Indeed, there was little effect on the secretion of Yops when pYscP was introduced into the ΔP1 mutant (Fig. 5, compare ΔP1/PT7 and ΔP1); however, the ΔP2 mutant was fully complemented by this plasmid and secreted Yops and V antigen similarly to the parent (Fig. 5, compare ΔP2/PT7 and parent). This indicated that pYscP in fact did express some functional YscP. In a separate experiment, the putative YscP (ca. 60 kDa) and YscP1 (ca. 48 kDa) were observed in fractions of Y. pestis KIM5-3001.17 carrying pYscP (ΔP1/PT7) (results not shown). It may be that low-level extra expression of YscP is tolerated without a significant effect on Yop secretion. Accordingly, Yop secretion by Y. pestis KIM5-3001.17 (ΔP1) was similar to that by ΔP1 carrying pYscP in trans (ΔP1/PT7) (Fig. 5). In contrast, in Y. pestis KIM5-3001.17 carrying yscP oriented with the lac promoter (which is constitutively expressed in Y. pestis) (ΔP1/P), YscP and YscP1 were detected in whole cells (data not shown) but none was secreted (Fig. 7, ΔP1/P, lane e), and Yops were not secreted (Fig. 5, ΔP1/P). Indeed, we have found that the lac promoter provides stronger expression of YscP than does the putative yscP promoter (data not shown). The data suggest that YscP expression is tightly regulated in Y. pestis and show that excess YscP blocks secretion. The block in secretion could be due to excess YscP sterically blocking the secretion pore. Alternatively, YscP could associate with other Y. pestis proteins, and in strains expressing excess YscP, the extra YscP could sequester one or more factors into nonfunctional complexes, resulting in a loss of function and no Yop secretion. Either alternative explains the results obtained for ΔP1 or the parent carrying pYscP or pYscP.2 and also explains the complementation of ΔP2 by pYscP but not pYscP.2
Secretion of V antigen and YopM expressed from plasmids with non-LCR-inducible promoters.
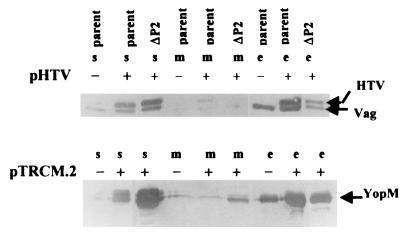
The data suggest that the secretion defect of Y. pestis KIM5-3001.18 (ΔP2) could be due to the effect of YscP on the secretion mechanism. However, in the LCR, maximal yop expression is dependent upon a functional Ysc. We have previously shown that mutations in yscC, yscD, yscG, and yscO indirectly affect expression by directly blocking secretion (37, 42). To distinguish these two potential effects of yscP and to test the hypothesis that yscP affects secretion directly, secretion was analyzed in Y. pestis KIM5-3001 (parent) and Y. pestis KIM5-3001.18 (ΔP2), each carrying V antigen or YopM expressed in trans from an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible, non-LCR regulated promoter on, respectively, pHT-V, which produces histidine-tagged V antigen (HT-V) or pTRCM.2, which encodes YopM. Five hours prior to harvest, the expression of YopM or HT-V was induced by the addition of IPTG. The expression and secretion of YopM and HT-V were monitored by immunoblotting (Fig. 8).
FIG. 8.
YscP directly affects the secretion of YopM and HT-V. An immunoblot analysis of YopM, V antigen, and HT-V in the soluble (s), membrane (m), and culture medium (e) fractions from Y. pestis KIM5-3001 (parent) and Y. pestis KIM5-3001.17 (ΔP2) with (+) and without (−) pHT-V or pTRCM.2 is shown. Y. pestis strains were grown in the defined medium TMH at 37°C in the absence of Ca2+; expression of HT-V from pHT-V and YopM from pTRCM.2 was induced by addition of IPTG to 1 mM at 5 h prior to harvest. Proteins from each fraction were separated by SDS-PAGE (12% [wt/vol] acrylamide) and transferred to Immobilon P. Polyclonal antibody to HT-V or YopM was used to detect HT-V and YopM, respectively. Secondary antibody was conjugated to alkaline phosphatase. In the lower panel, YopM was detected as two closely migrating species.
When pTRCM.2 was present, the parent strain expressed and secreted higher levels of YopM than it did without pTRCM.2. With pHT-V, the parent strain expressed both native V antigen and HT-V, which ran slightly above the native V antigen due to its His6-containing leader sequence. The ΔP2 mutant carrying pHT-V expressed HT-V and secreted some of it, but most of the HT-V was retained in the soluble fraction. Similarly, despite the high levels of YopM expressed in the mutant from pTRCM.2, over half of the YopM expressed was retained in the soluble fraction of the bacteria. These results indicate that the mutation in Y. pestis KIM5-3001.18 (ΔP2) causes a defect in some aspect of the secretion process and supports a direct role for yscP in the secretion of LCR virulence proteins by Y. pestis.
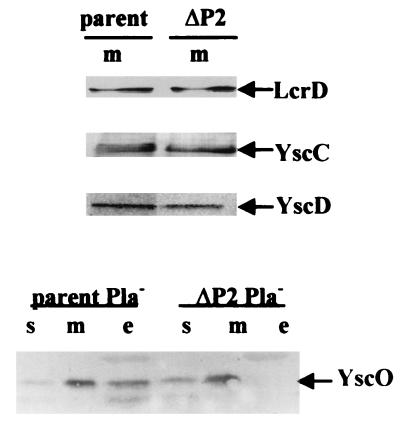
It is possible that yscP affects the expression or localization of other Ysc proteins. However, immunoanalysis of proteins from membrane fractions of the parent and the yscP mutant Y. pestis KIM5-3001.18 (ΔP2) showed that at least LcrD, YscC, YscD, and YscO were present in comparable amounts in both strains (Fig. 9). YscO normally is secreted under inductive conditions (37), but the ΔP2 mutant did not secrete any (Fig. 9). This suggests that YscO, like YscP, depends on the Ysc for its own secretion. We do not yet know the significance of the secretion of YscO; however, it is not likely that the ΔP2 phenotype reflects nonfunctional YscO, because a yscO null mutant has a completely inactive Ysc (37) whereas the ΔP2 strain still secretes some Yops.
FIG. 9.
Detection of LcrD, YscD, YscC, and YscO in yscP Y. pestis. Y. pestis strains were grown in the defined medium TMH at 37°C in the absence of Ca2+. The proteins in the soluble (s), membrane (m), and culture medium (e) fractions were separated by SDS-PAGE (12% [wt/vol] acrylamide), transferred to Immobilon P, and analyzed by immunoblotting. (Top) Pla+ Y. pestis KIM5-3001 (parent) and Pla+ Y. pestis KIM5-3001.18 (ΔP2) were analyzed with antibodies specific to LcrD, YscC, or YscD. (Bottom) Y. pestis KIM8-3002 (parent Pla−) and Y. pestis KIM8-3002.4 (ΔP2 Pla−) were analyzed with anti-YscO. The secondary antibody used was conjugated to alkaline phosphatase. Arrows indicate each protein.
Effect of extra copies of yscP in other Y. pestis backgrounds.
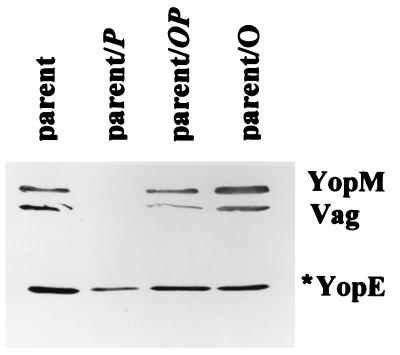
We performed several tests to identify a potential target for the secretion-blocking effect of excess YscP. First we tested whether YscP would still block secretion when overexpressed along with YscO, the other Ysc protein that lacks sequence homology to Spa counterparts in Salmonella and Shigella and hence that may have the same Yersinia-specific adaptation that we postulate for YscP. Plasmids pYscP.2, pYscOP.2, and pYscO.2 were introduced into the parent Y. pestis KIM5-3001, creating the strains parent/P, parent/OP, and parent/O, respectively, and the culture medium of each strain was analyzed for the secretion of YopM, V antigen, and YopE (Fig. 10). Secretion of these proteins was similar in the parent and the parent carrying either pYscO.2 (parent/O) or pYscOP.2 (parent/OP). In contrast, the parent expressing yscP secreted less YopE than did the parent, and levels of YopM and V antigen were barely detectable, a phenotype similar to that of the ΔP2 mutant. This indicates that, as in the phenotypically wild-type ΔP1 mutant, overexpression of YscP in the parent strain imposes a secretion defect. However, it is not extra YscP per se but extra YscP unbalanced by YscO that causes the defect, because overexpression of YscO together with YscP did not affect secretion of V antigen and Yops. This may indicate that an interaction occurs between YscP and YscO.
FIG. 10.
Overexpression of YscP does not block secretion if YscO also is overexpressed. An immunoblot analysis of proteins expressed and secreted from the Pla+ Y. pestis KIM5-3001 (parent) and the parent expressing yscO, yscP, or both in trans (parent/O, parent/P, and parent/OP, respectively) is shown. Bacteria were grown in TMH at 37°C without Ca2+. Proteins from bacterial fractions were separated by SDS-PAGE (12% acrylamide gels) and transferred to Immobilon P. Proteins from the culture medium were detected with polyclonal antibodies specific to YopM, V antigen (Vag), and YopE. The secondary antibody used was conjugated to alkaline phosphatase. Due to the activity of Pla, only the major degradation product of YopE (YopE*) was observed. The proteins are identified by labels to the right.
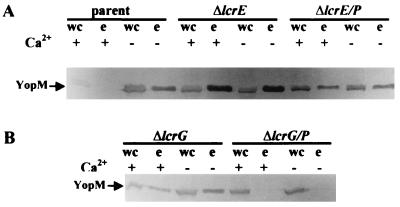
We next determined where the secretion block from YscP fits in the secretion/regulatory pathway by testing if the negative effect of excess YscP would be dominant over an lcrG or lcrE defect. Yersiniae with mutations of lcrE or lcrG are constitutively induced for the LCR because they constitutively secrete the negative regulator(s). Plasmid pYscP.2 was introduced into both mutants, and the resulting strains were checked to see if extra YscP would block secretion (Fig. 11). As expected, both mutants expressed and secreted YopM irrespective of Ca2+ and expression of YopM was higher in the mutants than in the parent irrespective of Ca2+. Introducing yscP in trans into the lcrE mutant (ΔlcrE/P) decreased the expression of YopM to the parental level but did not block secretion. This shows that excess YscP could not have its full secretion-blocking effect in the absence of lcrE function. However, introduction of yscP in trans into the lcrG mutant (ΔlcrG/P) resulted in a block in the secretion of YopM, showing that the effect of extra YscP did not require functional LcrG. In a current model for secretion, loss of either LcrG or LcrE would open the secretion pore at the inner or outer membrane, respectively, allowing the secretion of LcrQ and resulting in the upregulation of Yop expression. Our results show that the secretion block due to excess YscP can occur in the absence of LcrG but not of LcrE and suggest the hypothesis that the normal function of YscP involves an interaction with the mechanism of LcrE for modulating secretion system activity. YscP may act in conjunction with YscO and LcrE to control the opening of the secretion “pore.”
FIG. 11.
Effects of YscP overexpression in lcrE and lcrG Y. pestis. An immunoblot analysis of proteins expressed and secreted from Y. pestis strains carrying yscP in trans is shown. Bacteria were grown in TMH at 37°C without Ca2+. Proteins from whole cells (wc) and the culture medium (e) were separated by SDS-PAGE (12% polyacrylamide gels) and transferred to Immobilon P. Proteins from the culture medium were detected with polyclonal antibodies specific to YopM. The secondary antibody used was conjugated to alkaline phosphatase. Arrows denote YopM. (A) Y. pestis KIM5-3001 (parent), KIM5-3001.6 (ΔlcrE), and KIM5-3001.6 carrying yscP in trans on pYscP.2 (ΔlcrE/P). (B) Y. pestis KIM5-3001.5 (ΔlcrG) and KIM5-3001.5 carrying pYscP.2 (ΔlcrG/P).
Interactions of YscP and other Y. pestis proteins.
We performed a direct test of the postulated interactions of YscP with other proteins, such as YscO and LcrE. We cross-linked bacterial fractions and growing whole cells of the Pla+ parent, Y. pestis KIM5-3001, and the Pla− parent, Y. pestis KIM8-3002, by using two cross-linkers, one that was sulfhydryl reactive and one that was amine reactive. No novel bands were observed in any cross-linked reactions with either cross-linker at any of the concentrations tested (data not shown).
Targeting of Yops in the ΔP2 mutant.
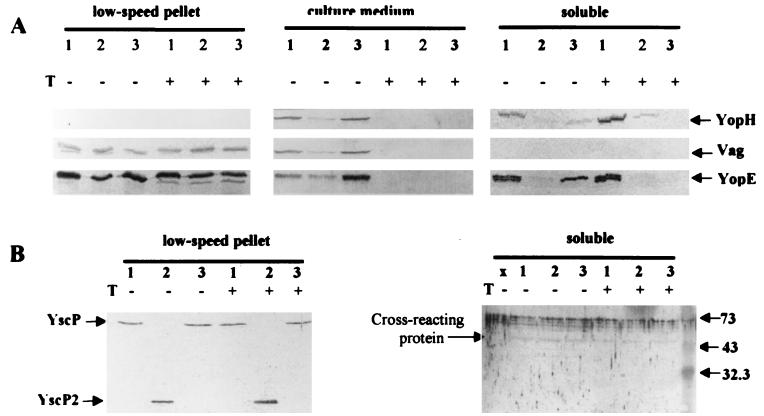
To gain a more complete picture of the role of YscP in Yop deployment by Y. pestis, we wanted to determine if the mutation in yscP affected the targeting of Yops into HeLa and J774 cells. We found that the ΔP2 mutant was not as strongly cytotoxic as the parent, and when we tested for the presence of YopE and YopH in the soluble fraction of infected HeLa cells, we found that less had been targeted by the ΔP2 mutant than by the parent (Fig. 12A, soluble, compare lanes 1 and 2 [data not shown for J774 cells]). It was surprising that there was a significant effect on the targeting of YopE, since we had seen little effect of the ΔP2 mutation on YopE secretion. However, during the course of these experiments, separate studies in our laboratory suggested that V-antigen secretion may be required for targeting (34, 35); therefore, the reduction in the amount of Yops targeted by this mutant could be an indirect effect due to the defect in V-antigen secretion by the ΔP2 Y. pestis. YscP and YscP2 themselves were detected in the Yersinia-containing low-speed pellet of eukaryotic cells infected with the respective Y. pestis strains but not in the corresponding soluble fractions (Fig. 12B, data shown for HeLa cells). This shows that YscP is expressed in the tissue culture infection model but that YscP itself is not targeted.
FIG. 12.
YscP is not targeted into eukaryotic cells. The Pla− Y. pestis strains KIM8-3002 (parent Pla−) (lanes 1), KIM8-3002.4 (ΔP2 Pla−) (lanes 2), and KIM8-3002.1 (ΔyopB Pla−) (lanes 3) were used to infect HeLa cells. After 4 h, trypsin (T) was added to a duplicate culture for each strain to assess the protease resistance of proteins in the low-speed pellet (pellet containing yersiniae, obtained after centrifugation of the H2O-lysed cells), in the culture medium, and in the soluble fraction (Yops that are targeted). Protein samples were separated by SDS-PAGE (12% [wt/vol] acrylamide) and analyzed with primary antibody against YopH, V antigen, YopE, or YscP and secondary antibody conjugated with alkaline phosphatase. (A) Arrows denote YopH, V antigen (Vag), and YopE. (B) Arrows show YscP and YscP2 in the low-speed pellet. They were not detected in the soluble fraction; however, a ∼73-kDa artifactual band was detected in all wells including those containing only electrophoresis buffer (lane x), and a cross-reacting band was seen at ca. 45 kDa. Numbers to the right of the gel loaded with soluble fraction represent molecular masses in kilodaltons.
Virulence of the yscP mutants in mice.
To determine if the yscP mutants were defective in virulence, a test was performed for lethality in BALB/c mice by using doses of 103 and 105 of each yscP mutant. As expected, a nominal dose of 103 (800 CFU) of the parent, Y. pestis KIM5-3001, was lethal for all mice tested. (The intravenous 50% lethal dose of the parent strain is 4.2 × 101 [27].) The 50% lethal dose of Y. pestis KIM5-3001.17 (ΔP1) was near 103 (880 CFU), since three of five mice died from infection with this dose. All five mice infected with the higher dose died. Mice infected with ΔP1 succumbed to infection 1 to 2 days later than did those infected with the parent (data not shown). In contrast, all mice survived infection with Y. pestis KIM5-3001.18 (ΔP2) at either dose 103 (3 × 103 CFU) or 105 (3.7 × 105). Since Y. pestis KIM5-3001.17 (ΔP1) expressed and secreted Yops similarly to the parent Y. pestis KIM5-3001, the small difference in virulence may suggest that ΔP1 has defects in the secretion of proteins not detected by our analysis. The lack of virulence of Y. pestis KIM5-3001.18 (ΔP2) was not surprising, in light of its defects in V antigen and Yop secretion and targeting.
DISCUSSION
The present study found that YscP is a secreted protein and that it is necessary for the normal secretion of Yops and V antigen by Y. pestis. The secretion defect in the yscP mutant Y. pestis KIM8-3002.4 (ΔP2 Pla−) also caused decreased targeting of YopE and YopH and probably other Yops. These effects are believed to be due directly or indirectly to a mutation in yscP and not to a polar effect on downstream genes.
YscP was expressed under both inductive and noninductive conditions, as are other components of the Ysc, and its expression increased under LCR-inductive conditions, as has been the case for several other ysc gene products. When Ca2+ was present, YscP itself was present only in the whole-cell fraction of the culture, with most being in the soluble fraction; and in the absence of Ca2+, it was mostly secreted, a distribution similar to that of Yops. Additionally, the secretion of YscP was dependent on a functional Ysc, since it was expressed but not secreted in a secretion-negative mutant. However, we do not think that YscP is purely an antihost, LCR effector protein like the six targeted Yops, since YscP itself was not targeted into HeLa or J774 cells. Moreover, a mutation in yscP (ΔP2) affected growth and secretion, and these effects have not been attributed to such Yops. Other LCR secreted proteins such as YscO, LcrV, LcrG, LcrE, YopB, YopD, and YopK have the cellular distribution of YscP in yersiniae grown in TMH, even though they function in LCR regulation and Yop targeting. Because YscP itself is secreted by the Ysc, as are YscO (37) and the Ysc-regulatory proteins LcrE (16) and LcrG (49), its own mobility may be an essential part of its function: it is a moving part of the Ysc. The role of YscP in the Ysc may be related to the function of the corresponding type III protein in Salmonella, SpaN/InvJ. SpaN/InvJ is a secreted protein that is essential for the secretion of Salmonella invasion proteins (Sips) (9). This is in contrast to the corresponding protein in Shigella, Spa32, which is associated with the outer membrane and is necessary for the release of Ipas from the bacterial surface but not for their transport to the surface.
Our further characterization of the ΔP2 mutation by complementation led to the finding that too much YscP inactivates the Ysc whereas co-overexpression of YscO with YscP prevented YscP from blocking secretion, as though YscO needs to be present for YscP to function. Possibly, excess YscP forms abnormal complexes with other Ysc proteins and these are unable to promote Yop secretion. In ΔP2/P, most of the YscP and YscP2 is membrane localized (data not shown). In ΔP2/PT7, which did not show blocked secretion, the YscP was expressed from a multicopy plasmid but from the weak putative yscP promoter (data not shown). The low level of wild-type YscP might alleviate the secretion defect of the ΔP2 strain by displacing YscP2 from any interactions with other Ysc components and reestablishing the normal mechanism for opening the secretion channel.
It is interesting that overexpression of YscP in an lcrE mutant had little effect on altering the constitutive secretion of Yops by that mutant. However, secretion was blocked in strains expressing LcrE and making extra YscP (ΔP2/P, parent/P, ΔP1/P, and ΔlcrG/P). This may indicate that YscP normally acts at the level of the secretion-modulating mechanism of LcrE: YscP, working in conjunction with YscO, might be necessary for the secretion channel to open under inductive conditions, and this affects the secretion of some Yops more than others.
We hypothesize that YscP may be part of a complex; however, we did not detect YscP interactions with other Yersinia proteins by chemical cross-linking, and we did not detect any specific interaction of YscO and YscP in an affinity blot by using the soluble fusion protein GST-YscO as a probe against proteins of the parent Y. pestis separated by electrophoresis (data not shown). We are aware that interactions in the Sec system were difficult to detect before means were found to lock in a conformation of this dynamic mechanism, and we have not exhausted all methods to detect protein interactions. Strains with secretion blocked by excess YscP may provide one locked-in secretion conformation and will be useful for future probing of interactions among components of the Ysc. Therefore, we do not rule out the possibility that YscP functions as part of a larger complex, and we speculate that YscP and YscO may participate together as LCR-adapted components of the Ysc type III secretion mechanism.
The phenotype of the ΔP2 strain itself is open to more than one interpretation. First, the finding that Yops are still secreted by the ΔP2 mutant could indicate that YscP is a nonessential component of the secretion machinery under the conditions of our experiments. We cannot rule out the possibility that the secretion defect in this strain was due to the accumulation of YscP2 in membranes, perhaps nonspecifically partially plugging the secretion channel. Second, the phenotype of a strain with a complete deletion of yscP might be identical to that of the ΔP2 mutant, and the effect of YscP on secretion might be analogous to that of YscW (VirG). A yscW mutant secreted less than wild-type levels of YopB, YopD, and V antigen but a normal level of YopH (1). Third, it is possible that a yscP null mutant would be unable to secrete any Yops and that YscP2 has partial function because it is stable and contains 42% of YscP. A similar phenotype was observed for the partially functional protein, YscFmod. An N-terminal truncation of YscF, with its first 12 residues replaced by 8 vector-encoded amino acids, impaired the secretion of YopB and YopD but did not affect the secretion of YopH, which was in contrast to the phenotype of a yscF null mutant, which did not secrete any Yops (2). Hence, the partial secretion defect by yscFmod Y. enterocolitica was due to a partially functional YscF protein. Similarly, YscP2 may have partial function but would lack any functions stemming from its own secretion, since it was not secreted. We presently favor the second hypothesis, because disruption of YscP function by overexpression of wild-type YscP had the same effect on secretion of Yops as did the ΔP2 mutation. However, this must be tested by making a true yscP null mutant.
In conclusion, we have shown that YscP is a mobile component of the Ysc that acts at the level of the LcrE modulation of secretion system activity. These data add to our previous evidence that the type III Ysc mechanism has a dynamic moving core, and perhaps YscP is part of this.
ACKNOWLEDGMENTS
This study was supported by Public Health Service grant AI21017.
We thank Gregory V. Plano (University of Miami, Miami, Fla.) for a generous gift of rabbit anti-YopE, and we thank Gerard P. Andrews and Arthur M. Friedlander (USAMRIID, Ft. Dietrick, Md.) for a generous gift of mouse anti-YopE and anti-YopH.
REFERENCES
- 1.Allaoui A, Scheen R, Lambert de Rouvroit C, Cornelis G R. VirG, a Yersinia enterocolitica lipoprotein involved in Ca2+ dependency, is related to ExsB of Pseudomonas aeruginosa. J Bacteriol. 1995;177:4230–4237. doi: 10.1128/jb.177.15.4230-4237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allaoui A, Schulte R, Cornelis G R. Mutational analysis of the Yersinia enterocolitica virC operon: characterization of yscE, F, G, I, J, K required for Yop secretion and yscH encoding YopR. Mol Microbiol. 1995;18:343–355. doi: 10.1111/j.1365-2958.1995.mmi_18020343.x. [DOI] [PubMed] [Google Scholar]
- 3.Allaoui A, Woestyn S, Sluiters C, Cornelis G R. YscU, a Yersinia enterocolitica inner membrane protein involved in Yop secretion. J Bacteriol. 1995;176:4534–4542. doi: 10.1128/jb.176.15.4534-4542.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 5.Barve S, Straley S C. lcrR, a low-Ca2+-response locus with dual Ca2+-dependent functions in Yersinia pestis. J Bacteriol. 1990;172:4661–4671. doi: 10.1128/jb.172.8.4661-4671.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergman T, Erickson K, Galyov E, Persson C, Wolf-Watz H. The lcrB (yscN/U) gene cluster of Yersinia pseudotuberculosis is involved in Yop secretion and shows high homology to the spa gene clusters of Shigella flexneri and Salmonella typhimurium. J Bacteriol. 1994;176:2619–2626. doi: 10.1128/jb.176.9.2619-2626.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collazo C M, Galan J E. Requirement for exported proteins in secretion through the invasion-associated type III system of Salmonella typhimurium. Infect Immun. 1996;64:3524–3531. doi: 10.1128/iai.64.9.3524-3531.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collazo C M, Zierler M K, Galan J E. Functional analysis of the Salmonella typhimurium invasion genes invI and invJ and identification of a target of the protein secretion apparatus encoded in the inv locus. Mol Microbiol. 1995;15:25–38. doi: 10.1111/j.1365-2958.1995.tb02218.x. [DOI] [PubMed] [Google Scholar]
- 10.Cornelis G R, Boland A, Boyd A P, Geuijen C, Iriarte M, Neyt C, Sory M-P, Stainier I. The virulence plasmid of Yersinia, an antihost genome. Microbiol Mol Biol Rev. 1998;62:1315–1352. doi: 10.1128/mmbr.62.4.1315-1352.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis R H, Botstein D, Roth J R. Advanced bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1980. [Google Scholar]
- 12.Ferber D M, Brubaker R. Plasmids in Yersinia pestis. Infect Immun. 1981;27:839–841. doi: 10.1128/iai.31.2.839-841.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fields, K. A., and S. C. Straley. Unpublished data.
- 14.Fields K A, Plano G V, Straley S C. A low-Ca2+ response (LCR) secretion (ysc) locus lies within the lcrB region of the LCR plasmid in Yersinia pestis. J Bacteriol. 1994;176:569–579. doi: 10.1128/jb.176.3.569-579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fields K A, Williams A W, Straley S C. Failure to detect binding of LcrH to the V antigen of Yersinia pestis. Infect Immun. 1997;65:3954–3957. doi: 10.1128/iai.65.9.3954-3957.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forsberg A, Viitanen A-M, Skurnik M, Wolf-Watz H. The surface-located YopN protein is involved in calcium signal transduction in Yersinia pseudotuberculosis. Mol Microbiol. 1991;5:977–986. doi: 10.1111/j.1365-2958.1991.tb00773.x. [DOI] [PubMed] [Google Scholar]
- 17.Fowler J M, Brubaker R R. Physiological basis of the low calcium response in Yersinia pestis. Infect Immun. 1994;62:5234–5241. doi: 10.1128/iai.62.12.5234-5241.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goguen J D, Yother J, Straley S C. Genetic analysis of the low calcium response in Yersinia pestis Mu d1 (Ap lac) insertion mutants. J Bacteriol. 1984;160:842–848. doi: 10.1128/jb.160.3.842-848.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groisman E A, Ochman H. Cognate gene clusters govern invasion of host epithelial cells by Salmonella typhimurium and Shigella flexneri. EMBO J. 1993;12:3779–3787. doi: 10.1002/j.1460-2075.1993.tb06056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haddix P L, Straley S C. The structure and regulation of the Yersinia pestis yscBCDEF operon. J Bacteriol. 1992;174:4820–4828. doi: 10.1128/jb.174.14.4820-4828.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hakansson S, Schesser K, Persson C, Galyov E E, Rosqvist R, Homble F, Wolf-Watz H. The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell plasma membrane disrupting activity. EMBO J. 1996;15:5812–5823. [PMC free article] [PubMed] [Google Scholar]
- 22.Holmstroem A, Pettersson J, Rosqvist R, Hakansson S, Tafazoli F, Faellman M, Magnusson K E, Wolf-Watz H, Forsberg A. YopK of Yersinia pseudotuberculosis controls translocation of Yop effectors across the eukaryotic cell membrane. Mol Microbiol. 1997;24:73–91. doi: 10.1046/j.1365-2958.1997.3211681.x. [DOI] [PubMed] [Google Scholar]
- 23.Hueck C J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iriarte M, Sory M-P, Boland A, Boyd A P, Mills S D, Lambermont I, Cornelis G R. TyeA, a protein involved in control of Yop release and in translocation of Yersinia Yop effectors. EMBO J. 1998;17:1907–1918. doi: 10.1093/emboj/17.7.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kado C I, Liu S T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981;145:1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 27.Lindler L E, Klempner M S, Straley S C. Yersinia pestis pH 6 antigen: genetic, biochemical, and virulence characterization of a protein involved in the pathogenesis of bubonic plague. Infect Immun. 1990;58:2569–2577. doi: 10.1128/iai.58.8.2569-2577.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maniatis T E, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 29.Michiels T, Vanooteghem J-C, Lambert de Rouvroit C, China B, Gustin A, Boudry P, Cornelis G. Analysis of virC, an operon involved in the secretion of Yop proteins by Yersinia enterocolitica. J Bacteriol. 1991;173:4994–5009. doi: 10.1128/jb.173.16.4994-5009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mullis K B, Faloona F A. Specific synthesis of DNA in vitro via a polymerase catalyzed chain reaction. Methods Enzymol. 1978;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- 31.Nakajima R, Motin V L, Brubaker R R. Suppression of cytokines in mice by protein A-V antigen fusion peptide and restoration of synthesis by active immunization. Infect Immun. 1995;63:3021–3029. doi: 10.1128/iai.63.8.3021-3029.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nedialkov Y A, Motin V L, Brubaker R R. Resistance to lipopolysaccharide mediated by the Yersinia pestis V antigen-polyhistidine fusion peptide: amplification of interleukin-10. Infect Immun. 1997;65:1196–1203. doi: 10.1128/iai.65.4.1196-1203.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nemeth J, Straley S C. Effect of Yersinia pestis YopM on experimental plague. Infect Immun. 1997;65:924–930. doi: 10.1128/iai.65.3.924-930.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nilles M L, Fields K A, Straley S C. The V antigen of Yersinia pestis regulates Yops vectorial targeting as well as Yops secretion through effects on YopB and LcrG. J Bacteriol. 1997;180:3410–3420. doi: 10.1128/jb.180.13.3410-3420.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nilles, M. L., and S. C. Straley. Unpublished data.
- 36.Nilles M L, Williams A W, Skrzypek E, Straley S C. Yersinia pestis LcrV forms a stable complex with LcrG and may have a secretion-related regulatory role in the low-Ca2+ response. J Bacteriol. 1997;179:1307–1316. doi: 10.1128/jb.179.4.1307-1316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Payne P L, Straley S C. YscO of Yersinia pestis is a mobile core component of the Yop secretion system. J Bacteriol. 1998;180:3882–3890. doi: 10.1128/jb.180.15.3882-3890.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perry R D, Pendrak M L, Schuetze P. Identification and cloning of a hemin storage locus involved in the pigmentation phenotype of Yersinia pestis. J Bacteriol. 1990;172:5929–5937. doi: 10.1128/jb.172.10.5929-5937.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perry R D, Straley S C, Fetherston J D, Rose D J, Gregor J, Blattner F R. DNA sequencing and analysis of the low-Ca2+-response plasmid pCD1 of Yersinia pestis KIM5. Infect Immun. 1998;66:4611–4623. doi: 10.1128/iai.66.10.4611-4623.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pettersson J R, Nordfelth E, Dubinina T, Bergman T, Gustavsson M, Magnusson K-E, Wolf-Watz H. Modulation of virulence factor expression by pathogen target cell contact. Science. 1996;273:1231–1233. doi: 10.1126/science.273.5279.1231. [DOI] [PubMed] [Google Scholar]
- 41.Plano G V, Straley S C. Multiple effects of lcrD mutations in Yersinia pestis. J Bacteriol. 1993;175:3536–3545. doi: 10.1128/jb.175.11.3536-3545.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plano G V, Straley S C. Mutations in yscC, yscD, and yscG prevent high-level expression and secretion of V antigen and Yops in Yersinia pestis. J Bacteriol. 1995;177:3843–3854. doi: 10.1128/jb.177.13.3843-3854.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Protsenko O A, Anisimov P I, Mosharov O T, Konnov N P, Popov Y A, Kokushkin A M. Detection and characterization of Yersinia pestis plasmids determining pesticin I, fraction I antigen, and “mouse” toxin synthesis. Sov Genet. 1983;19:838–846. [PubMed] [Google Scholar]
- 44.Rimpilainen, Forsberg A, Wolf-Watz H. A novel protein, LcrQ, involved in the low-calcium response of Yersinia pseudotuberculosis shows extensive homology to YopH. J Bacteriol. 1992;174:3355–3363. doi: 10.1128/jb.174.10.3355-3363.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sasakawa C, Komatsu K, Tobe T, Suzuki T, Yoshikawa M. Eight genes in region 5 that form an operon are essential for invasion of epithelial cells by Shigella flexneri 2a. J Bacteriol. 1993;175:2334–2346. doi: 10.1128/jb.175.8.2334-2346.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skrzypek E, Cowan C, Straley S C. Vectorial targeting and trafficking of the Yersinia pestis YopM protein to the nucleus of HeLa cells. Mol Microbiol. 1997;30:1051–1065. doi: 10.1046/j.1365-2958.1998.01135.x. [DOI] [PubMed] [Google Scholar]
- 47.Skrzypek E, Haddix P L, Plano G V, Straley S C. New suicide vector for gene replacement in yersiniae and other gram-negative bacteria. Plasmid. 1993;29:160–163. doi: 10.1006/plas.1993.1019. [DOI] [PubMed] [Google Scholar]
- 48.Skrzypek E, Straley S C. Differential effects of deletions in lcrV on secretion of V antigen, regulation of the low-Ca2+ response, and virulence of Yersinia pestis. J Bacteriol. 1995;177:2530–2542. doi: 10.1128/jb.177.9.2530-2542.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skrzypek E, Straley S C. LcrG, a secreted protein involved in negative regulation of the low-calcium response in Yersinia pestis. J Bacteriol. 1993;175:3520–3528. doi: 10.1128/jb.175.11.3520-3528.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sodeinde O A, Sample A K, Brubaker R R, Goguen J D. Plasminogen activator/coagulase gene of Yersinia pestis is responsible for degradation of plasmid-encoded outer membrane proteins. Infect Immun. 1988;56:2749–2752. doi: 10.1128/iai.56.10.2749-2752.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Straley S C, Bowmer W S. Virulence genes regulated at the transcriptional level by Ca2+ in Yersinia pestis include structural genes for outer membrane proteins. Infect Immun. 1986;51:445–454. doi: 10.1128/iai.51.2.445-454.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Straley S C, Plano G V, Skrzypek E, Haddix P L, Fields K A. Regulation by Ca2+ in the Yersinia low-Ca2+ response. Mol Microbiol. 1993;8:1005–1010. doi: 10.1111/j.1365-2958.1993.tb01644.x. [DOI] [PubMed] [Google Scholar]
- 53.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedures and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Une T, Brubaker R R. In vivo comparison of avirulent Vwa− and Pgm− or Pstr phenotypes of yersiniae. Infect Immun. 1984;43:895–900. doi: 10.1128/iai.43.3.895-900.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williams A W, Straley S C. YopD of Yersinia pestis plays a role in the negative regulation of the low-calcium response in addition to its role in the translocation of Yops. J Bacteriol. 1998;180:350–358. doi: 10.1128/jb.180.2.350-358.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woestyn S, Allaoui A, Wattiau P, Cornelis G R. YscN, the putative energizer of the Yersinia Yop secretion machinery. J Bacteriol. 1994;176:1561–1569. doi: 10.1128/jb.176.6.1561-1569.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yother J, Goguen J D. Isolation and characterization of Ca2+-blind mutants of Yersinia pestis. J Bacteriol. 1985;164:704–711. doi: 10.1128/jb.164.2.704-711.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]