Abstract
After nearly four decades of research, a safe and effective HIV-1 vaccine remains elusive. There are many reasons why the development of a potent and durable HIV-1 vaccine is challenging, including the extraordinary genetic diversity of HIV-1 and its complex mechanisms of immune evasion. HIV-1 envelope glycoproteins are poorly recognized by the immune system, which means that potent broadly neutralizing antibodies (bnAbs) are only infrequently induced in the setting of HIV-1 infection or through vaccination. Thus, the biology of HIV-1–host interactions necessitates novel strategies for vaccine development to be designed to activate and expand rare bnAb-producing B cell lineages and to select for the acquisition of critical improbable bnAb mutations. Here we discuss strategies for the induction of potent and broad HIV-1 bnAbs and outline the steps that may be necessary for ultimate success.
Subject terms: RNA vaccines, Translational research
There are many reasons why the development of a potent and durable vaccine to HIV-1 is exceptionally challenging, including the large genetic diversity of the virus and its complex mechanisms of immune evasion. In this Review, Haynes et al. discuss strategies for the induction of potent broadly neutralizing antibodies for HIV-1 and the steps that may be necessary for ultimate success.
Introduction
HIV-1 was discovered in 1983 (ref.1) and subsequently shown to be the cause of acquired immune deficiency syndrome (AIDS)2–4. Effective antiretroviral drug therapy has converted AIDS from a uniformly fatal disease to a chronic disease with a near normal lifespan5. Nonetheless, ~1.5 million people each year acquire HIV-1 (ref.6). Thus, even with antiretroviral drug therapy as prevention or treatment7,8, there is an urgent need for a safe and effective HIV-1 vaccine.
The development of an effective HIV-1 vaccine is particularly challenging owing to the exceptional and increasing genetic diversity of the HIV-1 lentivirus9,10, its complex mechanisms of immune evasion11–14 and the ability of HIV-1 to integrate into host immune cells to become resistant to host immunity and treatment regimens15,16.
The first generation of vaccines tested in clinical trials utilized gp120 as antigen for eliciting neutralizing antibodies, whereas more recent trials tested vaccines designed to elicit CD8+ T cell responses and non-neutralizing antibodies17–19. Out of eight HIV-1 vaccine efficacy trials completed so far, all but one failed (Table 1). The only trial that showed a small degree of estimated efficacy in reducing HIV-1 transmission (31.2%) was the RV144 (NCT00223080) trial of the CRFAE_01 canarypox/gp120 vaccine in Thailand20,21. This trial showed that high levels of antibodies binding to the HIV-1 variable loop 2 (V2) and low levels of IgA specific for the envelope (Env) protein correlated with decreased transmission and guided the design of subsequent clinical trials21. However, of two phase IIb/III clinical trials designed to improve on the RV144 trial — HIV-1 Vaccine Trials Network (HVTN) 702 (NCT02968849)18 and HVTN 705 (NCT03060629)15,19 — neither showed significant efficacy19, suggesting that the RV144 trial may not have been a harbinger of vaccine success. However, an ongoing phase III trial is further exploring the concept of inducing high titres of non-neutralizing antibodies (HVTN 706, NCT03964415).
Table 1.
HIV-1 vaccine efficacy trials completed or in progress
| Trial | Start | End | Vaccine | Location | Result | Refs. |
|---|---|---|---|---|---|---|
| VAX004 (NCT00002441) | 1999 | January 2000 | Bivalent clade B gp120 in alum | United States, Europe | No efficacy | 190–192 |
| VAX003 (NCT00006327) | March 1999 | August 2000 | Bivalent CRF_01AE/B gp120 in alum | Thailand | No efficacy | 193–195 |
| HVTN 502 (Step Study) (NCT00095576) | November 2004 | September 2009 | Adenovirus type 5 clade B gag/pol/nef | United States | No efficacy; increased infection in vaccinees | 169,196–201 |
| HVTN 503 (Phambili study) (NCT00413725) | December 2006 | July 2015 | Adenovirus type 5 clade B gag/pol/nef | South Africa | No efficacy; increased infection in male vaccinees | 170,202–205 |
| RV144 (NCT00223080) | September 2005 | April 2009 | ALVAC with gag/pro/Env; bivalent CRF_01AE/B gp120 in alum | Thailand | Estimated 31.2% vaccine efficacy at 42 months; 12-month efficacy, 60% | 20,21,206–215 |
| HVTN 505 (NCT00865566) | May 2009 | October 2017 | DNAs with clade B gag/pol/nef and DNAs with clade A, B, C Envs; adenovirus type 5 with gag/pol and clade A, B, C Envs | United States | No efficacy | 196,216–222 |
| HVTN 703/HPTN 081 (NCT02568215) | May 2016 | March 2021 | Antibody Mediated Protection (AMP) trial of VRC01 neutralizing antibody infusion IV | Sub-Saharan Africa | No overall efficacy; protection from only highly sensitive HIV-1 strains | 111,223,224 |
| HVTN 704/HPTN085 (NCT02716675) | April 2016 | December 2020 | Antibody Mediated Protection (AMP) trial of VRC01 neutralizing antibody infusion IV | North America, South America, Switzerland | No overall efficacy; protection from only highly sensitive HIV-1 strains | 111,223,224 |
| HVTN 702 Uhambo (NCT02968849) | October 2016 | September 2021 | ALVAC-C with gag/pol/Env; bivalent gp120s in MF59 | South Africa | No efficacy | 18,225 |
| HVTN 705 Imbokodo (NCT03060629) | November 2017 | August 2021 | Ad26, 4 valent T cell mosaic genes, boost with clade C gp140 Env | Sub-Saharan Africa | No efficacy | 226 |
| HVTN 706 Mosaico (NCT03964415) | October 2019 | Ongoing (est. March 2024) | Ad26, 4 valent T cell mosaic genes, boost with clade C gp140 Env + B cell mosaic gp140 Env | United States, Spain, Central/South America | Ongoing | 227 |
Adapted with permission from227. HVTN, HIV-1 Vaccine Trials Network; NA, not available.
Currently, the most successful non-HIV-1 vaccines in clinical use induce neutralizing antibodies as their primary mode of protection22. Thus, for HIV-1, the induction of antibodies that broadly protect against heterologous HIV-1 strains (called broadly neutralizing antibodies (bnAbs)) is a prime goal of HIV-1 vaccine development23–26.
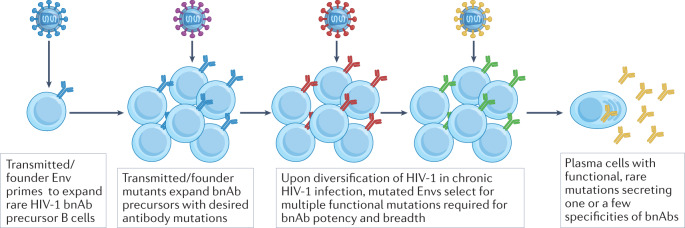
Importantly, sera from a substantial proportion of untreated individuals with HIV-1 will neutralize 50% or more of heterologous viruses, yet only a small subset of these individuals produce bnAbs with the high levels of neutralization breadth and potency that would be necessary for a protective vaccine response27–31. Moreover, in individuals infected with HIV-1 who produce bnAbs, these generally only develop after many months or years owing to infrequent germline B cell priming and the requirement for extensive antibody somatic hypermutation27–31. When bnAb activity in serum does develop, it is often mediated by a single clonal lineage (or, more rarely, two or three bnAb lineages) (Fig. 1), and bnAbs constitute a minor component of an individual’s overall HIV-1-specific antibody response32–36. Recent data suggest that in infants and children, high levels of bnAbs may develop over a shorter period of time and require fewer mutations than in adults, although the factors governing this remain unclear37,38.
Fig. 1. Model of broadly neutralizing antibody development in humans.
In individuals infected with HIV-1, viral diversification of envelope (Env) sequences was found to be required for broadly neutralizing antibody (bnAb) development33. From this work came the concept of transmitted/founder Envs that initiate the infection and B cell lineage design whereby sequential immunogens are chosen from autologous evolved viruses that induced bnAbs, or are structurally designed to have affinity gradients across maturing lineage members and to select for desired mutations to favour bnAb development. bnAb development follows a ‘jackpot effect’, where each individual with HIV-1 who makes bnAbs has only one or very few bnAb B cell lineages that have made it through a tortuous bnAb maturation pathway requiring multiple rare events stimulated by evolving virus.
Overall, it is clear that the induction of bnAbs is challenging owing to the unusual traits that are required to allow for breadth and neutralization capacity. These include the frequent presence of long heavy chain complementarity determining region 3 (HCDR3s)39–41 and extensive somatic hypermutation, including the selection of improbable antibody mutations that are necessary for bnAb activity as defined by structural and functional analysis42. The particular challenges for the induction of bnAbs are summarized in Box 1.
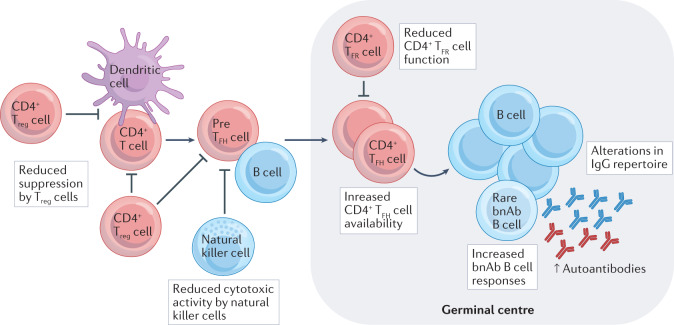
Individuals who do make potent bnAbs typically have moderate to high viral loads43 and share immunological characteristics such as high levels of circulating CD4+ T follicular helper (TFH) cells, fewer and less potent CD4+ regulatory T (Treg) cells and populations of dysregulated natural killer cells with reduced immunoregulatory activity39,44,45. The ability to make bnAbs in the setting of HIV-1 infection is also associated with a shift in the overall B cell repertoire, with higher frequencies of B cells that produce antibodies with autoantibody-like features46 (Box 1 and Fig. 2). Indeed, numerous studies have demonstrated that many bnAb-producing B cell lineages have features associated with polyreactivity (the ability of an antibody to bind to multiple structurally unrelated antigens47) or autoreactivity (antibody reacts with self-antigens48) that are associated with the development of HIV-1 neutralizing antibody breadth40,41. About 40% of early immature B cells in healthy humans express autoreactive and polyreactive B cell receptors (BCRs), but these are usually culled at the early stages of B cell development and only make up about 20% of the mature B cell repertoire49. The residual polyreactive BCRs augment the breadth and efficacy of normal humoral responses to microbial pathogens47. That HIV-1 bnAbs are frequently polyreactive or autoreactive can be an obstacle for both the induction and the durability of bnAb responses. Studies of the human antibody repertoire have suggested that bnAb naive B cell precursors with long HCDR3 regions required to bind conserved neutralizing HIV-1 epitopes are relatively rare50–52. Thus, a goal of HIV-1 vaccine design is to administer immunogens that bind to the BCRs of multiple specificities of B cells that are bnAb precursors53, with the aim to induce a broader polyclonal B cell response to bnAb Env epitopes than naturally occurs in individuals with HIV-1 infection (Fig. 3). Strategies for bnAb immunogen design are summarized in the legend to Fig. 4.
Fig. 2. Host immunoregulatory control abnormalities in individuals infected with HIV-1 who make broadly neutralizing antibodies.
In studies of cohorts of individuals positive for HIV-1, those who make broadly neutralizing antibodies (bnAbs) have high levels of circulating CD4+ T follicular helper (TFH) cells, low levels of CD4+ regulatory T (Treg) cells and circulating T follicular regulatory (TFR) cells, high levels of plasma autoantibodies and low levels of functional natural killer cells. Increased availability of CD4+ TFH cells and reductions in the numbers of functional Treg cells and natural killer cells, both of which constrain germinal centre responses to reduce autoantibody production, may enable enhanced B cell somatic hypermutation and repertoire diversification. In addition, individuals infected with HIV-1 who make bnAbs have perturbations in their B cell IgG repertoires such that B cell receptors (BCRs) with longer heavy chain complementarity determining region 3 (HCDR3s) and increased autoreactivity can expand. Thus, HIV-1 infection results in a permissive immunologic environment that favours eventual bnAb development.
Fig. 3. Complexity of a prototype HIV-1 vaccine for the induction of broadly neutralizing antibodies.
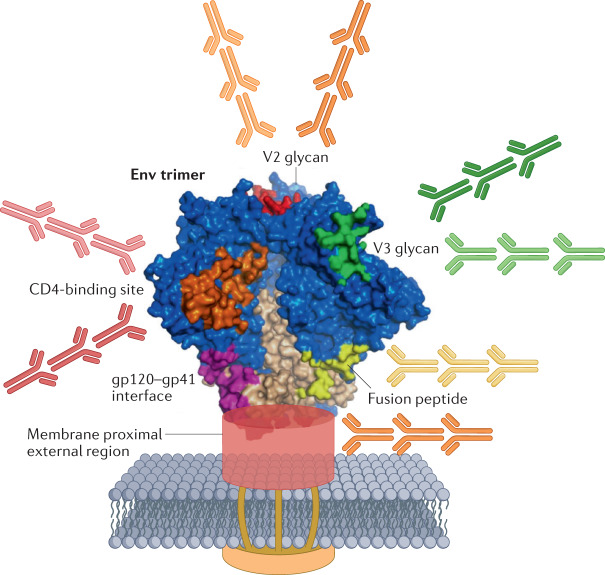
HIV-1 envelope (Env) trimer with targets for broadly neutralizing antibodies (bnAbs) shown in colour. A polyclonal multi-B cell lineage response requires development of bnAbs binding to the CD4 binding site and to at least two other epitopes, such as V2 glycan, V3-glycan patch, fusion domain or membrane proximal external region (MPER) sites. Fab-dimerized glycan (FDG) antibodies bind to high mannose residues at multiple sites on Env. Figure derived from structure described by Pancera et al.230.
Fig. 4. Strategies for the design of HIV-1-targeted broadly neutralizing antibody immunogens.
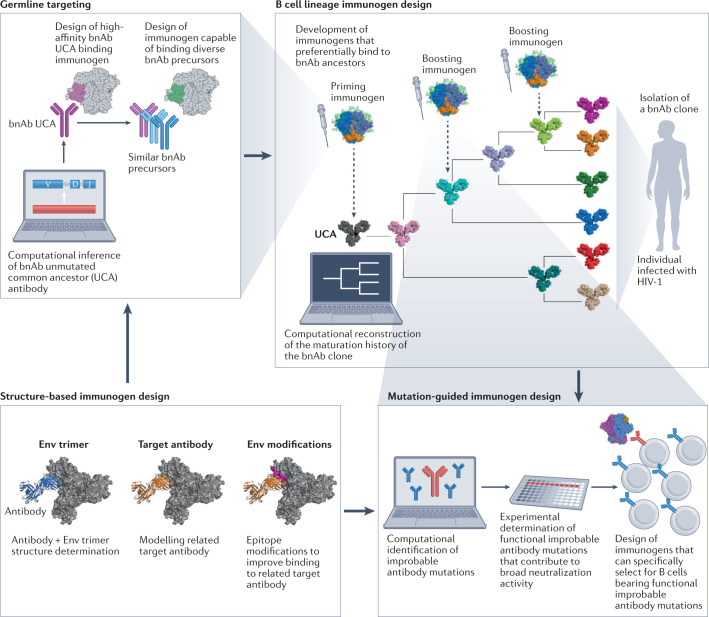
B cell lineage immunogen design25 (upper right) uses insights into HIV-1 virus/broadly neutralizing antibody (bnAb) B cell co-evolution to inform the design of envelope (Env)-based immunogens that can recapitulate generation of similar bnAbs by vaccination. B cell lineage immunogen design starts by isolating a bnAb-producing B cell clone from an individual infected with HIV-1, sequencing its B cell receptors (BCRs) to identify clonal members and, then computationally reconstructing the maturation history of the bnAb. The maturation pathway from the inferred unmutated common ancestor (UCA) antibody (representing the naive bnAb B cell receptor (BCR)) through inferred ancestral intermediates to the bnAb serves as a molecular guide for HIV-1 vaccine design. UCA and inferred antibody intermediates serve as templates for design of immunogens that bind with high affinity. Immunogens can be designed through germline-targeting (upper left), structure-based immunogen design (lower left) and mutation-guided immunogen design (lower right). The goal is for sequentially administered immunogens to provide a selective advantage in the germinal centre to B cells that follow similar desired evolutionary trajectories. Because bnAb UCAs rarely bind with high affinity to unmodified autologous Envs, priming immunogens frequently must be designed with epitope modifications (for example, the shortening of variable loops, or removal of key glycans) in an approach called germline-targeting (upper left). Germine-targeting is based on selection of a transmitted/founder virus or a specifically selected or designed Env immunogen that can bind to a UCA25,51,77,78,90. This can be achieved through in vitro selection techniques where UCAs are used to bind and select high-affinity Env antigens from a library of Env variants. These Env ligands are then used to isolate antibodies from the human immunoglobulin repertoire to identify a polyclonal mixture of putative bnAb precursors. A germline-targeting Env antigen can then be re-designed to improve its affinity for many of the isolated putative bnAb precursors using mutation-guided immunogen design and structure-based immunogen design. Mutation-guided immunogen design42 aims to identify the improbable mutations in bnAbs that are not routinely generated by somatic hypermutation but are critical for broad neutralization. These are then used to inform the design of immunogens that can specifically select for these mutations. Structure-based immunogen design91 is based on the determination of bnAb–Env complexes that provide atomic-level information that is necessary to computationally model specific bnAbs as templates for immunogen design. These inform the modifications for improving Env immunogen binding to bnAbs or bnAb precursors. Structure-based immunogen design is utilized to inform all stages of the vaccine design strategy.
Durable antibody responses depend on the induction of long-lived plasma cells that persist in bone marrow niches and maintain antibody responses for years or even decades54. Scheid et al. have demonstrated that more HIV-1-specific memory B cells are polyreactive than are bone marrow plasma cells with comparable specificity, suggesting that polyreactive bnAb B cells are more likely to develop into memory B cells than long-lived plasma cells55. As a large percentage of the HIV-1-targeted antibody response is mediated by polyreactive or autoreactive B cells40,41,47, the reduced presence of polyreactive bone marrow plasma cells may contribute to the inability of current HIV-1 vaccines to induce durable Env-targeted antibody responses20,56,57. Novel vaccine platforms such as integrase-deficient lentiviral vectors have shown improved HIV-1-specific serum antibody durability compared with protein immunization58. Additionally, nucleoside-modified mRNAs in lipid nanoparticles (LNPs) have been shown to induce durable low levels of plasma Zika virus and HIV-1 Env-targeted antibody levels in rhesus macaques59–61, and a TLR7 and TLR8 agonist adjuvant has induced durable HIV-1-specific plasma cell responses in macaques62.
HIV-1 infection has been shown to reduce the normal tolerance controls that constrain the development and maturation of polyreactive or autoreactive B cells, thereby creating an immune environment permissive for bnAb development39,46. Strategies for HIV-1 vaccine-induced bnAb induction in individuals negative for HIV-1 must be able to safely recreate, as much as possible, the permissive immune microenvironment that occurs in HIV-1 infection. One way to achieve this may be by selecting adjuvants that preferentially induce CD4+ TFH cell differentiation, high frequencies of which are needed for bnAb development39,45, and to minimize the activation of regulatory cells such as CD4+ Treg cells and natural killer cells. The nucleoside-modified mRNA–LNP vaccine platform may be advantageous in this regard as it has been shown to elicit potent CD4+ TFH cell responses to vaccine immunogens59,63,64.
Another major difficulty in inducing bnAb development is the large number of mutations that are required for bnAb maturation in germinal centres42. Mutations that are likely to be introduced by the activity of activation-induced cytidine deaminase (AID) arise frequently, a phenomenon termed ‘intrinsic mutability’ of immunoglobulin genes42,65–69. However, bnAbs are highly enriched in mutations that are rare during affinity maturation. The acquisition of such ‘improbable’ mutations can be guided by BCR ligands that specifically promote the expansion of B cells that have acquired such mutations42,70,71. Thus, the acquisition of improbable mutations in germinal centres can be rate-limiting for bnAb development, as the selection of improbable mutations requires specific Env epitopes to be present at the time the rare mutation arises.
Exploring the natural pathways of bnAb generation has provided key insights into the requirements for the induction of protective neutralizing antibody responses33,65,70–75. The HIV-1 vaccine field is essentially having to learn how to ‘engineer’ the adaptive immune system to generate bnAb B cell lineages that are highly disfavoured in the setting of vaccination51,76. Success in the induction of multiple probable and improbable mutations in a bnAb B cell lineage in humanized mouse models has been achieved, but the critical mutations that are required generally occur sporadically and independently, reflecting unlinked mutation rather than focused accumulation of combinations of mutations in a single B cell lineage76. Thus, a major task of HIV-1 vaccine development is to keep bnAb B cell lineages in germinal centres, or ensure they recirculate back to germinal centres, where they may accumulate the improbable mutations required for bnAb activity. Moreover, a successful bnAb-inducing vaccine will need to reduce the time needed for bnAb affinity maturation by providing optimal Env epitopes that are required to select for improbable bnAb mutations. In antibody–virus co-evolution studies in individuals infected with HIV-1, it was found that bnAb development requires viral diversification, suggesting the need for sequential immunizations with diverse Env epitopes that are capable of selecting for key mutations32–34,65,70,72,73,75,77,78. In this Review, we discuss current strategies for HIV-1 bnAb vaccine development, reflect on progress made to date in induction of bnAb lineages in animals and humans, and describe the different classes on HIV-1 bnAbs identified. Moreover, we explain the need for combined T cell and B cell vaccines and discuss the requirements that need to be met for the development of a practical HIV-1 vaccine.
Box 1 Roadblocks in the induction of HIV-1 broadly neutralizing antibodies.
HIV-1 sequence diversity continues to increase.
Early in infection, HIV-1 integrates into the host genome and can become ‘invisible’ to the immune system, thus requiring a successful HIV-1 vaccine to induce sterilizing immunity to prevent sustained HIV-1 infection originating from integrated HIV-1 genomes.
Broadly neutralizing antibodies (bnAbs) identified to date have unusual traits such as long heavy chain complementarity determining region 3 (HCDR3s), a high level of improbable mutations and polyreactivity with host or environmental antigens. These are required for bnAb activity.
The unusual traits required for bnAb activity can be disfavoured by tolerance mechanisms of the immune system such as deletion or anergy.
V3 glycan, V2 glycan and membrane proximal external region (MPER)-targeted bnAbs have long HCDR3 regions. Precursors of B cells capable of producing such bnAbs are usually deleted in the bone marrow owing to autoreactivity. Thus, precursors for such bnAbs may be extremely rare.
The HIV-1 envelope (Env) protein, the primary target for bnAbs, is heavily glycosylated and bnAb epitopes are cloaked in both high mannose and complex glycans that are poorly immunogenic.
Env has dominant non-neutralizing epitopes that compete with subdominant bnAb epitopes in immune germinal centres for B cells and T follicular helper cells (TFH cells).
Owing to lack of HIV-1 transmitted/founder or intermediate virus Envs that are capable of activating B cell lineages that encode unmutated common ancestors (UCAs), Env immunogens must be designed that can bind with high affinity to bnAb B cell lineage UCAs and early intermediates.
For a vaccine to be protective, multiple specificities of bnAb precursors need to be induced simultaneously.
The bar for protective neutralization breadth, potency and durability is very high, as determined in the Antibody Mediated Protection (AMP) trial.
The vaccine will need to protect against both blood and mucosal HIV-1 transmission.
bnAb biology: implications for vaccines
Detailed studies of HIV-1-neutralizing antibody co-evolution have been carried out to elucidate the ‘arms race’ between the evolving virus and B cell lineages that mature to produce bnAbs32–34,65,70,72,73,75. These studies have identified clonal ‘trees’ of bnAb B cells, their inferred bnAb unmutated common ancestors (UCAs)79 and the transmitted/founder viruses or their progeny that are bound by the BCRs of lineage members80. Current bnAb vaccine approaches are based on the hypothesis that priming B cells with Env molecules that are capable of binding to and activating naive B cells that express UCAs, followed by sequential Env immunogens that can select for improbable mutations and guide otherwise disfavoured bnAb B cell lineages to develop into full bnAb capability, can be ultimately successful25,42,78,81. An additional requirement for the design of sequential vaccines is the creation of affinity gradients of the Env immunogens across stages of bnAb B cell lineage development25,76,82, in order to create an ‘affinity pull’ across the lineage such that a ceiling of affinity is not reached until the B cell lineage achieves full maturation83. Moreover, vaccine-induced acquisition of fast on-rates is a critical attribute for at least a subset of bnAb B cell lineages to mature84–89. Given the complex characteristics that a successful HIV-1 vaccine will require and the physiological factors that disfavour bnAb induction (Box 1), numerous strategies for sequential immunogen design (B cell lineage immunogen design25, germline-targeting25,51,77,78,90, mutation-guided immunogen design42 and structure-based immunogen design91) have been developed (Fig. 4).
With continued exposure to sequential Env immunogens, bnAb B cell lineages can either remain on the evolutionary track to bnAb maturation or go off-track by the accumulation of mutations that disable Env reactivity70,92,93. In particular, the autoreactivity of some bnAb B cells raises the possibility that bnAb B cell maturation may be subjected to the process of ‘antibody redemption’, whereby autoreactive B cell lineages that have escaped central and peripheral tolerance checkpoints are rendered less or non-autoreactive during their somatic evolution in germinal centres94,95. In the case of some bnAbs, the loss of autoreactivity is tantamount with concomitant loss of bnAb activity as autoreactivity can be required for bnAb potency40,41,47,96.
To guide otherwise disfavoured bnAb B cell lineages to affinity mature to acquire full neutralization capacity, sequential protein Env immunogens should be formulated with adjuvants that promote TFH cell responses and disfavour Treg cell induction in order to overcome peripheral tolerance mechanisms such as B cell anergy. The engineering required to design immunogens that induce bnAb precursor-producing B cell lineages to affinity mature in the face of multiple developmental roadblocks is unprecedented in vaccinology and requires an in-depth understanding of B cell development and differentiation in the setting of vaccination. This contrasts with the development of COVID-19 vaccines in less than a year, owing both to the dramatically different host–virus interactions of SARS-CoV-2 compared with HIV-1 and to the extreme viral diversity of HIV-1 and the relative conservation of SARS-CoV-2 (refs.9,97).
Animal models are critical for the preclinical work towards the development of an HIV-1 vaccine that induces bnAbs. The two most useful models for validating immunogen design are simian-human immunodeficiency viruses (SHIVs) for infection of rhesus macaques (Box 2 and Fig. 5) and humanized mouse models (Box 3).
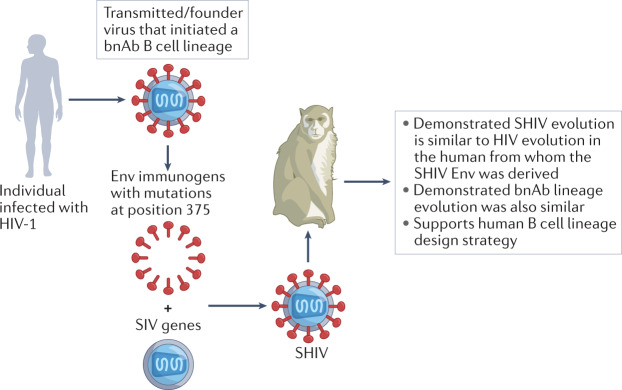
Fig. 5. Construction of simian-human immunodeficiency viruses containing clinically relevant primary HIV-1 Env immunogens.
Critical to simian-human immunodeficiency virus (SHIV) design is selection of primary or transmitted/founder HIV-1 envelope (Env) immunogens that exhibit features of clinically relevant viruses. For SHIV construction, a mutation at Env position 375 is made to facilitate macaque CD4+ T cell engagement. A HIV transmitted/founder or other Env immunogen is engineered to be expressed on the surface of a simian immunodeficiency virus (SIV) virion to form a chimeric SHIV. In this manner, virtually any HIV-1 Env can be used to produce a SHIV for testing for the ability to induce broadly neutralizing antibodies (bnAbs) in the setting of lentiviral infection.
Box 2 Construction of simian-human immunodeficiency viruses containing clinically relevant primary HIV-1 Env immunogens.
Shaw and colleagues engineered simian-human immunodeficiency viruses (SHIVs) by mutating the CD4 binding site of primary or transmitted/founder HIV-1 envelopes (Envs)80 to make them more infectious in rhesus macaques138,231,232 (Fig. 5). Remarkably, when SHIVs were constructed with Env immunogens derived from transmitted/founder viruses from individuals who made broadly neutralizing antibodies (bnAbs), and used to infect monkeys, the Env evolution in monkeys was frequently quite similar to Env evolution in the human infected with HIV-1 from whom the transmitted/founder Envs were derived138. Moreover, among 150 SHIV-infected macaques, ~15% of animals developed bnAbs after 3–24 months of infection. These bnAbs targeted canonical bnAb epitopes on the HIV-1 trimer, including the V2 glycan, V3-glycan patch, CD4 binding site and fusion peptide, and bore a remarkable resemblance to human bnAbs in structure, immunogenetics and antibody sequences needed for epitope recognition138. One rhesus bnAb (RHA1) was strikingly similar in structure and paratope–epitope interactions to the human V2 bnAbs PCT64 and PGT145 (ref.138). Thus, the SHIV-infected rhesus macaque provides a robust experimental model for evaluating common pathways of bnAb development. Most recently, Fab-dimerized glycan (FDG) bnAbs (DH851, DH1003) that bind to the Env glycan shield were isolated from SHIV-infected macaques, extending the value of the SHIV model156. Finally, a considerable advantage of the SHIV infection model is that bnAb lineages can be followed from before induction and throughout the affinity maturation process, culminating with the appearance of bnAbs. Identification of viral Envs that guide bnAb maturation can inform novel immunogen designs.
Box 3 Humanized mouse models for study of broadly neutralizing antibody B cell development.
The first broadly neutralizing antibody (bnAb) knock-in mouse model studied was the 2F5 VH knock-in mouse, in which B cells expressed the variable heavy chains of the bnAb 2F5. It demonstrated that B cells expressing the 2F5 B cell receptor (BCR) were mostly deleted at the pre-B cell stage in the bone marrow, and B cells with the 2F5 VH BCR that made it to the periphery were anergic144. Knock-in mice with a mutated 2F5 VH and knock-in mice that expressed the VH and VL regions of the bnAb 4E10 had similar defects87,110,233. Interestingly, the first-generation CD4 binding site bnAb 1b12 was found to be autoreactive, but it did not induce bone marrow B cell precursor deletion or anergy in peripheral B cells in knock-in mouse models234. By contrast, the CD4 binding site bnAb 3BNC60, also with a VH1-2*02 similar to VRC01 but paired with a different VL, when this VH + VL was knocked into mice, did demonstrate deletion, anergy and receptor editing, suggesting different fates dictated by VL usage110.
These initial bnAb knock-in mice were engineered to express the rearranged mature VH and VL or the rearranged VH and VL sequences of the unmutated common ancestor (UCA) of the respective bnAbs. The expression of pre-rearranged VH and VL sequences inhibited the rearrangement and expression of the endogenous mouse immunoglobulin loci. This type of mouse model is useful for testing whether the designed immunogen is capable of activating B cells that express particular bnAb precursors and whether precursor antibodies can mature to functional bnAbs. However, bnAb precursors are present in these mouse models at supra-physiologic frequencies. Immunization under such conditions cannot assess the specificity of immunogens for rare bnAb precursors in complex B cell repertoires235. To overcome this limitation, adoptive transfer of B cells that express bnAb precursors to wild type recipient mice can be used to reconstitute B cell compartments with physiological bnAb precursor frequencies236.
Recently, the groups of Alt, Tian and colleagues have developed HIV-1 bnAb germline-rearranging mice that developmentally generate diverse repertoires of bnAb VRC01-class precursors106. This type of mouse model is designed to assess the specificity of an immunogen for bnAb precursors as well as its ability to mature B cell lineages that produce related precursor antibodies towards the production of bnAbs. The latter function is important for immunogen efficacy in the human population, where individuals may have B cell lineages producing various bnAb precursors. In this mouse model, the human variable heavy 1–2 (VH1–2) gene segment recombines with mouse D and JH gene segments or mouse D segments and human JH segments to form a diverse range of humanized BCR heavy chains that express a VH1–2 region in association with a multitude of different complementary determining region 3 (CDR3) segments106. This model was revolutionary in that it provided for a more diverse repertoire of bnAb-producing B cells, and with a much lower frequency of precursors, resulting in a more physiologic testing environment for potential vaccine immunogens. Indeed, in this model, the eOD-GT8 prime (an immunogen designed to bind to VRC01 germline B cell receptors77), coupled with the 426c Env immunogen104 in a boost regimen, was successful in expanding VRC01 bnAb B cell precursors106,107. Mouse models are now being generated that mimic physiological conditions more closely, in which both VH and VL rearranging gene segments result in an immense diversity of humanized mouse BCR repertoires. Moreover, new models have been designed to circumvent B cell developmental blocks in the bone marrow by expressing bnAb precursors conditionally in mature B cells237.
Types of bnAbs
Numerous bnAbs have been identified in individuals with HIV-1, and have undergone in-depth characterization. These bind to several different conserved epitopes on the HIV-1 Env (Fig. 3 and Table 1). In general, Env epitopes for vaccine design have been chosen on the basis of the breadth and potency of bnAbs isolated from individuals infected with HIV-1 that bind the respective Env epitopes.
CD4 binding site-targeted bnAbs
There are two types of bnAbs that bind the CD4 binding site of Env: CD4 mimic bnAbs and HCDR3-binder CD4 binding site bnAbs (HCDR3-binder bnAbs). These are prime targets of HIV-1 vaccine development efforts. bnAbs of the first type mimic the CD4 contact residues for the CD4 binding site of Env via their HCDR2 regions and rely less on HCDR3 contacts with Env compared with the second type. Examples for CD4 mimic bnAbs are the VRC01 class and the 8ANC131/CH235 class, and an example of an HCDR3-binder bnAb is CH103 (Table 1).
CD4 mimic bnAbs
VRC01-class antibodies contain the variable heavy 1–2 (VH1–2) chain and require the use of a five amino acid light chain complementarity determining region 3 (LCDR3)75,98–101. However, one VH1–2 bnAb in this class (IOMA) has a normal length LCDR3 and fewer mutations and insertions or deletions than other VRC01-class antibodies102. VRC01 bnAbs are among the most potent and broad of all bnAbs isolated to date and their precursors are more common than other bnAb precursors77,98. However, a major roadblock for VRC01-class bnAb induction is the requirement for many improbable mutations, as well as antibody insertions and deletions, in order to acquire full breadth42,86. The VRC01 class is represented by 11 prototypic B cell lineages, which range from 80% neutralization breadth of heterologous primary HIV-1 strains (VRC-PG20) to 100% breadth (bnAb N49P7) (Table 1).
A germline-targeting immunogen, termed eOD-GT8, with picomolar affinity for the inferred UCA of VRC01 (ref.77) has been designed, and binds to most, but not all, putative UCAs of other members of the VRC01 class of bnAbs75,103. In VRC01 knock-in mice, eOD-GT8 primes and expands VRC01 precursors, and in combination with sequential boosting immunogens104 can select for B cells with VRC01 BCRs that have a degree of neutralization breadth105–107. eOD-GT8 is immunogenic in macaques, which respond more rapidly to subcutaneous rather than intramuscular injections108. In the IAVI G001 human trial, eOD-GT8 expanded B cells bearing BCRs with characteristics of VRC01 precursors109.
In addition to eOD-GT8, glycan-deleted derivatives of the clade C HIV-1 426c Env have been designed as immunogens that bind to VRC01-class bnAbs78,90,104,110. Initially, these designs focused on the strategic removal of glycans, as glycans at Env positions 276, 460 and 463 were found to be major obstacles for binding to VRC01-class precursors90. Comparison of eODGT8 and 426c Env immunogens in VRC01 UCA heavy chain knock-in mice showed that the two immunogens engage different VRC01 bnAb precursors104. However, the Antibody Mediated Protection (AMP) trials in South Africa, which studied the protective efficacy of passively administered VRC01 bnAb, showed no overall protection from HIV-1 infection111–113. Further study showed that for HIV-1 isolates sensitive to VRC01, the level of VRC01 bnAb required for preventing transmission was ~1 : 200 (ref.111). This indicated that a successful HIV-1 vaccine will require high levels of induced bnAbs that are maintained at protective levels over long periods of time, and will require bnAb induction to multiple Env epitopes to achieve neutralization breadth114,115. Moreover, during the AMP trial, HIV-1 diversification was greater than anticipated111, and the continuing evolution of HIV-1 will need to be considered.
The second type of CD4 mimic bnAbs, the 8ANC131/CH235 class, exclusively uses the variable heavy chain 1–46 (VH1–46) region but does not require the five amino acid LCDR3 light chain found in VRC01-class antibodies65,116–119. The 8ANC131/CH235 class includes the bnAbs CH235, 8ANC131, 1-18 and 1B2530, and their neutralization breadth ranges from 97% neutralization for the bnAb 1-18 to 71% for the bnAb 8ANC131 (Table 1). An individual (CH505) in the CHAVI001 acute HIV-1 infection cohort made both the CH235 bnAb as well as a member of the second type of CD4 binding site bnAb, the HCDR3-binder CH103 (refs.33,65). In this individual, the bnAb response required cooperation between the two neutralizing antibody lineages, whereby an early autologous neutralizing antibody of the CH235 lineage selected for viral escape mutants that were sensitive to the CH103 bnAbs produced by affinity matured B cells32. A similar mechanism of cooperation between neutralizing lineages was observed in an individual infected with HIV-1 (CH848) who made the DH270 V3-glycan bnAb70. In addition, 8ANC131/CH235 class bnAb 1-18 is particularly resistant to HIV-1 escape117. Most HCDR3-binder bnAb lineages that bind the CD4 binding site, such as the CH103 bnAb lineage, do not have the same level of neutralization breadth as the CD4 mimicking bnAb lineages (for example, the CH103 bnAb lineage achieves only 67% neutralization breadth)33.
Rhesus macaques have an orthologue for the human VH1–46 region and can make CH235-like antibodies76. In addition, Env proteins have been designed that both potently induce the expansion of CH235 precursors and select for a subset of improbable functional mutations that are necessary for acquiring heterologous neutralizing breadth in both UCA knock-in mice and in rhesus macaques76,120. As observed for VRC01-class bnAbs, the frequency of 8ANC131/CH235-class bnAb precursors does not appear to be limiting, but the very high frequency of required improbable mutations appears to be the predominant factor disfavouring induction of this bnAb class. Unlike VRC01-class bnAbs, most CH235 bnAbs do not require insertions or deletions (rare events in B cell maturation) for acquisition of bnAb breadth65,102.
HCDR3-binder CD4 binding site bnAbs
For the induction of HCDR3-binder bnAbs, a transmitted/founder gp120 Env termed CH505 T/F trimer (derived from individual CH505 from the CHAVI001 acute HIV-1 infection cohort) with relatively low affinity for binding to the CH103 UCA was designed and tested as a priming immunogen in the HVTN115 trial (NCT03220724)33. Although the CH505 T/F gp120 monomer had expanded CH103 UCA B cells in UCA knock-in mice93, it did not expand CH103 precursors in the HVTN 115 clinical trial121 (M. Sobieszczyk, W. Williams, B. F. Haynes, J. Kobie, unpublished results). As a follow-up to test the effect of affinity in mediating bnAb precursor expansion, a near-native stabilized CH505 T/F trimer with higher affinity for the CH103 UCA is now being tested in humans in the HVTN 300 clinical trial (NCT04915768). An additional factor limiting induction of HCDR3-binder or other CD4 binding site bnAbs may be the acquisition of autoreactivity at various stages in the bnAb B cell lineages11,33.
V3 glycan-targeted bnAbs
A second major bnAb epitope of Env, the V3-glycan patch, is located at the base of its V3 loop region at the ‘GDIR’ sequence (amino acids 324–327), located between two N-linked glycans at positions 301 and 332 (refs.122,123). Unlike CD4 mimic bnAbs that utilize VH1 heavy chains and have normal length HCDR3s, bnAbs targeted at V3 glycans are encoded by multiple VH gene segments and have long HCDR3s (18–24 amino acids). There are 6 prototypic V3-glycan bnAb lineages that range in neutralization breadth from 51 to 68% (Table 1). Their long HCDR3s are required to reach deep between glycans to bind to the Env polypeptide backbone123. However, for vaccine development, the requirement for a long HCDR3 region means that V3-glycan precursors are very rare due to the deletion of B cells with BCRs with long HCDR3s by immune tolerance mechanisms51,124,125. For example, V3-glycan precursors of the BG18 bnAb B cell lineage have been found to be present at a frequency of only 1 in 53 million51. V3-glycan germline-targeting Envs have been shown to expand these precursors in UCA knock-in mice51,126,127, while also selecting for improbable mutations required for heterologous breadth76,121. Moreover, in mice with a knock-in of a minimally mutated bnAb precursor, sequential immunization with Env immunogens induced antibody affinity maturation128. Immunization of macaques with the Env immunogen RC1, which has mutations in its V1 region and glycan deletions that result in increased accessibility of the V3 glycan site, elicited V3 glycan-targeted antibodies that were dependent on the glycan at amino acid position 332 of Env, but structural analysis revealed differences in antibody orientation compared with other V3-glycan bnAbs129. Boosting of macaques with sequential heterologous Env immunogens induced low levels of heterologous neutralizing antibodies targeting the V3-glycan patch, but also promoted the development of antibodies that bound off-target and offered minimal protection following SHIV challenge130. Besides the requirement for long HCDR3 loops, V3-glycan bnAbs have high levels of rare mutations required for acquisition of bnAb breadth42.
V2 glycan-targeted bnAbs
The V2-glycan epitope includes an N-linked glycan at position 160 and a lysine-rich carboxy-terminal strand (around positions 168–171). There are five prototypic V2-glycan bnAb B cell lineages termed PG9, PGDM1400, VRC26.25, CH01 and PCT64 (Table 1). Many V2 glycan-targeted bnAbs utilize their long negatively charged HCDR3 loops and sulfated tyrosines to penetrate the Env glycan shield and interact with the positively charged C-terminal strand131. The HCDR3 loops of V2-glycan bnAbs are typically even longer (24–36 amino acids) than those found in V3-glycan bnAbs (Table 1). This requirement makes precursors of V2-glycan bnAbs exceedingly rare. The long HCDR3s of many V2-glycan antibodies (including the prototypic bnAbs PG9, CH01 and PCT64, as well as the bnAbs CAP256.VRC26 and PG16) are characterized by the presence of a YYD tyrosine sulfation motif131–134.
In one study, antibodies with HCDR3 regions similar to the PG9 bnAb were found by ultra-deep sequencing in only 2 out of 70 individuals naive for HIV-1 (ref.52). In UCA knock-in mice, a series of immunogens were used to stimulate V2-glycan bnAb precursors135, and one study detected V2-glycan bnAb induction by vaccination with a series of engineered Env immunogens in a rhesus macaque136. To date, V2-glycan germline-targeting immunogens have yet to be studied in clinical trials, but a chimpanzee V2-glycan UCA-binding Env called MT145KdV5 is in Good Manufacturing Practice (GMP) production for testing in a phase I clinical trial135.
Although V2-glycan bnAb precursors are rare, one study including a cohort of people of sub-Saharan African descent with primary infection with HIV-1 detected V2-glycan bnAbs in 14% of individuals who had high serum levels of bnAbs, whereas the most common bnAb type was V3-glycan bnAbs, which were observed in 38% of subjects with high levels of bnAbs137. V2-glycan bnAbs have also been observed in SHIV-infected macaques138. Thus, either HIV-1 or SHIV infection induces a permissive immunologic milieu that facilitates the induction of bnAbs with rare long HCDR3s or the precursor frequency of potential V2-glycan bnAb BCRs in humans and macaques is greater than previously estimated. Precisely why retroviral infection leads to bnAb induction in only a subset of humans and macaques is not completely understood. The reasons are likely multifactorial, including shifts in the B cell repertoire that favour the expression of long HCDR3s in those that make bnAbs and the relaxation of immune tolerance mechanisms due to retroviral infection39,46.
bnAbs targeting the membrane proximal external region of gp41
Two different regions of the membrane proximal external region (MPER) of gp41 (the transmembrane domain of Env) are also targets of bnAbs and have both been found to be immunogenic in individuals infected with HIV-1. bnAbs targeting these regions are represented by six prototypic B cell lineages (Table 1). bnAbs targeted at the distal MPER, such as 10E8 and DH511.2-K3, are the most potent members of this class and both of these bnAbs use the same VH3-15 gene segment, have a 24 amino acid HCDR3 and, similar to most other MPER bnAbs, are of the IgG3 isotype34,139 (Table 1). Notably, the bnAbs 10E8 and DH511.2-K3 are also among the broadest (~99%) HIV-1 neutralizing antibodies isolated to date, making them attractive vaccine design targets. Other distal MPER bnAbs such as VRC42.1, PGZL1 and 4E10 are encoded by the VH1–69 gene segment with the variable light chain VK3–20 (ref.140) (Table 1). Most MPER bnAbs share the traits of long HCDR3s, have high levels of VH mutations and bind to a membrane proximal binding epitope that includes binding both the gp41 MPER and viral membranes (Table 2). Indeed, MPER bnAbs must also bind lipids in order to neutralize HIV-1, as this is thought to tether the bnAb to the virus lipid bilayer to be available for binding of the MPER after epitope exposure during receptor-mediated Env activation96,141. MPER bnAbs are among the most polyreactive of HIV-1 bnAbs and have been shown to bind, in addition to lipids40, host proteins including the splicing factor SF3B3 (4E10 bnAb) and kynureninase. Kynureninase carries a dimerization motif (ELDKWA) that is identical to an epitope in the proximal MPER bound by the bnAb 2F5 (ref.142). Mice engineered to express mature MPER bnAbs or UCAs of MPER bnAbs characteristically exhibit deletion or receptor editing of bnAb precursors in the bone marrow and anergy of residual bnAb precursor B cell lineages in the periphery143–146. For 2F5 bnAb knock-in mice, this central tolerance checkpoint appears not to be driven by interaction with lipids, as B cell compartments rescued by receptor editing or anergy lose the capacity to bind kynureninase but retain lipid reactivity145. Interestingly, in opossums, a point mutation in the kynureninase gene results in a dimerization motif (ELEKWA) that differs from that in humans by the single D → E replacement. In contrast, the SF3B3 proteins of humans and opossums are identical142. When opossums were immunized with gp41 MPER immunogens, they responded robustly to the ELDKWA epitope, presumably in the absence of tolerance imposed by the endogenous ELEKWA motif, but had little or no response to the SF3B3 protein they share with humans142. Thus, in humans, tolerance to kynureninase and SF3B3 likely limits the response to both the proximal and distal MPER bnAb epitopes.
Table 2.
Broadly neutralizing antibodies and their characteristics
| HIV-1 epitope | bnAb | Breadth (%)a (potency (μg ml–1)b) | VH/VL gene | HCDR3 length | LCDR3 length | VH mutation frequency (%) | VL mutation frequency (%) | Germline-targeting priming immunogen |
|---|---|---|---|---|---|---|---|---|
| CD4 binding site | N49P7 | 100 (0.10) | VH1–2/VL2–11 | 19 | 5 | 24.5 | 14.1 | NA |
| N6 | 99 (0.062) | VH1–2/VK1–33 | 13 | 5 | 30.2 | 22.4 | ||
| 12A12 | 93 (0.221) | VH1–2/VK1–33 | 13 | 5 | 21.9 | 15.5 | eOD-GT8 (ref.77) | |
| VRC01 | 91 (0.377) | VH1–2/VK3–20 | 12 | 5 | 31.6 | 17.2 | 426c.TM4ΔV1-3 (ref.110), BG505 SOSIP 4.1 GT1 (ref.53), eOD-GT8 | |
| 3BNC117 | 89 (0.116) | VH1–2/VK1–33 | 10 | 5 | 23.7 | 14.8 | eOD-GT8 | |
| VRC-CH31 | 84 (0.321) | VH1–2/VK1–33 | 13 | 5 | 20.2 | 15.2 | 426c.TM4ΔV1-3, eOD-GT8 | |
| PCIN63.71I | 84 (0.46) | VH1–2/VK1–6 | 13 | 5 | 14.6 | 12.5 | ||
| VRC-PG04 | 81 (0.317) | VH1–2/VK3–40 | 14 | 5 | 28.6 | 15.2 | eOD-GT8 | |
| VRC-PG20 | 80 (0.226) | VH1–2/VL2–14 | 13 | 5 | 24.0 | 14.8 | 426c.TM4ΔV1-3, eOD-GT8 | |
| IOMA | 49 (2.33) | VH1–2/VL2–23 | 15 | 8 | 25.4 | 23.3 | ||
| 1-18 | 97 (0.048) | VH1–46/VK3–20 | 16 | 9 | 26.4 | 20.2 | ||
| CH235.12 | 89 (0.70) | VH1–46/VK3–15 | 13 | 8 | 25.0 | 14.8 | CH505.M5.G458Y.GNTI- (ref.120) | |
| 1B2530 | 72 (3.62) | VH1–46/VL1–47 | 16 | 11 | 27.8 | 15.7 | ||
| 8ANC131 | 71 (1.78) | VH1–46/VK3–20 | 16 | 9 | 25.7 | 17.2 | ||
| CH103 | 67 (2.28) | VH4–59/VL3–1 | 13 | 10 | 16.9 | 11.1 | CH505 T/F33 | |
| V3 glycan | PGT128 | 68 (0.064) | VH4–39/VL2–8 | 19 | 10 | 19.1 | 7.0 | |
| PGT121 | 66 (0.072) | VH4–59/VL3–21 | 24 | 12 | 19.6 | 16.5 | MD39-11MUTb (ref.126), RC1-4Fill (ref.129) | |
| BG18 | 61 (0.032) | VH4–4/VL3–25 | 21 | 11 | 21.5 | 17.6 | MD39-11MUTb, N332 GT5 (ref.51) | |
| BF520.1 | 53c (7.31) | VH1–2/VK3–15 | 18 | 11 | 6.6 | 5.3 | ||
| PGDM12 | 54 (0.14) | VH3–11/VK2–24 | 19 | 9 | 19.1 | 14.3 | ||
| DH270.6 | 51 (0.21) | VH1–2/VL2–23 | 18 | 10 | 12.8 | 6.7 | CH848 SOSIP 10.17 DT (ref.76) | |
| PCDN76-33A | 46 (0.50) | VH4–34/VK3–20 | 20 | 8 | 13.0 | 11.6 | ||
| V2 apex | PG9 | 87 (0.154) | VH3–33/VL2–14 | 28 | 10 | 12.6 | 6.3 | BG505 SOSIP 4.1 GT1, BG505 SOSIP 4.1 GT1.1 (ref.228), ZM233 (ref.132), KER2018 (ref.133), BB201.B42 (ref.133) |
| PGDM1400 | 83 (0.02) | VH1–8/VK2–28 | 32 | 9 | 26.4 | 11.8 | ||
| VRC26.25 | 70 (0.004) | VH3–30/VL1–51 | 36 | 12 | 12.2 | 8.6 | ||
| CH01 | 54 (1.38) | VH3–20/VK3–20 | 24 | 9 | 16.7 | 11.2 | BG505 SOSIP 4.1 GT1, BG505 SOSIP 4.1 GT1.1, ZM233, CM244 (ref.132), Q23.17 (ref.132), WITO132, T250 (ref.131) | |
| PCT64-35Md | 35 (0.41) | VH3–15/VK3–20 | 23 | 8 | 11.2 | 4.9 | ||
| MPER | 10E8 | 98 (0.356) | VH3–15/VL3–19 | 20 | 12 | 21.4 | 13.4 | NA |
| DH511.2 | 98 (0.943) | VH3–15/VK1–39 | 21 | 11 | 19.8 | 14.0 | ||
| 4E10 | 98 (1.81) | VH1–69/VK3–20 | 18 | 9 | 6.9 | 4.1 | ||
| VRC42.1 | 96 (4.09) | VH1–69/VK3–20 | 15 | 9 | 10.8 | 5.6 | ||
| VRC43.1 | 63 (1.34) | VH4–4/VL7–43 | 19 | 9 | 11.1 | 8.5 | ||
| PGZL1 | 84 (6.06) | VH1–69/VK3–20 | 15 | 9 | 20.9 | 11.8 | ||
| 2F5 | 58 (2.83) | VH2–5/VK1–13 | 22 | 9 | 13.1 | 11.0 | MPER liposome87 | |
| Fusion peptide | PGT151 | 73 (0.04) | VH3-30/VK2D–20 | 26 | 9 | 20.8 | 11.5 | NA |
| VRC34 | 47 (0.32) | VH1–2/VK1–9 | 13 | 9 | 14.9 | 8.7 | ||
| Silent face | SF12 | 62 (0.20) | VH4–59/VK3-20 | 21 | 6 | 16.3 | 13.9 | NA |
| Fab dimer glycan | 2G12e | 21 (3.75) | VH3–21/VK1–5 | 14 | 9 | 21.1 | 11.7 | V3-glycopeptide156 |
The following bnAbs met the inclusion criteria but were not included for space considerations: 3BNC55, VRC13, VRC16, VRC18, VRC27. Adapted with permission from ref.227. bnAb, broadly neutralizing antibody; MPER, membrane proximal external region; NA, not available; VH1–2, variable heavy 1–2; HCDR3, heavy chain complementarity determining region 3; LCDR3, light chain complementarity determining region 3. aOnly bnAbs that have >45% breadth on a multiclade panel of >50 viruses (from CATNAP database229), except where noted. bGeometric mean of detected. cBF520.1 neutralization was only tested on 15 viruses but is included here because it notably was induced in an infant38. dPCT64 included because it is only one of two V2 lineages profiled from acute HIV-1 infection to bnAb breadth. e2G12 does not meet >45% breadth threshold but is included because expected high precursor frequency of natural Fab-dimerized glycan (FDG) antibodies makes this class an attractive target for design.
Nonetheless, immunization of monkeys and UCA knock-in mice with an MPER peptide liposome containing the proximal and distal MPER bnAb epitopes induced precursors of 2F5-like antibodies to expand87. In monkeys, a 2F5-like bnAb B cell lineage expanded and underwent affinity maturation but was limited in its capacity to undergo full bnAb maturation by the lack of an improbable proline mutation in the HCDR3 (ref.87). Thus, in mice and monkeys, it was shown that antibodies binding the proximal MPER epitope can be induced with germline-targeting immunogens.
The human immunization trial HVTN133 (NCT03934541) tested an MPER peptide liposome as an immunogen to determine whether B cell precursors targeting the proximal 683LDKW686 epitope of gp41 can be expanded, and bnAb precursors that target this epitope have indeed been identified in clinical trial vaccinees (W. Williams, L. Baden, B. F. Haynes, unpublished observations). However, to date, success has not been achieved in any model system that stimulates the precursors of the more potent distal MPER bnAbs such as 10E8 or DH511-K3. This may be due, in part, to the close proximity of the distal MPER epitope to the viral membrane and to the extraordinary autoreactivity of some of the distal MPER antibodies. For example, 4E10 cross-reacts with SF3B3, has strong reactivity to lipids such as cardiolipin and has lupus anticoagulant activity40,142. Moreover, it can prolong the partial thromboplastin time when administered to humans in vivo147. In general, however, it is thought that autoreactive HIV-1 bnAbs are not pathogenic. Rather, the polyreactive or autoreactive nature of bnAbs reflects their requirement to have unusual traits such as long HCDR3s for bnAb activity.
Fusion domain-targeted bnAbs
The fusion domain peptide is exposed on the surface of the HIV-1 Env trimer, and bnAbs targeting this epitope, such as the VRC34 lineage, have been identified. Vaccination of mice or monkeys with fusion domain peptides conjugated to carrier proteins (to enhance immunogen valency and to provide a source of T cell epitopes for CD4+ TFH cell induction) has been shown to induce antibodies that neutralize heterologous HIV-1 strains148–151. Whereas the general strategy for vaccine induction of most HIV-1 bnAbs has been to employ some form of B cell lineage design by targeting UCAs or germline naive B cells, followed by sequential boosts to guide favoured bnAb B cell lineages, a different strategy is pursued for fusion domain-targeted bnAbs. Here, vaccination with a fusion domain linear peptide sequence is followed by immunofocusing152,153 with fusion domain peptides. Although fusion domain-targeted bnAbs are not as potent or broad as other types of HIV-1 bnAbs, fusion domain-targeted antibodies could potentially be an important component of a vaccine-induced polyclonal and multi-epitope neutralizing antibody response to Env. There are currently no known immune mechanisms that disfavour the formation of fusion domain-targeted antibodies, although, to date, potent fusion domain bnAbs have been difficult to induce. An analysis of the fusion peptide-targeted bnAb lineage VRC34 revealed an accumulation of improbable mutations during affinity maturation. In particular, a Y → P mutation at position 33 was a key functional improbable mutation that occurred during affinity maturation and regulated the interaction between VRC34 intermediate antibodies and the fusion peptide149,151. This mutation occurred in the clade of the lineage that acquired broad neutralizing activity, but did not occur in other weakly neutralizing clades of the VRC34 lineage151. Therefore, the requirement for acquisition of improbable mutations may be one impediment to the development of neutralization breadth by this antibody class42,151.
Fab-dimerized glycan bnAbs
The glycan-reactive bnAb 2G12 was isolated from an individual infected with HIV-1 in 1996 (ref.154) and shown to have a unique VH domain swap conformation that formed an ‘I-shaped’ bnAb with an expanded Fab-dimerized paratope capable of Env glycan binding, called Fab-dimerized glycan (FDG) antibodies155. Recently, Williams et al. isolated two new FDG-targeted bnAbs with the characteristic ‘I-shape’, but without the VH domain swap found in the bnAb 2G12, from SHIV-infected macaques156. Macaques immunized with a glycosylated V3 peptide and boosted with scaffolded mannose glycans expand FDG precursor antibodies, and FDG bnAbs have also been isolated from SHIV-infected monkeys (bnAbs DH851 and DH1003)156. Interestingly, analysis of human FDG precursor antibodies that were isolated with high mannose-containing ‘hooks’ demonstrated that they were predominantly of the IgM isotype and were present in the CD27+IgM+IgD– marginal zone B cell pool, which is thought to be a source of natural antibody-producing B cells157,158. In contrast, in the context of SHIV infection, FDG precursor antibodies that have bnAb activity are of the IgG isotype, whereas FDG precursor antibodies of the IgM isotype have no heterologous neutralizing activity. FDG bnAbs can target multiple glycan sites on HIV-1 Env and are, therefore, a promising component of a polyclonal bnAb response, despite the current lack of neutralization breadth of any particular FDG bnAb156. Thus, one strategy to elicit FDG antibodies (and perhaps other glycan-reactive HIV-1 bnAbs) with vaccines is to stimulate the pool of glycan-reactive natural antibodies to class-switch from IgM to IgG and become bnAbs156. Natural glycan-reactive antibodies generally develop in a T cell-independent manner158. However, as bnAbs generally develop in germinal centres with help provided by CD4+ TFH cells, these data suggest that a successful HIV-1 vaccine strategy for some types of bnAbs will be to recruit the precursors of extrafollicular natural glycan antibody-producing B cells into germinal centres to interact with TFH cells for maturation to neutralization breadth. Studies are underway to test this hypothesis, and novel vaccine delivery regimens are being explored that prolong the germinal centre response and promote CD4+ TFH cell responses159–162 (Box 4).
Box 4 Env immunogen optimization and delivery regimens.
A major advance in the HIV-1 vaccine field was the design and structural definition of a cleaved, stabilized envelope (Env) trimer by addition of the T > C mutation at position 605, A > C mutation at position 501 and I > P mutation at position 559, called the SOSIP.664 envelope gp140 (SOSIP Env trimer)238–241. SOSIP Env trimers have been used to select for broadly neutralizing antibodies (bnAbs) targeting the quaternary structure of the Env trimer apex242. It is also becoming clear that multimeric, stabilized Env immunogens decorating protein nanoparticles may be more immunogenic than a combination of Env immunogens with small-molecule adjuvants76,243,244. This is both because of improved movement of nanoparticles into germinal centres and because of the need to stabilize Env immunogens to prevent the formation of non-neutralizing, diverting epitopes that can take bnAb B cell lineages off-target245–247. For example, insufficient glycosylation of Env, resulting in so-called ‘glycan holes’, can cause this problem248–252. The base of the Env trimer also contains diverting epitopes that can impede bnAb development and promote trimer disassembly253. Thus, both stabilization and minimization of non-neutralizing, diverting epitopes are important for HIV-1 vaccine immunogen design.
Another aspect to consider in immunogen design is the fact that almost all bnAbs have high levels of mutations, suggesting that B cells either remain in germinal centres for prolonged periods of time or recirculate back into germinal centres to accumulate functional mutations required for bnAb maturation. Therefore, immunization regimens need to prolong germinal centre responses and induce CD4+ T follicular helper cells (TFH cells) to support robust bnAb development in germinal centres39,45. In this regard, it was recently demonstrated that modulating the quantity of HIV-1 Env-specific CD4+ T cell help can promote rare B cell responses in mice160. In individuals with HIV-1 infection, there is a constant supply of antigenic variants of Env that may, over time, select for rare functional bnAb mutations. This results in bnAb development over 2–5 years in approximately 50% of adults infected with HIV-127–31,33,70. Two studies have found that vaccine strategies that include delivery of antigen either by continuous administration via subcutaneous pumps or by administration of escalating doses of antigen over time result in enhanced antibody responses159,162. In macaques, such a regimen sustained germinal centre responses for up to 29 weeks, and with late boosting at 30 weeks, low titres of heterologous HIV-1 neutralizing antibodies were induced161. Finally, vaccines based on modified mRNAs in lipid nanoparticles (LNPs) potently stimulate TFH cells59,64. After intramuscular immunization, these are rapidly distributed to the liver, spleen and systemic lymph nodes, with prolonged expression of antigen over days189. Work has now begun to formulate complex stabilized Env trimer nanoparticle multimers either encoded in mRNAs or delivered as mRNA LNPs127,188, with some success reported in bnAb unmutated common ancestor (UCA) knock-in mice and in monkeys188,254. Thus, development efforts are ongoing to define the optimum platform for delivery of a multicomponent HIV-1 bnAb vaccine.
Combined T cell and B cell vaccines
T cell responses can contribute to HIV-1 vaccine efficacy in two ways. First, the induction of strong CD4+ TFH cell responses is required to support vaccine-mediated HIV-1 bnAb induction39,45. It was shown that LNP-enclosed mRNA can induce potent TFH cell responses. Moreover, ionizable LNPs can be used as an adjuvant in combination with protein vaccines to promote CD4+ T cell help59,64. Second, T cells can act as direct effectors of vaccine-mediated protection by mechanisms including cytolysis of HIV-1-infected cells by CD8+ T cells163–166. In acute HIV-1 infection, HLA class Ia-restricted T cell responses play an important role in the containment of viraemia and drive a rapid selection for HIV-1 escape mutants167, and they make a key contribution to sustained control of viral replication in HIV-1 elite controllers163. However, they fail to eradicate HIV-1 after viral integration has occurred167. Vaccines that are primarily designed to induce HLA class Ia-restricted CD8+ T cell responses have failed to prevent HIV-1 transmission to date. Although limited success has been achieved with CD8+ T cell-inducing vaccines in reducing set point viral loads after infection in preclinical models168, clinical trials conducted to date with such vaccines have not succeeded in reducing viraemia169,170. Thus, although HLA class Ia-restricted CD8+ T cells clearly have some degree of anti-HIV-1 activity in animal models, they have not yet been shown to protect against infection in the setting of vaccination in humans, nor are they able to clear infection in the setting of acute HIV-1 infection.
However, experiments in rhesus macaques have shown that a vaccine that induces HIV-1-reactive CD8+ T cells restricted by the MHC class Ib molecule MHC-E shows some protection from infection, where it eradicated simian immunodeficiency virus (SIV)-infected cells early in infection in ~55% of animals164. The vaccine that achieved this unprecedented protective effect employed a rhesus cytomegalovirus (RhCMV) vector with fortuitous gene deletion that allows it to induce MHC-E-restricted CD8+ T cells164,165,171,172. Although this vaccine did not prevent initial infection, it did eliminate virus-infected cells before a durable latent pool of infected CD4+ T cells was established, at least in ~55% of the animals. In vitro experiments have shown that it is possible to prime human HIV-1-targeted HLA-E-restricted CD8+ T cells166, and the observation that insertion of orthologous human (H)CMV genes into RhCMV generates a vector that induces the same level of protection upon SIV challenge as the original RhCMV vector in rhesus macaques172 suggests that it may be possible to develop vaccines that induce HLA-E restricted anti-HIV-1 T cells in humans. An HCMV-vectored HIV-1 vaccine is currently in development (NCT0472587).
Notably, experiments in macaques have shown that the combination of a potent T cell vaccine, which stimulates classical HLA-Ia-restricted CD8+ T cells, with a B cell vaccine that induces autologous neutralizing antibodies resulted in protection from infection at lower bnAb titres compared with the B cell vaccine alone173. Moreover, combination of a T cell immunogen with a B cell immunogen did not compromise the potency of either vaccine in an animal model174.
Therefore, the combination of a next-generation T cell vaccine with a bnAb-inducing vaccine may be more effective than either alone. Moreover, the combination of a T cell vaccine that can induce anti-HIV-1 HLA-E-restricted responses with CD4+ TFH cell-inducing and bnAb-inducing vaccine constructs may hold promise.
Outlook
Much progress has been made in B cell lineage immunogen design, germline-targeting, immunofocusing and structure-based immunogen design. Overall, the field is using an immunisation strategy where immunogens target naive BCRs, followed by sequential immunogens that select for memory B cells with BCRs with improbable mutations, these become activated, proliferate and continue to acquire functional mutations, resulting in acquisition of bnAb breadth25,42,51,76,79,81,175,176. Although the specific design of sequential Env immunogens for a practical vaccine remains to be determined, the desired attributes are now known, and there is a better understanding of the immunobiology of bnAb development and the roadblocks that prevent their induction11 (Box 1 and Fig. 1).
The requirements for a multicomponent HIV-1 vaccine that is capable of inducing bnAb lineages are daunting. Sequential immunogens need to keep bnAb maturation on track and avoid the selection of antibody mutations that interfere with binding to the Env bnAb epitope. If a single Env protein is to be used as a priming immunogen, it must bind to many diverse bnAb UCAs. Similarly, studies are underway to determine whether many steps of a sequential vaccine can be administered simultaneously to simplify vaccination regimens. As noted above, bnAb-inducing vaccines also need to elicit potent CD4+ TFH cell responses in order to stimulate high levels of highly mutated and long-lived bnAbs, and the final version of a practical HIV-1 vaccine will likely also need a component that can induce broadly cross-reactive CD8+ T cell responses to kill HIV-1-infected CD4+ T cells. A key attribute of a successful HIV-1 vaccine will be to prevent infection by the majority of circulating HIV-1 strains. HIV-1 has continued to diversify in terms of within-clade evolution177, and such increasing diversity can have an impact on the sensitivity to bnAb neutralization177,178. In addition, the complexity of new recombinant forms of HIV-1 and the diversity of clades and recombinant forms within local geographic populations are increasing9. The panels of HIV-1 pseudoviruses currently in use to evaluate vaccine-induced neutralizing antibody responses are based on older HIV-1 strains, and are in the process of being updated to better represent the contemporary diversity of HIV-1 as well as physiologically relevant transmitted/founder viruses.
Despite the utility of the bnAb UCA knock-in mouse model and SHIV challenges in rhesus macaques, studies of complex HIV-1 vaccines in humans are necessary to learn the precise rules for shaping the B cell response to disfavoured Env bnAb epitopes and for determining the response of the outbred human B cell repertoire to experimental vaccine components and full practical vaccines. To accomplish this evaluation of bnAb vaccines in vivo, the HVTN has established the HVTN Experimental Medicine Trials protocol for testing immunogens in small groups of human volunteers so that rapid vaccine design iteration can occur81. In this manner, multiple experimental vaccines can be tested in the context of the human immune system.
A protective HIV-1 vaccine will likely be the most complex vaccine ever designed, employing novel vaccine platform technologies such as modified mRNAs in LNPs or novel vectors. Early after infection, HIV-1 provirus can integrate into the host genome as latent virus without producing viral proteins179,180, becoming, in effect, invisible to the immune system. For this reason, an effective HIV-1 bnAb vaccine that aims to prevent transmission with sterilizing immunity will need to be essentially 100% effective for both blood and mucosal exposure, an extraordinary bar that no vaccine has yet achieved16,181. Finally, the production of multicomponent protein vaccines will be challenging owing to both the difficulty and time necessary to produce multiple proteins by GMP and the cost of such a vaccine. The modified mRNA and LNP technology that has been so successful for COVID-19 vaccines182,183 may be more amenable for the production of multicomponent vaccines compared to protein-based vaccines127,184–189.
The hope is that once a vaccine is designed and shown to be effective, the immune correlates of protection of a successful bnAb vaccine will be identified and the complex nature of the immunogen can be simplified and made practical for administration in countries around the globe.
Acknowledgements
The authors thank I. Pedroza-Pacheco for Fig. 1, and W. Edwards Beck and S. Devine for editorial assistance, S. Shapiro for National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID) and Division of AIDS (DAIDS) programme support and K. Cuttle for Consortia for HIV/AIDS Vaccine Development (CHAVD) management. Supported by the NIH, NIAID and DAIDS CHAVD grant UM1 AI144371, the NIH, NIAID and DAIDS grant R01 AI147778 and UM1 grants AI142596 and AI135902. P.B. and A.J.M. are Jenner Institute Investigators.
Glossary
- Transmitted/founder viruses
One or a few HIV-1 strains that establish transmission after HIV-1 mucosal exposure.
- On-rates
The rate constants (Kon or Ka) of association of an antibody to the immunogen.
- Receptor-mediated Env activation
The events that transpire subsequent to CD4 binding the HIV-1 Env.
- Partial thromboplastin time
A test that measures the time it takes for a blood sample to clot. The partial thromboplastin time can be prolonged in the presence of anti-cardiolipin antibodies.
- Fusion domain peptide
A short peptide at the amino terminus of gp41 that inserts into the host cell membrane after receptor-mediated Env activation and affects virus and host cell fusion. The fusion peptide is a target for HIV-1 broadly neutralizing antibodies (bnAbs).
- Carrier proteins
Molecules added to small non-immunogenic molecules such as peptides to make them more immunogenic. Examples of carrier proteins are keyhole limpet haemocyanin and tetanus toxoid.
- Immunofocusing
The design of immunogens to induce antibodies with a focused, defined specificity.
- Paratope
The binding region of an antibody for an antigen.
- Natural antibodies
Antibodies produced by B1 or marginal zone B cells that are present before antigen stimulation and act early in host defence prior to the adaptive, secondary B cell response.
- CD4+ TFH cell responses
(CD4+ T follicular helper cell responses). CD4+ helper T cell responses in the germinal centre that promote somatic hypermutation and affinity maturation of B cells.
- Set-point viral loads
The residual viral loads that result in chronic HIV-1 infection after the peak viral load that occurs during acute HIV-1 infection.
- Immune correlates of protection
The immune responses induced by a vaccine that are responsible for vaccine efficacy.
Author contributions
B.F.H. researched and wrote the first draft of the paper. All authors contributed to the content and edited the manuscript.
Peer review
Peer review information
Nature Reviews Immunology thanks the anonymous reviewers for their contribution to the peer review of this work.
Competing interests
K.O.S. and B.F.H. are inventors on International Patent Application PCT/US2018/020788 submitted by Duke University that covers the composition and use of CH848 HIV-1 Envs for induction of HIV-1 antibodies. K.O.S., K.W. and B.F.H. are inventors on International Patent Application PCT/US2018/03477 submitted by Duke University that covers the composition and use of CH505 HIV-1 Envs for induction of HIV-1 antibodies.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
3/1/2023
A Correction to this paper has been published: 10.1038/s41577-023-00854-0
References
- 1.Barré-Sinoussi F, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 2.Gallo RC, et al. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984;224:500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- 3.Popovic M, Sarngadharan MG, Read E, Gallo RC. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984;224:497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- 4.Sarngadharan MG, Popovic M, Bruch L, Schüpbach J, Gallo RC. Antibodies reactive with human T-lymphotropic retroviruses (HTLV-III) in the serum of patients with AIDS. Science. 1984;224:506–508. doi: 10.1126/science.6324345. [DOI] [PubMed] [Google Scholar]
- 5.Saag MS, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2020 recommendations of the International Antiviral Society-USA panel. JAMA. 2020;324:1651–1669. doi: 10.1001/jama.2020.17025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.UNAIDS. Fact sheet 2021. UNAIDShttps://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf (2021).
- 7.Kumi Smith M, Jewell BL, Hallett TB, Cohen MS. Treatment of HIV for the prevention of transmission in discordant couples and at the population level. Adv. Exp. Med. Biol. 2018;1075:125–162. doi: 10.1007/978-981-13-0484-2_6. [DOI] [PubMed] [Google Scholar]
- 8.Cohen MS, Gamble T, McCauley M. Prevention of HIV transmission and the HPTN 052 study. Annu. Rev. Med. 2020;71:347–360. doi: 10.1146/annurev-med-110918-034551. [DOI] [PubMed] [Google Scholar]
- 9.Fischer W, et al. HIV-1 and SARS-CoV-2: patterns in the evolution of two pandemic pathogens. Cell Host Microbe. 2021;29:1093–1110. doi: 10.1016/j.chom.2021.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korber B, Hraber P, Wagh K, Hahn BH. Polyvalent vaccine approaches to combat HIV-1 diversity. Immunol. Rev. 2017;275:230–244. doi: 10.1111/imr.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haynes BF, Verkoczy L. Host controls of HIV neutralizing antibodies. Science. 2014;344:588–589. doi: 10.1126/science.1254990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelsoe G, Haynes BF. Host controls of HIV broadly neutralizing antibody development. Immunol. Rev. 2017;275:79–88. doi: 10.1111/imr.12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirchhoff F. Immune evasion and counteraction of restriction factors by HIV-1 and other primate lentiviruses. Cell Host Microbe. 2010;8:55–67. doi: 10.1016/j.chom.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe Y, Bowden TA, Wilson IA, Crispin M. Exploitation of glycosylation in enveloped virus pathobiology. Biochim. Biophys. Acta Gen. Subj. 2019;1863:1480–1497. doi: 10.1016/j.bbagen.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitney JB, et al. Prevention of SIVmac251 reservoir seeding in rhesus monkeys by early antiretroviral therapy. Nat. Commun. 2018;9:5429. doi: 10.1038/s41467-018-07881-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes BF. The immune response during acute HIV-1 infection: clues for vaccine development. Nat. Rev. Immunol. 2010;10:11–23. doi: 10.1038/nri2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang Y, et al. Effect of rAd5-vector HIV-1 preventive vaccines on HIV-1 acquisition: a participant-level meta-analysis of randomized trials. PLoS ONE. 2015;10:e0136626. doi: 10.1371/journal.pone.0136626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gray GE, et al. Vaccine efficacy of ALVAC-HIV and bivalent subtype C gp120-MF59 in adults. N. Engl. J. Med. 2021;384:1089–1100. doi: 10.1056/NEJMoa2031499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson & Johnson and Global Partners. Johnson & Johnson and Global Partners announce results from phase 2b Imbokodo HIV vaccine clinical trial in young women in sub-Saharan Africa; https://www.jnj.com/johnson-johnson-and-global-partners-announce-results-from-phase-2b-imbokodo-hiv-vaccine-clinical-trial-in-young-women-in-sub-saharan-africa (2021).
- 20.Rerks-Ngarm S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 21.Haynes BF, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 2012;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plotkin SA. Updates on immunologic correlates of vaccine-induced protection. Vaccine. 2020;38:2250–2257. doi: 10.1016/j.vaccine.2019.10.046. [DOI] [PubMed] [Google Scholar]
- 23.Burton DR. Advancing an HIV vaccine; advancing vaccinology. Nat. Rev. Immunol. 2019;19:77–78. doi: 10.1038/s41577-018-0103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burton DR, et al. A blueprint for HIV vaccine discovery. Cell Host Microbe. 2012;12:396–407. doi: 10.1016/j.chom.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haynes BF, Kelsoe G, Harrison SC, Kepler TB. B-cell-lineage immunogen design in vaccine development with HIV-1 as a case study. Nat. Biotechnol. 2012;30:423–433. doi: 10.1038/nbt.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haynes BF, et al. HIV–host interactions: implications for vaccine design. Cell Host Microbe. 2016;19:292–303. doi: 10.1016/j.chom.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray ES, et al. The neutralization breadth of HIV-1 develops incrementally over four years and is associated with CD4+ T cell decline and high viral load during acute infection. J. Virol. 2011;85:4828–4840. doi: 10.1128/JVI.00198-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hraber P, et al. Prevalence of broadly neutralizing antibody responses during chronic HIV-1 infection. AIDS. 2014;28:163–169. doi: 10.1097/QAD.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mikell I, et al. Characteristics of the earliest cross-neutralizing antibody response to HIV-1. PLoS Pathog. 2011;7:e1001251. doi: 10.1371/journal.ppat.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simek MD, et al. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J. Virol. 2009;83:7337–7348. doi: 10.1128/JVI.00110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomaras GD, et al. Polyclonal B cell responses to conserved neutralization epitopes in a subset of HIV-1-infected individuals. J. Virol. 2011;85:11502–11519. doi: 10.1128/JVI.05363-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao F, et al. Cooperation of B cell lineages in induction of HIV-1-broadly neutralizing antibodies. Cell. 2014;158:481–491. doi: 10.1016/j.cell.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liao HX, et al. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature. 2013;496:469–476. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams LD, et al. Potent and broad HIV-neutralizing antibodies in memory B cells and plasma. Sci. Immunol. 2017 doi: 10.1126/sciimmunol.aal2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonsignori M, et al. Two distinct broadly neutralizing antibody specificities of different clonal lineages in a single HIV-1-infected donor: implications for vaccine design. J. Virol. 2012;86:4688–4692. doi: 10.1128/JVI.07163-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sajadi MM. Identification of near-pan-neutralizing antibodies against HIV-1 by deconvolution of plasma humoral responses. Cell. 2018;173:1783–1795. doi: 10.1016/j.cell.2018.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nduati EW, et al. Coordinated Fc-effector and neutralization functions in HIV-infected children define a window of opportunity for HIV vaccination. Aids. 2021;35:1895–1905. doi: 10.1097/QAD.0000000000002976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simonich CA, et al. HIV-1 neutralizing antibodies with limited hypermutation from an infant. Cell. 2016;166:77–87. doi: 10.1016/j.cell.2016.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moody MA, et al. Immune perturbations in HIV-1-infected individuals who make broadly neutralizing antibodies. Sci. Immunol. 2016;1:aag0851. doi: 10.1126/sciimmunol.aag0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haynes BF, et al. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308:1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- 41.Mouquet H, et al. Polyreactivity increases the apparent affinity of anti-HIV antibodies by heteroligation. Nature. 2010;467:591–595. doi: 10.1038/nature09385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiehe K, et al. Functional relevance of improbable antibody mutations for HIV broadly neutralizing antibody development. Cell Host Microbe. 2018;23:759–765.e6. doi: 10.1016/j.chom.2018.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore PL, Williamson C, Morris L. Virological features associated with the development of broadly neutralizing antibodies to HIV-1. Trends Microbiol. 2015;23:204–211. doi: 10.1016/j.tim.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bradley T, et al. RAB11FIP5 expression and altered natural killer cell function are associated with induction of HIV broadly neutralizing antibody responses. Cell. 2018;175:387–399.e17. doi: 10.1016/j.cell.2018.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Locci M, et al. Human circulating PD-1+CXCR3–CXCR5+ memory TFH cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity. 2013;39:758–769. doi: 10.1016/j.immuni.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roskin KM, et al. Aberrant B cell repertoire selection associated with HIV neutralizing antibody breadth. Nat. Immunol. 2020;21:199–209. doi: 10.1038/s41590-019-0581-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mouquet H, Nussenzweig MC. Polyreactive antibodies in adaptive immune responses to viruses. Cell Mol. Life Sci. 2012;69:1435–1445. doi: 10.1007/s00018-011-0872-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elkon K, Casali P. Nature and functions of autoantibodies. Nat. Clin. Pract. Rheumatol. 2008;4:491–498. doi: 10.1038/ncprheum0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wardemann H, et al. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 50.Havenar-Daughton C, et al. The human naive B cell repertoire contains distinct subclasses for a germline-targeting HIV-1 vaccine immunogen. Sci. Transl. Med. 2018 doi: 10.1126/scitranslmed.aat0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steichen JM, et al. A generalized HIV vaccine design strategy for priming of broadly neutralizing antibody responses. Science. 2019 doi: 10.1126/science.aax4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Willis JR, et al. Long antibody HCDR3s from HIV-naive donors presented on a PG9 neutralizing antibody background mediate HIV neutralization. Proc. Natl Acad. Sci. USA. 2016;113:4446–4451. doi: 10.1073/pnas.1518405113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Medina-Ramírez M, et al. Design and crystal structure of a native-like HIV-1 envelope trimer that engages multiple broadly neutralizing antibody precursors in vivo. J. Exp. Med. 2017;214:2573–2590. doi: 10.1084/jem.20161160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slifka MK, Ahmed R. Long-lived plasma cells: a mechanism for maintaining persistent antibody production. Curr. Opin. Immunol. 1998;10:252–258. doi: 10.1016/S0952-7915(98)80162-3. [DOI] [PubMed] [Google Scholar]
- 55.Scheid JF, et al. Differential regulation of self-reactivity discriminates between IgG+ human circulating memory B cells and bone marrow plasma cells. Proc. Natl Acad. Sci. USA. 2011;108:18044–18048. doi: 10.1073/pnas.1113395108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bonsignori M, et al. HIV-1 envelope induces memory B cell responses that correlate with plasma antibody levels after envelope gp120 protein vaccination or HIV-1 infection. J. Immunol. 2009;183:2708–2717. doi: 10.4049/jimmunol.0901068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morris L, et al. HIV-1 antigen-specific and -nonspecific B cell responses are sensitive to combination antiretroviral therapy. J. Exp. Med. 1998;188:233–245. doi: 10.1084/jem.188.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blasi M, et al. Immunogenicity, safety, and efficacy of sequential immunizations with an SIV-based IDLV expressing CH505 Envs. NPJ Vaccines. 2020;5:107. doi: 10.1038/s41541-020-00252-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pardi N, et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J. Exp. Med. 2018;215:1571–1588. doi: 10.1084/jem.20171450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pardi N, et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature. 2017;543:248–251. doi: 10.1038/nature21428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saunders KO, et al. Lipid nanoparticle encapsulated nucleoside-modified mRNA vaccines elicit polyfunctional HIV-1 antibodies comparable to proteins in nonhuman primates. NPJ Vaccines. 2021;6:50. doi: 10.1038/s41541-021-00307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kasturi SP, et al. 3M-052, a synthetic TLR-7/8 agonist, induces durable HIV-1 envelope-specific plasma cells and humoral immunity in nonhuman primates. Sci. Immunol. 2020 doi: 10.1126/sciimmunol.abb1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lederer K, et al. SARS-CoV-2 mRNA vaccines foster potent antigen-specific germinal center responses associated with neutralizing antibody generation. Immunity. 2020;53:1281–1295.e5. doi: 10.1016/j.immuni.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alameh MG, et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity. 2021;54:2877–2892.e7. doi: 10.1016/j.immuni.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bonsignori M, et al. Maturation pathway from germline to broad HIV-1 neutralizer of a CD4-mimic antibody. Cell. 2016;165:449–463. doi: 10.1016/j.cell.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hwang JK, et al. Sequence intrinsic somatic mutation mechanisms contribute to affinity maturation of VRC01-class HIV-1 broadly neutralizing antibodies. Proc. Natl Acad. Sci. USA. 2017;114:8614–8619. doi: 10.1073/pnas.1709203114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yeap LS, et al. Sequence-intrinsic mechanisms that target AID mutational outcomes on antibody genes. Cell. 2015;163:1124–1137. doi: 10.1016/j.cell.2015.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Betz AG, Neuberger MS, Milstein C. Discriminating intrinsic and antigen-selected mutational hotspots in immunoglobulin V genes. Immunol. Today. 1993;14:405–411. doi: 10.1016/0167-5699(93)90144-A. [DOI] [PubMed] [Google Scholar]
- 69.Neuberger MS, et al. Monitoring and interpreting the intrinsic features of somatic hypermutation. Immunol. Rev. 1998;162:107–116. doi: 10.1111/j.1600-065X.1998.tb01434.x. [DOI] [PubMed] [Google Scholar]
- 70.Bonsignori M, et al. Staged induction of HIV-1 glycan-dependent broadly neutralizing antibodies. Sci. Transl. Med. 2017 doi: 10.1126/scitranslmed.aai7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bonsignori M, et al. Antibody–virus co-evolution in HIV infection: paths for HIV vaccine development. Immunol. Rev. 2017;275:145–160. doi: 10.1111/imr.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Doria-Rose NA, et al. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature. 2014;509:55–62. doi: 10.1038/nature13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.MacLeod DT, et al. Early antibody lineage diversification and independent limb maturation lead to broad HIV-1 neutralization targeting the Env high-mannose patch. Immunity. 2016;44:1215–1226. doi: 10.1016/j.immuni.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McCoy LE, Burton DR. Identification and specificity of broadly neutralizing antibodies against HIV. Immunol. Rev. 2017;275:11–20. doi: 10.1111/imr.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Umotoy J, et al. Rapid and focused maturation of a VRC01-class HIV broadly neutralizing antibody lineage involves both binding and accommodation of the N276-glycan. Immunity. 2019;51:141–154.e6. doi: 10.1016/j.immuni.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saunders KO, et al. Targeted selection of HIV-specific antibody mutations by engineering B cell maturation. Science. 2019 doi: 10.1126/science.aay7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jardine JG, et al. HIV-1 broadly neutralizing antibody precursor B cells revealed by germline-targeting immunogen. Science. 2016;351:1458–1463. doi: 10.1126/science.aad9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stamatatos L, Pancera M, McGuire AT. Germline-targeting immunogens. Immunol. Rev. 2017;275:203–216. doi: 10.1111/imr.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kepler TB, et al. Reconstructing a B-cell clonal lineage. II. Mutation, selection, and affinity maturation. Front. Immunol. 2014;5:170. doi: 10.3389/fimmu.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Keele BF, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl Acad. Sci. USA. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Haynes BF, Burton DR, Mascola JR. Multiple roles for HIV broadly neutralizing antibodies. Sci. Transl. Med. 2019 doi: 10.1126/scitranslmed.aaz2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jardine J, et al. Rational HIV immunogen design to target specific germline B cell receptors. Science. 2013;340:711–716. doi: 10.1126/science.1234150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Batista FD, Neuberger MS. Affinity dependence of the B cell response to antigen: a threshold, a ceiling, and the importance of off-rate. Immunity. 1998;8:751–759. doi: 10.1016/S1074-7613(00)80580-4. [DOI] [PubMed] [Google Scholar]
- 84.Kong R, et al. Antibody lineages with vaccine-induced antigen-binding hotspots develop broad HIV neutralization. Cell. 2019;178:567–584.e19. doi: 10.1016/j.cell.2019.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kelsoe G, Haynes BF. What are the primary limitations in B-cell affinity maturation, and how much affinity maturation can we drive with vaccination? Breaking through immunit’s glass ceiling. Cold Spring Harb. Perspect. Biol. 2018 doi: 10.1101/cshperspect.a029397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kepler TB, et al. Immunoglobulin gene insertions and deletions in the affinity maturation of HIV-1 broadly reactive neutralizing antibodies. Cell Host Microbe. 2014;16:304–313. doi: 10.1016/j.chom.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang R, et al. Initiation of immune tolerance-controlled HIV gp41 neutralizing B cell lineages. Sci. Transl. Med. 2016;8:336ra362. doi: 10.1126/scitranslmed.aaf0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Henderson R, et al. Selection of immunoglobulin elbow region mutations impacts interdomain conformational flexibility in HIV-1 broadly neutralizing antibodies. Nat. Commun. 2019;10:654. doi: 10.1038/s41467-019-08415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hossain MA, et al. B cells discriminate HIV-1 Envelope protein affinities by sensing antigen binding association rates. Cell Rep. 2022 doi: 10.1016/j.celrep.2022.111021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McGuire AT, et al. Engineering HIV envelope protein to activate germline B cell receptors of broadly neutralizing anti-CD4 binding site antibodies. J. Exp. Med. 2013;210:655–663. doi: 10.1084/jem.20122824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Burton DR. What are the most powerful immunogen design vaccine strategies? Reverse vaccinology 2.0 shows great promise. Cold Spring Harb. Perspect. Biol. 2017 doi: 10.1101/cshperspect.a030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Verkoczy L, et al. Induction of HIV-1 broad neutralizing antibodies in 2F5 knock-in mice: selection against membrane proximal external region-associated autoreactivity limits T-dependent responses. J. Immunol. 2013;191:2538–2550. doi: 10.4049/jimmunol.1300971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Williams WB, et al. Initiation of HIV neutralizing B cell lineages with sequential envelope immunizations. Nat. Commun. 2017;8:1732. doi: 10.1038/s41467-017-01336-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sabouri Z, et al. Redemption of autoantibodies on anergic B cells by variable-region glycosylation and mutation away from self-reactivity. Proc. Natl Acad. Sci. USA. 2014;111:E2567–E2575. doi: 10.1073/pnas.1406974111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reed JH, Jackson J, Christ D, Goodnow CC. Clonal redemption of autoantibodies by somatic hypermutation away from self-reactivity during human immunization. J. Exp. Med. 2016;213:1255–1265. doi: 10.1084/jem.20151978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dennison SM, et al. Induction of antibodies in rhesus macaques that recognize a fusion-intermediate conformation of HIV-1 gp41. pLoS ONE. 2011;6:e27824. doi: 10.1371/journal.pone.0027824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Haynes BF. SARS-CoV-2 and HIV-1 — a tale of two vaccines. Nat. Rev. Immunol. 2021;21:543–544. doi: 10.1038/s41577-021-00589-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou T, et al. Multidonor analysis reveals structural elements, genetic determinants, and maturation pathway for HIV-1 neutralization by VRC01-class antibodies. Immunity. 2013;39:245–258. doi: 10.1016/j.immuni.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee JH, et al. Vaccine genetics of IGHV1-2 VRC01-class broadly neutralizing antibody precursor naive human B cells. NPJ Vaccines. 2021;6:113. doi: 10.1038/s41541-021-00376-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu X, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou T, et al. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science. 2010;329:811–817. doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gristick HB, et al. Natively glycosylated HIV-1 Env structure reveals new mode for antibody recognition of the CD4-binding site. Nat. Struct. Mol. Biol. 2016;23:906–915. doi: 10.1038/nsmb.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bonsignori M, et al. Inference of the HIV-1 VRC01 antibody lineage unmutated common ancestor reveals alternative pathways to overcome a key glycan barrier. Immunity. 2018;49:1162–1174.e8. doi: 10.1016/j.immuni.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lin YR, et al. HIV-1 VRC01 germline-targeting immunogens select distinct epitope-specific B cell receptors. Immunity. 2020;53:840–851.e6. doi: 10.1016/j.immuni.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Huang D, et al. B cells expressing authentic naive human VRC01-class BCRs can be recruited to germinal centers and affinity mature in multiple independent mouse models. Proc. Natl Acad. Sci. USA. 2020;117:22920–22931. doi: 10.1073/pnas.2004489117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tian M, et al. Induction of HIV neutralizing antibody lineages in mice with diverse precursor repertoires. Cell. 2016;166:1471–1484.e18. doi: 10.1016/j.cell.2016.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen X, et al. Vaccination induces maturation in a mouse model of diverse unmutated VRC01-class precursors to HIV-neutralizing antibodies with >50% breadth. Immunity. 2021;54:324–339.e8. doi: 10.1016/j.immuni.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Havenar-Daughton C, et al. Rapid germinal center and antibody responses in non-human primates after a single nanoparticle vaccine immunization. Cell Rep. 2019;29:1756–1766.e8. doi: 10.1016/j.celrep.2019.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.IAVI. First-in-human clinical trial confirms novel HIV vaccine approach developed by IAVI and Scripps Research. IAVIhttps://www.iavi.org/news-resources/press-releases/2021/first-in-human-clinical-trial-confirms-novel-hiv-vaccine-approach-developed-by-iavi-and-scripps-research (2021). This work is the press release for a clinical trial of induction of HIV-1 CD4 binding site precursors in humans.
- 110.McGuire AT, et al. Specifically modified Env immunogens activate B-cell precursors of broadly neutralizing HIV-1 antibodies in transgenic mice. Nat. Commun. 2016;7:10618. doi: 10.1038/ncomms10618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Corey L, et al. Two randomized trials of neutralizing antibodies to prevent HIV-1 acquisition. N. Engl. J. Med. 2021;384:1003–1014. doi: 10.1056/NEJMoa2031738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gilbert PB, et al. Basis and statistical design of the passive HIV-1 antibody mediated prevention (AMP) test-of-concept efficacy trials. Stat. Commun. Infect. Dis. 2017 doi: 10.1515/scid-2016-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Magaret CA, et al. Prediction of VRC01 neutralization sensitivity by HIV-1 gp160 sequence features. pLoS Comput. Biol. 2019;15:e1006952. doi: 10.1371/journal.pcbi.1006952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kong R, et al. Improving neutralization potency and breadth by combining broadly reactive HIV-1 antibodies targeting major neutralization epitopes. J. Virol. 2015;89:2659–2671. doi: 10.1128/JVI.03136-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wagh K, et al. Optimal combinations of broadly neutralizing antibodies for prevention and treatment of HIV-1 clade C infection. PLoS Pathog. 2016;12:e1005520. doi: 10.1371/journal.ppat.1005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chuang GY, et al. Residue-level prediction of HIV-1 antibody epitopes based on neutralization of diverse viral strains. J. Virol. 2013;87:10047–10058. doi: 10.1128/JVI.00984-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schommers P, et al. Restriction of HIV-1 escape by a highly broad and potent neutralizing antibody. Cell. 2020;180:471–489.e22. doi: 10.1016/j.cell.2020.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Scheid JF, et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333:1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhou T, et al. Structural repertoire of HIV-1-neutralizing antibodies targeting the CD4 supersite in 14 donors. Cell. 2015;161:1280–1292. doi: 10.1016/j.cell.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.LaBranche CC, et al. Neutralization-guided design of HIV-1 envelope trimers with high affinity for the unmutated common ancestor of CH235 lineage CD4bs broadly neutralizing antibodies. pLoS Pathog. 2019;15:e1008026. doi: 10.1371/journal.ppat.1008026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Saunders, K. O. et al. Protective HIV-1 neutralizing antibody titers and epitope specificities in vaccinated macaques Sci. Transl. Med. (in press).
- 122.Daniels CN, Saunders KO. Antibody responses to the HIV-1 envelope high mannose patch. Adv. Immunol. 2019;143:11–73. doi: 10.1016/bs.ai.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Doores KJ, et al. Two classes of broadly neutralizing antibodies within a single lineage directed to the high-mannose patch of HIV envelope. J. Virol. 2015;89:1105–1118. doi: 10.1128/JVI.02905-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Verkoczy L. Humanized immunoglobulin mice: models for HIV vaccine testing and studying the broadly neutralizing antibody problem. Adv. Immunol. 2017;134:235–352. doi: 10.1016/bs.ai.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Meffre E, et al. Immunoglobulin heavy chain expression shapes the B cell receptor repertoire in human B cell development. J. Clin. Invest. 2001;108:879–886. doi: 10.1172/JCI13051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Steichen JM, et al. HIV vaccine design to target germline precursors of glycan-dependent broadly neutralizing antibodies. Immunity. 2016;45:483–496. doi: 10.1016/j.immuni.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mu Z, et al. mRNA-encoded HIV-1 Env trimer ferritin nanoparticles induce monoclonal antibodies that neutralize heterologous HIV-1 isolates in mice. Cell Rep. 2022;38:110514. doi: 10.1016/j.celrep.2022.110514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Escolano A, et al. Sequential immunization elicits broadly neutralizing anti-HIV-1 antibodies in Ig knockin mice. Cell. 2016;166:1445–1458.e12. doi: 10.1016/j.cell.2016.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Escolano A, et al. Immunization expands B cells specific to HIV-1 V3 glycan in mice and macaques. Nature. 2019;570:468–473. doi: 10.1038/s41586-019-1250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Escolano A, et al. Sequential immunization of macaques elicits heterologous neutralizing antibodies targeting the V3-glycan patch of HIV-1 Env. Sci. Transl. Med. 2021;13:eabk1533. doi: 10.1126/scitranslmed.abk1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Andrabi R, et al. Identification of common features in prototype broadly neutralizing antibodies to HIV envelope V2 apex to facilitate vaccine design. Immunity. 2015;43:959–973. doi: 10.1016/j.immuni.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bonsignori M, et al. Analysis of a clonal lineage of HIV-1 envelope V2/V3 conformational epitope-specific broadly neutralizing antibodies and their inferred unmutated common ancestors. J. Virol. 2011;85:9998–10009. doi: 10.1128/JVI.05045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gorman J, et al. Structures of HIV-1 Env V1V2 with broadly neutralizing antibodies reveal commonalities that enable vaccine design. Nat. Struct. Mol. Biol. 2016;23:81–90. doi: 10.1038/nsmb.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Walker LM, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Andrabi R, et al. The chimpanzee SIV envelope trimer: structure and deployment as an HIV vaccine template. Cell Rep. 2019;27:2426–2441.e6. doi: 10.1016/j.celrep.2019.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Saunders KO, et al. Vaccine induction of heterologous tier 2 HIV-1 neutralizing antibodies in animal models. Cell Rep. 2017;21:3681–3690. doi: 10.1016/j.celrep.2017.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Landais E, et al. Broadly neutralizing antibody responses in a large longitudinal sub-Saharan HIV primary infection cohort. PLoS Pathog. 2016;12:e1005369. doi: 10.1371/journal.ppat.1005369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Roark RS, et al. Recapitulation of HIV-1 Env-antibody coevolution in macaques leading to neutralization breadth. Science. 2021 doi: 10.1126/science.abd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Huang J, et al. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature. 2012;491:406–412. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang L, et al. An MPER antibody neutralizes HIV-1 using germline features shared among donors. Nat. Commun. 2019;10:5389. doi: 10.1038/s41467-019-12973-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Haynes BF, Nicely NI, Alam SM. HIV-1 autoreactive antibodies: are they good or bad for HIV-1 prevention? Nat. Struct. Mol. Biol. 2010;17:543–545. doi: 10.1038/nsmb0510-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yang G, et al. Identification of autoantigens recognized by the 2F5 and 4E10 broadly neutralizing HIV-1 antibodies. J. Exp. Med. 2013;210:241–256. doi: 10.1084/jem.20121977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Doyle-Cooper C, et al. Immune tolerance negatively regulates B cells in knock-in mice expressing broadly neutralizing HIV antibody 4E10. J. Immunol. 2013;191:3186–3191. doi: 10.4049/jimmunol.1301285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Verkoczy L, et al. Autoreactivity in an HIV-1 broadly reactive neutralizing antibody variable region heavy chain induces immunologic tolerance. Proc. Natl Acad. Sci. USA. 2010;107:181–186. doi: 10.1073/pnas.0912914107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Finney J, et al. Cross-reactivity to kynureninase tolerizes B cells that express the HIV-1 broadly neutralizing antibody 2F5. J. Immunol. 2019;203:3268–3281. doi: 10.4049/jimmunol.1900069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen Y, et al. Common tolerance mechanisms, but distinct cross-reactivities associated with gp41 and lipids, limit production of HIV-1 broad neutralizing antibodies 2F5 and 4E10. J. Immunol. 2013;191:1260–1275. doi: 10.4049/jimmunol.1300770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mehandru S, et al. Adjunctive passive immunotherapy in human immunodeficiency virus type 1-infected individuals treated with antiviral therapy during acute and early infection. J. Virol. 2007;81:11016–11031. doi: 10.1128/JVI.01340-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kong R, et al. Fusion peptide of HIV-1 as a site of vulnerability to neutralizing antibody. Science. 2016;352:828–833. doi: 10.1126/science.aae0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xu K, et al. Epitope-based vaccine design yields fusion peptide-directed antibodies that neutralize diverse strains of HIV-1. Nat. Med. 2018;24:857–867. doi: 10.1038/s41591-018-0042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ou L, et al. Preclinical development of a fusion peptide conjugate as an HIV vaccine immunogen. Sci. Rep. 2020;10:3032. doi: 10.1038/s41598-020-59711-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shen CH, et al. VRC34-antibody lineage development reveals how a required rare mutation shapes the maturation of a broad HIV-neutralizing lineage. Cell Host Microbe. 2020;27:531–543.e6. doi: 10.1016/j.chom.2020.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Brouwer PJM, et al. Immunofocusing and enhancing autologous tier-2 HIV-1 neutralization by displaying Env trimers on two-component protein nanoparticles. NPJ Vaccines. 2021;6:24. doi: 10.1038/s41541-021-00285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pantophlet R, Burton DR. Immunofocusing: antigen engineering to promote the induction of HIV-neutralizing antibodies. Trends Mol. Med. 2003;9:468–473. doi: 10.1016/j.molmed.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 154.Trkola A, et al. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 1996;70:1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Calarese DA, et al. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science. 2003;300:2065–2071. doi: 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- 156.Williams WB, et al. Fab-dimerized glycan-reactive antibodies are a structural category of natural antibodies. Cell. 2021;184:2955–2972.e25. doi: 10.1016/j.cell.2021.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Weill JC, Weller S, Reynaud CA. Human marginal zone B cells. Annu. Rev. Immunol. 2009;27:267–285. doi: 10.1146/annurev.immunol.021908.132607. [DOI] [PubMed] [Google Scholar]
- 158.Holodick NE, Rodríguez-Zhurbenko N, Hernández AM. Defining natural antibodies. Front. Immunol. 2017;8:872. doi: 10.3389/fimmu.2017.00872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cirelli KM, et al. Slow delivery immunization enhances HIV neutralizing antibody and germinal center responses via modulation of immunodominance. Cell. 2020;180:206. doi: 10.1016/j.cell.2019.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lee JH, et al. Modulating the quantity of HIV Env-specific CD4 T cell help promotes rare B cell responses in germinal centers. J. Exp. Med. 2021 doi: 10.1084/jem.20201254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lee JH, et al. Long-lasting germinal center responses to a priming immunization with continuous proliferation and somatic mutation. bioRxiv. 2021 doi: 10.1101/2021.12.20.473537. [DOI] [Google Scholar]
- 162.Tam HH, et al. Sustained antigen availability during germinal center initiation enhances antibody responses to vaccination. Proc. Natl Acad. Sci. USA. 2016;113:E6639–E6648. doi: 10.1073/pnas.1606050113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Collins DR, Gaiha GD, Walker BD. CD8+ T cells in HIV control, cure and prevention. Nat. Rev. Immunol. 2020;20:471–482. doi: 10.1038/s41577-020-0274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Picker LJ, Hansen SG, Lifson JD. New paradigms for HIV/AIDS vaccine development. Annu. Rev. Med. 2012;63:95–111. doi: 10.1146/annurev-med-042010-085643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Walker BD. CMV, MHC-E, and the quest for an unconventional AIDS vaccine. Sci. Immunol. 2021 doi: 10.1126/sciimmunol.abi5830. [DOI] [PubMed] [Google Scholar]
- 166.Yang H, et al. HLA-E-restricted, Gag-specific CD8+ T cells can suppress HIV-1 infection, offering vaccine opportunities. Sci. Immunol. 2021 doi: 10.1126/sciimmunol.abg1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Goonetilleke N, et al. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J. Exp. Med. 2009;206:1253–1272. doi: 10.1084/jem.20090365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Liu J, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009;457:87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Buchbinder SP, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gray GE, et al. Safety and efficacy of the HVTN 503/Phambili study of a clade-B-based HIV-1 vaccine in South Africa: a double-blind, randomised, placebo-controlled test-of-concept phase 2b study. Lancet Infect. Dis. 2011;11:507–515. doi: 10.1016/S1473-3099(11)70098-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Verweij MC, et al. Modulation of MHC-E transport by viral decoy ligands is required for RhCMV/SIV vaccine efficacy. Science. 2021 doi: 10.1126/science.abe9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Malouli D, et al. Cytomegaloviral determinants of CD8+ T cell programming and RhCMV/SIV vaccine efficacy. Sci. Immunol. 2021 doi: 10.1126/sciimmunol.abg5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Arunachalam PS, et al. T cell-inducing vaccine durably prevents mucosal SHIV infection even with lower neutralizing antibody titers. Nat. Med. 2020;26:932–940. doi: 10.1038/s41591-020-0858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wee EG, et al. Parallel induction of CH505 B cell ontogeny-guided neutralizing antibodies and tHIVconsvX conserved mosaic-specific T cells against HIV-1. Mol. Ther. Methods Clin. Dev. 2019;14:148–160. doi: 10.1016/j.omtm.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Dimitrov DS. Therapeutic antibodies, vaccines and antibodyomes. mAbs. 2010;2:347–356. doi: 10.4161/mabs.2.3.11779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chen, W. et al. in AIDS Vaccine 2008 Vol. 24 11–12 Abstract 0A03-03 (AIDS Research and Human Retroviruses, 2008).
- 177.Rademeyer C, et al. Features of recently transmitted HIV-1 clade C viruses that impact antibody recognition: implications for active and passive immunization. PLoS Pathog. 2016;12:e1005742. doi: 10.1371/journal.ppat.1005742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hraber P, et al. Impact of clade, geography, and age of the epidemic on HIV-1 neutralization by antibodies. J. Virol. 2014;88:12623–12643. doi: 10.1128/JVI.01705-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Coffin JM, Hughes SH. Clonal expansion of infected CD4+ T cells in people living with HIV. Viruses. 2021 doi: 10.3390/v13102078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Margolis DM, Garcia JV, Hazuda DJ, Haynes BF. Latency reversal and viral clearance to cure HIV-1. Science. 2016;353:aaf6517. doi: 10.1126/science.aaf6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cohen MS, Shaw GM, McMichael AJ, Haynes BF. Acute HIV-1 infection. N. Engl. J. Med. 2011;364:1943–1954. doi: 10.1056/NEJMra1011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Baden LR, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Polack FP, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Buschmann MD, et al. Nanomaterial delivery systems for mRNA vaccines. Vaccines. 2021 doi: 10.3390/vaccines9010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chaudhary N, Weissman D, Whitehead KA. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat. Rev. Drug Discov. 2021 doi: 10.1038/s41573-021-00283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tombácz I, Weissman D, Pardi N. Vaccination with messenger RNA: a promising alternative to DNA vaccination. Methods Mol. Biol. 2021;2197:13–31. doi: 10.1007/978-1-0716-0872-2_2. [DOI] [PubMed] [Google Scholar]
- 187.Whitley J, et al. Development of mRNA manufacturing for vaccines and therapeutics: mRNA platform requirements and development of a scalable production process to support early phase clinical trials. Transl. Res. 2022;242:38–55. doi: 10.1016/j.trsl.2021.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mu Z, Haynes BF, Cain DW. HIV mRNA vaccines — progress and future paths. Vaccines. 2021 doi: 10.3390/vaccines9020134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Pardi N, et al. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Control. Release. 2015;217:345–351. doi: 10.1016/j.jconrel.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Flynn NM, et al. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J. Infect. Dis. 2005;191:654–665. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 191.Gilbert P, et al. Magnitude and breadth of a nonprotective neutralizing antibody response in an efficacy trial of a candidate HIV-1 gp120 vaccine. J. Infect. Dis. 2010;202:595–605. doi: 10.1086/654816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gilbert PB, et al. Correlation between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 infection in a phase 3 HIV-1 preventive vaccine trial. J. Infect. Dis. 2005;191:666–677. doi: 10.1086/428405. [DOI] [PubMed] [Google Scholar]
- 193.Montefiori DC, et al. Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials. J. Infect. Dis. 2012;206:431–441. doi: 10.1093/infdis/jis367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Pitisuttithum P, et al. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J. Infect. Dis. 2006;194:1661–1671. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- 195.Sterrett S, et al. Low multiplicity of HIV-1 infection and no vaccine enhancement in VAX003 injection drug users. Open Forum Infect. Dis. 2014;1:ofu056. doi: 10.1093/ofid/ofu056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Boppana S, et al. HLA-I associated adaptation dampens CD8 T-cell responses in HIV Ad5-vectored vaccine recipients. J. Infect. Dis. 2019;220:1620–1628. doi: 10.1093/infdis/jiz368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Curlin ME, et al. Long-term mucosal T cell activation and homing phenotypes in recipients of an Ad5-vectored HIV vaccine. Vaccine. 2020;38:5814–5821. doi: 10.1016/j.vaccine.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Duerr A, et al. Extended follow-up confirms early vaccine-enhanced risk of HIV acquisition and demonstrates waning effect over time among participants in a randomized trial of recombinant adenovirus HIV vaccine (Step Study) J. Infect. Dis. 2012;206:258–266. doi: 10.1093/infdis/jis342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Fitzgerald DW, et al. An Ad5-vectored HIV-1 vaccine elicits cell-mediated immunity but does not affect disease progression in HIV-1-infected male subjects: results from a randomized placebo-controlled trial (the Step Study) J. Infect. Dis. 2011;203:765–772. doi: 10.1093/infdis/jiq114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Rolland M, et al. Genetic impact of vaccination on breakthrough HIV-1 sequences from the STEP trial. Nat. Med. 2011;17:366–371. doi: 10.1038/nm.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Janes H, et al. Vaccine-induced gag-specific T cells are associated with reduced viremia after HIV-1 infection. J. Infect. Dis. 2013;208:1231–1239. doi: 10.1093/infdis/jit322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Gray GE, et al. Recombinant adenovirus type 5 HIV gag/pol/nef vaccine in South Africa: unblinded, long-term follow-up of the phase 2b HVTN 503/Phambili study. Lancet Infect. Dis. 2014;14:388–396. doi: 10.1016/S1473-3099(14)70020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hertz T, et al. A study of vaccine-induced immune pressure on breakthrough infections in the Phambili phase 2b HIV-1 vaccine efficacy trial. Vaccine. 2016;34:5792–5801. doi: 10.1016/j.vaccine.2016.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Leitman EM, et al. Lower viral loads and slower CD4+ T-cell count decline in MRKAd5 HIV-1 vaccinees expressing disease-susceptible HLA-B*58:02. J. Infect. Dis. 2016;214:379–389. doi: 10.1093/infdis/jiw093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Moodie Z, et al. Continued follow-up of Phambili phase 2b randomized HIV-1 vaccine trial participants supports increased HIV-1 acquisition among vaccinated men. PLoS ONE. 2015;10:e0137666. doi: 10.1371/journal.pone.0137666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Robb ML, et al. Risk behaviour and time as covariates for efficacy of the HIV vaccine regimen ALVAC-HIV (vCP1521) and AIDSVAX B/E: a post-hoc analysis of the Thai phase 3 efficacy trial RV 144. Lancet Infect. Dis. 2012;12:531–537. doi: 10.1016/S1473-3099(12)70088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Akapirat S, et al. Characterization of HIV-1 gp120 antibody specificities induced in anogenital secretions of RV144 vaccine recipients after late boost immunizations. PLoS ONE. 2018;13:e0196397. doi: 10.1371/journal.pone.0196397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Gottardo R, et al. Plasma IgG to linear epitopes in the V2 and V3 regions of HIV-1 gp120 correlate with a reduced risk of infection in the RV144 vaccine efficacy trial. PLoS ONE. 2013;8:e75665. doi: 10.1371/journal.pone.0075665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Gray GE, et al. Immune correlates of the Thai RV144 HIV vaccine regimen in South Africa. Sci. Transl. Med. 2019 doi: 10.1126/scitranslmed.aax1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Dommaraju K, et al. CD8 and CD4 epitope predictions in RV144: no strong evidence of a T-cell driven sieve effect in HIV-1 breakthrough sequences from trial participants. PLoS ONE. 2014;9:e111334. doi: 10.1371/journal.pone.0111334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gartland AJ, et al. Analysis of HLA A*02 association with vaccine efficacy in the RV144 HIV-1 vaccine trial. J. Virol. 2014;88:8242–8255. doi: 10.1128/JVI.01164-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Huang Y, et al. Predictors of durable immune responses six months after the last vaccination in preventive HIV vaccine trials. Vaccine. 2017;35:1184–1193. doi: 10.1016/j.vaccine.2016.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Pitisuttithum P, et al. Safety and reactogenicity of canarypox ALVAC-HIV (vCP1521) and HIV-1 gp120 AIDSVAX B/E vaccination in an efficacy trial in Thailand. PLoS ONE. 2011;6:e27837. doi: 10.1371/journal.pone.0027837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhao LP, et al. Landscapes of binding antibody and T-cell responses to pox-protein HIV vaccines in Thais and South Africans. PLoS ONE. 2020;15:e0226803. doi: 10.1371/journal.pone.0226803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zolla-Pazner S, et al. Vaccine-induced IgG antibodies to V1V2 regions of multiple HIV-1 subtypes correlate with decreased risk of HIV-1 infection. PLoS ONE. 2014;9:e87572. doi: 10.1371/journal.pone.0087572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.deCamp AC, et al. Sieve analysis of breakthrough HIV-1 sequences in HVTN 505 identifies vaccine pressure targeting the CD4 binding site of Env-gp120. PLoS ONE. 2017;12:e0185959. doi: 10.1371/journal.pone.0185959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Fong Y, et al. Modification of the association between T-cell immune responses and human immunodeficiency virus type 1 infection risk by vaccine-induced antibody responses in the HVTN 505 trial. J. Infect. Dis. 2018;217:1280–1288. doi: 10.1093/infdis/jiy008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hammer SM, et al. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N. Engl. J. Med. 2013;369:2083–2092. doi: 10.1056/NEJMoa1310566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Janes HE, et al. Higher T-cell responses induced by DNA/rAd5 HIV-1 preventive vaccine are associated with lower HIV-1 infection risk in an efficacy trial. J. Infect. Dis. 2017;215:1376–1385. doi: 10.1093/infdis/jix086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Li SS, et al. Fcγ receptor polymorphisms modulated the vaccine effect on HIV-1 risk in the HVTN 505 HIV vaccine trial. J. Virol. 2019 doi: 10.1128/jvi.02041-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Neidich SD, et al. Antibody Fc effector functions and IgG3 associate with decreased HIV-1 risk. J. Clin. Invest. 2019;129:4838–4849. doi: 10.1172/JCI126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Williams WB, et al. HIV-1 VACCINES. Diversion of HIV-1 vaccine-induced immunity by gp41-microbiota cross-reactive antibodies. Science. 2015;349:aab1253. doi: 10.1126/science.aab1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Edupuganti S, et al. Feasibility and successful enrollment in a proof-of-concept HIV prevention trial of VRC01, a broadly neutralizing HIV-1 monoclonal antibody. J. Acquir. Immune Defic. Syndr. 2021;87:671–679. doi: 10.1097/QAI.0000000000002639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Mgodi NM, et al. A phase 2b study to evaluate the safety and efficacy of VRC01 broadly neutralizing monoclonal antibody in reducing acquisition of HIV-1 infection in women in sub-Saharan Africa: baseline findings. J. Acquir. Immune Defic. Syndr. 2021;87:680–687. doi: 10.1097/QAI.0000000000002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Laher F, et al. Willingness to use HIV prevention methods among vaccine efficacy trial participants in Soweto, South Africa: discretion is important. BMC Public. Health. 2020;20:1669. doi: 10.1186/s12889-020-09785-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Barouch DH, et al. Evaluation of a mosaic HIV-1 vaccine in a multicentre, randomised, double-blind, placebo-controlled, phase 1/2a clinical trial (APPROACH) and in rhesus monkeys (NHP 13-19) Lancet. 2018;392:232–243. doi: 10.1016/S0140-6736(18)31364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Haynes, B. F., Wiehe, K., Acharya, P. & Saunders, K. O. in Vaccines 8th edn, Ch. 31 (eds Plotkin, S. A. et al.) (Elsevier, in press).
- 228.Mayer CT, et al. The microanatomic segregation of selection by apoptosis in the germinal center. Science. 2017 doi: 10.1126/science.aao2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Yoon H, et al. CATNAP: a tool to compile, analyze and tally neutralizing antibody panels. Nucleic Acids Res. 2015;43:W213–W219. doi: 10.1093/nar/gkv404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Pancera M, et al. Structure and immune recognition of trimeric pre-fusion HIV-1 Env. Nature. 2014;514:455–461. doi: 10.1038/nature13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Li H, et al. New SHIVs and improved design strategy for modeling HIV-1 transmission, immunopathogenesis, prevention and cure. J. Virol. 2021 doi: 10.1128/JVI.00071-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Li H, et al. Envelope residue 375 substitutions in simian-human immunodeficiency viruses enhance CD4 binding and replication in rhesus macaques. Proc. Natl Acad. Sci. USA. 2016;113:E3413–E3422. doi: 10.1073/pnas.1606636113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Finton KA, et al. Autoreactivity and exceptional CDR plasticity (but not unusual polyspecificity) hinder elicitation of the anti-HIV antibody 4E10. PLoS Pathog. 2013;9:e1003639. doi: 10.1371/journal.ppat.1003639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ota T, et al. B cells from knock-in mice expressing broadly neutralizing HIV antibody b12 carry an innocuous B cell receptor responsive to HIV vaccine candidates. J. Immunol. 2013;191:3179–3185. doi: 10.4049/jimmunol.1301283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Verkoczy L, Alt FW, Tian M. Human Ig knockin mice to study the development and regulation of HIV-1 broadly neutralizing antibodies. Immunol. Rev. 2017;275:89–107. doi: 10.1111/imr.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Abbott RK, et al. Precursor frequency and affinity determine B cell competitive fitness in germinal centers, tested with germline-targeting HIV vaccine immunogens. Immunity. 2018;48:133–146.e6. doi: 10.1016/j.immuni.2017.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Tian M, et al. Conditional antibody expression to avoid central B cell deletion in humanized HIV-1 vaccine mouse models. Proc. Natl Acad. Sci. USA. 2020;117:7929–7940. doi: 10.1073/pnas.1921996117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Julien JP, et al. Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science. 2013;342:1477–1483. doi: 10.1126/science.1245625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Lyumkis D, et al. Cryo-EM structure of a fully glycosylated soluble cleaved HIV-1 envelope trimer. Science. 2013;342:1484–1490. doi: 10.1126/science.1245627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Sanders RW, et al. A next-generation cleaved, soluble HIV-1 Env trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing but not non-neutralizing antibodies. pLoS Pathog. 2013;9:e1003618. doi: 10.1371/journal.ppat.1003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Sanders RW, Moore JP. Virus vaccines: proteins prefer prolines. Cell Host Microbe. 2021;29:327–333. doi: 10.1016/j.chom.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Sok D, et al. Recombinant HIV envelope trimer selects for quaternary-dependent antibodies targeting the trimer apex. Proc. Natl Acad. Sci. USA. 2014;111:17624–17629. doi: 10.1073/pnas.1415789111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Martin JT, et al. Targeting HIV Env immunogens to B cell follicles in nonhuman primates through immune complex or protein nanoparticle formulations. NPJ Vaccines. 2020;5:72. doi: 10.1038/s41541-020-00223-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Tokatlian T, et al. Innate immune recognition of glycans targets HIV nanoparticle immunogens to germinal centers. Science. 2019;363:649–654. doi: 10.1126/science.aat9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kulp DW, et al. Structure-based design of native-like HIV-1 envelope trimers to silence non-neutralizing epitopes and eliminate CD4 binding. Nat. Commun. 2017;8:1655. doi: 10.1038/s41467-017-01549-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Nogal B, et al. HIV envelope trimer-elicited autologous neutralizing antibodies bind a region overlapping the N332 glycan supersite. Sci. Adv. 2020;6:eaba0512. doi: 10.1126/sciadv.aba0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Torrents de la Peña A, et al. Similarities and differences between native HIV-1 envelope glycoprotein trimers and stabilized soluble trimer mimetics. PLoS Pathog. 2019;15:e1007920. doi: 10.1371/journal.ppat.1007920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Derking R, et al. Enhancing glycan occupancy of soluble HIV-1 envelope trimers to mimic the native viral spike. Cell Rep. 2021;35:108933. doi: 10.1016/j.celrep.2021.108933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kumar S, et al. Neutralizing antibodies induced by first-generation gp41-stabilized HIV-1 envelope trimers and nanoparticles. mBio. 2021;12:e0042921. doi: 10.1128/mBio.00429-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ringe RP, et al. Closing and opening holes in the glycan shield of HIV-1 envelope glycoprotein SOSIP trimers can redirect the neutralizing antibody response to the newly unmasked epitopes. J. Virol. 2019 doi: 10.1128/jvi.01656-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Schorcht A, et al. The glycan hole area of HIV-1 envelope trimers contributes prominently to the induction of autologous neutralization. J. Virol. 2022;96:e0155221. doi: 10.1128/JVI.01552-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wagh K, et al. Completeness of HIV-1 envelope glycan shield at transmission determines neutralization breadth. Cell Rep. 2018;25:893–908.e7. doi: 10.1016/j.celrep.2018.09.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Turner HL, et al. Disassembly of HIV envelope glycoprotein trimer immunogens is driven by antibodies elicited via immunization. Sci. Adv. 2021 doi: 10.1126/sciadv.abh2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Zhang P, et al. A multiclade env–gag VLP mRNA vaccine elicits tier-2 HIV-1-neutralizing antibodies and reduces the risk of heterologous SHIV infection in macaques. Nat. Med. 2021;27:2234–2245. doi: 10.1038/s41591-021-01574-5. [DOI] [PubMed] [Google Scholar]