Abstract
The present study aimed to evaluate the association between obesity and COVID-19 mortality and length of stay in ICU patients, and how these associations were modified by age groups. We performed a retrospective multicenter cohort study with data obtained from a hospital-based registry. The sample consisted of 8183 ICU hospitalized patients who tested positive for SARS-CoV-2. Cox proportional models were used to evaluate the association between BMI categories and COVID-19 mortality and generalized linear models for the length of stay in the ICU. After adjusting for confounders, those in the younger group with severe obesity had an increased risk of COVID-19 mortality compared to those with normal/overweight (HR 1.27; 95% CI 1.01–1.61). An increased risk of death was also observed for patients with underweight (HR 3.74; 95% CI 1.39–10.07). For patients aged ≥ 60 year, mild/moderate obesity was associated with reduced mortality risk (HR 0.87; 95% CI 0.78–0.97). For the age group < 60 year, the length of stay in ICU for those patients with severe obesity was 35% higher compared to the normal/overweight category (eβ 1.35; 95% CI 1.21–1.51). Conversely, for the survivors in the underweight category, the length of stay in ICU was 51% lower compared to the normal/overweight group (eβ 0.49; 95% CI 0.31–0.78). In the age group ≥ 60 year, mild/moderate obesity was associated with an increased length of stay in the ICU (eβ 1.10; 95% CI 1.01–1.21), adjusting for confounders. These findings could be helpful for health professionals to identify subgroups at higher risk for worse outcomes.
Subject terms: Epidemiology, Infectious diseases
Introduction
In March 2020, the World Health Organization declared the COVID-19 pandemic, and by May 31, 2021, over 170 million cases and nearly 4 million deaths were reported worldwide1. Brazil was one of the most affected countries, counting more than 555,000 deaths, in the same period2.
Since then, many studies have demonstrated that the elderly and people living with diabetes, cardiovascular disease, and respiratory or kidney diseases were associated with an increased risk of adverse outcomes and poor COVID-19 prognosis3,4. Also, there is a consistent finding that obesity is associated with worse COVID-19 outcomes5, with several systematic reviews and meta-analyses showing that obesity was associated with a higher risk of infection5,6, intensive care unit (ICU) admission5,7–9, severe COVID-195,7,8,10, invasive mechanical ventilation5,7,9, and mortality4–6,8,10,11.
However, most meta-analyses showed a large heterogeneity for the combined effect related to the association between obesity and COVID-19 mortality, and only a few tried to explain this issue10,12. Two meta-analyses with metaregression suggested that age could be an effect modifier between obesity and COVID-19 mortality10,12, showing that increased age was associated with a decrease in the magnitude of the association. Similar results were demonstrated in another meta-analysis that conducted a subgroup analysis by age. The authors showed that among individuals younger than 60 years old, the combined odds ratio was 3.30 (95% CI 2.13–5.10), and for the elderly 1.52 (95% CI 1.05–2.19)13. Other studies also demonstrated that the association between BMI and adverse COVID-19 outcomes was stronger in younger individuals14–16.
In relation to critically ill COVID-19 patients, Dana et al. was the first study to report that in-hospital mortality was significantly lower for patients admitted to the ICU with BMI 30–39.9 kg/m2, compared to those with normal weight, overweight or severe obesity17. From other respiratory diseases, some studies also showed reduced ICU and mortality rates in overweight or obese people compared to normal BMI, suggesting the so-called ‘obesity paradox’18–20. Although older individuals are at a greater risk of COVID-19 mortality, and some studies observed an effect modification by age for the association between obesity and COVID-19 mortality, the interaction of obesity and age in critically COVID-19 patients is still unclear. A recent systematic review of COVID-19 in-hospital mortality concluded that obesity was only associated with mortality in studies that included fewer critical patients21.
Another important aspect related to COVID-19 infection is the length of stay in the ICU. Some authors have already shown that COVID-19 demands a prolonged length of stay in ICU, with the oldest patients spending the longest period22; however, little is known about the association between BMI status and length of stay in ICU in patients with COVID-19.
Therefore, the primary objective was to evaluate the association between obesity and mortality in patients with COVID-19 admitted to the ICU in Brazil. The secondary objective was to evaluate the association between obesity and length of stay in the ICU among the survivors and to assess the potential role of age as a modifier of these associations. We hypothesized that severe obesity would be associated with increased mortality risk and a greater length of stay in intensive care units among younger patients and associated with reduced mortality risk and length of stay in intensive care units among older patients, in comparison to normal/overweight.
Methods
Study design and setting
This is a retrospective multicenter cohort study of a hospital-based registry oriented to clinical and administrative purposes23. The registry is utilized by ICUs pertaining to a network of 32 private hospitals in Brazil in the states of São Paulo, Rio de Janeiro, Ceará, Pernambuco, and the Federal District.
Participants’ recruitment
In this registry, patients are consecutively recruited at ICU admission and followed up until hospital discharge. For this study, we selected all patients aged ≥ 18 years, admitted to ICU with SARS-CoV2 infection confirmed by reverse transcription-polymerase chain reaction (RT-PCR), testing between March 01, 2020, and May 31, 2021. We included patients who were admitted directly from the emergency department, transferred from a hospital ward, or referred from another hospital. According to a network-wide clinical protocol, patients with COVID-19 were admitted to ICU care when oxygen delivery exceeded 4 L per minute, when invasive ventilation or face-mask non-invasive ventilation was indicated, or whenever any associated organ dysfunction was present. All patients with missing data for body weight and/or height were excluded from the analyses since BMI calculation was not possible for these patients.
Theoretical model
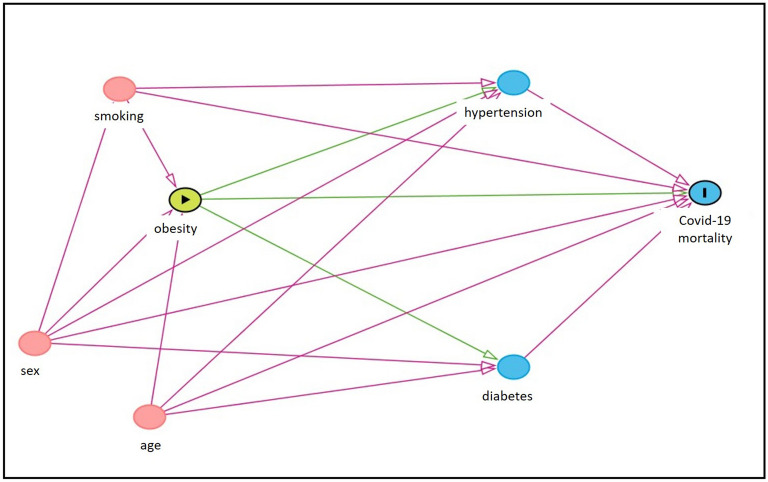
Figure 1 shows the theoretical model examining the association between obesity (exposure) and COVID-19 mortality (outcome). The red arrows represent the back-door paths, and the green arrows represent the direct and indirect pathways between obesity and COVID-19 mortality24. The model postulate that smoking, sex, and age are potential common causes of both exposure and outcome. Also, hypertension and diabetes are mediator variables through the causal path between obesity and COVID-19 mortality.
Figure 1.
Directed Acyclic Graph presenting the potential relationship between obesity (exposure), COVID-19 mortality (outcome), and the covariates, for age groups < 60 years and ≥ 60 years.
Data measurements and variables
Data collection from electronic medical records was performed daily during working days by trained nurses. Demographic variables, smoking status (yes/no), comorbidities (yes/no), and medical conditions were self-reported or informed by relatives at the moment of their admission to the hospitals. BMI was calculated as a ratio between body weight (kg) and squared height (m2), and categorized into four groups: underweight (BMI < 18.5 kg/m2), normal/overweight (18.5–29.9 kg/m2), mild/moderate obesity (30–39.9 kg/m2), and severe obesity (≥ 40 kg/m2). These categories were defined following a clinical rationale based on previous studies17,25.
The primary outcome was COVID-19 mortality during ICU stay, and the secondary outcome was the length of stay in ICU, defined as the time elapsed between ICU admission and ICU discharge, in days.
Statistical analyses
For descriptive analysis, means and standard deviations or median and interquartile range for continuous variables and frequencies (percentage) for categorical variables were calculated for variables of interest at baseline. To compare the characteristics across BMI categories, Kruskal–Wallis test for continuous, and Chi-squared and Fisher’s exact tests for categorical variables were used.
To evaluate the association between BMI categories and COVID-19 outcomes we tested three models: (1) unadjusted model; (2) adjusted for confounders; (3) adjusted for confounders + mediators. Confounding variables were selected through a Directed Acyclic Graphs (DAG)24, and the minimum sufficient adjustment set for the identification of the total association of obesity on COVID-19 mortality or length of stay in the intensive care unit included the variables age, sex, and smoking (model 2), as depicted in Fig. 1. The variables set was identified using the DAGitty application26. Additional analysis (model 3) was performed including mediator variables (hypertension and diabetes mellitus) to investigate the direct association between the exposure and outcomes, or other possible pathways not presented in the DAG.
To evaluate the association between BMI categories and COVID-19 in-hospital mortality, Cox proportional hazards models were performed. The association between BMI categories and length of stay in ICU among the survivors was evaluated using generalized linear models, with gamma distribution and log link function. All analyses were also stratified by age below or above 60 years old.
For the elderly, sensitivity analysis grouping patients according to different BMI categories were also performed, as follows: underweight (< 22.0 kg/m2), normal weight (22.0–26.9 kg/m2), overweight (27.0–29.9 kg/m2), and obesity (≥ 30 kg/m2)27. Additional sensitivity analyses were performed to evaluate the effect of missing data on the variables hypertension, diabetes mellitus, and smoking status on mortality. Firstly, all missing data were considered as cases, and then, as non-cases28. To evaluate the possible influence of the vaccination period, we excluded older individuals (> 60 years) admitted to ICU between February 01 to May 31, 2021. This refers to the vaccination period during the study, which mostly included older adults for such period.
All analyses were performed using SAS On-demand for Academics, and the statistical significance was set at p < 0.05.
Ethics approval
The study was analyzed and approved by the Research Ethics Committee of Pró Cardiaco Hospital with a waiver of informed consent (CAAE number: 43739321.3.0000.5533).
Results
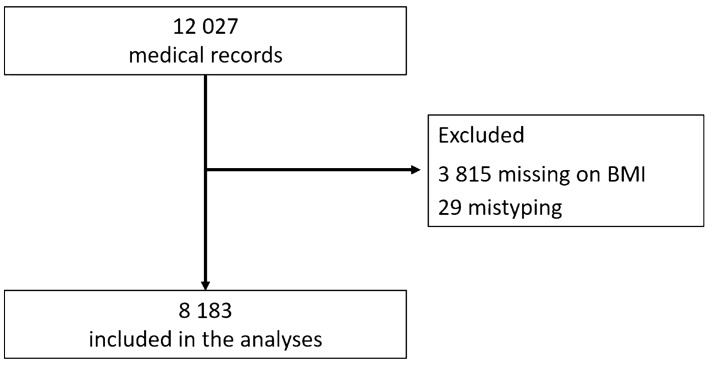
Of the 12,027 medical registries in the original database, 3815 had missing data for BMI, 29 had implausible data for BMI (1 BMI value below 10 kg/m2 and 26 BMI values above 100 kg/m2), and two negative values for ICU length of stay, being excluded from the analyses. Therefore, the final sample included in the present study consists of 8183 ICU hospitalized patients (Fig. 2).
Figure 2.
Flowchart of study participants.
Tables 1 and 2 describe the baseline characteristics of the patients. For the 4130 patients aging < 60 years (Table 1), the mean age was 47.0 years old, with 2670 (64.7%) men, and 56.3% with some degree of obesity (BMI > 30 kg/m2). The most prevalent comorbidity was hypertension (55.0%), followed by diabetes mellitus (31.4%). For those aging ≥ 60 years (Table 2), the mean age was 72.0 years old, with a slight predominance of men (56.3%). The proportion of individuals with obesity was lower (35.0%) among the elderly in comparison to younger individuals. However, the prevalence of comorbidities in the older group was greater, being hypertension (77.9%) and diabetes mellitus (48.7%) the most reported. The proportion of death in the age group < 60 years was 18.7% with a median ICU length of stay of 7.4 days. In contrast, in the older age group, 43.9% of the patients died, with a median ICU length of stay of 8.8 days.
Table 1.
Baseline characteristics of study patients aged < 60 years.
| Variables | Overall (n = 4130) | BMI categories (kg/m2) | p-value* | |||
|---|---|---|---|---|---|---|
| < 18.5 (n = 25) | 18.5–29.9 (n = 1780) | 30.0–39.9 (n = 1882) | ≥ 40.0 (n = 443) | |||
| Age (years) | 47.0 (14.0) | 34.0 (25.0) | 48.0 (14.0) | 47.0 (14.0) | 42.0 (14.0) | < 0.001 |
| Sex | < 0.001 | |||||
| Men | 2670 (64.7) | 12 (0.5) | 1163 (43.6) | 1240 (46.4) | 255 (9.6) | |
| Women | 1460 (35.3) | 13 (0.9) | 617 (42.3) | 642 (44.0) | 188 (12.9) | |
| Hypertensionª | 1584 (55.0) | 6 (0.4) | 583 (36.8) | 756 (47.7) | 239 (15.1) | 0.12 |
| Diabetes mellitusª | 904 (31.4) | 5 (0.6) | 351 (38.8) | 421 (46.6) | 127 (14.1) | 0.25 |
| Smokingª | 114 (4.0) | 0 (0.0) | 52 (45.6) | 49 (43.0) | 13 (11.4) | 0.16 |
| Chronic kidney diseaseª | 140 (4.9) | 3 (2.1) | 83 (59.3) | 46 (32.9) | 8 (5.7) | < 0.001 |
| Myocardial infarctionª | 54 (1.9) | 0 (0.0) | 23 (42.6) | 26 (48.2) | 5 (9.3) | 0.57 |
| Previous strokeª | 40 (1.4) | 0 (0.0) | 25 (62.5) | 13 (32.5) | 2 (5.0) | 0.005 |
| Dementiaª | 12 (0.4) | 0 (0.0) | 7 (58.3) | 4 (33.3) | 1 (8.3) | 0.37 |
| Atrial fibrillationª | 15 (0.5) | 0 (0.0) | 5 (33.3) | 8 (53.3) | 2 (13.3) | 1.00 |
| Hospital mortality§ | 747 (18.7) | 6 (0.8) | 281 (37.6) | 342 (45.8) | 118 (15.8) | < 0.001 |
| ICU length of stay¥ | 7.4 (10.4) | 4.4 (6.0) | 6.6 (9.6) | 7.7 (10.5) | 10.2 (12.8) | < 0.001 |
For the variables age and ICU length of stay, data are median (IQR).
*Kruskal–Wallis test for continuous and Chi-squared and Fisher’s exact test for categorical variables.
§The variable hospital mortality presented 141 missing data.
¥The variable ICU length of stay presented 60 missing data.
ªThe variables hypertension, diabetes mellitus, smoking, chronic kidney disease, previous myocardial infarction, previous stroke, dementia, and atrial fibrillation have 1249 missing data.
Table 2.
Baseline characteristics of study patients aged ≥ 60 years.
| Variables | Overall (n = 4053) | BMI categories (kg / m2) | p-value* | |||
|---|---|---|---|---|---|---|
| < 18.5 (n = 47) | 18.5–29.9 (n = 2594) | 30.0–39.9 (n = 1264) | ≥ 40.0 (n = 148) | |||
| Age (years) | 72.0 (13.0) | 81.0 (15.0) | 73.0 (15.0) | 69.0 (11.0) | 68.0 (8.5) | < 0.001 |
| Sex | < 0.001 | |||||
| Men | 2282 (56.3) | 23 (1.0) | 1567 (68.7) | 641 (28.1) | 51 (2.2) | |
| Women | 1771 (43.7) | 24 (1.4) | 1027 (58.0) | 623 (35.2) | 97 (5.5) | |
| Hypertensionª | 2937 (77.9) | 30 (1.0) | 1781 (60.6) | 995 (33.9) | 131 (4.5) | < 0.001 |
| Diabetes mellitusª | 1838 (48.7) | 17 (0.9) | 1093 (59.5) | 639 (34.8) | 89 (4.8) | < 0.001 |
| Smokingª | 192 (5.1) | 2 (1.0) | 127 (66.2) | 57 (29.7) | 6 (3.1) | 0.80 |
| Chronic kidney diseaseª | 408 (10.8) | 9 (2.2) | 278 (68.1) | 110 (27.0) | 11 (2.7) | 0.01 |
| Myocardial infarctionª | 230 (6.1) | 4 (1.7) | 158 (68.7) | 64 (27.8) | 4 (1.7) | 0.11 |
| Previous strokeª | 222 (5.9) | 4 (1.8) | 177 (79.7) | 37 (16.7) | 4 (1.8) | < 0.001 |
| Dementiaª | 291 (7.7) | 17 (5.8) | 228 (78.4) | 40 (13.8) | 6 (2.1) | < 0.001 |
| Atrial fibrillationª | 175 (4.7) | 3 (1.7) | 126 (72.0) | 39 (22.3) | 7 (4.0) | 0.04 |
| Hospital mortality§ | 1738 (43.9) | 22 (1.3) | 1146 (65.9) | 500 (28.8) | 70 (4.0) | 0.02 |
| ICU length of stay¥ | 8.8 (12.6) | 4.7 (10.1) | 8.4 (12.2) | 9.6 (13.2) | 9.1 (12.8) | < 0.001 |
For the variables age and ICU length of stay, data are median (IQR).
ªThe variables hypertension, diabetes mellitus, smoking, chronic kidney disease, previous myocardial infarction, previous stroke, dementia, and atrial fibrillation have 282 missing data.
*Kruskal–Wallis test for continuous and Chi-squared test for categorical variables.
§The variable hospital mortality presented 91 missing data.
¥The variable ICU length of stay presented 19 missing data.
For the entire sample (Table 3), stratifying by BMI categories and adjusting for age, sex, and smoking status, those patients with severe obesity showed an increased risk of COVID-19 mortality (HR 1.21; 95% CI 1.03–1.43) compared to those with normal/overweight. No difference was observed for the mild/moderate obesity (HR 0.91; 95% CI 0.83–1.00) and the underweight (HR 1.21; 95% CI 0.80–1.81) categories. In addition, for the survivors in the highest BMI category (≥ 40 kg/m2), the length of stay in ICU was 31% higher compared to those in the normal/overweight category (eβ 1.31; 95% CI 1.17–1.45). An increased length of stay in ICU was also observed for BMI between mild/moderate obesity (eβ 1.09; 95% CI 1.03–1.16) in comparison to normal/overweight. No difference was detected for the underweight group (eβ 0.86; 95% CI 0.64–1.15).
Table 3.
Hazard ratios (HR) for COVID-19 mortality and Length of stay in ICU, according to BMI categories, for the entire sample.
| BMI categories | COVID-19 mortality (n = 6474) | Length of stay in ICU (n = 4343) | ||||||
|---|---|---|---|---|---|---|---|---|
| n | HR | 95% CI | p-value | n | eβ | 95% CI | p-value | |
| Underweight | 63 | 1.21 | 0.80–1.81 | 0.37 | 39 | 0.86 | 0.64–1.15 | 0.30 |
| Normal/overweight | 3337 | Ref | – | – | 2108 | Ref | – | – |
| Mild/moderate obesity | 2543 | 0.91 | 0.83–1.00 | 0.06 | 1830 | 1.09 | 1.03–1.16 | 0.005 |
| Severe obesity | 531 | 1.21 | 1.03–1.43 | 0.02 | 366 | 1.31 | 1.17–1.45 | < 0.001 |
Adjusted for age, sex, smoking status.
Table 4 presents the association between BMI categories and in-hospital mortality, according to age groups. For the age group < 60 year, in the unadjusted model (model 1), severe obesity was associated with an increased risk of death (HR 1.27; 95% CI 1.02–1.57) compared to those individuals in the normal/overweight category. After adjusting for the confounders (model 2), the result was similar (HR 1.27; 95% CI 1.01–1.61), and when potential mediator variables were also introduced in the model (model 3), a borderline association was observed (HR 1.25; 95% CI 0.99–1.58). For the underweight category (BMI < 18.5 kg/m2), an increased risk for mortality was observed in the crude model (model 1) (HR 2.30; 95% CI 1.03–5.17), and also in model 2, adjusting for confounders (HR 3.74; 95% CI 1.39–10.07), and model 3, after adjusting for confounders and mediator variables (HR 3.71; 95% CI 1.37–9.99). No difference was observed for the mild/moderate obesity category.
Table 4.
Hazard ratios (HR) for COVID-19 mortality according to age groups and BMI categories.
| < 60 years | Model 1 (n = 3989) | Model 2 (n = 2783) | Model 3 (n = 2783) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | HR | 95%CI | p-value | n | HR | 95%CI | p-value | n | HR | 95%CI | p-value | |
| Underweight | 24 | 2.30 | 1.03–5.17 | 0.04 | 18 | 3.74 | 1.39–10.07 | 0.009 | 18 | 3.71 | 1.37–9.99 | 0.01 |
| Normal/overweight | 1731 | Ref | – | – | 1017 | Ref | – | – | 1017 | Ref | – | – |
| Mild/moderate obesity | 1812 | 1.12 | 0.96–1.32 | 0.15 | 1353 | 1.10 | 0.92–1.32 | 0.29 | 1353 | 1.11 | 0.92–1.33 | 0.28 |
| Severe obesity | 422 | 1.27 | 1.02–1.57 | 0.03 | 395 | 1.27 | 1.01–1.61 | 0.04 | 395 | 1.25 | 0.99–1.58 | 0.06 |
| Model 1 (n = 3961) | Model 2 (n = 3690) | Model 3 (n = 3690) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Underweight | 47 | 1.41 | 0.92–2.15 | 0.11 | 45 | 1.03 | 0.66–1.61 | 0.89 | 45 | 1.03 | 0.66–1.60 | 0.91 |
| Normal/overweight | 2532 | Ref | – | – | 2319 | Ref | – | – | 2319 | Ref | – | – |
| Mild/moderate obesity | 1240 | 0.82 | 0.74–0.91 | < 0.001 | 1190 | 0.87 | 0.78–0.97 | 0.02 | 1190 | 0.87 | 0.78–0.97 | 0.01 |
| Severe obesity | 142 | 1.01 | 0.79–1.28 | 0.94 | 136 | 1.19 | 0.93–1.53 | 0.17 | 136 | 1.17 | 0.91–1.51 | 0.22 |
Model 1: unadjusted.
Model 2: adjusted for age, sex, smoking status.
Model 3: adjusted for age, sex, smoking status, hypertension, diabetes.
For those aging ≥ 60 year in the unadjusted analysis (model 1), mild/moderate obesity was associated with a reduced risk of death (HR 0.82; 95% CI 0.74–0.91), compared to the normal/overweight group. After adjusting for the confounders (model 2) and mediation variables (model 3), the reduced mortality risk was still observed (HR 0.87; 95% CI 0.78–0.97, and HR 0.87; 95% CI 0.78–0.97, respectively). No differences were observed for underweight and severe obesity for all three models (Table 4) in comparison to those normal/overweight. Results were similar when considering alternative BMI thresholds among older participants (Table S1 Supplementary Material).
Sensitivity analyses showed similar results when missing data for hypertension, diabetes, and smoking were categorized as cases (model 1), and as non-cases (model 2) (Table S2 Supplementary Material).
Table 5 shows the length of stay in ICU among the survivors, according to age group and BMI categories. For the age group < 60 year, in the unadjusted model, the length of stay in ICU for those patients with severe obesity was 35% higher compared to the normal/overweight category (eβ 1.35; 95% CI 1.21–1.51). Even after adjustments for confounding (model 2), and potential mediators (model 3), the results were similar (eβ 1.34; 95% CI 1.19–1.51 and eβ 1.34; 95% CI 1.19–1.51, respectively). Conversely, for the survivors in the underweight category, the length of stay in ICU was 36% lower compared to the normal/ overweight group, in the unadjusted model (eβ 0.64; 95% CI 0.42–0.97), and 51% lower after adjusting for confounders (eβ 0.49; 95% CI 0.31–0.78) and also after adding the mediators in the model (eβ 0.49; 95% CI 0.31–0.78) (Table 5). For the mild/moderate obesity group, the length of stay in ICU was 7% higher compared to the normal/overweight group (eβ 1.07; 95% CI 1.00–1.14), although a borderline association was detected. After adjusting for the confounding (model 2) and mediator (model 3) variables, no difference was observed (eβ 1.05; 95% CI 0.97–1.14 and eβ 1.06; 95% CI 0.97–1.14, respectively).
Table 5.
Length of stay in ICU, among the survivors, according to age groups and BMI categories.
| < 60 years | Model 1 (n = 3323) | Model 2 (n = 2235) | Model 3 (n = 2235) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | eβ | 95% CI | p-value | n | eβ | 95% CI | p-value | n | eβ | 95% CI | p-value | |
| Underweight | 18 | 0.64 | 0.42–0.97 | 0.04 | 14 | 0.49 | 0.31–0.78 | 0.002 | 14 | 0.49 | 0.31–0.78 | 0.002 |
| Normal/overweight | 1480 | Ref | – | – | 827 | Ref | – | – | 827 | Ref | – | – |
| Mild/moderate obesity | 1507 | 1.07 | 1.00–1.14 | 0.04 | 1102 | 1.05 | 0.97–1.14 | 0.20 | 1102 | 1.06 | 0.97–1.14 | 0.18 |
| Severe obesity | 318 | 1.35 | 1.21–1.51 | < 0.001 | 292 | 1.34 | 1.19–1.51 | < 0.001 | 292 | 1.34 | 1.19–1.51 | < 0.001 |
| Model 1 (n = 2296) | Model 2 (n = 2108) | Model 3 (n = 2108) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Underweight | 25 | 1.13 | 0.76–1.67 | 0.55 | 25 | 1.23 | 0.83–1.82 | 0.31 | 25 | 1.23 | 0.83–1.82 | 0.31 |
| Normal/overweight | 1432 | Ref | – | – | 1281 | Ref | – | – | 1281 | Ref | – | – |
| Mild/moderate obesity | 761 | 1.10 | 1.01–1.20 | 0.04 | 728 | 1.10 | 1.01–1.21 | 0.03 | 728 | 1.10 | 1.00–1.21 | 0.04 |
| Severe obesity | 78 | 1.19 | 0.95–1.49 | 0.14 | 74 | 1.14 | 0.90–1.45 | 0.26 | 74 | 1.13 | 0.89–1.43 | 0.30 |
Model 1: unadjusted.
Model 2: adjusted for age, sex, smoking status.
Model 3: adjusted for age, sex, smoking status, hypertension, diabetes.
In the age group ≥ 60 year, the length of stay in ICU for the mild/moderate obesity category was about 10% higher compared to the normal/overweight category, in all three models. However, we did not observe any differences in length of stay in ICU for the survivor’s patients categorized into underweight or severe obesity categories (Table 5).
To investigate the possible influence of the vaccination, a sensitivity analysis excluding those older individuals admitted to ICU between February 01 to May 31, 2021, demonstrated similar results for COVID-19 mortality and length of stay in ICU among the survivors (Tables S3, S4—Supplementary Material).
Discussion
The present study demonstrated that, in the entire sample, severe obesity (BMI ≥ 40 kg/m2) was positively associated with COVID-19 mortality, compared to the normal/overweight category; however, when stratified by age groups, the increased risk of mortality was only observed for the younger category (< 60 years), that also showed an increased risk of death for those in the underweight group. Among the elderly, mild/moderate obesity showed a reduced risk of mortality. For the survivors in the younger group, an increased length of stay in the ICU was observed for those with severe obesity; however, being underweight showed a reduced length of stay in the ICU, when compared to the normal/overweight group. For the survivors in the older group, being mild/moderate obese showed an increased length of stay in the ICU.
Considering that the prevalence of obesity is increasing worldwilde29, identifying the groups at higher risk of worse COVID-19 outcomes becomes a priority. In Brazil, according to data from the last National Health Survey, conducted in 2019, the prevalence of obesity is 25.5%, being higher among women (29.5%) when compared to men (21.8%)30.
In agreement with our findings, recent systematic reviews4,5,10,11 and large cohort studies31,32 have demonstrated a positive association between obesity and COVID-19 outcomes. At least five biological mechanisms could explain this association. First, obesity changes the mechanical properties of the lungs and chest wall and can predispose patients to develop respiratory failure in the case of lung infection33. Second, obesity can increase the inflammation process which decreases innate and adaptive immunity contributing to worse outcomes in patients with COVID-1934. Third, obesity is associated with hypercoagulability, increasing the risk of arterial thrombosis and venous thromboembolism, being one of the most important causes of COVID-19 complication35. Fourth, the higher expression of angiotensin-converting enzyme 2 in adipose tissue increases the susceptibility to SARS-CoV-2 infection and the risk of severe disease36. Finally, obesity is associated with several comorbidities which are linked with poor COVID-19 outcomes4,9,37.
In our study, patients with severe obesity had an increased risk of COVID-19 mortality only for those in the younger group, after adjusting for confounders. Although other studies found an increased mortality risk, a weaker magnitude of the association was also demonstrated for the elderly. In a Spain cohort study, involving approximately 2 million Catalans, the risk of poor COVID-19 outcomes related to increased BMI was higher for those individuals aged ≤ 59 years, compared to the older age group32. Another study conducted in the United States also found a similar result, with severe obesity independently associated with COVID-19 mortality (adjusted OR 5.1; 95% CI 2.3–11.1), for the younger population. For the older population, severe obesity was also independently associated with mortality to a lesser extent (adjusted odds ratio 1.6; 95% CI 1.2–2.3)38. Finally, a recent Brazilian study16, including 313 898 hospitalized patients, observed the mortality risk for obese patients was greater in younger individuals. Gao et al. demonstrated that the mortality risk for each increase in BMI unit decreased progressively, with increasing age becoming non-significant in the 80 years and older age group15. Despite few patients, we also observed that being underweight was associated with an increased risk of COVID-19 mortality, among the younger group, as already previously demonstrated15.
For the elderly, mild/moderate obesity presented a reduced risk of COVID-19 mortality in our study. Although people with obesity have an increased risk for obesity-related diseases39, they present a reduced mortality risk for some respiratory diseases, especially during critical health conditions20,40. This situation has been called as “obesity paradox” and might explain how some degree of obesity can provide a protective effect among older patients. According to the paradox hypothesis, a greater fat accumulation provides an additional energy reserve to resist a catabolic environment that usually occurs in ICU41,42. Another hypothesis that could explain the obesity paradox is assistance. Older patients with obesity are usually assumed with a poor prognosis, resulting in earlier admission and more aggressive monitoring and management43. Finally, elderly patients with obesity are more likely to have hypertension and cardiovascular disease. These comorbidities are commonly treated with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, decreasing the risk of mortality44. Therefore, the “obesity paradox” may also be applied to the COVID-19 scenario.
Consistent findings have already shown an increased risk for ICU admission in obese individuals5; however, only a few studies investigated the association between obesity and length of stay in ICU. For this analysis, we included only the survivors, and the results showed that patients with severe obesity in the younger group, and mild/moderate obesity in the older group, had significantly longer ICU length of stay than normal/overweight patients. In the elderly, although the magnitude of the association seems to be greater for the severe obesity patients, it was not statistically significant, probably due to the small sample size in the category. Sjögren and colleagues in Sweden also demonstrated among the survivors that obesity was associated with a doubled risk of ICU length of stay ≥ 14 days, compared to normal BMI group45; however, the analysis was not stratified by age groups. This is an important issue because long periods in ICU are related to poor rehabilitation, and increase the risk of opportunistic infection46. Also, it demands time from family members, impacts mental health, and promote increased cost for families and the health system47. Surprisingly, although patients with underweight showed an increased mortality risk in the younger group, the survivors presented a 51% lower length of stay in ICU, compared to normal/overweight patients.
This study has some limitations. First, body weight and height were self-reported or informed by relatives at the moment of hospital admission. Although this method can lead to measurement error (mainly among older adults due to recall bias), the self-reported method is a valid alternative to determine weight status48. Second, some covariates had missing data, which could influence the results; however, we tried to deal with this problem by conducting sensitivity analyses. Third, some variables, identified as a confounder based on the Causal Diagram Theory, may not have been measured in this study and could contribute to residual confounding. Fourth, we do not have access to patients’ clinical conditions, and delays in medical care at the time of admission. Both situations are strongly associated with mortality risk and could influence the results49. Fifth, the results of the present study can be generalized only to those individuals who have access to private medical units (most of them with health insurance). However, it could be interpreted as a strength of our work, as most research in Brazil is conducted on public health services. Finally, individuals readmitted to the ICU after hospital discharge were included as new patients; however, according to Todt et al.50, the readmission rate is low, and we suppose it did not influence the results.
In summary, our study showed a significant association between severe obesity and an increased risk for COVID-19 mortality and greater length of stay in ICU, in the younger group. Patients with underweight also presented an increased mortality risk; however, the survivors had a reduced length of stay in the ICU. Among the elderly, mild/moderate obesity was associated with reduced COVID-19 mortality risk, corroborating the obesity paradox in this age group, but also showed an increase in length of stay in the ICU. These results could be helpful for public health policy-making, especially in countries affected by a high prevalence of obesity.
Supplementary Information
Author contributions
V.B.P. conceived and designed the manuscript, conducted the statistical analysis, interpreted the results, drafted and revised the manuscript, and approved the final version. T.H.L. conceived and designed the manuscript, interpreted the results, drafted and revised the manuscript, and approved the final version. M.F.F.M. designed the manuscript, conducted the statistical analysis, interpreted the results, revised the manuscript, and approved the final version. R.S. conceived and designed the manuscript, interpreted the results, revised the manuscript, and approved the final version. G.A.S. designed the manuscript, interpreted the results, revised the manuscript, and approved the final version. V.C. acquired data, revised the manuscript, and approved the final version. A.B. acquired data, revised the manuscript, and approved the final version. E.S. acquired data, revised the manuscript, and approved the final version. BAMPB acquired data, revised the manuscript, and approved the final version. R.C.M. acquired data, revised the manuscript, and approved the final version. C.E.B. acquired data, revised the manuscript, and approved the final version. D.C.K.G. designed the manuscript, interpreted the results, revised the manuscript, and approved the final version. C.A.G. designed the manuscript, interpreted the results, revised the manuscript, and approved the final version. P.C. designed the manuscript, acquired data, interpreted the results, revised the manuscript, and approved the final version.
Funding
This research was supported by the State Research Foundation of Rio de Janeiro (FAPERJ), Number 255937 (Support for Projects in Research Networks on SARS-CoV-2/COVID-19), and a fellowship from Brazilian National Research Foundation (CNPq), Number 381166/2020-1. The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Data availability
The dataset used and/or analyzed during the current study is available from the corresponding author on reasonable request. The data collection procedure was performed in accordance with relevant guidelines and regulations.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-17197-w.
References
- 1.World Health Organization. WHO Coronavirus (COVID-19) Dashboard [Internet]. https://covid19.who.int/. Accessed June 2022.
- 2.Brazil, H. M. Coronavírus Brasil. https://covid.saude.gov.br/. Accessed July 2022.
- 3.Figliozzi S, Masci PG, Ahmadi N, Tondi L, Koutli E, Aimo A, et al. Predictors of adverse prognosis in COVID-19: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2020;50(10):e13362. doi: 10.1111/eci.13362. [DOI] [PubMed] [Google Scholar]
- 4.Noor FM, Islam MM. Prevalence and associated risk factors of mortality among COVID-19 patients: A meta-analysis. J. Community Health. 2020;45(6):1270–1282. doi: 10.1007/s10900-020-00920-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang J, Tian C, Chen Y, Zhu C, Chi H, Li J. Obesity aggravates COVID-19: An updated systematic review and meta-analysis. J. Med. Virol. 2021;93(5):2662–2674. doi: 10.1002/jmv.26677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho JSY, Fernando DI, Chan MY, Sia CH. Obesity in COVID-19: A systematic review and meta-analysis. Ann. Acad. Med. Singapore. 2020;49(12):996–1008. doi: 10.47102/annals-acadmedsg.2020299. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, Lewis AM, Moley JR, Brestoff JR. A systematic review and meta-analysis of obesity and COVID-19 outcomes. Sci. Rep. 2021;11(1):7193. doi: 10.1038/s41598-021-86694-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoong CWS, Hussain I, Aravamudan VM, Phyu EE, Lin JHX, Koh H. Obesity is associated with poor Covid-19 outcomes: A systematic review and meta-analysis. Horm. Metab. Res. 2021;53(2):85–93. doi: 10.1055/a-1326-2125. [DOI] [PubMed] [Google Scholar]
- 9.Földi M, Farkas N, Kiss S, Zádori N, Váncsa S, Szakó L, et al. Obesity is a risk factor for developing critical condition in COVID-19 patients: A systematic review and meta-analysis. Obes. Rev. 2020;21(10):e13095. doi: 10.1111/obr.13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du Y, Lv Y, Zha W, Zhou N, Hong X. Association of body mass index (BMI) with critical COVID-19 and in-hospital mortality: A dose-response meta-analysis. Metabolism. 2021;117:154373. doi: 10.1016/j.metabol.2020.154373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Y, Lu Y, Huang Y-M, Wang M, Ling W, Sui Y, et al. Obesity in patients with COVID-19: A systematic review and meta-analysis. Metabolism. 2020;113:154378. doi: 10.1016/j.metabol.2020.154378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao X, Gang X, He G, Li Z, Lv Y, Han Q, et al. Obesity increases the severity and mortality of influenza and COVID-19: A systematic review and meta-analysis. Front. Endocrinol. 2020;11:595109. doi: 10.3389/fendo.2020.595109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu Y, Yang J, Shi J, Zhang P, Wang X. Obesity is associated with increased severity of disease in COVID-19 pneumonia: A systematic review and meta-analysis. Eur. J. Med. Res. 2020;25(1):64. doi: 10.1186/s40001-020-00464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sattar N, Ho FK, Gill JM, Ghouri N, Gray SR, Celis-Morales CA, et al. BMI and future risk for COVID-19 infection and death across sex, age and ethnicity: Preliminary findings from UK biobank. Diabetes Metab. Syndr. 2020;14(5):1149–1151. doi: 10.1016/j.dsx.2020.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao M, et al. Associations between body-mass index and COVID-19 severity in 6.9 million people in England: A prospective, community-based, cohort study. Lancet Diabetes Endocrinol. 2022;9(6):350–359. doi: 10.1016/S2213-8587(21)00089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Discacciati MG, Siani S, Campa A, Nakaya HI. Why should obese youth be prioritized in COVID-19 vaccination programs? A nationwide retrospective study. Lancet Reg. Health Am. 2022;7:100167. doi: 10.1016/j.lana.2021.100167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dana R, Bannay A, Bourst P, Ziegler C, Losser MR, Gibot S, et al. Obesity and mortality in critically ill COVID-19 patients with respiratory failure. Int. J. Obes. (Lond.) 2021;45(9):2028–2037. doi: 10.1038/s41366-021-00872-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pickkers P, De Keizer N, Dusseljee J, Weerheijm D, Van Der Hoeven JG, Peek N. Body mass index is associated with hospital mortality in critically ill patients: An observational cohort study. Crit. Care Med. 2013;41(8):1878–1883. doi: 10.1097/CCM.0b013e31828a2aa1. [DOI] [PubMed] [Google Scholar]
- 19.Hutagalung R, Marques J, Kobylka K, Zeidan M, Kabisch B, Brunkhorst F, et al. The obesity paradox in surgical intensive care unit patients. Intens. Care Med. 2011;37(11):1793–1799. doi: 10.1007/s00134-011-2321-2. [DOI] [PubMed] [Google Scholar]
- 20.Guo Z, Wang X, Wang Y, Xing G, Liu S. “Obesity paradox” in acute respiratory distress syndrome: A systematic review and meta-analysis. PLoS ONE. 2016;11(9):e0163677. doi: 10.1371/journal.pone.0163677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mesas AE, Cavero-Redondo I, Álvarez-Bueno C, Cabrera MAS, de Andrade SM, Sequí-Dominguez I, et al. Predictors of in-hospital COVID-19 mortality: A comprehensive systematic review and meta-analysis exploring differences by age, sex and health conditions. PLoS ONE. 2020;15(11):e0241742. doi: 10.1371/journal.pone.0241742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shryane N, Pampaka M, Aparicio-Castro A, Ahmad S, Elliot MJ, Kim J, et al. Length of stay in ICU of Covid-19 patients in England, March–May 2020. Int. J. Popul. Data Sci. 2021;5(4):1411. doi: 10.23889/ijpds.v5i4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zampieri FG, Soares M, Borges LP, Figueira Salluh JI, Ranzani OT. The epimed monitor ICU database®: A cloud-based national registry for adult intensive care unit patients in Brazil. Rev. Bras. Ter. Intens. 2017;29(4):418–426. doi: 10.5935/0103-507X.20170062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenland S, Pearl JMR. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48. doi: 10.1097/00001648-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Sawadogo W, Tsegaye M, Gizaw A, Adera T. Overweight and obesity as risk factors for COVID-19-associated hospitalisations and death: Systematic review and meta-analysis. BMJ Nutr. Prev. Health. 2022;2:e000375. doi: 10.1136/bmjnph-2021-000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Textor J, van der Zander B, Gilthorpe MS, Liśkiewicz M, Ellison GT. Robust causal inference using directed acyclic graphs: The R package “dagitty”. Int. J. Epidemiol. 2016;45(6):1887–1894. doi: 10.1093/ije/dyw341. [DOI] [PubMed] [Google Scholar]
- 27.Senpe SE de NP y E, SEGG SE de G y G. Valoración Nutricional del Anciano, 1st edn (2011).
- 28.Paravidino VB, Sichieri R, Gomes DCK, e Silva GA. High discrepancies in the mortality of hospitalized patients with COVID-19 in the two most economically important states in Brazil. Rev. Bras. Epidemiol. 2021 doi: 10.1590/1980-549720210056. [DOI] [PubMed] [Google Scholar]
- 29.Di Cesare M, Bentham J, Stevens GA, Zhou B, Danaei G, Lu Y, et al. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387(10026):1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Statistics BI of Group and Painel de Indicadores—PNS. https://www.pns.icict.fiocruz.br/painel-de-indicadores-mobile-desktop/. Accessed July 2022.
- 31.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. OpenSAFELY: Factors associated with COVID-19 death in 17 million patients. Nature. 2020;584(7821):430. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Recalde M, Pistillo A, Fernandez-Bertolin S, Roel E, Aragon M, Freisling H, et al. Body mass index and risk of COVID-19 diagnosis, hospitalization, and death: A cohort study of 2524926 catalans. J. Clin. Endocrinol. Metab. 2021 doi: 10.1210/clinem/dgab546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dixon AE, Peters U. The effect of obesity on lung function. Expert Rev. Respir. Med. 2018;12(9):755–767. doi: 10.1080/17476348.2018.1506331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malavazos AE, Corsi Romanelli MM, Bandera F, Iacobellis G. Targeting the adipose tissue in COVID-19. Obesity (Silver Spring) 2020;28(7):1178–1179. doi: 10.1002/oby.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samad F, Ruf W. Inflammation, obesity, and thrombosis. Blood. 2013;122(20):3415–3422. doi: 10.1182/blood-2013-05-427708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higham A, Singh D. Increased ACE2 expression in bronchial epithelium of COPD patients who are overweight. Obesity (Silver Spring) 2020;28(9):1586–1589. doi: 10.1002/oby.22907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vera-Zertuche JM, Mancilla-Galindo JM-G, Tlalpa-Prisco M, Aguilar-Alonso P, Aguirre-García MM, Segura-Badilla O, et al. Obesity is a strong risk factor for short-term mortality and adverse outcomes in Mexican patients with COVID-19: A National Observational Study. Epidemiol. Infect. 2021;149:109. doi: 10.1017/S0950268821001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klang E, Kassim G, Soffer S, Freeman R, Levin MA, Reich DL. Severe obesity as an independent risk factor for COVID-19 mortality in hospitalized patients younger than 50. Obesity (Silver Spring) 2020;28(9):1595–1599. doi: 10.1002/oby.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karki S, Muscatello DJ, Banks E, MacIntyre CR, McIntyre P, Liu B. Association between body mass index and laboratory-confirmed influenza in middle aged and older adults: A prospective cohort study. Int. J. Obes. (Lond.) 2018;42(8):1480–1488. doi: 10.1038/s41366-018-0029-x. [DOI] [PubMed] [Google Scholar]
- 40.Ni YN, Luo J, Yu H, Wang YW, Hu YH, Liu D, et al. Can body mass index predict clinical outcomes for patients with acute lung injury/acute respiratory distress syndrome? A meta-analysis. Crit. Care. 2017;21(1):3. doi: 10.1186/s13054-017-1615-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oreopoulos A, Kalantar-Zadeh K, Sharma AM, Fonarow GC. The obesity paradox in the elderly: Potential mechanisms and clinical implications. Clin. Geriatr. Med. 2009;25(4):643–659. doi: 10.1016/j.cger.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 42.El Moheb M, Jia Z, Qin H, El Hechi MW, Nordestgaard AT, Lee JM, et al. The obesity paradox in elderly patients undergoing emergency surgery: A nationwide analysis. J. Surg. Res. 2021;265:195–203. doi: 10.1016/j.jss.2021.02.008. [DOI] [PubMed] [Google Scholar]
- 43.Schetz M, De Jong A, Deane AM, Druml W, Hemelaar P, Pelosi P, et al. Obesity in the critically ill: A narrative review. Intens. Care Med. 2019;45(6):757–769. doi: 10.1007/s00134-019-05594-1. [DOI] [PubMed] [Google Scholar]
- 44.Zhang X, Yu J, Ya PL, Yin JH. ACEI/ARB use and risk of infection or severity or mortality of COVID-19: A systematic review and meta-analysis. Pharmacol. Res. 2020;158:104927. doi: 10.1016/j.phrs.2020.104927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sjögren L, Stenberg E, Thuccani M, Martikainen J, Rylanderid C, Wallenius V, et al. Impact of obesity on intensive care outcomes in patients with COVID-19 in Sweden—A cohort study. PLoS ONE. 2021;16:e0257891. doi: 10.1371/journal.pone.0257891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castro AAM, Calil SR, Freitas SA, Oliveira AB, Porto EF. Chest physiotherapy effectiveness to reduce hospitalization and mechanical ventilation length of stay, pulmonary infection rate and mortality in ICU patients. Respir. Med. 2013;107(1):68–74. doi: 10.1016/j.rmed.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 47.Goldfarb MJ, Bibas L, Bartlett V, Jones H, Khan N. Outcomes of patient- and family-centered care interventions in the ICU: A systematic review and meta-analysis. Crit. Care Med. 2017;45(10):1751–1761. doi: 10.1097/CCM.0000000000002624. [DOI] [PubMed] [Google Scholar]
- 48.Moreira NF, Luz VG, Moreira CC, Pereira RA, Sichieri R, Ferreira MG, et al. Self-reported weight and height are valid measures to determine weight status: Results from the Brazilian National Health Survey (PNS 2013) Cad. Saude Publica. 2018 doi: 10.1590/0102-311x00063917. [DOI] [PubMed] [Google Scholar]
- 49.Mancilla-Galindo J, Kammar-García A, Martínez-Esteban A, Meza-Comparán HD, Mancilla-Ramírez J, Galindo-Sevilla N. COVID-19 patients with increasing age experience differential time to initial medical care and severity of symptoms. Epidemiol. Infect. 2021 doi: 10.1017/S095026882100234X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Todt BC, Szlejf C, Duim E, Linhares AOM, Kogiso D, Varela G, et al. Clinical outcomes and quality of life of COVID-19 survivors: A follow-up of 3 months post hospital discharge. Respir. Med. 2021;184:106453. doi: 10.1016/j.rmed.2021.106453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset used and/or analyzed during the current study is available from the corresponding author on reasonable request. The data collection procedure was performed in accordance with relevant guidelines and regulations.