Abstract
Messenger RNAs (mRNAs) serve as blueprints for protein synthesis by the molecular machine the ribosome. The ribosome relies on hydrogen bonding interactions between adaptor aminoacyl-transfer RNA molecules and mRNAs to ensure the rapid and faithful translation of the genetic code into protein. There is a growing body of evidence suggesting that chemical modifications to mRNA nucleosides impact the speed and accuracy of protein synthesis by the ribosome. Modulations in translation rates have downstream effects beyond protein production, influencing protein folding and mRNA stability. Given the prevalence of such modifications in mRNA coding regions, it is imperative to understand the consequences of individual modifications on translation. In this review we present the current state of our knowledge regarding how individual mRNA modifications influence ribosome function. Our comprehensive comparison of the impacts of 16 different mRNA modifications on translation reveals that most modifications can alter the elongation step in the protein synthesis pathway. Additionally, we discuss the context dependence of these effects, highlighting the necessity of further study to uncover the rules that govern how any given chemical modification in an mRNA codon is read by the ribosome.
Keywords: Translation, RNA modification, mRNA modification, Kinetics
1. Introduction
RNA molecules serve a variety of essential roles in the cellular protein synthesis machinery. Ribosomal RNAs (rRNAs) form the core of the ribosome, messenger RNAs (mRNAs) act as templates for the ribosome to ensure that amino acids are added in the correct order, and transfer RNAs (tRNAs) bring amino acids into the ribosome (Fig. 1A). These diverse functions are accomplished despite the relatively redundant chemical properties of the four nucleoside building blocks (cytidine, uridine, guanosine and adenosine) used to make all RNAs (Fig. 1B). One strategy that cells use to overcome the monotonous nature of the standard nucleosides is to enzymatically modify their structures after they are linked together to form RNAs. In all organisms post-transcriptional modifications increase the topologies, chemistries and functionalities available to RNA molecules [1,2,3,4,5,6,7,8]. While the significance of modified nucleosides in tRNA and rRNAs have been well-known for decades, mRNA modifications are only recently gaining recognition as modulators of mRNA maturation, structure, stability and translation [9,10,3,11,5,12,7]. Consistent with the idea that mRNA modifications have the potential to play important biological roles, the dysregulation of mRNA incorporating enzymes is linked to development a variety of diseases, including intellectual disorders and cancers [13,14, 15,16,17,18,19,20,21,22,23,24,25,26,27]. In this review we present data suggesting that most modifications influence how quickly and accurately the ribosome decodes an mRNA. We also discuss the limitations in our current capacity to predict which sites of mRNA modification are likely to promote biologically significant perturbations to protein synthesis.
Fig. 1.
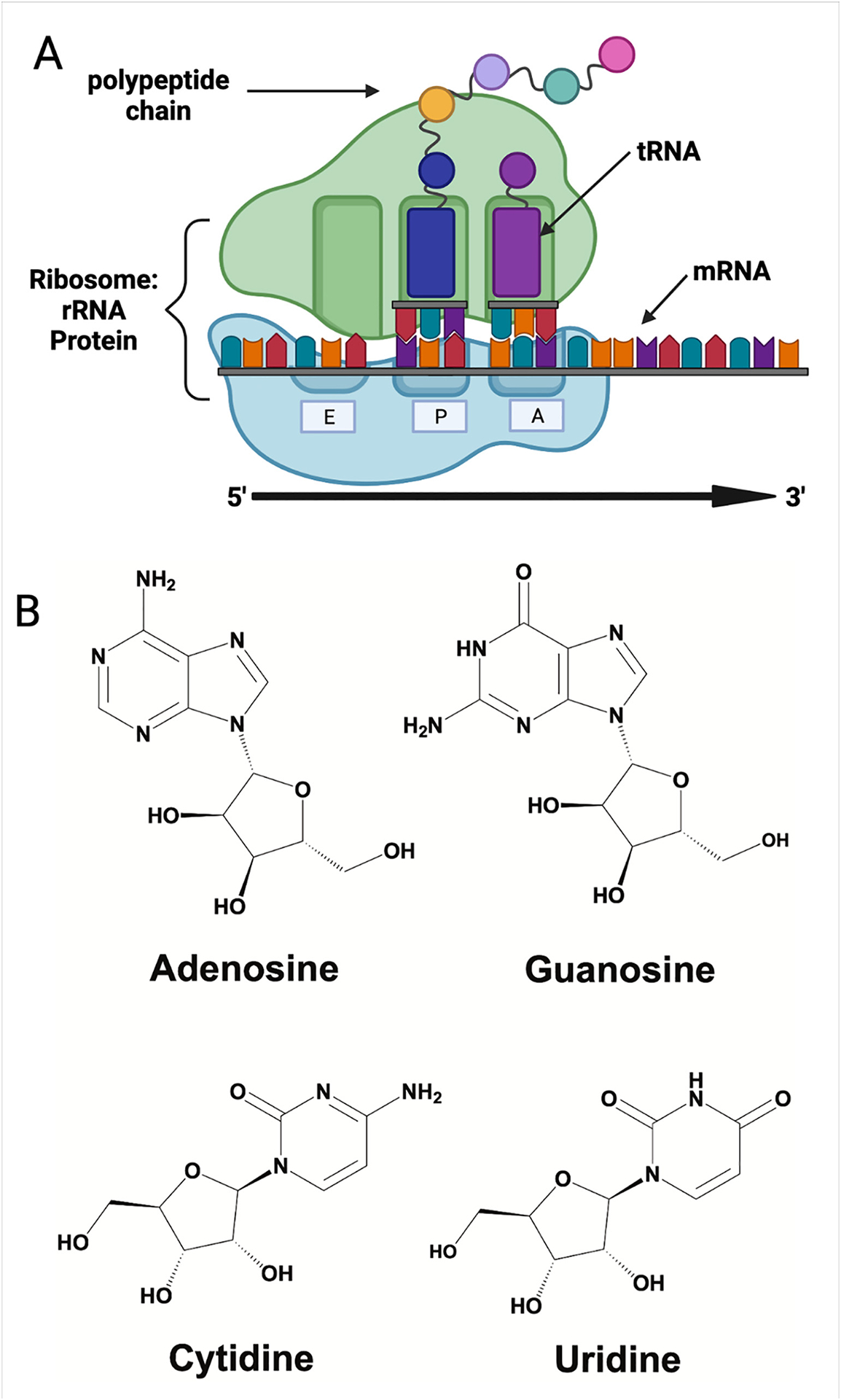
RNAs form the basis of the protein synthesis machinery. (A) Depiction of the basic components of the translational machinery, with the three central RNA species highlighted (rRNA, tRNA and mRNA). (B) The four chemical building blocks of RNA: adenosine (A), guanosine (G), cytidine (C) and uridine (U).
To date > 20 chemical modifications have been detected in eukaryotic protein coding mRNAs [28,3,4] (Fig. 2). Modifications can be added by enzymes or through non-enzymatic damage, such as alkylation or oxidation [28,29,30]. RNA-seq based technologies enabled the development of maps reporting where 13 enzymatically incorporated modifications can reside in all RNAs within a cell: N6-methyladenosine (m6A), pseudouridine (Ψ), dihydrouridine (D), N4-acetylcytidine (ac4C), N1-methyladenosine (m1A), N7-methylguanosine (m7G), 2′O-methyl modifications (Cm, Am, Gm, Um), 5-methylcytidine (m5C), and 5-hydroxymethylcytidine (hm5C), and inosine (I) [31,32,1,33,34,35,36,37,38,39,40]. Apart from the m7G cap incorporated into the 5′ end of all eukaryotic mRNAs, quantitative liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) studies reveal that N6-methyladenosine (m6A), inosine (I) and pseudouridine (Ψ) are the most common modifications incorporated into mRNA by enzymes (Fig. 2A) [3]. m6A, Ψ and I exist in concentrations 10 to 100-fold above those of other reported modifications [3]. mRNA modifications resulting from oxidation, alkylation, or UV damage are typically present at lower levels than enzymatically incorporated modifications [30]. Such damage to RNA bases is usually found at the most chemically reactive groups (N- or O-) in the purine and pyrimidine rings of all four nucleosides [e.g. 8-oxoguanosine, N3-methylcytidine, O4-methyluridine and 1-methyladenosine] (Fig. 2B) [30]. Regardless of their origin, all nucleoside chemical modifications have the potential to impact how ther ribosome decodes an mRNA (Fig. 3A).
Fig. 2.
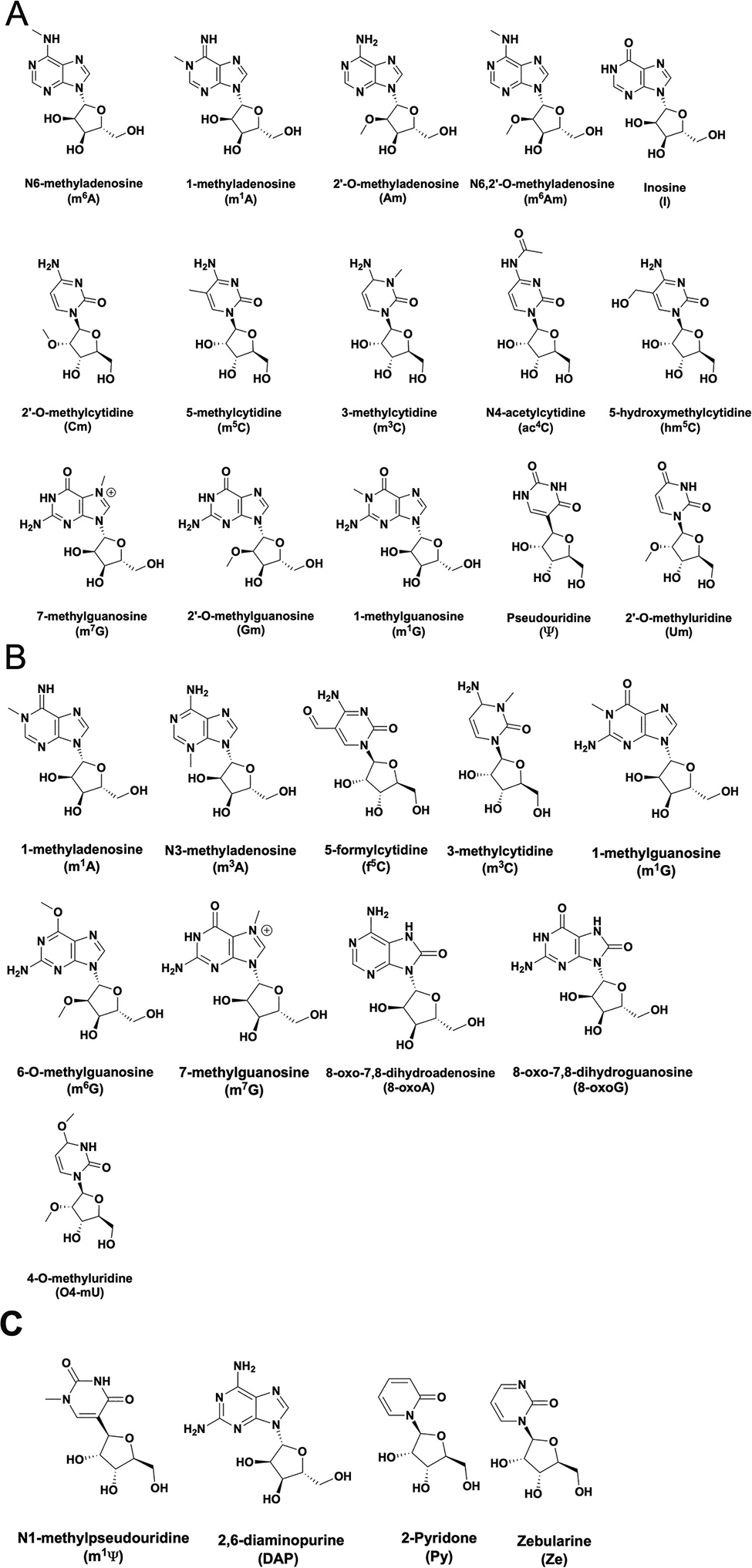
Modifications in mRNAs. (A) Modifications reported to be enzymatically incorporated into mRNAs. (B) Modifications that can be incorporated as a result of RNA damage. (C) Modifications not found naturally found in mRNAs that are either incorporated into mRNA vaccines (m1Ψ) or have been used to probe translation termination.
Fig. 3.
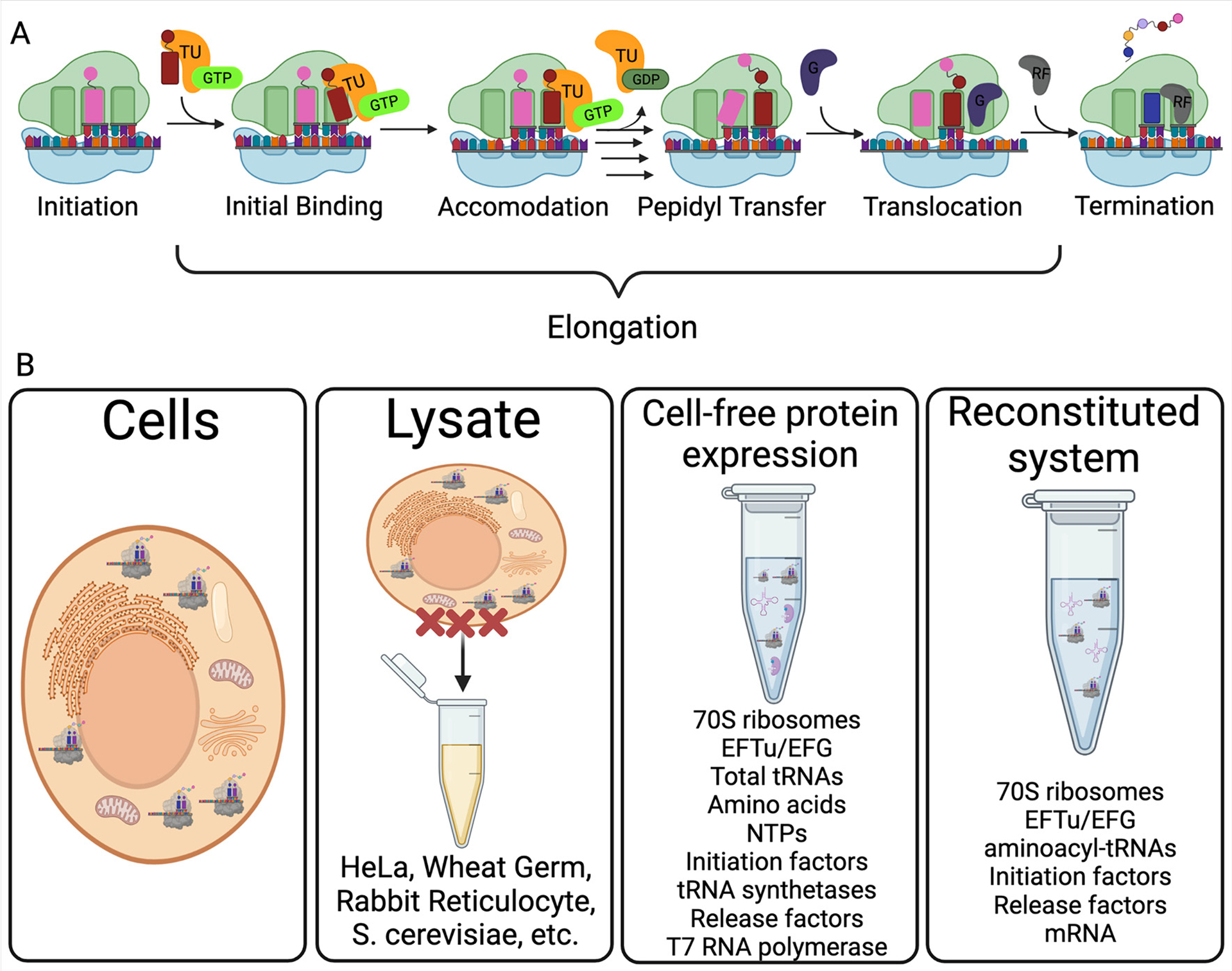
Assessing protein synthesis. (A) Schematic of the steps in bacterial protein synthesis In the first step (initiation), initiation factors (IFs) help the 70S ribosome form on an AUG start codon with fMet-tRNAfMet bound in the ribosome P site. During the elongation phase of translation, aminoacyl-tRNAs (aa-tRNAs) are brought into the ribosome A site by EF-Tu:GTP (initial binding). The aa-tRNA is then positioned properly in the A site during accommodation, and EF-Tu exits following GTP hydrolysis. The amino acid (or peptide) on the P site tRNA is then transferred to the aa-tRNA in the ribosome A site during peptidyl-transfer. After a new peptide bond is catalyzed by the ribosome, EF-G binds and moves (translocates) the ribosome and associated tRNAs to the next codon. The cycle of elongation continues until the ribosome reaches a stop codon (UAA, UGA or UAG), and release factors (RFs) bind to the A site to catalyze the hydrolysis of the complete polypeptide. (B) Common approaches used to study translation. The kinetic resolution and mechanistic detail possible to attain increases as with the purification level of the translation system (from cells to reconstituted translation).
In addition to the modifications present in cellular mRNAs, there is an emerging need to understand the consequences on translation of including non-naturally occurring modifications into mRNA nucleosides. This information is essential because such modifications are required components of many RNA-based therapeutics [41,42,43,44,45,46]. For mRNA therapeutics, non-naturally occurring modifications, such as N1-methylpseudouridine (m1Ψ), enable the mRNA sequences evade degradation by the innate immune response [41]. Indeed, incorporation of such modifications was crucial to the success of the recent COVID-19 mRNA vaccines [43]. Developing a comprehensive understanding of how RNA modifications influence translation will be essential for the continued rapid development of therapeutic mRNA technologies.
Discerning how individual modifications impact protein synthesis is challenging in cells because most of the enzymes that incorporate mRNA modifications also catalyze the addition of modifications into multiple non-coding RNAs essential to translation (rRNA, tRNA; Fig. 1A) [28,3]. This makes it difficult to determine the discrete causes of observed changes to protein production when modifying enzymes are depleted. Furthermore, mRNA modifications are generally incorporated at sub-stoichiometric frequencies, and a mixed population of modified and unmodified mRNAs of the same sequence exist in cells [47,48,49,50,51]. Therefore, our most direct understanding of how mRNA modifications directly impact translation comes from studies investigating protein production from in vitro modified mRNA transcripts in translationally active lysates or fully purified reconstituted translation systems (Fig. 3B). With this in mind, below we examine how 16 individual mRNA modifications can influence the elongation and termination steps in protein synthesis (Fig. 4A), emphasizing work conducted in lysate and purified translation systems (Table 1).
Fig. 4.
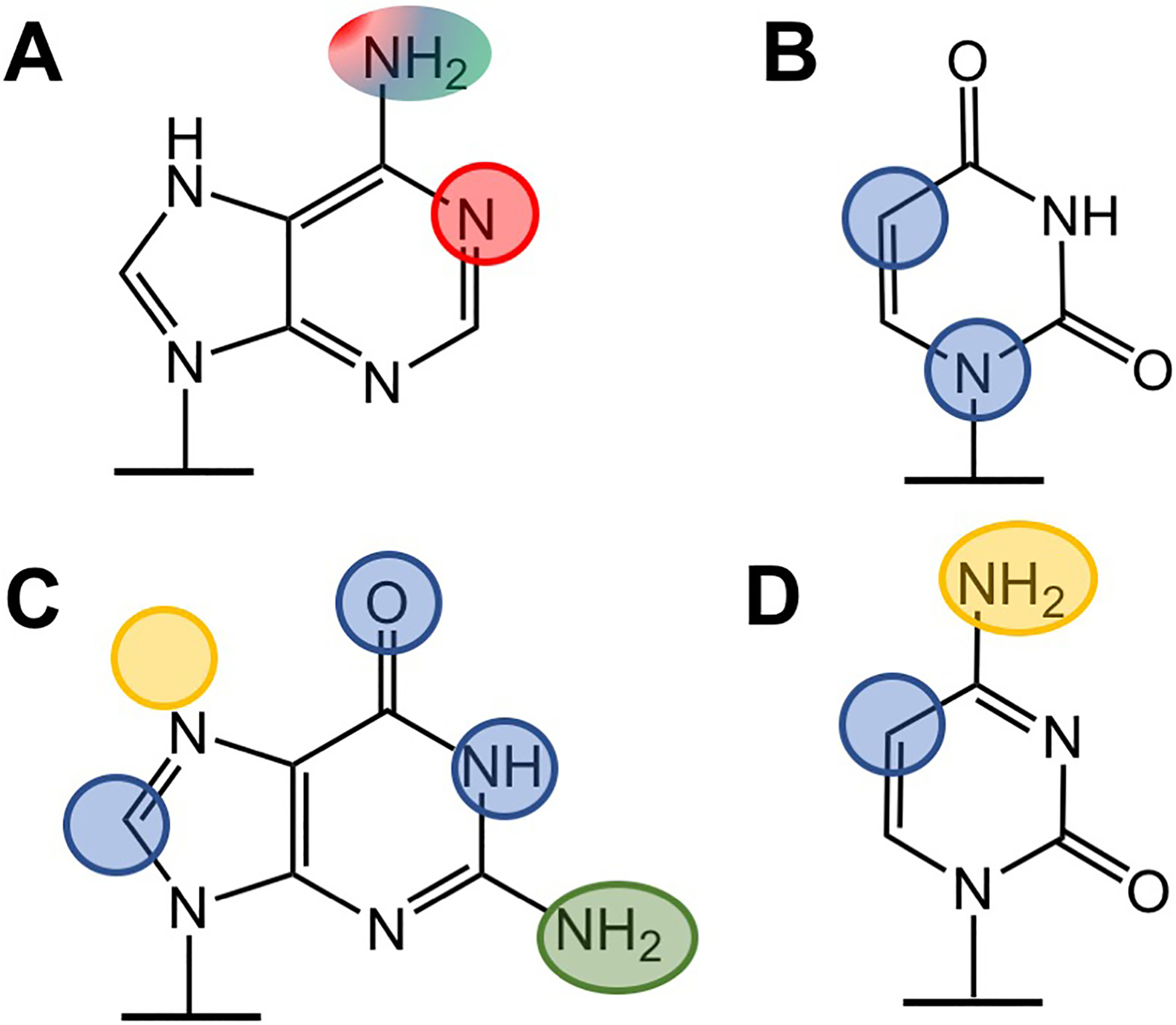
Modification of nucleobase positions impacts translation. Positions in adenosine (A), uridine (B), guanosine (C) and cytidine (D) that impede only elongation rates (red), elongation rates and amino acid mis-incorporation (blue), translation termination (green) are circled. The data are too preliminary to confidently assign impacts to positions highlighted in orange. (For interpretation of the references to colour in this figure legend, the reader is referred to Table 1 and the web version of this article.)
Table 1.
Summary of modifications and their impact on translation.
| Modification | Reported Consequence on Translation | References |
|---|---|---|
| Adenosine | ||
| N6-methyladenosine (m6A) | In cells: Increases protein expression, may increase cap-independent translation. In vitro: Modestly slows translation elongation. Impedes tRNA binding and accommodation. |
[52,53,54,55,56,22,57,58,59,60,61,62,63] |
| 1-methyladenosine (m1A) | Generally represses translation, marks mRNA for degradation. | [64,60,63,65] |
| Inosine (I) | Alters translation accuracy of elongation and termination in some contexts, slows elongation. | [66,67,68] |
| Uridine | ||
| Pseudouridine (Ψ) | Enhances protein production, modestly decreases elongation rates, promotes initiation, can promote low level amino acid mis-incorporation in some contexts | [69,62,59,10,70,68] |
| Guanosine | ||
| 7-methylguanosine (m7G) | 5′ m7G cap: Enhances mRNA maturation, nuclear export, prevents degradation, promotes translation initiation. |
[71] |
| 1-methylguanosine (m1G) | Abolishes translation when present at 1st or 2nd position of a codon, reduces ribosome accuracy and protein production | [63] |
| 6-O-methylguanosine (m6G) | Reduces the rate constant for amino acid addition and ribosome accuracy in a position dependent manner. | [72,63] [61] |
| 8-oxo-7,8-dihydroguanosine (8-oxo) | Stalls elongation, decreases yield of protein product. | [73,74] |
| Cytidine | ||
| 5-methylcytidine (m5C) | In cells:Can repress and enhance translation. In vitro: Decreases protein production, enhances miscoding |
[59,75] |
| N4-acetylcytidine (ac4C) | Increases translation efficiency. | [76] |
| Ribose | ||
| 2′-O-methylations (Am, Gm, Um, Cm) | Decreases translational efficiency in position and sequence dependent manner. | [59] |
| Non-naturally occurring | ||
| N1-methylpseudouridine (m1Ψ) | Increases protein yield, slows elongation, increases initiation. | [77,78,79,80,81,68] |
| 2-Pyridone (Py) | Abolishes stop codon recognition by release factors. | [82] |
| Zebularine (Ze) | Abolishes stop codon recognition, promotes read through. | [82] |
| Purine (P) | Decreases stop codon recognition by RF2, but not RF1, in a position dependent manner. | [82] |
| 2,6-diaminopurine (DAP) | Decreases stop codon recognition by RF2, but not RF1, in a position dependent manner. | [82] |
2. Adenosine modifications
Post-transcriptional chemical additions to adenosine represent the most well-studied class of mRNA modifications. Three enzymatically incorporated adenosine modifications that change Watson-Crick face of nucleobases have been reported: N6-methyladenosine (m6A), N1-methyladenosine (m1A) and inosine (I). Notably, m1A can also be added non-enzymatically, as the result of alkylative damage. Each of these modifications has the potential to change hydrogen bonding interactions between an A site mRNA codon and an incoming aa-tRNA or release factor. Given that the ribosome relies heavily on precise hydrogen bonding patterns to ensure rapid and accurate translation, it is unsurprising that all three of these modifications perturb mRNA decoding by the ribosome. However, the severity of these effects varies widely between m6A, m1A and I, suggesting that additional factors beyond the simple disruption of mRNA:tRNA basepairs contribute to their consequences on translation.
2.1. N6-methyladenosine (m6A)
mA can be incorporated at thousands of locations in the transcriptome [83,84,85,86]. It was the first mRNA modification whose location was mapped and is a clear modulator of mRNA stability [87,88,6,89]. While m6A has been observed throughout mRNA transcripts, it is enriched in mRNA 3′ untranslated regions (3’ UTR) and coding sequences (CDS) around stop codons [86,7]. Initial studies speculated that these sites enhance translation [52]. Consistent with this, recent ribosome profiling, RIP-seq and reporter assays suggest that m6A binding proteins (YTHDF1, 3 and METTL3) increase the translation efficiency of m6A-containing mRNAs in cells and may promote cap independent translation [56,53,22,57,54,55]. While these correlative studies suggest the possibility that m6A increases protein expression, investigations with modified mRNAs in lysates and bacterial reconstituted translation systems reach different conclusions.
Studies with in vitro transcribed mRNAs in translationally active lysates and purified translation systems reveal that the elongation of growing polypeptides by the ribosome slows on m6A containing codons [58,59,60,61,62,90,63]. The impact of m6A on translation elongation dependents upon the position of the modification within a given codon. The largest reductions in peptide formation are observed when m6A is incorporated into the first position of a codon, while the smallest effects are seen when m6A is in the 3rd position [58,59,63]. The reduced impact on elongation at the 3rd position could possibly be attributed to the permissive base-pairing between the tRNA and mRNA tolerated by the ribosome at the wobble-position [91]. The detrimental influence of m6A on translation elongation has been observed in both bacterial and eukaryotic cells, though the reported effects are most significant in the fully purified E. coli translation system [59,60,63]. Mechanistic studies of purified E. coli ribosomes reveal m6A disrupts a crucial step in ribosome decoding, the hydrolysis of GTP by EF-Tu (Fig. 3A) [58]. These findings are consistent with NMR studies reporting that m6A destabilizes duplex A–U base pairs [92]. Despite the potential of m6A to influence mRNA:tRNA base-pairing, and its ability to perturb tRNA binding and accommodation, it does not increase the propensity of the ribosome to select the wrong tRNAs during elongation [70,61]. These data suggest the possibility that the inclusion of m6A into codons may present cells with a way to transiently slow the ribosome. Programmed transitory pauses \ could conceivably help to facilitate co-translational processes, such as nascent protein folding and modification, without detrimentally impacting the accuracy of protein production.
2.2. N1-methyladenosine (m1A)
The prevalence of m1A in mRNA transcripts has been controversial, with some groups reporting that it exists at low frequency within thousands of eukaryotic transcripts and others suggesting that m1A is rarely incorporated into a handful cytoplasmic mRNAs and a single mitochondrial mRNA [93,94,64, 95]. Establishing the location and abundance of m1A is complicated both by the ability of m1A to be added non-enzymatically under alkylative stress, and the apparent influence of tissue type and cellular conditions on m1A incorporation [64,29,30]. Regardless of when m1A is present or how it is incorporated, when the ribosome encounters the modification it has the potential to impact translation.
m1A contains a methyl group attached the N1 position of the ring, causing the nucleobase to be positively charged (Fig. 2). Relative to adenosine, m1A has significantly disrupted hydrogen bonding accepting potential, and as such, can be reasonably expected to disrupt tRNA: mRNA interactions [96]. Consistent with this, polysome fractionation experiments and luciferase-based reporter assays in human cells demonstrate that m1A containing mRNAs are translationally repressed [64]. These findings are supported by observations that the insertion of m1A robustly inhibits mRNA translation in bacterial, yeast and wheat germ lysate based translation systems [60,63]. Pre-steady state kinetic analyses of cognate and near-cognate amino acid addition on m1A modified codons reveal that the modification generally inhibits the ability of adenosine to form base pairs [65]. In light of this, it makes sense that when the ribosome decodes m1A modified mRNAs in E. coli it activates the mRNA quality control pathway (tmRNA) that targets damaged mRNAs for degradation [65]. We expect similar quality control mechanisms, such as No-Go Decay (NGD), will be triggered in eukaryotes when the ribosome encounters m1A containing codons [97].
2.3. Inosine (I)
The deamination of adenosine to form inosine within mRNAs is catalyzed by a family of enzymes called adenosine deaminases acting on RNA (ADARs) [98]. Inosine was among the first modifications to be discovered in mRNA, and the consequences of inosine incorporation change depending on where it is localized within a transcript [99]. The majority of inosine sites are located in mRNA untranslated regions where they modulate mRNA stability, structure and localization [100,101]. Nonetheless, over 1000 sites have been reported in mRNA coding regions, suggesting that inosine is regularly encountered by the ribosome in cells [102].
While inosine is created from adenosine, and lacks an amine group at the C2 position, its chemical structure also closely resembles that of guanosine – with a carbonyl at C6 (Fig. 2A). Because its structure is intermediate between adenosine and guanosine, inosine has the ability to base pair with adenosine, cytidine, and uridine - though it preferentially binds to cytidine [101]. The significance of this property is highlighted by the observation that inosine modifications in tRNA anticodons can be recognized as guanosine by the ribosome during translation [103]. As such, it is unsurprising the incorporation of inosine into mRNA codons can lead to the incorporation of a variety of amino acids at the modified position [66]. It is hypothesized that ability to incorporate non-cognate amino acids could be a way to alter protein activities. Indeed, this has been shown to happen in the glutamate receptor GluR-B, where the presence of an inosine in an mRNA leads to the incorporation of arginine instead of glutamine (B. [104]). This is a functionally significant change, influencing the receptor efficiency and selectivity. However, inosine does not always cause the re-coding of an mRNA. The ability to promote alternative amino acid incorporation depends strongly on the position of the modification within a codon and context of the surrounding sequence. Some codons containing inosine modifications promote amino acid substitution < 1% of the time, and others result in substitutions nearly every time [66]. While the ribosome appears to mis-incorporate amino acids most often when inosine as at the 1st position in a codon, the parameters dictating context dependent still remain to be established.
Not only does inosine have the ability to change the identity of protein products, iterated inosines also cause ribosomes to stall and generate truncated peptide products [67,66]. Changes to multiple aspects of the translation pathway likely contribute to these stalling events, including inosine impacting EF-Tu binding and anti-codon/codon interactions [67]. Furthermore, the substitution of A for I in UAG, UGA and UAA stop codons can change the specificity of bacterial release factors, with UIG and UGI substitutions increasing stop-codon read-through (by up to 90%) [82,105,66]. Together these findings suggest that the promiscuous nature of inosine base pairing can impact all steps of translation that depend on hydrogen bonding to ensure accuracy.
3. Uridine modifications
In contrast to modifications of other nucleosides, uridine modifications have been generally more difficult to discover and quantify in mRNAs in part due to the lower limits of detection possible for these nucleosides on mass-spectrometry in LC-MS/MS. Nonetheless, the two most common uridine modifications in non-coding RNAs, pseudouridine (Ψ) and dihydrouridine (D), have also been reported in mRNAs [106,31,107,108,109]. D was discovered only recently, and the loss of the enzymes that incorporate it into mRNAs (which also target tRNAs) appears to slow translation [31]. Ψ is the second most abundant naturally occurring modification in mRNAs, and is also notable because it is related to the 1-methylpseudouridine (m1Ψ) modification included in many mRNA vaccines and therapeutics [41,43] (Fig. 4B, and ‘non-natural mRNA modifications’ section below). While decades of studies demonstrate that Ψs present in tRNA make important contributions to translation, it has only recently come to light that Ψ in mRNA may also have significant consequences on protein synthesis.
3.1. Pseudouridine (Ψ)
Pseudouridine is unique among mRNA modifications because it is the only isomer of a nucleoside base that is incorporated (Fig. 2). Studies mapping the position of Ψ in the transcriptome indicate that it is present in hundreds of mRNA transcripts, with the majority of Ψ sites being localized in mRNA introns and CDS regions [106,107,108,110,111,109]. Initial reporter studies in cells suggested that the inclusion of Ψ in mRNA codons enhances translation and promotes protein production [69,62]. However, in vitro studies reached contradictory conclusions, indicating that Ψ slows translation [70,59,60]. These findings have since been reconciled by a recent study demonstrating that Ψ containing mRNAs slow elongation to induce stalling events, but still generate high levels of protein because they exhibit increased levels of ribosome loading onto transcripts. The impact of Ψ on translation termination has also been investigated. The insertion of Ψ has been reported to suppress translation termination at stop codons, though the degree to which this occurs in cells remains controversial [70,112,113,114]. Taken together these studies demonstrate that Ψ can influence multiple steps along the translation pathway.
Ψ has long been studied for its ability to form non-canonical base pairs. Similar to inosine - though to a lesser degree - Ψ also promotes the addition of near-cognate amino acids in a highly context dependent manner [70]. In vitro translation studies reveal that the inclusion of Ψ increases the rate constant for valine mis-incorporation on phenylalanine UUU codons when Ψ is at the first and third codon positions. Investigations of amino acid mis-incorporation into luciferase reporters produced from Ψ substituted mRNAs in HEK293 cells support these findings. Luciferase peptides generated from fully Ψ substituted mRNAs have much higher (> 20-fold) levels of mis-incorporated amino acids than peptides made from mRNAs only containing canonical uridine nucleosides [70]. However, consistent with the context dependence observed in in vitro translation studies, amino acid addition was not observed on every Ψ substituted codon. The position dependent modulations in ribosome accuracy by Ψ is akin to what has been reported for inosine. Understanding the rules that govern these context dependent effects will be important for researchers seeking to identify which Ψ-modified mRNA sites have the greatest potential to translationally control gene expression.
4. Guanosine modifications
Methylations account for all of the mRNA modifications reported in guanosine nucleosides to date. These modifications result from either enzymatic reactions (N7-methylguanosine) and non-specific RNA damage (6-O-methylguanosine, 1-methylguanosine, 8-oxoguanosine) (Fig. 2). Similar to analogous purine methylations in adenosine, guanosine modifications impact the speed and fidelity of translation (Fig. 4C). As might be expected, the promiscuously incorporated RNA damage products all strongly reduce the ability of the ribosome to catalyze protein synthesis.
4.1. N7-methylguanosine (m7G)
The N7-methylguanasine cap present at the 5′ of all eukaryotic mRNAs is perhaps the most well studied mRNA modification. In contrast to the other modifications discussed thus far, the m7G cap is attached to mRNAs by a tri-phosphate linkage [115]. The m7G cap is a key feature of eukaryotic mRNAs, making crucial contributions to mRNA maturation, nuclear export, stability and translation initiation [71]. Recently m7G modifications were also discovered in mRNAs outside of the 5′ cap, in the 3′ UTR and CDS regions of mRNAs [116,71,117]. As the many functions of the m7G cap have been widely reviewed elsewhere [118,119], we will limit our brief discussion to the new class of internal mRNA m7G modifications.
Like other methylations, the location of m7G sites within mRNA transcripts redistributes under varying stress conditions [71]. Analysis of human ribosome profiling data suggest that the inclusion of m7G increases the translation efficiency of modified mRNAs [71]. However, whether the observed impact is mediated by a cellular protein, occurs at initiation, or is a direct consequence of modifications at discrete sites on translation elongation or termination remains to be seen. Given the known ability of m7G in rRNAs and tRNAs to alter duplex RNA structure and dynamics [120,121,122] we anticipate that direct studies with purified components (Fig. 3B) will reveal that m7G impedes tRNA binding and accommodation, similar to other methylations that change RNA structure and dynamics. Knowing this information would allow future studies to direct their investigations towards identifying RNA-protein interactions that may help to improve translation efficiency, akin to the proposed role YTHDF reader proteins in enhancing the translation of m6A-modified transcripts [53].
4.2. 1-methylguanosine (m1G)
1-methylguanosine (m1G) is among the least abundant modifications reported in mammalian mRNAs [3]. Translation studies in wheat germ lysates and a reconstituted E. coli translation system suggest that, much like m1A, m1G can have dramatic effects on protein synthesis [63]. The incorporation of m1G into the 1st and 2nd codon positions abolishes protein production from reporter mRNAs (Fig. 3B), though protein output is unaffected when m1G was instead present in the 3rd position (wobble). The introduction of m1G into the first position of an mRNA codon also reduces protein production and ribosome accuracy (being decoded as either uridine or cytidine) in translationally active wheat germ lysates. Amino acid substitution on m1G codons is also observed, though to a more dramatic extent, in the peptides produced from the E. coli translation system. m1G changes translation in a similar way as m1A, suggesting that methylations to the N1 position of purine rings generally disrupts ribosome function [65].
4.3. 6-O-methylguanosine (m6G)
While the alkylation of adenosine at the N6 position is accomplished enzymatically, the analogous modification in guanosine (6-O-methylguanosine (m6G)) occurs through RNA damage [61,29,30]. Despite its discovery over 30 years ago the impact of m6G on translation has only been recently assessed [72,63] [61]. m6G changes in the guanosine Watson-Crick basepairing face and permits the nucleoside to be decoded as an adenosine. This results in the increased mis-incorporation of amino acids by both bacterial and eukaryotic ribosomes [61,63]. In cells, this change in basepairing has the potential to alter protein composition, or impact the overall rate of protein synthesis.
Investigations across multiple translation systems (Fig. 3B) demonstrate that m6G perturbs protein synthesis in a position dependent manner. When poised in the first position of a codon, m6G reduces the rate constants for amino acid addition by the ribosome and impedes the cognate tRNA selection [61]. Substitution of m6G for G at the second position in a codon also reduces peptide bond formation with cognate aa-tRNA by 1000-fold, but unlike subsitution at the first position, it does not alter tRNA selection to permit non-cognate aa-tRNA to react [61,63]. In contrast, the presence of m6G at the 3rd position in a codon does not appear to affect cognate aa-tRNA interactions with the ribosome, but does enhance the incorporation of near cognate amino acids [61,63]. Interestingly, the ability of m6G to promote miscoding when in the 1st and 3rd positions of a codon, but not in the second, is reminiscent of the effects of Ψ. This differs markedly from the behavior of m6A, suggesting that adding an electron donating methyl-group to the hydrogen bond acceptor of a nucleobase is more detrimental than adding a methyl group to an hydrogen bond donor in N6 position of a purine ring.
4.4. 8-oxoguanine (8-oxoG)
It is estimated that 8-oxoG is generated once per every 105 unmodified guanosine nucleosides. It results from oxidative damage, and mRNAs are particularly susceptible to such damage due to their single stranded structures [123,124]. 8-oxoG is among the most abundant RNA damage products and significantly disrupts RNA structure [125,126]. Consistent with its ability to perturb RNA structure, the inclusion of 8-oxoG in mRNAs has been shown to stall translation elongation, decreasing the yield of protein product in both eukaryotic lysates and fully purified bacterial translation systems [73,74]. Consistent with this, kinetic studies in a reconstituted translation system demonstrate the inclusion of 8-oxoG in an mRNA codon reduces the rate constants for amino acid addition by up to four orders of magnitude [74]. In contrast to many other modifications, the extent to which 8-oxoG changes amino acid addition is independent of where the modification is localized within a codon. 8-oxoG is recognized by near-cognate tRNAs, though the peptide bond formation efficiency for these reactions is low, and the rate constants for near-cognate incorporation on codons containing 8-oxoG are only slightly faster (< 10%) than on unmodified codons [74]. As such, it is unlikely that 8-oxoG causes miscoding in the cell. The steric clash between the oxygen at the 8th position of the base and the phosphate group at the 5th, which changes the base pairing potential of 8-oxoG, likely contributes to the observed uniformity of 8-oxoG mediated disruptions to translation. Given the large magnitude of disruptions to translation caused by 8-oxoG, we expect ribosomes to stall significantly, resulting in ribosome collisions that ultimately lead to the degradation of 8-oxoG containing transcripts by co-translational mRNA surveillance mechanisms [127,128,129].
5. Cytidine modification
While a variety cytidine modifications have been reported in mRNAs (ac4C, m3C, hm5C, m5C, Cm), the consequences of only two nucleobase modifications, 5-methylcytidine and N4-actylcytidine, have been investigated. These initial studies reveal that the activities of enzymes incorporating m5C and ac4C into non-coding RNAs and mRNAs are important for translation. They also raise the possibility that cytidine mRNA modifications may have the unique ability to both slow and enhance translation elongation.
5.1. 5-methylcytidine (m5C)
The most common cytidine modification in both non-coding and protein coding mRNAs is m5C. The presence of m5C in tRNA influences aminoacylation, structure, stability and codon recognition [130] [131,132]. The ability of m5C in tRNA to effect codon:anti-codon interactions suggests that it may have a similar impact when present in mRNA codons. Much like other common modifications, m5C was first identified within mRNAs several decades ago, though its location within transcripts has only recently been mapped [133,134,135][75]. The abundance and frequency of m5C in mRNAs is not yet clear, though transcriptome wide maps of m5C sites indicate that the modification is more common in mRNA UTRs than coding regions [75].
Ribosome profiling studies have reached opposing conclusions about the effect of m5C in mRNA transcripts on translation, with some studies suggesting that these modifications enhance translation, and others indicating that they either repress, or do not change protein synthesis [75,136,137]. For example, a key m5C modifying enzyme, Nsun2 [75], has been reported to both increase and decrease the levels of translation in eukaryotic cells137] [60]. Studies in a fully reconstituted E. coli translation system found that the insertion of a single m5C into an mRNA reporter decreases the production of peptide in a reconstituted E. coli translation system by ~40% and increases amino acid mis-incorporation levels [59]. Such significant effects on both the extent and accuracy of protein production may help to rationalize why m5C is more commonly mapped in mRNA UTRs than in coding regions. Additional studies will be needed to deconvolute the impacts of m5C in UTRs and CDS, as it is possible that m5C in UTR regions enhance translation, while that in CDS regions may repress elongation.
5.2. N4-actylcytidine (ac4C)
The only acetylation reported in mRNA nucleosides is found on the N4 position of cytidine. Ac4C is common in non-coding RNAs and has recently also been observed in yeast, human and hyperthermophilic archaea mRNAs [76,138,139]. The N-acetyltransferase 10 (Nat10 in humans) enzyme is responsible for incorporating ac4C in yeast and humans [76,139]. One recent study conducted an in-depth investigation into the role of ac4C in mRNA [76]. This work used transcriptome-wide approaches to compare the stability and translation of mRNAs in wild-type nat10Δ cells. Their findings suggest that the presence of ac4C in mRNA increases translation efficiency, which the authors attribute to stabilizations in mRNA structure. However, the fact that Nat10 catalyzes ac4C addition not only into mRNAs, but also into tRNAs and rRNAs, and the prediction that most ac4C sites have relatively low frequencies of incorporation, present challenges in the interpretation of these data. Additional biochemical studies will be required to verify these initial studies and fully detangle the contributions of ac4C in mRNA and non-coding RNAs during translation.
6. Ribose modifications (2′ OMe)
2′-O-methylations have been reported on all four standard nucleosides (Am, Gm, Um, Cm) and some modified bases (m6Am) in mRNAs [140]. When present in mRNA CDS regions, 2′OMe modifications decrease translational efficiency in a position and sequence dependent manner [141,59]. Kinetic analyses, together with x-ray crystallography studies, demonstrate that 2′OMe modifications disrupt the ribosome decoding center, perturbing tRNA accommodation during translation elongation [141]. NMR studies reveal that 2′OMe modifications have large impacts on RNA dynamics, suggesting the possibility that these modifications modulate the secondary structure ensembles of mRNAs in the ribosome active site – perhaps subtly favoring conformations that are not optimal for translation [142].
7. Non-naturally occurring mRNA modifications
There is growing interest in understanding the impact of mRNA modifications not present in nature on translation. The inclusion of modifications, such as N1-methylpseudouridine (m1Ψ), in mRNAs vaccines and therapeutics, underscores the need to be able to predict how changing individual positions in a nucleobase will impact translation [43]. Below we present what is known about the influence of m1Ψ on translation, and discuss investigations of a series of non-naturally occurring modifications that revealed the chemical basis for stop-codon recognition by release factors during translation termination.
7.1. N1-methylpseudouridine (m1Ψ)
m1Ψ is present in both tRNA and rRNA, but has not yet been detected in mRNA [28,143,144]. However, it is decoded by the ribosome when it is included in therapeutic mRNA, such as the SARS-CoV-2 mRNA vaccines [43]. Protein yield from m1Ψ-modified mRNAs is increased because the modification helps transcripts to evade the cellular immune response [41,77]. Studies of fully m1Ψ modified mRNA reporters in cell-free rabbit reticulocyte lysate translation systems indicate that m1Ψ also impacts protein synthesis. Like Ψ, m1 Ψ induces ribosome stalling to slow polypeptide elongation, while still creating large amounts of protein product [78]. Similar to Ψ, these two observations can be reconciled by increased translation initiation rates and the ability of cellular membranes to prevent ribosome collisions [68]. Moreover, the ability of m1Ψ to stabilize RNA structure may also account for some of the observed effects of m1Ψ on mRNA half-life and translation [77]. Structure generally increases RNA half-life, and stabilization of some structures in mRNA coding regions can enhance protein expression [79,78].
Though it seems counterintuitive, there are multiple ways that modest decreases in ribosome elongation speed could lead to increase levels of protein product [78]. Slowed progression of the ribosome along an mRNA can enhance co-translational protein folding (and therefore stability) [78,145], provide time for important RNA binding proteins to interact with a transcript [78,80], or the ribosome itself can protect an mRNA from endonucleases [78,81]. Furthermore, because m1Ψ reduces eIF2α-phosphorylation levels, slowed elongation might be beneficial in preventing ribosome collisions that might otherwise occur with rapidly loaded ribosomes. These possibilities can help to rationalize the observed impact of incorporating m1Ψ into mRNAs on the ultimate levels of their resulting protein products.
7.2. Pyridone (Py), zebularine (Ze), 2,6-diaminopurine (DAP), purine (P)
Pyridone (Py), zebularine (Ze), 2,6-diaminopurine (DAP) and purine (P) have all been introduced into stop codons (UAA, UAG, UGA) to assess the chemical requirements of nucleobases essential for translation termination. At the stringently monitored U1 position, removal of the ability of stop-codon nucleobases to donate and accept hydrogen bonds by the inclusion of Py and Ze abolishes the recognition of stop-codons release factors [82]. However, these modifications had differential effects – with Ze promoting translational readthrough, and Py leading to stalled ribosomes. In contrast to the U1 pyrimidine modifications, the bacterial release factor 1 (RF1) still robustly catalyzes release on codons containing DAP and Purine at the 2nd and 3rd position [82]. However, release factor 2 (RF2) is only able to perform peptide hydrolysis when Purine is in the 3rd codon position, and DAP is in the 2nd position of a stop codon. These studies reveal that RF1 more flexibly recognizes stop-codons than RF2. Additionally, the accuracy of RF2 stop codon recognition is enhanced in the presence of an amino or carbonyl group at the second stop-codon nucleotide, and N6 in the last nucleoside of a stop codon. Analogous studies with non-natural nucleosides in other codons could be informative for gaining insight into how modified nucleosides in tRNA anticodons interact with mRNA in the ribosome A site.
8. Conclusions
Post-transcriptional modifications to mRNA nucleosides have been observed at thousands of sites in the eukaryotic transcriptome [106,84,107,108,109,117,51]. Although all of the modifications investigated so far can alter translation (Fig. 4), they do so to different degrees. The largest impacts on amino acid addition rate constants are observed for methylations to the N1 positions on purine nucleobases that abolish the ability of the base to form hydrogen bonds at this crucial position (m1G, m1A). This is in contrast to modifications made to the adjacent O6 and N6 functional groups (m6A, O6G, inosine), which alter tRNA binding and accommodation by the ribosome, but still permit amino acid addition. In general, non-methyl modifications to the 6-membered ring in purines and pyrimidines appear to effect ribosome accuracy (inosine, Ψ), likely because such modifications can significantly change strength and pattern of possible hydrogen bonding interactions between codons and tRNA anti-codons. Notably, merely changing the strength of Watson-Crick hydrogen bonding does not necessarily promote the misreading of codons. Illustrative of this, both inosine and m6A disrupt slow translation elongation, but only inosine promotes amino acid mis-incorporation. This suggests that changing, not simply abrogating, the hydrogen bonding potential of nucleobases has a large influence on tRNA selection. These observations are consistent with what is already known about the fundamental principles that shape mRNA:tRNA interactions, and alterations in Watson-Crick pairing have consequences on translation regardless of whether modifications are incorporated onto tRNA anticodons or mRNA codons.
The parameters that dictate how modifications not on the Watson-Crick face of nucleobases impact translation are less clear. It is possible that such modifications, like Ψ, m5C or 8-oxoG, change nucleobase ring electronics to perturb the strength of the hydrogen bond donors and acceptors involved in base pairing. Additionally, modifications have the capacity to change intra-molecular interactions with an mRNA, or interactions between rRNA and mRNA within the A site. There is growing evidence that such factors, and not only anticodon: codon interactions, have a bigger contribution to translation elongation than previously recognized. This idea is supported by the common conclusion reached in studies of multiple modifications that the effect of a given modification on amino acid addition and translation accuracy largely depends on the sequence context in which the modification is placed.
The in vitro work discussed in this review assesses translation on mRNAs that have every site of interest 100% modified. In cells this is rarely the case. While there are not yet measurements available of the occupancy of most modified sites, we do know that the two most common mRNA modifications, m6A and Ψ, are incorporated at sub-stoichiometric levels (ranging from ~5–80%) [146,147,109,51]. The biological impact of any given modified site on translation will therefore depend largely on its occupancy. This is analogous to what has been observed for protein post-translational modifications, which are also incorporated at similarly sub-stoichiometric levels [148]. We anticipate that sites with higher levels of modification incorporation are likely to have more significant impacts on protein production. Future systematic biochemical and computational studies will be needed to uncover both the stoichiometry of modified mRNA sites as well as the context dependence of translational effects. This information will be broadly useful as researchers seek to identify which of the many chemically-modified positions reported mRNA codons are the most likely to have consequences for translation in cells.
Acknowledgements
We would like to acknowledge Joshua D. Jones for his thoughtful discussions regarding LC-MS/MS studies, as well as Tyler J. Smith and Jeremy Monroe for their careful reading the manuscript. We also thank the National Institutes of Health (NIGMS R35 GM128836) and National Science Foundation (CAREER Award 2045562) for their support. Bio-Render software was used to generate Figs. 1A and 3.
Footnotes
CRediT authorship contribution statement
Monika K. Franco: Conceptualization, Writing – original draft, Writing – review & editing. Kristin S. Koutmou: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Gilbert WV, Bell TA, Schaening C, Messenger RNA modifications: form, distribution, and function, Science (New York, N.Y.) 352 (6292) (2016) 1408–1412, 10.1126/science.aad8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jackman JE, Alfonzo JD, Transfer RNA modifications: Nature’s combinatorial chemistry playground: transfer RNA modifications, Wiley Interdisc. Rev. RNA 4 (1) (2013) 35–48, 10.1002/wrna.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jones JD, Monroe J, Koutmou KS, A molecular-level perspective on the frequency, distribution, and consequences of messenger RNA modifications, Wiley Interdisc. Rev. RNA 11 (4) (2020), e1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].McCown PJ, Ruszkowska A, Kunkler CN, Breger K, Hulewicz JP, Wang MC, Springer NA, Brown JA, Naturally occurring modified ribonucleosides, WIREs RNA 11 (5) (2020), e1595, 10.1002/wrna.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nachtergaele S, He C, The emerging biology of RNA post-transcriptional modifications, RNA Biol. 14 (2) (2017) 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nachtergaele S, He C, Chemical modifications in the life of an mRNA transcript, Annu. Rev. Genet 52 (2018) 349–372, 10.1146/annurev-genet-120417-031522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Roundtree IA, Evans ME, Pan T, He C, Dynamic RNA modifications in gene expression regulation, Cell 169 (7) (2017) 1187–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang X, He C, Dynamic RNA modifications in posttranscriptional regulation, Mol. Cell 56 (1) (2014) 5–12, 10.1016/j.molcel.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Harcourt EM, Kietrys AM, Kool ET, Chemical and structural effects of base modifications in messenger RNA, Nature 541 (7637) (2017) 339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hoernes TP, Hüttenhofer A, Erlacher MD, mRNA modifications: dynamic regulators of gene expression? RNA Biol. 13 (9) (2016) 760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Meyer KD, Jaffrey SR, Rethinking m6A readers, writers, and erasers, Annu. Rev. Cell Dev. Biol 33 (2017) 319–342, 10.1146/annurev-cellbio-100616-060758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Peer E, Rechavi G, Dominissini D, Epitranscriptomics: regulation of mRNA metabolism through modifications, Curr. Opin. Chem. Biol 41 (2017) 93–98. [DOI] [PubMed] [Google Scholar]
- [13].Angelova MT, Dimitrova DG, Dinges N, Lence T, Worpenberg L, Carré C, Roignant J-Y, The emerging field of epitranscriptomics in neurodevelopmental and neuronal disorders, Front. Bioeng. Biotechnol 6 (2018) 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ben-Haim MS, Moshitch-Moshkovitz S, Rechavi G, FTO: linking m 6 a demethylation to adipogenesis, Cell Res. 25 (1) (2015) 3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chen X-Y, Zhang J, Zhu J-S, The role of m 6 A RNA methylation in human cancer, Mol. Cancer 18 (1) (2019) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cui QI, Shi H, Ye P, Li L, Qu Q, Sun G, Sun G, Lu Z, Huang Y, Yang C-G, M6A RNA methylation regulates the self-renewal and tumorigenesis of glioblastoma stem cells, Cell Rep. 18 (11) (2017) 2622–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Du T, Rao S, Wu L, Ye N, Liu Z, Hu H, Xiu J, Shen Y, Xu Q, An association study of the m6A genes with major depressive disorder in Chinese Han population, J. Affect. Disord 183 (2015) 279–286. [DOI] [PubMed] [Google Scholar]
- [18].Bykhovskaya Y, Casas K, Mengesha E, Inbal A, Fischel-Ghodsian N, Missense mutation in pseudouridine synthase 1 (PUS1) causes mitochondrial myopathy and sideroblastic anemia (MLASA), Am. J. Hum. Genet 74 (6) (2004) 1303–1308, 10.1086/421530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hsu PJ, Shi H, He C, Epitranscriptomic influences on development and disease, Genome Biol. 18 (1) (2017) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jonkhout N, Tran J, Smith MA, Schonrock N, Mattick JS, Novoa EM, The RNA modification landscape in human disease, Rna 23 (12) (2017) 1754–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Knight SW, Vulliamy TJ, Heiss NS, Matthijs G, Devriendt K, Connor JM, D’Urso M, Poustka A, Mason PJ, Dokal I, 1.4 Mb candidate gene region for X linked dyskeratosis congenita defined by combined haplotype and X chromosome inactivation analysis, J. Med. Genet 35 (12) (1998) 993–996, 10.1136/jmg.35.12.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lin S, Choe J, Du P, Triboulet R, Gregory RI, The m6A methyltransferase METTL3 promotes translation in human cancer cells, Mol. Cell 62 (3) (2016) 335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mangum JE, Hardee JP, Fix DK, Puppa MJ, Elkes J, Altomare D, Bykhovskaya Y, Campagna DR, Schmidt PJ, Sendamarai AK, Lidov HGW, Barlow SC, Fischel-Ghodsian N, Fleming MD, Carson JA, Patton JR, Pseudouridine synthase 1 deficient mice, a model for mitochondrial myopathy with sideroblastic anemia, exhibit muscle morphology and physiology alterations, Sci. Rep 6 (2016) 26202, 10.1038/srep26202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Patton JR, Bykhovskaya Y, Mengesha E, Bertolotto C, Fischel-Ghodsian N, Mitochondrial myopathy and sideroblastic anemia (MLASA): missense mutation in the pseudouridine synthase 1 (PUS1) gene is associated with the loss of tRNA pseudouridylation, J. Biol. Chem 280 (20) (2005) 19823–19828, 10.1074/jbc.M500216200. [DOI] [PubMed] [Google Scholar]
- [25].Ruggero D, Grisendi S, Piazza F, Rego E, Mari F, Rao PH, Cordon-Cardo C, Pandolfi PP, Dyskeratosis congenita and cancer in mice deficient in ribosomal RNA modification, Science (New York, N.Y.) 299 (5604) (2003) 259–262, 10.1126/science.1079447. [DOI] [PubMed] [Google Scholar]
- [26].Wang X, Li Z, Kong B, Song C, Cong J, Hou J, Wang S, Reduced m6A mRNA methylation is correlated with the progression of human cervical cancer, Oncotarget 8 (58) (2017) 98918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhao X, Yang Y, Sun B-F, Shi Y, Yang X, Xiao W, Hao Y-J, Ping X-L, Chen Y-S, Wang W-J, FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis, Cell Res. 24 (12) (2014) 1403–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Boccaletto P, Machnicka MA, Purta E, Piatkowski P, Baginski B, Wirecki TK, de Cŕecy-Lagard V, Ross R, Limbach PA, Kotter A, Helm M, Bujnicki JM, MODOMICS: a database of RNA modification pathways, 2017 update, Nucleic Acids Res. 46 (D1) (2018) D303–D307, 10.1093/nar/gkx1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wurtmann EJ, Wolin SL, RNA under attack: cellular handling of RNA damage, Crit. Rev. Biochem. Mol. Biol 44 (1) (2009) 34–49, 10.1080/10409230802594043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yan LL, Zaher HS, How do cells cope with RNA damage and its consequences? J. Biol. Chem 294 (41) (2019) 15158–15171, 10.1074/jbc.REV119.006513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dai W, Li A, Yu NJ, Nguyen T, Leach RW, Wuhr M, Kleiner RE, Activity-based RNA-modifying enzyme probing reveals DUS3L-mediated dihydrouridylation, Nat. Chem. Biol 17 (2021) 1178–1187, 10.1038/s41589-021-00874-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Finet O, Yague-Sanz C, Kruger LK, Migeot V, Ernst FG, Lafontaine DL, Tran P, Wéry M, Morillon A, Hermand D, Transcription-Wide Mapping of Dihydrouridine (D) Reveals that mRNA Dihydrouridylation is Essential for Meiotic Chromosome Segregation, 2022. Available at SSRN 3569550. [DOI] [PMC free article] [PubMed]
- [33].Grozhik AV, Jaffrey SR, Distinguishing RNA modifications from noise in epitranscriptome maps, Nat. Chem. Biol 14 (3) (2018) 215–225, 10.1038/nchembio.2546. [DOI] [PubMed] [Google Scholar]
- [34].Helm M, Motorin Y, Detecting RNA modifications in the epitranscriptome: predict and validate, Nat. Rev. Genet 18 (5) (2017) 275–291, 10.1038/nrg.2016.169. [DOI] [PubMed] [Google Scholar]
- [35].Li X, Xiong X, Yi C, Epitranscriptome sequencing technologies: decoding RNA modifications, Nat. Methods 14 (1) (2016) 23–31, 10.1038/nmeth.4110. [DOI] [PubMed] [Google Scholar]
- [36].Limbach PA, Paulines MJ, Going global: the new era of mapping modifications in RNA, Wiley Interdisc. Rev. RNA 8 (1) (2017), 10.1002/wrna.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Motorin Y, Helm M, Methods for RNA modification mapping using deep sequencing: established and new emerging technologies, Genes 10 (1) (2019) E35, 10.3390/genes10010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sarkar A, Gasperi W, Begley U, Nevins S, Huber SM, Dedon PC, Begley TJ, Detecting the epitranscriptome, WIREs RNA 12 (6) (2021), e1663, 10.1002/wrna.1663. [DOI] [PubMed] [Google Scholar]
- [39].Schwartz S, Cracking the epitranscriptome, RNA (New York, N.Y.) 22 (2) (2016) 169–174, 10.1261/rna.054502.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Schwartz S, Motorin Y, Next-generation sequencing technologies for detection of modified nucleotides in RNAs, RNA Biol. 14 (9) (2017) 1124–1137, 10.1080/15476286.2016.1251543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Andries O, Mc Cafferty S, De Smedt SC, Weiss R, Sanders NN, Kitada T, N(1)-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice, J. Control. Release 217 (2015) 337–344, 10.1016/j.jconrel.2015.08.051. [DOI] [PubMed] [Google Scholar]
- [42].Bumcrot D, Manoharan M, Koteliansky V, Sah DWY, RNAi therapeutics: a potential new class of pharmaceutical drugs, Nat. Chem. Biol 2 (12) (2006) 711–719, 10.1038/nchembio839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Nance KD, Meier JL, Modifications in an emergency: the role of N1-methylpseudouridine in COVID-19 vaccines, ACS Cent. Sci 7 (5) (2021) 748–756, 10.1021/acscentsci.1c00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Pardi N, Weissman D, Nucleoside modified mRNA vaccines for infectious diseases, Method Mol. Biol. (Clifton, N.J.) 1499 (2017) 109–121, 10.1007/978-1-4939-6481-9_6. [DOI] [PubMed] [Google Scholar]
- [45].Sahin U, Karikó K, Türeci Ö, mRNA-based therapeutics—developing a new class of drugs, Nat. Rev. Drug Discov 13 (10) (2014) 759–780, 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- [46].Seyhan AA, RNAi: a potential new class of therapeutic for human genetic disease, Hum. Genet 130 (5) (2011) 583–605, 10.1007/s00439-011-0995-8. [DOI] [PubMed] [Google Scholar]
- [47].Liu N, Parisien M, Dai Q, Zheng G, He C, Pan T, Probing N6-methyladenosine RNA modification status at single nucleotide resolution in mRNA and long noncoding RNA, Rna 19 (12) (2013) 1848–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Meyer KD, DART-seq: an antibody-free method for global m 6 A detection, Nat. Methods 16 (12) (2019) 1275–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Molinie B, Wang J, Lim KS, Hillebrand R, Lu ZX, Van Wittenberghe N, 620 Howard BD, Daneshvar K, Mullen AC, Dedon P, Xing Y, Giallourakis CC. 621 2016. M (6) A-LAIC-seq reveals the census and complexity of the m (6) A 622 epitranscriptome, Nat. Methods 13 (2022) 692–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Tegowski M, Flamand MN, Meyer KD, ScDART-seq reveals distinct m6A signatures and mRNA methylation heterogeneity in single cells, Mol. Cell (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zhang W, Eckwahl MJ, Zhou KI, Pan T, Sensitive and quantitative probing of pseudouridine modification in mRNA and long noncoding RNA, Rna 25 (9) (2019) 1218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Heilman KL, Leach RA, Tuck MT, Internal 6-methyladenine residues increase the in vitro translation efficiency of dihydrofolate reductase messenger RNA, Int. J. Biochem. Cell Biol 28 (7) (1996) 823–829, 10.1016/1357-2725(96)00014-3. [DOI] [PubMed] [Google Scholar]
- [53].Li A, Chen Y-S, Ping X-L, Yang X, Xiao W, Yang Y, Sun H-Y, Zhu Q, Baidya P, Wang X, Cytoplasmic m 6 A reader YTHDF3 promotes mRNA translation, Cell Res. 27 (3) (2017) 444–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Shi H, Wang X, Lu Z, Zhao BS, Ma H, Hsu PJ, Liu C, He C, YTHDF3 facilitates translation and decay of N 6-methyladenosine-modified RNA, Cell Res. 27 (3) (2017) 315–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, Weng X, Chen K, Shi H, He C, N6-methyladenosine modulates messenger RNA translation efficiency, Cell 161 (6) (2015) 1388–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Coots RA, Liu X-M, Mao Y, Dong L, Zhou J, Wan J, Zhang X, Qian S-B, M6A facilitates eIF4F-independent mRNA translation, Mol. Cell 68 (3) (2017) 504–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Meyer KD, Patil DP, Zhou J, Zinoviev A, Skabkin MA, Elemento O, Pestova TV, Qian S-B, Jaffrey SR, 5′ UTR m6A promotes cap-independent translation, Cell 163 (4) (2015) 999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Choi J, Ieong K-W, Demirci H, Chen J, Petrov A, Prabhakar A, O’Leary SE, Dominissini D, Rechavi G, Soltis SM, Ehrenberg M, Puglisi JD, N(6)-methyladenosine in mRNA disrupts tRNA selection and translation-elongation dynamics, Nat. Struct. Mol. Biol 23 (2) (2016) 110–115, 10.1038/nsmb.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hoernes TP, Clementi N, Faserl K, Glasner H, Breuker K, Lindner H, Hüttenhofer A, Erlacher MD, Nucleotide modifications within bacterial messenger RNAs regulate their translation and are able to rewire the genetic code, Nucleic Acids Res. 44 (2) (2016) 852–862, 10.1093/nar/gkv1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hoernes TP, Heimdörfer D, Köstner D, Faserl K, Nußbaumer F, Plangger R, Kreutz C, Lindner H, Erlacher MD, Eukaryotic translation elongation is modulated by single natural nucleotide derivatives in the coding sequences of mRNAs, Genes 10 (2) (2019) E84, 10.3390/genes10020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hudson BH, Zaher HS, O6-Methylguanosine leads to position-dependent effects on ribosome speed and fidelity, RNA (New York, N.Y.) 21 (9) (2015) 1648–1659, 10.1261/rna.052464.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Karikó K, Muramatsu H, Welsh FA, Ludwig J, Kato H, Akira S, Weissman D, Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability, Mol. Ther 16 (11) (2008) 1833–1840, 10.1038/mt.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].You C, Dai X, Wang Y, Position-dependent effects of regioisomeric methylated adenine and guanine ribonucleosides on translation, Nucleic Acids Res. 45 (15) (2017) 9059–9067, 10.1093/nar/gkx515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Safra M, Sas-Chen A, Nir R, Winkler R, Nachshon A, Bar-Yaacov D, Erlacher M, Rossmanith W, Stern-Ginossar N, Schwartz S, The m1A landscape on cytosolic and mitochondrial mRNA at single-base resolution, Nature 551 (7679) (2017) 251–255, 10.1038/nature24456. [DOI] [PubMed] [Google Scholar]
- [65].Thomas EN, Kim KQ, McHugh EP, Marcinkiewicz T, Zaher HS, Alkylative damage of mRNA leads to ribosome stalling and rescue by trans translation in bacteria, ELife 9 (2020), 10.7554/eLife.61984 e61984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Licht K, Hartl M, Amman F, Anrather D, Janisiw MP, Jantsch MF, Inosine induces context-dependent recoding and translational stalling, Nucleic Acids Res. 47 (1) (2019) 3–14, 10.1093/nar/gky1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Hoernes TP, Faserl K, Juen MA, Kremser J, Gasser C, Fuchs E, Shi X, Siewert A, Lindner H, Kreutz C, Micura R, Joseph S, Höbartner C, Westhof E, Hüttenhofer A, Erlacher MD, Translation of non-standard codon nucleotides reveals minimal requirements for codon-anticodon interactions, Nat. Commun 9 (1) (2018) 4865, 10.1038/s41467-018-07321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Svitkin YV, Gingras A-C, Sonenberg N, Membrane-dependent relief of translation elongation arrest on pseudouridine- and N1-methyl-pseudouridine-modified mRNAs, Nucleic Acids Res. (2021), 10.1093/nar/gkab1241 gkab1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Anderson BR, Muramatsu H, Nallagatla SR, Bevilacqua PC, Sansing LH, Weissman D, Karikó K, Incorporation of pseudouridine into mRNA enhances translation by diminishing PKR activation, Nucleic Acids Res. 38 (17) (2010) 5884–5892, 10.1093/nar/gkq347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Eyler DE, Franco MK, Batool Z, Wu MZ, Dubuke ML, Dobosz-Bartoszek M, Jones JD, Polikanov YS, Roy B, Koutmou KS, Pseudouridinylation of mRNA coding sequences alters translation, Proc. Natl. Acad. Sci. U. S. A 116 (46) (2019) 23068–23074, 10.1073/pnas.1821754116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Malbec L, Zhang T, Chen Y-S, Zhang Y, Sun B-F, Shi B-Y, Zhao Y-L, Yang Y, Yang Y-G, Dynamic methylome of internal mRNA N7-methylguanosine and its regulatory role in translation, Cell Res. 29 (11) (2019) 927–941, 10.1038/s41422-019-0230-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Kersten H, Albani M, Männlein E, Praisler R, Wurmbach P, Nierhaus KH, On the role of ribosylthymine in prokaryotic tRNA function, Eur. J. Biochem 114 (2) (1981) 451–456, 10.1111/j.1432-1033.1981.tb05166.x. [DOI] [PubMed] [Google Scholar]
- [73].Shan X, Chang Y, Lin CG, Messenger RNA oxidation is an early event preceding cell death and causes reduced protein expression, FASEB J. 21 (11) (2007) 2753–2764, 10.1096/fj.07-8200com. [DOI] [PubMed] [Google Scholar]
- [74].Simms CL, Hudson BH, Mosior JW, Rangwala AS, Zaher HS, An active role for the ribosome in determining the fate of oxidized mRNA, Cell Rep. 9 (4) (2014) 1256–1264, 10.1016/j.celrep.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Huang T, Chen W, Liu J, Gu N, Zhang R, Genome-wide identification of mRNA 5-methylcytosine in mammals, Nat. Struct. Mol. Biol 26 (5) (2019) 380–388, 10.1038/s41594-019-0218-x. [DOI] [PubMed] [Google Scholar]
- [76].Arango D, Sturgill D, Alhusaini N, Dillman AA, Sweet TJ, Hanson G, Hosogane M, Sinclair WR, Nanan KK, Mandler MD, Acetylation of cytidine in mRNA promotes translation efficiency, Cell 175 (7) (2018) 1872–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Svitkin YV, Cheng YM, Chakraborty T, Presnyak V, John M, Sonenberg N, N1-methyl-pseudouridine in mRNA enhances translation through eIF2α-dependent and independent mechanisms by increasing ribosome density, Nucleic Acids Res. 45 (10) (2017) 6023–6036, 10.1093/nar/gkx135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Mauger DM, Cabral BJ, Presnyak V, Su SV, Reid DW, Goodman B, Link K, Khatwani N, Reynders J, Moore MJ, McFadyen IJ, MRNA structure regulates protein expression through changes in functional half-life, Proc. Natl. Acad. Sci. U. S. A 116 (48) (2019) 24075–24083, 10.1073/pnas.1908052116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Ding Y, Tang Y, Kwok CK, Zhang Y, Bevilacqua PC, Assmann SM, In vivo genome-wide profiling of RNA secondary structure reveals novel regulatory features, Nature 505 (7485) (2014) 696–700, 10.1038/nature12756. [DOI] [PubMed] [Google Scholar]
- [80].Re A, Joshi T, Kulberkyte E, Morris Q, Workman CT, RNA-protein interactions: an overview, Method Mol. Biol. (Clifton, N.J.) 1097 (2014) 491–521, 10.1007/978-1-62703-709-9_23. [DOI] [PubMed] [Google Scholar]
- [81].Schoenberg DR, Mechanisms of endonuclease-mediated mRNA decay, Wiley Interdisc. Rev. RNA 2 (4) (2011) 582–600, 10.1002/wrna.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Hoernes TP, Clementi N, Juen MA, Shi X, Faserl K, Willi J, Gasser C, Kreutz C, Joseph S, Lindner H, Hüttenhofer A, Erlacher MD, Atomic mutagenesis of stop codon nucleotides reveals the chemical prerequisites for release factor-mediated peptide release, Proc. Natl. Acad. Sci. U. S. A 115 (3) (2018) E382–E389, 10.1073/pnas.1714554115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Chen K, Lu Z, Wang X, Fu Y, Luo G-Z, Liu N, Han D, Dominissini D, Dai Q, Pan T, High-resolution N6-methyladenosine (m6A) map using photo-crosslinking-assisted m6A sequencing, Angew. Chem 127 (5) (2015) 1607–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M, Topology of the human and mouse m 6 A RNA methylomes revealed by m 6 A-seq, Nature 485 (7397) (2012) 201–206. [DOI] [PubMed] [Google Scholar]
- [85].Linder B, Grozhik AV, Olarerin-George AO, Meydan C, Mason CE, Jaffrey SR, Single-nucleotide resolution mapping of m6A and m6Am throughout the transcriptome, Nat. Methods 12 (8) (2015) 767–772, 10.1038/nmeth.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR, Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons, Cell 149 (7) (2012) 1635–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Ke S, Pandya-Jones A, Saito Y, Fak JJ, Vågbø CB, Geula S, Hanna JH, Black DL, Darnell JE, Darnell RB, M 6 A mRNA modifications are deposited in nascent pre-mRNA and are not required for splicing but do specify cytoplasmic turnover, Genes Dev. 31 (10) (2017) 990–1006, 10.1101/gad.301036.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Lavi U, Fernandez-Mufioz R, Darnell JE, Content of N-6 methyl adenylic acid in heterogeneous nuclear and messenger RNA of HeLa cells, Nucleic Acids Res. 4 (1) (1977) 63–69, 10.1093/nar/4.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Sommer S, Salditt-Georgieff M, Bachenheimer S, Darnell JE, Furuichi Y, Morgan M, Shatkin AJ, The methylation of adenovirus-specific nuclear and cytoplasmic RNA, Nucleic Acids Res. 3 (3) (1976) 749–766, 10.1093/nar/3.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Smith T, Tardu M, Khatri HR, Koutmou KS, MRNA and tRNA Modification States Influence Ribosome Frame Maintenance During Poly(lysine) Peptide Synthesis, Submitted, 2022. [DOI] [PMC free article] [PubMed]
- [91].Agris PF, Decoding the genome: a modified view, Nucleic Acids Res. 32 (1) (2004) 223–238, 10.1093/nar/gkh185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Liu B, Merriman DK, Choi SH, Schumacher MA, Plangger R, Kreutz C, Horner SM, Meyer KD, Al-Hashimi HM, A potentially abundant junctional RNA motif stabilized by m6A and Mg2, Nat. Commun 9 (1) (2018) 2761, 10.1038/s41467-018-05243-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Dominissini D, Nachtergaele S, Moshitch-Moshkovitz S, Peer E, Kol N, Ben-Haim MS, Dai Q, Di Segni A, Salmon-Divon M, Clark WC, Zheng G, Pan T, Solomon O, Eyal E, Hershkovitz V, Han D, Doŕe LC, Amariglio N, Rechavi G, He C, The dynamic N(1)-methyladenosine methylome in eukaryotic messenger RNA, Nature 530 (7591) (2016) 441–446, 10.1038/nature16998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Grozhik AV, Olarerin-George AO, Sindelar M, Li X, Gross SS, Jaffrey SR, Antibody cross-reactivity accounts for widespread appearance of m1A in 5′UTRs, Nat. Commun 10 (1) (2019) 5126, 10.1038/s41467-019-13146-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Schwartz S, m1A within cytoplasmic mRNAs at single nucleotide resolution: a reconciled transcriptome-wide map, RNA (New York, N.Y.) 24 (11) (2018) 1427–1436, 10.1261/rna.067348.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Zhou H, Kimsey IJ, Nikolova EN, Sathyamoorthy B, Grazioli G, McSally J, Bai T, Wunderlich CH, Kreutz C, Andricioaei I, Al-Hashimi HM, M(1)A and m (1)G disrupt A-RNA structure through the intrinsic instability of Hoogsteen base pairs, Nat. Struct. Mol. Biol 23 (9) (2016) 803–810, 10.1038/nsmb.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Meydan S, Guydosh NR, A cellular handbook for collided ribosomes: surveillance pathways and collision types, Curr. Genet 67 (1) (2021) 19–26, 10.1007/s00294-020-01111-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Bass BL, Nishikura K, Keller W, Seeburg PH, Emeson RB, O’Connell MA, Samuel CE, Herbert A, A standardized nomenclature for adenosine deaminases that act on RNA, RNA (New York, N.Y.) 3 (9) (1997) 947–949. [PMC free article] [PubMed] [Google Scholar]
- [99].Bass BL, Weintraub H, An unwinding activity that covalently modifies its double-stranded RNA substrate, Cell 55 (6) (1988) 1089–1098, 10.1016/0092-8674(88)90253-x. [DOI] [PubMed] [Google Scholar]
- [100].Hundley HA, Bass BL, ADAR editing in double-stranded UTRs and other noncoding RNA sequences, Trends Biochem. Sci 35 (7) (2010) 377–383, 10.1016/j.tibs.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Srinivasan S, Torres AG, Ribas de Pouplana L, Inosine in biology and disease, Genes 12 (4) (2021) 600, 10.3390/genes12040600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Picardi E, Manzari C, Mastropasqua F, Aiello I, D’Erchia AM, Pesole G, Profiling RNA editing in human tissues: towards the inosinome atlas, Sci. Rep 5 (2015) 14941, 10.1038/srep14941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Basilio C, Wahba AJ, Lengyel P, Speyer JF, Ochoa S, Synthetic polynucleotides and the amino acid code. V, Proc. Natl. Acad. Sci. U. S. A 48 (1962) 613–616, 10.1073/pnas.48.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Sommer B, Köhler M, Sprengel R, Seeburg PH, RNA editing in brain controls a determinant of ion flow in glutamate-gated channels, Cell 67 (1) (1991) 11–19, 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- [105].Ito K, Uno M, Nakamura Y, A tripeptide “anticodon” deciphers stop codons in messenger RNA, Nature 403 (6770) (2000) 680–684, 10.1038/35001115. [DOI] [PubMed] [Google Scholar]
- [106].Carlile TM, Rojas-Duran MF, Zinshteyn B, Shin H, Bartoli KM, Gilbert WV, Pseudouridine profiling reveals regulated mRNA pseudouridylation in yeast and human cells, Nature 515 (7525) (2014) 143–146, 10.1038/nature13802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Li X, Zhu P, Ma S, Song J, Bai J, Sun F, Yi C, Chemical pulldown reveals dynamic pseudouridylation of the mammalian transcriptome, Nat. Chem. Biol 11 (8) (2015) 592–597, 10.1038/nchembio.1836. [DOI] [PubMed] [Google Scholar]
- [108].Lovejoy AF, Riordan DP, Brown PO, Transcriptome-wide mapping of pseudouridines: Pseudouridine synthases modify specific mRNAs in S. cerevisiae, PLoS One 9 (10) (2014), e110799, 10.1371/journal.pone.0110799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Schwartz S, Bernstein DA, Mumbach MR, Jovanovic M, Herbst RH, Leon-Ricardó BX, Engreitz JM, Guttman M, Satija R, Lander ES, Fink G, Regev A, Transcriptome-wide mapping reveals widespread dynamic-regulated pseudouridylation of ncRNA and mRNA, Cell 159 (1) (2014) 148–162, 10.1016/j.cell.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Martinez NM, Su A, Burns MC, Nussbacher JK, Schaening C, Sathe S, Yeo GW, Gilbert WV, Pseudouridine synthases modify human pre-mRNA co-transcriptionally and affect pre-mRNA processing, Mol. Cell (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Nakamoto MA, Lovejoy AF, Cygan AM, Boothroyd JC, MRNA pseudouridylation affects RNA metabolism in the parasite toxoplasma gondii, RNA (New York, N.Y.) 23 (12) (2017) 1834–1849, 10.1261/rna.062794.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Fernández IS, Ng CL, Kelley AC, Wu G, Yu Y-T, Ramakrishnan V, Unusual base pairing during the decoding of a stop codon by the ribosome, Nature 500 (7460) (2013) 107–110, 10.1038/nature12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Karijolich J, Yu Y-T, Converting nonsense codons into sense codons by targeted pseudouridylation, Nature 474 (7351) (2011) 395–398, 10.1038/nature10165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Svidritskiy E, Madireddy R, Korostelev AA, Structural basis for translation termination on a pseudouridylated stop codon, J. Mol. Biol 428 (10 Pt B) (2016) 2228–2236, 10.1016/j.jmb.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Shatkin AJ, Capping of eucaryotic mRNAs, Cell 9 (4, Part 2) (1976) 645–653, 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- [116].Chu J-M, Ye T-T, Ma C-J, Lan M-D, Liu T, Yuan B-F, Feng Y-Q, Existence of internal N7-methylguanosine modification in mRNA determined by differential enzyme treatment coupled with mass spectrometry analysis, ACS Chem. Biol 13 (12) (2018) 3243–3250, 10.1021/acschembio.7b00906. [DOI] [PubMed] [Google Scholar]
- [117].Zhang L-S, Liu C, Ma H, Dai Q, Sun H-L, Luo G, Zhang Z, Zhang L, Hu L, Dong X, He C, Transcriptome-wide mapping of internal N7-methylguanosine methylome in mammalian mRNA, Mol. Cell 74 (6) (2019) 1304–1316.e8, 10.1016/j.molcel.2019.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Filipowicz W, Functions of the 5′-terminal m7G cap in eukaryotic mRNA, FEBS Lett. 96 (1) (1978) 1–11, 10.1016/0014-5793(78)81049-7. [DOI] [PubMed] [Google Scholar]
- [119].Ramanathan A, Robb GB, Chan S-H, mRNA capping: biological functions and applications, Nucleic Acids Res. 44 (16) (2016) 7511–7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Enroth C, Poulsen LD, Iversen S, Kirpekar F, Albrechtsen A, Vinther J, Detection of internal N7-methylguanosine (m7G) RNA modifications by mutational profiling sequencing, Nucleic Acids Res. 47 (20) (2019), e126, 10.1093/nar/gkz736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Kastrup RV, Schmidt PG, 1H NMR of valine tRNA modified bases. Evidence for multiple conformations, Nucleic Acids Res. 5 (1) (1978) 257–269, 10.1093/nar/5.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Sierzputowska-Gracz H, Gopal HD, Agris PF, Comparative structural analysis of 1-methyladenosine, 7-methylguanosine, ethenoadenosine and their protonated salts IV: 1H, 13C, and 15N NMR studies at natural isotope abundance, Nucleic Acids Res. 14 (19) (1986) 7783–7801, 10.1093/nar/14.19.7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Hofer T, Badouard C, Bajak E, Ravanat J-L, Mattsson A, Cotgreave IA, Hydrogen peroxide causes greater oxidation in cellular RNA than in DNA, Biol. Chem 386 (4) (2005) 333–337, 10.1515/BC.2005.040. [DOI] [PubMed] [Google Scholar]
- [124].Parsa I, Friedman S, Cleary CM, Visualization of O6-methylguanine in target cell nuclei of dimethylnitrosamine-treated human pancreas by a murine monoclonal antibody, Carcinogenesis 8 (6) (1987) 839–846, 10.1093/carcin/8.6.839. [DOI] [PubMed] [Google Scholar]
- [125].Hsu GW, Ober M, Carell T, Beese LS, Error-prone replication of oxidatively damaged DNA by a high-fidelity DNA polymerase, Nature 431 (7005) (2004) 217–221, 10.1038/nature02908. [DOI] [PubMed] [Google Scholar]
- [126].Sampoli Benítez B, Barbati ZR, Arora K, Bogdanovic J, Schlick T, How DNA polymerase X preferentially accommodates incoming dATP opposite 8-oxoguanine on the template, Biophys. J 105 (11) (2013) 2559–2568, 10.1016/j.bpj.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Bicknell AA, Ricci EP, When mRNA translation meets decay, Biochem. Soc. Trans 45 (2) (2017) 339–351, 10.1042/BST20160243. [DOI] [PubMed] [Google Scholar]
- [128].Karousis ED, Mühlemann O, Nonsense-mediated mRNA decay begins where translation ends, Cold Spring Harb. Perspect. Biol 11 (2) (2019), a032862, 10.1101/cshperspect.a032862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].van Hoof A, Wagner EJ, A brief survey of mRNA surveillance, Trends Biochem. Sci 36 (11) (2011) 585–592, 10.1016/j.tibs.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Agris PF, Bringing order to translation: the contributions of transfer RNA anticodon-domain modifications, EMBO Rep. 9 (7) (2008) 629–635, 10.1038/embor.2008.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Anderson JT, Wang X, Nuclear RNA surveillance: no sign of substrates tailing off, Crit. Rev. Biochem. Mol. Biol 44 (1) (2009) 16–24, 10.1080/10409230802640218. [DOI] [PubMed] [Google Scholar]
- [132].Helm M, Post-transcriptional nucleotide modification and alternative folding of RNA, Nucleic Acids Res. 34 (2) (2006) 721–733, 10.1093/nar/gkj471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Dubin DT, Taylor RH, The methylation state of poly A-containing messenger RNA from cultured hamster cells, Nucleic Acids Res. 2 (10) (1975) 1653–1668, 10.1093/nar/2.10.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Squires JE, Patel HR, Nousch M, Sibbritt T, Humphreys DT, Parker BJ, Suter CM, Preiss T, Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA, Nucleic Acids Res. 40 (11) (2012) 5023–5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Squires JE, Preiss T, Function and detection of 5-methylcytosine in eukaryotic RNA, Epigenomics 2 (5) (2010) 709–715, 10.2217/epi.10.47. [DOI] [PubMed] [Google Scholar]
- [136].Tang H, Fan X, Xing J, Liu Z, Jiang B, Dou Y, Gorospe M, Wang W, NSun2 delays replicative senescence by repressing p27 (KIP1) translation and elevating CDK1 translation, Aging 7 (12) (2015) 1143–1158, 10.18632/aging.100860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Xing J, Yi J, Cai X, Tang H, Liu Z, Zhang X, Martindale JL, Yang X, Jiang B, Gorospe M, Wang W, NSun2 promotes cell growth via elevating cyclin-dependent kinase 1 translation, Mol. Cell. Biol 35 (23) (2015) 4043–4052, 10.1128/MCB.00742-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Sas-Chen A, Thomas JM, Matzov D, Taoka M, Nance KD, Nir R, Bryson KM, Shachar R, Liman GLS, Burkhart BW, Gamage ST, Nobe Y, Briney CA, Levy MJ, Fuchs RT, Robb GB, Hartmann J, Sharma S, Lin Q, Schwartz S, Dynamic RNA acetylation revealed by quantitative cross-evolutionary mapping, Nature 583 (7817) (2020) 638–643, 10.1038/s41586-020-2418-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Tardu M, Jones JD, Kennedy RT, Lin Q, Koutmou KS, Identification and quantification of modified nucleosides in Saccharomyces cerevisiae mRNAs, ACS Chem. Biol 14 (7) (2019) 1403–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Ayadi L, Galvanin A, Pichot F, Marchand V, Motorin Y, RNA ribose methylation (2′-O-methylation): occurrence, biosynthesis and biological functions, Biochim. Et Biophys. Acta. Gene Regul. Mech 1862 (3) (2019) 253–269, 10.1016/j.bbagrm.2018.11.009. [DOI] [PubMed] [Google Scholar]
- [141].Choi J, Indrisiunaite G, DeMirci H, Ieong K-W, Wang J, Petrov A, Prabhakar A, Rechavi G, Dominissini D, He C, Ehrenberg M, Puglisi JD, 2′-O-methylation in mRNA disrupts tRNA decoding during translation elongation, Nat. Struct. Mol. Biol 25 (3) (2018) 208–216, 10.1038/s41594-018-0030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Abou Assi H, Rangadurai AK, Shi H, Liu B, Clay MC, Erharter K, Kreutz C, Holley CL, Al-Hashimi HM, 2′-O-methylation can increase the abundance and lifetime of alternative RNA conformational states, Nucleic Acids Res. 48 (21) (2020) 12365–12379, 10.1093/nar/gkaa928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Brand RC, Klootwijk J, Planta RJ, Maden BE, Biosynthesis of a hypermodified nucleotide in Saccharomyces carlsbergensis 17S and HeLa-cell 18S ribosomal ribonucleic acid, Biochem. J 169 (1) (1978) 71–77, 10.1042/bj1690071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Edmonds CG, Crain PF, Gupta R, Hashizume T, Hocart CH, Kowalak JA, Pomerantz SC, Stetter KO, McCloskey JA, Posttranscriptional modification of tRNA in thermophilic archaea (Archaebacteria), J. Bacteriol 173 (10) (1991) 3138–3148, 10.1128/jb.173.10.3138-3148.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Rauscher R, Ignatova Z, Timing during translation matters: synonymous mutations in human pathologies influence protein folding and function, Biochem. Soc. Trans 46 (4) (2018) 937–944, 10.1042/BST20170422. [DOI] [PubMed] [Google Scholar]
- [146].Garcia-Campos MA, Edelheit S, Toth U, Safra M, Shachar R, Viukov S, Winkler R, Nir R, Lasman L, Brandis A, Deciphering the “m6A code” via antibody-independent quantitative profiling, Cell 178 (3) (2019) 731–747. [DOI] [PubMed] [Google Scholar]
- [147].Purchal MK, Eyler DE, Tardu M, Franco MK, Korn MM, Khan T, McNassor R, Giles R, Lev K, Sharma H, Monroe J, Mallik L, Koutmos M, Koutmou KS, Pseudouridine synthase 7 is an opportunistic enzyme that binds and modifies substrates with diverse sequences and structures, Proc. Natl. Acad. Sci 119 (4) (2022), e2109708119, 10.1073/pnas.2109708119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Prus G, Hoegl A, Weinert BT, Choudhary C, Analysis and interpretation of protein post-translational modification site stoichiometry, Trends Biochem. Sci 44 (11) (2019) 943–960, 10.1016/j.tibs.2019.06.003. [DOI] [PubMed] [Google Scholar]


