Abstract
Within the genome, expressed genes marked by “open” chromatin are often adjacent to silent, heterochromatic regions. There are also regions containing neighboring active genes with different programs of expression. In both cases, DNA sequence elements may function as insulators, either providing barriers that prevent the incursion of heterochromatic signals into open domains or acting to block inappropriate contact between the enhancer of one gene and the promoter of another. The mechanisms associated with insulation are diverse: Enhancer-blocking insulation is largely associated with the ability to stabilize the formation of loop domains within the nucleus. Barrier insulation is often associated with the ability to block propagation of silencing histone modifications. Here, we provide examples of both kinds of insulator action, derived initially from studies of the compound insulator element at the 5′ end of the chicken β-globin locus. Such elements appear to have more general regulatory roles in the genome that have been exploited to provide insulator function where necessary to demarcate separate domains within the nucleus.
INTRODUCTION
Broadly defined, an insulator element (IE) is a DNA sequence that interferes with interactions among distinct regions or sites in the genome (Dorman et al. 2007; Wallace and Felsenfeld 2007). Some elements can prevent a distal enhancer from “inappropriately” activating a gene; others prevent the spreading of a condensed (heterochromatic) chromatin domain into adjacent, more open and active regions. Cells have devised many strategies for performing these functions. Many of these have been discovered as a result of studies in Drosophila, as well as in Saccharomyces cerevisiae and Schizosaccharomyces pombe (Geyer and Corces 1992; Kellum and Schedl 1992; Cai and Levine 1995; Zhao et al. 1995; Donze and Kamakaka 2001; Noma et al. 2001, 2006). For a number of years, we have used the chicken β-globin locus as an experimental system in which to study the relationship between chromatin structure and patterns of gene expression. Some years ago, we identified a DNA sequence element, marked by a DNase hypersensitive site (5′HS4), that appeared to mark the 5′ boundary between the open chromatin region containing the four β-globin genes and an extended upstream heterochromatic region (Fig. 1A) (Chung et al. 1993, 1997). An initial test of its properties showed that an ~250-bp DNA IE present at 5′HS4 was able to block the stimulatory effects of enhancers on promoters when placed between them (Fig. 1B). Subsequently, we developed an assay to test the IE for the second kind of insulator function that prevents the spread of heterochromatin; the intact IE also possesses this activity (Fig. 1C) (Pikaart et al. 1998). To determine the protein(s) responsible for these activities, we first used DNase footprinting to identify five distinct protein-binding domains within the IE (Fig. 1D). In a series of studies still in progress, we have identified these proteins and explored their properties. In each case, we have discovered reactions and structures that have implications not only for insulator function but, more generally, for regulatory mechanisms.
Figure 1.

Properties of the chicken β-globin 5′HS4 compound insulator element (IE). (A) Diagram of the locus, showing the position of the insulator relative to the globin gene cluster, the condensed chromatin region upstream of the cluster, and further upstream the erythroid-specific folate receptor gene. (B) Enhancer-blocking assay using a locus control region (LCR) element as an enhancer to drive a neomycin-resistance gene (Neo) in the presence of an ~250-bp element from 5′HS4 (INS) or a small noninsulating DNA fragment derived from λ bacteriophage. Increased insulator activity results in fewer neomycin-resistant colonies (Chung et al. 1993). (C) Barrier function assay using fluorescence-activated cell sorter (FACS) analysis to detect the expression levels of a fragment of the IL2 receptor. The fragment was expressed from a reporter construct carrying a globin-specific promoter and enhancer, stably transformed in each case into the avian erythroid cell line 6C2. (Left panel) Uninsulated reporter, (right panel) reporter surrounded by two copies on each side of the ~250-bp IE from 5′HS4 (Pikaart et al. 1998). (D) The five distinct protein-binding domains within the IE, the proteins that bind to them, and their functions. At FIV, the USF1/USF2 heterodimer binds to DNA and recruits a protein complex containing SET1 and another containing PRMT1 (Huang et al. 2007). Histone acetyltransferases are also present in each complex, and the complexes are probably not bound simultaneously. The overlaps between factors shown are not intended to signify physical contacts between them.
ENHANCER-BLOCKING INSULATION AND THE FUNCTION OF CTCF
As shown in Figure 1B, when the IE is placed between a promoter and enhancer, it prevents the enhancer from activating the promoter. Dissection of the IE showed that the DNA sequence in footprint 2 (Fig. 1D) was necessary and sufficient for this function. We identified the protein binding to this site as CTCF, a known factor thought to have a variety of regulatory roles but not previously identified as having insulator activity (Bell et al. 1999). A direct role for this activity was soon demonstrated at the mouse and human Igf2/H19 imprinted locus: CTCF binds to the imprinted control region (ICR) on the maternal allele, preventing distal enhancers from activating Igf2 (Fig. 2) (Bell and Felsenfeld 2000; Hark et al. 2000; Kanduri et al. 2000). In contrast, CpG sites on the ICR of the paternal allele are methylated. This inhibits CTCF binding, and in the absence of insulating activity, the enhancers can activate Igf2.
Figure 2.
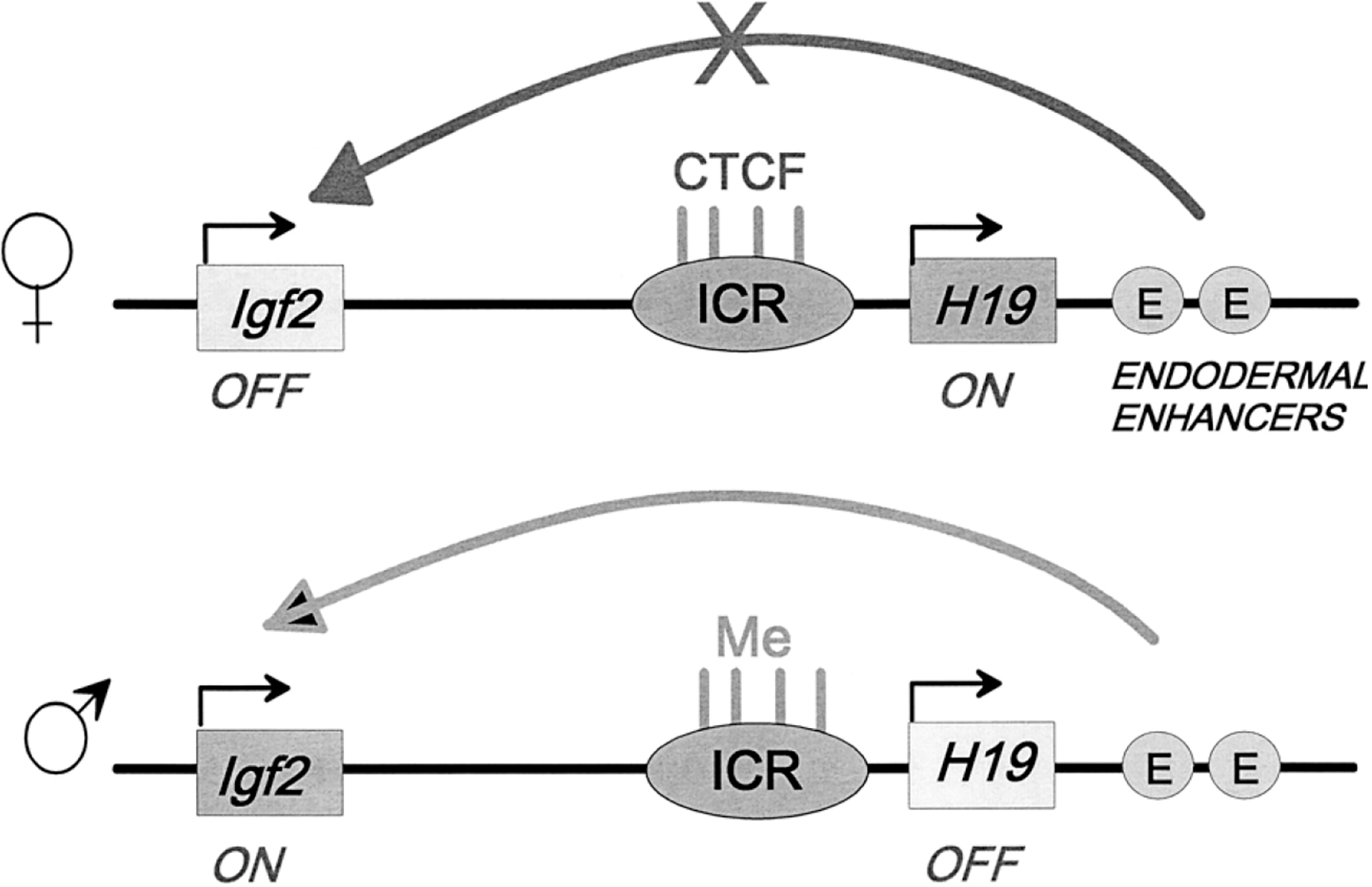
Schematic representation of the role of CTCF binding to the imprinted control region (ICR) at the Igf2/H19 locus in mice. On the maternally transmitted allele, four CTCF sites are occupied and constitute a strong insulator that blocks the ability of downstream enhancers to activate Igf2 expression. On the paternal allele, CpG sites within the ICR are methylated and CTCF does not bind. There is no insulator activity and Igf2 expression is activated on this allele. Similar mechanisms are found at the human locus (see Bell and Felsenfeld 2000; Hark et al. 2000; Kanduri et al. 2000).
We suggested that long-range interactions between widely separated bound CTCF molecules could create loop domains and that insulating activity would result when enhancer and promoter were in separate domains (Yusufzai and Felsenfeld 2004; Yusufzai et al. 2004). This model had been proposed earlier by Corces, and data in its support presented, to explain the insulator properties of the gypsy element in Drosophila (Labrador and Corces 2002). However, the proteins involved in stabilizing these interactions are quite different from those associated with CTCF insulator activity (Pai et al. 2004).
It has since become clear that a primary role of CTCF is to stabilize long-range interactions in the nucleus. Insulator activity is a consequence of a particular configuration in which the CTCF site lies between an enhancer and promoter, but in other configurations, the formation of long-range contacts may cause, for example, distant promoters and enhancers to be brought into contact, resulting in stimulation of transcription (Gaszner and Felsenfeld 2006; Phillips and Corces 2009; Ohlsson et al. 2010).
BARRIERS AGAINST THE SPREAD OF HETEROCHROMATIN
Histone Modifications
The remaining four DNA-binding domains in the β-globin IE do not appear to take part in the kind of interactions that give CTCF its properties as an enhancer-blocking insulator. However, they do have the important role of protecting “open” chromatin domains from being silenced by the spread of adjacent heterochromatic regions. Eukaryotes appear to have developed many mechanisms for achieving this important regulatory goal. Our recent results indicate that the β-globin IE makes use of at least two such mechanisms.
To study the barrier properties of this IE, we devised an assay in which a reporter expressing a protein that can be detected at the cell surface is stably integrated into a cell line (Pikaart et al. 1998). In most cases, expression from such a reporter is silenced after 50–100 d in culture, a manifestation of “position effects” in which integration into the typically heterochromatic regions of the genome results in silencing. In contrast, when the reporter is surrounded by two copies of the β-globin IE, expression is maintained in virtually every cell line (Fig. 1C).
We prepared a series of mutant IEs in which individual footprint regions were deleted and tested them in the position effect assay (Recillas-Targa et al. 2002). Elements in which footprint 4 was deleted were unable to protect against silencing; histones covering the reporter sequence lost histone acetylation and H3 lysine 4 methylation. We identified the protein binding to footprint 4 as a heterodimer of the factors USF1 and USF2 (West et al. 2004). These, in turn, recruited a large number of histone-modifying enzymes, all of which delivered “activating” modifications to the histones on adjacent nucleosomes (Huang et al. 2007). We identified two such complexes: one containing Set1, which methylates lysine 4 on histone H3, and another containing PRMT1, responsible for asymmetric methylation of Arg 3 on histone H4, an important signal for the subsequent recruitments of HATs. The effect of these modifications is to prevent the propagation of negative modifications from adjacent heterochromatic regions and thus block the advance of silencing signals. This is a more sophisticated version of a mechanism discovered earlier in S. cerevisiae, in which recruitment of HATs sets up a barrier of histone acetylation marks to prevent encroachment of silencing deacetylation (Donze and Kamakaka 2001).
DNA Methylation
Whereas deletion of footprint 4 leads to loss of activation-associated histone modifications, deletion of footprint 1, 3, or 5 results in high levels of DNA methylation within the promoter of the IL2R reporter gene; the gene is silenced. We showed that the protein associated with all three footprint sites is BGP1, a zinc-finger protein identified many years ago in our laboratory as having a strong affinity for G-rich DNA sequences (Dickson et al. 2010). Since that early work was done, the mouse homolog Vezf1 has been cloned (Xiong et al. 1999). The availability of a mouse embryonic stem cell line in which both copies of Vezf1 had been deleted made it possible to explore its biological effects that notably included loss of DNA methylation at many repeat elements (Line 1 and minor satellite repeats) as well as some imprinted loci (Gowher et al. 2008). The methylation loss could be ascribed to a decrease in levels of the de novo DNA methyltransferase Dnmt3b. This change, in turn, appeared to be correlated with a decrease in the splice variant that codes for the active form of this enzyme (Fig. 3). Interestingly, binding sites for Vezf1 at the Dnmt3b locus were found in a 3′ intron and in the 3′-untranslated region of the gene (Fig. 3). This led us to suggest that Vezf1/BGP1 has a role in attenuating the movement of elongating RNA polymerase II (Pol II) that, in turn, could affect the choice of splicing partners. A relationship between transcription elongation rates and splicing outcomes has been demonstrated (Kornblihtt 2006). Recent work from our laboratory supports this role for Vezf1/BGP1 (H Gowher et al., unpublished).
Figure 3.
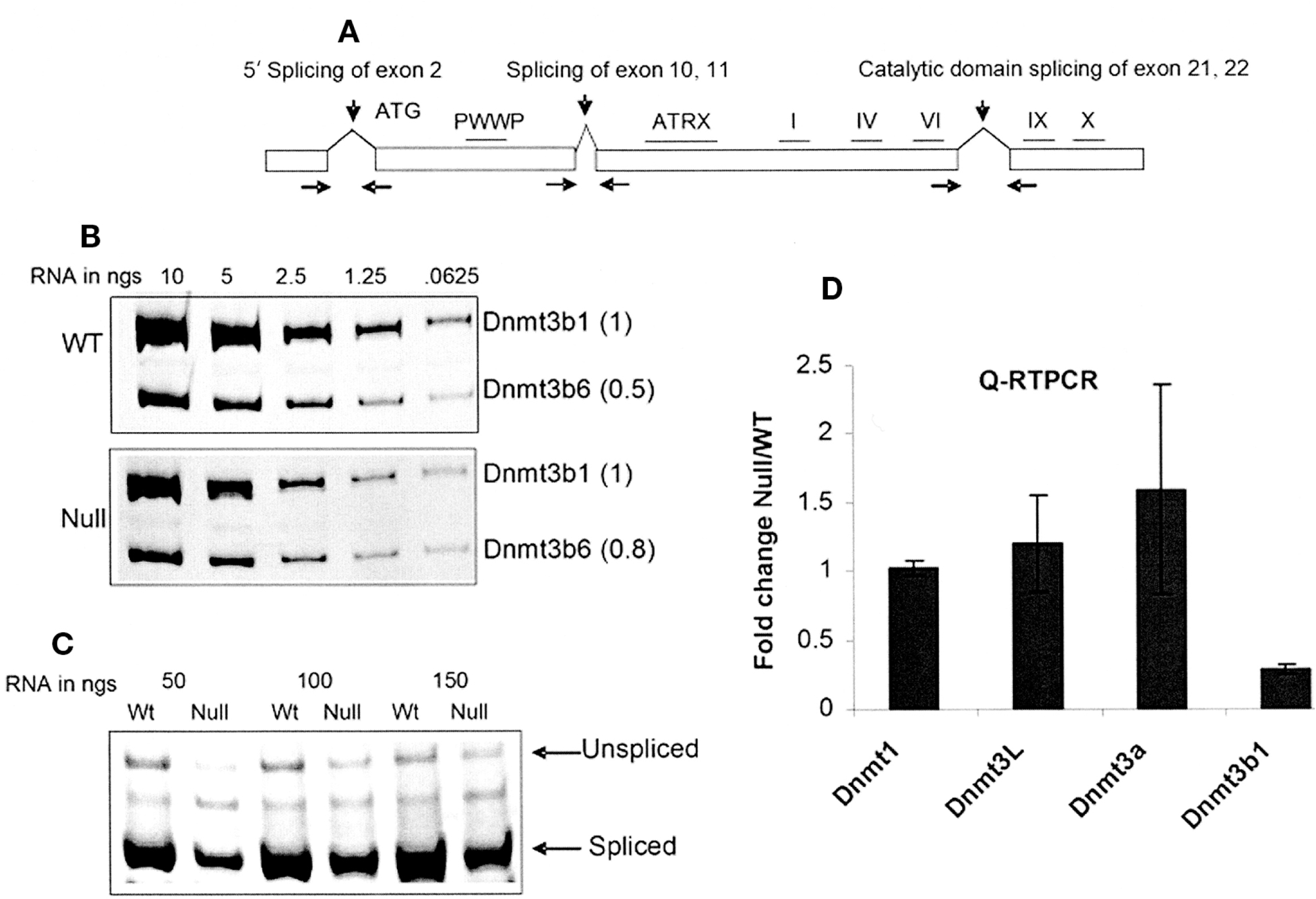
Analysis of the splice variants of Dnmt3b; Dnmt3b transcript is decreased in Vezf1−/− cells. (A) Map of Dnmt3b gene showing three characterized splicing events. To analyze alternative splicing events, total RNA from wild-type and Vezf1−/− ES cells was reverse-transcribed and amplified by PCR for 27 cycles. (B) Catalytic domain splicing. (C) 5′-end splicing. (D) Relative quantitative reverse-transcriptase (RT)–PCR analysis for the measurement of DNA methylases (MTases) using TaqMan expression assay. The Ct values for all MTases are normalized to that of β-actin. (Reprinted, with permission, from Gowher et al. 2008.)
Structure of a Condensed Chromatin Domain
To understand the possible connection between this behavior and function as an insulator, we asked what mechanisms might be involved in the formation of a heterochromatic domain and, in particular, the condensed chromatin region immediately upstream of the β-globin IE. This ~16-kb-long region had been the subject of a detailed hydrodynamic analysis in earlier studies (Ghirlando et al. 2004). For those studies, we took advantage of the fact that cytosines at CpG sites within the condensed region are highly methylated, whereas those outside are not. Digestion with Hpa II, which will not cut its CCGG site when the internal C is methylated, released the region from the nucleus intact. It was then possible, using polymerase chain reaction (PCR) detection, to determine the sedimentation constant and buoyant mass with high accuracy, permitting calculation of the frictional coefficient of the particle. The value of the frictional coefficient was consistent with a particle of rod-like shape.
We performed a control digestion experiment in which Hpa II was replaced by Msp I, which cuts the same site even when it is methylated (Ghirlando et al. 2004). To our surprise, the 16-kb region was relatively resistant to digestion: Although some fragmentation occurred, to a considerable extent the sites were inaccessible (Fig. 4A). This is a reflection of the compact nature of the structure, and it provided an important tool in subsequent experiments. We began by asking what relationship existed between this structure and its associated histone modifications: The region is marked by low levels of histone acetylation and high levels of histone methylation at histone H3 lysine 9, as expected for heterochromatin. We treated the avian cell line 6C2 with trichostatin A (TSA), a histone deacetylase (HDAC) inhibitor (Giles et al. 2010). We observed large increases in histone H4 acetylation over the 16-kb region (Fig. 4B). Furthermore, this treatment also resulted in increased susceptibility to attack by MspI, indicating that the chromatin structure had become less compact (Fig. 4A). Because we wanted to determine whether this heterochromatic structure was maintained or established by RNAi-dependent mechanisms related to those shown to function in S. pombe (Grewal and Rice 2004), we also measured transcript levels over the region, both in untreated and TSA treated cells. Low levels of transcript could be detected in these cells before exposure to TSA, and these increased significantly after addition of the HDAC inhibitor (Fig. 4C,D). We then performed analogous experiments with cells in which Dicer had been depleted by introduction of Dicer specific small interfering RNAs (siRNAs), with quite similar results (Giles et al. 2010). Dicer knockdown led to increased histone acetylation over the region, decreases in H3K9 dimethylation, and increases (Fig. 4E) in transcript levels. Furthermore, MspI accessibility increased, leading to the production of smaller chromatin fragments (Fig. 4F). We found that the compact structure also depended on the presence of Argonaute 2 (Ago2) that could be detected as being associated with the heterochromatic region in 6C2 cells, in a Dicer-dependent manner. These results show that mechanisms at least partly related to those present in S. pombe also function in vertebrate cells to maintain a heterochromatic structure. Recent studies of a human cell line in our laboratory (KE Giles and G Felsenfeld, in preparation) suggest that many other heterochromatic structures are controlled by the same mechanism.
Figure 4.

Effects of TSA and Dicer on structure of the 16-kb heterochromatin domain upstream of the β-globin cluster (see Fig. 1A). (A) Sucrose gradient sedimentation pattern of the 16-kb chromatin fragment (see Fig. 1A), liberated from 6C2 cell nuclei digested with Msp1. (Vertical arrow) Peak position of the intact fragment that has not been cut internally by the enzyme. Approximately 31% of the total chromatin is in this peak (see F). (B) Effects of treatment with trichostatin A (TSA) on levels of histone H4 acetylation over the 16-kb heterochromatin domain, showing large increases in acetylation. TSA was then removed and 17 h later, acetylation levels were again measured. (Light gray bars) Untreated, (dark gray bars) TSA added, (white bars) TSA removed. (C) Transcript RNA levels over the 16-kb domain in wild-type 6C2 cells. (D) Increase in transcript RNA after TSA treatment. (E) Effect of Dicer knockdown on transcript levels across the 16-kb domain. (F) Results of sucrose gradient experiments such as that in A, showing the fraction of Msp1-resistant intact 16-kb fragment present after knockdown of Ago2 or Dicer, compared to results for a mock knockdown experiment (shown in A). (Reprinted or replotted, with permission, from Giles et al. 2010 [Nature Publishing Group].)
How could this be related to the function of Vezf1/BGP1 at the β-globin IE? One possibility is that because Vezf1 may interfere with the progress of a transcribing RNA Pol II, it could also prevent low-level transcripts associated with heterochromatic regions from extending into adjacent open chromatin, thus serving as a barrier to the propagation of the heterochromatic structure.
Other Types of Barrier
In principle, barriers can be created by any mechanism that interferes with the propagation of a processive signal associated with silencing. Such elements have been identified in S. cerevisiae, adjacent to the HMR heterochromatic locus (Donze et al. 1999; Donze and Kamakaka 2001). In S. pombe, the IR (inverted repeat) boundary elements at the ends of the heterochromatic mating-type domain prevent spreading of heterochromatin into adjacent regions by recruiting the TFIIIC complex to B-box elements within the IR elements (Donze and Kamakaka 2001; Noma et al. 2006). In the case of the β-globin IE, at least two distinct pathways, one involving USF1/USF2 and the other Vezf1, may be exploited to block extension of heterochromatin in the 3′ direction (West et al. 2004; Huang et al. 2007). There does not appear to be an equivalent IE at the 5′ end of the 16-kb heterochromatic region, and the question remains as to whether a barrier exits there. One possibility is that the strong enhancer and promoter of the folate receptor gene, immediately upstream, are sufficient to maintain high levels of activating histone modifications. However, we have observed that this region is occupied by nucleosomes that are enriched for the double variants H3.3 and H2A.Z. We have reported that such nucleosomes are quite unstable and tend to lose the H2A.Z/H2B dimer (Jin et al. 2009). One proposed propagation mechanism for the extension of a heterochromatic domain is through the action of the two Polycomb response complexes PRC1 and PRC2. PRC2 methylates lysine 27 on histone H3; PRC1 recognizes this mark and, through its Ring1B subunit, ubiquitylates lysine 119 of histone H2A (Wang et al. 2004). Loss of the H2A.Z/H2B dimer would necessarily inhibit this reaction. However, a recent report shows that although Ring1B is required to compact the Hoxb and Hoxd gene loci in embryonic stem cells, its ubiquitylating activity is not (Eskeland et al. 2010).
CONCLUSIONS
It has become increasingly clear that the genome is organized into discrete physical domains within the nucleus and that some DNA sequence elements, and their associated factors, are important in establishing and maintaining these domains. Elements that recruit Suppressor of Hairy Wing (Labrador and Corces 2001) or BEAF (Zhao et al. 1995; Bushey et al. 2009) in flies, or CTCF in vertebrates and flies, can stabilize loop domains and in so doing may inhibit interactions between an enhancer and promoter or between different regulatory elements. Other proteins, such as GAGA-binding factor (encoded by Trithorax-like) in flies, appear to function similarly (Ohtsuki and Levine 1998; Melnikova et al. 2004a,b). As noted above, there are other possible consequences of bringing together distant regions of the genome, which are independent of insulator activity. Similarly, elements found in barriers against the mixing of active and heterochromatic domains may elsewhere be involved in more general regulatory mechanisms. It has been known for many years that a strong enhancer or locus control region (LCR) can help to protect a gene against heterochromatic silencing. The USF1/ USF2-binding site within the β-globin IE is found at other sites in the genome, e.g., within enhancer elements, where it also appears to serve the purpose of maintaining activating histone modifications on adjacent nucleosomes. The Vezf1/BGP1 site, as discussed above, may have a primary function of modulating transcriptional elongation. As the genome has become more complex, organisms have adapted existing regulatory elements to maintain both the independence of and orderly cooperation between neighborhoods.
ACKNOWLEDGEMENTS
This work was supported by the NIDDK Intramural Research Program.
REFERENCES
- Bell AC, Felsenfeld G. 2000. Methylation of a CTCF-dependent boundary controls imprinted expression of the Igf2 gene. Nature 405: 482–485. [DOI] [PubMed] [Google Scholar]
- Bell AC, West AG, Felsenfeld G. 1999. The protein CTCF is required for the enhancer blocking activity of vertebrate insulators. Cell 98: 387–396. [DOI] [PubMed] [Google Scholar]
- Bushey AM, Ramos E, Corces VG. 2009. Three subclasses of a Drosophila insulator show distinct and cell type-specific genomic distributions. Genes Dev 23: 1338–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Levine M. 1995. Modulation of enhancer-promoter interactions by insulators in the Drosophila embryo. Nature 376: 533–536. [DOI] [PubMed] [Google Scholar]
- Chung JH, Whiteley M, Felsenfeld G. 1993. A 5′ element of the chicken β-globin domain serves as an insulator in human erythroid cells and protects against position effect in Drosophila. Cell 74: 505–514. [DOI] [PubMed] [Google Scholar]
- Chung JH, Bell AC, Felsenfeld G. 1997. Characterization of the chicken β-globin insulator. Proc Natl Acad Sci 94: 575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson J, Gowher H, Strogantsev R, Gaszner M, Hair A, Felsenfeld G, West AG. 2010. VEZF1 elements mediate protection from DNA methylation. PLoS Genet 6: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donze D, Kamakaka RT. 2001. RNA polymerase III and RNA polymerase II promoter complexes are heterochromatin barriers in Saccharomyces cerevisiae. EMBO J 20: 520–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donze D, Adams CR, Rine J, Kamakaka RT. 1999. The boundaries of the silenced HMR domain in Saccharomyces cerevisiae. Genes Dev 13: 698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman ER, Bushey AM, Corces VG. 2007. The role of insulator elements in large-scale chromatin structure in interphase. Semin Cell Dev Biol 18: 682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskeland R, Leeb M, Grimes GR, Kress C, Boyle S, Sproul D, Gilbert N, Fan Y, Skoultchi AI, Wutz A, et al. 2010. Ring1B compacts chromatin structure and represses gene expression independent of histone ubiquitination. Mol Cell 38: 452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaszner M, Felsenfeld G. 2006. Insulators: Exploiting transcriptional and epigenetic mechanisms. Nat Rev Genet 7: 703–713. [DOI] [PubMed] [Google Scholar]
- Geyer PK, Corces VG. 1992. DNA position-specific repression of transcription by a Drosophila zinc finger protein. Genes Dev 6: 1865–1873. [DOI] [PubMed] [Google Scholar]
- Ghirlando R, Litt MD, Prioleau MN, Recillas-Targa F, Felsenfeld G. 2004. Physical properties of a genomic condensed chromatin fragment. J Mol Biol 336: 597–605. [DOI] [PubMed] [Google Scholar]
- Giles KE, Ghirlando R, Felsenfeld G. 2010. Maintenance of a constitutive heterochromatin domain in vertebrates by a Dicer-dependent mechanism. Nat Cell Biol 12: 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowher H, Stuhlmann H, Felsenfeld G. 2008. Vezf1 regulates genomic DNA methylation through its effects on expression of DNA methyltransferase Dnmt3b. Genes Dev 22: 2075–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal SIS, Rice JC. 2004. Regulation of heterochromatin by histone methylation and small RNAs. Curr Opin Cell Biol 16: 230–238. [DOI] [PubMed] [Google Scholar]
- Hark AT, Schoenherr CJ, Katz DJ, Ingram RS, Levorse JM, Tilghman SM. 2000. CTCF mediates methylation-sensitive enhancer-blocking activity at the H19/Igf2 locus. Nature 405: 486–489. [DOI] [PubMed] [Google Scholar]
- Huang S, Li X, Yusufzai TM, Qiu Y, Felsenfeld G. 2007. USF1 recruits histone modification complexes and is critical for maintenance of a chromatin barrier. Mol Cell Biol 27: 7991–8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Zang C, Wei G, Cui K, Peng W, Zhao K, Felsenfeld G. 2009. H3.3/H2A.Z double variant-containing nucleosomes mark “nucleosome-free regions” of active promoters and other regulatory regions. Nat Genet 41: 941–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanduri C, Pant V, Loukinov D, Pugacheva E, Qi CF, Wolffe A, Ohlsson R, Lobanenkov VV. 2000. Functional association of CTCF with the insulator upstream of the H19 gene is parent of origin-specific and methylation-sensitive. Curr Biol 10: 853–856. [DOI] [PubMed] [Google Scholar]
- Kellum R, Schedl P. 1992. A group of scs elements function as domain boundaries in an enhancer-blocking assay. Mol Cell Biol 12: 2424–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblihtt AR. 2006. Chromatin, transcript elongation and alternative splicing. Nat Struct Mol Biol 13: 5–7. [DOI] [PubMed] [Google Scholar]
- Labrador M, Corces VG. 2001. Protein determinants of insertional specificity for the Drosophila Gypsy retrovirus. Genetics 158: 1101–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrador M, Corces VG. 2002. Setting the boundaries of chromatin domains and nuclear organization. Cell 111: 151–154. [DOI] [PubMed] [Google Scholar]
- Melnikova L, Biessmann H, Georgiev P. 2004a. The vicinity of a broken chromosome end affects P element mobilization in Drosophila melanogaster. Mol Genet Genomics 272: 512–518. [DOI] [PubMed] [Google Scholar]
- Melnikova L, Juge F, Gruzdeva N, Mazur A, Cavalli G, Georgiev P. 2004b. Interaction between the GAGA factor and Mod(mdg4) proteins promotes insulator bypass in Drosophila. Proc Natl Acad Sci 101: 14806–14811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma K, Allis CD, Grewal SI. 2001. Transitions in distinct histone H3 methylation patterns at the heterochromatin domain boundaries. Science 293: 1150–1155. [DOI] [PubMed] [Google Scholar]
- Noma K, Cam HP, Maraia RJ, Grewal SI. 2006. A role for TFIIIC transcription factor complex in genome organization. Cell 125: 859–872. [DOI] [PubMed] [Google Scholar]
- Ohlsson R, Lobanenkov V, Klenova E. 2010. Does CTCF mediate between nuclear organization and gene expression? Bioessays 32: 37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuki S, Levine M. 1998. GAGA mediates the enhancer blocking activity of the eve promoter in the Drosophila embryo. Genes Dev 12: 3325–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai CY, Lei EP, Ghosh D, Corces VG. 2004. The centrosomal protein CP190 is a component of the gypsy chromatin insulator. Mol Cell 16: 737–748. [DOI] [PubMed] [Google Scholar]
- Phillips JE, Corces VG. 2009. CTCF: Master weaver of the genome. Cell 137: 1194–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikaart MJ, Recillas-Targa F, Felsenfeld G. 1998. Loss of transcriptional activity of a transgene is accompanied by DNA methylation and histone deacetylation and is prevented by insulators. Genes Dev 12: 2852–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recillas-Targa F, Pikaart MJ, Burgess-Beusse B, Bell AC, Litt MD, West AG, Gaszner M, Felsenfeld G. 2002. Position-effect protection and enhancer blocking by the chicken β-globin insulator are separable activities. Proc Natl Acad Sci 99: 6883–6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JA, Felsenfeld G. 2007. We gather together: Insulators and genome organization. Curr Opin Genet Dev 17: 400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wang L, Erdjument-Bromage H, Vidal M, Tempst P, Jones RS, Zhang Y. 2004. Role of histone H2A ubiquitination in Polycomb silencing. Nature 431: 873–878. [DOI] [PubMed] [Google Scholar]
- West AG, Huang S, Gaszner M, Litt MD, Felsenfeld G. 2004. Recruitment of histone modifications by USF proteins at a vertebrate barrier element. Mol Cell 16: 453–463. [DOI] [PubMed] [Google Scholar]
- Xiong JW, Leahy A, Lee HH, Stuhlmann H. 1999. Vezf1: A Zn finger transcription factor restricted to endothelial cells and their precursors. Dev Biol 206: 123–141. [DOI] [PubMed] [Google Scholar]
- Yusufzai TM, Felsenfeld G. 2004. The 5′-HS4 chicken β-globin insulator is a CTCF-dependent nuclear matrix-associated element. Proc Natl Acad Sci 101: 8620–8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusufzai TM, Tagami H, Nakatani Y, Felsenfeld G. 2004. CTCF tethers an insulator to subnuclear sites, suggesting shared insulator mechanisms across species. Mol Cell 13: 291–298. [DOI] [PubMed] [Google Scholar]
- Zhao K, Hart CM, Laemmli UK. 1995. Visualization of chromosomal domains with boundary element-associated factor BEAF-32. Cell 81: 879–889. [DOI] [PubMed] [Google Scholar]


