Abstract
The antioxidant, cholinergic, monoaminergic, and purinergic activities of flavonoid-rich extract from Dalbergiella welwitschii leaf (FEDW) were investigated on oxidative testicular injury (ex vivo) due to the local report on the use of this plant as anti-testicular injury. Flavonoid extract was obtained from FEDW using a standard procedure. Five male albino rats were used, testes harvested and incubated with FeSO4 for accessing the cholinergic, monoaminergic, and purinergic activities of the FEDW (ex vivo). Testicular tissues incubated with FeSO4 demonstrated a significant decrease in antioxidant biomarkers, arginase, ATPase, ENTPDase, 5ʹ-nucleotidase, and PDE-5 activities, as well as Zn and sialic acid levels with an upsurge in malondialdehyde (MDA), and NO levels, myeloperoxidase, cholinesterases, monoamine oxidase (MAO), and angiotensin-converting enzyme (ACE) activities. Treatment of testicular tissues incubated with FeSO4 via different concentrations of FEDW significantly increased the activities of antioxidant, arginase, ATPase, E-NTPDase, 5ʹ-nucleotidase, phosphodiesterase-5 (PDE-5), as well as Zn and sialic acid levels with a significant decrease in MDA, nitric oxide (NO), myeloperoxidase, cholinesterases, MAO, and ACE levels. Molecular docking revealed the molecular interactions of cyclooxygenase-2 (COX-2) with ellagic acid, piperine, and caffeine with piperine and caffeine obeyed the druggability and pharmacokinetic. These findings point to FEDW as a possible potential for the treatment of oxidative testicular injury.
Keywords: In silico; in vitro; ex vivo, inflammation; molecular docking; druggability; pharmacokinetic
Introduction
The majority of problems that endanger the male reproductive systems, particularly testicular function, are linked to free radical-induced oxidative stress. Life-threatening free radical attacks can cause arterial occlusion, severe damage to reproductive cells, and, as a result, defects in spermatogenesis. 1 This is due to the high concentration of unsaturated fatty acids in the testes and sperm cells, which are vulnerable to peroxidative damage. 2 In other words, oxidative stress is linked to a rise in the production of free radicals and peroxidants, and a weakened antioxidant defense system. 3
In other words, Erukainure et al 4 documented that the testes contain a variety of antioxidant systems composed of both enzymatic and non-enzymatic components. Conversely, in the presence of abundant free radicals, the antioxidant system is screwed, resulting in oxidative stress, which is disadvantageous to male fertility. Also, testicular oxidative damage is related to a decline in antioxidant activities and an increase in proinflammatory cytokines (COX-2, IL-1, etc) as a result of testicular inflammation, which results in a decrease in testosterone production and spermatogenesis.1,5,6
Cholinergic proteins have been shown to perform a crucial role in testicular function and fertility, as evidenced by their expression in Leydig and Sertoli cells. 7 Acetylcholine, for example, plays an important role in regulating Leydig cell functions; its deficiency causes increased acetylcholinesterase activity, which is a key factor in testicular dysfunction. 8 Besides, abnormal testicular activities of both purinergic and monoaminergic have been documented as a key repressor of spermatogenesis and impotence in males. 9 Hence, controlling of these enzyme activities can serve as an important factor in managing/treating male infertility. 10
Because of their accessibility, affordability, and availability, medicinal plants have been utilized to treat and manage a variety of conditions all over the world.11,12 Male fertility difficulties such as sperm abnormalities, erectile and ejaculatory dysfunctions, sexual asthenia, and low libido have all been treated and managed using several of these herbs. 11 These are attributed to their wide spectrum of phytochemicals. 13
Dalbergiella welwitschii is a shrub that is also known as blackwood in West Africa and Elemosoo among Yoruba-speaking communities in Nigeria. 14 D welwitschii leaves are used in folklore to treat cutaneous and subcutaneous parasitic infections. 14 Ofodile et al 15 reported the anthelmintic thin layer chromatographic fractions of the leaves of the herbal plant D welwitschii. It is also used locally in the management/treatment of oxidative stress diseases with little or no scientific backing. Due to this, the present study aimed to examine the inhibitory effect of D welwitschii leaves on cholinergic, monoaminergic, and purinergic enzyme activities.
Materials and Methods
Plant materials source, authentication, and processing
D welwitschii leaves were collected in an open location at the Federal Research Institute of Nigeria in Ibadan, Oyo State, Nigeria. Mr Odewo, S.A., a senior taxonomist at the Federal Research Institute of Nigeria (FRIN), Ibadan, Nigeria, identified and authenticated the plant with voucher number FHI: 113156. Hence, D welwitschii leaves were dried for 2 weeks at room temperature. An electric blender was used to grind the air-dried leaves into powder.
Chemicals and reagents
Alcoholic solution of DPPH, butylated hydroxytoluene (BHT), ABTS solution, potassium persulfate, methanol, sodium nitroprusside, Griess reagent, naphthyl ethylenediamine dichloride, galantamine, acetylthiocholine iodide, DTNB (5,5′-dithiobis-2-nitrobenzoic acid), buffer-MeOH, 10% TCA, Ellman’s reagent, diethylenetriaminepentaacetic acid (DETAPAC), 6-hydroxydopamine (6-HD), H2O2, sodium phosphate buffer, ammonium molybdate, 8.1% SDS solution, 0.25% thiobarbituric acid (TBA), KCl, 1.25% ammonium-molybdate, Tris-HCl buffer, ascorbic acid, CaCl2, EDTA, glucose, sucrose, ATP, MgSO4, and AMP were acquired from Sigma-Aldrich, Chemie GmbH (Steinheim, Germany), while all the reagents used were of analytical grade.
Extraction of flavonoid from D welwitschii leaf
The powder was defatted in 95% methanol, then 30 g of the residue was dissolved in 200 mL of 10% H2SO4 and hydrolyzed in a water bath at 100°C for 30 minutes. The flavonoid aglycones were precipitated by placing the mixture on ice for 15 minutes. After that, the flavonoid aglycones were dissolved in 50 mL of 95% ethanol, filtered, and concentrated using a rotary evaporator. 16 The sample was then named FEDW.
In vitro antioxidant
Determination of 2-diphenyl-1-picrylhydrazil (DDPH) scavenging ability
The approach of Atolani and Olatunji 17 was used to determine this free radical scavenging activity. 500 μL of 0.3 mM alcoholic DPPH solution was applied to 2.5 mL of test samples at various doses. The samples were incubated in the dark for 30 minutes before being measured with a UV-visible spectrophotometer at 518 nm. As a positive control, butylated hydroxytoluene (BHT), a synthetic antioxidant, was used. The studies were done in triplicates, and the scavenging activity was calculated as a percentage inhibition using the formula
where AbScontrol means absorbance of the control and AbSsample means absorbance of the sample.
Determination of 2,2-Azino-bis-3-ethylbenzothiazoline-6-sulfonate (ABTS) scavenging ability
The ABTS reagent was first dissolved to a concentration of 7 mM via deionized water, and then a freshly prepared solution of 2.45 mM potassium persulfate was added at a 1:1 ratio and stored in the dark for 24 hours. After that, the ABTS solution was diluted in methanol at 1:25. Hence, 20 μL of samples (diluted 1:10) were mixed with 2 mL of ABTS+ solution, which was maintained at 30 °C. The absorbance was measured at 734 nm, 10 minutes after mixing the solution. 18 All of the tests were done in triplicate
Abscontrol means absorbance of the control while Abssample means the absorbance of the sample.
Determination of nitric oxide scavenging assay
The method outlined by Ebrahimzadeh et al 19 2 mL of the extracts at various concentrations were incubated for 2 hours at 27°C with 0.5 mL sodium nitroprusside. Then 1 mL of the incubated solution was aliquoted and added with 0.6 mL Griess reagent in 20% glacial acetic acid at room temperature for 5 minutes with 1 mL of naphthyl ethylenediamine dichloride. The absorbance was immediately measured at 550 nm.
Animals
Five male Wistar rats weighing 220 to 250 g were obtained from Afe Babalola University Animal Holding Unit, Ado-Ekiti, Ekiti State, Nigeria. The rats were fasted for 24 hours before sacrificed with halothane. The testes of each animal were harvested, washed in 0.9% NaCl solution, and homogenized in sodium phosphate buffer (50 mM; pH 7.5; with 10% Triton X-100). Using the procedure described by Salau et al 20 the homogenized tissues were centrifuged at 22 000 g for 10 minutes at 4°C (2020). Supernatants were collected in 2 mL Eppendorf tubes for ex vivo experiments, stored at −4°C, and named as sample. Approval was granted and selected animals were handled in accordance with the approval of the Animal Ethics Committee of Federal University Oye-Ekiti, Ekiti State, Nigeria which was strictly followed.
Ex vivo studies
A known volume (100 µL) of an aliquot from each prepared concentration of FEDW (31.25-1000 µg/mL) was incubated at 37°C for 30 minutes with 100 µL of testicular tissue homogenates and 30 µL of 0.1 mM FeSO4. The tissues incubated in the absence of a FEDW (only 30 µL of 0.1 mM FeSO4) were considered untreated. Whereas testicular tissues that were not exposed to 30 µL of 0.1 mM FeSO4, as well as an FEDW were named as the control (normal). 4
Oxidative stress biomarkers
Reduced glutathione (GSH) level was determined using the method of Ellman. 21 The catalase (CAT) activity of the testes was assessed using Aebi. 22 The procedure outlined by Kakkar et al 23 was used for assessing superoxide dismutase (SOD) activity while the Paglia and Valentine 24 method was employed in evaluating glutathione peroxidase activity. The level of lipid peroxidation measured using malondialdehyde (MDA) was carried out by Chowdhury and Soulsby. 25
Proinflammation
Determination of nitric oxide (NO) level
100 μL of the sample was left at 25°C for 30 minutes using a dark cupboard; in addition, an equal volume of Griess reagent was then mixed to the solution. At 548 nm, absorbance was measured. 26
Determination of myeloperoxidase activity
For 10 minutes, 100 μL of the sample was added to 100 μL of KCl and 25 μL of H2O2. After that, 50 μL of ammonium molybdate was added to the solution and left for 5 minutes at 25 C. At 405 nm, absorbance was read. 27
Cholinergic
Determination of acetylcholinesterase (AChE) inhibitory activity
Ellman et al 28 method was used in this assay. Briefly, 20 μL of the sample was left for 20 minutes at 25°C with 10 μL Ellman’s reagent and 50 μL of phosphate buffer. After that, 10 μL of acetylcholine iodide or butyrylcholine iodide was added to the mixture. Thus, at 3-min intervals, the absorbance was read at 412 nm.
Monoaminergic
Determination of monoamine oxidase (MAO) activity
A mixture of 0.025 M phosphate buffer, 0.0125 M semicarbazide, 10 mM benzylamine, 75 μL of MAO reagent, and 31.25 to 1000 μg/mL of the testicular homogenate were incubated for 30 min. Thereafter, acetic acid was mixed with a solution, boiled for 3 min in a water bath before centrifugation. Then 1 mL of the supernatant was added to the same volume of 2,4-DNPH and 1.25 mL of absolute benzene was added and incubated for 10 min at 25°C. This formed a benzene layer and separated; subsequently the same volume of 0.1 N NaOH was added, which formed an alkaline layer that was decanted and subjected to heat at 80°C for 10 min. At 450 nm absorbance was read via a spectrophotometer. 29
Determination of arginase activity
A total volume of 250 µL of Tris-HCl buffer, 250 µL of manganese chloride, 0.1 M arginine solution, 200 µL of FEDW leaf, and 100 µL of FeSO4 exposed testicular homogenate. The mixture was incubated in a water bath for 15 minutes at 37°C and finally, 2.5 mL of Ehrlich reagent was added. After 25 minutes at 450 nm the absorbance was measured. 30
Purinergic enzyme activities
Determination of ATPase activity
Erukainure et al 31 was used in this assay; 200 μL of the samples were combined with 200 μL of 5 mM KCl, 1300 μL of 0.1 M Tris-HCl buffer, and 40 μL of 50 mM ATP. In a shaker, the solution was shaken for 30 minutes at 37°C. To stop the reaction, 1 mL distilled water and 1 mL ammonium molybdate were added, respectively. After that, 1 mL of freshly made 9% ascorbic acid was added, and the combination was allowed to sit at room temperature for 30 minutes. The amount of inorganic phosphate (Pi) liberated/min/mg was estimated as the ATPase activity was measured at 660 nm.
Determination of ENTPDase activity
The procedure previously described by Schetinger et al 32 was employed. In brief, 20 μL of the sample was left at 37°C for 10 minutes with 200 μL of reaction buffer. The reaction mixture was then added 20 μL of 50 mM ATP and agitated for 20 minutes at 37°C. After stopping the reaction with 200 μL of 10% TCA, the reaction was incubated in ice for 10 minutes with 200 L of 1.25% ammonium molybdate and freshly prepared 9% ascorbic acid. At 600 nm, the absorbance was measured.
Determination of 5′-nucleotidase activity
20 μL of tissue sample were treated for 10 minutes at 37°C with 100 μL of 10 mM MgSO4 and 100 mM Tris-HCl buffer. Then, 2 mM AMP was added to the mixture and incubated at 37°C for 10 minutes. The reaction was halted using 200 μL of 10% TCA and allowed to cool on ice for 10 minutes. At 600 nm, the absorbance was read as reported by Heymann et al. 33
Determination of phosphodiesterase-5 (PDE-5)
PDE-5 activity was carried out using the procedure outlined by Oboh et al. 34 The reaction solution containing 300 µL of 5 mM of p-nitrophenyl phenyl phosphonate, sample (ie, testicular testes exposed to FeSO4), 250 µL of Tris buffer, and 250 µL of FEDW leaf (at different concentration) were placed in a water bath for 15 minutes at 37°C. Then the mixture was incubated for 10 minutes and the amount of p-nitrophenol formed was measured at 400 nm.
Determination of angiotensin-I-converting enzyme (ACE)
Varying concentrations of the flavonoid-rich extract from D welwitschii leaf as well as 50 µL of FeSO4 testicular homogenate were pre-incubated at 37°C for 10 minutes. Later, 200 µL of 8.33 mM ACE substrate in 125 mM of Tris-HCl buffer (pH 8.3) was added and placed on a water bath at 37°C for 25 minutes. The enzymatic reaction was terminated by adding 300 µL of 1 M hydrochloric acid. This led to the formation of hippuric acid (Bz-Gly), which was removed by adding 2 mL of ethyl acetate. This mixture was centrifuged to obtain filtrate, dried and reconstituted, then the absorbance was measured at 228 nm. 35
Determination of zinc level
This was done using the procedure of Homster and Zak. 36 A known volume of zinc reagent (1000 µL) was pipetted into a test tube labeled blank, standard, and sample (using different concentrations of FEDW). Then, 50 µL of sample and standard reagent were added separately into the respective test tube. Thereafter the solution was incubated for 5 minutes at 37°C, and the absorbance was evaluated at 560 nm as
where Asample is the absorbance of the sample, Ablank is the absorbance of the blank, and Astandard is the absorbance of the standard.
Determination of sialic acid
Briefly, 0.1 mL of distilled water, sample (at different concentrations of FEDW) and standard were pipetted into 3 test tubes named blank, sample, and standard. Thus, 4 mL of sialic reagent was added to each test tube and thoroughly mixed. Then, each test tube was incubated at 100°C for 15 minutes, cooled under running water, then centrifuged at 2325 g for 10 minutes. The supernatant was taken from each tube and the absorbance measured at 560 nm using a UV spectrophotometer as
where Asample = absorbance of sample, Astandard = absorbance of standard, Ablank = absorbance of blank, Std Conc = standard concentration, DF = Dilution factor, and mg prot = mg protein. 37
RP-HPLC characterization of flavonoid-rich extract from D welwitschii leaf
RP-HPLC analysis was done in gradient mode at room temperature (28°C-32°C). Chromatographic analysis was performed on a Bandapak C18 reversed-phase column (250 mm 4.6 mm) packed with 5-m diameter particles; the mobile phase was acetonitrile: water (60:40 v/v) stabilized with ethyl acetate. This mobile phase was ultrasonically deaerated and filtered through a 0.45-m membrane filter (millipore) before use. The injection volume was 10 μL and the mobile phase flow rate was 1 mL/min. In the FEDW leaf, several chemicals were discovered using appropriate detection wavelengths. By comparing the retention time (Rt) and UV spectra of the analytes with those of the reference standards, the chromatographic peaks of the analytes were confirmed. Following the analytical procedure, all chromatographic processes were performed at room temperature. 38
In silico study
Receptor protein
The 3-dimensional structure (3D) of human cyclooxygenase-2 (COX-2) (PDB ID: 5IKT) was recovered from the protein data bank repository (www.rcsb.org).
Preparation of protein
This was accomplished with the help of Maestro’s wizard version 11.5. Bond orders were assigned to the protein, missing hydrogen atoms were added, and water molecules beyond 5 were deleted. For optimizing the corrected structure, the proteins were refined by H-bond assignment via PROPKA (at pH: 7.0). Finally, using the OPLS3 force field, the corrected protein structure was minimized by converting heavy atoms to RMSD 0.30. 39 In addition, the receptor grid file was created by selecting the native ligand within the active site to generate coordinates.
Bioactive compounds retrieval
The five bioactive compounds in Table 1 were obtained from the flavonoid-rich extract of D welwitschii leaf. PubChem (https://pubchem.ncbi.nlm.nih.gov/) was used to retrieve the chemical structure of ellagic acid, gallic acid, caffeine, piperine, and homoharrington in structure-data file (SDF) form. Hence, these compounds were grouped and prepared by Ligprep panel to produce the corresponding low-energy 3D structures for use by programs such as Glide.
Table 1.
HPLC characterization of flavonoid-rich extract of Dalbergiella welwitschii leaf.
| Compounds | Quantity (mg/g) |
|---|---|
| Ellagic acid | 5.506 ± 0.01 |
| Gallic acid | 1.327 ± 0.01 |
| Vincamine | 0.089 ± 0.02 |
| Caffeine | 1.532 ± 0.01 |
| Galantamine | 0.131 ± 0.01 |
| Quinine | 0.089 ± 0.02 |
| Furostan | 0.134 ± 0.01 |
| Piperine | 1.468 ± 0.01 |
| Homoharrington | 5.810 ± 0.01 |
| Pyrogallic acid | 0.185 ± 0.02 |
| Psilocin | 0.127 ± 0.01 |
| Chelerythrine | 0.228 ± 0.01 |
| Vincamine | 0.214 ± 0.01 |
Extra precision (XP) docking, prediction of the compounds’ druggability and pharmacokinetics properties
The five compounds were docked against 5IKT (Cyclooxygenase-2) using extra precision docking. In addition, the five secondary metabolites obtained from the flavonoid-rich extract from D welwitschii leaf were screened for drug-likeness via Lipinski, Ghose, Veber, and Egan rules. In addition, the gastrointestinal (GI) absorption and effect on CYP450 principal isozyme were predicted by the SWISSADME server on the compounds.
Statistical analysis
The data were analyzed using 1-way analysis of variance (ANOVA) and presented as mean ± standard deviation (SD). Using Tukey’s multiple range post hoc test, significant differences between means were obtained at P < .05.
Results
Inhibitory ability of flavonoid-rich extract from D welwitschii leaf on DPPH, ABTS, and NO
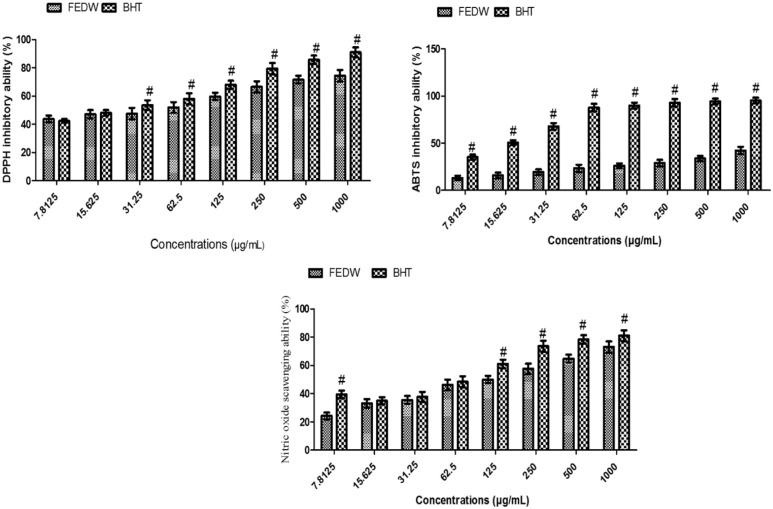
As shown in Figure 1, there was no significant (P > .05) difference in the DPPH inhibitory ability of flavonoid-rich extract from D welwitschii leaf (FEDW) and butylated hydroxytoluene (BHT) (the standard used) at 7.8125 and 15.625 µg/mL concentrations. However, at concentrations of 31.25 to 1000 µg/mL there was a significant (P < .05) difference in the DPPH ability of FEDW and BHT.
Figure 1.
Inhibitory ability of flavonoid-rich extract from Dalbergiella welwitschii leaf on DPPH, ABTS, and NO.
Data were expressed as mean ± SD, n = 3.
#P < .05 vs the FEDW.
BHT indicates butylated hydroxytoluene; FEDW, flavonoid-rich extract of Dalbergiella welwitschii leaf.
There was a significant (P < .05) difference in ABTS ability of FEDW and BHT in all the concentrations (7.8125-1000 µg/mL) (Figure 1). In addition, there was no significant (P > .05) difference in the nitric oxide (NO) inhibitory ability of FEDW and BHT at 7.8125 to 62.5 µg/mL concentrations, whereas a significant (P < .05) difference was exhibited in the NO inhibitory ability of FEDW and BHT at 12.5 to 1000 µg/mL concentrations (Figure 1).
Oxidative biomarkers of flavonoid-rich extract from D welwitschii leaf in oxidative testicular injury
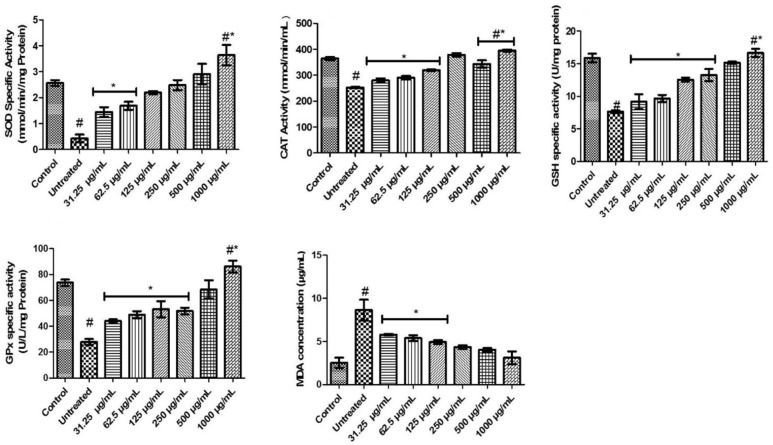
In Figure 2, there was a significant (P < .05) decline in SOD activities of untreated rats compared with normal and FeSO4 incubated testicular tissues treated with different concentrations (31.25-1000 µg/mL) of FEDW. FEDW at concentrations of 31.25 to 62. 5 µg/mL exhibited a noticeable difference when compared with control, untreated, and other concentrations of FEDW. FEDW at a concentration of 1000 µg/mL exhibited a significant (P < .05) increase in the SOD activity, compared with untreated, control, and other concentrations of the extract.
Figure 2.
Oxidative biomarkers of flavonoid-rich extract from Dalbergiella welwitschii leaf in oxidative testicular injury.
Data expressed as mean ± SD, n = 5.
#P < .05 vs the control, *P < .05 vs the untreated.
CAT indicates catalase; GPx, glutathione peroxidase; GSH, glutathione; MDA, malondialdehyde; SOD, superoxide dismutase.
The activity of CAT in the untreated was significantly (P < .05) decreased when compared with both control and different concentrations of FEDW (Figure 2). Also, the activity of CAT in FeSO4 incubated testicular tissues, treated with FEDW at concentrations of 31.25 to 12.5 µg/mL, was not significantly (P > .05) different from each other, but significantly (P < .05) different from concentrations (Figure 2). Testicular tissues incubated to FeSO4 treated with 250 to 500 µg/mL concentrations of FEDW exhibited no significant (P > .05) difference with control, while those treated with 1000 µg/mL demonstrated a significant (P < .05) rise when compared with control, untreated, and other concentrations of FEDW (Figure 2).
The untreated exhibited a significant (P < .05) decrease in GSH and GPx-specific activities when compared with control and all the concentrations of FEDW. The testicular tissues exposed to FeSO4 treated with 31.25 to 250 µg/mL concentrations of FEDW exhibited no significant (P > .05) difference in GSH and GPx-specific activities but showed a momentous difference when compared with control, untreated, and other concentrations of FEDW. The FEDW at 1000 µg/mL concentration revealed a significant (P < .05) increase in GSH and GPx-specific activities (Figure 2).
Also, in Figure 2, there was an (P < .05) increase in the MDA level of untreated compared with others. However, testicular tissues incubated with FeSO4 treated with 31.25 to 125 µg/mL concentrations of FEDW showed no significant (P > .05) difference in MDA level but revealed a significant (P < .05) difference when compared with untreated, control, and other concentrations of FEDW. Furthermore, FEDW concentrations of 250 to 1000 µg/mL were not momentously different from control.
Pro-inflammation biomarkers of flavonoid-rich extract from D welwitschii leaf in oxidative testicular injury
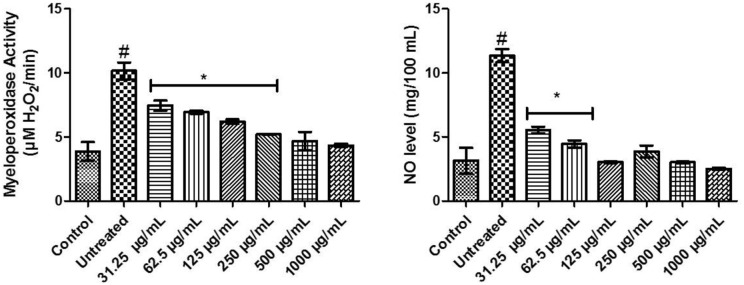
In Figure 3, there was an upsurge in the activity of myeloperoxidase of untreated compared with others. Testicular tissues incubated with FeSO4 treated with 31.25 to 250 µg/mL concentrations of FEDW were significantly (P < .05) different from control, untreated, and other concentrations of FEDW. FEDW at concentrations of 500 to 1000 µg/mL was not momentously different from control. Moreover, there was a rise in untreated nitric oxide levels when compared with control and different concentrations of FEDW. Testicular tissues incubated with FeSO4 treated with FEDW at concentrations of 31.25 to 62.5 µg/mL exhibited a noticeable difference when compared with control, untreated, and other concentrations of FEDW, whereas FEDW at concentrations of 125 to 1000 µg/mL were not significantly (P > .05) different from control.
Figure 3.
Pro-inflammation biomarkers of flavonoid-rich extract from Dalbergiella welwitschii leaf in oxidative testicular injury.
Data expressed as mean ± SD, n = 5.
#P < .05 vs the control, *P < .05 vs the untreated.
NO indicates nitric oxide.
Cholinergic enzyme activities of flavonoid-rich extract from D welwitschii leaf in oxidative testicular injury
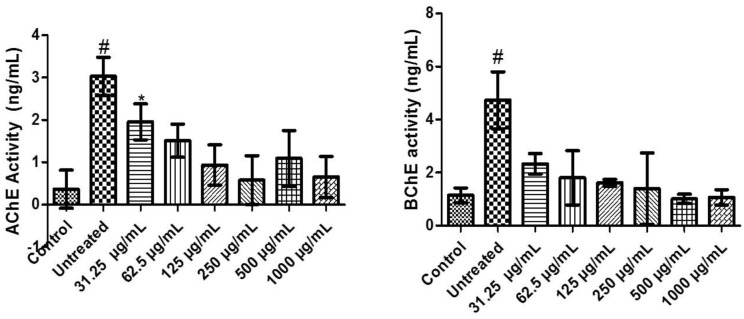
There was a significant (P < .05) increase in AChE activity of untreated compared with control and different concentrations of FEDW. Testicular tissues incubated with FeSO4 treated with 31.25 concentration of FEDW was significantly (P < .05) different from control, untreated, and concentrations of FEDW. But testicular tissues incubated with FeSO4 treated with 62.5 to 1000 µg/mL concentrations of FEDW revealed no significant (P > .05) difference from control (Figure 4).
Figure 4.
Cholinergic enzyme activities of flavonoid-rich extract from Dalbergiella welwitschii leaf in oxidative testicular injury.
Data expressed as mean ± SD, n = 5
#P < .05 vs the control, *P < .05 vs the untreated.
AChE indicates acetylcholinesterase; BChE, butyrylcholinesterase.
There was an upsurge in the activity of BChE of untreated compared with control and different concentrations of FEDW. Meanwhile, testicular tissues incubated with FeSO4 treated with 31.25 to 1000 µg/mL concentrations of FEDW showed no noticeable difference with control (Figure 4).
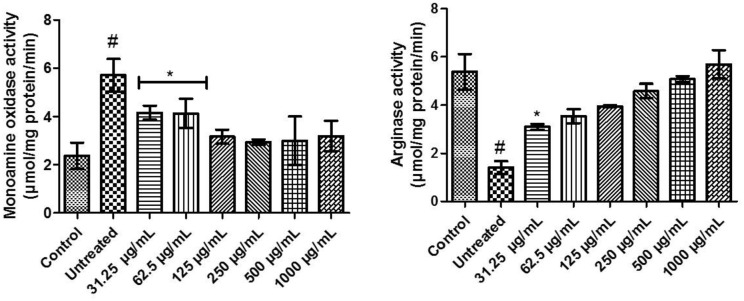
Monoaminergic enzyme activities of flavonoid-rich extract from D welwitschii leaf in oxidative testicular injury
The incubation of testicular tissue with FeSO4 significantly (P < .05) elevated the activity of monoamine oxidase (untreated) when compared with others. Testicular tissues incubated with FeSO4 treated with 31.25 and 62.5 µg/mL concentrations of FEDW were not significantly (P > .05) different from each other, but significantly (P < .05) different from control, untreated, and other concentrations of FEDW. There was no momentous difference in monoamine oxidase activity of FEDW at concentrations of 125 to 1000 µg/mL compared with control (Figure 5).
Figure 5.
Monoaminergic enzyme activities of flavonoid-rich extract from Dalbergiella welwitschii leaf in oxidative testicular injury.
Data expressed as mean ± SD, n = 5.
#P < .05 vs the control, *P < .05 vs the untreated.
Besides, Figure 5 shows a (P < .05) decline in the arginase activity (untreated) when compared with control and others. Testicular tissues exposed to FeSO4 treated with 31.25 µg/mL concentration of FEDW was significantly (P < .05) different in arginase activity when compared with control and other concentrations of FEDW. In addition, testicular tissues incubated with FeSO4 treated with 62.5 to 1000 µg/mL concentrations of FEDW showed no significant (P > .05) difference in arginase activity when compared with control.
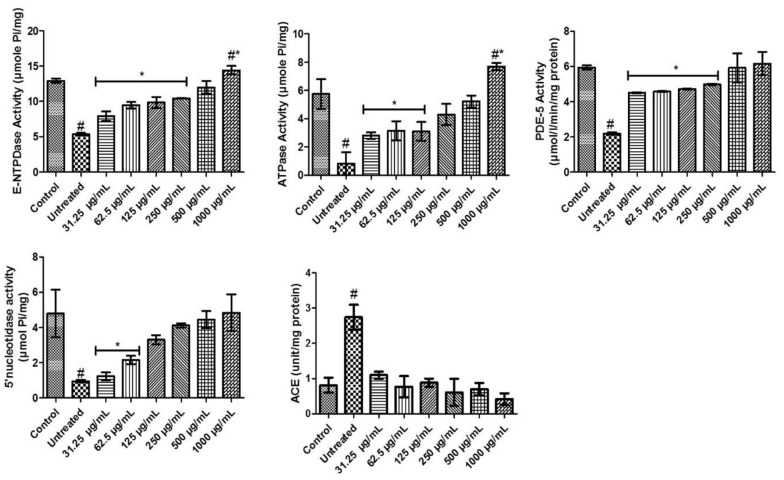
Purinergic enzyme activities of flavonoid-rich extract from D welwitschii leaf in oxidative testicular injury
Testicular tissues incubated with FeSO4 showed a significant (P < .05) reduction in E-NTPDase activity when compared with others. However, FEDW at concentrations of 31.25 to 250 µg/mL exhibited a significant (P < .05) difference when compared with control and other FEDW concentrations. Testicular tissues incubated with FeSO4 treated with FEDW at a concentration of 500 µg/mL showed no noticeable difference in E-NTPDase activity with control. And testicular tissues incubated with FeSO4 treated with FEDW at a concentration of 1000 µg/mL had the highest E-NTPDase activity (Figure 6).
Figure 6.
Purinergic enzyme activities of flavonoid-rich extract from Dalbergiella welwitschii leaf in oxidative testicular injury.
Data expressed as mean ± SD, n = 5
#P < .05 vs the control, *P < .05 vs the untreated.
ACE indicates angiotensin-converting enzyme; E-NTPDase, ectonucleotidase; PDE-5, phosphodiesterase-5.
The induction of oxidative testicular damage with FeSO4 (P < .05) decreased the activity of ATPase (untreated) when compared with control and FEDW concentrations. Testicular tissue incubated with FeSO4, treated with 31.25 to 125 µg/mL of FEDW concentrations, showed a noticeable decrease in ATPase activity when compared with control and other FEDW concentrations. Also, FEDW concentrations at 250 and 500 µg/mL show no significant (P > .05) difference in ATPase activity when compared with control. Testicular damage with FeSO4 that received the highest concentration of FEDW had the highest ATPase activity (Figure 6).
In Figure 6, testicular damage with FeSO4 declined the activity of PDE-5; however, treatment with 31.25 to 250 µg/mL of FEDW concentrations were significantly (P < .05) different from control, untreated, and other FEDW concentrations. Treatment of testicular tissues with 500 to 1000 µg/mL of FEDW concentrations showed no momentous difference in PDE-5 activity with control.
There was a (P < .05) decrease in the activity of 5ʹ-nucleotidase activity in testicular tissues incubated with FeSO4 (untreated). Treatment of testicular damage with 31.5 and 62.5 µg/mL of FEDW concentrations display a noticeable difference in 5ʹ-nucleotidase activity when compared with control, untreated, and other FEDW concentrations. In another vein, testicular tissues incubated with FeSO4 showed no momentous difference in FEDW concentrations of 125 to 1000 µg/mL of 5ʹ-nucleotidase activity when compared with control (Figure 6).
The testicular tissues incubated with FeSO4 increase the activity of ACE (untreated) compared with others. Meanwhile, testicular treatment with FEDW concentrations (31.25-1000 µg/mL) display no difference with control but exhibited differences with untreated (Figure 6).
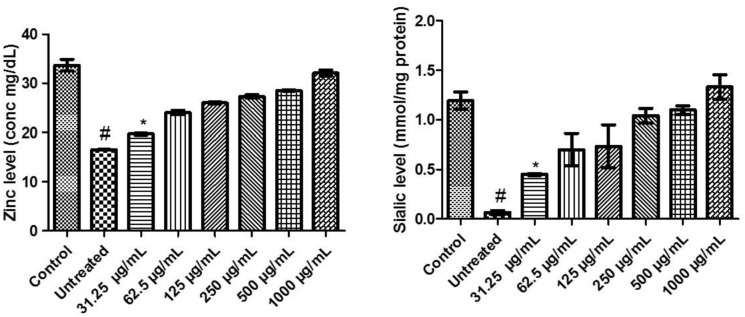
Zinc and sialic acid levels of flavonoid-rich extract from D welwitschii leaf in oxidative testicular injury
There was a significant (P < .05) decrease in the levels of zinc and sialic acid in testicular tissues incubated with FeSO4 (untreated). Treatment of testicular damage with 31.5 µg/mL of FEDW concentration showed a momentous difference in the levels of zinc and sialic acid compared with control and untreated. Conversely, treatment of testicular damage with 62.5 to 1000 µg/mL of FEDW concentrations showed no noticeable difference with the control on the levels of zinc and sialic acid (Figure 7).
Figure 7.
Zinc and sialic acid levels of flavonoid-rich extract from Dalbergiella welwitschii leaf in oxidative testicular injury.
Data expressed as mean ± SD, n = 5.
#P < .05 vs the control, *P < .05 vs the untreated.
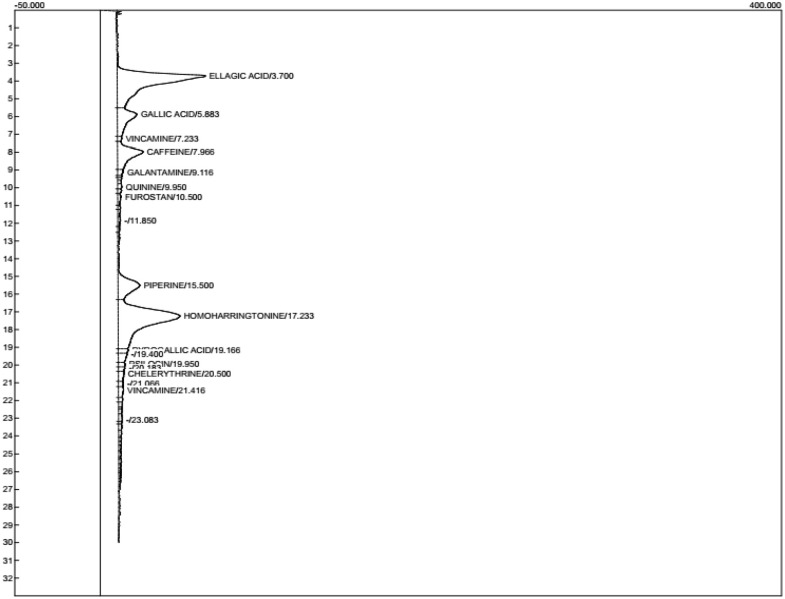
HPLC characterization of flavonoid-rich extract of D welwitschii leaf
As shown in Table 1 and Figure 8, a total of 13 compounds were present in the FEDW with homoharrington having the highest value of 5.810 ± 0.01; this was followed by ellagic acid (5.506 ± 0.01) and the least compounds were quinine and vincamine (0.089 ± 002).
Figure 8.
Chromatogram of flavonoid-rich extract from Dalbergiella welwitschii leaf.
Molecular docking
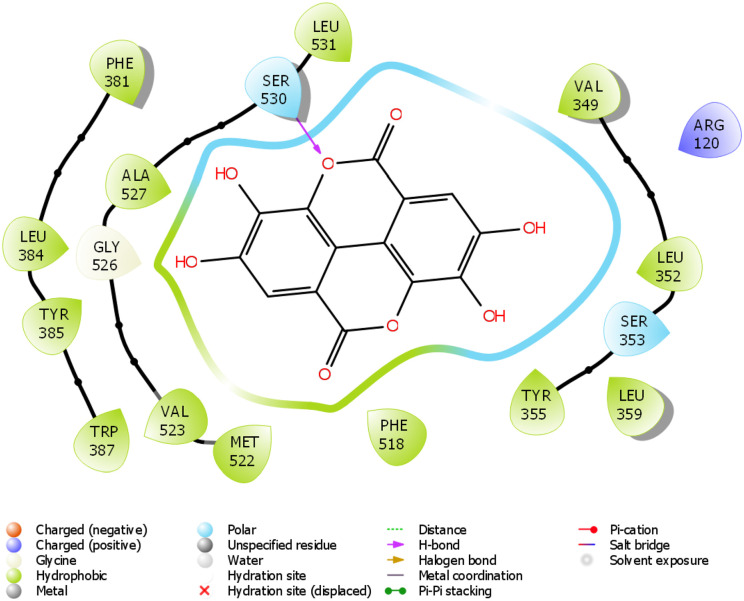
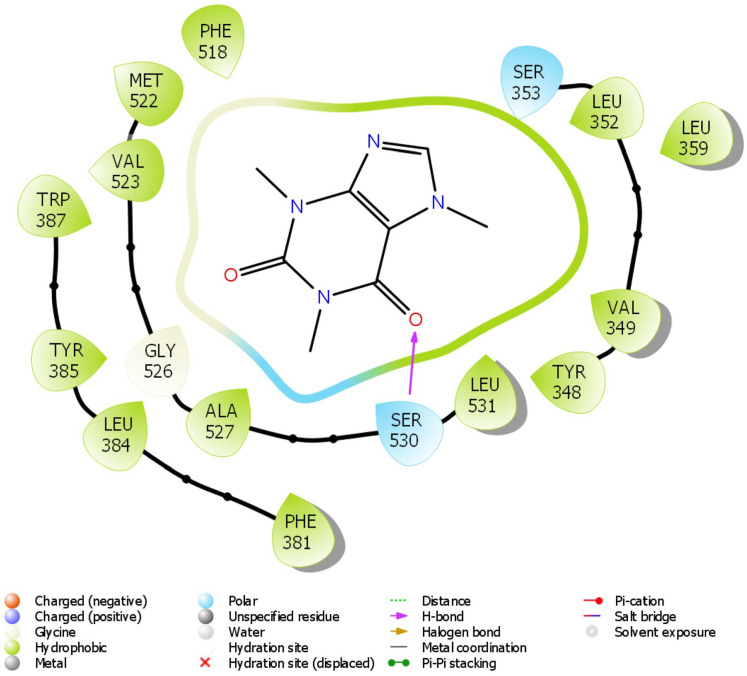
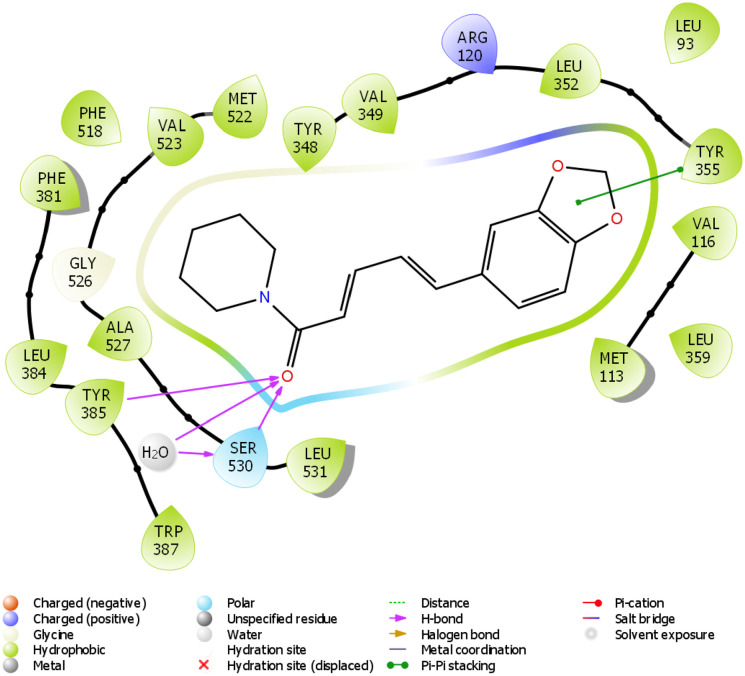
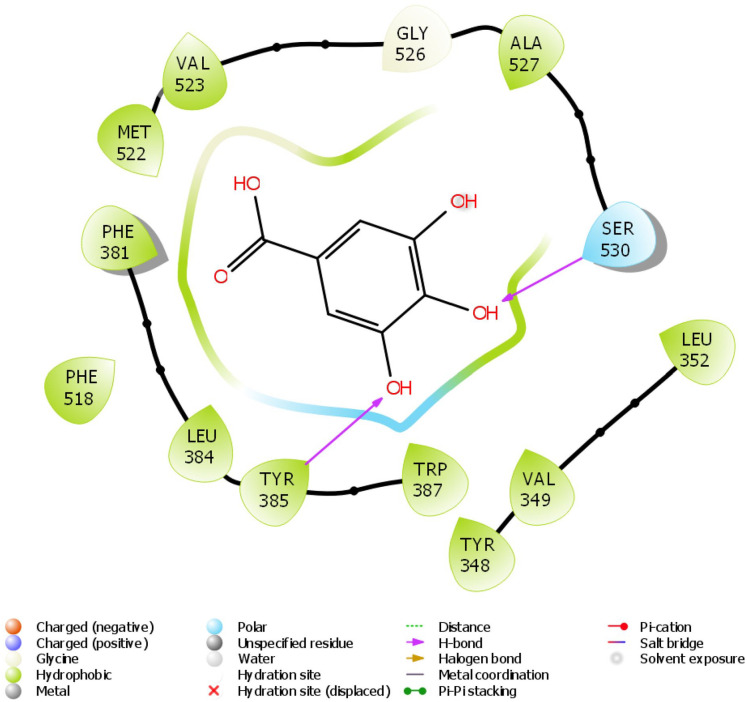
The docking scores of ellagic acid, piperine, gallic acid, and caffeine are shown in Table 2 against COX-2. Ellagic acid has the highest docking score of −9.909 Kcal/kJ, while the least was caffeine with −5.068 Kcal/mol. Figures 9 to 12 show the 2D interaction of the ligand with the protein (COX-2). The molecular interaction displayed hydrogen bonding, pi-pi stacked, and Van der Waals interactions, among others. Ellagic acid has H-bond interaction with SER 530, piperine with SER 530 and TYR 385, gallic acid has H-bond interaction with TYR 385, while caffeine has H-bond interaction SER 530.
Table 2.
Docking scores of some phytochemicals from flavonoid rich extract of Dalbergiella welwitschii leaf with COX-2.
| Compounds | Docking score (Kcal/mol) |
|---|---|
| Ellagic acid | −9.909 |
| Piperine | −8.683 |
| Gallic acid | −7.761 |
| Caffeine | −5.068 |
Figure 9.
2D molecular interaction of ellagic acid with amino acid residues within the pocket of COX-2.
Figure 12.
2D molecular interaction of caffeine with amino acid residues within the pocket of COX-2.
Table 3 shows the druggability results of the selected phytochemicals. Only piperine followed all of the rules (Lipinski, Ghose, Veber, Egan, and Muegge rules). Other phytochemicals were found to have broken one or more of the rules. In addition, the pharmacokinetic properties of the 4 selected phytochemicals are presented in Table 4. Ellagic acid, piperine, gallic acid, and caffeine had high GI absorption while only piperine can cross blood-brain barrier (BBB) permeant. Also, only piperine can serve as an inhibitor of CYP1A2, CYP2C19, and CYP2C9. Caffeine, on the contrary, was not an inhibitor of these cytochrome isoforms. Subsequently, ellagic acid, piperine, gallic acid, and caffeine were not P-gp substrates.
Table 3.
Druggability of some phytochemicals from flavonoid rich extract of Dalbergiella welwitschii leaf.
| Compounds | Lipinski | Ghose | Veber | Egan | Muegge |
|---|---|---|---|---|---|
| Ellagic acid | Yes; 0 violation | Yes | No; 1 violation: TPSA > 140 | No; 1 violation: TPSA > 131.6 | Yes; 0 violation |
| Piperine | Yes; 0 violation | Yes; 0 violation | Yes; 0 violation | Yes; 0 violation | Yes; 0 violation |
| Gallic acid | Yes; 0 violation | No; 2 violations: MR < 40, #atoms < 20 | Yes; 0 violation | Yes; 0 violation | No; 1 violation: MW < 200 |
| Caffeine | Yes; 0 violation | No; 1 violation: WLOGP <-0.4 | Yes; 0 violation | Yes; 0 violation | No; 1 violation: MW < 200 |
Abbreviations: MW, molecular weight; TPSA, topological polar surface area.
Table 4.
Pharmacokinetics properties of some phytochemicals from flavonoid-rich extract of Dalbergiella welwitschii leaf.
| Compounds | GI absorption | BBB permeant | P-gp substrate | CYP1A2 inhibitor | CYP2C19 inhibitor | CYP2C9 inhibitor | CYP2D6 inhibitor | CYP3A4 inhibitor | Log Kp (skin permeation) (cm/s) |
|---|---|---|---|---|---|---|---|---|---|
| Ellagic acid | High | No | No | Yes | No | No | No | No | −7.36 |
| Piperine | High | Yes | No | Yes | Yes | Yes | No | No | −5.58 |
| Gallic acid | High | No | No | No | No | No | No | Yes | −6.84 |
| Caffeine | High | No | No | No | No | No | No | No | −7.53 |
Discussion
The toxicity of the testicular tissues might result in male infertility. One of the major health problems globally, especially in developing countries (eg, Nigeria) is male infertility. There are different factors that can trigger testicular toxicity, one of which is oxidative injury. 4 In this study, in vitro antioxidant ability (DPPH, ABTS, and NO) (Figure 1) gives the clue for the antioxidant potential of the flavonoid-rich extract of D welwitschii leaf (FEDW). The scavenging potential of FEDW against DPPH, ABTS, and NO radicals provide information on the possible antioxidant nature of the extract. 40 Hence, the free radical’s mop-up ability of FEDW might protect the testicular organs.
Due to the high susceptibility of testicular polyunsaturated fatty acids (PUFAs) to free radical attack, oxidative stress has been linked to male reproductive system dysfunction. 26 The decrease observed in oxidative stress biomarkers such as GSH, CAT, SOD, and glutathione peroxidase (GPx) activities with a concomitant increase in MDA (Figure 2). GSH participates in many biological processes as an intra- and extracellular antioxidant. 41 It lowers the levels of lipid peroxidation by reducing hydrogen peroxide. 42 GSH is essential in cell defense against free oxygen radicals and other oxidative species. 41 Hence, a significant increase in GSH activity observed in FeSO4-exposed tissues treated with different concentrations of FEDW (particularly at 1000 µg/mL) supports its anti-free radical effect on testicular tissues. CAT is a tetrameric enzyme that translates hydrogen peroxide into oxygen and water. 43 CAT protects the cell from oxidative stress caused via reactive oxygen species (ROS). The increase in CAT activity in FeSO4-exposed tissues treated with various concentrations of FEDW (especially at 1000 µg/mL) illustrates a reduction in ROS levels in testicular cells.
SOD is an enzyme that plays an important role in the physiological protective mechanism by converting endogenous cytotoxic superoxide radicals to hydrogen peroxide as documented by Miaffo et al. 41 The increased activity of SOD in rats treated with FEDW shows a significant effect on the oxidative defense mechanism of the testicular tissues. GPx catalyzed detoxification of either hydrogen peroxide or lipid. 44 In this work, the activity of GPx increased in FeSO4-exposed tissues treated with various concentrations of FEDW (principally at 1000 µg/mL). Thus, FEDW has noticeable anti-oxidative stress potentials against testicular oxidative stress. The FeSO4-exposed rats triggered an increase in lipid peroxidation measured as MDA, which was observed in this study. A momentous decrease in MDA is elucidated by a decline in lipid peroxidation because of an increase in antioxidant enzyme activities. 41 FEDW has the ability to inhibit lipid peroxidation and enhance the antioxidant defense system of the testicular tissues. This study is in line with the previous work documented by Erukainure et al. 4
Proinflammation has also been related to the advancement of oxidative testicular toxicity, with ROS generation identified as a major mechanistic link. 45 The increased production of O2•- interacts with NO, resulting in the formation of peroxynitrite (ONOO−), a powerful free radical as documented by Erukainure et al. 5 The breakdown of H2O2 to hydroxyl radicals by myeloperoxidase reacts with hydrochloric acid (HCl) to produce hypochlorous acid (HOCl−). 5 The increased levels of NO and myeloperoxidase activity observed in FeSO4-exposed testicular tissues show a proinflammatory effect. Hence, these results support the anti-inflammatory effect of FEDW in oxidative testicular injury.
Andersson 46 reported that the upregulation of testicular acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) activities have been linked to the malfunctioning of testicular tissues that can corroborate infertility in men. The increased notice in the activities of AChE and BChE in FeSO4-exposed testicular tissues shows an abnormality in acetylcholine and butrylcholine levels, which reveals impairment of the testicular function. Akomolafe et al 8 reported that inhibition of AChE and BChE activities have been useful for treating/managing testicular injury. Surprisingly, the FeSO4-exposed tissues treated with various concentrations of FEDW ameliorated the activities of AChE and BChE, suggesting an improved testicular function that supports the antioxidant nature of the extract (Figure 4). Therefore, FEDW might serve as a cholinergic booster.
Monoamine oxidase (MAO) present in the mitochondria of testicular tissues plays an important role in ROS generation. 47 An increase in the activity of MAO is acknowledged by an increase in H2O2 production which was supported by FeSO4-exposed testicular tissues (Figure 5) and this can contribute to testicular malfunction. The findings in this study revealed that FeSO4-exposed testicular tissues treated with various concentrations of FEDW-inhibited MAO activity, probably because of the antioxidant booster of the FEDW as indicated in Figure 2. One of the key factors in male infertility is the alteration in the NO bioavailability in testicular damage. 34 Oboh et al, 34 among other scientists, have documented the aberration in arginase activity due to testicular injury, which is in accordance with the present study (Figure 5). However, amelioration of arginase activity increases penile NOS activity and cGMP levels, thereby restoring endothelial-derived NO vasodilatation and erectile function. 48 In this study, FeSO4-exposed testicular tissues treated with various concentrations of FEDW suggesting its anti-arginase properties probably linked to the antioxidative nature of the extract.
The suppressed purinergic activities in untreated FeSO4-exposed testicular tissues are shown by decreased ATPase, E-NTPDase, and 5ʹ-nucleotidase and increased ACE activities. Purinergic enzyme activities play crucial roles in the physiological activities of the testes. ATPase, E-NTPDase, and 5ʹ-nucleotidase enzymes phospho-hydrolyze adenosine triphosphate (ATP) and adenosine monophosphate (AMP) for the generation of endogenous signaling nucleotide, adenosine which serves as a potential dilator and a key factor in bioenergetics of the testes. 49 The decrease in ATPase, 5ʹ- nucleotidase, and E-NTPDase activities shows an alteration in testicular nucleotide metabolism. This encourages a decrease in ATP and adenosine levels of the testes. However, FeSO4-exposed testicular tissues treated with various concentrations of FEDW increased ATPase, 5ʹ-nucleotidase and E-NTPDase activities, which shows a normal ATP level and ameliorates an altered bioenergetic potential. Hence, FEDW improved bioenergetics. These results are in agreement with the previous work of Salau et al. 20
Phosphodiesterase-5 (PDE-5) is a key enzyme in the NO/cGMP signaling pathway, which performs an important series of functions in the corpus cavernosum. 10 The rate of synthesis of cGMP by guanylate cyclase and its degradation by PDEs governs the amount of cGMP in corpus cavernosum. 50 The ability of FEDW at different concentrations on FeSO4-exposed testicular tissues support its action as a PED-5 booster.
ACE catalyzes the hydrolysis of angiotensin I to angiotensin II, which is associated with hypertension, an important agent of testicular malfunction. 10 It has been reported by Jin 51 that increase in NADPH oxidase activity, production of ROS, and inhibition of nitric oxide synthase activity in testicular tissues might be because of a rise in the levels of angiotensin-II. This was noticed in FeSO4-exposed testicular tissues (Figure 6). Hence, FeSO4-exposed testicular tissues treated with various concentrations of FEDW inhibit the generation of angiotensin II and this might be useful for treating/managing testicular injury. This act of FEDW might be linked to its antioxidative nature.
Zinc is a trace element that is required for life. Zinc influences critical processes such as cell proliferation, immune function, and defense against free radicals. 52 It activates the antioxidant system, which protects cells from damage.52,53 Zinc is a component of the oxidant defense system and performs numerous functions. 54 It is believed to have antioxidant properties by maintaining an adequate level of metallothionein, and it is an essential component of Cu/Zn SOD. 54 Zinc is said to promote spermatogenesis and influence sperm motility. Zinc deficiency has been linked to testicular damage, increased levels of lipid peroxidation, and decrease in antioxidant enzyme activities. Therefore, different concentrations of FEDW revealed its potential to enhance zinc levels in FeSO4-exposed testicular tissues (Figure 7). This is in line with a previous study conducted by Bashandy et al. 54
Sialic acid is an N-acetylmannose derivative that is an important component of glycolipids and glycoproteins. 55 The obtained results showed that there was a significant decrease in testicular sialic acid level in untreated FeSO4-incubated testicular tissues (Figure 7). This decrease in testicular sialic acid levels could be attributed to a slowing of spermatogenesis. 55 Moreover, FeSO4-incubated testicular tissues treated with different concentrations of FEDW enhanced the levels of sialic acid, which shows that the extract can serve as a spermatogenesis enhancer. This could be linked to the antioxidant nature of the FEDW.
One of the main mechanisms of testicular injury is the accumulation of free radicals, which was mimicked in this study via FeSO4 induction. Hence, the anti-testicular injury of the FEDW could be linked to its constituent flavonoids. These include ellagic acid, gallic acid, vincamine, caffeine, galantamine, quinine, furostan, piperine, homoharrington, pyrogallic acid, psilocin, and chelerythrine (Figure 8 and Table 1) and have been documented to possess anti-inflammatory, antioxidant properties among others. Thus, FEDW can serve as a good anti-testicular injury, making it useful in male infertility boosters.
The in silico study was carried out on some compounds identified by HPLC in FEDW to find out the hit compound(s). The target protein used in this case was an anti-inflammatory marker named COX-2. This is important as testicular injury can be encouraged by inflammation. According to Boittier et al, 56 docking is a computational approach for predicting the binding pose and affinity of a ligand (phytochemicals) and protein. This plays a crucial role in rational drug design. In this study, ellagic acid has the highest docking score followed by piperine (Table 2). Ellagic acid and piperine have been documented as an antioxidant, anti-inflammatory, anti-apoptotic,57,58 among others. This implies that ellagic acid and piperine can be useful in designing drugs for testicular injury. The molecular interactions ellagic acid, piperine, gallic acid, and caffeine with COX-2 pocket (Figure 9-12) revealed the presence of H-bond, charged ions, pi cation, pi-pi stacking, etc in interacting with the COX-2 structure. These occur between the ligands and COX-2, which played a significant role in the repressive activity on COX-2 as well as attractive charge.
Figure 10.
2D molecular interaction of piperine with amino acid residues within the pocket of COX-2.
Figure 11.
2D molecular interaction of gallic acid with amino acid residues within the pocket of COX-2.
The process of drug development and production is aided by assessing druggability. To pass through the biological barrier, a drug’s topological polar surface area (TPSA) and molecular weight must be addressed. The lower the TPSA and molecular weight numbers, the less penetration the drug candidate has, and vice versa. 59 Furthermore, Lipinski’s RO5 demonstrates that an effective drug molecule should have properties that fall within the acceptable range of the 5 Lipinski guidelines; this was obeyed by ellagic acid, piperine, gallic acid, and caffeine (Table 3). However, it was only piperine that obeys all the rules (Lipinski, Ghose, Veber, Egan, and Muegge). Hence, piperine can be recommended as a hit compound in the development of a drug for testicular injury.
The pharmacokinetics properties of ellagic acid, piperine, gallic acid, and caffeine demonstrated that they all have high gastrointestinal tract (GIT) absorption (Table 4). This implies that all the compounds have the ability of proper GIT absorption on oral administration, which is an important criterion for drug discovery. 60 The metabolism of the substances was predicted using 5 cytochrome P450 (CYP) monooxygenase isoforms, namely, CYP1A2, CYP2C19, CYP2C9, CYP2D6, and CYP3A4. The monooxygenase cytochrome P450 plays a crucial function in drug metabolism and elimination. The fact that the discovered compounds have no inhibitory effect on these enzymes suggests that they are very likely to be converted and hence accessible when taken orally. 60 Hence, the inhibition of some CYP isomers by some of the compounds might affect the bioavailability probably due to the accumulation of their toxic side. 61
Dixon et al 62 claim that skin is a selective barrier that permits different chemicals to enter in different ways, depending on their physicochemical qualities. Thus, skin permeability; determined as LogKp is a parameter used in assessing molecules/compounds that require transdermal administration. Ellagic acid, piperine, gallic acid, and caffeine were impermeable as they had negative LogKp values (Table 4), meaning that they could be efficiently administered via the skin. 63 ADMET (absorption, distribution, metabolism, and excretion) predictions were evaluated so as to ascertain the ability of a drug molecule inside a biological system after its pharmacological and pharmacodynamic properties. Furthermore, the crucial factor for the drugs that mainly target the brain cells is their ability to cross the BBB, only piperine has this potential. This also makes piperine useful for the management of male reproductive malfunction, as the brain is also involved.
Added to this, it is known that a large quantity of drugs are administered orally, such drugs are digested by the intestinal tissue. In lieu of this, the plasma membrane ATP-binding cassette transporter, called P-glycoprotein (P-gp), aids in transporting drugs. 59 The inability of the ellagic acid, piperine, gallic acid, and caffeine (Table 4) for this act make these compounds more useful as a potential testicular drug.
Conclusions
In vitro, ex vivo, and in silico investigations of FEDW as a potential treatment agent for oxidative testicular injury. The findings reveal that FEDW has therapeutic and protective properties against oxidative testicular injury, as evidenced by its ability to reduce oxidative stress, proinflammation, and cholinergic, monoaminergic, and purinergic dysfunction. As a result, FEDW could be used as a pharmacological adjuvant in the treatment and management of testicular damage subsequent to in vivo study.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was sponsored by the authors.
Declaration Of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Conceptualization, optimization, and validation of the method used as well as supervision of the research were done by BOA, BEO, OAO, and ORA. Analysis and interpretation were done by AOA, OEL, BAB, and YAJ. Draft and approval of the original draft of the manuscript were performed by all the authors.
Availability of Data and Materials: Only on special request from the corresponding author.
Ethical Approval: Approval was granted and selected animals were handled in accordance with the approval of the Animal Ethics Committee of Federal University Oye-Ekiti, Ekiti State, Nigeria.
References
- 1. Asadi N, Bahmani M, Kheradmand A, Rafieian-Kopaei M. The impact of oxidative stress on testicular function and the role of antioxidants in improving it: a review. J Clin Diagnost Res. 2017;11:IE01-IE05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aitken RJ, Roman SD. Antioxidant systems and oxidative stress in the testes. Oxid Med Cell Longev. 2008;1:15-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2006;97:1634-1658. [DOI] [PubMed] [Google Scholar]
- 4. Erukainure OL, Atolani O, Banerjee P, et al. Oxidative testicular injury: effect of Lleucine on redox, cholinergic and purinergic dysfunctions, and dysregulated metabolic pathways. Amino Acids. 2021;53:359-380. [DOI] [PubMed] [Google Scholar]
- 5. Erukainure OL, Hafizur R, Kabir N, et al. Suppressive effects of clerodendrum volubile P Beauv. [Labiatae] methanolic extract and its fractions on type 2 diabetes and its complications. Front Pharm. 2018;9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guazzone VA, Jacobo P, Theas MS, Lustig L. Cytokines and chemokines in testicular inflammation: a brief review. Microsc Res Tech. 2009;72:620-628. [DOI] [PubMed] [Google Scholar]
- 7. Mor I, Soreq H. Cholinergic toxicity and the male reproductive system. In: Gupta RC, ed. Reproductive and Developmental Toxicology. Amsterdam, The Netherlands: Elsevier; 2011:863-870. [Google Scholar]
- 8. Akomolafe S, Oboh G, Olasehinde T, Oyeleye S, Ogunsuyi O. Modulatory effects of Aqueous extract from Tetracarpidium conophorum leaves on key enzymes linked to erectile dysfunction and oxidative stress-induced lipid peroxidation in penile and testicular tissues. J Appl Pharm Sci. 2017;7:51-56. [Google Scholar]
- 9. Olofinsan KA, Salau VF, Erukainure OL, Islam MS. Ocimum tenuiflorum mitigates iron-induced testicular toxicity via modulation of redox imbalance, cholinergic and purinergic dysfunctions, and glucose metabolizing enzymes activities. Andrologia. 2021;53:e14179. [DOI] [PubMed] [Google Scholar]
- 10. Ojo OA, Ojo AB, Oyinloye BE, et al. Ocimum gratissimum Linn. Leaves reduce the key enzymes activities relevant to erectile dysfunction in isolated penile and testicular tissues of rats. BMC Complement Alter Med. 2019;19:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nantia E, Moundipa P, Monsees T, Carreau S. Medicinal plants as potential male anti-infertility agents: a review. Basic Clin Androl. 2009;19:148-158. [Google Scholar]
- 12. Sharma AK, Gupta K. Anti-hyperglycemic activity of aqueous extracts of some medicinal plants on Wistar rat. J Diabet Metabol. 2007;8:752. [Google Scholar]
- 13. Xu DP, Li Y, Meng X, et al. Natural antioxidants in foods and medicinal plants: extraction, assessment and resources. Int J Molec Sci. 2017;18:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Adediwura F, Oluwakemi A. Phytochemical and Anti-inflammatory activities of the methanol extract and fraction of Dalbergiella welwitschii baker (Baker.f) leaves. Res J Pharmaceut Biol Chem Sci. 2013;4:1191-1200. [Google Scholar]
- 15. Ofodile L, Olusegun-Joseph T, Ogundele G, Emmanuel A. Anthelmintic thin layer chromatographic fractions of the leaves of the herbal plant Dalbergiella welwitschii. Austr J Basic Appl Sci. 2019;13:93-98. [Google Scholar]
- 16. Obafemi TO, Akinmoladun AC, Olaleye MT, Stephen OA, Amos AO. Potentials of flavonoid-rich extract. J Ayurveda Integ Med. 2017;8:238-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Atolani O, Olatunji GA. Chemical composition, antioxidant and cytotoxicity potential. Turkish J Pharmaceutical Sci. 2016;13:84-94. [Google Scholar]
- 18. Atolani O, Olatunji G, Fabiyi OA, Adeniji JA. Phytochemicals from Kigelia pinnata leaves show antioxidant and anticancer potential on human cancer cell line. J Med Food. 2013;16:878-885. [DOI] [PubMed] [Google Scholar]
- 19. Ebrahimzadeh MA, Nabavi SF, Nabavi SM. Antioxidant activities of methanol extract of Sambucus ebulus L. flower. Pak J Bio Sci. 2009;12:447. [DOI] [PubMed] [Google Scholar]
- 20. Salau VF, Erukainure OL, Islam MS. Caffeic acid protects against iron-induced cardiotoxicity by suppressing angiotensin-converting enzyme activity and modulating lipid spectrum, gluconeogenesis and nucleotide hydrolyzing enzyme activities. Biol Trace Elem Res. 2020;199:1052-1061. [DOI] [PubMed] [Google Scholar]
- 21. Ellman GL. Tissuesulfhydryl groups. Arch Biochem Biophys. 1959;82:70-77. [DOI] [PubMed] [Google Scholar]
- 22. Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121-126. [DOI] [PubMed] [Google Scholar]
- 23. Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130-132. [PubMed] [Google Scholar]
- 24. Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158-169. [PubMed] [Google Scholar]
- 25. Chowdhury P, Soulsby M. Lipid peroxidation in rat brain. Ann Clin Lab Sci. 2002;32:188-192. [PubMed] [Google Scholar]
- 26. Erukainure OL, Reddy R, Islam MS. Raffia palm (Raphia hookeri) wine extenuates redox imbalance and modulates activities of glycolytic and cholinergic enzymes in hyperglycemia-induced testicular injury in type 2 diabetes rats. J Food Biochem. 2019;43:e12764. [DOI] [PubMed] [Google Scholar]
- 27. Granell S, Gironella M, Bulbena O, Penes J, Maur M, Closa D. Myeloperoxidase activity. Crit Care Med. 2003;31:525-530. [DOI] [PubMed] [Google Scholar]
- 28. Ellman GL, Courtney KD, Andres V, Jr, Featherstone RM. A new and rapid colometric determination of Acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88-95. [DOI] [PubMed] [Google Scholar]
- 29. Nwanna EE, Adebayo AA, Oboh G, Ogunsuyi OB, Ademosun AO. Modulatory effects of alkaloid extract from Gongronema latifolium (utazi) and Lasianthera Africana (editan) on activities of enzymes relevant to neurodegeneration. J Diet Suppl. 2018;16:27-39. [DOI] [PubMed] [Google Scholar]
- 30. Kaysen GA, Strecker HJ. Purification and properties of arginase of rat kidney. Biochem J. 1973;133:779-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Erukainure O, Okafor O, Obode O, Ajayi A, Oluwole O, Oke O. Blend of roselle calyx and selected fruit modulates testicular redox status and sperm quality of diabetic rats. J Diab Metab. 2017;3:2. [Google Scholar]
- 32. Schetinger MRC, Morsh VN, Bonan CD, Wyse AT. NTPDase activities in physiological and disease conditions. Pharm Res. 2007;31:77-98. [DOI] [PubMed] [Google Scholar]
- 33. Heymann D, Reddington M, Kreutzberg GW. Subcellular localization of 5-nucleotidase in rat brain. J Neurochem. 1984;43:971-978. [DOI] [PubMed] [Google Scholar]
- 34. Oboh G, Adebayo AA, Ademosun AO, Boligon AA. In vitro inhibition of phosphodiesterase-5 and arginase activities from rat penile tissue by two Nigerian herbs (Hunteria umbellata and Anogeissus leiocarpus). J Basic Clin Physiol Pharmacol. 2017;28:393-401. [DOI] [PubMed] [Google Scholar]
- 35. Cushman DW, Cheung HS. Spectrometric assay and properties of the angiotensin—converting enzyme of rat. Biochem Pharmacol. 1971;20:163-148. [DOI] [PubMed] [Google Scholar]
- 36. Homster R, Zak B. Diagnostic reagent for determination of Zinc concentration.Clin Chem. 1985;1310-1313. [PubMed] [Google Scholar]
- 37. Traving C, Schauer R. Structure, function and metabolism of sialic acid. J Cell Molec Life Sci. 1998;54:1330-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ojo OA, Ojo AB, Ajiboye BO, et al. Chromatographic fingerprint analysis, antioxidant properties, and inhibition of cholinergic enzymes (acetylcholinesterase and butyrylcholinesterase) of phenolic extracts from Irvingia gabonensis (Aubry-Lecomte ex O’Rorke) Baill bark. J Basic Clin Physiol Pharmacol. 2018;29:217-224. [DOI] [PubMed] [Google Scholar]
- 39. Oyinloye BE, Iwaloye O, Ajiboye BO. Polypharmacology of Gongronema latifolium leaf secondary metabolites against protein kinases implicated in Parkinson’s disease and Alzhemier’s disease. Sci African. 2021;12:e00826. [Google Scholar]
- 40. Ajiboye BO, Ojo OA, Fatoba B, et al. In vitro antioxidant and enzyme inhibitory properties of the n-butanol fraction of Senna podocarpa (Guill. and Perr.) leaf. J Basic Clin Physiol Pharmacol. 2020;31:20190123. [DOI] [PubMed] [Google Scholar]
- 41. Miaffo D, Kamgue OG, Tebou NL, Temhoul CMM, Kamanyi A. Antidiabetic and antioxidant potentials of Vitellaria paradoxa barks in alloxan-induced diabetic rats. Clin Phytosci. 2019;5:44. [Google Scholar]
- 42. Arivazhagan S, Balasenthil S, Nagini S. Garlic and neem leaf extracts enhance hepatic glutathione and glutathione dependent enzymes during N-methyl-Nnitrosoguanidine (MNNG)-induced gastric carcinogenesis. Phytother Res. 2000;14:291-293. [DOI] [PubMed] [Google Scholar]
- 43. Chelikani P, Fita I, Loewen PC. Diversity of structure and properties among catalases. Cell Mol Life Sci. 2004;61:192-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Aktaş M, Değirmenci U, Ercan SK, Tamer L, Atik U. Redükte glutatyon ölçümünde HPLC ve spektrofotometrik yöntemlerin karşılaştırılması. Türk Klinik Biyokimya Derg. 2005;3:95-99. [Google Scholar]
- 45. Azenabor A, Ekun AO, Akinloye O. Impact of inflammation on male reproductive tract. J Reprod Infertil. 2015;16:123-129. [PMC free article] [PubMed] [Google Scholar]
- 46. Andersson KE. Mechanisms of penile erection and basis for pharmacological treatment of erectile dysfunction. Pharmacol Rev. 2011;63:811-859. [DOI] [PubMed] [Google Scholar]
- 47. Ademosun AA, Ayokunle O, Adebayo G, Oboh1. Modulatory effect of some citrus (Citrus lemon, Citrus reticulata, Citrus maxima) peels on monoamine oxidase, phosphodiesterase-5 and angiotensin-1 converting enzyme activities in rat heart homogenate. J Complemen Integrat Med. 2018;2018:20180067. [DOI] [PubMed] [Google Scholar]
- 48. Goswami SK, Inamdar MN, Jamwal R, Dethe S. Effect of Cin-namomum cassia methanol extract and sildenafil on arginase and sexual function of young male Wistar rats. J Sex Med. 2014;11:1475-1483. [DOI] [PubMed] [Google Scholar]
- 49. Burnstock G. Purinergic signaling in the cardiovascular system. Circ Res. 2017;120:207-228. [DOI] [PubMed] [Google Scholar]
- 50. Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Ther. 2006;109:366-398. [DOI] [PubMed] [Google Scholar]
- 51. Angiotensin JL, II. Signaling and its implication in erectile dysfunction. J Sexual Med. 2009;6:302-310. [DOI] [PubMed] [Google Scholar]
- 52. Cruz KJ, de Oliveira AR, Marreiro D. Antioxidant role of zinc in diabetes mellitus. W J Diabet. 2015;6:333-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Prasad AS. Zinc deficiency. BMJ. 2003;326:409-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bashandy SA, Enayat AA, Omara HE, Mohamed MA, Mahmoud SS. Role of zinc as an antioxidant and anti-inflammatory to relieve cadmium oxidative stress induced testicular damage in rats. Asian Pac J Trop Biomed. 2016;6:1056-10641. [Google Scholar]
- 55. Mohamed EAK, Saddek EA. The protective effect of taurine and/or vanillin against renal, testicular and hematological alteration induced by potassium bromate toxicity in rats. JOBAZ. 2019;80:3. [Google Scholar]
- 56. Boittier I, Eric D, Tang YY, et al. Assessing molecular docking tools to guide targeted drug discovery of CD38 inhibitors. Int J Mol Sci. 2020;21:5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen P, Chen F, Zhou B. Antioxidative, anti-inflammatory and anti-apoptotic effects of ellagic acid in liver and brain of rats treated by D-galactose. Sci Rep. 2018;8:1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Abdel-Daim AA, Mohamed MS, Ahmed A, et al. Piperine enhances the antioxidant and anti-inflammatory activities of thymoquinone against microcystin-lr-induced hepatotoxicity and neurotoxicity in mice. Oxidat Med Cell Long. 2019;2019:1309175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ojo OA, Adegboyega AE, Johnson GI, et al. Deciphering the interactions of compounds from Allium sativum targeted towards identification of novel PTP 1B inhibitors in diabetes treatment: a computational approach. Inform Med Unlock. 2021;26:100719. [Google Scholar]
- 60. Chow HH, Garland LL, Hsu CH, et al. Resveratrol modulates drug-and carcinogen metabolizing enzymes in a healthy volunteer study. Cancer Prev Res (Phila). 2010;3:1168-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Khan MF, Bari MA, Islam MK, et al. The natural anti-tubercular agents: in silico study of physicochemical, pharmacokinetic and toxicological properties. J App Pharm Sci. 2017;7:34-38. [Google Scholar]
- 62. Dixon SL, Smondyrev AM, Knoll EH, Rao SN, Shaw DE, Friesner RA. PHASE: a new engine for pharmacophore perception, 3D QSAR model development, and 3D database screening: 1 methodology and preliminary results. J Comput Aided Mol Des. 2006;20:647-671. [DOI] [PubMed] [Google Scholar]
- 63. Sahin S, Benet LZ. The operational multiple dosing half-life: a key to defining drug accumulation in patients and to designing extended release dosage forms. Pharm Res. 2008;25:2869-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]