Abstract
Background:
COVID-19 pandemic causes severe acute respiratory syndrome and requires rapid action. The development of effective safe vaccines become a global priority for achieving herd immunity. Vaccination is expected to form specific antibodies against the SARS-CoV-2 spike protein which can neutralize the virus, preventing the virus from binding with ACE 2 receptors.
Objective:
Evaluating and to know if there any differences of kinetics antibody levels from recipient’s anti-IgG S-RBD and NAb with complete second dose CoronaVac Vaccine, to determine the antibody response in preventing SARS-CoV-2.
Method:
A prospective-cohort study using observational analytics was conducted from January-April 2021 at Dr. Soetomo Hospital, Surabaya. A total of 50 subjects are healthcare workers who received two doses of CoronaVac. The IgG S-RBD and NAb levels were measured on Maglumi 800 device (SNIBE, China). Differences in IgG S-RBD and NAb levels before vaccination and after second dose CoronaVac vaccination on 14th day, on 28th day, ware tested using Friedman and Wilcoxon tests.
Result:
Mean values of IgG S-RBD and NAb have fluctuated. There was a significant difference between IgG S-RBD and NAb levels on day-0 (0.090 vs 18.630; p < 0.001) and day-28 (141.266 vs 116.640; p = 0.037). The median value showed the IgG S-RBD level on day-28 was much better than NAb value (141,266 v 116,640).
Conclusion:
CoronaVac will form persistent antibodies. Despite antibody development, the acquired humoral immunity decreased at 28 days after full CoronaVac immunization. Kinetics of antibody NAb decreased more rapidly than IgG S-RBD.
Keywords: SARS-CoV-2, vaccination, coronaVac, IgG S-RBD, NAb
Introduction
Coronavirus Disease 2019 (COVID-19) is an infectious disease caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Coronavirus Disease (COVID-19) spread rapidly throughout the world, to the point where the World Health Organization (WHO) declared this incident a pandemic on March 11, 2020. Globally, the COVID-19 pandemic has resulted in a high rate of morbidity and mortality. 1 The Indonesian Ministry of Health reported that as of March 21, 2021, there were 1,460,184 total confirmed cases of COVID-19, with 39,550 deaths and 5,533,379 who had received vaccinations.2–4
The development of effective safe vaccines and new therapies has become a global priority. Vaccination aims to reduce the transmission of COVID-19, reduce morbidity and mortality due to COVID-19, and achieve herd immunity.5,6 COVID-19 vaccines induce both innate and adaptive immune responses through a variety of mechanisms. It involves both a cellular response (T cells) and an antibody response (B cells), which results in the production of antibodies directed against the various SARS-CoV-2 antigens. After vaccination, the body is expected to produce specific antibodies against the SARS-CoV-2 spike protein, which are capable of neutralizing the virus and preventing it from binding to its specific receptor (ACE 2 receptor).7,8
SARS-CoV-2 is an enveloped non-segmented positive-sense RNA (COVID-19) virus. SARS-CoV-2 contains several structural proteins, including the spike (S), the envelope (E), the membrane (M), and the nucleocapsid (N). Spike protein (S), and nucleocapsid protein are two proteins known to act as immunogens (N). Protein S plays an important role in the attachment, fusion, transmission, and entry of viruses. The S protein consists of the S1 subunit which is responsible for binding to the viral receptor and the S2 subunit which is responsible for the fusion of the viral cell membrane. S1 is further subdivided into an N-terminal domain (NTD) and a receptor-binding domain (RBD). RBD has a high affinity for the ACE 2 receptor on the cell surface membrane. 9
Response immune after SARS-CoV-2 vaccination is very important to describe the effectiveness and determination of immunogenicity of vaccination. The IgA response occurs slightly earlier than IgM and IgG. Seroconversion to IgA, IgM, and IgG generally occurs thereafter approximately 2 weeks after onset. Neutralizing antibody (NAb) seropositive rates can reach 100% within 20 days of disease onset. Another study said NAb increased significantly on day-14 and reached its peak at 28 days after vaccination. Some of the antibodies produced have a neutralizing effect on Neutralizing antibody (NAb) and specific IgG-binding antibody titer. Based on the explanation above, the subject’s samples were taken three times, first day before vaccination (day-0), after the second dose of CoronaVac vaccination on the 14th day (day-14), and on the 28th day (day-28) to monitor antibody levels.10,11
Serological diagnostic tests for COVID-19 have been developed, but antibody responses in vaccinated subjects especially using CoronaVac remain largely unknown. This study aimed to evaluate the levels of anti-IgG S-RBD and NAb kinetics in a prospective-cohort study, so it can describe antibody response to help prevent SARS-CoV-2 disease.
Design and methods
Clinical procedures
This research is an observational analytical study with a prospective cohort approach. The study was carried out in the Clinical Pathology laboratory unit, Central Laboratory Installation, RSUD Dr. Soetomo Surabaya-Indonesia, from January to April 2021. The samples were taken consecutively only on health workers at RSUD Dr. Soetomo Surabaya, who received two doses of the SARS-CoV-2 (CoronaVac) vaccine for the period January 2021–March 2021 and never had a history of COVID-19 illness based on history, medical history, and also attach negative PCR swab results before taking vaccinations.
Antibody measurement
Subjects will be to phlebotomy three times, were before first day before vaccination (day-0) and after second dose CoronaVac vaccination on the 14th day (day-14), on the 28th day (day-28). Measurement of IgG S-RBD and NAb before vaccination (day 0), was used to ensure that the subjects in this study has been infected with COVID-19 before and to know the first levels. The SST (serum separator tube) tube is used to collect the blood Serum samples were classified according to their time of collection (days). The levels of IgG S-RBD and NAb will be determined in all subjects. Maglumi 800 (Snibe) can quantitatively measure SARS-CoV-2 antibodies with two assays from different reagents, namely IgG S-RBD and NAb. The measured NAb is not specific only to IgG but also measures IgE and IgM levels. Many kinds of literature say that the highest neutralizing effect is only on IgG S-RBD. The measurement of IgG S-RBD levels was carried out by the indirect chemiluminescence immunoassay method and NAb levels by the competitive chemiluminescence method on the Maglumi 800 device (SNIBE—Shenzhen New Industries Biomedical Engineering Co., Ltd, Shenzhen, China).12,13 The unit of measure for each level under the latest notification received from the World Health Organization (WHO).
Statistical analysis
All statistical analyses were performed using IBM SPSS 25.0 statistical software. The Kolmogorov-Smirnov test was used to determine the normality of the collected data. The median and mean are used to express quantitative variables. The Friedman and Wilcoxon tests were used to determine differences in IgG S-RBD titer levels before and after booster CoronaVac on days 14 and 28 using a non-parametric test. p-Value ≤0.05 indicates statistical significance.
Research ethics
The study has been reviewed by the Health Research Ethics Committee of Dr. Soetomo General Academic Hospital.
Result
A total of 50 subjects were included in this study. The average age of the subjects was 35.74 ± 6.99 years. The gender characteristics of the patients in this study were found to be more female than a male with 31 (62%) female subjects and 19 (32%). The mean age in this study was 35.74 ± 6.99 years, with a median of 34.5 years and the lowest age being 25 years and the highest age being 57 years. There is a history of comorbidities in several subjects, known to have a history of diabetes mellitus 3 (6%), obesity 7 (14%), hypertension 10 (25%), hypercholesterolemia 10 (25%) (Table 1).
Table 1.
Characteristics of research subjects.
| Variable | Value (n) |
|---|---|
| Number of subjects | 50 |
| Age | |
| Mean ± SD | 35.74 ± 6.99 |
| Median | 34.50 |
| Range | 25–57 |
| Gender (n (%)) | |
| Male | 19 (38) |
| Female | 31 (12) |
| Diabetes mellitus (n (%)) | |
| Yes | 3 (6) |
| No | 47 (94) |
| Obesity (n (%)) | |
| Yes | 7 (14) |
| No | 43 (86) |
| Hyperkolesterolemia (n (%)) | |
| Yes | 10 (20) |
| No | 40 (80) |
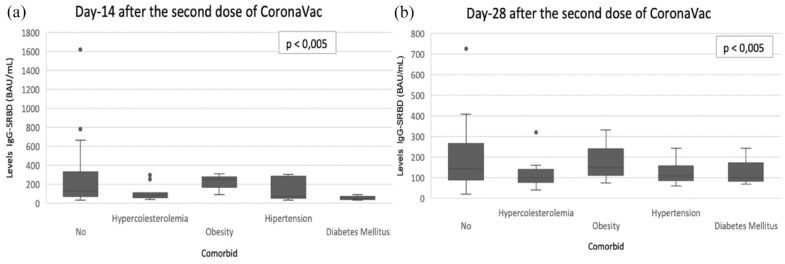
Comparative analysis of post-vaccination IgG S-RBD levels based on comorbidities (DM, hypercholesterolemia, obesity, and hypertension) was performed using the Mann-Whitney test. Based on the results of the analysis on each comorbidity, there was no significant difference between the comorbid group and the no comorbid group on day 14, day 28 (p > 0.05) (Figure 1).
Figure 1.
Levels IgG S-RBD at measurement days (14, 28) divided according to the medical history of the subjects participating in the study. There was no significant difference individuals with comorbid compared to individuals with no comorbidities on day 14, day 28 (p > 0.05) all other cases, no statistically significant differences were observed. (a) Day-14; (b) Day-28.
Levels of IgG S-RBD and NAb after measuring IgG S-RBD levels 3 times, 1 day- before vaccination (day-0) and after second dose CoronaVac vaccination on the 14th day (day-14), on the 28th day (day-28) (Table 2). According to Table 2, it is known that the overall median value has increased. In contrast to the fluctuating NAb levels. On day-0 (pre-vaccination) the median value of NAb data was 18.63 IU/mL (IQR 7.70–157.14) then increased on day–14 to 166.66 IU/mL (IQR 28.35–1221.48 ) and decreased on day–28 to 116.64 IU/mL (IQR 17.82–515.16).
Table 2.
Levels of IgG S-RBD and NAb pre-vaccination and post-vaccination SARS-CoV-2 (CoronaVac).
| Variable | n | Minimum | Maximum | Median | Mean | SD |
|---|---|---|---|---|---|---|
| IgG S-RBD (BAU/mL) # | ||||||
| Day-0 | 50 | 0.09 | 52.19 | 0.09 | 2.57 | 10.16 |
| Day-14 | 50 | 30.71 | 1619.42 | 109.25 | 204.31 | 252.81 |
| Day-28 | 50 | 19.38 | 1158.28 | 141.27 | 200.25 | 195.16 |
| NAb (IU/mL) $ | ||||||
| Day-0 | 50 | 7.70 | 157.14 | 18.63 | 27.18 | 29.67 |
| Day-14 | 50 | 28.35 | 1221.48 | 166.66 | 223.74 | 205.14 |
| Day-28 | 50 | 17.82 | 515.16 | 116.64 | 154.54 | 120.00 |
IgG S-RBD units in BAU/mL (international standards from WHO).
NAb units in IU/mL (international standards from WHO).
In this study, IgG and NAb antibody kinetics were assessed to determine fluctuations in antibody level. The trend of both IgG S-RBD and NAb showed upward levels on day-14 and then downward on day-28. The kinetics of NAb in describing the antibody response after vaccination was found to decrease faster than the IgG S-RBD antibody kinetics. The line graph shown in (Figure 2) for IgG S-RBD and NAb levels on day-14 and day-28. The average speed of decrease from IgG S-RBD day-14 to day-28 was 2%per 14-day evaluation this was different from NAb which decreased faster, which was 30.9% per 14-day evaluation.
Figure 2.
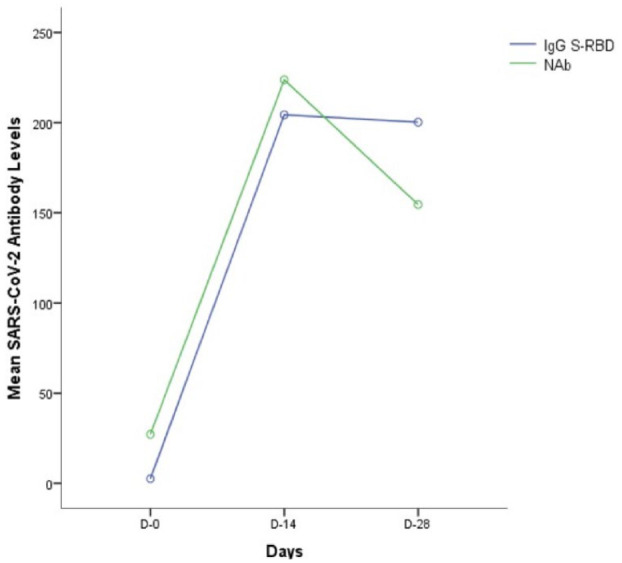
Antibody response of SARS-CoV-2 vaccine recipients. Line graph of thebkinetics of IgG S-RBD and NAb at day-0 (prevaccination), day-14 and day-28 after vaccination of the second dose of CoronaVac.
The results of the data distribution test using the Shapiro-Wilks test for IgG S-RBD and NAb levels of SARS-CoV-2 vaccine recipients were not normally distributed (p < 0.001). Thus, the Friedman and Wilcoxon tests will be used to compare levels. The complete results of the comparison test are illustrated in (Tables 3 and 4). There ware a significant difference in IgG S-RBD levels in SARS-CoV-2 (CoronaVac) vaccine recipients on day-0, day-14 and day-28 (p < 0.001). In the Wilcoxon test, there was a significant difference in IgG S-RBD levels on day-14 with day-0 and on day-28 with day-0 (p < 0.001). There was no significant difference between IgG S-RBD levels on day-28 and day-14 (p = 0.626).
Table 3.
Differences in prevaccinated and postvaccinated IgG S-RBD and NAb levels.
| Variable | n | Median | p |
|---|---|---|---|
| IgG S-RBD (BAU/mL) | |||
| Day-0 | 50 | 0.09 | <0.001 # |
| Day-14 | 50 | 109.25 | |
| Day-28 | 50 | 141.27 | |
| NAb (IU/mL) | |||
| Day-0 | 50 | 18.63 | <0.001 # |
| Day-14 | 50 | 166.66 | |
| Day-28 | 50 | 116.64 | |
Friedman test.
Table 4.
Differences in prevaccinated and postvaccinated IgG S-RBD and NAb levels for each observation.
| Results | IgG S-RBD | NAb | ||
|---|---|---|---|---|
| versus Day-14 | versus Day-28 | versus Day-14 | versus Day-28 | |
| Day-0 | ||||
| Z | −6.154 | −6.154 | −6.144 | −6.125 |
| p-value # | <0.001 | <0.001 | <0.001 | <0.001 |
| Day-14 | ||||
| Z | −0.487 | −4.803 | ||
| p-value # | 0.626 | <0.001 | ||
Wilcoxon tests.
The results of the Friedman test for the comparative test on NAb on day-0, day-14 and day-28 also obtained a value (p < 0.001) which was smaller than the 0.05 determination so that there was a significant difference in the NAb value in patients receiving vaccines on day-0, day-14, and day-28. The results were tested in the Wilcoxon test, and it was revealed that there was a significant difference in NAb levels on day-0, day-14, and day-28, with p-levels < 0.001.
This study also looks for a comparison of pre vaccination and postvaccination antibody levels between IgG S-RBD and NAb with the Wilcoxon test every day of observation (Table 5). There was a significant difference between IgG S-RBD and NAb levels on day-0 (0.090 vs 18.630; p < 0.001) and day-28 (141.266 vs 116.640; p = 0.037). On day-14, there was no significant difference between IgG S-RBD and NAb levels (109.246 vs 166.658; p = 0.282). The results of the median value showed that the final IgG S-RBD level on day-28 was much better than the NAb value (141,266 vs 116,640) although previously on day-0 and day-14 the median NAb level was much higher.
Table 5.
Comparison of prevaccinated and postvaccinated antibody levels between IgG S-RBD and Nab.
| IgG S-RBD (BAU/mL) | Median | Z | p | |
|---|---|---|---|---|
| IgG S-RBD (n = 50) | NAb (n = 50) | |||
| Prevaccinated | ||||
| Day − 0 | 0.090 | 18.630 | −6.155 | <0.001 $ |
| Postvaccinated | ||||
| Day-14 | 109.246 | 166.658 | −1.076 | 0.707 $ |
| Day-28 | 141.266 | 116.640 | −2.090 | 0.326 $ |
Wilcoxon test every day of observation.
Safety assessment
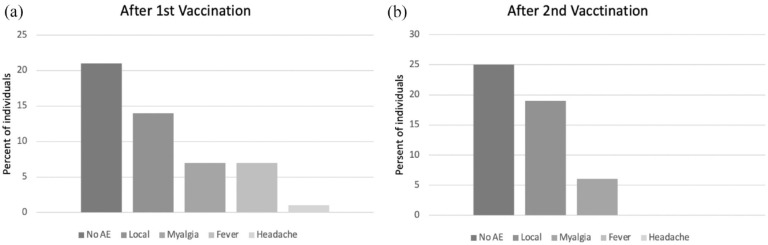
Regarding the safety profile, about half of the subjects (56%) had an adverse event after the first vaccination dose. After the second shot, the percentage of subjects with at least one adverse event was 50%. The influence of various factors such as gender, BMI, previous COVID-19, and age on the nature of adverse events was studied after both the first and second vaccination. However, no significant contribution of any factor was found in any of these cases. The type of adverse events, after the first and second vaccination, are shown in (Figure 3).
Figure 3.
Type of adverse events after vaccination. The most common adverse event was local manifestations (e.g. pain and/or swelling at the vaccination site, mild limitation of hand movement), followed by myalgia, fever, and headache: (a) adverse events after first vaccination and (b) adverse events after second vaccination.
Discussion
The SARS-CoV-2 infection is one of the most serious global health threats because of its severity and rapid spread throughout the world. As a result, vaccines have been rapidly developed to combat the spread of SARS-CoV-2 among populations. The determination of specific antibody levels serves as the foundation for determining vaccine efficacy. 8
Although SARS-CoV-2 infection can induce the production of antibodies that recognize different viral antigens, antibodies directed against RBD are the most relevant because of their neutralizing power. Therefore, most of the SARS-CoV-2 vaccines have been developed to induce the production of antibodies against SARS-CoV-2 infection.14,15
Measurement of circulating levels of IgG S-RBD and NAb can provide valuable information regarding acquired immunity to SARS-CoV-2 vaccination. 15 Maglumi 800 (Snibe) can quantitatively measure SARS-CoV-2 antibodies with two assays from different reagents, namely IgG S-RBD and also NAb. The measured NAb is not specific only to IgG, but also measures IgE and IgM levels. 16 According to research Galipeau et al. some of the antibodies produced have a neutralizing effect on IgM, IgE and IgG. These antibodies play a role in determining the immunogenicity of the SARS-CoV-2 vaccine.17,18
This study examined the kinetics of IgG S-RBD and NAb against SARS-CoV-2 from the recipient’s anti-IgG S-RBD and NAb with complete second dose CoronaVac Vaccine, to determine the antibody response in preventing SARS-CoV-2 disease. Subjects will be to phlebotomy three times, were before 1 day- before vaccination (day-0) and after second dose CoronaVac vaccination on the 14th day (day-14), on the 28th day (day-28) to monitor antibody levels.
There is a history of comorbidities in several subjects, known to have a history of diabetes mellitus 3 (6%), obesity 7 (14%), hypertension 10 (25%), hypercholesterolemia 10 (25%). This study did not find any difference between comorbid subjects and those without comorbidities. Where the presence of comorbidities may impair the vaccine’s immunogenicity, like a study by Ali et al. consisted of 81 diabetic subjects and 181 non-diabetic subjects, the post-vaccination SARS-CoV-2 NAb and IgG titers of SARS-CoV-2 with vaccine-type BNT162b2 were lower in the diabetes mellitus group than in the group without a history of diabetes mellitus (79.7% ± 19.5% vs 87.1% ± 11.6% for NAb). 19 Similar to diabetes mellitus, other metabolic syndromes such as hypertension, obesity, and hypercholesterolemia have also been shown to interfere with the immune response after COVID-19 vaccination. The study by Watanabe et al. showed that waist circumference was negatively correlated with the mean post-vaccination SARS-CoV-2 mRNA-type antibody (Pfizer-BioNTech), measured at 1–4 weeks postvaccination (R = −0.324; p = 0.004). In addition, the study also proved that the population with hypertension was also shown to have lower antibody titers than the normotensive population. 20
There was no significant difference in antibody levels in each comorbid group with the group without a comorbid history in this study may be due to the low number of subjects with comorbid diabetes mellitus, obesity, hypertension, and hypercholesterolemia.
The result of ours study on day-14, the highest levels of increase in IgG S-RBD and NAb was observed. On the day-28, statistically significant decreases in both types of antibodies were observed. Similarly, Favresse et al. recently reported a decrease in NAb levels at day-56 following administration of another type of platform vaccine, BNT162b2. 21
The results of our study showed an increase in IgG S-RBD and NAb levels, with the highest median at 14 days after receiving the second CoronaVac vaccination. The decrease in antibody levels began on day-28. The speed of reduction from day-14 to day-28 was seen faster in NAb (30.9% per 14 days average rate of reduction). This result supports previous research by Terpos et al. who discovered that maximum protection occurs 2 weeks after the second vaccination. Following that, there was a steady and gradual reduction in NAbs levels, consistent with data from other studies on the durability of antibody responses at 3 and 6 months following two-dose vaccination with a mRNA-1273 vaccine. 22 Although SARS-CoV-2 antibody binding and neutralization responses decreased over time, they remained well above the positive threshold level. 23
We believe that based on the results and explanations above, IgG S-RBD measurement is more representative of the SARS CoV-2 post-vaccination antibody response than NAb measurement. This is because the average rate of IgG S-RBD reduction is slower than that of NAb.
On the day-28, there was a significant difference in the decrease in NAb levels compared to IgG S-RBD. Where the kinetics of the decrease in NAb levels is sharper on the line graph. Researchers thought that because NAb measures IgA, IgM, and IgG, it will decrease faster than measuring only IgG S-RBD levels. This is following theory and research from18,22 et al and Post et al. showing the early response of the humoral immune system to COVID-19 infection. IgM was consistently detected before IgG in the included studies, peaking at weeks two to five and decreasing for 3–5 weeks after the onset of symptoms depending on the patient group. IgG peaks around week 3–7 weeks of post-symptom onset then levels off, generally persisting for at least 8 weeks. IgA with time will decrease at a lower level than IgG.18,22
NAb is not specific to describe the neutralizing effect on SARS-CoV-2, because NAb not only measures specific IgG but also measures IgE and IgM levels. The increase is influenced by the response of IgM and IgE, where IgE will also increase in allergic conditions. Many kinds of literature say that the highest neutralizing effect is only on IgG S-RBD.10,11
According to Favresse et al. rate of reduction remains constant in BNT162b2 vaccine, 5–6 months after the second vaccination, the IgG S-RBD and NAb titer will reach a critical level at which time another vaccination or additional booster may be required. 21
This study has the advantage of having no dropouts during the study; all subjects participated in the study from before vaccination until 28 days or 1 month after receiving the full dose of vaccination.
This study only includes subjects receiving the CoronaVac vaccine from Surabaya–Indonesia, one of the most interesting phenomena that has emerged for the global COVID-19 pandemic is the inter-country variability in COVID-19 morbidity and mortality. Observations of each subject were only included for 1 month or 28 days after receiving the second dose of vaccine. Ideally, additional observation should continue until the sixth month to gain a better understanding of the vaccine’s kinetics and immunogenicity.
Conclusion
The antibody response after a full dose of SARS-CoV-2 vaccination will evoke a humoral immune response of both IgG S-RBD and NAb. We report a persistent antibody but declining anti-SARS-CoV-2 humoral immunity following vaccination with CoronaVac. NAb describes the total antibody which decreased more rapidly at 28 days after full CoronaVac, compared to the more stable IgG S-RBD where this antibody was more specific in describing the neutralizing effect of SARS-CoV-2. This decrease may be a consideration for other vaccinations or additional boosters.
Acknowledgments
We would like to thank all staffs at the Department of Clinical Pathology, Faculty of Medicine, Airlangga University, Dr. Soetomo General Academic Hospital, and Dr. Soetomo Surabaya’s ethics committees.
Footnotes
Author Contributions: Jusak Nugraha, Cynthia Ayu Permatasari, Munawaroh Fitriah, Betty Agustina Tambunan, M. Robi’ul Fuadi conceived the study and approved the final draft. Cynthia Ayu Permatasari contributed to collecting samples. Jusak Nugraha, Cynthia Ayu Permatasari, and Munawaroh Fitriah drafted the manuscript and critically revised the manuscript for important intellectual content. Jusak Nugraha, Cynthia Ayu Permatasari, Munawaroh Fitriah, Betty Agustina Tambunan, M. Robi’ul Fuadi facilitated all project-related tasks. All authors agree with the content of the manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Universitas Airlangga through the Grant Faculty’s Flagship Research 2021 and the Annual Budget Activity Plan 2021.
Ethical approval: The study has been reviewed by the Health Research Ethics Committee of Dr. Soetomo General Academic Hospital with the number 0141/KEPK/II/2021.
Significance for public health: COVID-19 pandemic causes severe acute respiratory syndrome and requires rapid action. The development of effective safe vaccines become a global priority for achieving herd immunity. This research will be useful as a reference in knowing the antibodies produced from the second dose vaccination, especially in countries where the population gets the most CoronaVac vaccine. Through understanding the characteristics of these antibodies, and the estimation of these antibodies starting to decrease, it is hoped that stakeholders, governments, and policymakers can take the best approach to consider giving booster vaccinations with the same vaccine or with other types of vaccines.
Availability of data and materials: All data generated or analyzed during this study are included in this published article.
ORCID iDs: Jusak Nugraha  https://orcid.org/0000-0001-6700-9921
https://orcid.org/0000-0001-6700-9921
Cynthia Ayu Permatasari  https://orcid.org/0000-0002-9375-8982
https://orcid.org/0000-0002-9375-8982
Munawaroh Fitriah  https://orcid.org/0000-0002-7617-9874
https://orcid.org/0000-0002-7617-9874
Betty Agustina Tambunan  https://orcid.org/0000-0002-3214-993X
https://orcid.org/0000-0002-3214-993X
Muhamad Robi’ul Fuadi  https://orcid.org/0000-0002-8416-6862
https://orcid.org/0000-0002-8416-6862
References
- 1. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun 2020; 109: 102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kemenkes RI. KMK No. HK.01.07-MENKES-413-2020 tentang Pedoman Pencegahan dan Pengendalian COVID-19. 2020. [Google Scholar]
- 3. WHO. COVID 19 Public Health Emergency of International Concern (PHEIC). 2020. [Google Scholar]
- 4. KPCPEN. Data Vaksinasi Covid-19 Update [Internet]. https://covid19.go.id/p/berita/data-vaksinasi-covid-19-update-21-maret-2021) (2021, accessed 22 March 2022)
- 5. Jeyanathan M, Afkhami S, Smaill F, et al. Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol 2020; 20(10): 615–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kolifarhood G, Aghaali M, Saadati HM, et al. 19; a narrative review. Clin Asp COVID 2020; 8(1): 41. [PMC free article] [PubMed] [Google Scholar]
- 7. Nile SH, Nile A, Qiu J, et al. COVID-19: Pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev 2020; 53: 66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xia S, Duan K, Zhang Y, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA 2020; 324(10): 951–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Amanat F, Stadlbauer D, Strohmeier S, et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med 2020; 26(7): 1033–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Natarajan H, Crowley AR, Butler SE, et al. SARS-CoV-2 antibody signatures robustly predict diverse antiviral functions relevant for convalescent plasma therapy. medRxiv Prepr Serv Heal Sci 2020; 9922: 1–27. [Google Scholar]
- 11. Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis 2021; 21: 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lo Sasso B, Giglio RV, Vidali M, et al. Evaluation of anti-sars-cov-2 s-rbd igg antibodies after covid-19 mrna bnt162b2 vaccine. Diagnostics 2021; 11(7): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Padoan A, Cosma C, Sciacovelli L, et al. Analytical performances of a chemiluminescence immunoassay for SARS-CoV-2 IgM/IgG and antibody kinetics. Clin Chem Lab Med 2020; 58(7): 1081–1088. [DOI] [PubMed] [Google Scholar]
- 14. Azkur AK, Akdis M, Azkur D, et al. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy 2020; 75(7): 1564–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu Z, Xu W, Xia S, et al. RBD-Fc-based COVID-19 vaccine candidate induces highly potent SARS-CoV-2 neutralizing antibody response. Signal Transduct Target Ther 2020; 5(1): 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Snibe Diagnostic. MAGLUMI®️_SARS_CoV_2_Neutralizing_Antibody_CLIA_. 2021. [Google Scholar]
- 17. Galipeau Y, Greig M, Liu G, et al. Humoral responses and serological assays in SARS-CoV-2 infections. Front Immunol 2020; 11: 610688–610719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Post N, Eddy D, Huntley C, et al. Antibody response to SARS-CoV-2 infection in humans: a systematic review. PLoS One 2020; 15(12): e0244126–e0244127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ali H, Alterki A, Sindhu S, et al. Robust antibody levels in both diabetic and Non-Diabetic individuals after BNT162b2 mRNA COVID-19 vaccination. Front Immunol 2021; 12: 752233–752239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Watanabe M, Caruso D, Tuccinardi D, et al. Visceral fat shows the strongest association with the need of intensive care in patients with COVID-19. Metabolism 2020; 111: 154319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Favresse J, Gillot C, Di Chiaro L, et al. Neutralizing antibodies in covid-19 patients and vaccine recipients after two doses of bnt162b2. Viruses 2021; 13(7): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Terpos E, Trougakos IP, Karalis V, et al. Kinetics of anti-SARS-CoV-2 antibody responses 3 months post complete vaccination with bnt162b2; a prospective study in 283 health workers. Cells 2021; 10(8): 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gillot C, Douxfils J, Cadrobbi J, et al. An original elisa-based multiplex method for the simultaneous detection of 5 sars-cov-2 igg antibodies directed against different antigens. J Clin Med 2020; 9(11): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]