Abstract
Background
In areas highly endemic for malaria, Plasmodium falciparum infection prevalence peaks in school-age children, adversely affecting health and education. School-based intermittent preventive treatment reduces this burden but concerns about cost and widespread use of antimalarial drugs limit enthusiasm for this approach. School-based screening and treatment is an attractive alternative. In a prospective cohort study, we evaluated the impact of school-based screening and treatment on the prevalence of P. falciparum infection and anemia in 2 transmission settings.
Methods
We screened 704 students in 4 Malawian primary schools for P. falciparum infection using rapid diagnostic tests (RDTs), and treated students who tested positive with artemether-lumefantrine. We determined P. falciparum infection by microscopy and quantitative polymerase chain reaction (qPCR), and hemoglobin concentrations over 6 weeks in all students.
Results
Prevalence of infection by RDT screening was 37% (9%–64% among schools). An additional 9% of students had infections detected by qPCR. Following the intervention, significant reductions in infections were detected by microscopy (adjusted relative reduction [aRR], 48.8%; P < .0001) and qPCR (aRR, 24.5%; P < .0001), and in anemia prevalence (aRR, 30.8%; P = .003). Intervention impact was reduced by infections not detected by RDT and new infections following treatment.
Conclusions
School-based screening and treatment reduced P. falciparum infection and anemia. This approach could be enhanced by repeating screening, using more-sensitive screening tests, and providing longer-acting drugs.
Clinical Trials Registration
Keywords: chemoprevention, anemia, schoolchildren, adolescent, intervention
In this cohortstudy of primary school students in 2 malaria transmission settings, school-based screening and treatment was associated with decreased prevalence of Plasmodium falciparuminfection and anemia. Impact was reduced by infections not detected and new infections following treatment.
Despite global declines in malaria morbidity and mortality over the last 2 decades, this disease remains a significant public health burden in sub-Saharan Africa [1]. Malaria surveillance and research has largely focused on children younger than 5 years and pregnant women, the groups at highest risk of adverse outcomes. Expansion of research to other age groups has revealed an underappreciated burden of Plasmodium falciparum infection in school-age children [2]. Although severe disease occurs less frequently in school-age children compared to younger children, P. falciparum infection in school-age children is associated with anemia [3–9], increased school absenteeism [10–12], and lower performance on cognitive testing [3, 12–15].
School-based preventive treatment of malaria is a promising strategy to reduce P. falciparum infection, clinical malaria, and anemia in school-age children [16]. Preventive treatment includes both intermittent preventive treatment (IPT), in which all students are periodically treated regardless of infection status, and screen-and-treat, in which students are screened for infection with a point-of-care test and immediately treated if positive. To our knowledge, the only clinical trial evaluating the malaria screen-and-treat approach in schoolchildren demonstrated no decrease in prevalence of P. falciparum infection or of anemia [17]. One explanation for the lack of efficacy in that large, cluster-randomized trial is the use of a relatively short-acting treatment, artemether-lumefantrine (AL) (duration of prevention 13.8 days [18]). Also, in that study, students were screened once per school term, allowing substantial time for reinfection. Furthermore, malaria rapid diagnostic tests (RDTs) used for screening are not sensitive in detecting low-density P. falciparum infections, which are common in school-age children [19]. Nevertheless, a screen-and-treat approach has multiple advantages over IPT, including decreasing the cost and adverse effects of medication and reducing the selective pressure for drug resistance by limiting treatment to test-positive children. Given these advantages, more evidence that evaluates screen-and-treat should help characterize the value of this approach in improving health.
We conducted a school-based, prospective, cohort study of P. falciparum infections in school-age children and determined the impact of the screen-and-treat approach on P. falciparum infection and anemia prevalence in 2 different transmission settings in southern Malawi. We hypothesized that RDTs commonly fail to detect low-parasite–density infections, and that these infections contribute to the disease burden of P. falciparum infection in school-age children. We further hypothesized that school-age children are frequently reinfected following antimalarial treatment, necessitating repeated screening and treatment. We also explored the effect of transmission setting on intervention efficacy, because this may determine the proportion of infections that are subpatent and the frequency of reinfection following treatment. Our ultimate goal is to further develop malaria control interventions that will improve the health of school-age children.
METHODS
Study Sites
School-based, prospective, cohort studies of students were conducted in 4 schools selected from malaria surveillance sites in southern Malawi [20]. Previous studies showed that 2 schools (in Maseya and Makhuwira) had high P. falciparum prevalence in school-age children (>40% in the rainy and dry seasons of 2012–2014 and less than a 2-fold prevalence difference between seasons). The other 2 schools (in Bvumbwe and Ngowe) had lower, seasonal transmission (<15% prevalence in the rainy and dry seasons of 2012–2014 and at least a 2-fold difference between seasons). All cohorts were followed during 2 periods in 2015: at the end of the rainy season (April–May) and during the dry season (September–October), when P. falciparum transmission typically is high and low, respectively. Routine malaria control measures in place during our study included use of long-lasting insecticide treated nets (LLINs) and treatment (with AL) in government-operated health facilities. LLINs had been distributed nationally in 2012. AL treatment was freely available upon RDT-based malaria diagnosis at local health facilities.
Study Design
Prior to initiating the study, sensitization meetings were held with community leaders, school leaders, and district and local government officials, who provided permission to conduct the study. Using a random number generator, 15 students in each grade level (standard 1–8), were invited to participate. Random selection was done independently for the rainy and dry season cohorts. Sample size calculations are provided in the Supplementary Material. Students were excluded if a parent/guardian was unavailable to provide consent, or they were younger than 5 years old, older than 15 years, would not be attending the school for the duration of the study, had a known allergy to AL, or did not provide assent. To evaluate the intervention separately in each season, students enrolled in the rainy season cohort were excluded from participating in the dry season cohort.
School-based study visits were conducted at baseline (1–2 weeks after enrollment), and 1, 2, and 6 weeks later. At the baseline visit, a finger prick blood sample was obtained for point-of-care RDT detection of P. falciparum infection using a histidine-rich protein 2-based (PfHRP2) RDT in accordance with manufacturer instructions (Paracheck Orchid Biomedical Systems or SD Bioline, Standard Diagnostics, Inc). In addition, microscopy and molecular detection tests for P. falciparum were performed, as was measurement of hemoglobin (Hemocue). Students with infection detected by RDT were treated with AL (Novartis Pharma, AG or Ajanta Pharma, Ltd) using weight-based dosing (Supplementary Text 2). At each follow-up visit, a finger prick blood sample was obtained. A smear and dried blood spot on filter paper was prepared for later microscopy and molecular detection of parasites. At all visits a study team member conducted a one-on-one structured questionnaire interview with each student about their bed net use the night prior, current or recent illness, and use of antimalarial treatment. At the final visit, RDT and hemoglobin tests were repeated, treatment was administered to RDT-positive students, and intercurrent fever or malaria treatment was identified by parent interview and review of portable medical records (health passports). Data were collected on android-based tablets using OpenDataKit and REDCap [21]. The study is registered at Clinicaltrials.gov (NCT04858087).
Laboratory Methods
Thick-smear microscopy slides were stained with Giemsa stain and read by trained, blinded microscopists, limit of detection approximately 80 parasites/μL. Quality control was conducted using published procedures [22]. Molecular detection of parasites was conducted using real-time polymerase chain reaction (PCR) to detect P. falciparum lactate dehydrogenase (PfLDH) DNA, as described previously, with a limit of detection of approximately 6 parasites/μL [23]. Plates were run in duplicate and the sample was considered positive if a positive result was detected in at least 1 run.
Statistical Analysis
P. falciparum infections detected by microscopy and PCR were treated as binary (present/absent) and were analyzed separately. Subpatent infections were defined as those that were PCR positive but RDT negative. Treatment groups were defined based on RDT result at baseline. RDT-positive students with negative PCR were considered negative for active infection, but were included in the treatment group for analysis because they received AL treatment. Anemia was defined as hemoglobin less than 11.0 g/dL. Nonstudy malaria treatment administered at baseline or during follow-up was defined as any treatment with an effective antimalarial drug that was reported by the student, elicited during the parent interview, or identified in the student’s health passport.
Baseline characteristics were compared between study sites and by RDT status. Associations between baseline characteristics and infection detected by RDT at baseline were assessed using log-binomial models for unadjusted prevalence ratios and 95% confidence intervals (CI).
We assessed changes in prevalence of PCR-detected P. falciparum infection, microscopy-detected P. falciparum infection, and anemia using log-binomial generalized estimating equation (GEE) models, with compound symmetry covariance and clustering within students nested in schools. GEE models were used to estimate prevalence at baseline and at the 6-week follow-up visit, for all schools, and stratified by transmission setting. Linear GEE models were used to assess change in hemoglobin over follow-up. All models were adjusted a priori for factors known to be associated with malaria risk: age 5 to 10 years versus 11 to 15 years and season. Models for anemia and hemoglobin concentration were adjusted for baseline PCR results. Relative difference in prevalence was calculated as the difference between prevalence at baseline and after 6 weeks divided by baseline prevalence. Analyses were conducted using SAS statistical software version 9.3 (SAS Institute).
Human Subjects
Written informed consent and assent (from students aged 13 years and older) were obtained in person from guardians and children in accordance with Good Clinical Practice guidelines. Approval for this study was obtained from the University of Malawi College of Medicine Research and Ethics Committee and the institutional review board of the University of Maryland, Baltimore.
RESULTS
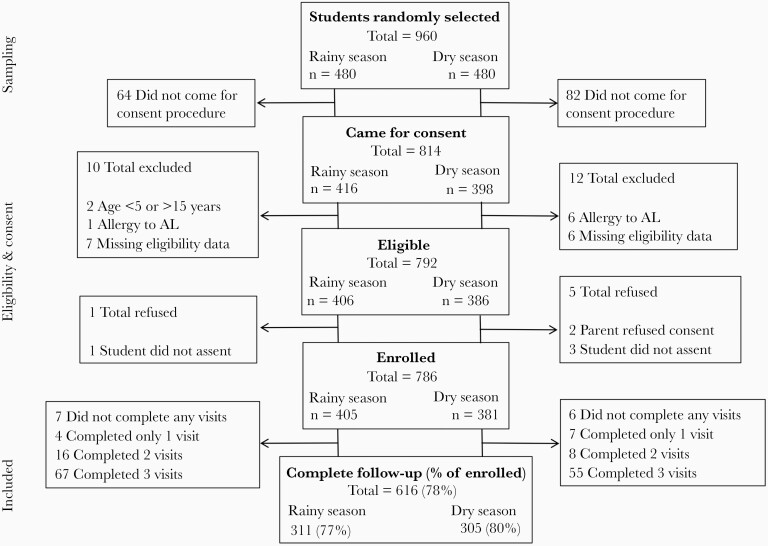
A total of 786 students were enrolled: 405 in the rainy season and 381 in the dry season (Figure 1). Screening and treatment was conducted at baseline for 704 (89.6%) students, and complete follow-up was obtained for 616 (78.4%) students. The remaining 82 students who were not present at baseline were excluded from all analyses. Baseline characteristics, including sex, bed net use, anemia, recent fever, and prevalence of P. falciparum infection, varied significantly by school (Table 1). In total, 259 students (37%) were RDT positive at baseline and were treated with AL. Having an RDT-detected infection, and thus receiving treatment, was more common among students who attended a higher-prevalence school, who were part of the rainy season cohort, were male, were anemic, did not sleep under a bed net the previous night, or had recently taken an antimalarial medication (Table 2).
Figure 1.
Enrollment and follow-up of participants in school-based cohorts. Abbreviation: AL, artemether-lumefantrine.
Table 1.
Characteristics at the Time of Study Enrollment
| Characteristic |
Total n = 704 |
Low Transmission | High Transmission | P a | ||
|---|---|---|---|---|---|---|
| Bvumbwe n = 184 |
Ngowe n = 186 |
Maseya n = 185 |
Makhuwira n = 149 |
|||
| Female, n (%) | 382 (54.3) | 118 (64.1) | 94 (50.5) | 92 (49.7) | 78 (52.4) | .02 |
| Older school age, >10 y, n (%) | 435 (61.8) | 105 (57.2) | 109 (58.6) | 119 (64.3) | 102 (68.5) | .12 |
| Slept under bed net last night,b n (%), missing = 23 |
309 (49.3) | 62 (53.9) | 124 (68.5) | 65 (35.3) | 58 (39.5) | <.0001 |
| Rainy season, n (%) | 364 (51.7) | 99 (53.8) | 100 (53.8) | 97 (52.4) | 68 (45.6) | .41 |
| Anemic,c n (%), missing = 1 |
106 (15.1) | 9 (4.9) | 35 (18.8) | 34 (18.4) | 28 (18.8) | .0002 |
| Hemoglobin, g/dL, mean (SD), missing = 1 |
12.3 (1.5) | 13.1 (1.3) | 12.0 (1.5) | 12.0 (1.3) | 12.0 (1.3) | <.0001 |
| Fever last 48 h,d n (%), missing = 13 |
192 (27.8) | 28 (15.5) | 53 (29.6) | 64 (34.8) | 47 (32.0) | .0002 |
| Recent malaria treatment,e n (%), missing = 9 |
121 (17.4) | 25 (13.7) | 33 (18.2) | 35 (19.0) | 28 (19.1) | .48 |
| P. falciparum infection | ||||||
| RDT positive, n (%) | 259 (36.8) | 16 (8.7) | 56 (30.1) | 91 (49.2) | 96 (64.4) | <.0001 |
| Subpatent infection,f n (%) | 63 (9.0) | 12 (6.5) | 10 (5.4) | 22 (11.9) | 19 (12.8) | .03 |
| Proportion of infections subpatent | 0.20 | 0.43 | 0.15 | 0.19 | 0.16 | .01 |
Abbreviations: PCR, polymerase chain reaction; RDT, rapid diagnostic test.
P value of differences across school, χ2 test used for categorical variables and ANOVA used for hemoglobin.
Net use data for Bvumbwe was only available in the dry season.
Defined as hemoglobin < 11.0 g/dL.
Fever in the last 48 hours reported or measured temperature ≥37.5ºC.
Recent treatment defined as student or parent reported receipt of an effective antimalarial drug in the 2 weeks preceding the interview.
At baseline, 63 children had a negative RDT result but had a positive result for malaria PCR (n = 61) or microscopy (n = 2).
Table 2.
Associations With Plasmodium falciparum Infections Detected by RDT Among Enrolled Students at Baselinea
| Predictor | n (%)b | Prevalence Ratio (95% CI) |
|---|---|---|
| School | ||
| Bvumbwe | 16 (8.7) | Ref. |
| Ngowe | 56 (30.1) | 3.5 (2.1–5.8) |
| Maseya | 91 (49.2) | 5.7 (3.5–9.2) |
| Makhuwira | 96 (64.4) | 7.4 (4.6–12.0) |
| Season | ||
| Rainy | 159 (43.7) | Ref. |
| Dry | 100 (29.4) | 0.7 (0.6–0.8) |
| Age, y | ||
| 5 to 10 | 89 (33.1) | Ref. |
| 11 to 15 | 170 (39.1) | 1.2 (1.0–1.5) |
| Sex | ||
| Male | 141 (43.8) | Ref. |
| Female | 118 (30.9) | 0.7 (0.6–0.9) |
| Anemicc | ||
| No | 193 (32.3) | Ref. |
| Yes | 65 (61.3) | 1.9 (1.6–2.3) |
| Fever last 48 hd | ||
| No | 170 (34.1) | Ref. |
| Yes | 84 (43.8) | 1.3 (1.0–1.6) |
| Recent medicatione | ||
| No | 193 (33.6) | Ref. |
| Yes | 62 (51.2) | 1.5 (1.2–1.9) |
| Slept under bed net last night | ||
| No | 144 (45.3) | Ref. |
| Yes | 109 (35.3) | 0.8 (0.6–0.9) |
Abbreviations: RDT, rapid diagnostic test; Ref., reference.
Log-binomial models for bivariate association between covariates and RDT positivity at baseline.
Number and percent of children with a positive RDT result within each strata; children with a positive RDT result at baseline were treated and children with a negative RDT result at baseline were not treated.
Defined as hemoglobin less than 11.0 g/dL.
Fever in the last 48 hours reported or measured temperature ≥37.5ºC.
Recent treatment defined as student or parent reported receipt of an effective antimalarial drug in the 2 weeks preceding the interview.
Infections not detected by RDT were common: 63 students (9% of all students at baseline) had infection detected by PCR but not RDT (24% of all infections detected by PCR or microscopy). While these subpatent infections were more likely to occur in the high-transmission schools (Supplementary Table 1), they made up a larger proportion of all infections in Bvumbwe, the school with the lowest prevalence of infection (42.8% vs 17.3% [P = .003] of infections were not detected by RDT in Bvumbwe vs the other 3 schools combined; Table 1). Compared to microscopy and PCR, respectively, RDTs had a sensitivity of 91.1% and 76.3%, and a specificity of 76.1% and 85.9%.
Impact of Screening and Treatment on Prevalence of Infection After 6 Weeks
We evaluated the impact of screening and treatment on P. falciparum infections detected by microscopy, to facilitate comparisons with prior studies and assess impact on infections more likely to be clinically relevant, and also by PCR, to assess impact that included lower-density infections. Six weeks after screening and treatment, infection prevalence among all enrolled students was 48.8% and 24.5% lower by microscopy and PCR, respectively (both P < .001; Table 3). In analyses stratified by transmission setting, the prevalence of infection detected by microscopy decreased by 41.9% in high-transmission schools (P < .001) and by 66.5% in low-, seasonal-transmission schools (P = .003). For infections detected by PCR, we observed a 35.2% decrease in prevalence in the high-transmission schools (P < .001), but a nonstatistically significant 12.2% increase in prevalence in the low-, seasonal-transmission schools (P = .40). Results by school are shown in Supplementary Figure 1.
Table 3.
Adjusted Prevalence and Mean of Infection Outcomes at Baseline and 6 Weeks After the Interventiona
| Outcome | Baseline (95% CI) |
6 Wk (95% CI) |
Adjusted Relative Difference, %b | P Value Comparing 6 Wk to Baseline |
|---|---|---|---|---|
| Prevalence | ||||
| Microscopy positive, n (%) | 135 (19.2) | 59 (8.6) | <.0001 | |
| Adjusted prevalence, all schools | 19.5 (12.1–29.9) | 10.0 (7.6–13.0) | −48.8 | <.0001 |
| High transmissionc | 27.8 (17.5–41.2) | 16.1 (12.3–20.9) | −41.9 | <.0001 |
| Lower, seasonal transmissiond | 11.3 (2.8–36.5) | 3.8 (1.9–7.5) | −66.5 | .008 |
| PCR positive, n (%) | 257 (36.5) | 184 (26.9) | <.0001 | |
| Adjusted prevalence, all schools | 33.9 (30.4–37.7) | 25.6 (22.5–29.0) | −24.5 | <.0001 |
| High transmission | 55.7 (50.4–61.7) | 36.1 (31.3–41.7) | −35.2 | <.001 |
| Lower, seasonal transmission | 14.0 (10.9–18) | 15.7 (12.4–19.9) | +12.2 | .40 |
| Anemic, n (%)e | 106 (15.1) | 72 (10.5) | .01 | |
| Adjusted prevalence, all schools | 12.5 (10–15.7) | 8.7 (6.6–11.4) | −30.8 | .003 |
| High transmission | 16.1 (11.4–22.8) | 12.1 (9–16.3) | −36.6 | .007 |
| Lower, seasonal transmission | 10.3 (7.6–13.9) | 8.0 (5.5–11.5) | −22.4 | .15 |
| Mean | ||||
| Hemoglobin, g/dL, mean (SD) | 12.28 (1.46) | 12.56 (1.36) | .0002 | |
| Adjusted mean,f all schools | 12.35 (12.2–12.5) | 12.64 (12.5–12.8) | 2.4 | <.0001 |
| High transmission | 11.9 (11.9–12.1) | 12.2 (12.2–12.4) | 3.1 | <.0001 |
| Lower, seasonal transmission | 12.6 (12.6–12.7) | 12.8 (12.8–12.9) | 1.8 | .003 |
Abbreviations: CI, confidence interval; GEE, generalized estimating equation; PCR, polymerase chain reaction.
Log binomial GEE models for adjusted prevalence adjusted a priori for sex, age, season, and visit, including nested clustering by individual and school.
Relative difference in prevalence is the difference between prevalence at baseline and after 6 weeks divided by baseline prevalence.
High transmission schools were in Maseya and Makhuwira.
Lower, seasonal transmission schools were in Bvumbwe and Ngowe.
Defined as hemoglobin less than 11.0 g/dL. Anemia models adjusted for PCR results for Plasmodium falciparum at baseline.
Adjusted mean hemoglobin and 95% CIs estimated using linear GEE models.
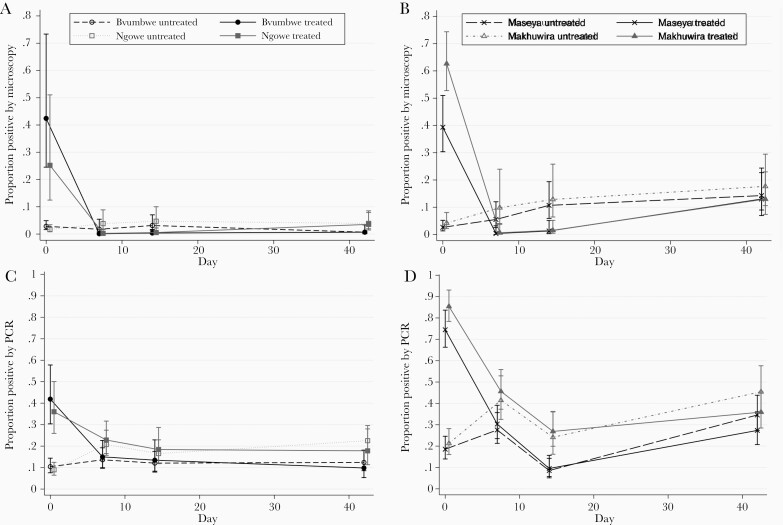
To further evaluate patterns of infection and the effect of screening and treatment in different transmission settings, we examined school-specific differences in the prevalence of infection during follow-up among treated and untreated students (Figure 2). In the higher-transmission schools, RDTs detected most infections, and treatment effectively cleared those infections at the 1- and 2-week follow-up visits. However, prevalence of infection among both treated and untreated students increased between 2 and 6 weeks after the intervention (Figure 2B and 2D).
Figure 2.
Proportion of Plasmodium falciparum infections stratified by school, treatment status, and detection method. Low, seasonal-transmission schools (Bvumbwe and Ngowe) infections detected by microscopy (A) and polymerase chain reaction (PCR) (C). High-transmission schools (Maseya and Makhuwira) infections detected by microscopy (B) and PCR (D). Treated students, solid lines; untreated students, dashed lines. Error bars are 95% Confidence Intervals.
In the schools with lower, seasonal transmission, 38% (22/58) of infections at baseline were not detected by RDTs and therefore not treated. Treatment cleared infections that were detected by microscopy in the students who received treatment (Figure 2A). However, there was no overall reduction in infections detected by PCR due to both persistence of infections detected by PCR in untreated students, and either uncleared or new infections detected by PCR in the treated students (Figure 2C).
Impact of Screening and Treatment on Anemia
At baseline, the mean hemoglobin among all students was 12.3 g/dL; 15.1% of students were anemic (Hb < 11 g/dL; Table 1). Anemia at baseline was less common among students in Bvumbwe compared to other schools, and less common among girls. However, baseline anemia was more common among students with a positive RDT and students with recent fever (Supplementary Table 2). Six weeks after screening and treatment, the mean hemoglobin concentration had increased by 0.29 g/dL (P < .001), corresponding to a 30.8% reduction in anemia prevalence (P = .003; Table 3). In analyses stratified by transmission setting, the increase in hemoglobin was significant in both transmission settings while the decrease in the prevalence of anemia only reached statistical significance in the high-transmission schools where anemia was more prevalent.
Clinical and Parasitological Outcomes of Subpatent Infections
To evaluate the consequences of subpatent infections, we compared clinical and parasitological outcomes among students with subpatent infection to uninfected students, defined as students with negative RDT, microscopy, and PCR results at screening. Subpatent infections at screening were not associated with adverse clinical outcomes at the 6-week follow-up, including fever (adjusted odds ratio [aOR], 1.05; 95% CI, .64–1.72), antimalarial treatment (aOR, 0.97; 95% CI, .40–2.32), anemia (aOR, 1.05; 95% CI, .38–2.85), or mean difference in hemoglobin concentration (aOR, 0.04; 95% CI, −.31 to .39) (Supplementary Table 3). However, baseline subpatent infections persisted, with these infections increasing in density leading to detection by microscopy (aOR, 9.54; 95% CI, 5.42–16.8) and PCR (aOR, 8.03; 95% CI, 5.50–11.7) during follow-up. Thirty-four percent (20/59) of students with subpatent infections at baseline had positive RDTs at the 6-week follow-up, suggesting that repeated screening and treatment may further reduce prevalence of infection.
DISCUSSION
School-based screening and treatment is an attractive approach to decreasing the burden of P. falciparum infection and malaria disease in school-age children. Our results suggest this intervention may decrease infection and anemia prevalence, nearly halving those infections detected by microscopy. However, the success of RDTs in detecting infections differed by transmission intensity, contributing to variability in the impact of the intervention. In lower-, seasonal-transmission settings, a large proportion of infections went untreated because they were subpatent. In higher-transmission settings, however, the duration of screening and treatment benefit was limited by new infections within the 6-week period.
Overall, our results provide insights into the design of screening and treatment interventions to deliver school-based preventive treatment. The only other published clinical trial evaluating school-based screening and treatment showed no long-term impact on P. falciparum infection, anemia, or cognition [17]. However, that cluster-randomized trial occurred in a setting that was similar to the lower-, seasonal-prevalence schools in our study, where RDTs failed to detect a large proportion of infections. While subpatent infections were not associated with clinical disease in our study, they likely contributed to the lack of long-term impact in the clinical trial, which had longer follow-up. The lack of efficacy of malaria screening and treatment for other target populations, including pregnant women [24], and in community-wide interventions [25, 26] has also been attributed to the low sensitivity of currently available RDTs. The sensitivity and specificity of RDTs in our study were similar to the prior school-based screen-and-treat trial and other community-based studies [17, 27–29]. The RDTs used in this study, and for routine clinical diagnosis in Malawi, detect PfHRP2. While there is increasing concern about the spread of parasites with HRP2/3 gene deletions, limited data from Malawi and neighboring Mozambique do not suggest widespread deletions in the region [30, 31]. However, the prevalence of HRP2/3 deletions should be considered in future studies.
Our study identified different patterns of infection between treated and untreated students in both transmission settings, suggesting important implications for the design of school-based malaria control interventions. In settings with moderate-to-high transmission, the screen-and-treat approach using currently available screening tools may be effective in identifying and treating most infections, but the duration of the impact may be short lived due to frequent new infection. Thus, in these settings the intervention could be improved by either repeating screening and treatment, or by using a longer-acting drug, such as dihydroartemisinin-piperaquine.
In lower-transmission settings where a high proportion of infections fall below the level of detection by commonly used point-of-care tests, a screening test with higher sensitivity than standard RDTs should improve the effectiveness of the screen-and-treat approach. Newly approved point-of-care tests that are undergoing evaluation may be suitable for use in school-based interventions, particularly in low-transmission settings [32]. Alternatively, frequent repeat testing may detect subpatent infections that persist and increase in density to reach patency.
The alternative to screening and treatment is providing treatment to all students without screening, as in IPT, an approach used to prevent malaria during pregnancy and among children living in areas of highly seasonal malaria transmission. In recent meta-analyses of preventive treatment in schoolchildren, the majority of studies provided treatment to all students enrolled in the intervention arm, and showed substantial reductions of both P. falciparum infection prevalence and clinical malaria [16]. This approach has the advantages of treating subpatent infections and providing prophylaxis to all students regardless of their infection status. Furthermore, IPT is easier to implement as no blood collection or time for testing is required. However, there are concerns about the cost-benefit of this approach if prevalence is low, and if selective pressure on drug-resistant parasites results from such widespread administration of antimalarial drugs.
While our findings provide new insights into the utility of school-based screen-and-treat approaches, there are some limitations. We designed our observational study to investigate the dynamics of infections in treated and untreated children after screening. Randomized trials provide more definitive conclusions about the efficacy of the intervention; however, our approach enabled an intensive and standardized assessment of the effect of the intervention on parasite prevalence in a range of transmission settings. Although we were unable to control for secular trends and complex confounders (eg, nutritional status, age and integrity of bed nets, household vector density) in this analysis, our results still provide useful insights into the dynamics of infections among students in different transmission settings. Lastly, the study involved only 6 weeks of follow-up, limiting our ability to comment on the longer-term impacts of subpatent infections and potential increases in hemoglobin.
The value of a screen-and-treat approach using currently available RDTs must be assessed based on the intervention goal(s), for example, reducing clinical disease, improving educational outcomes, and/or reducing overall infection prevalence to decrease transmission. Screening and treatment reduced the prevalence of infections detected by microscopy, and subpatent infections were not associated with clinical disease over the course of follow-up in our study population, suggesting that this approach effectively reduced clinical malaria. The intervention also reduced anemia and, therefore, may improve cognitive function and educational achievement. However, the impact on infections detected by PCR was more modest, suggesting the reduction in overall P. falciparum prevalence was more limited. The impact of preventive treatment against malaria on these end points may be increased by targeting all students with antimalarial medication through IPT and/or combining malaria preventive treatment with other interventions, such as deworming and nutrition programs. There are interrelated, multifactorial etiologies of anemia, decreased cognitive function, and lower educational achievement among school children in the malaria-endemic world. Combining malaria treatment with existing school feeding and deworming programs could synergistically enhance the effect of each intervention.
While the primary aim of school-based malaria control is to decrease the adverse consequences of P. falciparum infection in students, there may also be indirect effects on transmission in the community [33, 34]. Increasing evidence suggests that infected school-age children with persistent asymptomatic infections contribute significantly to malaria dynamics [35–37]. Thus, school-based programs to treat and prevent malaria infections may have indirect effects on malaria parasite transmission in the community, and future studies should include this outcome.
Given the high prevalence of P. falciparum infection and the negative impacts of infection on the health and educational achievement of school-age children, improved strategies to decrease this burden is critical. School-based screening and treatment effectively reduced P. falciparum infection and anemia in our study, suggesting this as an attractive strategy, because treatment is targeted to students with documented infection. Further randomized studies are needed to evaluate this approach in different transmission settings, to assess the impact of using more sensitive screening tests, and/or longer-acting treatment drugs on outcomes, as well as to compare screen-and-treat to IPT.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Heidi Fancher and the administrative team at the Malaria Alert Centre for their support in executing the study. We are grateful for the hard work and dedication of the field team, study nurses, teachers, and school administrators who made the study possible. Most importantly, we thank the participants for their commitment and patience.
Financial support. This work was supported by the National Institutes of Health (grant numbers U19AI089683 and K24AI114996 to M. K. L., and K23AI135076 to L. M. C.); the Thrasher Research Fund Early Career Award (to L. M. C.); and the Burroughs Wellcome Fund/American Society of Tropical Medicine and Hygiene Postdoctoral Fellowship in Tropical Infectious Diseases (to L. M. C.).
Contributor Information
Lauren M Cohee, Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore, Maryland, USA.
Ingrid Peterson, Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore, Maryland, USA.
Andrea G Buchwald, Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore, Maryland, USA.
Jenna E Coalson, Department of Epidemiology, School of Public Health, University of Michigan, Ann Arbor, Michigan, USA.
Clarissa Valim, Department of Global Health, Boston University School of Public Health, Boston, Massachusetts, USA.
Moses Chilombe, Malaria Alert Centre, Kamuzu University of Health Sciences, Blantyre, Malawi.
Andrew Ngwira, Malaria Alert Centre, Kamuzu University of Health Sciences, Blantyre, Malawi.
Andy Bauleni, Malaria Alert Centre, Kamuzu University of Health Sciences, Blantyre, Malawi.
Sarah Schaffer-DeRoo, University of Maryland School of Medicine, Baltimore, Maryland, USA.
Karl B Seydel, College of Osteopathic Medicine, Michigan State University, East Lansing, Michigan, USA.
Mark L Wilson, Department of Epidemiology, School of Public Health, University of Michigan, Ann Arbor, Michigan, USA.
Terrie E Taylor, College of Osteopathic Medicine, Michigan State University, East Lansing, Michigan, USA.
Don P Mathanga, Malaria Alert Centre, Kamuzu University of Health Sciences, Blantyre, Malawi.
Miriam K Laufer, Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore, Maryland, USA.
References
- 1. World Health Organization (WHO). World malaria report 2019. Geneva: WHO, 2019. https://www.who.int/publications/i/item/9789241565721. Accessed 1 March 2020. [Google Scholar]
- 2. Nankabirwa J, Brooker SJ, Clarke SE, et al. . Malaria in school-age children in Africa: an increasingly important challenge. Trop Med Int Health 2014; 19:1294–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clarke SE, Jukes MC, Njagi JK, et al. . Effect of intermittent preventive treatment of malaria on health and education in schoolchildren: a cluster-randomised, double-blind, placebo-controlled trial. Lancet 2008; 372:127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gitonga CW, Edwards T, Karanja PN, Noor AM, Snow RW, Brooker SJ.. Plasmodium infection, anaemia and mosquito net use among school children across different settings in Kenya. Trop Med Int Health 2012; 17:858–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barger B, Maiga H, Traore OB, et al. . Intermittent preventive treatment using artemisinin-based combination therapy reduces malaria morbidity among school-aged children in Mali. Trop Med Int Health 2009; 14:784–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nankabirwa J, Cundill B, Clarke S, et al. . Efficacy, safety, and tolerability of three regimens for prevention of malaria: a randomized, placebo-controlled trial in Ugandan schoolchildren. PLoS One 2010; 5:e13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nankabirwa JI, Wandera B, Amuge P, et al. . Impact of intermittent preventive treatment with dihydroartemisinin-piperaquine on malaria in Ugandan schoolchildren: a randomized, placebo-controlled trial. Clin Infect Dis 2014; 58:1404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clarke SE, Rouhani S, Diarra S, et al. . Impact of a malaria intervention package in schools on Plasmodium infection, anaemia and cognitive function in schoolchildren in Mali: a pragmatic cluster-randomised trial. BMJ Glob Health 2017; 2:e000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yimgang DP, Buchwald AG, Coalson JE, et al. . Population attributable fraction of anemia associated with Plasmodium falciparum infection in children in southern Malawi. Am J Trop Med Hyg 2021; 104:1013–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brooker S, Guyatt H, Omumbo J, Shretta R, Drake L, Ouma J.. Situation analysis of malaria in school-aged children in Kenya—what can be done? Parasitol Today 2000; 16:183–6. [DOI] [PubMed] [Google Scholar]
- 11. King N, Dewey C, Borish D.. Determinants of primary school non-enrollment and absenteeism: results from a retrospective, convergent mixed methods, cohort study in rural Western Kenya. PLoS One 2015; 10:e0138362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thuilliez J, Sissoko MS, Toure OB, Kamate P, Berthélemy JC, Doumbo OK.. Malaria and primary education in Mali: a longitudinal study in the village of Donéguébougou. Soc Sci Med 2010; 71:324–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nankabirwa J, Wandera B, Kiwanuka N, Staedke SG, Kamya MR, Brooker SJ.. Asymptomatic Plasmodium infection and cognition among primary schoolchildren in a high malaria transmission setting in Uganda. Am J Trop Med Hyg 2013; 88:1102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fernando D, de Silva D, Carter R, Mendis KN, Wickremasinghe R.. A randomized, double-blind, placebo-controlled, clinical trial of the impact of malaria prevention on the educational attainment of school children. Am J Trop Med Hyg 2006; 74:386–93. [PubMed] [Google Scholar]
- 15. Fernando D, Silva de D, Wickremasinghe R.. Short-term impact of an acute attack of malaria on the cognitive performance of school children living in a malaria-endemic area of Sri Lanka. Trans R Soc Trop Med Hyg 2003; 97:633–9. [DOI] [PubMed] [Google Scholar]
- 16. Cohee LM, Opondo C, Clarke SE, et al. . Preventive malaria treatment among school-aged children in sub-Saharan Africa: a systematic review and meta-analyses. Lancet Glob Health 2020; 8:e1499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Halliday KE, Okello G, Turner EL, et al. . Impact of intermittent screening and treatment for malaria among school children in Kenya: a cluster randomised trial. PLoS Med 2014; 11:e1001594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Okell LC, Cairns M, Griffin JT, et al. . Contrasting benefits of different artemisinin combination therapies as first-line malaria treatments using model-based cost-effectiveness analysis. Nat Commun 2014; 5:5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Okell LC, Bousema T, Griffin JT, Ouédraogo AL, Ghani AC, Drakeley CJ.. Factors determining the occurrence of submicroscopic malaria infections and their relevance for control. Nat Commun 2012; 3:1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Walldorf JA, Cohee LM, Coalson JE, et al. . School-age children are a reservoir of malaria infection in Malawi. PLoS One 2015; 10:e0134061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG.. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. RTS,S Clinical Trials Partnership. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med 2012; 367:2284–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coalson JE, Walldorf JA, Cohee LM, et al. . High prevalence of Plasmodium falciparum gametocyte infections in school-age children using molecular detection: patterns and predictors of risk from a cross-sectional study in southern Malawi. Malar J 2016; 15:527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Desai M, Hill J, Fernandes S, et al. . Prevention of malaria in pregnancy. Lancet Infect Dis 2018; 18:e119–32. [DOI] [PubMed] [Google Scholar]
- 25. Tiono AB, Sirima SB, Hamed K.. Fulani show decreased susceptibility to Plasmodium falciparum infection versus Mossi: data from a community-wide screening and treatment of asymptomatic carriers in Burkina Faso. Malar J 2013; 12:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Samuels AM, Odero NA, Odongo W, et al. . Impact of community-based mass testing and treatment on malaria infection prevalence in a high-transmission area of western Kenya: a cluster randomized controlled trial. Clin Infect Dis 2021; 72:1927–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tiono AB, Ouédraogo A, Diarra A, et al. . Lessons learned from the use of HRP-2 based rapid diagnostic test in community-wide screening and treatment of asymptomatic carriers of Plasmodium falciparum in Burkina Faso. Malar J 2014; 13:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ruizendaal E, Schallig HDFH, Scott S, et al. . Evaluation of malaria screening during pregnancy with rapid diagnostic tests performed by community health workers in Burkina Faso. Am J Trop Med Hyg 2017; 97:1190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nankabirwa JI, Yeka A, Arinaitwe E, et al. . Estimating malaria parasite prevalence from community surveys in Uganda: a comparison of microscopy, rapid diagnostic tests and polymerase chain reaction. Malar J 2015; 14:528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Parr JB, Belson C, Patel JC, et al. . Estimation of Plasmodium falciparum transmission intensity in Lilongwe, Malawi, by microscopy, rapid diagnostic testing, and nucleic acid detection. Am J Trop Med Hyg 2016; 95:373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Plucinski MM, Candrinho B, Dimene M, et al. . Assessing performance of HRP2 antigen detection for malaria diagnosis in Mozambique. J Clin Microbiol 2019; 57:e00875-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. World Health Organization. WHO technical consultation on research requirements to support policy recommendations on highly sensitive point-of-care diagnostics for P. falciparum malaria, 2018. https://www.who.int/malaria/mpac/mpac-october2018-session7-report-high-sensitive-poct.pdf. Accessed 18 October 2020.
- 33. Staedke SG, Maiteki-Sebuguzi C, Rehman AM, et al. . Assessment of community-level effects of intermittent preventive treatment for malaria in schoolchildren in Jinja, Uganda (START-IPT trial): a cluster-randomised trial. Lancet Glob Health 2018; 6:e668–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McCann RS, Cohee LM, Goupeyou-Youmsi J, Laufer MK.. Maximizing impact: can interventions to prevent clinical malaria reduce parasite transmission? Trends Parasitol 2020; 36:906–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barry A, Bradley J, Stone W, et al. . Higher gametocyte production and mosquito infectivity in chronic compared to incident Plasmodium falciparum infections. Nat Commun 2021; 12:2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stone W, Gonçalves BP, Bousema T, Drakeley C.. Assessing the infectious reservoir of falciparum malaria: past and future. Trends Parasitol 2015; 31:287–96. [DOI] [PubMed] [Google Scholar]
- 37. Andolina C, Rek JC, Briggs J, et al. . Sources of persistent malaria transmission in a setting with effective malaria control in eastern Uganda: a longitudinal, observational cohort study. Lancet Infect Dis 2021; 21:1568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.