Abstract
Background
Group A Streptococcus (GAS) causes superficial pharyngitis and skin infections as well as serious autoimmune sequelae such as acute rheumatic fever (ARF) and subsequent rheumatic heart disease. ARF pathogenesis remains poorly understood. Immune priming by repeated GAS infections is thought to trigger ARF, and there is growing evidence for the role of skin infections in this process.
Methods
We utilized our recently developed 8-plex immunoassay, comprising antigens used in clinical serology for diagnosis of ARF (SLO, DNase B, SpnA), and 5 conserved putative GAS vaccine antigens (Spy0843, SCPA, SpyCEP, SpyAD, Group A carbohydrate), to characterize antibody responses in sera from New Zealand children with a range of clinically diagnosed GAS disease: ARF (n = 79), GAS-positive pharyngitis (n = 94), GAS-positive skin infection (n = 51), and matched healthy controls (n = 90).
Results
The magnitude and breadth of antibodies in ARF was very high, giving rise to a distinct serological profile. An average of 6.5 antigen-specific reactivities per individual was observed in ARF, compared to 4.2 in skin infections and 3.3 in pharyngitis.
Conclusions
ARF patients have a unique serological profile, which may be the result of repeated precursor pharyngitis and skin infections that progressively boost antibody breadth and magnitude.
Keywords: acute rheumatic fever, antibodies, autoimmunity, group A Streptococcus, pathogenesis, serology
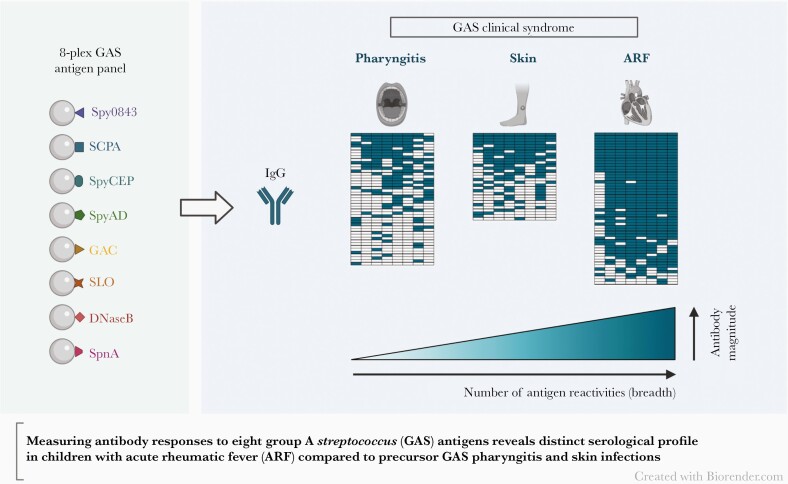
A multiantigen analysis using a novel 8-plex assay revealed a distinct serological profile in rheumatic fever patients, characterized by increases in both the magnitude and breadth of group A Streptococcusantibodies, compared to children with precursor pharyngeal and skin infections.
Graphical Abstract
Graphical Abstract.
Clinical manifestations of Streptococcus pyogenes (group A Streptococcus; GAS) infection are varied and include superficial pharyngitis (throat) and pyoderma (skin) infections, serious invasive diseases, and autoimmune sequalae such as acute rheumatic fever (ARF), which is associated with carditis and can lead to chronic rheumatic heart disease (RHD) [1, 2]. ARF and RHD are substantial causes of morbidity and mortality in low- and middle-income countries, as well as in certain communities within high-income countries [3, 4]. In New Zealand, the burden of ARF is almost exclusively carried by people of Māori and Pacific ethnicity, who have some of the highest rates of the disease in the world [5].
Treating GAS pharyngitis with antibiotics is the mainstay for ARF and RHD prevention, as although candidate vaccines are being developed, none have reached licensure [6]. The limited understanding of ARF pathogenesis presents a major hurdle for vaccine development from a perceived safety perspective, despite contemporary clinical trials showing no link between GAS vaccines and autoimmune complications [7]. The historical molecular mimicry hypothesis for ARF postulates that antibodies generated during the immune response to a GAS infection are able to cross-react with similarly structured human proteins [2, 4, 8, 9]. It is also thought that repeated infections are required to “prime” the immune system, eventually leading to loss of tolerance and autoimmune pathogenesis [2, 10].
The immune priming hypothesis is supported by epidemiological evidence showing that precursor GAS pharyngitis and skin infections are frequent in children younger than 5 years [11], while the peak age for ARF is in school children aged 5–14 years [5]. Recently, serological profiling to retrospectively map GAS exposures has shown that a minimum of 2 preceding GAS infections are associated with first-episode ARF, based on type-specific antibody reactivities to the GAS T-antigen and the M protein (encoded by the emm gene) [10, 12]. While certain GAS strains (emm-types) were historically linked to ARF and considered rheumatogenic, recent global analyses have observed a broad range of ARF associated emm-types [13], suggesting the concept of rheumatogenicity should be reconsidered. There is also growing evidence for skin infections in ARF pathogenesis [14]. High rates of GAS skin infections and low rates of pharyngitis have been observed in tropical regions with a high ARF burden, such as in the Northern Territories of Australia [15] and Fiji [16]. In New Zealand, there is a predominance of skin-associated GAS strains in children with ARF [17], and a recent study has shown that ARF risk is elevated 5-fold in the 3-month period following a GAS-positive throat or skin swab compared to children with a GAS-negative swab [18].
Robust, high-throughput assays based on advanced multiplex technology can facilitate serological profiling for GAS disease [19]. Elevated antibody titers are used clinically as evidence of a preceding GAS infection for the diagnosis of ARF [20–22], as well as to distinguish serologically confirmed GAS pharyngitis from carriage in epidemiology studies [23, 24]. Our laboratory recently developed a triplex immunoassay to measure antibody responses to streptolysin O (SLO), deoxyribonuclease B (DNaseB), and S. pyogenes nuclease A (SpnA) with improved efficiency and accuracy compared to clinical serological assays [25]. This assay has now been expanded to an 8-plex that encompasses putative GAS vaccine targets (S. pyogenes leucine-rich repeat domain-containing protein [Spy0843/LRRP], streptococcal C5a peptidase [SCPA], S. pyogenes cell envelope protease [SpyCEP], S. pyogenes adhesion and division protein [SpyAD], and the group A carbohydrate [GAC]) [19]. These conserved antigens, carried by > 99% of strains (irrespective of emm-type) [26], elicit protective antibody responses in animal models and are included in various multicomponent GAS vaccines under development [27–29]. Serological responses to several of these antigens are yet to be well characterized, particularly following skin infections and in ARF.
The aim of this study was to use the 8-plex assay to profile the GAS immune response in ARF compared to precursor pharyngitis and skin infections. Antibody responses were measured in sera from New Zealand children with a range of GAS clinical syndromes (ARF, GAS-positive pharyngitis, and GAS-positive skin infection) and compared with antibody responses in closely matched healthy controls. The assay was validated against commercial serological assays, and antibody dynamics based on ethnic group identification, time since infection, and disease state were considered.
METHODS
Study Samples
Sera were obtained from two New Zealand studies approved by the Health and Disability Ethics Committee (HDEC) and written informed consent was provided by all participants or their parents/guardians. Participants with suspected GAS infections were recruited as part of a GAS skin/throat study (ethics 17/NTA/262), conducted in Auckland between 2018 and 2019 [30]. This included participants with symptomatic pharyngitis and a GAS-positive throat culture and participants with a GAS-positive skin infection. Convalescent sera (n = 145) collected at the follow-up visit (3–6 weeks after initial presentation) were analyzed in this study to ensure sufficient time had lapsed for seroconversion. As with a prior study on this cohort [23], only participants with a GAS-negative throat swab at the follow-up visit were included to exclude those that were recolonized between visits. Convalescent GAS-positive pharyngitis (n = 94) and skin infection (n = 51) sera was collected 24–34 days and 24–45 days after the initial positive swab, respectively.
ARF cases (n = 79) were recruited as part of the Rheumatic Fever Risk Factors (RF RISK) case-control study (ethics 14/NTA/53) [31]. Cases were diagnosed according to the New Zealand modification of the Jones criteria [32, 33] between 2014 and 2017 and classified into early or late groups based on the number of days from hospital admission that their serum sample was taken (early group range 2–26 days [n = 54]; late group range 28–62 days [n = 25]) as previously published [25]. Twelve of the ARF cases had a GAS strain obtained via throat swab at hospital admission that was emm-typed using standard protocols [17]. Healthy, asymptomatic control sera from the GAS skin/throat study (n = 15) and the RF RISK study (n = 75) were also available (total n = 90). The controls from the RF RISK study were closely matched to the ARF cases by age and social deprivation.
Antibody levels were initially measured in all samples (n = 314) to validate the 8-plex assay method. Focused analyses were subsequently performed on a subset of participants, with specific inclusion criteria applied to ensure groups were closely matched according to their ethnic group identification and estimated time since infection (Supplementary Figure 1 and Table 1). Participants in the focused analyses cohort comprised the early ARF group (n = 54), GAS-positive pharyngitis (n = 65), GAS-positive skin infection (n = 37), and healthy controls (n = 73).
Table 1.
Demographics of the Participants Included in the Validation Phase (Validation Cohort) and the Focused Analysis Comparing Antibody Levels Between Different Disease States (Focused Analyses Cohort)
| Validation Cohort | ||||
|---|---|---|---|---|
| Characteristic | Healthy | GAS-Positive Pharyngitis/Skin Infection | ARF | |
| Total No. | 90 | 145 | 79 | |
| Study | RF RISK, GAS skin/throat | GAS skin/throat | RF RISK | |
| Age, y, median (range) | 11 (6–19) | 9 (5–14) | 11 (5–19) | |
| Sex, M/F | 53/37 | 80/65 | 55/24 | |
| Ethnic group | ||||
| Māori/Pacific | 73 | 102 | 79 | |
| Othera | 17 | 43 | … | |
| Focused Analyses Cohort | ||||
| Characteristic | Healthy | GAS-Positive Pharyngitis | GAS-Positive Skin Infection | ARF |
| Total No. | 73 | 65 | 37 | 54 |
| Study | RF RISK | GAS skin/throat | GAS skin/throat | RF RISK |
| Age, y, median (range) | 12 (6–19) | 9 (5–14) | 8 (5–14) | 11 (5–19) |
| Sex, M/F | 45/28 | 36/29 | 16/21 | 38/16 |
| Ethnic group, Māori/Pacific | 73 | 65 | 37 | 54 |
| Time since infection, d, median (range) | … | 29 (24–34) | 30 (25–45) | … |
| Time since hospital admission, d, median (range) | … | … | … | 13 (2–26)b |
Abbreviations: ARF, acute rheumatic fever; GAS-positive, group A Streptococcus-culture positive; RF, rheumatic fever.
Participants belonging to ethnic group “Other” (non-Māori and non-Pacific ethnic background, primarily New Zealand European).
Assuming ARF occurs 2–4 weeks after a GAS infection, the median days from the precursor infection in the early ARF group was estimated to correspond to between 27 and 41 days.
Antibody Quantification
Antigen-specific IgG antibody responses were measured using the 8-plex assay as published [19]. Median fluorescence intensity signals for each sample were measured with a MagPix instrument (Luminex Corporation) and data were collected using xPonent software version 4.2 (Luminex Corporation). Standard curves were generated from pooled human immunoglobulin (intravenous immunoglobulin; Privigen; CSL Behring), that was buffer exchanged into phosphate buffered saline, and fitted using a 5-parameter logistic regression with Belysa software (Merck). Antigen-specific IgG concentrations were quantified in μg/mL by interpolation.
Comparison with Commercially Determined Titers
A subset of sera (n = 235) comprising 90 healthy controls and 145 GAS-positive pharyngitis and skin infections had anti-SLO and anti-DNaseB data available from commercial serological methods [23]: anti–SLO from a turbidimetric assay on a SPAplus analyzer (The Binding Site); and anti-DNaseB titers from the enzyme inhibition assay method (bioMerieux). Midtiter values of 12.5 IU/mL for anti-SLO and 50 U/mL for anti-DNaseB were used when inexact ranges for low-titer samples were given.
Determination of Serologically Confirmed GAS Infections
To determine seropositivity in the focused analyses, cutoffs were established for each antigen (in μg/mL) using the nonparametric upper limit of normal (ULN) approach in Microsoft Excel, as previously described [25, 34]. The antigen-specific ULN were calculated as the 80th percentile of antibody levels in 73 healthy participants from the RF RISK study (with non-Māori and non-Pacific participants excluded to enable closely matched comparisons). Immunological evidence of a GAS infection was defined as a seropositive response above ULN for at least 1 antigen, as previously described [24]. A total of 47/65 GAS-positive pharyngitis and 31/37 GAS-positive skin infections were classified as serologically confirmed GAS infections based on this criterion (Supplementary Figure 1).
Data Analysis
Statistical analyses were performed using GraphPad Prism version (version 8.0) and RStudio (version 4.0.3) [35]. Data did not follow a normal distribution and nonparametric methods were used to determine strength of correlations (Spearman r) and differences in antibody responses between groups. Mann-Whitney (Wilcoxon) tests were used to calculate statistical significance between 2 groups. Kruskal–Wallis followed by Dunn test (with a Bonferroni adjustment to correct for multiple comparisons) was used to calculate statistical significance between 3 or more groups. χ2 tests were used to determine differences in rates of seropositivity. A P value of ≤ .05 was considered statistically significant. Box and whisker plots (Tukey) represent median, 25th and 75th percentiles, and the smallest and largest values extending no further than 1.5 × interquartile range from the 25th and 75th percentiles, respectively. Hierarchical clustering was performed using Euclidean distance with the pheatmap package [36].
RESULTS
Antibody Levels Determined With 8-Plex Assay Confirm and Expand Previous Findings
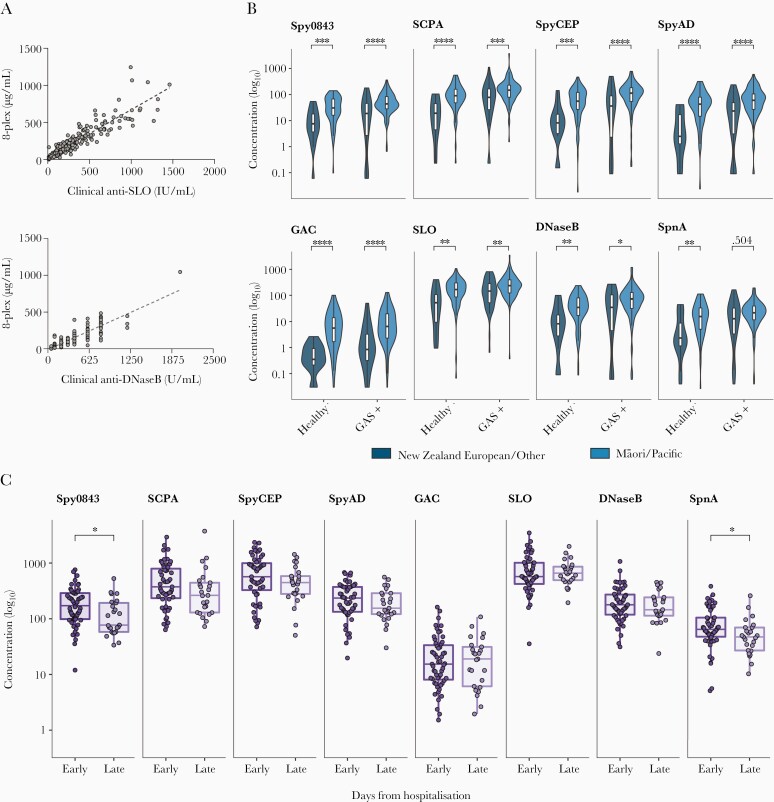
Antibody responses to GAS antigens Spy0843, SCPA, SpyCEP, SpyAD, GAC, SLO, DNaseB, and SpnA were measured using an in-house 8-plex assay [19]. To validate the 8-plex assay format, initial investigations were conducted that confirmed and built on prior observations for selected antigens. Our former triplex assay (SLO, DNaseB, and SpnA) was validated against commercially available anti-SLO and anti-DNaseB clinical serology assays [25]. Similarly, anti-SLO and anti-DNaseB antibody levels determined in the 8-plex format correlated highly with commercially determined anti-SLO and anti-DNaseB titers (Figure 1A).
Figure 1.
Data comprising samples from the validation cohort. A, Scatter plots showing the correlation between the in-house 8-plex GAS assay and commercial assays for antibodies to SLO (n = 233, Spearman r = 0.936) and DNaseB (n = 233, Spearman r = 0.927). P values were < .001 for both antigens. The linear regression equations are represented by the dashed lines (SLO, R2 = 0.829; DNaseB, R2 = 0.7944). B, Box-and-whisker plots overlayed onto violin plots showing the concentration (in μg/mL) of antibodies to each of the 8 GAS antigens between participants of European/other ethnicity (green) and Māori and Pacific participants (blue). Antibodies at baseline (healthy participants) and following GAS culture-positive (GAS+) infection (pharyngitis/skin) are shown. C, Plots showing the concentration of antibodies to each of the 8 GAS antigens in acute rheumatic fever cases, split by days since hospital admission; early group, median days from hospitalization = 13 (interquartile range = 10–17); late group, median days = 34 (interquartile range = 29–39). P values determined by Mann-Whitney tests. **** < .001; *** < .005; ** .005–.01; *.01–.05. Abbreviations: DNaseB, deoxyribonuclease B; GAC, group A carbohydrate; GAS, group A Streptococcus; SCPA, streptococcal C5a peptidase; SLO, streptolysin O; SpnA, S. pyogenes nuclease A; Spy0843, S. pyogenes leucine-rich repeat domain-containing protein; SpyAD, S. pyogenes adhesion and division protein; SpyCEP, S. pyogenes cell envelope protease.
Ethnicity-associated differences in anti-SLO and anti-DNaseB titers were recently demonstrated between children of Māori/Pacific and European/other ethnic group identification in the GAS study cohort using commercial assays [23]. This finding was confirmed using the 8-plex assay, with Māori and Pacific children (n = 73) having significantly higher SLO and DNaseB antibody levels than non-Māori and Pacific participants (n = 17) at baseline (healthy control group). This pattern extended to each of the other 6 antigens in the 8-plex assay, with significantly elevated antibody levels observed in Māori and Pacific children (all P values < .01; Figure 1B and Supplementary Table 1). Additionally, Māori and Pacific children (n = 102) had significantly higher antibody levels than non-Māori/Pacific participants (n = 43) to 7/8 antigens following GAS infection (all P values < .05; Figure 1B and Supplementary Table 1).
As final verification of the 8-plex format, antigen-specific immunoglobulin G (IgG) concentrations in ARF were compared to prior observations from the triplex assay (SLO, DNaseB, and SpnA), which had shown that antibodies to SpnA appeared to wane faster than antibodies to SLO and DNaseB when cases were stratified into early and late groups [25]. In the 8-plex format, with ARF cases likewise stratified into early (n = 54) and late (n = 25) groups following hospital admission, the median level of SpnA antibodies was significantly reduced in the late ARF group (P value = .018; Figure 1C). Amongst the wider antigen panel, antibodies to GAC, SLO, and DNaseB remained stable, and trended downwards for SCPA, SpyCEP, and SpyAD in the late ARF group but the differences were not significant. The median level of Spy0843 antibodies was significantly lower in the late ARF group (P value = .0104). Taken together, these results highlight differences in immunokinetics between the 8 antigens.
Magnitude of the Antibody Response Is Associated With GAS Disease State
To compare antibody responses for the 8 antigens between GAS clinical syndromes, a focused analysis was performed on participants matched according to their ethnic group identification (all Māori/Pacific) and time since onset of infection (Supplementary Figure 1 and Table 1). This comprised healthy controls (n = 73), GAS-positive pharyngitis (n = 65), GAS-positive skin (n = 37), and early ARF cases (n = 54). The median days from infection for GAS-positive pharyngitis and skin infections was 29 (range, 24–34) and 30 (range, 24–45), respectively. The median days from hospitalization in the ARF early group was 13 (range, 2–26). As ARF is assumed to occur 2–4 weeks after a GAS infection [2], the median days from precursor infection in the early ARF group was estimated to be 27–41 days.
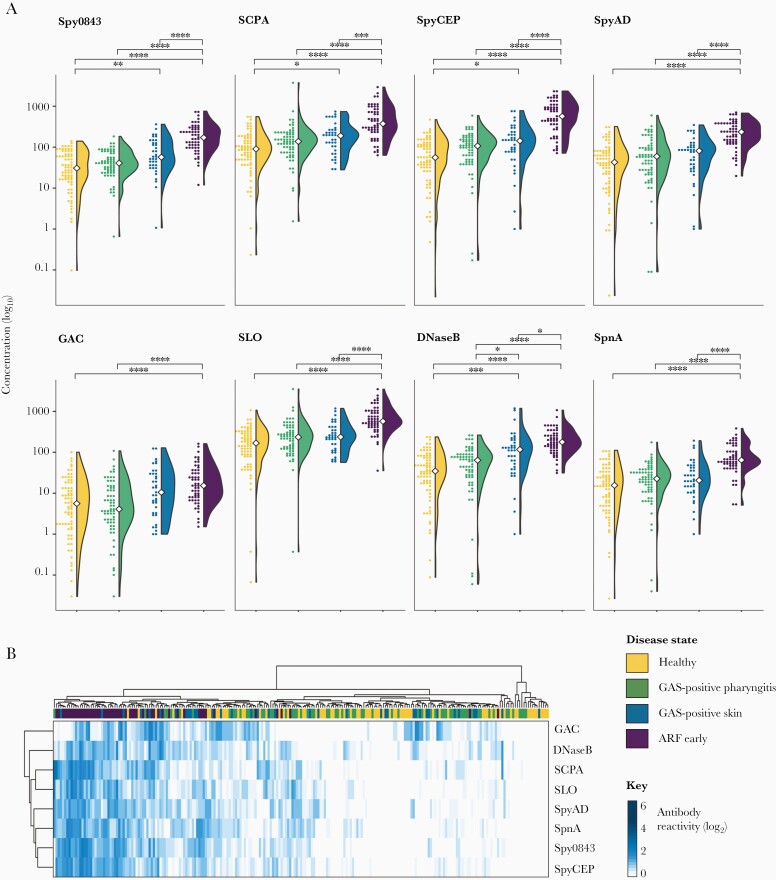
Although median antibody levels to some antigens were higher in the GAS-positive pharyngitis group than in matched healthy controls, the differences were not significant (Figure 2A and Supplementary Table 2). In contrast, significant differences were observed between the GAS-positive skin infection group and healthy controls for Spy0843, SCPA, SpyCEP, and DNaseB (P values < .05). Antibodies to DNaseB in skin infections were also higher than in pharyngitis (P value = .0227). Most notably, antibody levels in ARF cases were significantly elevated above matched healthy controls and GAS-positive pharyngitis/skin infections for all 8 antigens (P values < .0001), except the GAC in participants with skin infections (P value = .615). Total antibody levels against the GAC were lower in general compared with the protein antigens.
Figure 2.
Comparison of antigen-specific antibodies in different disease states: healthy participants (yellow, n = 73), GAS-positive pharyngitis cases (green, n = 65), GAS-positive skin cases (blue, n = 37), and early ARF cases (purple, n = 54). A, Violin plots combined with dot plots showing the distribution of antibody concentration (in μg/mL) to each of the 8 GAS antigens within the 4 disease groups. White circles indicate median antibody values. B, Heat map showing the magnitude of antigen-specific antibody responses. Each column represents an individual participant, color coded by clinical syndrome. Antibody levels were log2 transformed and scaled by the maximum value of each row to allow comparison between each antigen. Dendrograms represent hierarchical clustering that was performed to test for similarities in antibody reactivity between antigens (by row) and individual participants (by columns). P values determined by Kruskal–Wallis followed by Dunns test with a Bonferroni adjustment: **** < .001; *** < .005; **.005–.01; *.01–.05. Abbreviations: ARF, acute rheumatic fever; DNaseB, deoxyribonuclease B; GAC, group A carbohydrate; GAS, group A Streptococcus; SCPA, streptococcal C5a peptidase; SLO, streptolysin O; SpnA, S. pyogenes nuclease A; Spy0843, S. pyogenes leucine-rich repeat domain-containing protein; SpyAD, S. pyogenes adhesion and division protein; SpyCEP, S. pyogenes cell envelope protease.
Unsupervised hierarchical clustering based on antibody responses (Figure 2B) showed clear differentiation of ARF cases, while GAS-positive pharyngitis/skin infections and matched healthy controls were intermixed indicating the differences between these groups are more subtle. This differentiation, attributable to the ARF cases reacting to multiple antigens and having higher levels of circulating antibodies to these antigens, suggests a unique antibody signature in ARF compared to the other clinical syndromes. In terms of antigen clustering, GAC was distinct from the 7 other antigens as evidenced by the separated branch on the dendrogram, indicating that responses to the carbohydrate differ from those to the protein antigens in the 8-plex panel.
Breadth of the Antibody Response Is Associated With GAS Disease State
To further explore the breadth of the antibody response in ARF and precursor infections, seropositivity criteria were applied to the 8 GAS antigens. ULN cutoff values were defined as the 80th centile in the matched healthy control group (n = 73). An antibody response above these experimentally determined values for at least 1 antigen was used as a criterion for a serologically confirmed GAS infection. There were 18/65 (27.6%) participants in the GAS-positive pharyngitis group and 6/37 (16.2%) in the skin infection group that did not have a positive serological response to a single antigen and were thus excluded (Supplementary Figure 1).
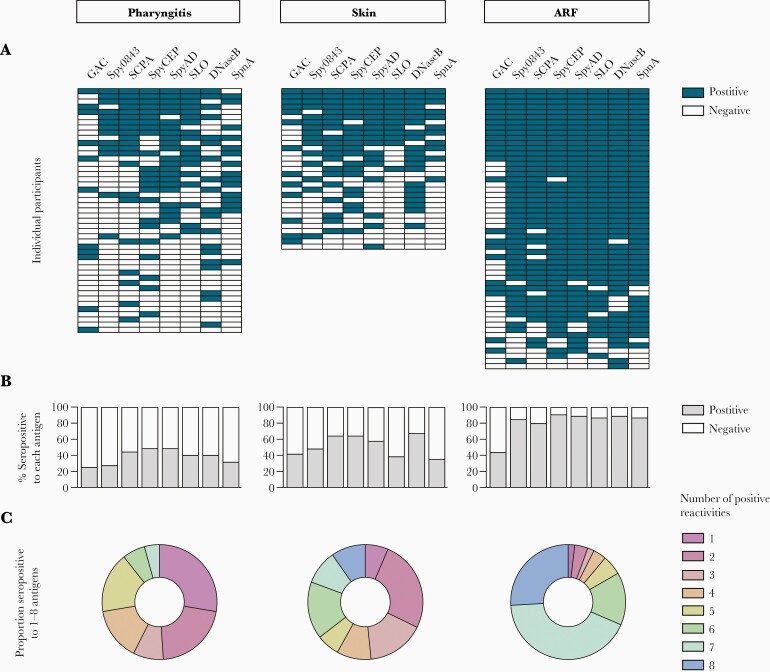
The frequency of positive responses to any given antigen in serologically confirmed GAS pharyngitis participants (n = 47) was quite low (< 50%) and no participant had a response to all 8 antigens (Figure 3A and 3B and Supplementary Table 3). The frequency of positive responses was slightly higher in serologically confirmed GAS skin infection group (n = 31), with the most common responses observed for DNaseB (67.7%, 21/31), SpyCEP, and SCPA (both 64.5%, 20/31). For both skin and pharyngitis infections, no single antigen was elevated in the majority of participants, nor was there any obvious synchronicity in response between particular antigens (Figure 3A).
Figure 3.
Serological profile of antibodies in different disease states: GAS pharyngitis cases (n = 47), GAS skin infection cases (n = 31), and early ARF cases (n = 54). A, Barcodes showing a binary positive (dark blue) or negative (white) antibody response to each GAS antigen, grouped by clinical syndrome. Experimentally determined upper limits of normal were used as positivity cutoffs. One horizontal row equates to the responses of an individual participant. B, Bar graphs showing, for each antigen, the percentage of participants in each group that had a positive (gray) or negative (white) response. C, Donut charts quantifying the breadth of the immune response in each disease group. Each color represents the proportion of participants that were seropositive to either 1 (light purple), 2 (dark pink), 3 (pink), 4 (orange), 5 (yellow), 6 (green), 7 (aqua), or 8 (blue) antigens. Abbreviations: ARF, acute rheumatic fever; DNaseB, deoxyribonuclease B; GAC, group A carbohydrate; GAS, group A Streptococcus; SCPA, streptococcal C5a peptidase; SLO, streptolysin O; SpnA, S. pyogenes nuclease A; Spy0843, S. pyogenes leucine-rich repeat domain-containing protein; SpyAD, S. pyogenes adhesion and division protein; SpyCEP, S. pyogenes cell envelope protease.
The profile of antigen-specific seropositivity in ARF cases was notably distinct to that of the precursor skin and pharyngeal infections (Figure 3A and 3B). The frequency of seropositivity was over 79% for all antigens except the GAC, which elicited a positive response in less than half of the participants and for which there was no significant difference compared to the precursor infections (Supplementary Table 3). Positive responses to SpyCEP were most common in ARF, with 90.7% (49/54) of participants above the cutoff, followed by SpyAD and DNaseB at 88.9% (48/54), and SpnA and SLO at 87% (47/54). A greater proportion of ARF cases also had positive responses to multiple antigens (Figure 3C) with an average of 6.5 antigen-specific reactivities per individual, compared to 4.2 in skin infections and 3.3 in pharyngitis. Indeed, significant differences were observed between ARF and the precursor infections when the cumulative seropositivity rates to increasing numbers of antigens were assessed (P values < .01; Supplementary Table 4), with a high proportion of ARF cases (83.3%) having a response to 6 or more of the antigens tested (compared to 36% in skin and 11% in pharyngitis). Each of the GAS isolates (n = 12) obtained from ARF cases at hospital admission belonged to a unique emm-type (Supplementary Figure 2), highlighting the diversity of ARF-associated strains in New Zealand as previously shown [17], and suggesting seropositivity to the conserved antigens in the 8-plex assay is unlikely to be influenced by emm-type.
DISCUSSION
This study investigated the breadth and magnitude of antibody responses to GAS antigens in New Zealand children presenting with a range of clinical syndromes that were closely matched for age, ethnic group identification, and estimated time since infection. The use of a validated 8-plex assay enabled robust quantification of antibodies to conserved GAS antigens utilized in both clinical serology and as vaccine targets (Spy0843, SCPA, SpyCEP, SpyAD, GAC, SLO, DNaseB, and SpnA) and uncovered striking differences in the serological profile for ARF compared to precursor skin and pharyngeal infections.
The distinct antibody profile in ARF was characterized by an increase in both breadth and magnitude to the 8 antigens tested. Increased levels of circulating antibodies to some of these antigens have been demonstrated previously in ARF. They include GAC, SLO, and DNaseB in a study comparing ARF to GAS pharyngitis [37], SCPA in ARF compared with GAS skin infections [38], and our prior investigation of ARF and closely matched controls with the triplex assay (SLO, DNaseB, and SpnA) [25]). These prior analyses focused on group comparisons, whereas the application of hierarchal clustering and barcoding of individual responses in this study has illuminated a distinct ARF profile of antibody reactivity, with the majority of ARF patients seropositive to 6 or more antigens. In contrast, antibody responses to the same 8 antigens were unable to distinguish GAS-positive pharyngitis and skin infections from matched healthy controls, and there was no clear pattern of responses to particular antigens in these precursor infections. This finding is in agreement with previous studies of GAS pharyngitis that observed significant heterogeneity of serological responses to GAS antigens [39, 40].
The antibody profile in ARF revealed in this study further supports immune priming contributing to pathogenesis. Recent serological studies provided evidence for multiple prior GAS exposures in ARF based on the presence of antibodies to 2 or more type-specific antigens [10, 12]. Here, the comparison of antibody reactivity to conserved GAS antigens suggests that each precursor infection progressively boosts and broadens the GAS antibody response, resulting in ARF patients reacting to significantly more GAS antigens in the 8-plex panel. Indeed, the increase of reactivity from an average 3.3 antigens in GAS pharyngitis and 4.2 in GAS skin infections to 6.5 in ARF is difficult to explain without repeated infections, especially given the groups were closely matched and comprised Māori and Pacific children who bear the major burden of ARF in New Zealand [5]. Expansions in pathogen-specific antibody repertoires have been observed in other infectious diseases such as malaria, where repeated Plasmodium falciparum infections result in gradual, stepwise acquisition and boosting of merozoite antibodies [41, 42]. Similarly, Streptococcus pneumoniae human challenge studies found bacterial carriage resulted in both expansion and boosting of prior antipneumococcal protein antibodies [43], and repeated minor invasions associated with Staphylococcus aureus colonization contribute to increases in antistaphylococcal antibodies [44]. However, the consequences of repeated infections vary by pathogen and GAS is particularly complex. While GAS immunity is eventually acquired in adulthood, with pharyngitis and skin infections considered childhood diseases [45], repeated GAS infections can also lead to immune dysregulation and ARF in susceptible individuals.
There have been limited data on antibody responses in GAS skin infections compared with pharyngitis. This study adds to recent investigations showing skin infections induce detectable antibody responses to selected GAS antigens, including type-specific M-protein peptides [46] and traditional serological antigens SLO and DNaseB [23]. DNaseB antibodies have been observed to be elevated following skin infections compared with SLO antibodies [23, 47], and this has been confirmed in this study. Beyond these traditional antigens, this study also shows GAS skin infections can induce antibody responses to all 8 antigens in the panel, and the breadth of antibody responses is increased for skin infections compared to pharyngitis. This observation, combined with recent studies showing that GAS skin infections are markedly higher in Māori and Pacific peoples in New Zealand who are most at risk of ARF [48, 49], further corroborates the role of GAS skin infections as priming events for ARF.
In summary, this study has generated additional data on the utility of an 8-plex assay for assessing serological responses to GAS infections. Application of the assay uncovered distinct serological responses by GAS-related clinical syndrome, emphasizing the value of multiantigen analysis when investigating GAS disease. The broad ARF antibody repertoire observed, likely due to repeated prior GAS infections, highlights the importance of comprehensive strategies that prevent both GAS pharyngitis and skin infections rather than strategies that target only one of these clinical syndromes. It follows that an effective vaccine for ARF will need to interrupt GAS priming events to avert immune dysregulation.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We are grateful to the Māori and Pacific governance groups of the Rheumatic Fever Risk Factors (RF RISK) and Group A Streptococcus (GAS) skin/throat studies for cultural advice on study design and operation. We thank Dr Danilo Moriel of the GSK Vaccines Institute for Global Health for providing the SpyCEP, SpyAD, and GAC antigens for the 8-plex assay development. GSK Vaccines Institute for Global Health Srl is an affiliate of GlaxoSmithKline Biologicals SA.
Financial support. This work was supported by the Maurice Wilkins Centre for Biodiscovery, University of Auckland. A. L. W. is supported by a University of Auckland Doctoral Scholarship. The RF RISK study from which samples were obtained was funded by the Health Research Council of New Zealand (HRC) Rheumatic Fever Research Partnership fund (Ministry of Health, Te Puni Kōkiri, Cure Kids, Heart Foundation, and HRC (grant number 13/959)). The GAS skin/throat study was supported by the HRC (grant number 16/005).
Contributor Information
Alana L Whitcombe, School of Medical Sciences and Maurice Wilkins Centre, University of Auckland, Auckland, New Zealand.
Reuben McGregor, School of Medical Sciences and Maurice Wilkins Centre, University of Auckland, Auckland, New Zealand.
Julie Bennett, Department of Public Health, University of Otago, Wellington, New Zealand.
Jason K Gurney, Department of Public Health, University of Otago, Wellington, New Zealand.
Deborah A Williamson, University of Melbourne at The Peter Doherty Institute for Infection and Immunity, Melbourne, Victoria, Australia.
Michael G Baker, Department of Public Health, University of Otago, Wellington, New Zealand.
Nicole J Moreland, School of Medical Sciences and Maurice Wilkins Centre, University of Auckland, Auckland, New Zealand.
References
- 1. Walker MJ, Barnett TC, McArthur JD, et al. . Disease manifestations and pathogenic mechanisms of group A Streptococcus. Clin Microbiol Rev 2014; 27:264–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carapetis JR, Beaton A, Cunningham MW, et al. . Acute rheumatic fever and rheumatic heart disease. Nat Rev Dis Primers 2016; 2:15084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Watkins DA, Johnson CO, Colquhoun SM, et al. . Global, regional, and national burden of rheumatic heart disease, 1990–2015. NEJM 2017; 377:713–22. [DOI] [PubMed] [Google Scholar]
- 4. Karthikeyan G, Guilherme L.. Acute rheumatic fever. Lancet 2018; 392:161–74. [DOI] [PubMed] [Google Scholar]
- 5. Bennett J, Zhang J, Leung W, et al. . Rising ethnic inequalities in acute rheumatic fever and rheumatic heart disease, New Zealand, 2000–2018. Emerg Infect Dis 2021; 27:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dale JB, Walker MJ.. Update on group A streptococcal vaccine development. Curr Opin Infect Dis 2020; 33:244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vekemans J, Gouvea-Reis F, Kim JH, et al. . The path to group A Streptococcus vaccines: World Health Organization research and development technology roadmap and preferred product characteristics. Clin Infect Dis 2019; 69:877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tandon R, Sharma M, Chandrashekhar Y, Kotb M, Yacoub MH, Narula J.. Revisiting the pathogenesis of rheumatic fever and carditis. Nat Rev Cardiol 2013; 10:171–7. [DOI] [PubMed] [Google Scholar]
- 9. Cunningham MW. Rheumatic fever revisited. Nat Rev Cardiol 2014; 11:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Raynes JM, Frost HRC, Williamson DA, et al. . Serological evidence of immune priming by group A streptococci in patients with acute rheumatic fever. Front Microbiol 2016; 7:1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Steer AC, Danchin MH, Carapetis JR.. Group A streptococcal infections in children. J Paediatr Child Health 2007; 43:203–13. [DOI] [PubMed] [Google Scholar]
- 12. Lorenz N, Ho TKC, McGregor R, et al. . Serological profiling of group A Streptococcus infections in acute rheumatic fever. Clin Infect Dis 2021; 73:2322–5. [DOI] [PubMed] [Google Scholar]
- 13. Crombrugghe G de, Baroux N, Botteaux A, et al. . The limitations of the rheumatogenic concept for group A Streptococcus: systematic review and genetic analysis. Clin Infect Dis 2020; 70:1453–60. [DOI] [PubMed] [Google Scholar]
- 14. Parks T, Smeesters PR, Steer AC.. Streptococcal skin infection and rheumatic heart disease. Curr Opin Infect Dis 2012; 25:145–53. [DOI] [PubMed] [Google Scholar]
- 15. McDonald MI, Towers RJ, Andrews RM, Benger N, Currie BJ, Carapetis JR.. Low rates of streptococcal pharyngitis and high rates of pyoderma in Australian aboriginal communities where acute rheumatic fever is hyperendemic. Clin Infect Dis 2006; 43:683–9. [DOI] [PubMed] [Google Scholar]
- 16. Steer AC, Jenney AWJ, Kado J, et al. . High burden of impetigo and scabies in a tropical country. PLoS Negl Trop Dis 2009; 3:e467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Williamson DA, Smeesters PR, Steer AC, et al. . M-protein analysis of Streptococcus pyogenes isolates associated with acute rheumatic fever in New Zealand. J Clin Microbiol 2015; 53:3618–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oliver J, Bennett J, Thomas S, et al. . Preceding group A Streptococcus skin and throat infections are individually associated with acute rheumatic fever: evidence from New Zealand. BMJ Glob Health 2021; 6:e007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Whitcombe AL, Han F, McAlister SM, et al. . An eight-plex immunoassay for group A Streptococcus serology and vaccine development. J Immunol Methods 2021; 500: 113194. [DOI] [PubMed] [Google Scholar]
- 20. Shet A, Kaplan EL.. Clinical use and interpretation of group A streptococcal antibody tests: a practical approach for the pediatrician or primary care physician. Pediatr Infect Dis J 2002; 21:420–6; quiz 427. [DOI] [PubMed] [Google Scholar]
- 21. Parks T, Smeesters PR, Curtis N, Steer AC.. ASO titer or not? When to use streptococcal serology: a guide for clinicians. Eur J Clin Microbiol Infect Dis 2015; 34:845–9. [DOI] [PubMed] [Google Scholar]
- 22. Steer AC, Smeesters PR, Curtis N.. Streptococcal serology: secrets for the specialist. Pediatr Infect Dis J 2015; 34:1250–2. [DOI] [PubMed] [Google Scholar]
- 23. Bennett J, Moreland NJ, Williamson DA, et al. . Comparison of group A streptococcal titres in healthy children and those with pharyngitis and skin infections. J Infect 2022; 84:24–30. [DOI] [PubMed] [Google Scholar]
- 24. Oliver J, Wadu EM, Pierse N, Moreland NJ, Williamson DA, Baker MG.. Group A Streptococcus pharyngitis and pharyngeal carriage: a meta-analysis. PLoS Negl Trop Dis 2018; 12:e0006335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Whitcombe AL, Hanson-Manful P, Jack S, et al. . Development and evaluation of a new triplex immunoassay that detects group A Streptococcus antibodies for the diagnosis of rheumatic fever. J Clin Microbiol 2020; 58:e0030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Davies MR, McIntyre L, Mutreja A, et al. . Atlas of group A streptococcal vaccine candidates compiled using large-scale comparative genomics. Nat Genet 2019; 51:1035–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Di Benedetto R, Mancini F, Carducci M, et al. . Rational design of a glycoconjugate vaccine against group A Streptococcus. Int J Mol Sci 2020; 21:8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reglinski M, Lynskey NN, Choi YJ, Edwards RJ, Sriskandan S.. Development of a multicomponent vaccine for Streptococcus pyogenes based on the antigenic targets of IVIG. J Infect 2016; 72:450–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rivera-Hernandez T, Pandey M, Henningham A, et al. . Differing efficacies of lead group A streptococcal vaccine candidates and full-length M protein in cutaneous and invasive disease models. mBio 2016; 7:e00618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bennett J, Moreland NJ, Oliver J, et al. . Understanding group A streptococcal pharyngitis and skin infections as causes of rheumatic fever: protocol for a prospective disease incidence study. BMC Infect Dis 2019; 19:633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baker MG, Gurney J, Oliver J, et al. . Risk factors for acute rheumatic fever: literature review and protocol for a case-control study in New Zealand. Int J Environ Res Public Health 2019; 16:4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Atatoa-Carr P, Lennon D, Wilson N; New Zealand Rheumatic Fever Guidelines Writing Group. . Rheumatic fever diagnosis, management, and secondary prevention: a New Zealand guideline. N Z Med J 2008; 121:59–69. [PubMed] [Google Scholar]
- 33. Heart Foundation New Zealand. New Zealand guidelines for rheumatic fever: 1. Diagnosis, management and secondary prevention of acute rheumatic fever and rheumatic heart disease: 2014 Update. https://www.heartfoundation.org.nz/shop/marketing/non-stock-resources/diagnosis-management-rheumatic-fever-guideline.pdf. Accessed 21 May 2019.
- 34. Steer AC, Vidmar S, Ritika R, et al. . Normal ranges of streptococcal antibody titers are similar whether streptococci are endemic to the setting or not. Clin Vaccine Immunol 2009; 16:172–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2019. [Google Scholar]
- 36. Kolde R. pheatmap: pretty heatmaps. R package version 1.0.12, 2019. https://CRAN.R-project.org/package=pheatmap. Accessed 23 November 2021.
- 37. Martins TB, Hoffman JL, Augustine NH, et al. . Comprehensive analysis of antibody responses to streptococcal and tissue antigens in patients with acute rheumatic fever. Int Immunol 2008; 20:445–52. [DOI] [PubMed] [Google Scholar]
- 38. Karmarkar MG, Santosh K, Vineetha V, et al. . Human immune response to streptococcal C5a peptidase in Indian children and adults. Int Congr 2006; 1289:54–7. [Google Scholar]
- 39. Hysmith ND, Kaplan EL, Cleary PP, Johnson DR, Penfound TA, Dale JB.. Prospective longitudinal analysis of immune responses in pediatric subjects after pharyngeal acquisition of group A streptococci. J Pediatric Infect Dis Soc 2017; 6:187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Johnson DR, Kurlan R, Leckman J, Kaplan EL.. The human immune response to streptococcal extracellular antigens: clinical, diagnostic, and potential pathogenetic implications. Clin Infect Dis 2010; 50:481–90. [DOI] [PubMed] [Google Scholar]
- 41. Weiss GE, Traore B, Kayentao K, et al. . The Plasmodium falciparum-specific human memory B cell compartment expands gradually with repeated malaria infections. PLoS Pathog 2010; 6:e1000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yman V, White MT, Asghar M, et al. . Antibody responses to merozoite antigens after natural Plasmodium falciparum infection: kinetics and longevity in absence of re-exposure. BMC Med 2019; 17:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ferreira DM, Neill DR, Bangert M, et al. . Controlled human infection and rechallenge with Streptococcus pneumoniae reveals the protective efficacy of carriage in healthy adults. Am J Respir Crit Care Med 2013; 187:855–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Meyer TC, Michalik S, Holtfreter S, et al. . A comprehensive view on the human antibody repertoire against Staphylococcus aureus antigens in the general population. Front Immunol 2021; 12:651619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cannon JW, Zhung J, Bennett J, et al. . The economic and health burdens of diseases caused by group A Streptococcus in New Zealand. Int J Infect Dis 2021; 103:176–81. [DOI] [PubMed] [Google Scholar]
- 46. Frost HR, Laho D, Sanderson-Smith ML, et al. . Immune cross-opsonization within emm clusters following group A Streptococcus skin infection: broadening the scope of type-specific immunity. Clin Infect Dis 2017; 65:1523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kaplan EL, Anthony BF, Chapman SS, Ayoub EM, Wannamaker LW.. The influence of the site of infection on the immune response to group A streptococci. J Clin Invest 1970; 49:1405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Oliver J, Upton A, Jack SJ, Pierse N, Williamson DA, Baker MG.. Distribution of streptococcal pharyngitis and acute rheumatic fever, Auckland, New Zealand, 2010–2016. Emerg Infect Dis 2020; 26:1113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thomas S, Bennett J, Jack S, et al. . Descriptive analysis of group A Streptococcus in skin swabs and acute rheumatic fever, Auckland, New Zealand, 2010–2016. Lancet Reg Health West Pac 2021; 8:100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.