Abstract
Introduction and objectives
Dexmedetomidine (DEX) has been associated with a decrease in postoperative cognitive and behavioral dysfunction in patients submitted to general anesthesia, whether inhalation or total intravenous anesthesia. Consequently, the DEX effects on postoperative agitation and delirium in patients submitted to general anesthesia for non-cardiac surgery have been investigated.
Method
A systematic review and meta-analysis of randomized and double-blind clinical trials (RCTs) was undertaken assessing adults submitted to elective procedures under general anesthesia receiving DEX or placebo. The search included articles published in English in the Pubmed and Web of Science databases using keywords such as dexmedetomidine, delirium, and agitation. Duplicate publications, studies involving cardiac surgery or using active control (other than saline solution) were included. A random effects model was adopted using the DerSimonian-Laird method and estimate of Odds Ratio (OR) for dichotomous variables, and weighted mean difference for continuous variables, with their respective 95% Confidence Intervals (95% CI).
Results
Of the 484 articles identified, 15 were selected comprising 2,183 patients (1,079 and 1,104 patients in the DEX and control group, respectively). The administration of DEX was considered a protective factor for postoperative cognitive and behavioral dysfunction (OR = 0.36; 95% CI 0.23–0.57 and p < 0.001), regardless of the anesthesia technique used.
Conclusion
Dexmedetomidine administration reduced by at least 43% the likelihood of postoperative cognitive and behavioral dysfunction in adult patients submitted to general anesthesia for non-cardiac surgery.
Keywords: Dexmedetomidine, Delirium, Psychomotor agitation, General anesthesia, Meta-analysis
Introduction
Delirium is an acute inability to sustain attention associated with cognitive dysfunction, impairments in mental status and in sleep-wake cycle, as well as in behavior, which can lead the patient into a hypo or hyperactive state.1, 2 It has risk factors such as older age, postoperative period, past comorbidities, previous neurological deficits and sensory decline.1, 3 As reported both in pivotal studies and in more updated publications, delirium is associated with longer hospital length of stay, functional reduction, decreased likelihood of regaining autonomy, and greater morbidity and mortality.4, 5, 6 It is estimated that approximately 20% of hospitalized patients over 65 years of age develop postoperative delirium.1
Agitation is a mental state in which the patient is restless, uncooperative, and incoherent.7 It can be related to a paranoid ideation associated with time disorientation and misinterpretation of neurosensory stimuli, resulting from residual effect of anesthetic drugs.8 Postoperative agitation is associated with adverse events such as surgical site bleeding, accidental removal of drains or vascular catheters, and even damage to the performed surgery.9
Dexmedetomidine (DEX) is an α2 adrenoceptor agonist, with a selectivity ratio of 1600:1 (α2:α1). It acts on α2 receptors of the locus coeruleus promoting sedation, and on the spinal cord dorsal horn, decreasing the release of substance P and producing analgesia.10 DEX provides excellent sedation and analgesia, with minimal respiratory depression.11, 12 DEX is also associated with reduction in delirium and postoperative stress, and increased patient satisfaction.13
Decreased postoperative agitation14, 15 and delirium16, 17 has been attributed to DEX. The drug promotes a beneficial effect on morbidity and mortality associated with both conditions, which, in the present study, will be denominated postoperative cognitive and behavioral dysfunction. Therefore, the aim of this study was to evaluate the effects of dexmedetomidine on postoperative agitation and delirium in patients submitted to general anesthesia for non-cardiac surgery.
Method
A systematic review and meta-analysis of randomized and double-blind clinical trials (RCTs) was undertaken on the effects of DEX on postoperative cognitive and behavioral dysfunction in adult patients submitted to general anesthesia for non-cardiac surgery. This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines18 . The search in PubMed and Web of Science database included English language articles published between January 2013 and March 2020. The query was performed using the keywords “dexmedetomidine, delirium and agitation” or their synonyms, separated by AND and OR interlocutors with the following search strategy: (((((Deliri *) OR agitat *) AND dexmedetomidine))). A supplementary manual search of the references of the studies that complied with the inclusion criteria was performed, aiming to detect original articles not recovered previously.
Inclusion criteria comprised randomized, double-blind clinical trials with participants over 18 years of age, submitted to elective procedures under general anesthesia and receiving DEX or placebo. Duplicate articles, studies comprising cardiac surgery patients or studies that used active control (other than saline solution) were excluded.
Two independent investigators carried out a preliminary assessment of the titles/abstracts and extracted the data. After selecting the articles in conformity with the inclusion and exclusion criteria, a full-text reading was carried out. In case of disagreement, a third investigator made the final arbitration. Using a standardized form previously prepared by the authors, data were recorded on patient age, anesthesia technique, dosage and method administration of DEX, type of procedures and outcomes. For this study, the primary outcome was postoperative cognitive and behavioral dysfunction, comprising delirium and/or agitation (present or absent). Secondary outcomes included the awakening time and tracheal extubation time, estimated in minutes.
Sensitivity analysis was conducted to explore sources of heterogeneity among studies in the overall and in the subgroup analysis that evaluated cognitive outcome regarding patient age and anesthesia technique. Statistical heterogeneity was calculated using the chi-square method (χ2) and the Higgins test (I2).19 The presence of heterogeneity was deemed to occur when p < 0.05 and I2 ≥ 50%. The Odds Ratio (OR), with a 95% Confidence Interval (95% CI), was used to quantify the statistical difference between groups for dichotomous variables and Mean Difference (MD) for continuous variables (time in minutes). Following the qualitative analysis of the studies and statistical heterogeneity assessment, the random effects model was implemented using the DerSimonian-Laird20 method, and the statistical analysis was performed using the Comprehensive Meta-analyses® software v.3.3. Assessment of potential publication bias was performed by visual analysis of the funnel plot and by using the Begg21 and Egger22 tests. The statistical significance adopted was 5%. To assess the impact of the study, the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) tool was applied, using the GRADEpro GDT® software according to the methodological guidelines of the GRADE system provided by the Brazilian Ministry of Health.23
Results
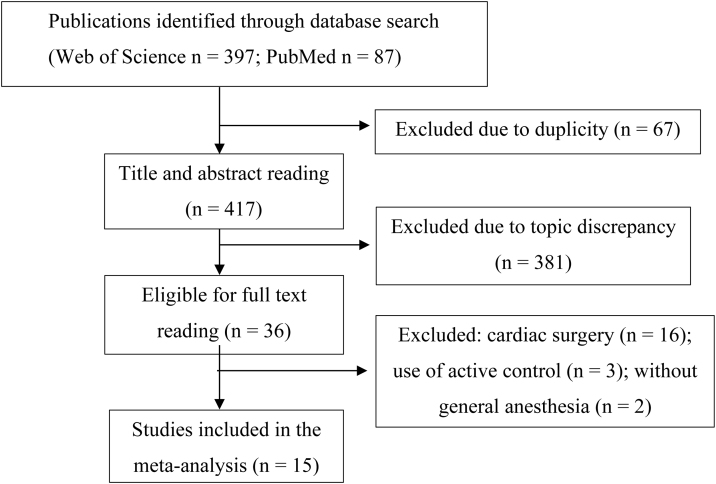
As depicted in Figure 1, 484 studies (397 on Web of Science and 87 on PubMed) were identified, from which 15 were selected to constitute this meta-analysis.
Figure 1.
Flowchart of the selected studies.
The 15 selected trials comprised 2,183 patients (1,079 in the intervention group and 1,104 in the control group). The characteristics are detailed in Table 1 14, 15, 16, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35 Seven studies were carried out in China, six in South Korea, one in the United States, and one in Iran.
Table 1.
Description of the selected studies.
| Year of publication of the study | Intervention | n | Age range (years) | Procedure |
|---|---|---|---|---|
| Kim 201324 | DEX 0.4 mcg.kg−1.h−1 | 50 | 20–58 | Nose surgery |
| Saline | 50 | |||
| Ham 201425 | DEX 0.1 mcg.kg−1 | 34 | 20–45 | Orthognathic surgery |
| Saline | 36 | |||
| Kim 201515 | DEX 0.4 mcg.kg−1.h−1 | 57 | ≥65 | Orthopedic surgery |
| Saline | 58 | |||
| Yang 201526 | DEX 0.5 mcg.kg−1.h−1 and 0.2–0.7 mcg.kg−1.h−1 | 39 | 18–80 | Oral and maxillofacial surgery |
| Saline | 40 | |||
| Ding 201527 | DEX 0.8 mcg.kg−1.h−1 | 20 | 45–80 | Cystectomy |
| Saline | 20 | |||
| Liu 201616 | DEX 0.2–0.4 mcg.kg−1.h−1 | 100 | 65–80 | Orthopedic surgery |
| Saline | 100 | |||
| Li 201628 | DEX 0.6 mcg.kg−1.h−1 | 30 | 18–65 | Open gastrectomy |
| Saline | 30 | |||
| Lee 201629 | DEX 0.1 mcg.kg−1.h−1 | 50 | ≥20 | Thoracoscopy |
| Saline | 50 | |||
| Kwon 201614 | DEX 0.5 mcg and 0.5 mcg.kg−1.h−1 | 30 | 30–80 | Transurethral resection of the prostate |
| Saline | 30 | |||
| Moshiri 201630 | DEX 0.5 mcg.kg−1.h−1 | 25 | 18–50 | Electroconvulsive therapy |
| Saline | 25 | |||
| Song 201631 | DEX 0.5 mcg.kg−1.h−1 | 25 | 18–60 | Craniotomy |
| Saline | 27 | |||
| Deiner 201732 | DEX 0.5 mcg.kg−1.h−1 | 189 | ≥68 | Non-cardiac elective surgery |
| Saline | 201 | |||
| Lee 201833 | DEX 0.1 mcg.kg−1 and 0.2–0.7 mcg.kg−1.h−1 | 95 | ≥65 | Non-cardiac surgery |
| Saline | 109 | |||
| Tang 201834 | DEX 0.1 mcg.kg−1 and 0.3 mcg.kg−1.h−1 | 54 | 18–70 | Neurosurgery |
| Saline | 52 | |||
| Sun 201935 | DEX 0.1 mcg.kg−1.h−1 | 281 | ≥65 | Non-cardiac surgery |
| Saline | 276 | |||
| Total | 2,183 | |||
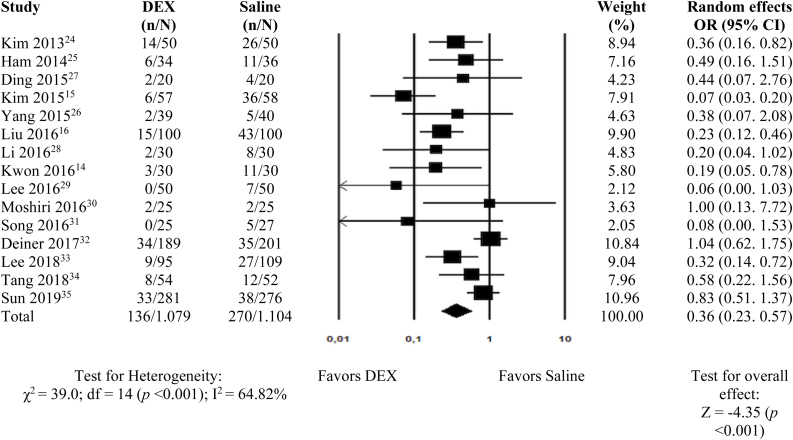
Postoperative cognitive and behavioral dysfunctions were assessed in the 15 studies,14, 15, 16, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35 and as shown in Fig. 2, the administration of DEX was considered to be a protective factor (OR = 0.36; 95% CI 0.23–0.57 and p < 0.001). Postoperative delirium and agitation were separately evaluated, and DEX was effective in reducing both complications (6 studies16, 27, 32, 33, 34, 35; OR = 0.53; 95% CI 0.31–0.92 and p = 0.023; and 9 studies14, 15, 24, 25, 26, 28, 29, 30, 31; OR = 0.24; 95% CI 0.13–0.42 and p < 0.001, respectively).
Figure 2.
Meta-analysis of the effect of dexmedetomidine on postoperative cognitive and behavioral dysfunction in adults submitted to general anesthesia for non-cardiac surgery. df, degrees of freedom.
Fig. 3 depicts the age subgroup analysis (adults under 60-years old vs. elderly above 60 years old), and it shows that the administration of DEX reduces the rate of postoperative cognitive and behavioral dysfunction in adults under 60-years of age (4 studies24, 25, 30, 31; OR = 0.40; 95% CI 0.22–0.75 and p = 0.004), and in the elderly (5 studies15, 16, 32, 33, 35; OR = 0.52; 95% CI 0.39–0.69 and p = 0.018).
Figure 3.
Meta-analysis of the effect of dexmedetomidine on postoperative cognitive and behavioral dysfunction according to age subgroups and type of anesthesia in adults submitted to general anesthesia for non-cardiac surgery. df, degrees of freedom.
Regarding anesthesia technique subgroups (intravenous vs. inhalational), two studies28, 35 were excluded because they involved patients undergoing both techniques indistinctly. Conversely, one study15 was included in the sub-analysis of both techniques, as they were discriminated between groups. DEX was shown to be beneficial against postoperative cognitive and behavioral dysfunction in both procedures: intravenous anesthesia (5 studies15, 16, 28, 30, 31; OR = 0.18; 95% CI 0.08–0.40 and p < 0.001) and inhalational anesthesia (9 studies14, 15, 24, 25, 26, 27, 29, 33, 34 OR = 0.28; 95% CI 0.17–0.47 and p < 0.001) (Fig. 3).
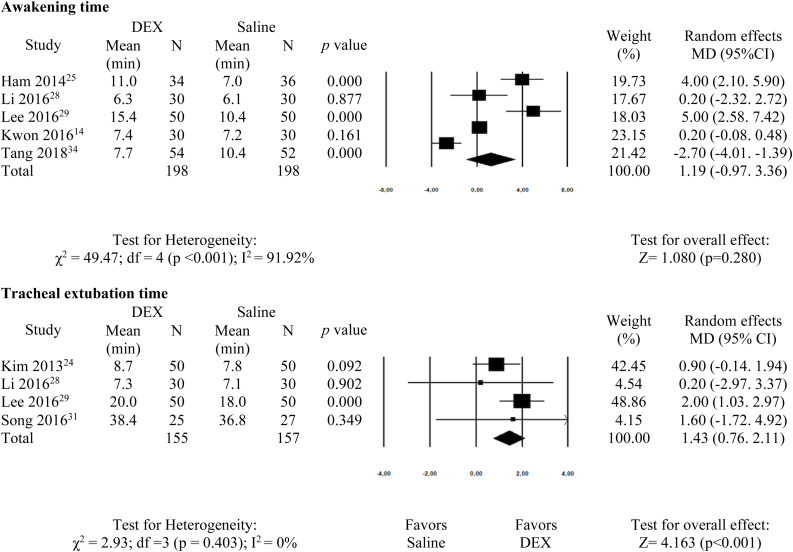
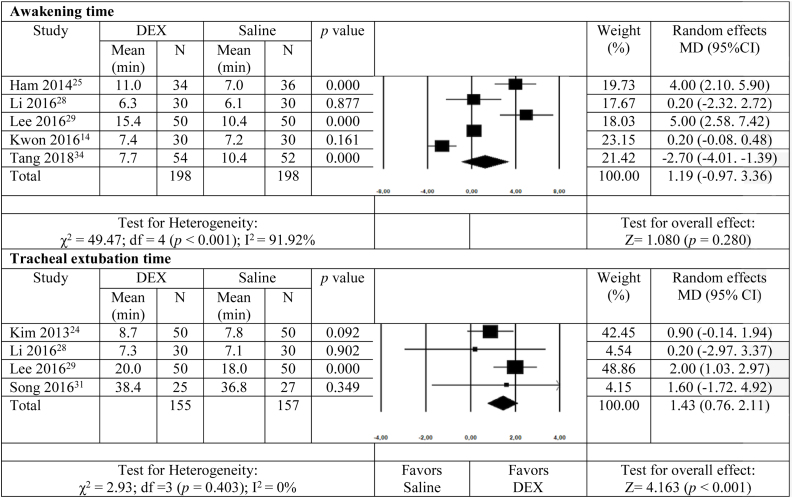
Awakening time was assessed in five studies14, 25, 28, 29, 34 and there was no significant difference between groups (MD = 1.19; 95% CI −0.97–3.36; p = 0.280). Fig. 4 shows that tracheal extubation time was different between groups, with a lengthier duration for the DEX group (4 studies24, 28, 29, 31; MD = 1.43; 95% CI 0.76–2.11; p < 0.001).
Figure 4.
Meta-analysis of the effect of dexmedetomidine on awakening and tracheal extubation time in adults submitted to general anesthesia for non-cardiac surgery. df, degrees of freedom.
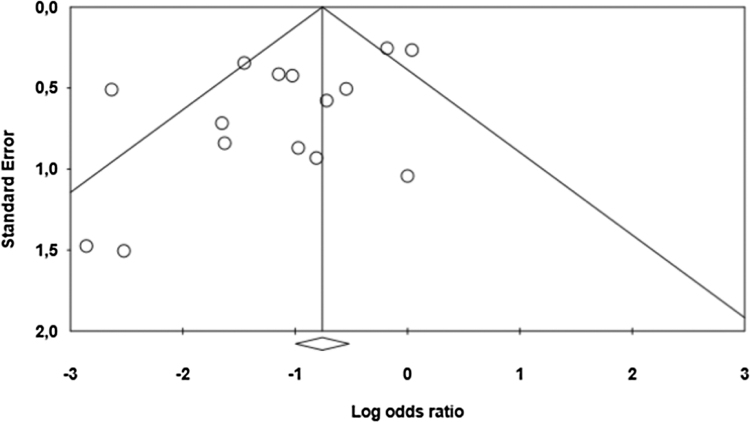
The funnel plot analysis (Fig. 5) shows an asymmetry with an absence of studies with small and medium samples to the right of the summary of findings. However, the Begg (p = 0.458) and Egger (p = 0.050) tests refute the hypothesis of publication bias. The evaluation by the GRADE protocol demonstrated that the study has a high quality of evidence.
Figure 5.
Funnel plot of the effect of dexmedetomidine on cognitive and behavioral dysfunction in adults submitted to general anesthesia for non-cardiac surgery.
Discussion
This meta-analysis included 15 randomized clinical trials published between 2013 and 2019, evaluating the effect of DEX on cognitive and behavioral dysfunction in the postoperative period of adult patients submitted to general anesthesia.
Variations in the dosage and method administration of DEX were observed, with studies administering DEX throughout the intraoperative period, while others used it only at anesthetic induction, and others that maintained the drug postoperatively. Such studies have achieved similar results regarding the incidence rate of postoperative cognitive and behavioral dysfunction, suggesting that there is no recommended time for the administration of DEX regarding its beneficial effect against such events.
Delirium has multiple causes and the most effective prevention strategies comprise pharmacological and non-pharmacological approaches.1, 2, 3 Among the pharmacological options, DEX was effective in reducing the delirium rate when compared to placebo.16, 33
This meta-analysis shows DEX as a protective factor against delirium and postoperative agitation in adults submitted to general anesthesia for non-cardiac surgery and a similar result has been found by other authors.15, 24, 26, 28 Although the actual mechanism for this effect remains unknown, the analgesic and sedative effects of DEX are thought to be contributing factors.13, 36 Studies have suggested a perioperative anti-inflammatory activity of DEX, as they reported a decrease in plasma concentration of interleukin 6 and tumor necrosis factor alpha.37 There is also evidence that perioperative administration of DEX decreases plasma concentration of neuron-specific enolase and S100β protein, both neural injury biomarkers , and promotes a neuroprotective effect.34 Therefore, one should consider that DEX, additionally to its known action at the locus coeruleus, has inherent neurocytological protection activity,28, 34, 37 reducing postoperative agitation and delirium and thus contributing to preservation of preoperative neurological status.32
Elderly people are more prone to delirium and cognitive dysfunction postoperatively, nonetheless DEX acted as a beneficial anesthetic adjunct agent as it prevented these events in both age subgroups analyzed, adults under 60-years old and the elderly . This result was also found elsewhere, but this time in trials assessing the DEX effect in patients submitted to cardiac and non-cardiac surgery.38 Thus, DEX effectiveness in preventing cognitive and behavioral dysfunction revealed to be age independent.
Regarding the analysis by subgroups of anesthetic technique, DEX showed to act as a protective factor for cognitive and behavioral dysfunction in both intravenous and inhalation general anesthesias. This finding reinforces the concepts that lessening the endocrine-metabolic stress and providing analgesia play a more prominent role than choosing the anesthetic technique itself.39
This study revealed no difference in awakening time with administration of DEX, which is in conflict with the literature.25, 29 Due to its elimination half-life of 2 to 2.5 hours and an upward context-sensitive half-life, DEX sustains sedative activity through its sympatholytic properties.40 Additionally, due to DEX capability of intensifying the effects of other analgesics simultaneously administered,41 there exists the likelihood of extending anesthesia effects and thus prolonging awakening time. Conversely, a significant difference was found in tracheal extubation time, but with an irrelevant clinical significance, a difference of less than two minutes. This effect coincides with the literature.38 The reason for not observing a greater difference may lie in the heterogeneity of the methods and timing of drug administration described in the primary studies included here.
The quality of a meta-analysis depends on the selection of relevant studies, heterogeneity, and detection bias.18 Despite the different strategies adopted in this study to minimize possible biases, they cannot be ruled out. A search was performed using two important databases, complemented by manual search, and the selected works were submitted to the appreciation of two independent investigators. Also, only clinical, double-blind, randomized trials were included. The present study adds an important contribution in demonstrating the protective effect of DEX against cognitive and behavioral dysfunction in patients submitted to non-cardiac surgery. Other systematic reviews with meta-analysis can be found in the literature, however, they include patients admitted in intensive care units, undergoing cardiac surgery or only in elderly patients.38, 42, 43
The authors of this meta-analysis considered that the studies were heterogeneous, due to the variation of DEX dose between groups, and the type of surgery the patients were submitted to. Nonetheless, the heterogeneity was tested and confirmed with a high χ2 value and an I2 greater than 64%, indicating medium heterogeneity.
As according to the GRADE protocol,23 the authors considered that the study has no significant publication bias, since the query was conducted using two important databases and was complemented by manual analysis of the references. The GRADE outcome indicates that the study has a considerable impact and high consistency concerning the DEX protection effect on postoperative cognitive and behavioral dysfunction.
DEX provides reliable hemodynamic stability and has been increasingly administered in anesthetic practice due to its straightforward use in the pre-anesthetic, intraoperative and even postoperative period, as well as its wide range of dosage formulations.11 In addition to the beneficial effects on cognitive and behavioral dysfunction evaluated in this study, there is a reported sparing effect on the use of volatile agents and opioids.31
Conclusion
This systematic review with meta-analysis reveals that the administration of dexmedetomidine reduced from 43% to 77% the likelihood of cognitive and behavioral dysfunction in the postoperative period of adult patients submitted to general anesthesia for non-cardiac surgery.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Inouye S.K. Delirium in older persons. N Engl J Med. 2006;354:1157–1165. doi: 10.1056/NEJMra052321. [DOI] [PubMed] [Google Scholar]
- 2.Fitzpatrick S., Owen K. 2018. Postoperative cognitive disorders: postoperative delirium and postoperative cognitive dysfunction. Anaesthesia tutorial of the week. Available at: https://www.wfsahq.org/components/com_virtual_library/media/8c6f8f69fda03c9f78a3eca904980fa6-atow-385-00-01.pdf. [Google Scholar]
- 3.Müller A., Lachmann G., Wolf A., et al. Peri- and postoperative cognitive and consecutive functional problems of elderly patients. Curr Opin Crit Care. 2016;22:406–411. doi: 10.1097/MCC.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 4.Moller J.T., Cluitmans P., Rasmussen L.S., et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International study of post-operative cognitive dysfunction. Lancet. 1998;351:857–861. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 5.Schenning K.J., Deiner S.G. Postoperative delirium in the geriatric patient. Anesthesiol Clin. 2015;33:505–516. doi: 10.1016/j.anclin.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iamaroon A., Wongviriyawong T., Sura-arunsumrit P., et al. Incidence of and risk factors for postoperative delirium in older adult patients undergoing noncardiac surgery: a prospective study. BMC Geriatr. 2020;20:40. doi: 10.1186/s12877-020-1449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vlajkovic G.P., Sindjelic R.P. Emergence delirium in children: many questions, few answers. Anesth Analg. 2007;104:84–91. doi: 10.1213/01.ane.0000250914.91881.a8. [DOI] [PubMed] [Google Scholar]
- 8.Wells L.T., Rasch D.K. Emergence “delirium” after sevoflurane anesthesia: a paranoid delusion? Anesth Analg. 1999;88:1308–1310. doi: 10.1097/00000539-199906000-00020. [DOI] [PubMed] [Google Scholar]
- 9.Singh R., Sood N., Chatterji C., et al. Comparative evaluation of incidence of emergence agitation and post-operative recovery profile in paediatric patients after isoflurane, sevoflurane and desflurane anaesthesia. Indian J Anaesth. 2012;56:156–161. doi: 10.4103/0019-5049.96325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villela N.R., Nascimento Junior P. Uso de dexmedetomidina em anestesiologia. Rev Bras Anestesiol. 2003;53:97–113. doi: 10.1590/s0034-70942003000100013. [DOI] [PubMed] [Google Scholar]
- 11.Cooper L., Candiotti K., Gallagher C., et al. A randomized, controlled trial on dexmedetomidine for providing adequate sedation and hemodynamic control for awake, diagnostic transesophageal echocardiography. J Cardiothorac Vasc Anesth. 2011;25:233–237. doi: 10.1053/j.jvca.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Candiotti K.A., Bergese S.D., Bokesch P.M., et al. Monitored anesthesia care with dexmedetomidine: a prospective, randomized, double-blind, multicenter trial. Anesth Analg. 2010;110:47–56. doi: 10.1213/ane.0b013e3181ae0856. [DOI] [PubMed] [Google Scholar]
- 13.Shehabi Y., Grant P., Wolfenden H., et al. Prevalence of delirium with dexmedetomidine compared with morphine based therapy after cardiac surgery: a randomized controlled trial (DEXmedetomidine Compared to Morphine-DEXCOM Study) Anesthesiology. 2009;111:1075–1084. doi: 10.1097/ALN.0b013e3181b6a783. [DOI] [PubMed] [Google Scholar]
- 14.Kwon S.Y., Joo J.D., Cheon G.Y., et al. Effects of dexmedetomidine infusion on the recovery profiles of patients undergoing transurethral resection. J Korean Med Sci. 2016;31:125–130. doi: 10.3346/jkms.2016.31.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim D.J., Kim S.H., So K.Y., et al. Effects of dexmedetomidine on smooth emergence from anaesthesia in elderly patients undergoing orthopaedic surgery. BMC Anesthesiol. 2015;15:139. doi: 10.1186/s12871-015-0127-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y., Ma L., Gao M., et al. Dexmedetomidine reduces postoperative delirium after joint replacement in elderly patients with mild cognitive impairment. Aging Clin Exp Res. 2016;28:729–736. doi: 10.1007/s40520-015-0492-3. [DOI] [PubMed] [Google Scholar]
- 17.Su X., Meng Z.T., Wu X.H., et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet. 2016;388:1893–1902. doi: 10.1016/S0140-6736(16)30580-3. [DOI] [PubMed] [Google Scholar]
- 18.Moher D., Liberati A., Tetzlaff J., et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 20.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 22.Egger M., Davey Smith G., Schneider M., et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brasil . Ministério da Saúde; Brasília: 2014. Ministério da Saúde. Secretaria de Ciência, Tecnologia e Insumos Estratégicos. Diretrizes metodológicas: sistema GRADE – Manual de graduação da qualidade da evidência e força de recomendação para tomada de decisão em saúde; p. 72. http://bvsms.saude.gov.br/bvs/publicacoes/diretrizes_metodologicas_sistema_grade.pdf [acessado em 18 de outubro de 2019] [Google Scholar]
- 24.Kim S.Y., Kim J.M., Lee J.H., et al. Efficacy of intraoperative dexmedetomidine infusion on emergence agitation and quality of recovery after nasal surgery. Br J Anaesth. 2013;111:222–228. doi: 10.1093/bja/aet056. [DOI] [PubMed] [Google Scholar]
- 25.Ham S.Y., Kim J.E., Park C., et al. Dexmedetomidine does not reduce emergence agitation in adults following orthognathic surgery. Acta Anaesthesiol Scand. 2014;58:955–960. doi: 10.1111/aas.12379. [DOI] [PubMed] [Google Scholar]
- 26.Yang X., Li Z., Gao C., et al. Effect of dexmedetomidine on preventing agitation and delirium after microvascular free flap surgery: a randomized, double-blind, control study. J Oral Maxillofac Surg. 2015;73:1065–1072. doi: 10.1016/j.joms.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Ding L., Zhang H., Mi W., et al. Effects of dexmedetomidine on anesthesia recovery period and postoperative cognitive function of patients after robot-assisted laparoscopic radical cystectomy. Int J Clin Exp Med. 2015;8:11388–11395. [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y., Wang B., Zhang L.L., et al. Dexmedetomidine combined with general anesthesia provides similar intraoperative stress response reduction when compared with a combined general and epidural anesthetic technique. Anesth Analg. 2016;122:1202–1210. doi: 10.1213/ANE.0000000000001165. [DOI] [PubMed] [Google Scholar]
- 29.Lee S.H., Lee C.Y., Lee J.G., et al. Intraoperative dexmedetomidine improves the quality of recovery and postoperative pulmonary function in patients undergoing video-assisted thoracoscopic surgery: a CONSORT-prospective, randomized, controlled trial. Medicine. 2016;95:e2854. doi: 10.1097/MD.0000000000002854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moshiri E., Modir H., Bagheri N., et al. Premedication effect of dexmedetomidine and alfentanil on seizure time, recovery duration, and hemodynamic responses in electroconvulsive therapy. Ann Card Anaesth. 2016;19:263–268. doi: 10.4103/0971-9784.179618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song J., Ji Q., Sun Q., et al. The opioid-sparing effect of intraoperative dexmedetomidine infusion after craniotomy. J Neurosurg Anesthesiol. 2016;28:14–20. doi: 10.1097/ANA.0000000000000190. [DOI] [PubMed] [Google Scholar]
- 32.Deiner S., Luo X., Lin H.M., et al. Intraoperative infusion of dexmedetomidine for prevention of postoperative delirium and cognitive dysfunction in elderly patients undergoing major elective noncardiac surgery: a randomized clinical trial. JAMA Surg. 2017;152:e171505. doi: 10.1001/jamasurg.2017.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee C., Lee C.H., Lee G., et al. The effect of the timing and dose of dexmedetomidine on postoperative delirium in elderly patients after laparoscopic major non-cardiac surgery: a double blind randomized controlled study. J Clin Anesth. 2018;47:27–32. doi: 10.1016/j.jclinane.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Tang C., Juan L., Zhe-tao Z., et al. Neuroprotective effect of bispectral index-guided fast-track anesthesia using sevoflurane combined with dexmedetomidine for intracranial aneurysm embolization. Neural Regen Res. 2018;13:280–288. doi: 10.4103/1673-5374.226399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun Y., Jiang M., Ji Y., et al. Impact of postoperative dexmedetomidine infusion on incidence of delirium in elderly patients undergoing major elective noncardiac surgery: a randomized clinical trial. Drug Des Dev Ther. 2019;13:2911–2922. doi: 10.2147/DDDT.S208703. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Amorim M.A.S., Govêia C.S., Magalhães E., et al. Effect of dexmedetomidine in children undergoing general anesthesia with sevoflurane: a meta-analysis. Rev Bras Anestesiol. 2017;67:193–198. doi: 10.1016/j.bjan.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 37.Chen W., Bo L., Feng Z., et al. The effects of dexmedetomidine on post-operative cognitive dysfunction and inflammatory factors in senile patients. Int J Clin Exp Med. 2015;8:4601–4605. [PMC free article] [PubMed] [Google Scholar]
- 38.Duan X., Coburn M., Rossaint R., et al. Efficacy of perioperative dexmedetomidine on postoperative delirium: systematic review and meta-analysis with trial sequential analysis of randomised controlled trials. Br J Anaesth. 2018;121:384–397. doi: 10.1016/j.bja.2018.04.046. [DOI] [PubMed] [Google Scholar]
- 39.Aldecoa C., Bettelli G., Bilotta F., et al. European society of anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesthesiol. 2017;34:192–214. doi: 10.1097/EJA.0000000000000594. [DOI] [PubMed] [Google Scholar]
- 40.Scheinin H., Aantaa R., Antitila M., et al. Reversal of the sedative and sympatholytic effects of dexmedetomidine with a specific alpha2-adrenoceptor antagonist atipamezole: a pharmacodynamic and kinetic study in healthy volunteers. Anesthesiology. 1998;89:574–584. doi: 10.1097/00000542-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Lin T.F., Yeh Y.C., Lin F.S., et al. Effect of combining dexmedetomidine and morphine for intravenous patient-controlled analgesia. Br J Anaesth. 2009;102:117–122. doi: 10.1093/bja/aen320. [DOI] [PubMed] [Google Scholar]
- 42.Ng K.T., Shubash C.J., Chong J.S. The effect of dexmedetomidine on delirium and agitation in patients in intensive care: systematic review and meta-analysis with trial sequential analysis. Anaesthesia. 2019;74:380–392. doi: 10.1111/anae.14472. [DOI] [PubMed] [Google Scholar]
- 43.Janssen T.L., Alberts A.R., Hooft L., et al. Prevention of postoperative delirium in elderly patients planned for elective surgery: systematic review and meta-analysis. Clin Interv Aging. 2019;14:1095–1117. doi: 10.2147/CIA.S201323. [DOI] [PMC free article] [PubMed] [Google Scholar]