Abstract
The Physical Activity Guidelines recommend performing 150 minutes of moderate-to-vigorous intensity aerobic physical activity (MVPA) per week. These guidelines also recommend muscle-strengthening physical activity (MSPA) on ≥2 days per week for additional benefits including muscular fitness and bone health. The majority of the scientific evidence supporting the PA recommendations for health comes from studies of MVPA while the possible contributions of MSPA in these findings have been overlooked historically. Emerging evidence suggests that MSPA can independently protect against major cardiometabolic risk factors, chronic diseases, and mortality. Additional data from clinical trials indicate that many of the well-known health benefits of exercise, like improvements in cardiovascular disease risk factors, are more robust with combined MVPA and MSPA. This review will clarify the relative benefits of MSPA versus MVPA on health-related outcomes to determine the best type of PA for health.
Keywords: Aerobic, resistance, muscle-strengthening, physical activity, mortality
Summary Statement
Combined aerobic and muscle-strengthening physical activity provides superior and more complete health benefits than either activity alone.
Introduction
Individuals who meet the physical activity (PA) guidelines live 3–4 years longer on average than their inactive counterparts (1). While impressive, these longevity estimates are generated from studies that assessed mortality outcomes among people who actually met only half the PA guidelines—the aerobic half. The United States (US) PA guidelines recommend that adults perform 150 minutes per week of moderate-intensity (e.g., 3.0–5.9 metabolic equivalents); 75 minutes per week of vigorous-intensity (e.g., ≥6.0 metabolic equivalents); or an equivalent combination of moderate-to-vigorous intensity aerobic PA (MVPA; e.g. brisk walking, running, cycling) for substantial health benefits (2). However, there is another “muscle-strengthening half” of the guidelines that states, “muscle-strengthening activities provide additional benefits not found with aerobic activity [including] increased bone strength and muscular fitness” (2). While the musculoskeletal health benefits of muscle-strengthening PA (MSPA; weight or resistance exercise training) are undisputed, particularly for functional outcomes in older adults (3), emerging evidence suggests that meeting the MSPA guidelines of ≥2 days per week contributes to similar cardiometabolic health benefits as MVPA (4). Despite the new developments in MSPA research, several questions remain unanswered, particularly because MVPA and MSPA are usually studied in isolation. What are the health benefits of meeting just the “other MSPA half” of the guidelines? Can people who only perform MSPA still experience the well-known health benefits of MVPA? Is either type of PA sufficient on its own or do they act synergistically for maximal health benefits? This review will summarize the evidence from studies that have directly compared the associations of MVPA, MSPA, or combined MVPA and MSPA with major health outcomes to determine the best type of PA for optimal health.
MVPA versus MSPA on Mortality
Five prospective studies conducted in nationally representative samples have compared the associations between MVPA and MSPA and the risk of all-cause mortality. These studies found a comparable 15%–35% reduced risk of all-cause mortality among participants who met the MVPA guidelines only (5–9). These same studies indicate that there is a 10%–25% reduced risk of all-cause mortality among participants who met the MSPA guidelines only compared to those who met neither guideline (6–9). However, one study found no association between participation in MSPA only and mortality. This finding is possibly because the group who reported MSPA only at baseline was also more likely to report ≥1 chronic health condition (e.g., diabetes, hypertension) at baseline than the MVPA only or combined groups (5). Importantly, these studies show a 30–45% reduced risk of mortality among participants who met both guidelines compared to those who met neither guideline (5–10), suggesting the largest health benefits are observed with combined MSPA and MVPA.
Cardiovascular disease (CVD) and cancer are the two leading non-communicable causes of death in the US (11). A 20–35% reduced risk of CVD mortality has been found among groups who met the MVPA guidelines only compared to those who met neither guideline (6–9). Two studies have also reported a 20%–30% reduced risk of CVD mortality among groups who met only the MSPA guidelines (6,7). In contrast, two other studies reported non-significant associations between individuals who met only the MSPA guidelines and CVD mortality (8,9). However, one of these studies categorized their MSPA into groups that performed “any” or “no” MSPA as opposed to meeting the MSPA guidelines of ≥2 days per week, making it difficult to draw conclusions on meeting the MSPA guidelines with CVD mortality (8). Consistent with studies on all-cause mortality, the strongest relationships between PA patterns and CVD mortality were observed among those who met both PA guidelines, with risk reductions for CVD mortality ranging from 45–60% compared to those who met neither guideline (6–8,10).
The comparative associations between PA patterns and total cancer mortality in a general adult population are less consistent. A 20%–25% reduced risk of cancer mortality has been found among groups who met the MVPA guidelines only (6,7); although other studies have found trivial, non-significant associations between meeting MVPA guidelines and cancer mortality (8,9). Similarly, there was a 15%–35% reduced risk of cancer mortality among those who met the MSPA guidelines only compared to those who met neither guideline (6,7,9). Finally, among those who met both guidelines, there was a 30%–40% reduced risk of cancer mortality compared to those who met neither guideline (6,7,9). Kamada et al. found non-significant risk reductions of 3%–9% between any type of PA participation and cancer mortality (8). However, we urge caution when interpreting this finding, as it is likely that their relatively smaller sample size (N=28,879) created lower statistical power for rarer outcomes such as cancer, particularly as the other studies with significant findings had much larger samples (N= 80,306–479,856) (6,7,9).
In summary, the prospective associations between MVPA and MSPA and mortality outcomes have been largely consistent among the handful of studies conducted to date. Meeting either guideline has been associated with 10%–35% reduced risks of all-cause, CVD, and cancer mortality, although some studies have also found non-significant associations for either MVPA or MSPA alone for various mortality outcomes. However, as shown in Figure 1, meeting both guidelines has been associated consistently with the largest risk reductions for mortality.
Figure 1:
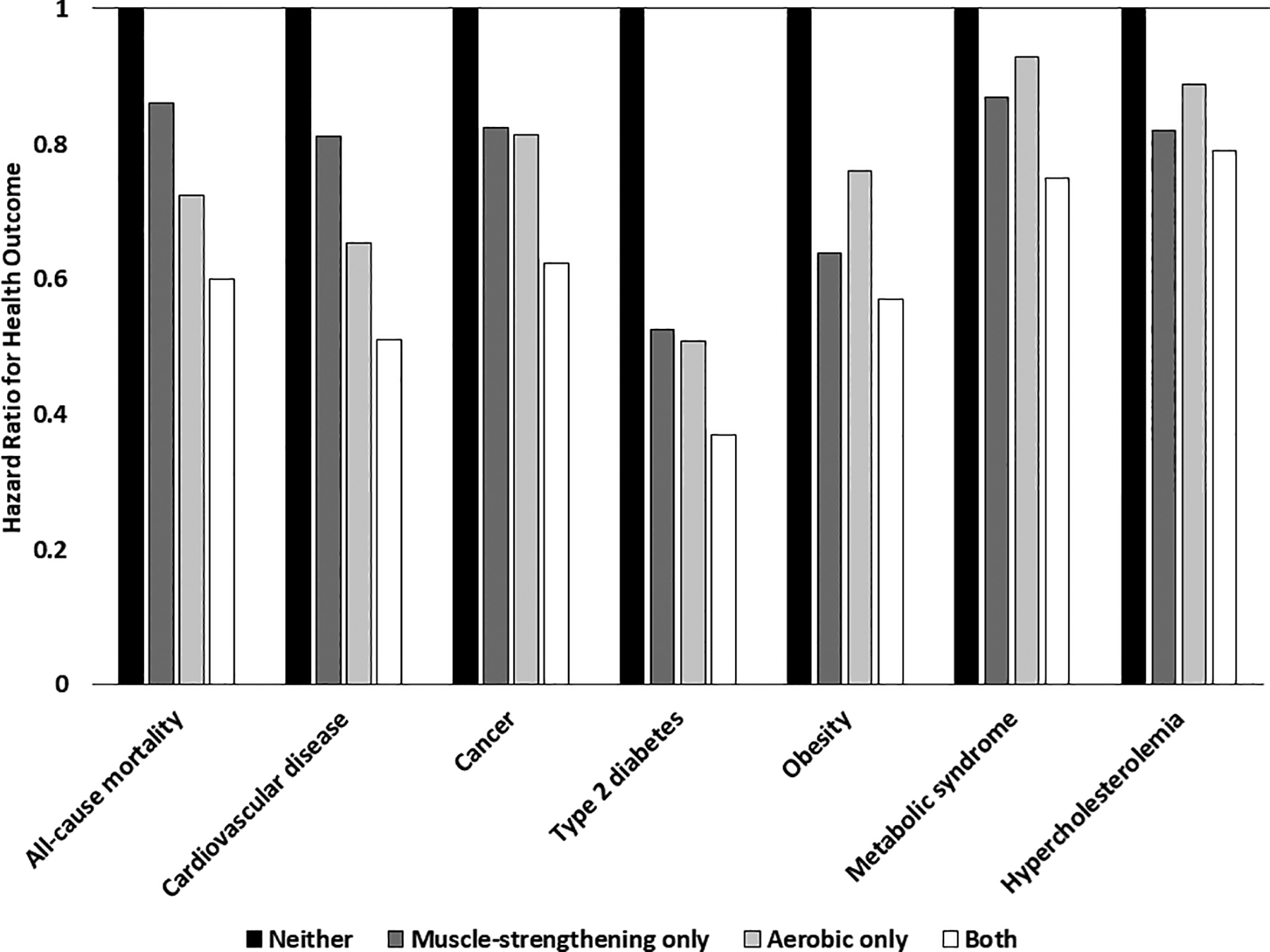
Associations of meeting different components of the physical activity guidelines with various health outcomes from prospective studies
MVPA versus MSPA on Chronic Diseases
Beyond mortality outcomes, cross-sectional studies have found that both MSPA and MVPA have similar, yet independent, associations with a variety of cardiometabolic risk factors. For example, there has been a lower prevalence of obesity reported among participants who met only the MSPA guidelines (11–14) or MVPA guidelines (12,14), while combined MVPA and MSPA has been dose-dependently associated with the lowest prevalence rates of obesity (15). There was also a lower prevalence of dyslipidemia (i.e., high low-density lipoprotein [LDL] cholesterol or low high-density lipoprotein [HDL] cholesterol), high blood pressure, or high triglycerides found among groups who met the MVPA only or both guidelines, but not among those who met only the MSPA guidelines (13,14,16). The prevalence of metabolic syndrome, which is a chronic condition that is defined by a cluster of cardiometabolic risk factors, was also lower among groups who met the MVPA only or both guidelines, but not MSPA only, compared to those who met neither guidelines (13,14). Although a causal relationship has yet to be established, these initial cross-sectional studies provide evidence that MVPA or combined MVPA and MSPA may be linked to more favorable cardiometabolic health than MSPA only.
Findings from prospective studies provide additional evidence that combined MVPA and MSPA may be superior for reducing the risk of chronic diseases. Obesity is a chronic condition that often precedes other chronic conditions including hypercholesterolemia, metabolic syndrome, and type 2 diabetes (17). Our recent longitudinal study showed that meeting either the MVPA only or MSPA only guidelines was associated with a 20–30% reduced risk of obesity defined by body mass index, waist circumference, or percent body fat (18). However, meeting both guidelines was associated with a 35–50% reduced risk of obesity (18). Interestingly, when comparing the group who met both guidelines with the group who met only the MVPA guidelines, there was a further 30% reduced risk of obesity defined by waist circumference or percent body fat, suggesting that among those who did MVPA, the addition of MSPA may help maintain a favorable body composition (18). Regarding other chronic disease risk factors, two longitudinal studies by Bakker et al. found that compared to the group that met neither guideline, only the group that met both guidelines, but not the MVPA only or MSPA only groups, had a 25% reduced risk of metabolic syndrome and a 21% reduced risk of hypercholesterolemia (19,20).
Type 2 diabetes is one of the fastest-growing chronic health conditions globally (21). Prospective studies with exposures based on meeting the different components of the PA guidelines have found a 40–67% reduced risk of type 2 diabetes among those who perform both MVPA and MSPA compared to inactivity in both types of PA (22–24). Similar risk reductions ranging from 40–60% have been reported in these studies among individuals who performed only MVPA (22–24). The associations among those who met only the MSPA guidelines of ≥2 days per week with type 2 diabetes were difficult to determine since the PA questionnaire items used in these studies did not capture the frequency of MSPA. When examining time spent in MSPA, there was a 50–60% reduced risk of diabetes among men who performed ≥150 minutes of MSPA per week, and this risk reduction was similar in men who did and did not meet MVPA guidelines (22). They also found a similar 50% risk reduction for diabetes among women who performed ≥60 minutes per week of MSPA (23). Conversely, a later study by Shiroma et al. found no significant associations for diabetes risk among a cohort of women who performed only MSPA (hazard ratio [95% confidence interval] = 0.21 [0.03–1.50]) (24). However, there was only one case of diabetes in this group during the follow-up period, thus making it difficult to perform any meaningful statistical analyses in this group (24). In summary, prospective studies indicate that participating in both MVPA and MSPA reduces the risk of obesity, hypercholesterolemia, metabolic syndrome, and type 2 diabetes, with slightly weaker and sometimes non-significant associations observed when either activity is performed alone.
MVPA versus MSPA in Clinical Exercise Trials
While observational studies suggest that combined MVPA and MSPA are associated with the most favorable health outcomes, there are several inherent limitations in these studies. First, reliance on self-reported PA, often collected at baseline only, continues to be a challenge, particularly due to the complete lack of assessment or crude level of detail captured for MSPA in many large cohorts. Second, the rapid incorporation of sophisticated aerobic PA monitors (e.g., Actigraph accelerometers) into large cohorts further widens this divide between the assessment of MVPA versus MSPA and limits the future development of more nuanced MSPA guidelines since there is no equivalent device to objectively measure MSPA. Finally, a large confounder not easily addressed in most observational research is that the groups meeting both guidelines are likely performing a greater total volume of exercise, which could explain the consistently stronger associations observed with combined PA. Total exercise volume may be a critical confounder when examining the associations between MSPA and MVPA with health outcomes that are related to energy balance such as obesity or diabetes, but it may be relatively less important to consider for other outcomes (e.g., blood pressure) (25). Beyond total exercise volume, it is also likely that individuals who meet both guidelines are more health-conscious in other areas (e.g., better diet quality, non-smoker) or may have better access to resources (e.g., financial means for a gym membership) that allow them to follow the guidelines fully (26), but these factors also greatly confound the relationship between PA and health. Because of these limitations in observational research, large clinical exercise trials generally provide the most reliable evidence to determine the comparative and combined effects of MVPA and MSPA on health.
The formative exercise trials described below all followed a similar design in that participants were randomly assigned to resistance exercise only (i.e., MSPA), aerobic exercise only (i.e., MVPA), combined aerobic and resistance exercise (i.e., MSPA and MVPA), or a no-exercise control group for ≥6 months. Exercise prescriptions were also comparable across these trials. In general, the resistance exercise groups attended 3 sessions per week and performed 2–3 sets of 8–12 repetitions on 7–9 machines targeting the upper and lower body with prescribed weights increasing when the participant could complete all repetitions with good form. The aerobic groups also attended 3 sessions per week and performed 45–60 minutes of aerobic exercise at a moderate-to-vigorous intensity (i.e., 65–80% of VO2peak or 75–85% of maximum heart rate). In 251 adults with type 2 diabetes, Sigal et al. found significant reductions in glycated hemoglobin (HbA1c; an indicator of long-term blood glucose levels) in all three exercise groups, with the greatest reductions observed in the combination group (27). Similar results were reported by Waters et al. in 160 older adults (28). Compared to a control group, insulin sensitivity improved significantly in all three exercise groups, yet there were significantly greater improvements in insulin sensitivity in the combined exercise group compared to either exercise group alone (28). A major limitation in both the Sigal et al. and Waters et al. studies is that the combined exercise group did the full aerobic exercise program plus the full resistance exercise program (i.e., double the exercise time), which may explain the superior results in the combined group. Addressing this limitation, Church et al. randomly assigned 262 adults with type 2 diabetes to time-matched aerobic, resistance, or combined exercise interventions (29). They found a significant reduction in HbA1c only in the combined exercise group but not in the aerobic only or resistance only groups (29). It is important to note that the Church et al. participants had a poorer health profile with a longer duration of diabetes and greater insulin use than the participants in the Sigal et al. or Waters et al. trials. Church et al. also permitted changes in hypoglycemic medications during the trial. Hence, these methodological and sample differences in Church et al. may explain why HbA1c reductions were smaller in response to either type of exercise alone. Thus, the results of three large trials indicate that combined exercise causes improvements in glucose regulation and insulin sensitivity, but that aerobic exercise only or resistance exercise only may be less effective, especially after considering total exercise time.
The aforementioned trials focused on a single clinical outcome; however, changes in a single outcome may not fully explain the comprehensive health effects of different types of exercise that are depicted in Figure 2. Furthermore, the risks for chronic diseases like CVD are typically determined not by one individual risk factor, but by the interactions among several major risk factors such as hypertension, hyperglycemia, hyperlipidemia, and excess adipose tissue (30). A few large studies have found beneficial effects of combined exercise, compared to aerobic exercise only or resistance exercise only, on body composition including reductions in fat mass and/or increases in lean mass (3,29,31). Yet surprisingly, most of the large and long-duration (i.e., ≥6 months) exercise trials that have compared the effects of aerobic, resistance, and combined exercise on various risk factors have found non-significant changes in individual CVD risk factors compared to a control group (27,29,32,33). More consistent effects of different types of exercise may emerge, however, when the outcome reflects a composite of changes in several CVD risk factors. Batemen and colleagues investigated the effects of 8 months of resistance, aerobic, or combined aerobic and resistance exercise on metabolic syndrome in 86 participants (33). Like with previous trials, there were no significant changes in most individual risk factors including HDL cholesterol, glucose, or systolic blood pressure among the exercise groups. However, the aerobic and combination exercise groups, but not the resistance exercise group, showed significant improvements in the metabolic syndrome z-score, a composite measure that included fasting glucose, waist circumference, mean arterial pressure, HDL cholesterol, and triglycerides (33). However, there were some limitations with this study. First, of the 196 participants initially randomized, only 86 were analyzed due to participant dropout or missing data in ≥1 metabolic syndrome z-score component. Thus, it was not an intention-to-treat analysis, and the final analytic sample included only 44% of the original participants. Second, there was no control group, so it is impossible to conclude that the improvements in the aerobic or combined groups, or even the lack of improvements in the resistance group, were significantly better or worse than no exercise for 8 months. Last, the groups were not time-matched, and the combination group did the full aerobic and resistance exercise interventions. Thus, while it appeared that combined exercise and possibly aerobic exercise were superior to resistance exercise only for comprehensive cardiometabolic health benefits, it is difficult to draw clear conclusions from this study. To address these methodological issues, we are currently conducting a large clinical trial in 406 adults who were randomly assigned to time-matched resistance only, aerobic only, combined aerobic and resistance exercise, or a no-exercise control group for 12 months (30). The primary outcome is a composite CVD risk score (including systolic blood pressure, fasting glucose, LDL cholesterol, and percent body fat), and the secondary outcomes are changes in each of the individual risk factors. The results from this large study may provide further clarity regarding the comprehensive health benefits of meeting different components of the PA guidelines while standardizing the amount of exercise time across groups. Overall, results from large RCTs suggest that combined exercise may provide slightly larger benefits for a wide variety of health outcomes compared to a control group than either type of exercise alone compared to a control group. However, the effects of combined exercise on health outcomes, while generally larger, are not statistically different from the effects of aerobic only or resistance only exercise in most studies especially when exercise durations are matched. Thus, it remains unclear whether the health benefits discussed are simply due to an increase in exercise volume or the physiological gestalt of combined MVPA and MSPA.
Figure 2:
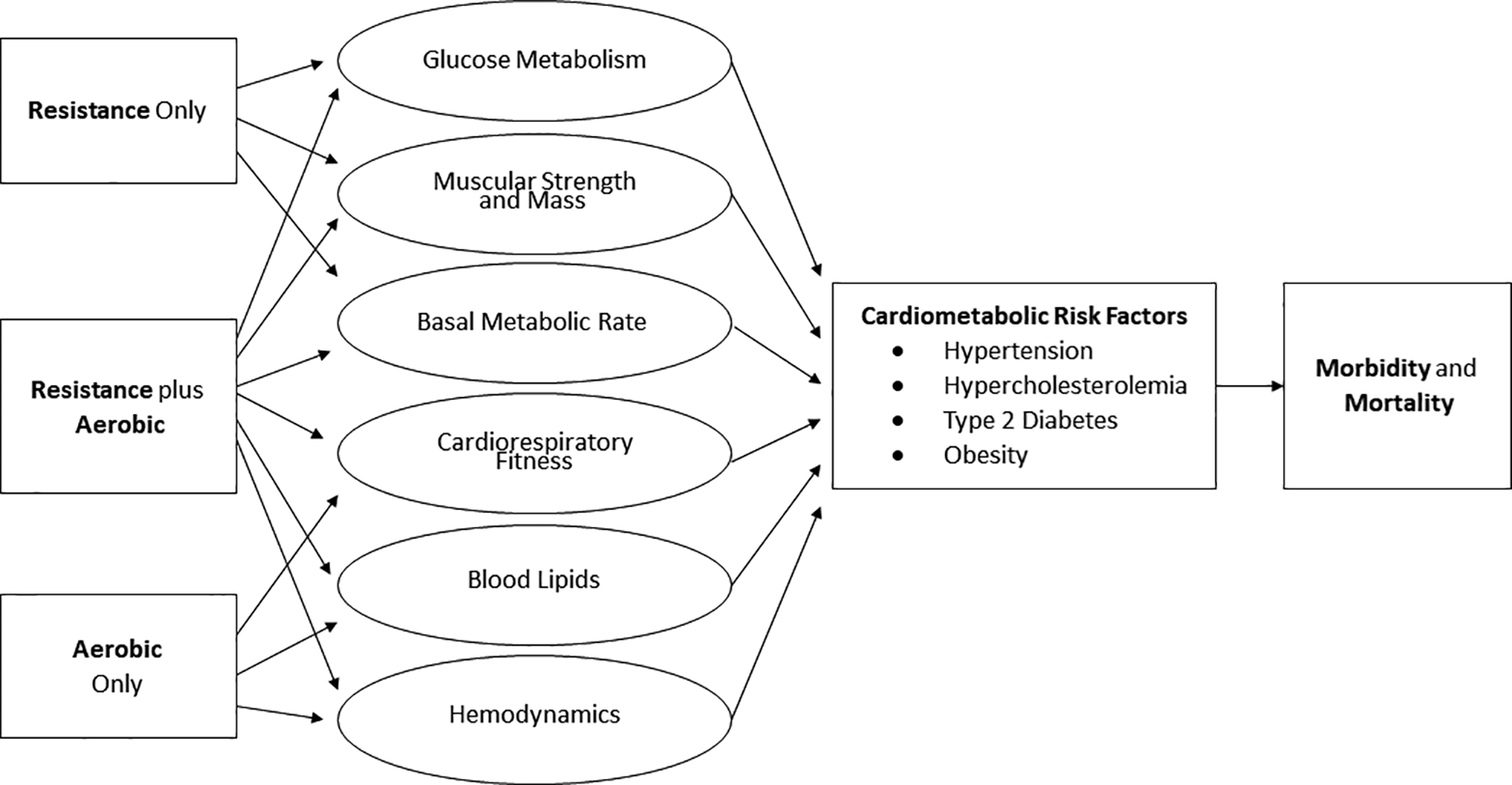
Mechanistic pathway between resistance, aerobic, or combined exercise training, major cardiometabolic risk factors, and mortality.
Other Considerations and Future Directions
The epidemiological and clinical data clearly indicate that engaging in both MSPA and MVPA offers health benefits compared to no exercise. However, the truly effective PA is the PA that people actually do. Population-level estimates based on self-report consistently show that individuals are much more likely to participate in MVPA (~50% of adults) than they are in MSPA (~30% of adults) (34,35). The estimates shift when they are subdivided into those who meet only certain components of the guidelines. For example, the prevalence of US adults reporting meeting only the MVPA guidelines increased from 26% to 30% from 2008 to 2018 (35). Conversely, the prevalence of US adults reporting meeting only the MSPA guidelines remained stagnant at around 3.5% over the same period (35). The prevalence of those meeting both guidelines grew the most, from 14% to 24% (35). While it is encouraging to see the greatest growth among those who meet both guidelines, the large gap between participation in either MVPA or MSPA only and the stagnation in MSPA participation suggests that MSPA is still seen as a secondary, rather than as an essential and synergistic, component to MVPA (36). Lower participation in MSPA may also indicate that there are more barriers to MSPA (e.g., unfamiliar terminology like “sets” and “reps”; lack of gym membership) compared to MVPA (e.g., a brisk walk outside) (26,37). Another advantage of aerobic PA is the ability to measure objectively the associations of even slight increases in aerobic PA (i.e., light intensity PA or reductions in sedentary time) and health (38). Therefore, it has become increasingly easier from a health promotion perspective to promote all types and intensities of aerobic PA. Without such nuanced data especially at the lower end of the MSPA spectrum, it remains challenging to construct MSPA guidelines that are highly achievable and accessible (i.e., less technical) for the majority of adults. However, the observational data consistently indicate that unlike with MVPA, there is less evidence of a dose-response relationship between MSPA and several health outcomes (39). The strongest associations are often observed at around one hour per week of MSPA, meaning that individuals may not need to perform as much MSPA as they do MVPA to get similar and substantial health benefits, although the apparent absence of additional health benefits beyond one hour per week of MSPA warrants further investigation (39). Nonetheless, compared to MVPA, it is possible that the time commitment for health-enhancing MSPA is lower, which may improve public health efforts to increase MSPA at the population level. Another limitation is that most of the observational studies and clinical trials discussed in this review did not account for sedentary time. Unmeasured sedentary time may confound the associations between MVPA and health outcomes, although it is still controversial whether sedentary time represents an independent health risk factor or merely reflects the low end of the MVPA spectrum (40). Furthermore, there is limited research on the possible interaction between sedentary time and MSPA.
In an opposite pattern to prevalence estimates, there are typically higher adherence and fewer dropouts in clinical trials in the resistance exercise only or combined exercise groups than in aerobic exercise groups (27,29,33). While more studies are needed, this may suggest that MSPA, once initiated, may be more sustainable long term. Partly explaining this may be the fact that, unlike aerobic exercise, resistance exercise contributes to rapid improvements in strength or muscle hypertrophy that can be appreciated directly by the participant and provide an immediate source of positive feedback. In contrast, improvements in aerobic fitness may be less noticeable since the prescribed heart rate range often stays the same throughout an intervention, even though participants may be performing the exercise at a higher incline or speed. Resistance exercise may also offer more opportunities for social interaction in a clinical trial setting, since participants often receive a higher degree of staff supervision to monitor safety and form. Resistance exercise participants also have the opportunity to interact with staff and other participants during the rest time between sets and equipment. This differs from aerobic exercise, where participants often use media (e.g., music, television) during their workouts and have a more difficult time interacting with staff due to the continuous nature of the exercise stimulus and stationary equipment (e.g., treadmill, bike). Finally, resistance exercise and certainly combined exercise may simply provide more variety and be more interesting than continuous aerobic exercise, especially in trials where total exercise time is matched between groups. However, we acknowledge the clear need for well-designed studies of different types of exercise on adherence to provide definitive data that will help get more people active.
This review focused specifically on comparisons of health benefits between different types of exercise behaviors. Critically, it did not address the health benefits of increases in cardiorespiratory fitness or muscular strength, which are the intended fitness outcomes of the behaviors. A large body of evidence suggests that cardiorespiratory fitness and muscular strength are more strongly associated with health-related outcomes than MVPA or MSPA, suggesting that improvements in these fitness indicators may underlie the observed associations between PA and health (41,42). Cardiorespiratory fitness and muscular strength reflect the physiological contributions of genetics, age, sex, diet, medications, and many other factors, but exercise is the most modifiable contributor (41). Individuals expect to become fitter and stronger when they start an exercise program, although this is not always the case. Clinical trial data suggest that 35–60% of adults who begin a supervised moderate-to-vigorous intensity aerobic exercise program do not significantly improve their cardiorespiratory fitness (i.e., “non-responders”) (43–45). It is still debated whether improvements in cardiorespiratory fitness are essential for improvements in other major risk factors or mortality (46,47). This debate is especially salient given the recent evidence from observational studies indicating that light-intensity PA or sedentary time is associated with health outcomes, although light-intensity PA and sedentary time arguably have negligible effects on improving cardiorespiratory fitness (38,46,48). It is possible that some health benefits (e.g., glucose regulation) could occur without significant improvements in fitness or strength (47–49). However, this is a moot point regarding resistance exercise since nearly 100% of novice participants who begin a resistance exercise program will improve their strength (50,51). Therefore, if the overall health benefits of exercise are partially dependent on improvements in physical fitness, then a combined exercise program should be prescribed to guarantee improvements in muscular strength and possibly cardiorespiratory fitness, too. Some studies have found equal or even greater improvements in cardiorespiratory fitness and muscular strength in combined exercise groups than in aerobic only or resistance only groups, which further suggests a synergistic (additive) rather than a competitive or detractive relationship between the two types of exercise (3,27,29). It is also possible to achieve significant gains in cardiorespiratory fitness and muscular strength without doubling exercise times. For example, a few small studies have reported significant improvements in VO2peak and muscular endurance have been found with very short-duration (4 minutes), hybrid exercise modalities such as bodyweight interval training where aerobic and strengthening exercises occur simultaneously (52,53). However, it remains unknown whether this type of training, despite its notable effects on cardiorespiratory fitness and muscular strength, is broadly applicable or sustainable in the long term to reduce the risk of chronic disease or mortality at the population level.
Finally, this review was limited to physical health, rather than mental health, in a general adult population. PA is also strongly recommended for mental health benefits, yet there have been only a few cross-sectional studies, and almost no prospective studies, that have examined the associations between MVPA and MSPA and mental health outcomes. Participation in MSPA only or MVPA only has been associated with a significantly lower prevalence of poor sleep, mild-to-severe depression symptoms, and even lower levels of clinical depression in a dose-dependent pattern (54–56). Similarly, levels of subclinical psychological distress (i.e., general symptoms of depression or anxiety) appear to be significantly lower among groups who participate in MVPA only or both MVPA and MSPA, but not MSPA only (57). Like with physical health outcomes, though, the strongest and most consistent associations between PA patterns and mental health outcomes have been observed among groups who meet both the MSPA and MVPA guidelines (54–57).
Clinical trial data comparing resistance and aerobic exercise on general mental health are also limited at this time. Three studies have found that combined exercise is generally better than either aerobic or resistance exercise alone for improving overall mental health and vitality in a nonclinical adult population (58–60). Regarding clinical mental health concerns like mood disorders, cognitive function, and chronic pain, recent meta-analyses that compiled data largely from aerobic exercise-only or resistance exercise-only interventions, concluded that both aerobic and resistance exercise led to improvements in symptoms of depression and anxiety, pain ratings, and cognitive outcomes compared to control groups, with no significant differences between aerobic and resistance exercise in the magnitude of the effects (61–65). Combined exercise has not been routinely investigated regarding depression or anxiety, but it has been studied extensively and appears to be particularly effective for cognitive function in older adults (64).
Conclusions
This paper provides an overview of the observational and clinical evidence on the health benefits of MVPA and MSPA. Overall, data from large prospective cohort studies suggest that engaging in either MVPA or MSPA alone significantly reduces the risk of all-cause, CVD, and cancer mortality, as well as many chronic diseases like type 2 diabetes. However, the largest health benefits, including an approximate 40% reduced risk of all-cause mortality and 50% reduced risk of CVD mortality, are observed among individuals who perform sufficient amounts of both MVPA and MSPA. An overview of clinical trial data further supports that the most comprehensive physical and mental health benefits occur through a combination of aerobic and resistance exercise. However, of note is the fact that combination groups in these studies have typically exercised for a greater amount of time. Importantly, both MVPA and MSPA also offer distinct and critical health benefits like improvements in cardiorespiratory fitness and muscular strength, respectively, which are associated more strongly with health outcomes than PA behaviors. Furthermore, the combination of MVPA and MSPA appears to have synergistic effects on health, elucidating additional benefits of exercise that were not always apparent with either type of exercise alone. Thus, we argue that there should be relatively less emphasis on promoting one type of exercise over the other and more emphasis on researching and promoting feasible, time-efficient ways to meet both guidelines.
Funding Disclosures/conflicts of interest:
The authors declare no conflicts of interest. This work was supported by NIH/NHLBI grant R01HL133069 and NIH/NIDDK grant R21 DK131429.
References
- 1.Lee DC, Brellenthin AG, Thompson PD, Sui X, Lee IM, Lavie CJ. Running as a key lifestyle medicine for longevity. Prog. Cardiovasc. Dis 2017; 60(1):45–55. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans. 2nd Edition. Washington DC; 2018. Available from: https://health.gov/paguidelines/second-edition/pdf/Physical_Activity_Guidelines_2nd_edition.pdf. [Google Scholar]
- 3.Villareal DT, Aguirre L, Gurney AB, et al. Aerobic or resistance exercise, or both, in dieting obese older adults. New Eng J Med [Internet]. 2017. May 18 [cited 2020 Aug 18];376(20):1943–55. Available from: https://pubmed.ncbi.nlm.nih.gov/28514618/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giovannucci EL, Rezende LFM, Lee DH. Muscle-strengthening activities and risk of cardiovascular disease, type 2 diabetes, cancer and mortality: A review of prospective cohort studies. J Internal Med. [Internet]. 2021. Oct 1 [cited 2022 Feb 1];290(4):789–805. Available from: https://pubmed.ncbi.nlm.nih.gov/34120373/. [DOI] [PubMed] [Google Scholar]
- 5.Schoenborn CA, Stommel M. Adherence to the 2008 adult physical activity guidelines and mortality risk. Am J Preventive Med. [Internet]. 2011. May [cited 2022 Feb 11];40(5):514–21. Available from: https://pubmed.ncbi.nlm.nih.gov/21496750/. [DOI] [PubMed] [Google Scholar]
- 6.Zhao M, Veeranki SP, Magnussen CG, Xi B. Recommended physical activity and all cause and cause specific mortality in US adults: prospective cohort study. BMJ [Internet]. 2020. Jul 1 [cited 2022 Feb 1];370. Available from: https://pubmed.ncbi.nlm.nih.gov/32611588/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nie J, Haberstroh M, Acosta T, Huang W, Wang Y, Barengo NC. Independent and joint associations between leisure time physical activity and strength activities with mortality outcomes in older adults at least 65 years of age: A prospective cohort study. J Gerontol. [Internet]. 2021. Dec 1 [cited 2022 Feb 8];76(12):2122–31. Available from: https://pubmed.ncbi.nlm.nih.gov/33858013/. [DOI] [PubMed] [Google Scholar]
- 8.Kamada M, Shiroma EJ, Buring JE, Miyachi M, Lee IM. Strength training and all-cause, cardiovascular disease, and cancer mortality in older women: A cohort study. J Am Heart Assoc. [Internet]. 2017. Nov 1 [cited 2022 Feb 8];6(11). Available from: https://pubmed.ncbi.nlm.nih.gov/29089346/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stamatakis E, Lee IM, Bennie J, et al. Does strength-promoting exercise confer unique health benefits? A pooled analysis of data on 11 population cohorts with all-cause, cancer, and cardiovascular mortality endpoints. Am J Epidemiol. [Internet]. 2018. May 1 [cited 2022 Feb 8];187(5):1102–12. Available from: https://pubmed.ncbi.nlm.nih.gov/29099919/. [DOI] [PubMed] [Google Scholar]
- 10.Saeidifard F, Medina-Inojosa JR, West CP, et al. The association of resistance training with mortality: A systematic review and meta-analysis. Eur J Preventive Cardiol. [Internet]. 2019. Oct 1 [cited 2022 Feb 8];26(15):1647–65. Available from: https://pubmed.ncbi.nlm.nih.gov/31104484/. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control. National Center for Health Statistics. FastStats - Leading Causes of Death [Internet]. [cited 2022 Feb 12]. Available from: https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm.
- 12.Bennie JA, de Cocker K, Pavey T, Stamatakis E, Biddle SJH, Ding D. Muscle strengthening, aerobic exercise, and obesity: A pooled analysis of 1.7 million US adults. Obesity [Internet]. 2020. Feb 1 [cited 2020 Aug 17];28(2):371–8. Available from: https://pubmed.ncbi.nlm.nih.gov/31709754/. [DOI] [PubMed] [Google Scholar]
- 13.Lim J, Park S, Kim J-S. Joint association of aerobic physical activity and muscle-strengthening activities with metabolic syndrome : the Korean National Health and Nutrition Examination Survey 2014–2015. Epidemiol Health. [Internet]. 2021. Nov 6 [cited 2022 Feb 8];43:e2021096. Available from: https://pubmed.ncbi.nlm.nih.gov/34773937/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dankel SJ, Loenneke JP, Loprinzi PD. The individual, joint, and additive interaction associations of aerobic-based physical activity and muscle strengthening activities on metabolic syndrome. Int J Behavioral Med. [Internet]. 2016. Dec 1 [cited 2022 Feb 8];23(6):707–13. Available from: https://pubmed.ncbi.nlm.nih.gov/27229520/. [DOI] [PubMed] [Google Scholar]
- 15.Bennie JA, Ding D, de Cocker K. Dose-dependent associations of joint aerobic and muscle-strengthening exercise with obesity: A cross-sectional study of 280,605 adults. J Sport Health Sci. 2021. Jan 9;S2095–2546(21)00002–8. doi: 10.1016/j.jshs.2021.01.002. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennie JA, Ding D, Khan A, Stamatakis E, Biddle SJH, Kim J. Run, lift, or both? Associations between concurrent aerobic-muscle strengthening exercise with adverse cardiometabolic biomarkers among Korean adults. Eur J Preventive Cardiol. [Internet]. 2020. May 1 [cited 2022 Feb 8];27(7):738–48. Available from: https://pubmed.ncbi.nlm.nih.gov/30861691/. [DOI] [PubMed] [Google Scholar]
- 17.Hamer M, Bell JA, Sabia S, Batty GD, Kivimäki M. Stability of metabolically healthy obesity over 8 years: The English Longitudinal Study of Ageing. Eur J Endocrinol. [Internet]. 2015. Nov 1 [cited 2021 Feb 9];173(5):703–8. Available from: https://pubmed.ncbi.nlm.nih.gov/26286585/. [DOI] [PubMed] [Google Scholar]
- 18.Brellenthin AG, Lee DC, Bennie JA, Sui X, Blair SN. Resistance exercise, alone and in combination with aerobic exercise, and obesity in Dallas, Texas, US: A prospective cohort study. PLoS Med. [Internet]. 2021. Jun 1 [cited 2022 Feb 13];18(6). Available from: https://pubmed.ncbi.nlm.nih.gov/34161329/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakker EA, Lee DC, Sui X, et al. Association of resistance exercise, independent of and combined with aerobic exercise, with the incidence of metabolic syndrome. Mayo Clinic Proceedings. [Internet]. 2017. Aug 1 [cited 2021 Jan 15];92(8):1214–22. Available from: https://pubmed.ncbi.nlm.nih.gov/28622914/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bakker EA, Lee DC, Sui X, et al. Association of resistance exercise with the incidence of hypercholesterolemia in men. Mayo Clinic Proceedings [Internet]. 2018. Apr [cited 2019 Oct 1];93(4):419–28. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29428677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Research and Clinical Practice [Internet]. 2019. Nov 1 [cited 2022 Feb 13];157. Available from: https://pubmed.ncbi.nlm.nih.gov/31518657/. [DOI] [PubMed] [Google Scholar]
- 22.Grøntved A, Pan A, Mekary RA, et al. Muscle-strengthening and conditioning activities and risk of type 2 diabetes: a prospective study in two cohorts of US women. PLoS Medicine [Internet]. 2014. Jan [cited 2022 Feb 8];11(1). Available from: https://pubmed.ncbi.nlm.nih.gov/24453948/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grøntved A, Rimm EB, Willett WC, Andersen LB, Hu FB. A prospective study of weight training and risk of type 2 diabetes mellitus in men. Archives Internal Med. [Internet]. 2012. Sep 24 [cited 2022 Feb 8];172(17):1306–12. Available from: https://pubmed.ncbi.nlm.nih.gov/22868691/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shiroma EJ, Cook NR, Manson JE, et al. Strength training and the risk of type 2 diabetes and cardiovascular disease. Med Sci Sports Exerc. [Internet]. 2017. Jan 1 [cited 2022 Feb 8];49(1):40–6. Available from: https://pubmed.ncbi.nlm.nih.gov/27580152/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Westcott WL, Winett RA, Annesi JJ, Wojcik JR, Anderson ES, Madden PJ. Prescribing physical activity: applying the ACSM protocols for exercise type, intensity, and duration across 3 training frequencies. Phys Sportsmed. [Internet]. 2009. Jun [cited 2022 May 17];37(2):51–8. Available from: https://pubmed.ncbi.nlm.nih.gov/20048509/. [DOI] [PubMed] [Google Scholar]
- 26.Schroeder EC, Welk GJ, Franke WD, Lee D-C. Associations of health club membership with physical activity and cardiovascular health. PloS One [Internet]. 2017. [cited 2019 Oct 16];12(1):e0170471. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28107459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sigal RJ, Kenny GP, Boulé NG, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Annals Internal Med. [Internet]. 2007. Sep 18 [cited 2021 Aug 27];147(6):357–69. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17876019. [DOI] [PubMed] [Google Scholar]
- 28.Waters DL, Aguirre L, Gurney B, et al. Effect of aerobic or resistance exercise, or both, on intermuscular and visceral fat and physical and metabolic function in older adults with obesity while dieting. J Gerontol. [Internet]. 2022. Jan 7 [cited 2022 Feb 9];77(1):131–9. Available from: https://academic.oup.com/biomedgerontology/article/77/1/131/6220316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Church TS, Blair SN, Cocreham S, et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: A randomized controlled trial. Journal of the Am Med Assoc. 2010;304(20):2253–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brellenthin AG, Lanningham-Foster LM, Kohut ML, et al. Comparison of the cardiovascular benefits of resistance, aerobic, and combined exercise (CardioRACE): Rationale, design, and methods. Am Heart J. 2019. Nov;217:101–111. doi: 10.1016/j.ahj.2019.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willis LH, Slentz CA, Bateman LA, et al. Effects of aerobic and/or resistance training on body mass and fat mass in overweight or obese adults. J Appl Physiol. (Bethesda, Md : 1985) [Internet]. 2012. Dec 15 [cited 2022 Feb 8];113(12):1831–7. Available from: https://pubmed.ncbi.nlm.nih.gov/23019316/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sillanpää E, Laaksonen DE, Häkkinen A, et al. Body composition, fitness, and metabolic health during strength and endurance training and their combination in middle-aged and older women. Eur J Appl Physiol. [Internet]. 2009. May 6 [cited 2018 Aug 27];106(2):285–96. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19266214. [DOI] [PubMed] [Google Scholar]
- 33.Bateman LA, Slentz CA, Willis LH, et al. Comparison of aerobic versus resistance exercise training effects on metabolic syndrome (from the Studies of a Targeted Risk Reduction Intervention Through Defined Exercise - STRRIDE-AT/RT). Am J Cardiol. [Internet]. 2011. Sep 15 [cited 2020 Aug 18];108(6):838–44. Available from: /pmc/articles/PMC3752599/?report=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennie JA, de Cocker K, Teychenne MJ, Brown WJ, Biddle SJH. The epidemiology of aerobic physical activity and muscle-strengthening activity guideline adherence among 383,928 U.S. adults. Int J Behavl Nutrition Phys Activity. 2019. Apr 18;16(1):34. doi: 10.1186/s12966-019-0797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hyde ET, Whitfield GP, Omura JD, Fulton JE, Carlson SA. Trends in meeting the physical activity guidelines: Muscle-strengthening alone and combined with aerobic activity, United States, 1998–2018. J Phys Activity Health. [Internet]. 2021. Aug 1 [cited 2022 Feb 6];18(S1):S37–44. Available from: https://pubmed.ncbi.nlm.nih.gov/34465652/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strain T, Fitzsimons C, Kelly P, Mutrie N. The forgotten guidelines: Cross-sectional analysis of participation in muscle strengthening and balance & co-ordination activities by adults and older adults in Scotland. BMC Public Health. [Internet]. 2016. Oct 21 [cited 2020 Aug 26];16(1). Available from: https://pubmed.ncbi.nlm.nih.gov/27769211/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rhodes RE, Lubans DR, Karunamuni N, Kennedy S, Plotnikoff R. Factors associated with participation in resistance training: a systematic review. Br J Sports Med. [Internet]. 2017. Oct 1 [cited 2022 Feb 8];51(20):1466–72. Available from: https://pubmed.ncbi.nlm.nih.gov/28404558/. [DOI] [PubMed] [Google Scholar]
- 38.Ekelund U, Tarp J, Steene-Johannessen J, et al. Dose-response associations between accelerometry measured physical activity and sedentary time and all-cause mortality: systematic review and harmonised meta-analysis. BMJ [Internet]. 2019. [cited 2022 Feb 1];366. Available from: https://pubmed.ncbi.nlm.nih.gov/31434697/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Momma H, Kawakami R, Honda T, Sawada SS. Muscle-strengthening activities are associated with lower risk and mortality in major non-communicable diseases: a systematic review and meta-analysis of cohort studies. Br J Sports Med. [Internet]. 2022. Feb 28 [cited 2022 May 17];bjsports-2021–105061. Available from: https://pubmed.ncbi.nlm.nih.gov/35228201/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stamatakis E, Ekelund U, Ding D, Hamer M, Bauman AE, Lee IM. Is the time right for quantitative public health guidelines on sitting? A narrative review of sedentary behaviour research paradigms and findings. Br J Sports Med. [Internet]. 2019. Mar 1 [cited 2022 May 17];53(6):377–82. Available from: https://pubmed.ncbi.nlm.nih.gov/29891615/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee DC, Artero EG, Sui X, Blair SN. Mortality trends in the general population: the importance of cardiorespiratory fitness. J Psychopharmacol. [Internet]. 2010. Nov [cited 2019 Dec 8];24(4 Suppl):27–35. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20923918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.García-Hermoso A, Cavero-Redondo I, Ramírez-Vélez R, et al. Muscular strength as a predictor of all-cause mortality in an apparently healthy population: A systematic review and meta-analysis of data from approximately 2 million men and women. Arch Physical Med Rehab. [Internet]. 2018. Oct 1 [cited 2022 Feb 8];99(10):2100–2113.e5. Available from: https://pubmed.ncbi.nlm.nih.gov/29425700/. [DOI] [PubMed] [Google Scholar]
- 43.Pandey A, Johnson JL, Slentz CA, et al. Short-term changes in cardiorespiratory fitness in response to exercise training and the association with long-term cardiorespiratory fitness decline: The STRRIDE reunion study. J Am Heart Assoc. 2019. Oct 15;8(20):e012876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sisson SB, Katzmarzyk PT, Earnest CP, Bouchard C, Blair SN, Church TS. Volume of exercise and fitness nonresponse in sedentary, postmenopausal women. Med Sci Sports Exerc. 2009. Mar;41(3):539–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ross R, Goodpaster BH, Koch LG, et al. Precision exercise medicine: Understanding exercise response variability. Br J Sports Med. 2019. Sep 1;53(18):1141–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steele J, Fisher J, Skivington M, et al. A higher effort-based paradigm in physical activity and exercise for public health: making the case for a greater emphasis on resistance training. BMC Public Health. [Internet]. 2017. Apr 5 [cited 2022 Feb 1];17(1). Available from: https://pubmed.ncbi.nlm.nih.gov/28381272/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pandey A, Swift DL, McGuire DK, et al. Metabolic effects of exercise training among fitness-nonresponsive patients with type 2 diabetes: The HART-D Study. Diabetes Care [Internet]. 2015. Aug [cited 2019 Dec 11];38(8):1494–501. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26084342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chastin SFM, de Craemer M, de Cocker K, et al. How does light-intensity physical activity associate with adult cardiometabolic health and mortality? Systematic review with meta-analysis of experimental and observational studies. Br J Sports Med. [Internet]. 2019. Mar 1 [cited 2022 Feb 20];53(6):370–6. Available from: https://bjsm.bmj.com/content/53/6/370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ekelund U, Franks PW, Sharp S, Brage S, Wareham NJ. Increase in physical activity energy expenditure is associated with reduced metabolic risk independent of change in fatness and fitness. Diabetes Care. [Internet]. 2007. Aug [cited 2021 Jan 20];30(8):2101–6. Available from: https://pubmed.ncbi.nlm.nih.gov/17536069/. [DOI] [PubMed] [Google Scholar]
- 50.Churchward-Venne TA, Tieland M, Verdijk LB, et al. There are no nonresponders to resistance-type exercise training in older men and women. J Am Medical Directors Association [Internet]. 2015. May 1 [cited 2022 Feb 13];16(5):400–11. Available from: https://pubmed.ncbi.nlm.nih.gov/25717010/. [DOI] [PubMed] [Google Scholar]
- 51.Hubal MJ, Gordish-Dressman H, Thompson PD, et al. Variability in muscle size and strength gain after unilateral resistance training. Med Sci Sports Exerc. [Internet]. 2005. [cited 2022 Feb 13];37:964–72. Available from: https://journals.lww.com/acsm-msse/Fulltext/2005/06000/Variability_in_Muscle_Size_and_Strength_Gain_after.10.aspx. [PubMed] [Google Scholar]
- 52.Menz V, Marterer N, Amin SB, Faulhaber M, Hansen AB, Lawley JS. Functional vs. running low-volume high-intensity interval training: Effects on VO2max and muscular endurance. J Sports Sci Med [Internet]. 2019. Sep 1 [cited 2022 May 17];18(3):497. Available from: /pmc/articles/PMC6683610/. [PMC free article] [PubMed] [Google Scholar]
- 53.Mcrae G, Payne A, Zelt JGE, et al. Extremely low volume, whole-body aerobic-resistance training improves aerobic fitness and muscular endurance in females. Appl Physiol Nutr Metab. [Internet]. 2012. Dec [cited 2022 May 17];37(6):1124–31. Available from: https://pubmed.ncbi.nlm.nih.gov/22994393/. [DOI] [PubMed] [Google Scholar]
- 54.Bennie JA, de Cocker K, Duncan MJ. Associations of muscle-strengthening and aerobic exercise with self-reported components of sleep health among a nationally representative sample of 47,564 US adults. Sleep Health. [Internet]. 2021. Apr 1 [cited 2022 Feb 8];7(2):281–8. Available from: https://pubmed.ncbi.nlm.nih.gov/33071201/. [DOI] [PubMed] [Google Scholar]
- 55.Bennie JA, Teychenne MJ, de Cocker K, Biddle SJH. Associations between aerobic and muscle-strengthening exercise with depressive symptom severity among 17,839 U.S. adults. Preventive Med. [Internet]. 2019. Apr 1 [cited 2022 Feb 8];121:121–7. Available from: https://pubmed.ncbi.nlm.nih.gov/30786252/. [DOI] [PubMed] [Google Scholar]
- 56.Bennie JA, de Cocker K, Biddle SJH, Teychenne MJ. Joint and dose-dependent associations between aerobic and muscle-strengthening activity with depression: A cross-sectional study of 1.48 million adults between 2011 and 2017. Depression Anxiety. [Internet]. 2020. Feb 1 [cited 2022 Feb 20];37(2):166–78. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/da.22986. [DOI] [PubMed] [Google Scholar]
- 57.de Cocker K, Teychenne M, White RL, Bennie JA. Adherence to aerobic and muscle-strengthening exercise guidelines and associations with psychological distress: A cross-sectional study of 14,050 English adults. Preventive Med. [Internet]. 2020. Oct 1 [cited 2022 Feb 8];139. Available from: https://pubmed.ncbi.nlm.nih.gov/32640287/. [DOI] [PubMed] [Google Scholar]
- 58.Myers VH, McVay MA, Brashear MM, et al. Exercise training and quality of life in individuals with type 2 diabetes: a randomized controlled trial. Diabetes Care. [Internet]. 2013. [cited 2022 Feb 9];36(7):1884–90. Available from: https://pubmed.ncbi.nlm.nih.gov/23404304/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Collins KA, Fos LB, Ross LM, et al. Aerobic, resistance, and combination training on health-related quality of life: The STRRIDE-AT/RT randomized trial. Front Sports Active Living. [Internet]. 2021. Feb 11 [cited 2022 Feb 9];2. Available from: https://pubmed.ncbi.nlm.nih.gov/33644749/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sillanpää E, Häkkinen K, Holviala J, Häkkinen A. Combined strength and endurance training improves health-related quality of life in healthy middle-aged and older adults. Int J Sports Med. [Internet]. 2012. [cited 2022 Feb 20];33(12):981–6. Available from: https://pubmed.ncbi.nlm.nih.gov/22782386/. [DOI] [PubMed] [Google Scholar]
- 61.Gordon BR, McDowell CP, Lyons M, Herring MP. The effects of resistance exercise training on anxiety: A meta-analysis and meta-regression analysis of randomized controlled trials. Sports Med. [Internet]. 2017. Dec 1 [cited 2022 Feb 8];47(12):2521–32. Available from: https://pubmed.ncbi.nlm.nih.gov/28819746/. [DOI] [PubMed] [Google Scholar]
- 62.Gordon BR, McDowell CP, Hallgren M, Meyer JD, Lyons M, Herring MP. Association of efficacy of resistance exercise training with depressive symptoms. JAMA Psychiatry [Internet]. 2018. Jun 1 [cited 2018 Jul 26];75(6):566. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29800984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miller KJ, Gonçalves-Bradley DC, Areerob P, Hennessy D, Mesagno C, Grace F. Comparative effectiveness of three exercise types to treat clinical depression in older adults: A systematic review and network meta-analysis of randomised controlled trials. Ageing Res Reviews. [Internet]. 2020. Mar 1 [cited 2022 Feb 8];58. Available from: https://pubmed.ncbi.nlm.nih.gov/31837462/. [DOI] [PubMed] [Google Scholar]
- 64.Northey JM, Cherbuin N, Pumpa KL, Smee DJ, Rattray B. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br J Sports Med. [Internet]. 2018. Feb 1 [cited 2022 Feb 14];52(3):154–60. Available from: https://pubmed.ncbi.nlm.nih.gov/28438770/. [DOI] [PubMed] [Google Scholar]
- 65.Owen PJ, Miller CT, Mundell NL, et al. Which specific modes of exercise training are most effective for treating low back pain? Network meta-analysis. Br J Sports Med. [Internet]. 2020. Nov 1 [cited 2022 Feb 14];54(21):1279. Available from: /pmc/articles/PMC7588406/. [DOI] [PMC free article] [PubMed] [Google Scholar]


