Abstract
Background
The effect of mild changes in CO2 levels to organ perfusion and tissue inflammation are well known, whereas an influence of hypercapnia under general anesthesia on adverse events as nausea and vomiting, or length of hospital stay is barely examined. The goal of our meta-analysis was to identify possibly positive effects of hypercapnia versus normocapnia in general anesthesia in adult patients.
Methods
We conducted a systematic review of parallel-arm randomised controlled trials comparing hypercapnia versus normocapnia in adult patients undergoing general anesthesia. In July 2018 and September 2019 we searched “CENTRAL‿, “MEDLINE‿, and “Embase‿, checked reference lists of all included studies and relevant systematic reviews for additional references to trials. Two review authors independently assessed trials for inclusion, extracted data, and completed a “Risk of bias‿ assessment for all included studies.
Results
Our search identified 297 records after abstract screening 30 full-text papers remained for further examination. Ten publications met our inclusion criteria and were used for narrative description of this systematic review. Three studies were eligible for the meta-analysis normocapnia versus hypercapnia with the outcomes: time to extubation and adverse events. On average, time to extubation was significantly reduced in the hypercapnia group with a mean difference 3.78 (95% CI 0.85 to 6.71). No difference was found regarding adverse events.
Conclusions
The findings of our study do not enable us to produce evidence of a positive influence of increased CO2 partial pressure levels during general anesthesia. A well-planned, adequately powered randomized controlled trial would be desirable in the future.
Keywords: General anesthesia, Hypercapnia, Normocapnia, Invasive ventilation, Adverse events, Time to extubation
Introduction
Targeting physiological ranges of end-tidal carbon dioxide is a cornerstone of what is called good anesthesia practitioning. The level of carbon dioxide influences several physiological pathways, especially organ perfusion, and interacts with pulmonary vasoconstriction and modulates inflammation.1 Especially in different comorbidities, even mild lower ranges of carbon dioxide are chosen to influence cerebral perfusion or cardiac output.2, 3 For practical reasons hypocapnia is often used to suppress the breathing reflex and possibly reduce the dose of anesthetics in general anesthesia.4, 5 Data from animal models investigating ventilator induced lung injury have demonstrated a protective effect of hypercapnia on the lung tissue and inflammation as well as in the diaphragm.6 Data suggest that hypercapnia even during regular anesthesia may influence wound healing, possibly via perfusion changes.7, 8, 9 The work of Wax and colleagues even pointed to a reduction in the hospital length of stay for patients after colon resection and open hysterectomy, which might be a profound influence for health care costs and morbidity by the simple measure of allowing hypercapnia to occur during general anesthesia.10 Besides this study there is only nebulous evidence about the influence of hypercapnia during general anesthesia on adverse events, or anesthesia-related factors, as time to extubation.
This systematic review and meta-analysis seeks to identify possibly positive effects of hypercapnia versus normocapnia in general anesthesia in adult patients. Especially effects on the occurrence of adverse events like wound healing, time to extubation or admission to the ICU are investigated.
Methods
The protocol of this systematic review is registered at Prospero: CRD42018104506. The reporting of this systematic review is in line with the PRISMA statement for the reporting of systematic reviews.11
Eligibility criteria
We included parallel-arm randomised controlled trials (RCTs) reported as full-text, those published as abstract only, and unpublished data. We included trials comparing hypercapnia versus normocapnia in adult patients (≥ 18 years of age) undergoing general anesthesia. Hypercapnia was defined as a carbon dioxide partial pressure (paCO2) > 45 mmHg and conditioned by this a respiratory acidosis with pH levels < 7.35.12 Normocapnia was characterised by a paCO2 between 35–45 mmHg. As no core outcome set for clinical studies investigating anesthetic care is available, the list of outcomes chosen is based on outcomes presumably most relevant to patients and measures from possible matching studies; we assessed time to extubation, hospital length of stay, incidence of wound healing complications, hospital mortality and adverse events (e.g. nausea, vomiting).
Systematic search
We identified trials through systematic searches of the following bibliographic databases: Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library, MEDLINE (Ovid, from 1946 onwards), Embase (Ovid, from 1980 onwards).
The preliminary search strategy for MEDLINE (Ovid) was adapted for use in the other databases. The search strategy was: hypercapnia[Title/Abstract] OR hypercarbia[Title/Abstract]) AND (anaesthesia[Title/Abstract] OR anesthesia[Title/Abstract]). Search results were limited to those that were randomised controlled trials and review. The Cochrane sensitivity-maximising RCT filter (Lefebvre 2011) was applied to MEDLINE (Ovid) and adaptations of it to the other databases, except CENTRAL. We searched all databases from their inception to the present, and we imposed no restriction on language of publication or publication status. We also checked reference lists of all included studies and any relevant systematic reviews identified for additional references to trials.
Selection of studies
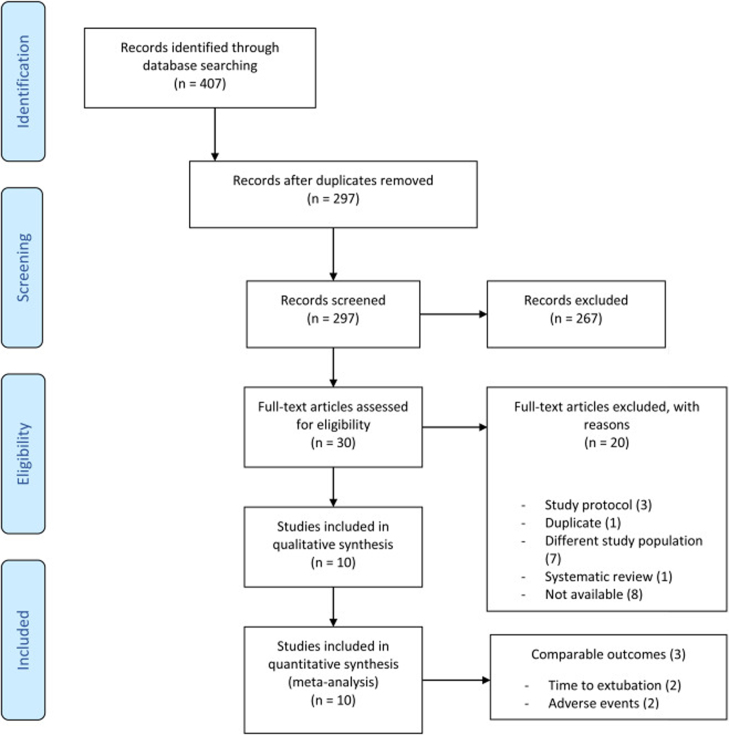
Two review authors (JP, CBr) independently screened titles and abstracts for inclusion of all the potential studies we identify as a result of the search and code them as “retrieve‿ or “do not retrieve‿. We retrieved the full-text study reports/publication and two review authors (JP, CBr) independently screened the full-text and identified studies for inclusion, and identified and recorded reasons for exclusion of the ineligible studies. We identified and excluded duplicates and collated multiple reports of the same study so that each study rather than each report is the unit of interest in the review. In September 2019 we updated our search. We describe the selection process in sufficient detail to complete a PRISMA flow diagram and “Characteristics of excluded studies‿ table (Figure 1 and Table 1).
Figure 1.
Flow diagram.
Table 1.
Overview characteristics of included studies.
| Study | Study design | Sex |
Intervention | Control | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients(n) |
Male (n) |
Female (n) |
Age (years) |
Weight (kg) |
|||||||||
| HC | NC | HC | NC | HC | NC | HC | NC | HC | NC | ||||
| Acka 2013 | Multicentre RCT | 590 | 616 | 303 | 310 | 270 | 288 | 51 | 53 | 77 | 78 | PE′CO2 ≈ 50 mmHg | PE′CO2 ≈ 35 mmHg |
| Baker 1976 | Single centre RCT | 42 | 16 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a | n.a. | PaCO2 > 60 torr; > 60 mmHg | PaCO2 = 25–60 torr; 25–60 mmHg |
| Enoki 2005 | RCT | 24 | 24 | 9 | 10 | 15 | 14 | 53 | 54 | 56 | 55 | etCO2 = 45 mmHg, expecting PaCO2 ≈ 50 mmHg | etCO2 = 35 mmHg, expecting PaCO2 ≈ 40 mmHg |
| Gao 2015 | Single centre RCT | 25 | 25 | 14 | 12 | 11 | 13 | 48.7 | 47.6 | 64.6 | 63.9 | PaCO2 = 60 to 70 mmHg | PaCO2 = 35 to 45 mmHg |
| Hager 2006 | Single centre RCT | 15 | 15 | 3 | 5 | 12 | 10 | 45 | 49 | 189 | 157 | etCO2 = 50 mmHg | etCO2 = 35 mmHg |
| Katznelson 2013 | RCT | 22 | 19 | n.a. | n.a. | n.a. | n.a. | 51.8 | 57.7 | n.a. | n.a. | 6% CO2 in O2 through the inspiratory relief valve (hypercapnia induced after skin closure) | Continued to breathe on the semi-open anesthesia |
| Kwak 2017 | Single centre RCT | 20 | 20 | 13 | 10 | 7 | 10 | 50 | 53 | 69 | 64 | etCO2 = 45 mmHg | etCO2 = 35 mmHg |
| Nakai 2013 | Single centre RCT | 15 | 15 | 8 | 7 | 7 | 8 | 57.2 | 55.6 | 64.8 | 62.3 | etCO2 < 55 mmHg | etCO2 = 33 mmHg |
| Nekhendzy 2007 | Single centre RCT | 60 | 60 | 33 | 30 | 27 | 30 | 47 | 53 | 86 | 77 | etCO2 > 60 mmHg | etCO2 = 37 mmHg |
| Son 2017 | Single centre RCT | 127 | 120 | 0 | 0 | 127 | 120 | 43 | 42 | 60 | 60 | PaCO2 = 46–50 mmHg | PaCO2 = 36–40 mmHg |
HC, hypercapnia; NC, normocapnia; RCT, randomized controlled trial; n.a., not applicable; etCO2 or pE’CO2, end tidal carbon dioxide level.
Data extraction process
We used a purposely pre-developed data collection form for study characteristics and outcome data, which we piloted on one study in the review. Three authors independently (JP, KA, CK) extracted the following study characteristics from the included studies (also compare Table 1). Methods (study design, number of study centres, total duration of study, date of study, withdrawals/drop-outs, inclusion criteria, exclusion criteria, experimental intervention, control, outcomes mentioned in “methods‿, funding for trial, and notable conflicts of interest of trial authors), patient characteristics (hypercapnia, normocapnia, sex, age, weight, BMI, American Society of Anesthesiologists (ASA) physical status, smoker, diabetes, diagnosis, operation method, paCO2, paO2, FiO2, end-tidal carbon dioxide levels, and intraoperative concomitant medication), and outcome data (time to extubation, hospital length of stay, hospital mortality, other reported outcomes mentioned). We double-checked the correctness of the data extraction. We transferred data into the Cochrane statistical software Review Manager.13 We contacted investigator or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data.
Assessment of risk of bias in included studies
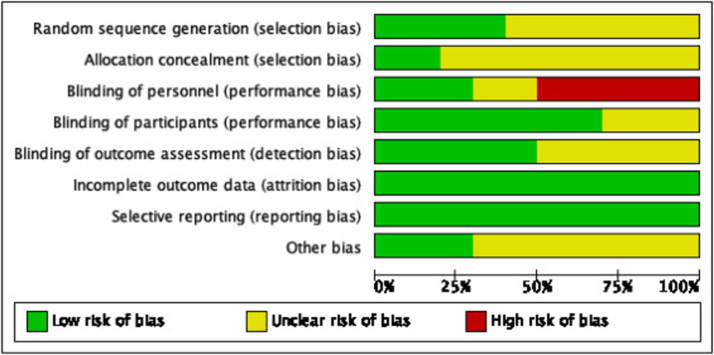
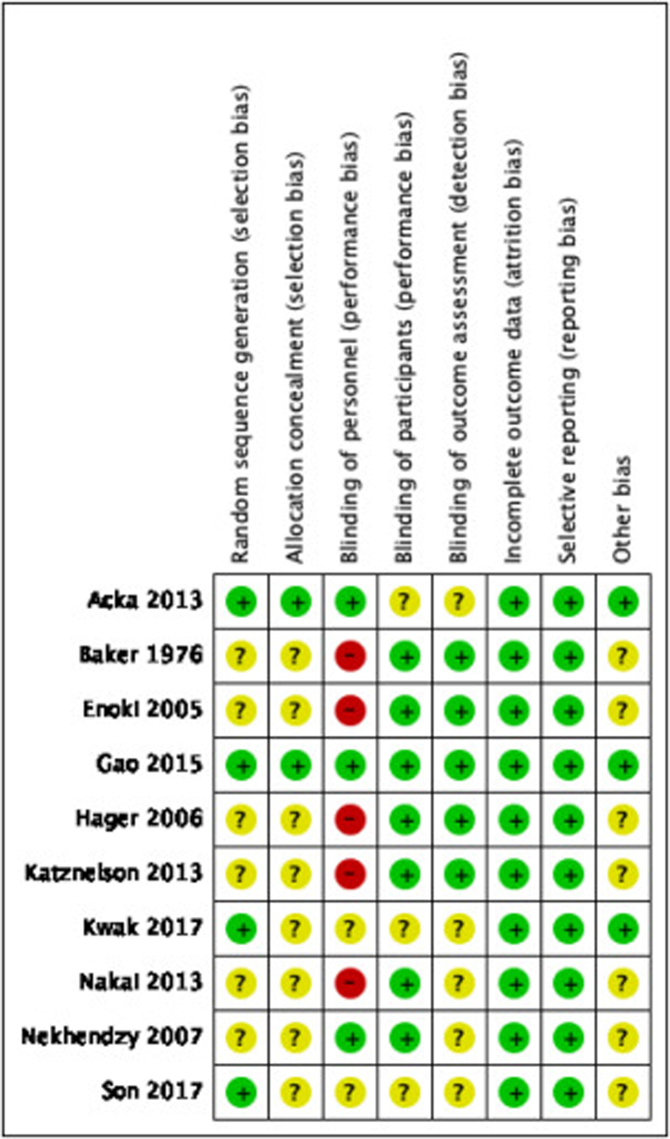
Two authors (JP, CBe) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions.14 We resolved any disagreements by discussion or by involving another author. We summarised the results of the “Risk of bias‿ assessment in both “Risk of bias‿ graph (Figure 2) and “Risk of bias‿ summary (Figure 3) for the following seven risk of bias domains: random sequence generation (checking for possible selection bias); blinding of participants and personnel (checking for possible performance bias); blinding of outcome assessment (checking for possible detection bias); allocation concealment (checking for possible selection bias); incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations); selective reporting (checking for reporting bias); and other bias (checking for other biases).
Figure 2.
Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
Figure 3.
Risk of bias summary: review authors’ judgements about each risk of bias item for each included study.
Measures of treatment effect
We analysed dichotomous data as risk ratios (RR) with 95% confidence intervals (CI). For continuous data, we used the mean difference with 95% CI for outcomes measured in the same way between trials. If pooling of data was not possible, we included a narrative summary of study results for the outcomes assessed in this systematic review.
Assessment of heterogeneity
Where we pooled data using meta-analysis, we assessed the presence heterogeneity by visual inspection of forest plots and by examining the X2 (Chi2) test for heterogeneity. We also assessed statistical heterogeneity in each meta-analysis using the Tau2 (tau-squared), I2 and Chi2 statistics. We regarded heterogeneity as substantial, if the I2 value was high (exceeding 30%); and either there is inconsistency between trials in the direction or magnitude of effects (judged visually) or there was a low p-value (<0.10) in the Chi2 test for heterogeneity; or the estimate of between-study heterogeneity (Tau2) was above zero.
Assessment of reporting biases
As we were not able to pool more than 10 trials, we did not create a funnel plot to explore possible small study biases.
Data synthesis
We undertook meta-analyses only where this was meaningful, i.e. the treatments, participants and the underlying clinical question were similar enough for pooling to make sense. Given the clinical heterogeneity in the setting of included studies (general anesthesia in a heterogenous patients), we used random-effects meta-analysis to produce an overall summary of average treatment effect across trials. We present results as the average treatment effect with its 95% confidence interval, and the estimates of T2 and I2.
Results
The search conducted on July 25, 2018 and on September 10, 2019 for an update identified 407 records. After duplicate removal, 297 records were screened during title-abstract screening of which 30 full-text papers were retrieved for further examination. Finally, 10 studies7, 8, 9, 15, 16, 17, 18, 19, 20, 21 met our inclusion criteria and were included in this systematic review. Katznelson and colleagues, Nakai and colleagues and Son and colleagues contributed data to the meta-analysis normocapnia vs. hypercapnia.17, 19, 21 The study flow diagram is shown in Figure 1.
Study characteristics
The characteristics of included studies table shows full details of the included studies (Table 1).
Trial centres and design: Two studies performed multicentre trial, Akca and colleagues involved 6 hospitals and Enoki and colleagues involved 2 hospitals.7, 16 The other 8 studies performed single centre trials from which all were randomized controlled trials.8, 9, 15, 17, 18, 19, 20
Participants
A total number of 1,794 patients were included, comprising data on 969 female (54.0%), 767 male (42.8%) and 58 undefined (3.2%) patients (Baker and colleagues did not categorize its patients into male and female15). Akca and colleagues, Nekhenzy and colleagues, and Son and colleagues included minimal 60 participants per group. Baker and colleagues included 42 patients in the hypercapnia group but only 16 in the normocapnia group.15
Son included only women, the other studies included male and female patients.21 In this systematic review we included the above mentioned studies comparing patients treated with hypercapnia or normocapnia. A total number of 901 patients were treated in a hypercapnia group (male 383; female 476; uncategorized 42) and 893 patients were treated in a normocapnia group (male 384; female 493; uncategorized 16).
Nakai and colleagues compared the influence of hypercapnia during surgery in elderly and middle-aged patients.19 To avoid clinical heterogeneity in pooling of the studies we focussed on the data from middle-aged patients and excluded the data from elderly patients. There were 30 patients included in the hypercapnia group and 30 patients in the normocapnia group during the study of Nakai and colleagues.19 From these 30 patients per group 15 patients were elderly people remaining 15 middle-aged participants in the hypercapnia and normocapnia group. The mean age of all patients was 50.6 (SD 4.5).
The mean age of the hypercapnia group was 49.6 (SD 4.3) and the mean age of the normocapnia patients was 51.7 (SD 4.7). There was significant clinical heterogeneity with regard to type of surgery among included studies, which has impact on the baseline characteristics of patients within this systematic review. For instance, the mean weight of all the patients is 80.2 kg (SD 37.7). However, without the obese patients by Hager and colleagues9 the mean weight is 67.0 (SD 9.2).
Risk of bias assessment
Table 1 provides details about the risk of bias in the included studies. Figure 2 shows a summary of our risk of bias judgements across studies and Figure 3 shows the risk of bias judgement more detailed per study.
Random sequence generation and allocation concealement (selection bias)
Random sequence generation was at low risk of bias in four trials7, 8, 18, 21 and unclear risk of bias in six trials.9, 15, 16, 17, 19, 20 Participants were randomised using computer-generated numbers7, 18, 21 or sequentially blocking based on a random number table.8 Allocation concealment was at low risk in two trials7, 8 and unclear risk of bias in eight studies9, 15, 16, 17, 18, 19, 20, 21 Acka and colleagues used sealed, sequentially numbered envelopes and Gao and colleagues divided the patients into two groups based on the blocking scheme.7, 8
Blinding (performance and detection bias)
Blinding of personnel was at low risk of bias in three trials,7, 8, 20 unclear risk of bias in two trials,18, 21 and high risk in five studies9, 15, 16, 17, 19 In the study of Acka and colleagues the anesthesiologists were not blinded to group assignments, but gas monitors were shielded to prevent surgeons from determining randomized group assignment.7 Attending surgeons, who were blinded to the randomized assignments, made hospital discharge decisions and wounds were evaluated daily throughout hospitalization by an investigator blinded to treatment. Gao and colleagues used two anesthesiologists, one with access to the randomization, and one anesthesiologist who did not have knowledge of the gas monitor and arterial blood gas analysis data, performed bronchoalveolar lavage.8 All surgeons were blinded during the study of Nekhendzy and colleagues.20 Blinding of participants was at low risk of bias in seven trials.8, 9, 15, 16, 17, 19, 20 During these studies, patients were not blinded but participants could not influence the outcome. Unclear risk of bias was found in three studies7, 18, 21 Blinding of outcome assessment was at low risk of bias in five trials8, 9, 15, 16, 17 and unclear risk of bias in five studies.7, 18, 19, 20, 21
Incomplete outcome data (attrition bias)
All included trials were at low risk of bias for incomplete outcome data. There was no missing data or less than 20% missing data which could be neglected.
Selective reporting (reporting bias)
All included trials were at low risk of bias for reporting bias. All studies described the results they mentioned in the methods section.
Other potential sources of bias
Other sources of bias were at low risk in three trials7, 8, 18 and unclear risk of bias in seven studies.9, 15, 16, 17, 19, 20, 21 Acka and colleagues, Gao and colleagues and Kwak and colleagues provided information about the funding of the study.7, 8, 18 Acka was supported in part by the Gheens Foundation (Louisville, KY, USA) and the Mater College for Postgraduate Research (Ireland).7 Viasys Healthcare (Wheeling, IL, USA) provided the Hi-Ox Oxygen masks. All personnel financial interests have been disclosed. Gao was supported by a grant from the Medjaden Academy and Research Foundation for Young Scientists (Hong Kong, China).8 All funding sources of Kwak were departmental and the authors declared to have no competing interests.18
Effects of interventions
Due to significant heterogeneity in outcome measurement and reporting, pooling of data for meta-analysis was only meaningful for two outcomes time to extubation and adverse events nausea/vomiting.
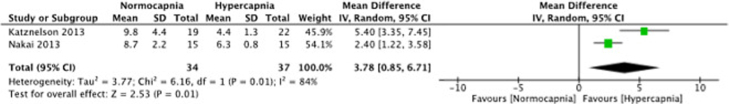
Time to extubation
Among the 10 trials that met the inclusion criteria of the meta-analysis, only two (Katznelson; Nakai) trials measured time to extubation.17, 19 On average, we found that the time to extubation was significantly reduced in the hypercapnia group with a mean difference 3.78 (95% CI 0.85 to 6.71); 2 studies; 71 patients; I2 = 84%; Figure 4. Heterogeneity was substantial as I2 was greater than 30%, the p-value for Chi2 was 0.01 and Tau2 was 3.77.
Figure 4.
Forest plot of comparison: 1 Normocapnia versus hypercapnia, outcome: 1.1 Time to extubation.
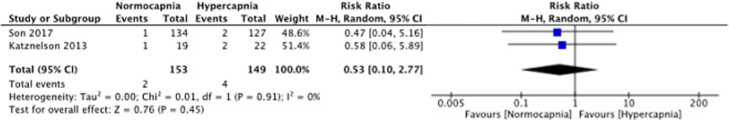
Adverse events (nausea, vomiting)
Two studies (Son and colleagues; Katznelson and colleagues) measured adverse events.17, 21 On average, no difference with regard to adverse events was reported among participants in the normocapnia or hypercapnia group with RR 0.52 (95%CI 0.10 to 2.76); 2 studies; 6 events; I2 = 0%; Figure 5. There was a low risk of heterogeneity as I2 was 0% and the p-value for Chi2 was 0.91.
Figure 5.
Forest plot of comparison: 1 Normocapnia versus hypercapnia, outcome: 1.2 Adverse events (nausea, vomiting).
Incidence of wound healing complications
The work of Akca and colleagues investigated the incidence of surgical site infections, but did not find a significant difference inbetween hypercapnic and normocapnic group.7
Hospital length of stay and hospital mortality
None of the included studies reported on hospital length of stay or mortality.
Discussion
Our systematic database research showed a very heterogenous set of studies. Following title and abstract screening we detected among the full-text articles assessed for eligibility a vast part with poor data according to our inclusion criteria. Due to that fact we were only able to pool a few studies helping to answer our research question, if hypercapnia during general anesthesia might have positive or negative effect on patient's outcome, morbidity and mortality.
Whereas physiological effects of mild changes in CO2 levels on organ perfusion, hemodynamics, heart function and respiration are well examined; Wax and colleagues’ study was the first to show an association of increased end-expiratory CO2 levels during general anesthesia and length of hospital stay.10 In our systematic review we found several studies examining intraoperative CO2 levels connected to a mass of diverse physiological and clinical parameters. Unfortunately, we were not able to find another work beside Wax and colleagues that could produce profound evidence of an impact of hypercapnia during general anesthesia on hard criteria as length of hospital stay or admission to ICU.10
Regional effects of hypercapnia
Among those studies eligible for full text analysis and data extraction several authors investigated cellular processes such as tissue perfusion, inflammatory response, and wound healing.
Akca and colleagues focused on decreased wound infections depending on increased tissue pO2 in hypercapnia.7 Despite a trend towards a decreased rate of surgical site infections in the hypercapnia group (11.2% vs. 13.3%) the study was interrupted by the Executive Committee in interim analysis (n = 1206) because a significant difference after full sample size of n = 2000 patients was not to be expected.
Hager and colleagues worked on improvement of tissue oxygenation by increased CO2 levels.9 Whereas paO2 was identical in hypercapnia and normocapnia group, subcutaneous tissue oxygenation was significantly greater in hypercapnia group (psqO2 mean ± SD [mmHg], 78 ± 31 vs. 56 ± 13).
Gao and colleagues examined suppression of inflammatory response in accordance to hypercapnia in one lung ventilation during lobectomy (8). The hypercapnia group showed a lower expression of tumor necrosis factor (BALF and Interleukins) and required lower respiratory peak (mean ± SD [cmH2O], 22.2 ± 2.9 vs. 29.8 ± 4.6) and plateau pressures (20.5 ± 2.4 vs. 27.1 ± 2.9) during operation resulting in higher dynamic compliance (mean ± SD [ml.cm−1 H2O], 46.6 ± 5.8 vs. 38.9 ± 6.5).
These data demonstrate a regional effect of hypercapnia on cellular pathways, tissue perfusion and local oxygenation, and suggest an influence on patient's recovery which is still to be subject of further investigations.
Hemodynamic effects
Another frequently investigated subject was the influence of mild hypercapnia on hemodynamics.
Enoki and colleagues observed arterial hypotension after induction of anesthesia.16 They were able to show a significantly lower rate of hypotension 15 minutes after induction of anesthesia with Thiopental-Isoflurane in the hypercapnia group (mean systolic arterial pressure, 116 vs. 103, p < 0.05), which was absent in propofol anesthesia).
Kwak and colleagues observed oxygenation and changes in hemodynamics due to hypercapnia.18 In sitting position during shoulder arthroscopy under general anesthesia the incidence of cerebral desaturation was significant lower in the hypercapnia group (0/20 vs. 5/20), regional cerebral oxygenation was significantly increased in the hypercapnia group (p < 0.05). Mean arterial pressure and heart rate were not influenced by hypercapnia.
In summary these data elaborate a CO2 dependence of hemodynamics, especially mild hypercapnia seems play a role in avoidance of hypotension and cerebral desaturation under general anesthesia.
Effects of hypercapnia on outcome parameters
Unfortunately, most of these collected parameters were not able to be connected with either adverse events or positive effects on patient recovery nor were these data comparable to be pooled. The studies mentioned above did not investigate an effect of their findings to further course of hospital stay but focused on intra- and perioperative settings (see above). Questioning several authors of unpublished data concerning our point of investigation was denied.
Finally, we were only able to pool three studies that worked on hypercapnia related effects on general anesthesia and measured the same parameters as time to extubation and adverse events (nausea/vomiting). We were able to compare the outcomes of Katznelson and colleagues and Nakai and colleagues on time to extubation17, 19 and the data of Katznelson and colleagues and Son and colleagues concerning adverse events.17, 21 In our meta-analysis we found a significant decreased time to extubation in the hypercapnia group. In the work of Katznelson and colleagues time to extubation was less than half in the hypercapnia group (4.4 ± 1.3 to 9.8 ± 4.4 min).17 It is well known that CO2 partial pressure appears to be the strongest physiological trigger for respiration in glomus caroticum and central nervous system.22 Patients with increased CO2 levels under general anesthesia show a much shorter time to extubation – in the trial of Katznelson and colleagues even almost half compared to the normocapnia group – probably due to an increased respiration drive.17 These findings could lead to a diminished overall time in the OR to sustain patients recovery and save clinical resources.
Whereas our meta-analysis of time to extubation consisted of an overall population of 71 patients, further trials with a larger set would be desirable in future.
The meta-analysis of adverse events contained a larger population of 302 patients. The pooled data of Katznelson and colleagues and Son and colleagues did not show a significant increased prevalence of adverse events as postoperative nausea and vomiting in the hypercapnia or normocapnia group.17, 21
Limitations
Ten studies matched the inclusion criteria of our systematic review, however, due to significant heterogeneity in outcome definition, measurement and reporting, we were only able to include few studies in our meta-analysis focusing on the influence of intraoperative CO2 partial pressure levels to patient's outcome. Also, patient population of the included studies consisted of relatively small sample sizes. However, based on the evidence that we have cited we are confident to have identified all studies in the field concerning this research question highlighting the need for a well planned and adequately powered randomized controlled trial.
Conclusion
These findings do not enable us to produce evidence of a positive influence of increased CO2 partial pressure levels during general anesthesia on morbidity or mortality. However, it could be shown that increased CO2 partial pressures leads to an earlier extubation in comparison to normal intraoperative CO2 levels.
Since only a limited number of patients were included in well randomized studies, a large prospective randomized trial is warranted/desireable to define the value of high PCO2-levels during surgery.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We acknowledge the authors of the primary studies included in the meta-analyses, who have kindly provided additional data and information regarding their studies.
References
- 1.Laffey J.G., Honan D., Hopkins N., et al. Hypercapnic acidosis attenuates endotoxin-induced acute lung injury. Am J Respir Crit Care Med. 2004;169:46–56. doi: 10.1164/rccm.200205-394OC. [DOI] [PubMed] [Google Scholar]
- 2.Kety S.S., Schmidt C.F. The Effects of active and passive hyperventilation one cerebral blood flow, cerebral oxygen consumption, cardia output, and blood pressure of normal young men. J Clin Invest. 1946;25:107–119. [PubMed] [Google Scholar]
- 3.Kety S.S., Schmidt C.F. The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J Clin Invest. 1948;27:484–492. doi: 10.1172/JCI101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forkin K.T., Nemergut E.C. Miller’s anesthesia, 8th edition. Anesthesiology: J Am Soc Anesthesiol. 2016;124:977–978. [Google Scholar]
- 5.Gropper M.A. 8th ed. Elsevier; Philadelphia: 2016. Miller’s anesthesia. [Google Scholar]
- 6.Ijland M.M., Heunks L.M., van der Hoeven J.G. Bench-to-bedside review: hypercapnic acidosis in lung injury – from “permissive‿ to “therapeutic‿. Crit Care. 2010;14:237. doi: 10.1186/cc9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akca O., Kurz A., Fleischmann E., et al. Hypercapnia and surgical site infection: a randomized trial. Br J Anaesth. 2013;111:759–767. doi: 10.1093/bja/aet233. [DOI] [PubMed] [Google Scholar]
- 8.Gao W., Liu D.D., Li D., et al. Effect of therapeutic hypercapnia on inflammatory responses to one-lung ventilation in lobectomy patients. Anesthesiology. 2015;122:1235–1252. doi: 10.1097/ALN.0000000000000627. [DOI] [PubMed] [Google Scholar]
- 9.Hager H., Reddy D., Mandadi G., et al. Hypercapnia improves tissue oxygenation in morbidly obese surgical patients. Anesth Analg. 2006;103:677–681. doi: 10.1213/01.ane.0000229715.71464.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wax D.B., Lin H.M., Hossain S., et al. Intraoperative carbon dioxide management and outcomes. Eur J Anaesthesiol. 2010;27:819–823. doi: 10.1097/EJA.0b013e32833cca07. [DOI] [PubMed] [Google Scholar]
- 11.Moher D., Liberati A., Tetzlaff J., et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. W64, W64. [DOI] [PubMed] [Google Scholar]
- 12.Klotz S., Boeken U. Zur S3-Leitlinie Invasive Beatmung und Einsatz extrakorporaler Verfahren bei akuter respiratorischer Insuffizienz. Zeitschrift für Herz-, Thorax- und Gefäßchirurgie. 2019;33:107–115. [Google Scholar]
- 13.5.3 ed. The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen: 2014. Review Manager (RevMan) [Google Scholar]
- 14.Higgins J.P.T. In: Cochrane handbook for systematic reviews of interventions. Cochrane, Version 6.0 (updated July 2019) Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A., editors. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker W.H., Rodman J.A., Barnes R.W., et al. An evaluation of hypocarbia and hypercarbia during carotid endarterectomy. Stroke. 1976;7:451–454. doi: 10.1161/01.str.7.5.451. [DOI] [PubMed] [Google Scholar]
- 16.Enoki T., Tsuchiya N., Shinomura T., et al. Effect of hypercapnia on arterial hypotension after induction of anaesthesia. Acta Anaesthesiol Scand. 2005;49:687–691. doi: 10.1111/j.1399-6576.2005.00681.x. [DOI] [PubMed] [Google Scholar]
- 17.Katznelson R., Djaiani G., Naughton F., et al. Post-operative hypercapnia-induced hyperpnoea accelerates recovery from sevoflurane anaesthesia: a prospective randomised controlled trial. Acta Anaesthesiol Scand. 2013;57:623–630. doi: 10.1111/aas.12093. [DOI] [PubMed] [Google Scholar]
- 18.Kwak H.J., Lee J.Y., Wha Lee J., et al. Effect of mild hypercapnia on lung oxygenation in sitting position during shoulder arthroscopy under general anesthesia. Med Sci Monit. 2017;23:843–849. doi: 10.12659/MSM.899801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakai K., Yoshida H., Hashimoto H., et al. Mild hypercapnia with hyperventilation attenuates recovery from anesthesia in elderly patients. J Anesth. 2013;27:712–719. doi: 10.1007/s00540-013-1617-5. [DOI] [PubMed] [Google Scholar]
- 20.Nekhendzy V., Lemmens H.J., Vaughan W.C., et al. The effect of deliberate hypercapnia and hypocapnia on intraoperative blood loss and quality of surgical field during functional endoscopic sinus surgery. Anesth Analg. 2007;105:1404–1409. doi: 10.1213/01.ane.0000282781.56025.52. table of contents. [DOI] [PubMed] [Google Scholar]
- 21.Son J.S., Oh J.Y., Ko S. Effects of hypercapnia on postoperative nausea and vomiting after laparoscopic surgery: a double-blind randomized controlled study. Surg Endosc. 2017;31:4576–4582. doi: 10.1007/s00464-017-5519-8. [DOI] [PubMed] [Google Scholar]
- 22.Haldane J.S., Priestley J.G. The regulation of the lung-ventilation. J Physiol. 1905;32:225–266. doi: 10.1113/jphysiol.1905.sp001081. [DOI] [PMC free article] [PubMed] [Google Scholar]