Abstract
BkdR is the transcriptional activator of the bkd operon, which encodes the four proteins of the branched-chain keto acid dehydrogenase multienzyme complex of Pseudomonas putida. In this study, hydroxyl radical footprinting revealed that BkdR bound to only one face of DNA over the same region identified in DNase I protection assays. Deletions of even a few bases in the 5′ region of the BkdR-binding site greatly reduced transcription, confirming that the entire protected region is necessary for transcription. In vitro transcription of the bkd operon was obtained by using a vector containing the bkdR-bkdA1 intergenic region plus the putative ρ-independent terminator of the bkd operon. Substrate DNA, BkdR, and any of the l-branched-chain amino acids or d-leucine was required for transcription. Branched-chain keto acids, d-valine, and d-isoleucine did not promote transcription. Therefore, the l-branched-chain amino acids and d-leucine are the inducers of the bkd operon. The concentration of l-valine required for half-maximal transcription was 2.8 mM, which is similar to that needed to cause half-maximal proteolysis due to a conformational change in BkdR. A model for transcriptional activation of the bkd operon by BkdR during enzyme induction which incorporates these results is presented.
Pseudomonas putida biotypes (20) are major players in the bioremediation of soil and water and metabolize a wide variety of natural and human-made compounds. P. putida possesses inducible pathways for the oxidation of many, if not all, amino acids even though they are required for protein synthesis. Branched-chain keto acid dehydrogenase is a multienzyme complex which catalyzes the oxidative decarboxylation of branched-chain keto acids resulting from transamination of l-branched-chain amino acids or oxidation of d-branched-chain amino acids. There is a good deal of interest in the structure and function of keto acid dehydrogenase multienzyme complexes (12) because of their tremendous size and because mutations in humans affecting the components of the complex result in serious genetic diseases (15).
The four proteins of the complex are encoded by the structural genes of the bkd operon, the expression of which is under positive control by BkdR (10). BkdR is a homologue of Lrp, which is a global transcriptional regulator in Escherichia coli (2). Lrp (for leucine-responsive protein) can act as an activator or a repressor in the presence of leucine or may be unaffected by the presence of l-leucine. In contrast, the only known function of BkdR is to activate transcription of the bkd operon. Expression of the bkd operon of P. putida is induced by growth on branched-chain amino or keto acids (11) and subject to catabolite repression by glucose, succinate, or ammonium ion (21). Branched-chain keto acid dehydrogenase is induced in media containing d- or l-branched-chain amino acids or branched-chain α-keto acids (11). Since BkdR is a transcriptional activator of the bkd operon, one would predict that the actual inducers would be required for BkdR-mediated transcription. Previous data implicated l-branched-chain amino acids as the inducers because addition of l-branched-chain amino acids to DNase I protection assays enhanced the appearance of hypersensitive sites in the protected region (9). However, l-branched-chain amino acids did not affect either the stoichiometry of the BkdR-DNA complex or its mobility (5). d-Valine and α-ketoisovalerate had no effect on the protection pattern. Enhancement of hypersensitive sites suggested that l-branched-chain amino acids effected a conformational change in BkdR, which was subsequently demonstrated by circular dichroism spectroscopy and limited proteolysis (8). Although l-branched-chain amino acids did not affect the migration of BkdR-DNA complexes in gel shift assays, they did affect bending of DNA caused by binding of BkdR (6); BkdR alone caused a bend angle of 92° while the angle formed by binding of BkdR plus l-valine was 76°.
These data predicted that l-valine and probably other l-branched-chain amino acids were the actual inducers of the bkd operon. The objective of this study was to identify the true inducers directly by determining the requirements for in vitro transcription of the bkd operon and to obtain additional information about the binding of BkdR.
MATERIALS AND METHODS
Bacterial strains and media.
For induction of branched-chain keto acid dehydrogenase, P. putida PpG2 was grown in 0.3% l-valine and 0.1% l-isoleucine mineral salt medium (11) with aeration at 30°C. This strain grows more rapidly in valine-isoleucine medium than in medium with l-valine alone because l-valine inhibits isoleucine synthesis. Mutants affected in bkdR or the bkd operon cannot metabolize branched-chain amino acids and are grown in valine-isoleucine medium plus a nonrepressing metabolizable substrate such as lactate or gluconate. E. coli DH5α was grown in 2× YT (18) medium with 200 μg of ampicillin/ml when carrying pUC plasmids. When P. putida carried pKRZ-1 plasmids, it was grown in medium with 90 μg of kanamycin/ml.
Reagents.
BkdR was prepared as described in reference 9. The samples of d-leucine were obtained from TCI and Aldrich Chemical Company. E. coli RNA polymerase holoenzyme, saturated with ς70, was obtained from Epicentre Technologies.
Plasmid constructions.
The plasmids used in this study are summarized in Table 1. pJRS168 was the template used for in vitro transcription of the bkd operon. For construction of pJRS168, an EcoRV/SalI fragment was transcloned from pJRS25 (7) to pBluescript II SK(+). This fragment contains nucleotides (nt) 1407 to 1753 (Fig. 1) and includes nt 1544, the transcriptional start site of the bkd operon. The EcoRV/SalI fragment was then transcloned into pJRS85, with EcoRI and KpnI pJRS85 contains the ρ-independent transcriptional terminator of the bkd operon on a KpnI/SphI fragment (nt 6497 to 6733 [GenBank sequence M57613]) in pUC19. This placed the terminator immediately downstream of the nucleotides encoding the transcript. The host for pJRS168 was E. coli DH5α (Gibco BRL), and DNA was purified with a QIAprep Spin Miniprep kit (Qiagen).
TABLE 1.
Plasmids used in this study
| Plasmid | Genotype or phenotypea | Reference or source |
|---|---|---|
| pBluescript II SK(+) | Phagemid cloning vector | Stratagene |
| pJRS25 | bkdR, intergenic region and part of bkdA1 in pUC19 | 7 |
| pJRS85 | ρ-independent terminator for bkd operon in pUC19 | This study |
| pJRS168 | EcoRV/SalI fragment (Fig. 1) plus the bkd operon ρ-independent terminator in pUC19 | This study |
| pJRS177 | nt 1434–1698 in pKRZ-1 | This study |
| pJRS178 | nt 1418–1698 in pKRZ-1 | This study |
| pJRS179 | nt 1449–1698 in pKRZ-1 | This study |
| pJRS185 | nt 1434–1698 plus ρ-independent terminator in pUC19 | This study |
| pJRS186 | nt 1418–1698 plus ρ-independent terminator in pUC19 | This study |
| pJRS187 | nt 1449–1698 plus ρ-independent terminator in pUC19 | This study |
| pKRZ-1 | Broad-host-range promoter probe vector | 17 |
| pUC19 | Cloning vector | 25 |
Nucleotide numbering is taken from GenBank sequence M57613.
FIG. 1.
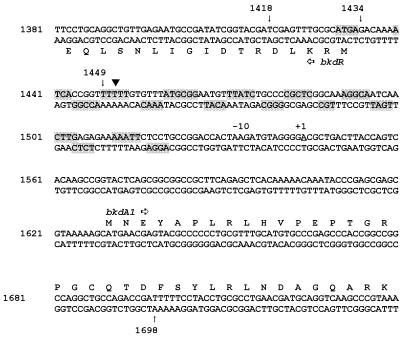
Nucleotide sequence of the DNA fragment studied in this report. Nucleotide numbering is taken from GenBank sequence M57613. The shaded bases are those protected from the action of hydroxyl radicals by binding of BkdR. Part of the coding sequences for bkdR and bkdA1 are translated to show the location of these genes. The arrows indicate the 5′ ends of the DNA fragments summarized in Table 2. The bend center is marked by the inverted triangle, and the transcriptional start is marked +1. There is no identifiable −35 hexamer.
Plasmids pJRS177 to -179 and pJRS185 to -187 were constructed from the same PCR fragments but were in different vectors. The vector for pJRS177, -178, and -179 was pKRZ-1 (17), while the vector for pJRS185, -186, and -187 was pUC19 (25). The fragments were created by PCR with pJRS82 (4) as the template as described in reference 6. The forward primers were 141, 142, and 142, and the reverse primer was 87 in all cases (Table 2). The PCR fragments were blunted with Klenow reagent and deoxynucleoside triphosphates and then cloned into the EcoRV site of pBluescript SK(+) (Stratagene). The fragments were released from pBluescript SK(+) with XbaI and HincII and cloned into the XbaI and SmaI sites of pKRZ-1, creating pJRS177, -178, and -179. Again, the host for these plasmids was E. coli DH5α, and the plasmids were introduced into P. putida PpG2 by triparental mating (19). For the data shown in Table 3, P. putida PpG2 containing pJRS177 to -179 was grown in valine-isoleucine medium to an A660 of 0.5, harvested, disrupted by sonic oscillation, centrifuged at 90,000 × g for 1 h, and then assayed for β-galactosidase (14).
TABLE 2.
Reverse and forward primers used for creation of PCR fragments
| Primer no. | Sequence | nta |
|---|---|---|
| Reverse primers | ||
| 142 | 5′-TCGAGTTTGCGCATGAGACAA-3′ | 1418–1437 |
| 141 | 5′-GACAAAATCACCGGTTTTTTG-3′ | 1434–1456 |
| 143 | 5′-TTTTTGTGTTTATGCGGAATG-3′ | 1449–1470 |
| Forward primer 87 | 5′-ATCGGTCTGGCAGCCTGG-3′ | 1681–1698 |
TABLE 3.
Effect of upstream deletions in the BkdR-binding region on bkd promoter activity
For the construction of pJRS185, -186, and -187, PCR fragments described in the previous paragraph were transcloned from the pBluescript SK(+) intermediate constructs into pUC19. The PCR fragments were released from the pBluescript intermediate constructs by digestion with EcoRI and XhoI and transcloned into pJRS168, also digested with EcoRI and XhoI. By this means, the insert of pJRS168 was replaced with the PCR fragments cloned into the pBluescript intermediate constructs. The host for pJRS185 to -187 was E. coli DH5α, and DNA was purified with a QIAprep Spin Miniprep kit (Qiagen).
DNase I and hydroxyl radical footprinting.
DNase I footprinting was performed as described in an earlier study (8). Hydroxyl radical footprinting was carried out as described in reference 22. Fragments A and B, previously used for DNase I footprinting (8), were also used for hydroxyl radical footprinting. Fragment A included nt 1300 to 1678 (Fig. 1), and fragment B included nt 1407 to 1678 (Fig. 1). Fragment A was labeled with [α-32P]dCTP and Klenow reagent at the 3′ end of the bottom strand shown in Fig. 1, and fragment B was labeled at the 3′ end of the top strand shown in Fig. 1 by the same procedure. Radioactivity was measured with a Molecular Dynamics phosphorimager.
In vitro transcription.
The volume of the reaction mixture was 35 μl. The buffer contained (final concentrations) 100 mM potassium glutamate, 50 mM KCl, 10 mM Tris-HCl (pH 8.0), 0.1 mM EDTA, 10 μg of bovine serum albumin/ml, 1 mM CaCl2, 5% glycerol, 1 mM dithiothreitol, and 5 mM MgCl2. Next, DNA template was added and the mixture was incubated for 5 min. Then 1.2 × 10−9 M RNA polymerase ς70 holoenzyme (Epicentre Technologies); 250 μM (each) ATP, GTP, and CTP; 25 μM [α-32P]UTP; and 50 μg of heparin per ml were added, and the mixture was incubated for an additional 5 min at 37°C. The reaction was chased with 250 μM UTP; incubated for 10 min; and then terminated with 2 volumes of ethanol, a 1/3 volume of 3 M sodium acetate, and 2 μl of 1% yeast tRNA as a carrier for the precipitation of RNA. The precipitate was dissolved in 6 μl of formamide dye mixture (75% formamide, 0.075% xylene cyanol, and 0.075% bromphenol blue) and then resolved in an 8% urea–6% polyacrylamide gel. The amount of radioactivity in the gel was determined by use of the phosphorimager.
RESULTS
Hydroxyl radical footprinting.
The footprint of BkdR on its substrate DNA, which was protected from the action of DNase I (9), extended from approximately nt 1420 to nt 1520 (Fig. 1). Addition of l-valine resulted in formation of hypersensitive sites at about nt 1453, 1475, and 1495 on the top strand and about nt 1455 and 1495 on the bottom strand. Hydroxyl radical footprinting was used to further characterize the binding of BkdR to substrate DNA (Fig. 2). Simultaneous chemical sequencing of DNA (13) identified specific nucleotides that were protected from hydroxyl radicals by binding of BkdR. The region of DNA covered by the hydroxyl radical footprint is nearly identical to that covered by the DNase I footprint (9). An interesting feature of the hydroxyl radical footprint is that protection is phased at about every 10 bases on both the top and bottom strands, which indicates that BkdR binds to one face of DNA (Fig. 1).
FIG. 2.
Hydroxyl radical footprint of the BkdR-DNA complex. (A) Sequence of the top strand in Fig. 1. (B) Sequence of the bottom strand in Fig. 1.
Upstream boundary of the region required for transcription.
In a previous study (6), it was shown that the downstream boundary of the region required for transcription of the bkd operon was very close to the transcriptional start site of the bkd operon (nt 1544 [Fig. 1]). In order to determine the upstream boundary of this region, several PCR-amplified fragments were constructed with 5′ ends at nt 1418, 1434, and 1449 and the 3′ end at nt 1698 (Fig. 1 and Materials and Methods). These fragments were cloned into pKRZ-1, with the bkd promoter oriented toward the promoterless lacZ reporter gene (17). The host for these plasmids was P. putida PpG2 grown in valine-isoleucine (inducing) medium as described in Materials and Methods. The results of the β-galactosidase assays are shown in Table 3. There was a noticeable decrease in the rate of transcription when the upstream nucleotide pair was 1434, which is just inside of the BkdR-binding region (Fig. 1). There was a much greater decrease, with only ∼4% of the activity retained, when the upstream boundary was nt 1449, which is near the bend center of the transcriptionally active DNA fragment (Fig. 1) (6) and in a region of six consecutive thymines. Therefore, the DNA fragment necessary for transcription of the bkd operon in vivo extends from the start of the BkdR-binding region to the transcriptional start site of the bkd operon.
In vitro transcription of the bkd operon.
The vector constructed for in vitro transcription studies of the bkd operon was pJRS168, which contains the entire region to which BkdR binds, fragments of bkdR and bkdA1, and a transcriptional terminator in pUC19. The terminator was the putative ρ-independent terminator of the bkd operon located downstream of lpdV, the final gene of the operon (1). The length of the transcript was calculated to be 368 bp, including restriction sites introduced during cloning.
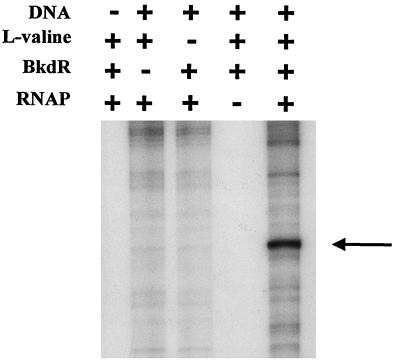
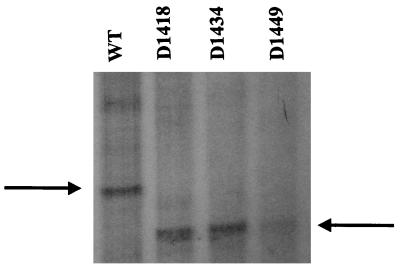
The factors required for transcription of the bkd operon were identified by the experiment whose results are shown in Fig. 3. Template DNA in the form of pJRS168, RNA polymerase containing ς70, BkdR, and l-valine all were required for transcription. There was very little expression of the bkd operon by RNA polymerase in the absence of BkdR, or RNA polymerase plus BkdR, but transcription was increased at least 8- to 10-fold over the background by the addition of l-valine. Therefore, l-valine is one of the inducers of the bkd operon. Some transcript was obtained with linear DNA, but transcription was much more efficient with pJRS168, which provided supercoiled DNA (data not shown). Since RNA polymerase used in these experiments was ς70 holoenzyme and since there is a −10 hexamer, TAAGAT, just upstream of the transcriptional start site of the bkd operon (nt 1544 [Fig. 1]), it can be concluded that the bkd operon is transcribed from a ς70 promoter. Because the transcript was the correct size, it can also be concluded that the presumed ρ-independent terminator functioned as predicted. The fragment of DNA protected from the action of DNase I (9) and hydroxyl radicals (Fig. 1) by BkdR and that is required for the expression of bkd operon-lacZ fusions (Table 3) is about 90 bp, which is unusually long. However, in vitro transcription experiments confirmed that this large fragment was necessary for transcription. A series of plasmids was constructed with the same PCR fragments as those shown in Table 3 plus the downstream ρ-independent terminator and used for in vitro transcription (Fig. 4). Very little transcript was obtained when the upstream end of the fragment was nt 1449, which is in the BkdR-binding region near the bend center (Fig. 1). When the upstream end of the PCR fragment was nt 1434 or 1418, the amount of transcript was similar to that obtained with pJRS168.
FIG. 3.
Factors required for in vitro transcription of the bkd operon. The DNA template was 1.8 × 10−10 M pJRS168, and the concentration of l-valine was 10 mM. The protocol and the concentrations of the other reactants are described in Materials and Methods. The arrow indicates the expected size of the message. RNAP, RNA polymerase.
FIG. 4.
Effect of deletions at the 5′ end of the BkdR-binding region on in vitro transcription. Because the PCR fragments were slightly shorter than the fragment in pJRS168, the size of the transcript was smaller, 321 compared with 368 bp for pJRS168. The arrows indicate the size of the expected transcript. WT, wild type.
Half-maximum transcription was obtained with a BkdR tetramer concentration of 2.5 × 10−9 M with pJRS168 as the DNA template. The calculated Km for l-valine in substrate saturation studies of transcription was 2.8 mM. The saturation curve was conventional, although there was some inhibition of transcription above 25 mM l-valine. Binding of l-valine causes a conformational change in BkdR, which enhances proteolysis of BkdR by trypsin (8). The half-maximal velocity of proteolysis was obtained at 2.5 mM l-valine, which is very close to the figure obtained in this study for in vitro transcription.
Other ligands which promote transcription.
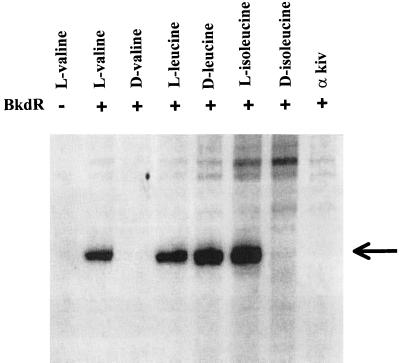
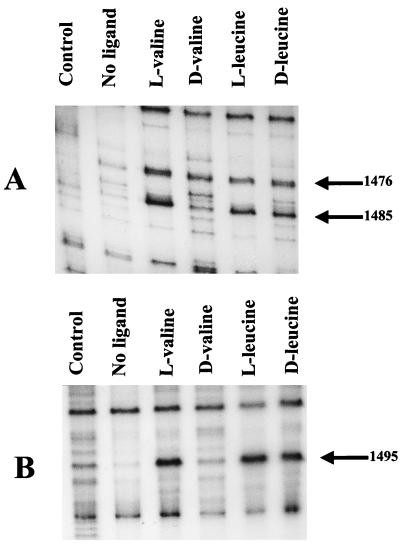
The l and d forms of valine, leucine, isoleucine, and α-ketoisovalerate were tested at 15 mM for their ability to promote transcription with pJRS168 as the DNA template (Fig. 5). The l forms of all three branched-chain amino acids stimulated transcription; therefore, l-leucine and l-isoleucine are inducers of the bkd operon in addition to l-valine. α-Ketoisovalerate and the d forms of valine and isoleucine had no effect and can be ruled out as inducers, even though branched-chain keto acid dehydrogenase is induced by growth in media with these compounds. Surprisingly, the d form of leucine was nearly as effective as l-leucine in stimulating transcription, a result that was obtained with d-leucine from two different suppliers. The Km for l-leucine was 4.4 mM, and the Km for d-leucine was 6.2 mM. The optical rotation obtained from both bottles of d-leucine was the same as the literature value, and so the bottles were not mislabeled.
FIG. 5.
Ability of l-branched-chain amino acids and d-leucine to initiate in vitro transcription of the bkd operon. The DNA template was pJRS168, and the potential ligands were tested at 15 mM. The arrow indicates the size of the expected transcript. kiv, ketoisovalerate.
Effect of d-leucine on DNase I footprint.
The addition of l-valine to BkdR and substrate DNA caused an enhancement of the hypersensitive sites when treated with DNase I (9). In view of the activity of d-leucine in transcription, and the failure of d-valine and d-isoleucine to stimulate transcription, the DNase I footprint obtained in the presence of d-leucine was compared with that obtained with l-valine (Fig. 6). The footprints were identical, so that the effect of d-leucine on transcription must be the same as that of the l-branched-chain amino acids and not an artifact. Therefore, d-leucine must also be an inducer of the bkd operon.
FIG. 6.
Effect of d-leucine on the DNase I protection assay. (A) Pattern obtained with the top strand in Fig. 1. (B) Pattern obtained with the bottom strand. Each of the ligands was tested at 15 mM, and the labeled fragments were the A and B fragments used in reference 9.
DISCUSSION
Transcription of the bkd operon during induction.
In noninducing media, BkdR binds to substrate DNA (5) but transcription does not take place because BkdR and DNA are not in the correct configuration. Transcriptionally inactive BkdR binds to one face of a 90-bp segment of DNA which extends from the initiating codon of bkdR to −25 relative to the transcriptional start site of the bkd operon (Fig. 1). When P. putida grows in an inducing medium, the intracellular concentration of l-branched-chain amino acids (or d-leucine) reaches the near-millimolar value, and several changes occur, the net result of which is transcription of the bkd operon. Induction obtained when P. putida is grown in media with d-valine, d-isoleucine, or branched-chain keto acids must be due to the conversion of these compounds to l-branched-chain amino acids (11). The concentration of l-valine which results in half-maximal transcription is high enough to prevent induction of branched-chain keto acid dehydrogenase in the absence of exogenous branched-chain amino acids. Conversely, the concentration of l-branched-chain amino acids in the pool is not high enough to cause induction.
The presence of inducers brings about two important changes that are essential for transcription of the bkd operon; BkdR undergoes a conformational change to active BkdR (8) and substrate DNA is bent to the correct angle (6). The conformational change exposes the hinge region of BkdR between the DNA-binding domain and the rest of BkdR, making it susceptible to proteolysis (8). The BkdR and RNA polymerase-binding regions are very close together, and so they can easily come in contact. However, the change in bend angle of DNA and change in conformation of BkdR are necessary to bring the transcriptional complex into the correct configuration for transcription.
The BkdR DNA-binding site.
BkdR binds one face of a long stretch of DNA between nt 1430 and 1520 (Fig. 1) (6). Lrp binds to one face of an Lrp-binding site in the leader region of the ilvGMEDA operon (16), which is discussed below. It is interesting that the moles percent guanine plus cytosine for the BkdR-binding site is 42% compared to 64% for the GenBank sequence M57613 which includes bkdR, the intergenic region, and the structural genes of the bkd operon of P. putida. The BkdR DNA-binding site very likely overlaps the transcriptional start site for bkdR, which would explain the repression of bkdR expression by BkdR (9). This is a rather large DNA-binding site, but since the stoichiometry of BkdR tetramer per mole of substrate DNA is 3:1 (5) and the Stokes radius is 32 Å (8), a large segment of DNA would be required. Binding of BkdR is probably cooperative since there was only one identifiable BkdR-DNA complex seen in gel shift assays (9), and cleavage of the binding site near the center of the bend angle resulted in total abolition of DNA binding (8). Inspection of the bases protected from hydroxyl radicals (Fig. 1) did not reveal any consistent BkdR-binding motif.
There is a difference between in vivo transcription and translation and in vitro transcription since less β-galactosidase than transcript was produced when the 5′ end of the DNA fragment was nt 1434 (Table 3 and Fig. 4). For some unknown reason, the entire in vivo transcript is not converted to translatable message. However, the two systems are not directly comparable, and there are many possible explanations for this result. For example, the message obtained from the lacZ fusion is not a normal message, and this transcript may not be as stable as the normal transcript.
Comparison of Lrp and BkdR.
Since BkdR and Lrp are homologous transcription factors, it is interesting to compare their properties and activities. The major functional difference is that Lrp is a global regulator while BkdR is a specific activator of the bkd operon. This is reflected in a 100-fold difference in copy numbers per cell: ca. 3,000 for Lrp (2) and 25 to 40 for BkdR (9). It is interesting, however, that Lrp can complement bkdR mutations in P. putida (10). Lrp can be either an activator or a repressor, and leucine may or may not have an effect on the action of Lrp (2). BkdR is strictly a transcriptional activator of the bkd operon, which requires l-branched-chain amino acids or d-leucine for expression. There is 36.5% sequence identity between Lrp and BkdR and 58% similarity. An interesting difference between the two proteins is that the pI for Lrp is 9.24 (24) and the pI for BkdR is 5.89 (10). Lrp is a dimer (24), while BkdR is a tetramer (8).
Lrp produces several complexes with DNA (3), while BkdR produces a single complex with a stoichiometry of three BkdR tetramers to one of substrate DNA (5). There are multiple DNA-binding sites for Lrp on the chromosome of E. coli (16, 23) but only one binding site for BkdR on the chromosome of P. putida (10). A consensus binding site for Lrp was reported in reference 3 as YAGHAWATTWT where Y = C or T, H = not G, W = A or T, D = not C, and R = A or G. Rhee et al. (16) found a high-affinity, 14-bp Lrp consensus sequence in the leader region of the ilvGMEDA operon of E. coli. Inspection of the binding region for BkdR (Fig. 1) did not reveal a clear Lrp consensus-binding site; however, there are several regions which are rich in A and T. Rhee et al. (16) also reported that Lrp bound to one face of the primary and secondary Lrp-binding sites, which is the case with BkdR as reported in this study (Fig. 1 and 2).
ACKNOWLEDGMENTS
This research was supported by Public Health Service grant DK 21737 and Presbyterian Health Foundation grant C5142801.
We extend our appreciation to Fritz Schmitz for the optical rotation analyses.
REFERENCES
- 1.Burns G, Brown T, Hatter K, Sokatch J R. Sequence analysis of the lpdV gene for lipoamide dehydrogenase of branched chain oxoacid dehydrogenase of Pseudomonas putida. Eur J Biochem. 1989;179:61–69. doi: 10.1111/j.1432-1033.1989.tb14521.x. [DOI] [PubMed] [Google Scholar]
- 2.Calvo J M, Mathews R G. The leucine-responsive regulatory protein, a global regulator of metabolism in Escherichia coli. Microbiol Rev. 1994;58:466–490. doi: 10.1128/mr.58.3.466-490.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui Y, Wang Q, Stormo G D, Calvo J M. A consensus sequence for binding of Lrp to DNA. J Bacteriol. 1995;177:4872–4880. doi: 10.1128/jb.177.17.4872-4880.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hester K, Luo J, Burns G, Braswell E H, Sokatch J R. Purification of active E1α2β2 of Pseudomonas putida branched-chain-oxoacid dehydrogenase. Eur J Biochem. 1995;233:828–836. doi: 10.1111/j.1432-1033.1995.828_3.x. [DOI] [PubMed] [Google Scholar]
- 5.Huang N, Madhusudhan K T, Sokatch J R. Stoichiometry of BkdR to substrate DNA in Pseudomonas putida. Biochem Biophys Res Commun. 1996;223:315–319. doi: 10.1006/bbrc.1996.0891. [DOI] [PubMed] [Google Scholar]
- 6.Madhusudhan K T, Hester K L, Friend V, Sokatch J R. Transcriptional activation of the bkd operon of Pseudomonas putida by BkdR. J Bacteriol. 1997;179:1992–1997. doi: 10.1128/jb.179.6.1992-1997.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madhusudhan K T, Huang G, Burns G, Sokatch J R. Transcriptional analysis of the promoter region of the Pseudomonas putida branched-chain keto acid dehydrogenase operon. J Bacteriol. 1990;172:5655–5663. doi: 10.1128/jb.172.10.5655-5663.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madhusudhan K T, Huang N, Braswell E H, Sokatch J R. Binding of l-branched-chain amino acids causes a conformational change in BkdR. J Bacteriol. 1997;179:276–279. doi: 10.1128/jb.179.1.276-279.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madhusudhan K T, Huang N, Sokatch J R. Characterization of BkdR-DNA binding in the expression of the bkd operon of Pseudomonas putida. J Bacteriol. 1995;177:636–641. doi: 10.1128/jb.177.3.636-641.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madhusudhan K T, Lorenz D, Sokatch J R. The bkdR gene of Pseudomonas putida is required for expression of the bkd operon and encodes a protein related to Lrp of Escherichia coli. J Bacteriol. 1993;175:3934–3940. doi: 10.1128/jb.175.13.3934-3940.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marshall V P, Sokatch J R. Regulation of valine catabolism in Pseudomonas putida. J Bacteriol. 1972;110:1073–1081. doi: 10.1128/jb.110.3.1073-1081.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mattevi A, de Kok A, Perham R N. The pyruvate dehydrogenase multienzyme complex. Curr Opin Struct Biol. 1992;2:877–887. [Google Scholar]
- 13.Maxam A M, Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65:499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- 14.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 15.Patel M S, Harris R A. Mammalian α-keto acid dehydrogenase complexes: gene regulation and genetic defects. FASEB J. 1995;9:1164–1172. doi: 10.1096/fasebj.9.12.7672509. [DOI] [PubMed] [Google Scholar]
- 16.Rhee K Y, Parekh B S, Hatfield G W. Leucine-responsive regulatory protein-DNA interactions in the leader region of the ilvGMEDA operon of Escherichia coli. J Biol Chem. 1998;271:26499–26507. doi: 10.1074/jbc.271.43.26499. [DOI] [PubMed] [Google Scholar]
- 17.Rothmel R K, Shinabarger D L, Parsek M R, Aldrich T L, Chakrabarty A M. Functional analysis of the Pseudomonas putida regulatory protein CatR: transcriptional studies and determination of the CatR DNA-binding site by hydroxyl-radical footprinting. J Bacteriol. 1991;173:4717–4724. doi: 10.1128/jb.173.15.4717-4724.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 19.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 20.Stanier R Y, Palleroni N J, Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966;43:159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- 21.Sykes P J, Burns G, Menard J, Hatter K, Sokatch J R. Molecular cloning of genes encoding branched-chain keto acid dehydrogenase of Pseudomonas putida. J Bacteriol. 1987;169:1619–1625. doi: 10.1128/jb.169.4.1619-1625.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tullius T D, Dombroski B A, Churchill M E A, Kam L. Hydroxyl radical footprinting: a high-resolution method for mapping protein-DNA contacts. Methods Enzymol. 1987;155:537–558. doi: 10.1016/0076-6879(87)55035-2. [DOI] [PubMed] [Google Scholar]
- 23.Wang Q, Calvo J M. Lrp, a global regulatory protein of Escherichia coli, binds co-operatively to multiple sites and activates transcription of ilvIH. J Mol Biol. 1993;229:306–318. doi: 10.1006/jmbi.1993.1036. [DOI] [PubMed] [Google Scholar]
- 24.Willins D A, Ryan C W, Platko J V, Calvo J M. Characterization of Lrp, an Escherichia coli regulatory protein that mediates a global response to leucine. J Biol Chem. 1991;266:10768–10774. [PubMed] [Google Scholar]
- 25.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]