Abstract
Purpose of Review:
People with HIV continue to have an excess burden of cardiovascular disease compared to the general population. The reasons for these disparities in cardiovascular disease include HIV-specific risk enhancers, traditional atherosclerotic cardiovascular disease risk factors, and sociodemographic disparities, all of which are ripe targets for intervention.
Recent Findings:
Accurate risk prediction of atherosclerotic cardiovascular disease remains difficult, and cardiovascular risk for people with HIV may be underestimated in the absence of HIV-specific risk enhancers. Despite this increased cardiovascular risk, people with HIV are undertreated and often placed on inadequate lipid lowering therapy. Structural racism and HIV-related stigma play a role, and provider-level and structural-level interventions to encourage early identification and treatment of persons at high risk are necessary.
Summary:
Persons with HIV should be screened with existing cardiovascular risk prediction tools, and those at high risk cardiovascular disease should be promptly referred for lifestyle and pharmacologic interventions as appropriate. System-level implementation research is ongoing in attempts to narrow the gap in cardiovascular care, particularly for vulnerable communities in low resource settings.
Keywords: disparities in cardiovascular disease, cardiovascular disease prevention, risk prediction, HIV-related stigma
Introduction
Dramatic progress in the treatment of HIV/AIDS has shifted the paradigm of HIV to a managed chronic disease. With this shift to chronic disease has been an attendant rise in the burden of cardiovascular disease (CVD) in people with HIV (PWH). Epidemiologic studies in a variety of care settings reveal significantly elevated risks of various CVD manifestations for PWH compared to controls, including myocardial infarction (MI), heart failure (HF), and sudden cardiac death (SCD), and stroke, even after accounting for common CVD risk factors.1–7
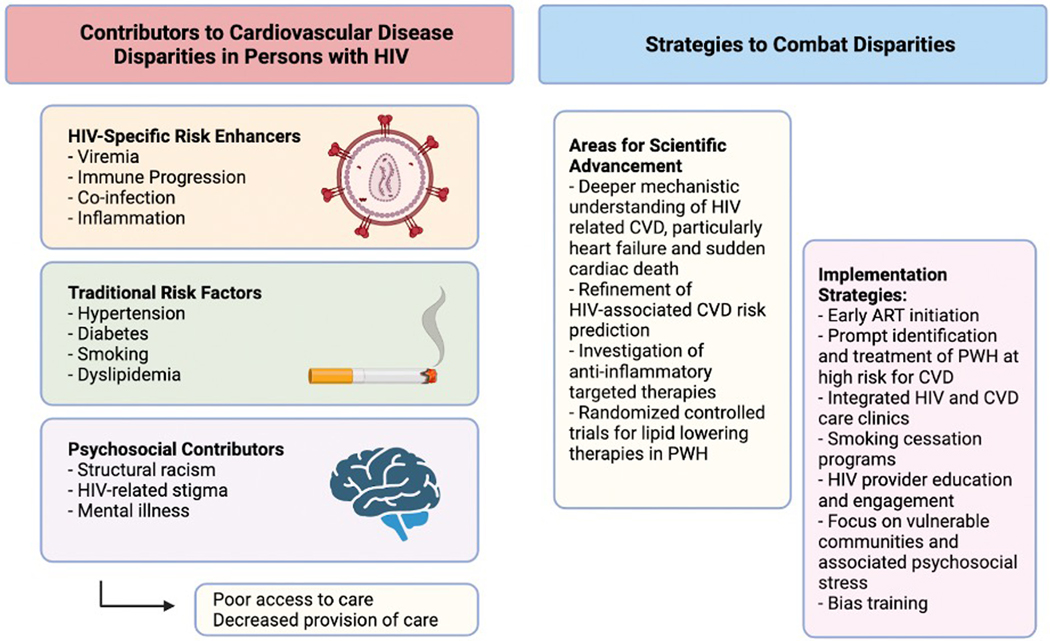
Efforts to understand the reasons for this elevated risk have identified several areas which contribute in unique but synergistic ways to CVD risks among PWH: (1) HIV-related factors such as sustained viremia, immunologic progression/non-response, viral co-infections, and related chronic immune activation;8,9 (2) traditional CVD risk factors elevated among PWH, ranging from dyslipidemia to cigarette smoking;10–12 and (3) sociodemographic disparities and HIV-related stigma, which contribute to psychosocial stress and adversely affect access to and provision of care for chronic noncommunicable conditions such as CVDs.13–18 Taken together, these factors contribute to elevated CVD risk but also increase barriers to CVD prevention and therapy among PWH. Understanding and addressing these factors in a comprehensive, integrated manner is necessary to achieve progress in CVD prevention and treatment of PWH.19 Accordingly, this review focuses on these unique areas of CVD risk among PWH, with a particular focus on major gaps and emerging practical targets to reduce CVD risk and morbidity among PWH (Figure 1).
Figure 1.
Factors leading to disparities in the burden of cardiovascular disease for people with HIV and potential strategies to combat them.
Incorporating HIV-Specific CVD Risk Enhancers into CVD Prevention and Treatment
Over the past 15 years, considerable evidence has accumulated outlining the contributions of sustained viremia, incomplete immunologic recovery (often marked by low CD4 count and CD4/CD8 ratio), and co-infection with viruses such as hepatitis C to a chronic inflammatory state in PWH. These factors are directly linked to increased risks of CVDs including myocardial infarction and stroke among PWH and thus have been termed HIV-related CVD risk enhancing factors.2,5,20,21 These HIV-related CVD risk enhancers and their contributions to CVD are covered elsewhere in this review series and have been previously discussed in detail.8,21,22 Therefore, our focus in this section is on briefly outlining these risk enhancers, followed by a discussion of how to address them in CVD risk-reducing interventions.
Fundamentally, HIV-related contributors to chronic inflammation derive from initial infection followed by the death and depletion of infected CD4 cells with variable and often incomplete immunologic recovery.8,23 These changes result in vulnerability to co-infection and unresolving inflammatory bias, with defects in inflammation-regulating immunity.22,24 This can affect the pathogenesis of diverse CVDs in a number of ways. HIV-associated inflammation, which can persist even in those with controlled HIV, accelerates progression of atherosclerosis and is linked with inflammatory markers such as IL-6 and D-dimer.8,9 Similarly, while the mechanistic link between HIV and cardiomyopathy remains incompletely understood, chronic inflammation has been linked to endothelial activation and high levels of cytokines that directly injure cardiomyocytes.25 PWH are thought to have an exaggerated response to ischemia engendered by the abnormal immune response, leading to increased cardiac damage and dysfunction after MI.26 Accordingly, cardiac magnetic resonance imaging (CMR) and autopsy studies of PWH have shown increased extracellular volume and interstitial fibrosis compared to HIV-uninfected controls.7,26,27
Although the precise underlying mechanisms by which inflammation drives end-organ disease pathogenesis may differ by CVD manifestation, there are common threads that are practically targetable for CVD risk reduction. Perhaps the most important mechanistically “upstream” means by which to address HIV-related CVD risk enhancers is to ensure immediate and sustained virologic suppression with ART. Despite widespread (though not ubiquitous) ART and sustained virologic suppression in the current era, PWH with sustained virologic suppression may still have histories of sustained viremia, incomplete immunologic recovery, and residual HIV/ART-related metabolic and lipid abnormalities that can contribute to residual CVD risk. An additional complicating factor is an evolving understanding of the metabolic and cardiovascular effects of newer antiretroviral therapy, such as potential implications of integrase inhibitor-associated weight gain.28–30
Incorporating these factors into practical clinical risk stratification remains challenging absent HIV-specific CVD risk stratification tools; general population CVD risk estimators underestimate CVD risk among PWH.31,32 Therefore, a reasonable interim approach to CVD risk stratification, as outlined in the 2019 American Heart Association (AHA) Scientific Statement on HIV and CVD, is to consider the presence of HIV-specific risk enhancing factors in evaluating the CVD risk of PWH. In the AHA statement, the authors proposed that with HIV-specific risk-enhancing factors, the actual CVD risk for a person with HIV may be 1.5- to 2-fold higher than predicted by general population estimators, whereas the risks may not differ much in absence of HIV-specific risk enhancing factors. Since the AHA statement was published, newer data from two distinct US based HIV cohorts indicate that the difference in MI risk for PWH vs HIV-uninfected risk factor matched controls has actually increased since 2010;33 indeed, PWH without risk enhancing factors may still have excess CVD risk, estimated to be approximately 1.1- to 1.2-fold. The purpose of incorporating HIV-specific risk enhancers into an overall estimate of CVD risk is to provide some guidance for CVD-preventive therapeutic interventions, discussed in the final section of this review. Regarding novel inflammation-targeted therapies, although these have been studied with varying effects in the general population,34 the optimal balance between benefit (reducing excess and deleterious inflammation) relative to risk (immunosuppression, infectious susceptibility) in PWH remains an open question.35–37
General Cardiovascular Risk Factors Enhanced in PWH
Traditional risk factors such as hypertension, diabetes, dyslipidemia, and smoking are highly prevalent among PWH. Rates of hypertension and diabetes in PWH on ART are higher than HIV-uninfected matched controls.38–40 The prevalence of tobacco smoking in persons with HIV ranges from 40–70%, about twice as high as the general population.41,42 Yet, hypertension and diabetes are severely undertreated in PWH: these contribute to low rates of overall CVD risk factor control among PWH, with <2% of PWH having optimally controlled CVD risk factors in a recent study.43
Dyslipidemia is also highly prevalent in PWH, related to a combination of chronic inflammation as well as off-target effects of specific antiretrovirals.44,45 Evidence-based and effective treatments for HIV-related dyslipidemia remain lacking and statins remain the default therapy. HIV-focused lipid optimization remains an area in need of substantial clinical and implementation research, as the target lipid class affected most by statins, low density lipoprotein (LDL)-cholesterol, is less affected in PWH than other lipid markers such as triglycerides (elevated in PWH) and HDL-cholesterol (decreased). Although several studies have evaluated statins and subclinical endpoints in HIV,46–48 no large clinical CVD endpoint-driven trials of statins among PWH have been completed. A large, international randomized controlled trial, REPRIEVE, is underway to compare statins versus placebo in PWH for primary prevention of atherosclerotic CVD (ASCVD).49 Studies are also evaluating the role of PCSK9 inhibition in reducing arterial inflammation in HIV.45,49
Other behavioral factors also may increase risk for CVD in PWH. Heavy alcohol use has been linked to a 30–40% increased risk of both ASCVD and heart failure among PWH, an association that was not as apparent in the general population.12 The number of studies evaluating the impact of other substance use on CVD in this population is sparse. One study observed a similar rate of MI and stroke in persons who inject drugs with HIV compared to those who did not inject drugs, but data on which injection drugs used were not collected.50 Morbidity and mortality from substance use may also be inaccurately labeled as cardiovascular disease. A recent autopsy study of PLWH with sudden cardiac death found that occult overdose was responsible for many of the presumed instances of sudden cardiac death, possibly leading to an over-inflation of the rates of SCD.7
Sociodemographic and Psychosocial Contributors to HIV-Associated CVD Risk
The overall social, demographic, and economic profile of PWH differs from the general population with particular enrichment for marginalized groups.51 For many Black persons with HIV, factors such as structural racism, geographic redlining, and increased physiologic stress may contribute CVD-related outcomes.52,53 Even among PWH, significant disparities exist by race. Data from the VACS showed that Black and Hispanic patients with HIV had higher mortality rates and a greater number of medical comorbidities, despite no significant differences in ART medication prescription and adherence.14 Reasons for these racial disparities are multifactorial, but one key component is undertreatment of co-morbidities.51 As discussed previously in this review, the prevalence of comorbid conditions such as diabetes and hypertension contributing to cardiovascular disease risk in PWH are high. A recent study of more than 20,000 veterans in the VACS cohort found that Black veterans with HIV were not only less likely to be prescribed ART and achieve viral control, but also had lower rates of hypertension and diabetes control, as well as decreased lipid monitoring.13 Additionally, in a separate study, Black and Hispanic PWH with clear indications for statin therapy were significantly less likely to receive appropriate statin therapy compared with white PWH.54
Persons with HIV have historically also had an elevated prevalence of other factors associated with social vulnerability, stigma, and chronic stress. Persons who identify as men who have sex with men (MSM) comprise only 4% of the US population based on community survey estimates but accounted for 69% of new HIV diagnoses in 2019 55,56. Similarly, persons who inject drugs accounted for 7% of new HIV infections but represented only 0.30% of the US population.50,55 Both MSM and persons who inject drugs have historically experienced significant social stigma associated with disparities in care,57 but evidence describing the intersection of these groups with HIV and CVD are limited.
Stigma and discrimination against PWH continue to be a significant challenge even in the current era.18 This stigma has been strongly linked to increased mental illness, poor access to and usage of care (discussed more in detail in the section below), and decreased adherence to antiretroviral therapy, all of which have been implicated in worse cardiovascular outcomes.58 Depression in particular has been strongly associated with worse cardiovascular health in VA cohorts; a diagnosis of MDD was linked with a 30% increased risk of incident MI and a 70% increased risk of incident HF in veterans with HIV, even after adjustment for confounders.10,59
Implementation Gap: Inadequate CVD Risk Factor Control among PWH
As discussed above, a combination of immune-inflammatory, traditional CVD-risk factor-driven, and social factors contribute to heightened risks for CVD among PWH. Unfortunately, further exacerbating these risks are individual and system-level inadequacies in CVD risk factor control. In a large analysis of over 200,000 outpatient visits, PWH who are at high risk for cardiovascular events were treated significantly less aggressively with statins and antiplatelet therapy when matched by propensity score with HIV-uninfected controls.60 Less than 25% of patients with HIV who met guideline thresholds for statin therapy such as existing atherosclerotic cardiovascular disease, diabetes mellitus, or dyslipidemia were on statin therapy at the time of their visit. Not only are PWH less likely to be prescribed statin therapy, but they also tend to be on lower potency statins after acute coronary syndrome. Most individuals were prescribed moderate intensity statins rather than high intensity, with worse lipid panels when compared with HIV-uninfected controls.16 Aside from HIV-related stigma, another postulated reason for pharmacologic inequity in this population is fear of drug-drug interactions with antiretroviral therapy. Even beyond pharmacologic therapy, PLWH are also much less likely to receive PCI after presenting with acute myocardial infarction and more likely to receive a bare metal stent over drug eluting stent.17
Strategies for Improvement
Given the multiple factors leading to health disparities in PWH as described in the previous sections, strategies to combat these disparities must be multi-pronged (Figure 1). The pathologic underpinning for ASCVD in HIV is relatively well elucidated, but mechanisms for the increased risk of HF, particularly heart failure with preserved ejection fraction, and SCD risk are less clear, perhaps related to the highly heterogeneous tissue-level pathology of each of these conditions. In any case, understanding these mechanisms is key to refining future targets for intervention. In the meantime, it is important to consider individual-level and system-level strategies to address major gaps in the provision of CVD prevention and treatment of PWH.
On an individual level, identifying, preventing, and intervening on diverse HIV-related risk factors is key. PWH who are at high risk for CVD based on traditional risk factors and HIV-specific risk enhancers must be better identified by providers. They must be screened and appropriately referred for lifestyle and pharmacologic interventions when appropriate. Smoking cessation should be a cornerstone of CVD prevention among PWH given its considerable population-attributable contribution to HIV-related CVD risk.11,42 The 2019 AHA Scientific Statement continues to provide useful guidance on risk stratification and the prevention and treatment of ASCVD for PWH, even with the recent data discussed above on underestimation of CVD risk in those without obvious HIV-related risk enhancers.21,33 In the absence of large-scale clinical endpoint-driven randomized controlled trial data, a reasonable interim approach remains consideration of statins for individuals at moderate or higher risk for ASCVD. Certainly, there are no indications that PWH with existing CVD should be treated less aggressively with guideline-directed therapies for atherosclerotic CVD (ASCVD) or heart failure than people without HIV. Considerations for drug-drug interactions do exist, particularly with potent boosters such as cobicistat, but the risks/benefits of measures such as halving the maximal statin dose in these PWH remain to be investigated. Likewise, the risks versus benefits of investigational inflammation-targeted therapies for PWH remain to be defined. An additional important but understudied consideration is the incorporation by providers of psychosocial factors such as substance use, housing instability, and chronic stigma-related stress that may drive risk.
In addition to provider-level interventions and maintenance of a low threshold to consider CVD-preventive interventions and/or diagnostics in PWH, system-level approaches to comprehensively incorporating CVD prevention and treatment into chronic care of PWH is necessary. Most prior focused interventions have targeted disparities in the use of ART and viral control. There are few existing implementation studies specifically targeting cardiovascular disparities in PLWH. The National Heart, Lung, and Blood Institute has taken note and is now funding five research teams conducting implementation studies to combat chronic comorbidities experienced by those with HIV, many of which are cardiovascular.61 There are a variety of interventions that include nursing led care coordination for blood pressure and cholesterol management, patient activation strategies for ASCVD risk reduction, and behavioral economics informed feedback for providers through peer comparisons of statin prescription.19,61 Another study, recognizing the contribution of mental illness to CVD in PLWH, addresses patients’ trauma history to increase engagement in care.61 Implementation-focused studies such as these will be critical in narrowing the gap in cardiovascular care.
Conclusion
There are significant cardiovascular disparities in ASCVD, heart failure, and sudden cardiac death for PLWH. Reasons for gaps in care are multifactorial and include HIV-related inflammation, specific antiretroviral therapies, behavioral and socioeconomic factors, and differences in provision of care. While ongoing research that seeks to further elucidate the link between HIV and cardiovascular disease is critical, known CV risk factors must be treated aggressively, with special attention paid to associated co-morbidities and unique socioeconomic factors. Individual-level and system-level approaches are needed to address this implementation gap and improve the cardiovascular health and longevity of PWH.
Key Points.
Further refinement of atherosclerotic cardiovascular disease risk prediction in people with HIV is essential for defining future targets and thresholds for intervention
Traditional cardiovascular risk factors such as smoking, hypertension, and diabetes are highly prevalent in people with HIV but remain undertreated due in part to HIV-related stigma, racial discrimination, and a lack of provider education
Implementation strategies are currently underway to reduce barriers to cardiovascular care using novel strategies such as nursing led coordination of hypertension and cholesterol management and an integrated focus on mental illness
Acknowledgements
Financial support and sponsorship: None
Footnotes
Conflicts of interest: None
References
- 1.Shah ASV, Stelzle D, Lee KK, et al. Global Burden of Atherosclerotic Cardiovascular Disease in People Living With HIV: Systematic Review and Meta-Analysis. Circulation. 2018;138(11):1100–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freiberg MS, Chang CCH, Skanderson M, et al. Association Between HIV Infection and the Risk of Heart Failure With Reduced Ejection Fraction and Preserved Ejection Fraction in the Antiretroviral Therapy Era: Results From the Veterans Aging Cohort Study. JAMA Cardiol. 2017;2(5):536–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tseng ZH, Secemsky EA, Dowdy D, et al. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol. 2012;59(21):1891–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freiberg MS, Duncan MS, Alcorn C, et al. HIV Infection and the Risk of World Health Organization-Defined Sudden Cardiac Death. J Am Heart Assoc. 2021;10(18):e021268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feinstein MJ, Steverson AB, Ning H, et al. Adjudicated Heart Failure in HIV-Infected and Uninfected Men and Women. J Am Heart Assoc. 2018;7(21):e009985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. **Tseng ZH, Moffatt E, Kim A, et al. Sudden Cardiac Death and Myocardial Fibrosis, Determined by Autopsy, in Persons with HIV. N Engl J Med. 2021;384(24):2306–2316. Annotation: This postmortem study demonstrated significantly elevated rates of sudden cardiac death and myocardial fibrosis for persons with HIV compared to HIV-uninfected individuals.
- 8.Hsue PY, Deeks SG, Hunt PW. Immunologic basis of cardiovascular disease in HIV-infected adults. J Infect Dis. 2012;205 Suppl 3:S375–S382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White JR, Chang CCH, So-Armah KA, et al. Depression and human immunodeficiency virus infection are risk factors for incident heart failure among veterans: Veterans Aging Cohort Study. Circulation. 2015;132(17):1630–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rasmussen LD, Helleberg M, May MT, et al. Myocardial infarction among Danish HIV-infected individuals: population-attributable fractions associated with smoking. Clin Infect Dis. 2015;60(9):1415–1423. [DOI] [PubMed] [Google Scholar]
- 12.Freiberg MS, McGinnis KA, Kraemer K, et al. The association between alcohol consumption and prevalent cardiovascular diseases among HIV-infected and HIV-uninfected men. J Acquir Immune Defic Syndr. 2010;53(2):247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richardson KK, Bokhour B, McInnes DK, et al. Racial Disparities in HIV Care Extend to Common Comorbidities: Implications for Implementation of Interventions to Reduce Disparities in HIV Care. J Natl Med Assoc. 2016;108(4):201–210.e3. [DOI] [PubMed] [Google Scholar]
- 14.McGinnis KA, Fine MJ, Sharma RK, et al. Understanding racial disparities in HIV using data from the veterans aging cohort 3-site study and VA administrative data. Am J Public Health. 2003;93(10):1728–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suchindran S, Regan S, Meigs JB, Grinspoon SK, Triant VA. Aspirin Use for Primary and Secondary Prevention in Human Immunodeficiency Virus (HIV)-Infected and HIV-Uninfected Patients. Open Forum Infect Dis. 2014;1(3):ofu076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boccara F, Miantezila Basilua J, Mary-Krause M, et al. Statin therapy and low-density lipoprotein cholesterol reduction in HIV-infected individuals after acute coronary syndrome: Results from the PACS-HIV lipids substudy. Am Heart J. 2017;183:91–101. [DOI] [PubMed] [Google Scholar]
- 17.Singh V, Mendirichaga R, Savani GT, et al. Coronary revascularization for acute myocardial infarction in the HIV population. J Interv Cardiol. 2017;30(5):405–414. [DOI] [PubMed] [Google Scholar]
- 18.Rueda S, Mitra S, Chen S, et al. Examining the associations between HIV-related stigma and health outcomes in people living with HIV/AIDS: a series of meta-analyses. BMJ Open. 2016;6(7):e011453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schexnayder J, Longenecker CT, Muiruri C, et al. Understanding constraints on integrated care for people with HIV and multimorbid cardiovascular conditions: an application of the Theoretical Domains Framework. Implement Sci Commun. 2021;2(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freiberg MS, Chang CCH, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173(8):614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feinstein MJ, Hsue PY, Benjamin LA, et al. Characteristics, Prevention, and Management of Cardiovascular Disease in People Living With HIV: A Scientific Statement From the American Heart Association. Circulation. 2019;140(2):e98–e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsue PY, Waters DD. HIV infection and coronary heart disease: mechanisms and management. Nat Rev Cardiol. 2019;16(12):745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsue PY, Hunt PW, Schnell A, et al. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS. 2009;23(9):1059–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382(9903):1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi H, Dey AK, Sharma G, et al. Etiology and pathophysiology of heart failure in people with HIV. Heart Fail Rev. 2021;26(3):497–505. [DOI] [PubMed] [Google Scholar]
- 26.Feinstein MJ, Mitter SS, Yadlapati A, et al. HIV-Related Myocardial Vulnerability to Infarction and Coronary Artery Disease. J Am Coll Cardiol. 2016;68(18):2026–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.*Wu KC, Haberlen SA, Plankey MW, et al. Human immunodeficiency viral infection and differences in interstitial ventricular fibrosis and left atrial size. Eur Heart J Cardiovasc Imaging. 2021;22(8):888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. **Kileel EM, Lo J, Malvestutto C, et al. Assessment of Obesity and Cardiometabolic Status by Integrase Inhibitor Use in REPRIEVE: A Propensity-Weighted Analysis of a Multinational Primary Cardiovascular Prevention Cohort of People With Human Immunodeficiency Virus. Open Forum Infect Dis. 2021;8(12):ofab537. Annotation: This investigation of a sub-sample of the REPRIEVE study demonstrated non-uniform differences in INSTI-associated weight gain, with women and non-white individuals with HIV being at the highest risk for weight and waist circumference increases.
- 29.O’Halloran JA, Sahrmann J, Butler AM, Olsen MA, Powderly WG. Brief Report: Integrase Strand Transfer Inhibitors Are Associated With Lower Risk of Incident Cardiovascular Disease in People Living With HIV. J Acquir Immune Defic Syndr. 2020;84(4):396–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huhn GD, Shamblaw DJ, Baril JG, et al. Atherosclerotic Cardiovascular Disease Risk Profile of Tenofovir Alafenamide Versus Tenofovir Disoproxil Fumarate. Open Forum Infect Dis. 2020;7(1):ofz472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feinstein MJ, Nance RM, Drozd DR, et al. Assessing and Refining Myocardial Infarction Risk Estimation Among Patients With Human Immunodeficiency Virus: A Study by the Centers for AIDS Research Network of Integrated Clinical Systems. JAMA Cardiol. 2017;2(2):155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Triant VA, Perez J, Regan S, et al. Cardiovascular Risk Prediction Functions Underestimate Risk in HIV Infection. Circulation. 2018;137(21):2203–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. **Silverberg MJ. Trends in Myocardial Infarction Risk by HIV Status in Two US Healthcare Systems. Presented at: Conference on Retroviruses and Opportunistic Infections; February 12–16 2022; Virtual. Accessed April 25, 2022 at: https://www.natap.org/2022/CROI/croi_43.htm Annotation: This analysis, presented at CROI, demonstrated that PWH did not experience a similar decrease in myocardial infarction risk as people without HIV from 2010–2017, resulting in a relative excess MI risk among PWH.
- 34.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377(12):1119–1131. [DOI] [PubMed] [Google Scholar]
- 35.Hsue PY, Li D, Ma Y, et al. IL-1β Inhibition Reduces Atherosclerotic Inflammation in HIV Infection. J Am Coll Cardiol. 2018;72(22):2809–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsue PY, Ribaudo HJ, Deeks SG, et al. Safety and Impact of Low-dose Methotrexate on Endothelial Function and Inflammation in Individuals With Treated Human Immunodeficiency Virus: AIDS Clinical Trials Group Study A5314. Clin Infect Dis. 2019;68(11):1877–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hays AG, Schär M, Barditch-Crovo P, et al. A randomized, placebo-controlled, double-blinded clinical trial of colchicine to improve vascular health in people living with HIV. AIDS. 2021;35(7):1041–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu Y, Chen X, Wang K. Global prevalence of hypertension among people living with HIV: a systematic review and meta-analysis. J Am Soc Hypertens. 2017;11(8):530–540. [DOI] [PubMed] [Google Scholar]
- 39.Hernandez-Romieu AC, Garg S, Rosenberg ES, Thompson-Paul AM, Skarbinski J. Is diabetes prevalence higher among HIV-infected individuals compared with the general population? Evidence from MMP and NHANES 2009–2010. BMJ Open Diabetes Res Care. 2017;5(1):e000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noubissi EC, Katte JC, Sobngwi E. Diabetes and HIV. Curr Diab Rep. 2018;18(11):125. [DOI] [PubMed] [Google Scholar]
- 41.Savès M, Chêne G, Ducimetière P, et al. Risk factors for coronary heart disease in patients treated for human immunodeficiency virus infection compared with the general population. Clin Infect Dis. 2003;37(2):292–298. [DOI] [PubMed] [Google Scholar]
- 42.Calvo-Sánchez M, Perelló R, Pérez I, et al. Differences between HIV-infected and uninfected adults in the contributions of smoking, diabetes and hypertension to acute coronary syndrome: two parallel case-control studies. HIV Med. 2013;14(1):40–48. [DOI] [PubMed] [Google Scholar]
- 43.Paisible AL, Chang CCH, So-Armah KA, et al. HIV infection, cardiovascular disease risk factor profile, and risk for acute myocardial infarction. J Acquir Immune Defic Syndr. 2015;68(2):209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maggi P, Di Biagio A, Rusconi S, et al. Cardiovascular risk and dyslipidemia among persons living with HIV: a review. BMC Infect Dis. 2017;17(1):551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Durstenfeld MS, Hsue PY. Mechanisms and primary prevention of atherosclerotic cardiovascular disease among people living with HIV. Curr Opin HIV AIDS. 2021;16(3):177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toribio M, Fitch KV, Sanchez L, et al. Effects of pitavastatin and pravastatin on markers of immune activation and arterial inflammation in HIV. AIDS. 2017;31(6):797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lo J, Lu MT, Ihenachor EJ, et al. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Lancet HIV. 2015;2(2):e52–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eckard AR, Jiang Y, Debanne SM, Funderburg NT, McComsey GA. Effect of 24 weeks of statin therapy on systemic and vascular inflammation in HIV-infected subjects receiving antiretroviral therapy. J Infect Dis. 2014;209(8):1156–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grinspoon SK, Fitch KV, Overton ET, et al. Rationale and design of the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE). Am Heart J. 2019;212:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lesko CR, Moore RD, Tong W, Lau B. Association of injection drug use with incidence of HIV-associated non-AIDS-related morbidity by age, 1995–2014. AIDS. 2016;30(9):1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sullivan PS, Satcher Johnson A, Pembleton ES, et al. Epidemiology of HIV in the USA: epidemic burden, inequities, contexts, and responses. Lancet. 2021;397(10279):1095–1106. [DOI] [PubMed] [Google Scholar]
- 52.Brothers RM, Fadel PJ, Keller DM. Racial disparities in cardiovascular disease risk: mechanisms of vascular dysfunction. Am J Physiol Heart Circ Physiol. 2019;317(4):H777–H789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferdinand KC, Yadav K, Nasser SA, et al. Disparities in hypertension and cardiovascular disease in blacks: The critical role of medication adherence. J Clin Hypertens. 2017;19(10):1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riestenberg RA, Furman A, Cowen A, et al. Differences in statin utilization and lipid lowering by race, ethnicity, and HIV status in a real-world cohort of persons with human immunodeficiency virus and uninfected persons. Am Heart J. 2019;209:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Statistics Overview. Published February 28, 2022. Accessed May 4, 2022. https://www.cdc.gov/hiv/statistics/overview/index.html
- 56.Grey JA, Bernstein KT, Sullivan PS, et al. Estimating the Population Sizes of Men Who Have Sex With Men in US States and Counties Using Data From the American Community Survey. JMIR Public Health Surveill. 2016;2(1):e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buttram ME, Kurtz SP. A mixed methods study of health and social disparities among substance-using African American/Black men who have sex with men. J Racial Ethn Health Disparities. 2015;2(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Strategies for Management of Antiretroviral Therapy (SMART) Study Group, El-Sadr WM, Lundgren JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355(22):2283–2296. [DOI] [PubMed] [Google Scholar]
- 59.Khambaty T, Stewart JC, Gupta SK, et al. Association Between Depressive Disorders and Incident Acute Myocardial Infarction in Human Immunodeficiency Virus-Infected Adults: Veterans Aging Cohort Study. JAMA Cardiol. 2016;1(8):929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ladapo JA, Richards AK, DeWitt CM, et al. Disparities in the Quality of Cardiovascular Care Between HIV-Infected Versus HIV-Uninfected Adults in the United States: A Cross-Sectional Study. J Am Heart Assoc. 2017;6(11). doi: 10.1161/JAHA.117.007107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gamble-George JC, Longenecker CT, Webel AR, et al. ImPlementation REsearCh to DEvelop Interventions for People Living with HIV (the PRECluDE consortium): Combatting chronic disease comorbidities in HIV populations through implementation research. Prog Cardiovasc Dis. 2020;63(2):79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]