Abstract
Background and objectives
Postoperative pain is still a major concern in several surgical procedures. Multimodal analgesia is best for postoperative pain management; however, opioid therapy is still the main treatment for pain after surgical procedures. Transdermal buprenorphine is a partial μ-agonist opioid widely used for chronic pain syndromes, with limited evidence for acute postoperative pain. A systematic review of studies examining transdermal buprenorphine for acute pain management after surgery was conducted.
Contents
Data from PubMed, Embase, The Cochrane Central Register of Controlled Trials (CENTRAL), CINAHL via EBSCOhost, and LILACS were reviewed, including randomized clinical trials that evaluated total postoperative pain, postoperative analgesic consumption, drug-related side effects and patient satisfaction with analgesia regimen. Data from nine studies (615 patients) were included in this review. Most studies initiated transdermal buprenorphine use 6 to 48 hours before surgery, maintaining use from 1 to 28 days after the procedure. Most studies showed lower or similar postoperative pain scores, postoperative analgesic consumption and patient satisfaction comparing buprenorphine to placebo, tramadol, celecoxib, flurbiprofen and parecoxib. The incidence of side effects varied between studies, with most showing no increase in drug-related side effects with buprenorphine use, except one study, which compared buprenorphine to oral tramadol, and one to transdermal fentanyl. However, most results were derived from evidence with an overall high or unclear risk of bias.
Conclusions
Although more studies are necessary, initial results show that transdermal buprenorphine seems to be an effective and safe opioid choice for management of acute postoperative pain.
Keywords: Buprenorphine, Cutaneous administration, Transdermal patch, Postoperative pain, Acute pain
Resumo
Justificativa e objetivos
A dor pós-operatória ainda é uma queixa importante em vários procedimentos cirúrgicos. A analgesia multimodal é a melhor conduta para a dor pós-operatória, embora a terapia com opioides ainda seja o principal tratamento para a dor após procedimentos cirúrgicos. A buprenorfina transdérmica é um opioide agonista μ amplamente prescrito nas síndromes de dor crônica, mas com limitada evidência do seu uso para dor aguda no pós-operatório. Realizamos revisão sistemática de estudos que examinaram o papel da buprenorfina transdérmica no tratamento da dor aguda pós-operatória.
Conteúdo
Revisamos os dados de PubMed, Embase, Registro Central de Ensaios Controlados Cochrane (CENTRAL), CINAHL via EBSCOhost e LILACS, incluindo estudos clínicos randomizados que avaliaram a dor pós-operatória total, consumo de analgésicos pós-operatórios, efeitos colaterais relacionados a medicamentos e satisfação do paciente com esquema de analgesia. Dados de nove estudos (615 pacientes) foram incluídos nesta revisão. A maioria dos estudos iniciou o uso transdérmico de buprenorfina 6 a 48 horas antes da cirurgia, mantendo o uso de 1 a 28 dias após o procedimento. A maioria dos estudos encontrou valores semelhantes ou menores para o escore de dor pós-operatória, consumo pós-operatório de analgésicos e satisfação do paciente quando a buprenorfina foi comparada ao placebo, tramadol, celecoxibe, flurbiprofeno e parecoxibe. A incidência de efeitos colaterais oscilou nos estudos, e a maioria não mostrou aumento de efeito colateral relacionado ao uso de buprenorfina, exceto em dois estudos, um que comparou buprenorfina ao tramadol oral e outro ao fentanil transdérmico. No entanto, a maioria dos resultados foi obtida a partir de evidências com um risco geral alto ou risco de viés impreciso.
Conclusões
Embora sejam necessários mais estudos, os resultados iniciais mostram que a buprenorfina transdérmica parece ser uma forma de administração segura e efetiva de opioide no tratamento da dor aguda pós-operatória.
Palavras-chave: Buprenorfina, Administração cutânea, Sistema transdérmico, Dor pós-operatória, Dor aguda
Introduction
In spite of recent developments in pain treatment, many patients still undergo moderate to severe pain after surgery. It is estimated that severe postoperative pain is reported by 20–40% of patients submitted to surgical procedures, especially abdominal, thoracic, orthopedic and pelvic surgeries.1 Pain in the first days after surgery can lead to delayed ambulation, increase in cardiopulmonary and thrombotic morbidity as well as the development of chronic pain.1, 2 Multimodal analgesia is currently the best treatment for acute postoperative pain, however opioid therapy is still the main approach for the management of moderate to severe postoperative pain.3 Despite extensive use, opioids can lead to side effects such as nausea, vomiting, prolonged ileus, sedation, urinary retention, respiratory depression and addiction. In fact, the need for high opioid doses in the postoperative period is related to higher incidence of side effects and risk of opioid abuse.4
Buprenorphine is a semisynthetic μ-opioid receptor (MOR) partial agonist and Kappa Opioid Receptor (KOR) antagonist. Its unique pharmacodynamics results in a lower incidence of opioid-related side effects and risk of abuse compared to other full MOR agonists. It also has a long duration of action due to its slow dissociation from MOR,5, 6, 7 being 75 to 100 times more potent than morphine, with a ceiling effect on respiratory depression, but not on analgesia.5, 8 It is metabolized in the liver by cytochrome P450 to its active metabolite (norbuprenorphine). However, it can be eliminated through the biliary and urinary tract, therefore there is evidence that buprenorphine can be safely used in patients with renal impairment and should be carefully considered in patients with impaired liver function.5, 7, 9
Buprenorphine has been used as an analgesic for chronic pain and opioid withdrawal syndrome,7, 9, 10, 11 but there is also evidence for use of buprenorphine in the postoperative period for the treatment of moderate to severe pain in a variety of surgical procedures. Most acute uses of buprenorphine include epidural, intrathecal, intravenous, sublingual, subcutaneous and intra-articular routes.5 Its high lipophilicity and low molecular weight make buprenorphine a suitable agent to use via the transdermal route.12, 13 The specific pharmacodynamics vary with each patch manufacturer, although most buprenorphine patches have an onset of 12 to 24 hours, achieving approximately stable plasma concentrations on the third day after use.14 Its duration of action is also prolonged after achieving a steady state, ranging from 3 to 7 days.5, 6, 9 Such a route has been used for the treatment of chronic pain conditions,9, 10, 12 with some recent studies investigating the use of perioperative transdermal buprenorphine for the treatment of postoperative pain.15, 16, 17, 18, 19, 20, 21, 22, 23
This article presents a systematic review regarding transdermal buprenorphine use in patients submitted to surgical procedures, compared to other analgesics commonly used in the perioperative period or placebo. Outcomes accessed were postoperative pain, rescue analgesic use, adverse effects and patient satisfaction.
Methods
Search strategy
Literature was retrieved from PubMed, Embase via Ovid SP, The Cochrane Central Register of Controlled Trials (CENTRAL), CINAHL via EBSCOhost, and LILACS. The last search was conducted on April 2, 2019 with no limit date. The search strategy included combinations of the keywords: “acute pain”; “postoperative pain”; “buprenorphine and other opioids commonly used for perioperative analgesia” (full strategy for MEDLINE in Appendix 1 – Supplementary Material). Free text words and controlled vocabulary/MeSH terms were combined without any limitation in the search period. The MEDLINE search terms were adapted for each database. Ad hoc searching was also performed; and the references from all included articles were manually searched to identify additional articles.
Study selection and data collection
The present review adhered to the recommendations of the Preferred Reporting Items for Systematic review and Meta-Analysis (PRISMA) protocol statement24 and is registered in PROSPERO database (CRD42019131666). Inclusion criteria were: randomized controlled trials with a population of 18 years-old or more, undergoing surgical procedures, use of transdermal buprenorphine in the perioperative period to treat acute pain, studies written in English or Spanish. Exclusion criteria included: case-reports, case series, animal model studies, observational and cohort studies, transdermal buprenorphine used for non-acute postoperative pain.
Two authors performed the search, selected the relevant articles according to the eligibility criteria, and performed data extraction and content analysis independently. Disagreements were discussed with a third author. Available data was collected from the articles and outcomes examined included postoperative pain, postoperative analgesic consumption, drug-related side effects and patient satisfaction. When limited relevant data was available, an attempt to contact the authors was made. Summary measures aimed to be collected were difference in means in postoperative pain, rescue analgesic use, adverse effects and patient satisfaction.
Risk of bias assessment
Risk of bias assessment was performed according to the following criteria for each study: selection bias (random sequence generation, allocation concealment); performance bias (blinding of participants and personnel); detection bias (blinding of outcome assessment); attrition bias (incomplete outcome data); reporting bias (selective reporting); and others.25 According to the Cochrane database, risk of bias can be graduated into high, low and unclear, with a high risk of bias considered when any of the previous items evaluated in the studies were not performed. When such items were assessed adequately, a low risk of bias was considered, whereas unclear risk of bias was considered when the available information was insufficient to classify each item as high or low risk of bias, or was not properly reported in the article.26, 27
Results
The initial search identified 386 potential studies. After duplicates and irrelevant titles exclusion, 143 articles titles and abstracts were reviewed. During abstract review, 110 studies were excluded for being case reports, case series, reviews, non-human research, language other than English or Spanish, or presenting data about non-transdermal or chronic buprenorphine use. The remaining 33 full-text articles were reviewed and a total of nine studies (615 patients) were included in the systematic review. The PRISMA process is detailed in Fig. 1 and a summary of studies contents is shown in Table 1. Surgical procedures included spine surgery,17, 19, 22 major15 and elective18 abdominal surgery, hysterectomy and myomectomy,20, 21 hip surgery,16 and hallux valgus23 corrections. Transdermal buprenorphine was started 6 to 48 hours before surgery in doses ranging from 5 mcg h−1 to 52.5 mcg h−115, 16, 17, 18, 22, 23 and maintained for 1–7 days after the procedure. Only one study initiated transdermal buprenorphine 36 hours after surgery and maintained it for 28 days after the procedure.17 Control groups received placebo,18, 19, 20 tramadol,16, 17 a different dosage of transdermal buprenorphine.18, 20, 21 Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)22, 23 or transdermal fentanyl.15 The risk of bias summary for included studies is shown in Fig. 2.
Figure 1.
PRISMA flow diagram. From: Moher D, Liberati A, Tetzlaff J, Altman DG, the PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Med 6(7): e1000097.
Table 1.
Studies characteristics.
| Author | Year | Surgical procedure | n | Buprenorphine doses | Form of administration | Control groups | Postoperative pain | Postoperative analgesic consumption | Drug-related side-effects | Patient satisfaction |
|---|---|---|---|---|---|---|---|---|---|---|
| Rivera-Ruiz et al. | 2018 | Abdominal hysterectomy | 45 | 17.5 mcg h−1 | From 24 h before surgery until 24 h PO | Placebo | VAS score higher for placebo at rest and movement. No differences in buprenorphine groups | X | Increasing somnolence with higher buprenorphine doses. Conflicting results for PONV | X |
| 26.25 mcg h−1 | ||||||||||
| 35 mcg h−1 | ||||||||||
| Xu et al. | 2018 | Hallux-valgus surgery | 90 | 10 mcg h−1 | From 2 days before surgery until POD5 | Flurbiprofen 50 mg I.V twice a day and celecoxib 200 mg orally twice a day | VAS score lower for buprenorphine and flurbiprofen in POD 1 compared to celecoxib. No difference after POD 1 | No difference in POD 1. On POD 2 and 3, buprenorphine and flurbiprofen groups had lower analgesic consumption | No significant differences | Higher satisfaction on buprenorphine group compared to both flurbiprofen and celecoxib |
| Desai et al. | 2017 | Hip surgery | 50 | 10 mcg h−1 | From 1 day before surgery until POD 7 | Tramadol 50 mg every 8 h | Pain at rest: no differences until 12 h PO but lower pain scores in buprenorphine group from 24 h PO to POD 7. Pain at movement: no differences until 24 h PO, but lower pain scores in buprenorphine group from POD 2 to 7 | Lower total analgesic consumption in buprenorphine group during the 7-days follow-up | Higher PONV incidence in tramadol group | Higher satisfaction on buprenorphine group |
| Kim et al. | 2017 | Spinal fusion | 69 | 5 mcg h−1 | From 36 h after surgery until POD 28 | 150-300 mg once a day controlled-release oral tramadol tablets | No differences in VAS score until POD 14 | No differences in analgesic consumption until POD 14 | No significant differences | X |
| 10 mcg h−1 | ||||||||||
| 15 mcg h−1 | ||||||||||
| 20 mcg h−1 | ||||||||||
| Niyogi et al. | 2017 | Spinal surgery | 70 | 10 mcg h−1 | From 24 h before surgery until 48 h PO | Placebo | VAS score lower in Buprenorphine group from 0 h to 48 h PO | Time of to first rescue analgesia were higher in buprenorphine group; frequency of use and total analgesic use were lower in brupenorphine group until 48 h PO | No significant differences | X |
| Tang et al. | 2017 | Lumbar discectomy | 96 | 5 mcg h−1 | From 2 days before surgery until POD 5 | Parecoxib 40 mg I.V. twice a day and celecoxib 200 mg orally twice a day | Better analgesia in buprenorphine and parecoxib groups until POD 1. No differences in POD 3 and 5 | No significant differences | No significant differences | Higher satisfaction on buprenorphine group compared to both parecoxib and celecoxib |
| Kumar et al. | 2016 | Elective abdominal surgery | 90 | 10 mcg h−1 | From night before surgery until POD 7 | Placebo | VAS score placebo group > buprenorphine 10 mg > buprenorphine 20 mg from end of surgery until POD 7 | Analgesic requirements on placebo group > buprenorphine 10 mg > buprenorphine 20 mg in the first 48 h PO. At POD 4, analgesic requirement was higher in placebo group but similar in both buprenorphine groups | Sedation scores with buprenorphine 20 mg > buprenorphine 10 mg > placebo until 12 h PO | X |
| 20 mcg h−1 | ||||||||||
| Arshad et al. | 2015 | Major abdominal surgery | 60 | 10 mcg h−1 | From 6 h before surgery until POD 3 | Transdermal fentanyl 25 mcg h−1 | Higher VAS score in Buprenorphine groups in POD 1, 2 and 3 | No significant differences | Higher sedation scores in buprenorphine groups in POD 1, 2 and 3 | X |
| Setti et al. | 2012 | Open hysterectomy, myomectomy | 45 | 17,5 mcg h−1 | From 12 h before surgery until 72 h PO | X | No significant differences | Analgesic requirements inversely proportional to buprenorphine dosage until 72 h PO | No significant differences | No significant differences |
| 35 mcg h−1 | ||||||||||
| 52,5 mcg h−1 |
Summary of studies included in the systematic review. PO, Postoperative; VAS, Visual Analog Scale; PONV, Postoperative Nausea and Vomiting; POD, Postoperative Day; IV, Intravenous.
Figure 2.
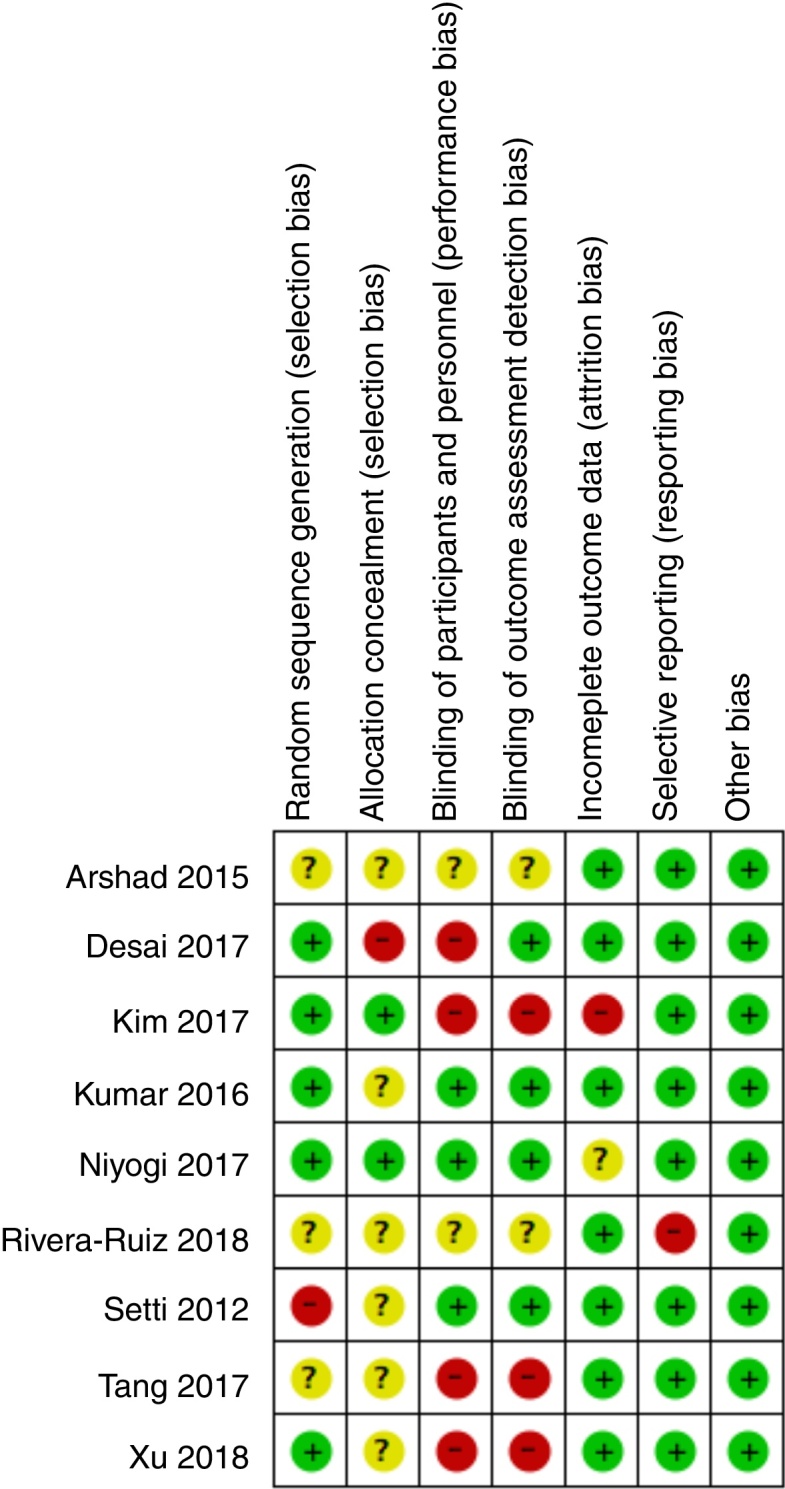
Risk of bias summary.
In six studies, buprenorphine was considered more effective than placebo,18, 19, 20 celecoxib22, 23 and tramadol16 for reducing postoperative pain scores. In three studies, buprenorphine was considered as effective as tramadol,17 parecoxib22 and flurbiprofen23 for reducing postoperative pain scores. In one study, buprenorphine led to higher VAS scores than transdermal fentanyl.15 One study compared 10 mg and 20 mg buprenorphine patches, finding lower pain scores in the higher dose buprenorphine group.18 Other study compared buprenorphine doses of 17.5 mcg h−1, 35 mcg h−1 and 52.5 mcg h−1, while another study compared buprenorphine doses of 17.5 mcg h−1, 26.25 mcg h−1 and 35 mcg h−1. There was no difference in postoperative pain scores with different buprenorphine dosages in both studies.20, 21
As for postoperative pain medications, five studies showed lower rescue analgesic dosages in transdermal buprenorphine groups compared to placebo,18, 19 tramadol16 and celecoxib.23 Four studies showed no difference in additional analgesia usage with transdermal buprenorphine compared to transdermal fentanyl,15 tramadol,17, 19 celecoxib22 and flurbiprofen.23 Two studies compared different doses of buprenorphine, in one the rescue analgesic consumption was higher in patients receiving 10 mcg h−1 than in patients receiving 20 mcg h−1,18 while in the other study rescue analgesic consumption decreased and transdermal buprenorphine dosage increased from 17.5 mcg h−1 to 32 mcg h−1 and 52.5 mcg h−1.21
Most studies reported a similar incidence of adverse effects between transdermal buprenorphine and placebo,18, 19 tramadol,17 NSAIDs,22, 23 or between different doses of transdermal buprenorphine (10 mg vs. 20 mg; 17.5 mcg h−1, 32 mcg h−1 or 52.5 mcg h−1).18, 21 One study showed higher Postoperative Nausea and Vomiting (PONV) in the tramadol control group than in the buprenorphine group,16 another reported more somnolence with buprenorphine 20 mg > buprenorphine 10 mg > placebo,20 whereas two studies showed deeper sedation scores in buprenorphine group compared to fentanyl15 or placebo.18
Only four studies evaluated overall patient satisfaction with analgesia, with three studies reporting that transdermal buprenorphine use led to higher patient satisfaction than tramadol16 and NSAIDs,22, 23 while one study showed no significant difference between different buprenorphine dosages ranging from 17.5 mcg h−1 to 52.5 mcg h−1.21
Discussion
This is the first review to assess transdermal buprenorphine use for acute postoperative pain, including data from 615 patients undergoing several surgical procedures in the qualitative analysis. Most studies initiated transdermal buprenorphine use 6–48 hours prior to surgery, which is consistent with a 12–24 hours latency time for transdermal buprenorphine patches.5, 6, 9 Time to achieve stable plasma concentrations would be ideal before a surgical procedure, however, initiation of opioid use far before a surgical procedure can raise ethical concerns regarding unnecessary opioid usage before pain stimuli. There was a large variation in the dosage of buprenorphine, ranging from 5 mcg h−1 to 52.5 mcg h−1. Nevertheless, most studies used 5 to 10 mcg h−1 buprenorphine patches, representing an equivalent oral morphine dose of up to 30 mg day−1. This dosage is compatible with most postoperative opioid requirements and can be achieved even with weak opioid intake regimens.1, 28, 29
All studies comparing transdermal buprenorphine with placebo showed lower postoperative pain scores and lower postoperative analgesic consumption in the buprenorphine groups.18, 19, 20 Two studies showed increased sedation scores,18 nausea or somnolence20 in the buprenorphine group, while one study showed no differences in drug-related side effects.19 It is well known that opioid usage reduces postoperative pain and postoperative analgesic consumption, while increasing drug-related side effects.1, 28, 29 In the present review, buprenorphine did improve pain management compared to placebo with evidence of increased nausea, somnolence and sedation scores, but no difference to placebo was reported in other common opioid-related side effects such as vomiting, pruritus, constipation, urinary retention and respiratory depression.
Studies comparing transdermal buprenorphine to tramadol, a weak opioid drug, favored buprenorphine or showed similar results regarding postoperative pain scores, analgesic consumption, drug side effects and patient satisfaction.16, 17 However, in the buprenorphine-favoring study, the buprenorphine patch was initiated 24 hours before surgery, while the other study initiated buprenorphine or tramadol 36 hours after surgery, with both groups using Patient Controlled Analgesia (PCA) with fentanyl. The onset of analgesia with buprenorphine is significantly slower compared to tramadol, therefore similar pain scores and PCA opioid consumption could favor buprenorphine.
When compared to NSAIDs, transdermal buprenorphine had similar postoperative pain scores to I.V. NSAIDs (flurbiprofen and parecoxib) and lower postoperative pain scores than oral celecoxib. Also, the buprenorphine group had similar postoperative analgesic consumption compared to flurbiprofen, parecoxib and celecoxib,22, 23 except in one study that showed lower consumption with buprenorphine compared to celecoxib.23 No differences in drug-related side effects were reported and both studies showed higher satisfaction in the buprenorphine group, suggesting that buprenorphine has similar or superior analgesic efficacy than flurbiprofen, parecoxib and celecoxib, with better patient satisfaction and similar drug-related side effects.
The only study that showed buprenorphine inferiority for postoperative pain compared transdermal buprenorphine 10 mcg h−1 and transdermal fentanyl 25 mcg h−1. However, in this study postoperative opioid consumption was similar and the buprenorphine group reported higher pain and sedation scores.15 Also, this study used non-equivalent buprenorphine and fentanyl dosages,9, 13, 30 both patches were applied 6 hours before surgery, allowing a wider transdermal fentanyl onset of action,31 but not an adequate buprenorphine onset of action.13
Three studies compared different dosages of buprenorphine.18, 20, 21 In one study comparing buprenorphine dosages of 10 mg vs. 20 mg, pain scores were higher in the 10 mg group,18 while there were no differences in pain scores20, 21 in two studies comparing higher buprenorphine dosages (17.5 mcg h−1 to 52.5 mcg h−1). Two studies reported postoperative opioid consumption comparing doses of transdermal buprenorphine ranging from 10 mcg h−1 to 52.5 mcg h−1 and, in both, analgesic requirements were inversely proportional to buprenorphine dosage. Studies also showed increasing somnolence20 or sedation scores18 with higher buprenorphine dosages, while one study showed no difference in side effects.21 One study reported no significant difference in patient satisfaction with buprenorphine dosages ranging from 17.5 mcg h−1 to 52.5 mcg h−1.21 These results suggest that transdermal buprenorphine dosages can increasingly reduce postoperative analgesic requirements, while possibly leading to higher drug-related side effects until 17.5 mcg h−1 to 20 mcg h−1 dosages. Higher dosages did not demonstrate an increased analgesic benefit. Moreover, no study reported severe or life-threatening side effects, suggesting that doses of 10 mcg h−1 to 52.5 mcg h−1 are relatively safe.
The reported results are derived from few clinical trials, so more studies are necessary to confirm the safety and efficacy of buprenorphine compared to other analgesics or different buprenorphine dosages for postoperative pain. Also, most multiple buprenorphine dosages studies used fractions of the buprenorphine patch,20, 21 which is not recommended by manufacturers.
Possible advantages of buprenorphine use over other opioids include less association with analgesic tolerance and dependency, less MOR related opioid-side effects, a ceiling effect on respiratory depression, evidence for safe use in elderly patients and patients with impaired renal function, less cognitive dysfunctions, no evidence of immunosuppressive or hypothalamic-pituitary-adrenal pathway side effects.9, 13, 32 Despite evidence for those advantages in chronic pain, several clinical trials for transdermal buprenorphine use in acute postoperative pain do not include elderly or renal-impaired patients and none evaluated tolerance, dependency, cognitive dysfunction, endocrine or immunosuppressive side effects. More clinical trials involving the mentioned populations should improve knowledge on transdermal buprenorphine use on acute pain.
Limitations of this systematic review include: 1) The high or unclear risk of bias from most included studies, which can contribute to an increase in the overall risk of bias for this review; 2) Surgical procedures analyzed had different nociceptive stimuli, probably reflecting different results on buprenorphine and control group comparisons; 3) Studies comparing different opioid treatments used non-equivalent dosages of transdermal buprenorphine and control group opioids, influencing the analyzed outcomes; 4) Most studies did not describe or did not use intermittent multimodal analgesia in the perioperative period, focusing only on rescue analgesia medications; 5) Other outcomes should be analyzed for a more thorough comparison of perioperative transdermal buprenorphine and other analgesic techniques; 6) Most studies had small sample sizes and/or did not provide a sample size or power of evidence calculations.
Summary
Postoperative pain is often treated with opioid agonists. Nevertheless, transdermal buprenorphine seems to be an effective and safe option for management of acute postoperative pain, showing an equivalent or superior effect compared to most control groups. However, these findings are based on few studies with a high or unclear risk of bias, which mostly did not compare buprenorphine with other opioids. Hence, further research is needed to investigate transdermal buprenorphine use in acute pain, specially comparing buprenorphine with other opioids commonly used in the postoperative period.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bjane.2020.06.009.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Gerbershagen H.J., Aduckathil S., Van Wijck A.J.M., et al. Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiology. 2013;118:934–944. doi: 10.1097/ALN.0b013e31828866b3. [DOI] [PubMed] [Google Scholar]
- 2.Kehlet H., Jensen T.S., Woolf C.J. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–1625. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 3.Chou R., Gordon D.B., De Leon-Casasola O.A., et al. Management of postoperative pain: a clinical practice guideline from the American pain society, the American society of regional anesthesia and pain medicine, and the American society of anesthesiologists’ committee on regional anesthesia, executive commi. J Pain. 2016;17:131–157. doi: 10.1016/j.jpain.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Brennan T.J. Pathophysiology of postoperative pain. Pain. 2011;152:S33–S40. doi: 10.1016/j.pain.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vadivelu N., Anwar M. Buprenorphine in postoperative pain management. Anesthesiol Clin. 2010;28:601–609. doi: 10.1016/j.anclin.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Macintyre P.E., Huxtable C.A. Buprenorphine for the management of acute pain. Anaesth Intensive Care. 2017;45:143–146. doi: 10.1177/0310057X1704500202. [DOI] [PubMed] [Google Scholar]
- 7.Khannaish K., Pillarisetti S. Buprenorphine – an attractive opioid with underutilized potential in treatment of chronic pain. J Pain Res. 2015;8:859–870. doi: 10.2147/JPR.S85951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahan A., Yassen A., Romberg R., et al. Buprenorphine induces ceiling in respiratory depression but not in analgesia. Br J Anaesth. 2006;96:627–632. doi: 10.1093/bja/ael051. [DOI] [PubMed] [Google Scholar]
- 9.Davis M.P., Pasternak G., Behm B. Treating chronic pain: an overview of clinical studies centered on the buprenorphine option. Drugs. 2018;78:1211–1228. doi: 10.1007/s40265-018-0953-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahn J.S., Lin J., Ogawa S., et al. Transdermal buprenorphine and fentanyl patches in cancer pain: a network systematic review. J Pain Res. 2017;10:1963–1972. doi: 10.2147/JPR.S140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sorge J., Sittl R. Transdermal buprenorphine in the treatment of chronic pain: results of a phase iii, multicenter, randomized, double-blind, placebo-controlled study. Clin Ther. 2004;26:1808–1820. doi: 10.1016/j.clinthera.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Kitzmiller J.P., Barnett C.J., Steiner N.S., et al. Buprenorphine: revisiting the efficacy of transdermal delivery system. Ther Deliv. 2015;6:419–422. doi: 10.4155/tde.15.3. [DOI] [PubMed] [Google Scholar]
- 13.Kress H.G. Clinical update on the pharmacology, efficacy and safety of transdermal buprenorphine. Eur J Pain. 2009;13:219–230. doi: 10.1016/j.ejpain.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Sastre J.A., Varela G., Lopez M., et al. Influence of uridine diphosphate-glucuronyltransferase 2B7 (UGT2B7) variants on postoperative buprenorphine analgesia. Pain Pract. 2013;15:22–30. doi: 10.1111/papr.12152. [DOI] [PubMed] [Google Scholar]
- 15.Arshad Z., Prakash R., Gautam S., et al. Comparison between transdermal buprenorphine and transdermal fentanyl for postoperative pain relief after major abdominal surgeries. J Clin Diagn Res. 2015;9:UC01–UC4. doi: 10.7860/JCDR/2015/16327.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desai S.N., Badiger S.V., Tokur S.B., et al. Safety and efficacy of transdermal buprenorphine versus oral tramadol for the treatment of post-operative pain following surgery for fracture neck of femur: a prospective, randomised clinical study. Indian J Anaesth. 2017;61:225–229. doi: 10.4103/ija.IJA_208_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim H.-J., Ahn H.S., Nam Y., et al. Comparative study of the efficacy of transdermal buprenorphine patches and prolonged-release tramadol tablets for postoperative pain control after spinal fusion surgery: a prospective, randomized controlled non-inferiority trial. Eur Spine J. 2017;26:2961–2968. doi: 10.1007/s00586-017-5213-5. [DOI] [PubMed] [Google Scholar]
- 18.Kumar S., Chaudhary A.K., Singh P.K., et al. Transdermal buprenorphine patches for postoperative pain control in abdominal surgery. J Clin Diagn Res. 2016;10:UC05–UC8. doi: 10.7860/JCDR/2016/18152.7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niyogi S., Bhunia P., Nayak J., et al. Efficacy of transdermal buprenorphine patch on post-operative pain relief after elective spinal instrumentation surgery. Indian J Anaesth. 2017;61:923–929. doi: 10.4103/ija.IJA_118_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rivera-ruiz A.P., Villegas-gómez R.M., Mejía-terrazas G.E. Buprenorfina transdérmica en dolor postoperatorio. Ensayo clinico controlado. Rev Mex Anestesiol. 2018;41:83–87. [Google Scholar]
- 21.Setti T., Sanfilippo F., Leykin Y. Transdermal buprenorphine for postoperative pain control in gynecological surgery: a prospective randomized study. Curr Med Res Opin. 2012;28:1597–1608. doi: 10.1185/03007995.2012.719864. [DOI] [PubMed] [Google Scholar]
- 22.Tang J., Fan J., Yao Y., et al. Application of a buprenorphine transdermal patch for the perioperative analgesia in patients who underwent simple lumbar discectomy. Medicine (Baltimore) 2017;96:e6844. doi: 10.1097/MD.0000000000006844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu C., Li M., Wang C., et al. Perioperative analgesia with a buprenorphine transdermal patch for hallux valgus surgery: a prospective, randomized, controlled study. J Pain Res. 2018;11:867–873. doi: 10.2147/JPR.S153456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moher D., Shamseer L., Clarke M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1–10. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins J.P.T., Altman D.G., Gotzsche P.C., et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guyatt G.H., Osoba D., Wu A.W., et al. Methods to explain the clinical significance of health status measures. Mayo Clin Proc. 2002;77:371–383. doi: 10.4065/77.4.371. [DOI] [PubMed] [Google Scholar]
- 27.Ferreira C.A., Loureiro C.A.S., Saconato H., et al. Validity of Qualis database as a predictor of evidence hierarchy and risk of bias in randomized controlled trials: a case study in dentistry. Clinics (Sao Paulo) 2011;66:337–342. doi: 10.1590/S1807-59322011000200025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peelen L.M., Ph D., Kalkman C.J., et al. Pain intensity on the first day after surgery. Anesthesiology. 2013;118:934–944. doi: 10.1097/ALN.0b013e31828866b3. [DOI] [PubMed] [Google Scholar]
- 29.Vetter T.R., Kain Z.N. Role of the perioperative surgical home in optimizing the perioperative use of opioids. Anesth Analg. 2017;125:1653–1657. doi: 10.1213/ANE.0000000000002280. [DOI] [PubMed] [Google Scholar]
- 30.Mc Pherson M. 2010. Demystifying opioid conversion calculations: a guide for effective dosing. Pharm AS of H-S, editor. Bethesda. [Google Scholar]
- 31.Nelson L., Schwaner R. Transdermal fentanyl: pharmacology and toxicology. J Med Toxicol. 2009;5:230–241. doi: 10.1007/BF03178274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis M.P. Twelve reasons for considering buprenorphine as a frontline analgesic in the management of pain. J Support Oncol. 2012;10:209–219. doi: 10.1016/j.suponc.2012.05.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



