Abstract
Background
Calcium carbide (CaC2) is a chemical primarily used in the production of acetylene gas. The misuse of CaC2 to induce fruit ripening is a global challenge with a potential adverse effects to human health. Additionally, CaC2 is known to contain some reasonable amount of arsenic and phosphorous compounds that are toxic and pose a danger to human health when ingested. The current study sought to characterize CaC2 toxicity and elucidate any protective effects by cyanocobalamin (vitamin B12), a well-established antioxidant and anti-inflammatory bio-molecule. Female Swiss white mice were randomly assigned into three groups; the first group was the control, while the second group was administered with CaC2. The third group received CaC2 followed by administration of vitamin B12. The mice were sacrificed at 60 days post treatment, hematological, biochemical, glutathione assay, cytokine ELISA and standard histopathology was performed.
Results
CaC2 administration did not significantly alter the mice body weight. CaC2 administration resulted in a significant decrease in packed cell volume (PCV), hemoglobin (Hb), red blood cells (RBCs) and RBC indices; indicative of CaC2-driven normochromic microcytic anaemia. Further analysis showed CaC2-driven leukopenia. Evidently, vitamin B12 blocked CaC2-driven suppression of PCV, Hb, RBCs and WBCs. Monocytes and neutrophils were significantly up-regulated by CaC2. CaC2-induced elevation of aspartate aminotransferase (AST), alanine aminotransferase (ALT) and bilirubin signaled significant liver damage. Notably, vitamin B12 stabilized AST, ALT and bilirubin in the presence of CaC2, an indication of a protective effect. Histopathological analysis depicted that vitamin B12 ameliorated CaC2-driven liver and kidney injury. CaC2 resulted in the depletion of glutathione (GSH) levels in the liver; while in the brain, kidney and lungs, the GSH levels were elevated. CaC2 administration resulted in elevation of pro-inflammatory cytokines TNF-α and IFN-γ. Vitamin B12 assuaged the CaC2-induced elevation of these pro-inflammatory cytokines.
Conclusions
These findings demonstrate for the first time that oral supplementation with vitamin B12 can protect mice against CaC2-mediated toxicity, inflammation and oxidative stress. The findings provide vital tools for forensic and diagnostic indicators for harmful CaC2 exposure; while providing useful insights into how vitamin B12 can be explored further as an adjunct therapy for CaC2 toxicity.
Keywords: Calcium carbide, Vitamin B12, Hematopoiesis, Toxicity
Background
Calcium carbide (CaC2) is used in the manufacture of many compounds including acetylene gas. CaC2 yields acetylene gas when dissolved in water. Prior studies have demonstrated that when ingested by human, acetylene produces free radicals that causes cellular and organ damage [1, 2]. These CaC2—induced free radicals cause cellular damage and accelerate the aging process [3, 4]. In addition, it also contains impurities of arsenic and phosphorous compounds that are relatively toxic to human and animals [5, 6]. The growing awareness of fruit safety in regard to chemical exposure has awakened research about hazards in regard to contamination with CaC2 and a repertoire of other chemicals used in fruit ripening process [7, 8]. Furthermore, CaC2 is inappropriately used to chemically induce fruit ripening in many countries [9]. CaC2, is cheap and readily available tempting farmers to harvest their fruits before maturation. Even though artificial ripeners quicken the rate of the ripening process, the nutritional quality in regard to sensory, and safety of the fruits is compromised [2]. Evidently, CaC2 causes food contamination, gastric irritation, mouth ulcers, cerebral oedema, seizures, and changes in vital hematological and biochemical processes [2, 10]. Further reports have shown that chemical substances used as artificial ripeners have negative effects on humans that include memory loss, cerebral oedema, prostate, changes in DNA and RNA [2]. It is well established that CaC2 impacts the sensory system by limiting oxygen supply to the brain, with potential consequence of neural damage. In addition, evidence shows involvement in lung failure, renal failure, dermal diseases and heart conditions [10, 11]. There is a need for detailed studies to elucidate and characterize the negative physiological and biochemical process affected by CaC2 in humans to aid in diagnosing exposure and development of pharmaceutical intervention. Previous investigations suggest involvement of oxidative stress and inflammation in CaC2-driven organ damage [4]. It therefore makes sense that a potent antioxidant and anti-inflammatory agent could mitigate CaC2 toxicity. Hence cyanocobalamin also known as vitamin B12, a potent antioxidant and anti-inflammatory molecules, usually taken as a supplement, was investigated to determine its potential to mitigate CaC2 toxicities. Vitamin B12 is vital in the synthesis of red blood cells, keeping nerve cells healthy and synthesis of genetic materials. The sources of vitamin B12 include meat, milk, fish, and shellfish [12]. Vitamin B12 is a methyl donor precursor, naturally occurring in the diet. Previous studies have demonstrated that when combined with folate, vitamin B12 down-regulated the pro inflammatory cytokines and low-grade systemic inflammation [13]. The current study sought to profile or characterize negative physiological and biochemical effects of CaC2 exposure in a mouse model. In addition; the study sought to establish the effectiveness of vitamin B12 in abrogating CaC2–induced negative effects.
Results
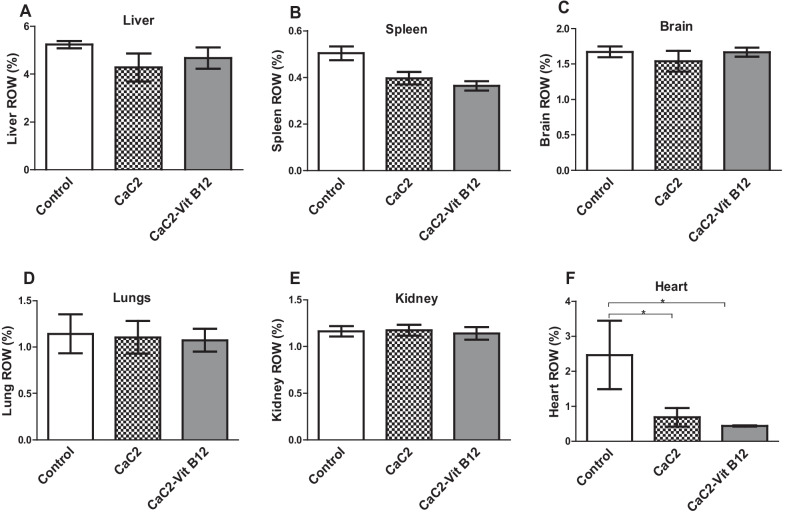
Effect of calcium carbide and vitamin B12 on body and organ weight
This study examined whether vitamin B12 administration would restore CaC2-induced alteration in physiological parameters by measuring changes in general body and relative organ weight. Determination of mice body weight showed a steady increase in weight of control group of mice compared to the CaC2 supplemented group of mice (Fig. 1). There was a marginal weight gain in the group of mice both supplemented with CaC2 and/or with vitamin B12 up to 40 days post-administration; and decreased thereafter up to 60 days post-administration. To determine if the weight change was due to alteration in organ weights, the relative organ weights were determined. This was vital in order to determine whether the effect of oral administration of CaC2 potentiated organ injury and whether administration of vitamin B12 could ameliorate such injury. The relative organ weight for liver, spleen, brain, lungs and kidney were unaffected upon CaC2 exposure (Fig. 2A–E) and a significant decrease in the weight of the heart (Fig. 2F) in the CaC2-exposed groups. This results suggest that exposure to CaC2 has minimal impact on the general body weight.
Fig. 1.
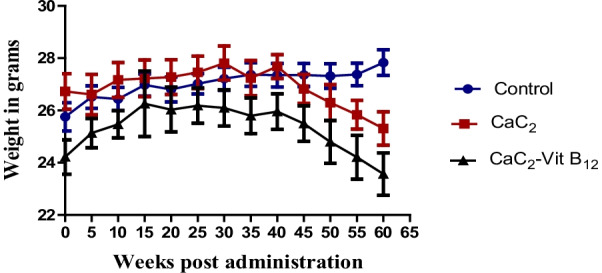
The effects of CaC2 and vitamin B12 on body weight. The change in body weight was analyzed by One-way ANOVA. n = 12. Bars presents ± SEM
Fig. 2.
The effect of CaC2 and vitamin B12 on relative organ weight. Bar graphs showing change in organ weight for, Liver (A), Spleen (B), Brain (C), Lungs (D), Kidney (E) and Heart (F). The relative organ weight was analyzed by One-way ANOVA, followed by Tukeys Post hoc test (*P ≤ 0.05). n = 12. Bars presents ± SEM
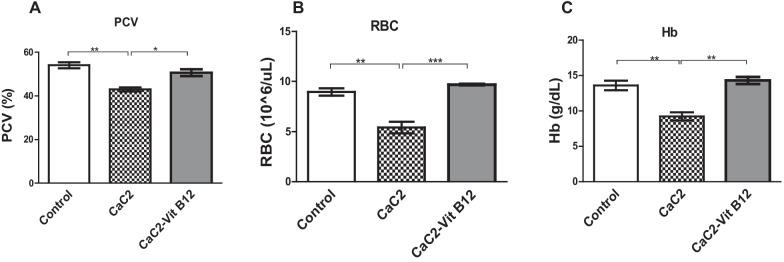
The effect of calcium carbide and vitamin B12 on packed cell volume (PCV), red blood cells (RBC) and haemoglobin (Hb)
This study aimed to investigate the modulating effect of vitamin B12 on PCV, RBC and Hb following CaC2 exposure. The altered blood levels of PCV, RBC and Hb were measured in the blood of CaC2 and vitamin B12 supplemented mice. The results showed that CaC2 significantly decreased (P < 0.0001) the levels of PCV (Fig. 3A). Notably, vitamin B12 when administered stabilized PCV levels. In the study, it was observed that CaC2- significantly depleted the levels of RBCs (Fig. 3B) with concomitant decrease in the levels of Hb (Fig. 3C); a clear indication of anaemia. These results suggest that, administration of vitamin B12 protected mice from CaC2-induced anemia is linked to its anti-oxidant properties.
Fig. 3.
The effect of CaC2 and vitamin B12 on PCV, RBC and Hb. PCV (A), RBC (B) and Hb (C) levels were evaluated. Mean comparison was analyzed by One-way ANOVA, followed by Turkeys Post hoc test (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001). n = 12. Bars presents ± SEM
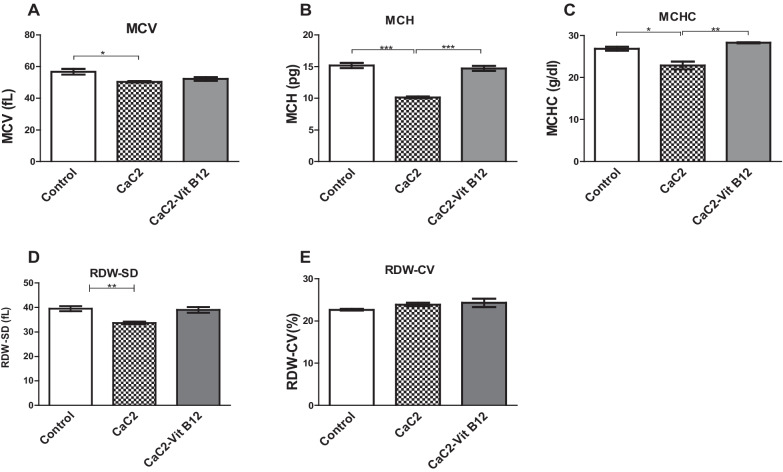
Effect of calcium carbide and vitamin B12 on red blood cell (RBC) indices
Next, this study sought to classify the type of anemia that was induced following CaC2 exposure. Classification was done by measuring various RBC indices in the blood. There was decrease in MCV levels upon exposure to CaC2 relative to control group of mice (Fig. 4A). Notably, vitamin B12 supplementation restored the significant CaC2-driven down-regulation of the mean corpuscular hemoglobin (MHC), the mean corpuscular hemoglobin concentration (MCHC) and ded cell distribution width standard deviation (RDW-SD) (Fig. 4B–D). However, the levels of red cell distribution width coefficient of variation (RDW-CV) (Fig. 4E) were comparable across all the groups. This result demonstrate the effect of protecting against CaC2-induced microcytic hypochromic anemia may be linked to the antioxidant impact of vitamin B12.
Fig. 4.
The effect of CaC2 and vitamin B12 on MCV (A), MCH (B), MCHC (C), RDW-SD (D) and RDW-CV (E). Mean comparison was analyzed by One-way ANOVA, followed by Turkeys Post hoc test (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001). n = 12. Bars presents ± SEM
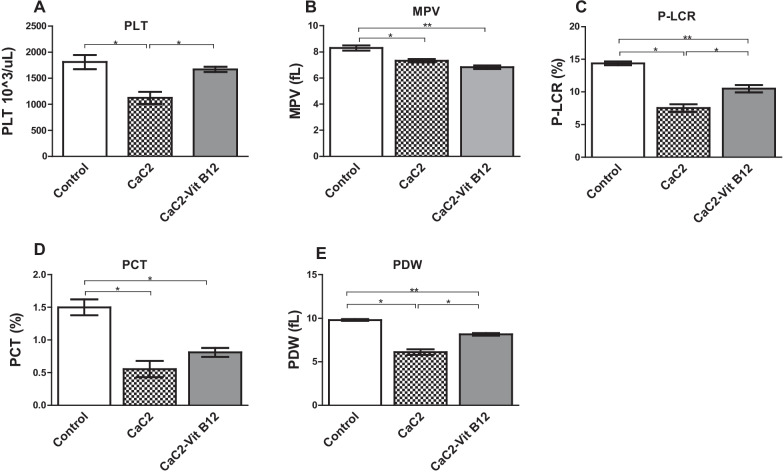
Effect of calcium carbide and vitamin B12 on platelets (PLT)
The levels of PLT and its indices were measured in the blood of CaC2 exposed mice to determine if supplementation with vitamin B12 can reverse any alteration that may be linked to thrombocytopenia. CaC2-induced thrombocytopenia was evident through suppression of platelet levels. Intriguingly, vitamin B12 administration appeared to aid the recovery of platelet levels (Fig. 5A). Further analysis of the platelet indices showed that mean platelet volume (MPV) levels (Fig. 5B) in mice exposed to calcium carbide was depleted when compared to the normal control. The platelet large cell ratio (P-LCR) was significantly reduced (P < 0.05) in mice receiving calcium carbide (Fig. 5C) when compared to either control or vitamin B12 supplemented group of mice. This was further validated by significant reduction in the plateletcrit (PCT) and the platelet distribution width (PDW) in comparison to the control group mice (P < 0.05; Fig. 5D, E). The findings from this study demonstrate that vitamin B12 aided the recovery of platelets in the presence of CaC2.
Fig. 5.
The effect of CaC2 and vitamin B12 on levels of platelets and platelet indices. The histogram shows levels of PLT (A), MPV (B), P-LCR (C), PCT (D) and PDW (E). Mean comparison of PLT and its sub-types were analyzed by One-way ANOVA, followed by Turkeys Post hoc test (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001). n = 12. Bars presents ± SEM
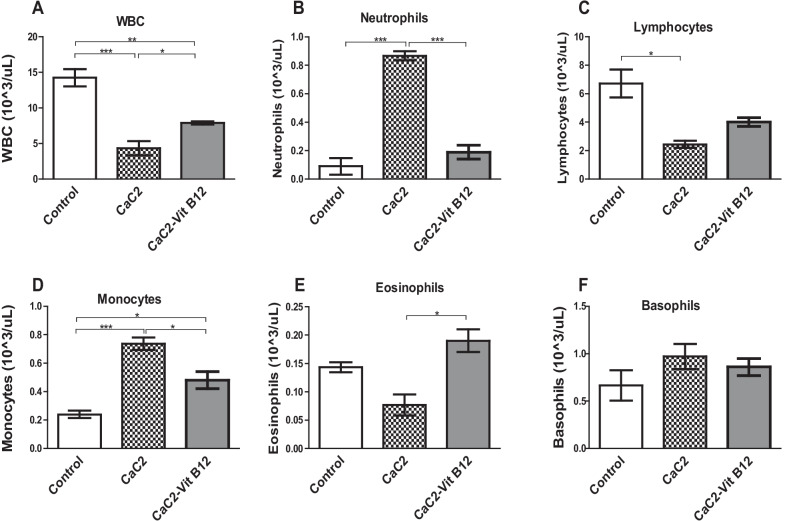
Effect of calcium carbide and vitamin B12 on white blood cells (WBCs) and its subtypes
An attempt to elucidate the putative effect of vitamin B12 to reverse the effects of CaC2-induced alteration of WBC was made. To address this question, the levels of WBC and WBC subtypes were measured in blood from the treatment group of mice. Normally, depletion of WBC numbers beyond a certain limit is an indication of leukopenia. Consistence with this, it was found out that the levels of WBCs were significantly decreased (P < 0.001) in mice administered with CaC2 compared to the control group (Fig. 6). Remarkably, exposure to vitamin B12 prevented CaC2-driven suppression of WBCs. Prior experiments have already shown that CaC2 has the capacity to induce leukopenia. Hence, this study sought to determine whether there was alteration in the composition of WBC phenotypes, in order to determine the extent of vulnerability of the mice to infections and signs of inflammation. Oral administration of CaC2 was found to significantly increase (P < 0.01) neutrophils with reduction in the lymphocytes (Fig. 6B, C respectively). However, exposure to CaC2 resulted in significant increase in monocyte levels with concomitant abrogation of eosinophil levels (Fig. 6D, E). Interestingly, vitamin B12 showed the ability to prevent the CaC2-induced elevation of monocytes and neutrophils and restored lymphocyte and eosinophils levels. On the other hand, the levels of basophils were comparable across all the treatment groups (Fig. 6F). These results suggests that vitamin B12 supplementation restored the CaC2-induced alteration in WBC and its subtypes.
Fig. 6.
The effect of CaC2 and vitamin B12 on WBCs and various subtypes of WBCs. Mice were monitored for WBCs (A), Neutrophils (B), Lymphocytes (C), Monocytes (D), eosinophils (E) and Basophils (F). Mean comparison of WBCs and its sub-types were analyzed by One-way ANOVA, followed by Turkeys Post hoc test (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001). n = 12. Bars presents ± SEM
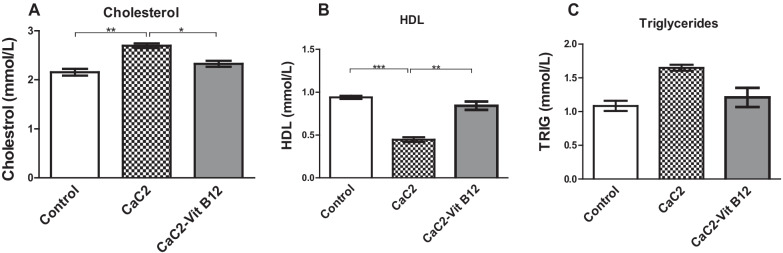
Effect of calcium carbide and vitamin B12 on the lipid profile
This study investigated whether vitamin B12 administration could restore the effect of CaC2-induced alteration of lipid profile by measuring the changes in the serum levels total cholesterol, high density lipoproteins and triglycerides. Results from this study further reveal that exposure of mice to CaC2 resulted in elevation of total cholesterol levels relative to either control or vitamin B12 administered mice (Fig. 7A). Notably, a significant decrease in high density lipoprotein (HDL) (P < 0.05) was observed in mice administered with CaC2 (Fig. 7B). On the contrary, the levels of triglycerides were comparable across all the treatment groups (Fig. 7C). This observation indicates that vitamin B12 was more effective in mitigating CaC2-induced elevation of cholesterol and reduction in high density lipoprotein, may be linked to the cellular protection.
Fig. 7.
The effect of CaC2 and vitamin B12 on the lipid profile. Levels of total Cholesterol (A), HDL (B) and triglycerides (C) were evaluated. Mean comparison of lipid profile was analyzed by One-way ANOVA, followed by Turkeys Post hoc test (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001). n = 12. Bars presents ± SEM
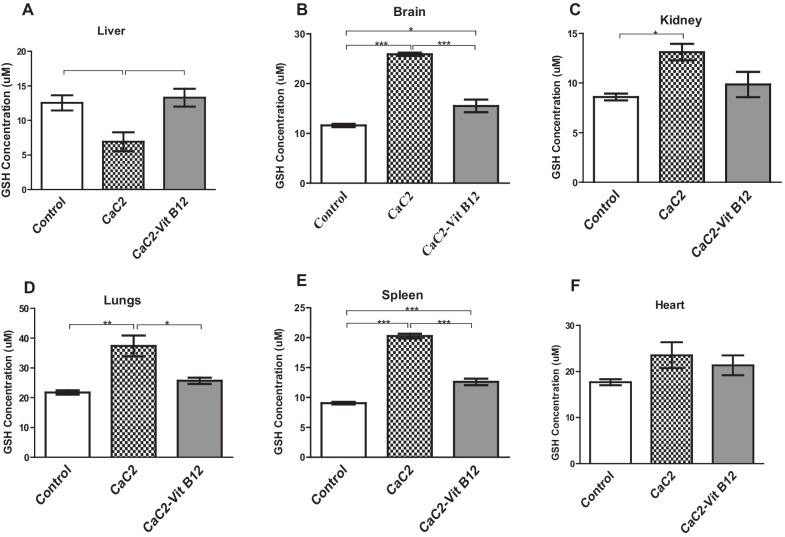
Effect of CaC2 and vitamin B12 on organ cellular reduced glutathione (GSH) concentration
Next we determined whether vitamin B12 supplementation had the capacity to modulate oxidative stress following exposure of mice with CaC2 by measuring the cellular levels of GSH in the liver, kidney, brain, spleen, lungs and heart. Reduced glutathione is one of the primary antioxidants involved in the quenching of reactive oxygen species (ROS) under abnormal physiological conditions. In the CaC2-treated group, there was significant decrease in the level of GSH in the liver relative to mice administered with vitamin B12 (Fig. 8A). Supplementation with vitamin B12 blocked suppression of the cellular GSH levels in the liver. Additionally, exposure of mice to CaC2 resulted in a significant increase (P < 0.05) in the concentration of cellular GSH levels in the brain, kidney, lungs and spleen (Fig. 8B–E). However, the cellular levels of in the heart were comparable across all the treatment groups (Fig. 8F). Vitamin B12 supplementation resulted in stabilization of GSH levels in this vital organs. These results is a clear indication of reduced oxidative stress in the presence of vitamin B12.
Fig. 8.
Effect of CaC2 and vitamin B12 on GSH levels in the liver (A), brain (B), kidney (C), lungs (D), spleen (E) and heart (F). Mean comparison of GSH levels was analyzed by One-way ANOVA, followed by Turkeys Post hoc test (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001). n = 12. Bars presents ± SEM
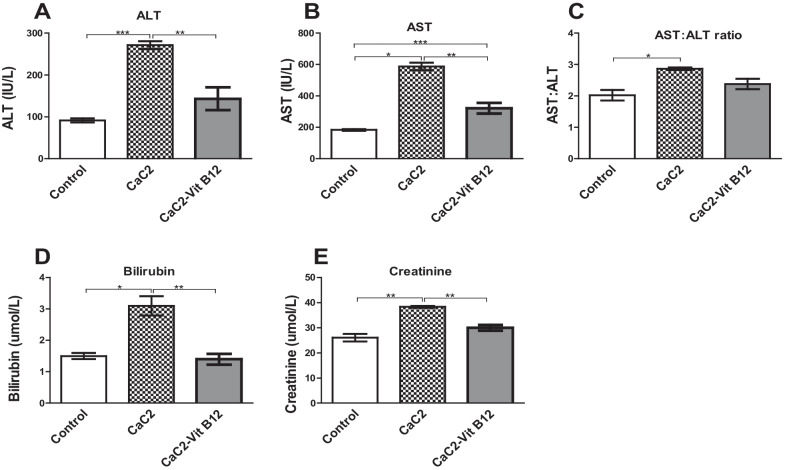
Effects of calcium carbide and vitamin B12 on the markers of liver and kidney injury
This study determined whether vitamin B12 supplementation would nullify CaC2-induced liver and kidney injury, by measuring serum levels of transaminases, total bilirubin and creatinine. Calcium carbide administration resulted in elevation of ALT and AST levels (Fig. 9A, B). Similarly, the result showed a ratio of more than 2:1 in regard to AST: ALT, indicative of liver damage (Fig. 9C). In the presence of vitamin B12, CaC2-driven rise in ALT and AST was blocked. Having clearly established the putative impact of oral administration of vitamin B12 on elevated on liver transaminases, in the presence of CaC2, it was important to determine the extent of CaC2-induced livery injury through the measurement of bilirubin. It was observed that administration of vitamin B12 after CaC2 exposure was effective in restoring CaC2-induced elevation of levels of total bilirubin (Fig. 9D). There was a significant increase (P < 0.05) in levels of the serum creatinine among mice treated with CaC2. Oral administration of vitamin B12 significantly abrogated CaC2-induced elevation of serum creatinine levels (Fig. 9E). These results suggest that vitamin B12 supplementation protected against CaC2-induced liver and kidney injury is associated with anti-inflammatory properties of vitamin B12.
Fig. 9.
Effect of CaC2 and vitamin B12 on ALT (A), AST (B), and AST: ALT (C) ratio and total bilirubin (D) and creatinine (E). Female Swiss white mice were orally administered with calcium carbide and vitamin B12. Comparison among the treatment groups was analyzed by One-way ANOVA, followed by Turkeys Post hoc test (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001). n = 12. Bars presents ± SEM
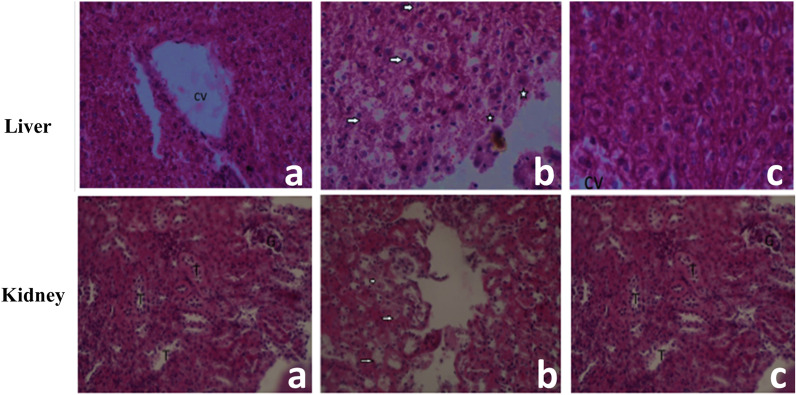
Histopathological analysis of liver and kidney
To establish presence of any deleterious effects of CaC2 administration on the liver cyto-architecture, we performed normal standard histopathological analysis. Exposure to CaC2 resulted in significant damage to the liver was characterized by diffuse hepatocyte swelling necrosis, and focal hemorrhages in the liver parenchyma (Fig. 10). It is noteworthy, that Vitamin B12 administration CaC2 assuaged CaC2-driven liver pathology. Furthermore, CaC2 administration resulted in kidney damage. Specifically, the injury was characterized by the presence of cytoplasmic vacuolation of tubular epithelial cells (Fig. 10). These results suggest that administration of vitamin B12 protected liver and kidney tissue from CaC2 -induced damage is associated with the anti-inflammatory properties of vitamin B12.
Fig. 10.
Effect of CaC2 and vitamin B12 on liver tissues from mice. Vitamin B12 was given to assess extent of protection against CaC2 exposure. Liver and kidney tissues from Control group (a), CaC2 group (b), and CaC2-Vitamin B12 group (c) were processed for histology with H&E staining. Images show representative liver sections with the hepatocyte necrosis (CV-Central vein, arrows indicated diffuse hepatocyte swelling and necrosis and stars are focal areas of hemorrhages in Liver parenchyma). From the kidney sections with the hepatocyte necrosis (G-Glomerulus; T-Renal tubules & Arrow- Cytoplasmic vacuolation of tubular epithelia cells). (Original magnification × 400)
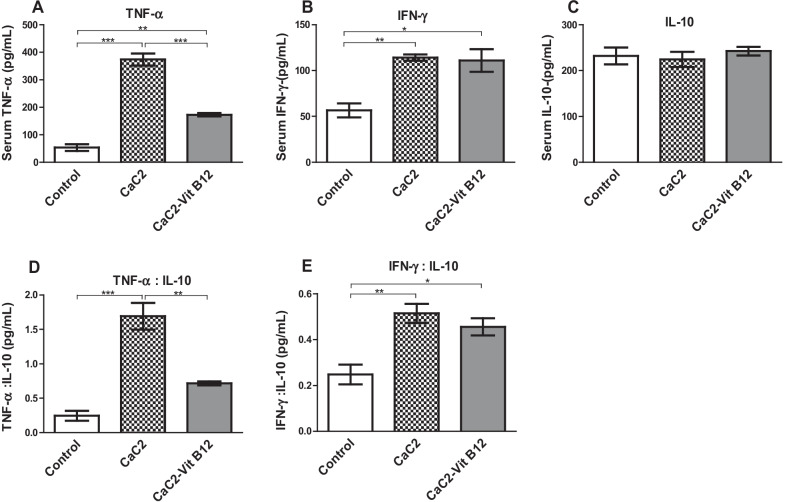
Effect of calcium carbide and vitamin B12 on cytokines
The levels of the pro-inflammatory cytokines tumor necrotic factor-alpha (TNF-α) and interferon gamma (IFN-γ), as well as the anti-inflammatory cytokine interleukin-10 (IL-10), were determined from serum samples to evaluate the ability of CaC2 to trigger inflammation. CaC2 markedly elevated levels of serum TNF-α and IFN-γ (P < 0.0001) (Fig. 11A, B). However, the levels of serum IL-10 were similar across the treatment groups (Fig. 11C). In the presence vitamin B12, the elevation of serum TNF-α and IFN-γ levels was abrogated. The ratios between pro-inflammatory and anti- inflammatory cytokines established the extent of active inflammation where CaC2 supplementation resulted in significant (P < 0.05) imbalance of both TNF-α: IL-10 ratio and IFN-γ: IL-10 ratios (Fig. 11D, E). However, vitamin B12 supplementation abrogated the CaC2-induced TNF-α: IL-10 imbalance. These results demonstrates the effect of protecting against CaC2-induced inflammation can be linked to the anti-inflammatory effect of vitamin B12.
Fig. 11.
Effect of CaC2 and vitamin B12 on pro -inflammation cytokines. Female Swiss white mice were orally administered with calcium carbide and vitamin B12. TNF-α (A), IFN-γ (B), and IL-10 (C), TNF-α: IL-10 (D) and IFN-γ: IL-10 (E) ratios were evaluated. Comparison of serum cytokine levels among the treatment groups was analyzed by One-way ANOVA, followed by Turkeys Post hoc test (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001). n = 12. Bars presents ± SEM
Discussion
Exposure to CaC2 causes serious detrimental effects that often can trigger cancer development, food poisoning, irritation of gastral tissues and mouth ulceration, cerebral oedema and seizures [2]. Moreover, ingestion of fruits ripened with CaC2 can cause alterations to vital physiological and biochemical processes [7]. Notably, the negative physiological and biochemical processes due to CaC2 exposure have not been well characterized to enable diagnostic and forensic determination of exposure, as well as allow development of detoxification or treatment strategies. In this study, we demonstrate for the first time that vitamin B12, a potent anti-inflammatory and antioxidant, assuaged CaC2-induced negative physiological and biochemical effects in a mouse model. Moreover, the findings from the present study established that exposure of mice to only vitamin B12 alone did not have any effect on both physiological, biochemical and inflammatory responses (data not provided). Notably, oral exposure to CaC2 resulted in marginal decrease in mice body weight, though not statistically significant. Other studies have reported significant CaC2–induced weight gain in wistar rats [7]. The marginal alteration in body weight due to CaC2 relative to the control indicates interference with normal physiological growth and development; which can be attributed to the impairment of biochemical processes critical for normal growth and loss of appetite. Notably, administration of vitamin B12 did not have any impact on the general body weight of mice due to CaC2 exposure, or that of its organs, probably due to its contribution in inhibition of adipocyte differentiation and lipid accumulation. Worthy of note is that the exposure to CaC2 resulted in significant decrease in the relative organ weight of the heart; which was not rescued by vitamin B12 supplementation. Indeed, increased levels of AST in the current study further shows the possibility of CaC2-induced toxicity in other organs such as the heart; given that AST is ubiquitously distributed in other major organs of the body. Recently it has been reported that cardiovascular dysfunction due to CaC2 is associated with inflammatory mediators and oxidative stress [14]. Indeed, this findings imply that exposure to CaC2 can result in a high risk of heart diseases This is a significant and profound phenomenon that warrants further investigations especially on the histopathological analysis of the heart.
Hematopoiesis is a very significant process that warranties effective and controlled supply of a range of blood cellular constituents [15]. This study reports clear evidence for CaC2-driven derangement of hematopoiesis. Reduction in PCV has been associated with oxidative stress and impaired osmoregulation [8]. In the current study, a significant CaC2-driven decrease in PCV was noted. Decrease in PCV may be attributed to harmful effect of calcium carbide on the bone marrow, alteration of micronutrients for RBC synthesis, inhibition of erythropoietin and elevated haemolysis of the red cells due to noxious consequence. A similar study in which mice were fed on fruits ripened by CaC2 showed reduced PCV levels [11]. Indeed, a decline in PCV is one of the key features of exposure to toxic chemicals that is associated with generation of ROS and lipid peroxidation [16]. Notably, administration of vitamin B12 significantly stabilized the PCV levels which may be attributed to its anti-inflammatory properties. Vitamin B12 scavenges free radicals and enhances membrane integrity by preventing lipid oxidation [17]. Indeed, lipid oxidation is interrelated with membrane fragmentation and ultimate cell death, then it is possible that vitamin B12 protected against CaC2-driven decline in PCV levels by quenching levels of free radicals in blood thus protecting the red blood cells membrane against oxidative damage.
There was a clear CaC2-induced reduction in the levels of the RBCs and hemoglobin; indicative of anemia. The present findings are consistent with prior study in which expose to CaC2 resulted in significant decrease in Red Blood Cell, Haemoglobin and Packed Cell Volume count [7]. CaC2 driven decrease in RBCs and Hb may be due to several factors including impaired erythropoiesis, accelerated RBC lysis and microcytic or normocytic anaemia [1]. Notably, CaC2 has been shown to obstruct elements and minerals important for erythropoiesis such as iron, folic acid and vitamin B12 [2]. It is noteworthy that decreased haemoglobin content may result from lyses of erythrocytes, erythropenia, haemopoiesis and hindrance of erythropoietin or disruption of haemoglobin production [1]. Previous studies have shown that vitamin B12 administration was more effective in stimulating erythropoiesis among premature infants [18]. A remarkable finding here was that administration of vitamin B12 protected mice from CaC2- induced suppression of RBCs and Hb levels. This results strongly supports the ameliorative effect of vitamin B12 in CaC2-induced anemia observed in the current study.
Changes in RBC indices (MCV, MCH, and MCHC) constitutes a key parameter applied in the classification of anaemia. Herein, it was observed that CaC2-induced microcytic hypochromic anaemia, as shown by the significant reduction in the levels of MCH, MCHC and RDW-SD. Note that, contrary findings have been reported in CaC2 exposure to rats; where RBC indices were up-regulated [7]. Once again, we demonstrated that vitamin B12 supplementation significantly restored the levels of red cell indices. This is not surprising given that vitamin B12 is vital for DNA synthesis and its deficiency can cause megaloblastic anaemia via abortive erythropoiesis and hyperbilirubinemias.
Platelets (PLT) play a critical role in blood clotting cascades, and are vital for preventing bleeding, support healing and have also been involved in inflammatory response and wound healing [19]. In the current study, CaC2 significantly suppressed platelet levels, possibly through the initiation of caspase-dependent apoptosis. Reduction in platelet count may also be an indication of thrombocytopenia, whose cause may be impaired hematopoiesis; which may constitute a serious health threat to people on blood thinning therapy [20, 21]. Vitamin B12 administration appeared to aid the recovery of platelet levels. Notably, vitamin B12 seems to stimulate thrombocytosis, possibly due to anti-inflammatory effects on vital molecules crucial for regulating platelet levels and modulate blood clotting cascades. This phenomena warrants further scrutiny.
White blood cells (WBCs) play an important role in immune function [10]. In the current study, a significant decrease in WBCs was recorded in mice administered with CaC2. Decrease in the WBCs also referred to as leukopenia due to CaC2 portends serious consequences in regard to the ability to fight infections as well as disease diagnostic tests that rely on WBC levels. Leukopenia in mice administered with CaC2 is indicative of CaC2-induced severe suppression of lymphoproliferative processes. Similar findings have been reported in other related studies [11]. Notably, other studies have shown contradictory findings in rats fed on fruits ripened with CaC2 [10]. Further, studies established the ability of vitamin B12 to prevent CaC2-driven down-regulation of WBCs when administered. We further evaluated the effect of CaC2 on the various WBC subtypes (lymphocytes, monocytes, basophil and neutrophils). Oral administration of CaC2 significantly increased the levels of neutrophils and resulted in reduced lymphocyte levels. CaC2-driven neutrophilia and monocytosis was noted. Notably, basophil levels were unchanged. However, in the presence of vitamin B12, lymphocyte levels were stabilized in mice administered with CaC2. Neutrophilia could be triggered due to CaC2-driven stress as well as inflammation and cellular damage. Neutrophilia, monocytosis and suppression of lymphocytes would definitely have detrimental implications in disease diagnosis. For example, elevation of neutrophils (neutrophilia) is characterized by laectanae infection. On the other hand, high lymphocytes and monocytes (monocytosis) traditionally signal an infection and could signal a serious disease such as leukemia. Moreover, eosinophilic is an indication for a parasitic infection, allergic reactions or cancer. The profound conclusion based on our findings is that CaC2-driven neutrophilia, monocytosis and suppression of lymphocytes can interfere with critical laboratory diagnostic data for serious bacterial or viral infections as well as diseases like cancer. Certainly, this phenomenon requires further scrutiny. Additionally, it is necessary to investigate this possibility in the future in order to determine the underlying mechanism through which CaC2 mediate the derangement of hematopoiesis process within the bone marrow.
Further investigations sought to elucidate the impact of CaC2 and vitamin B12 on lipid metabolism. Exposure of mice to CaC2, resulted in significant elevation of cholesterol. Notably, a significant decrease in high density lipoprotein (HDL) was observed in mice administered with CaC2. On the other hand, the levels of triglycerides were comparable across the groups. Previous studies have noted contradictory findings in regard to the effect of CaC2 on lipid metabolism. A previous study reported decreased plasma cholesterol and low-density lipoprotein (LDL) in rats fed on mangoes ripened with CaC2 [7]. Note that HDL stimulates efflux of excessive cholesterol from outlying tissues by reverting it back to the liver for biliary elimination [22]. Elevated serum levels of cholesterol, triglycerides (TG) and low density lipoprotein (LDL) is associated with dyslipidemia [23]. In pre-clinical studies, low vitamin B12 levels is linked to increased lipid accumulation in adipocytes that ultimately elicit dyslipidemia in mice [24]. On this basis, it can be concluded that CaC2 significantly affect lipid metabolism, with exposure to vitamin B12 counteracting this effect.
Glutathione (GSH) is a major antioxidant defense molecule produced in the body [25]. It is one of the most critical sources of reducing power and redox stabilization in cells [26]. Consequently, it provides first line cellular defense against free radicals. Reduced glutathione in the tissues is one of the primary antioxidants involved in the quenching of generated ROS under abnormal physiological conditions such as those induced by toxins [27, 28]. Hydroxamic acid, a known toxin, disrupts the antioxidant balance in the liver and spleen at higher doses in rats [27]. For this reason, changes in cellular GSH can be utilized as a marker for oxidative stress [29]. In the current study, a significant depletion of GSH was noted in the liver. Reduced levels of GSH in body tissues is suggestive of extreme oxidative stress. Calcium carbide administration resulted in a significant increase in the concentration of cellular GSH levels in the brain, kidney and lungs. However, the cellular levels of GSH in the spleen and heart were comparable across all the treatment groups. A rise in GSH levels may be due to induction of its synthesis via a cascade of enzymatic reactions; in response to rise in oxidative stress [26, 29]. On the other hand, decreased GSH may be caused by its overutilization during chronic oxidative stress due to its role as the prime intra cellular anti-oxidant [26]. Vitamin B12 supplementation resulted in stabilization of GSH in the presence of CaC2 in the liver, brain and kidney. This is not surprising given that Vitamin B12 is known to prevent deleterious effects associated with oxidative tissue injury due to its anti-apoptotic and anti-oxidative functions. It is noteworthy, to mention that other previous studies have demonstrated the role of vitamin B12 in stabilizing GSH levels [27, 30]. This may attribute to the ability of vitamin B12 to counter oxidative stress, thereby reducing the demand on GSH due to its anti-oxidant properties.
The liver is an important organ that plays a vital role in the metabolism of xenobiotics and is the primary target for CaC2 toxicity. Therefore, during CaC2 poisoning, liver damage is unavoidable.
Moreover, ALT and AST are important liver enzymes, whose levels in serum is an indicator of liver pathology. In the current study, ALT and AST levels were elevated upon exposure of mice to CaC2, but were stabilized upon administration of vitamin B12. Additional investigations on the liver showed CaC2-driven elevation of bilirubin. A bilirubin test is vital in diagnosing liver damage since bilirubin is a bile pigment formed from the breakdown of haemoglobin in RBCs. Increased bilirubin levels is indicative of hepatobiliary disorder with blockage of flow of bile through the bile duct [7]. Perhaps due to its antioxidant capabilities, vitamin B12 stabilized levels of AST, ALT and bilirubin. This a clear evidence for Vitamin B12-driven hepatocellular protection from CaC2-driven liver injury [31].
Additional investigations focused on the integrity of kidney function in the presence of CaC2 and vitamin B12. We demonstrate that CaC2 driven renal injury was ameliorated by administration of vitamin B12. Elevated creatinine levels suggest a reduction in the glomerular filtration rate; indicative of kidney function impairment. Similar findings have been noted in rat fed with CaC2-ripened mango [1]. Moreover, elevated creatinine levels is associated with reduced glomerular filtration, impaired elimination of waste products with potential kidney swelling, inflammation, and necrotic cell damage [16]. Once again, oral administration of vitamin B12 abrogated CaC2-induced rise in serum creatinine levels is linked to its anti-inflammatory properties.
Cytokines are biologically active proteins that mediate vital intercellular communication in the immune system and are secreted by different immune cell types. In addition, they participate in host defense, inflammatory and tissue repair activities [32, 33]. Pro-inflammatory cytokines are produced by activated macrophages effector cells that participated in adaptive immune system and play a significant role in exacerbation of inflammatory processes. Consequently, interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) are critical in fighting infections, and ultimately for cell survival mechanisms. In the current study, the levels of the pro-inflammatory cytokines tumor necrotic factor-alpha (TNF-α) and interferon gamma (IFN-γ), as well as the anti-inflammatory cytokine interleukin-10 (IL-10), were determined from serum samples to evaluate the ability of CaC2 to trigger inflammation. CaC2 supplementation resulted in markedly augmented levels of serum TNF-α and IFN-γ. Ordinarily, cytokines work in synergy with IFN-γ, and stimulates migration of immune cells to infection sites, leading to granuloma development, capable of regulating the immune response. The functions of IFN-γ in macrophage activation and stimulation of the antigen presentation cascades is well established [33, 34]. In the presence of vitamin B12, CaC2-driven elevation of serum TNF-α was blocked.
Anti-inflammatory cytokines such as interleukin 10 (IL-10), are immunoregulatory molecules that regulate the pro-inflammatory cytokine reaction. IL-10 is produced by leukocytes and is associated with inflammatory and autoimmune responses [35]. Furthermore, IL-10 exerts anti-inflammatory properties by inhibiting transcription factor; consequently targeting antigen-presenting cells and lymphocytes [32, 36]. Our findings demonstrated a CaC2-induced elevation of the pro-inflammatory cytokines, indicative of inflammation.
Vitamin B12 play a critical and vital role in the proper function of immune system chiefly as an immune-modulator [37]. Specifically, it has been observed that individuals who are deficient of Vitamin B12 have low levels of CD8+ T cells and impaired activity of NK cells [38]. Indeed, lambs put on vitamin B12 deficient diet were found to suffer most from Mycobacterium paratuberculosis due to low lymphoblastic proliferation response further highlighting the significance of vitamin B12 as an immune-stimulator [39]. Moreover, vitamin B12 has been reported to favor both humoral and cellular immunity by increasing the levels of serum IgG, IgA, and IgM [38].
It is worth noting that vitamin B12 may have had an immunomodulatory effect that attenuated CaC2 toxicity driven by inflammation. Moreover, Vitamin B12 reduces homocysteine levels and inflammation hence regulating production of TNF-alpha. Note that vitamin B12 has also been adversely linked to pro-inflammatory cytokines and low-grade systemic inflammation in some studies [13].
Abnormal and continuous secretion with concomitant accumulation of ROS during CaC2 metabolism is linked with aggravation in organ toxicities. This study further investigated the effect of CaC2 through histopathological analysis of the liver and kidney to support biochemical analysis data. Exposure to CaC2 resulted in significant damage to the liver. The liver injury was characterized by diffuse hepatocyte swelling, necrosis, and focal hemorrhages in liver parenchyma. The cytoplasm of the hepatocytes appeared normal with basophilic nucleus. Hepatocytes appeared apoptotic and shrunken. Some of the degenerating cells were shrunken and looked like minute, structure-less, hyaline masses. Vitamin B12 administration exposure assuaged the CaC2 -induced tissue and hepato-cellular damage.
The kidney is an essential organ controlling vital physiological and biochemical processes such as homeostasis, detoxification and elimination of lethal metabolites and drugs [40]. Histopathological analysis of the kidney revealed evidence for CaC2 -induced kidney injury, characterized by the presence of cytoplasmic vacuolation of tubular epithelial cells. It can only be concluded that kidney injury and nephrosis occurred due to calcium carbide and/or its metabolites. The findings are consistent with studies conducted on proliferative and nonproliferative lesions of the rat and mouse urinary systems due to calcium carbide on rat tissues [4]. It was notable that administration of vitamin B12 after CaC2 protected kidney tissue from CaC2-induced damage. The association between vitamin B12 and reduced inflammation and homocysteine which is associated with oxidative stress has widely been reported by a number of studies [41, 42]. The capacity of vitamin B12 to be well distributed in extracellular and cytosolic spaces is fundamental in its efficiency against CaC2 toxicity. Further research will be needed to further characterize CaC2-toxicity in order to elucidate the apparent molecular processes responsible for the ability of vitamin B12 to confer protection from CaC2 toxicity in mice and further determine the residue levels of CaC2 toxicity. Moreover, there is need for future studies to look at the impact of CaC2 exposure on male mice and compare the findings from studies on female mice to see whether there exist any apparent differences in terms of sex.
Conclusions
In this study we demonstrated a clear pattern of CaC2-induced interference with hematopoiesis, major organ function (liver, kidney), immune function, and oxidation status. A clear protective effect against these CaC2-driven assault by cyanocobalamin has been established. With further studies, a mitigation strategy against CaC2 toxicity using cyanocobalamin holds great promise.
Methods
Ethical statement
Experimental procedures and protocols involving use of mice adhered to International standards on laboratory animal use with strict adherence of the 3R rules and the ARRIVE checklist for handling animal research. The ethical clearance governing the use of mice in this study was approved by Institutional review for approval Committee (IRC) of the Institute of Primate Research Karen, Kenya (ISERC/08/2017).
Experimental animals
In this study 5–6-week-old female Swiss white mice were purchased were from Biotechnology Research Institute Muguga, Kenya and were left to acclimatize for one week before the start of the experiment. Mice were housed in standard clean cages under a controlled room temperature of 21–25 °C and a 12 h light/dark cycle. Mice had access to clean water ad libitum and mice were fed on standard chow diet (Unga Group Plc., Nairobi, Kenya).
Experimental design
Mice were randomly allocated into three treatment groups (n = 12). The treatments were administered orally at a dose of (100 mg/kg) Calcium carbide and (6 mg/kg) vitamin B12 all purchased from Sigma-Aldrich (Sigma-Aldrich Co., St. Louis, MO, USA). The calcium carbide sub-acute dosage was informed by previous findings that 500 mg/kg induced damage to vital organs [43]. A dose of 6 mg/kg was used for Vitamin B12 treatment in this study based on previous findings that 6 mg/kg enhance protection against xenobiotic induced toxicity [44]. Control group of mice received distilled water (vehicle), group two received calcium carbide dissolved in distilled water daily for 60 days, group three was administered with vitamin B12 after exposure to calcium carbide daily for 60 days. Mice were sacrificed at 60 days’ post treatment.
Sample collection
Mice from each group were sacrificed as per the experimental design (60 days post treatment) by administration of ketamine (50 mg/ml) and xylazine (100 mg/ml) (Merck KGaA., Darmstadt, Germany) in a ratio of 4:1 through intramuscular injection to euthanize the mice. Anaesthetized mice were intracardially perfused with sterile phosphate buffer solution (PBS) to clear both non-adhering and adhering blood lymphocytes and erythrocytes. Brain, lungs, heart, kidney, liver and spleen samples were extracted and placed in 1.5 µl eppendorf tubes and collagenase (Sigma-Aldrich Co., St. Louis, MO, USA).
Determination of body and organs weights
Change in general body weight was determined daily throughout the experimental period. After 60 days, mice from each group were anesthetized with 0.02 ml Ketamine and dissected to obtain liver, brain, lungs, heart, kidney and spleen. Analytical electronic balance (Mettler PM34, DoltaRange®., Mumbai, India) was used to measure the weight of the extracted organs. Individual relative organ weight were determined by dividing each animal’s organ weight by their body weight multiplied by 100%.
Determination of haematological indices and biochemical markers
For hematological analysis, blood samples were obtained intra-cardially and were placed in EDTA tubes while blood for biochemical assay was collected in non-heparinized tubes and then they were centrifuged to obtain serum. Full blood hemogram was analyzed using automated Benchman Coulter counter (Benchman, Indianapolis, USA). Blood was left to stand at room temperature then centrifuged at 10,000 rpm for 5 min at 4 °C. Serum obtained was used to measure the levels of aspartate amino transferase (AST), alanine amino transferase (ALT), total bilirubin (TBIL) and creatinine were measured using automated analyzer (COBAS Integra-400 plus analyzer, Basel, Switzerland).
Glutathione (GSH) assay determination
Snap frozen whole kidney, spleen, lungs, heart, brain and liver were homogenized using ice water at (4 °C) in 0.5 ml of 0.25 M sucrose, 5 mM Hepes-Tris, pH 7.5, with protease inhibitor cocktail whose concentration was 100% (w/v). Cellular levels of GSH (Sigma-Aldrich Co., St. Louis, MO, USA) was determined by employing the method of Griffith [45]. Briefly the cellular GSH from various organs was assessed by mixing the organ homogenates 5.5′ Dithiobis-2-nitrobenzoic acid (DNTB), with the absorbance of the resulting reaction product measured at 412 nm using a multi-detection microtitre plate reader (Thermo Fisher Scientific Inc., Wilmington, MA, USA).
Enzyme linked immunoassay (ELISA)
ELISA secreted cytokine levels in serum for TNF-α, IFN-γ and IL-10 EISA kits (Thermo Fisher Scientific Inc., California, USA) were employed according to the manufacturer’s protocols. Briefly, High binding ELISA plates were coated with capture antibody and incubated at 4 °C overnight and washed 3 times using washing buffer followed by blocking using ELISA diluent and incubated for 1 h at room temperature. The plates were then washed followed by additional of standard cytokines and samples to the appropriate wells. The plates were then incubated for 2 h at room temperature, and then washed after which detection antibody was added and then incubated for 1 h at room temperature. The plates were then washed and then secondary antibodies were added and the plates were incubated for 1 h, after which they were washed and the substrate was added and incubated for 15 min at room temperature. Serum levels of these cytokine were quantified by ELISA micro-titer reader (Thermo Fisher Scientific Inc., Wilmington, MA, USA) at absorbance of 450 nm.
Standard histopathology for the liver and kidney
Liver and kidney were harvested and rinsed in phosphate buffered saline and then fixed in 4% formaldehyde. Processing was done by dehydration at different concentration of alcohol and embedding them in paraffin wax. The tissues were then sectioned in thickness of 5 μm using HM 310 rotary microtome followed by staining of sectioned tissues with haematoxylin and eosin (H&E, Sigma-Aldrich Co.). The tissues sections were then analyzed by employing the use of compound microscope for pathological lesions.
Statistical analysis
One-way ANOVA was used to compare the treatment groups with controls. For internal comparisons, Turkey’s post-hoc test was used. The results were given as a ± SEM with significance set at P < 0.05. Statistical analysis was done using GraphPad prism software package (Version 5.0).
Acknowledgements
This research utilized the reagents and material that were initially from another research that had obtained funding from the Kenya National Innovation Agency (KENIA/ADM/I/Vol.1/ (91) for the utilization of via an innovation award in health research.
Abbreviations
- CaC2
Calcium carbide
- PCV
Packed cell volume
- Hb
Hemoglobin
- RBC
Red blood cells
- AST
Aspartate aminotransferase
- ALT
Alanine aminotransferase
- GSH
Reduced glutathione
- ROS
Reactive oxygen species
- TNF-α
Tumor necrotic factor-alpha
- IFN-γ
Interferon gamma
Author contributions
PAA, VKM and JNN performed experiments; PWA, AOI and JNN analyzed and interpreted the experimental results; PAA wrote the manuscript. VKM, PWA, AOI and JNN edited the manuscript. All authors read and approved the final manuscript.
Funding
This work didn’t receive any funding.
Availability of data and materials
All the data that support the findings of this study are available on request from the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bini M, Rajesh B, Babu TD. Acute and subacute toxicity evaluation of calcium carbide and ethylene glycol in Wistar albino rats. J Basic Clin Physiol Pharmacol. 2019;31(1):1–8. doi: 10.1515/jbcpp-2019-0126. [DOI] [PubMed] [Google Scholar]
- 2.Nura A, Dandago MA, Wali NR. Effects of artificial ripening of banana (Musa spp) using calcium carbide on acceptability and nutritional quality. J Postharvest Technol. 2018;6(2):14–20. [Google Scholar]
- 3.Filipek W, Broda K. Research on the concept of using calcium carbide as a source of energy for transport from the seabed. New Trends Prod Eng. 2018;1(1):277–284. doi: 10.2478/ntpe-2018-0034. [DOI] [Google Scholar]
- 4.Patoare Y, Hossain I, Islam MN. Effect of calcium carbide on rat tissue. Dhaka Univ J Pharm Sci. 2014;6(2):93–98. doi: 10.3329/dujps.v6i2.682. [DOI] [Google Scholar]
- 5.Fadairo O, Fadairo E. Studies of the biochemical effects of arsenic content of carbide induced ripe banana fruit in the rat. Orient J Chem. 2019;35(4):1–5. [Google Scholar]
- 6.Rasdi I, Abidin EZ, Praveena SM. Calcium carbide (CaC 2) exposure from fruit ripening process and health effects among fruit farmers: a research review. Int J Public Health. 2018;5(2):91–101. [Google Scholar]
- 7.Andrew GS, Simon UT, John AU, Godwin OO, Alexander NI, Ikagu YM. Studies on changes in some haematological and plasma biochemical parameters in wistar rats fed on diets containing calcium carbide ripened mango fruits. Int J Food Sci Nutr Eng. 2018;8(2):27–36. [Google Scholar]
- 8.Asif M. Physico-chemical properties and toxic effect of fruit-ripening agent calcium carbide. Ann Trop Med Public Health. 2012;5(3):150–157. doi: 10.4103/1755-6783.98602. [DOI] [Google Scholar]
- 9.Mahmood T, Saeed I, Anwer H, Mahmood I. Comparative study to evaluate the effect of calcium carbide ( cac 2) as an artificial ripening agent on shelf life, physio-chemical properties, iron containment and quality of prunus persica l. Batsch Eur J Res. 2013;1(5):685–700. [Google Scholar]
- 10.Essien EB, Onyegeme-okerenta BM, Harcourt P. Calcium carbide as an artificial fruit-ripening agent and its physiological effects on Wistar rats. Clin Exp Med. 2018;6(1):47–61. [Google Scholar]
- 11.Ogbuagu DH, Ujowundu CO, Izunobi LC. Calcium carbide-induced haematological alterations in the albino mice-Mus musculus. J Environ Sci Toxicol Food Technol. 2016;10(10):100–104. [Google Scholar]
- 12.Schmidt EA, Fee BE, Henry SC, Nichols AG, Shinohara ML, Rathmell JC, et al. Metabolic alterations contribute to enhanced inflammatory cytokine production in irgm1-deficient macrophages. J Biol Chem. 2017;292(11):4651–4662. doi: 10.1074/jbc.M116.770735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samblas M, Martínez JA, Milagro F. Folic acid improves the inflammatory response in LPS-activated THP-1 macrophages. Mediators Inflamm. 2018;2018:1312626. doi: 10.1155/2018/1312626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okeke ES, Okagu IU, Okoye CO, Ezeorba TPC. The use of calcium carbide in food and fruit ripening: Potential mechanisms of toxicity to humans and future prospects. Toxicology. 2022;468:153112. doi: 10.1016/j.tox.2022.153112. [DOI] [PubMed] [Google Scholar]
- 15.Poller W, Nahrendorf M, Swirski FK. Hematopoiesis and cardiovascular diseases. Circ Res. 2020;126(8):1061–1085. doi: 10.1161/CIRCRESAHA.120.315895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osakue JO, Okungbowa MAO. Calcium carbide ripened musa sapientum -induced reduction in white blood cell count in challenged albino Wistar rats. Trop J Nat Prod Res. 2018;2(3):114–117. doi: 10.26538/tjnpr/v2i3.2. [DOI] [Google Scholar]
- 17.van de Lagemaat EE, de Groot LCPGM, van den Heuvel EGHM. Vitamin B12 in relation to oxidative stress: A systematic review. Nutrients. 2019; 11(2):482. [DOI] [PMC free article] [PubMed]
- 18.Haiden N, Klebermass K, Cardona F, Schwindt J, Berger A, Kohlhauser-Vollmuth C, et al. A randomized, controlled trial of the effects of adding vitamin B12 and folate to erythropoietin for the treatment of anemia of prematurity. Pediatrics. 2006;118(1):180–188. doi: 10.1542/peds.2005-2475. [DOI] [PubMed] [Google Scholar]
- 19.Budak YU, Polat M, Huysal K. The use of platelet indices, plateletcrit, mean platelet volume and platelet distribution width in emergency non-traumatic abdominal surgery: a systematic review. Biochem Med (Zagreb) 2016;26(2):178–193. doi: 10.11613/BM.2016.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-samkari H, Kuter DJ. Thrombopoietin level predicts response to treatment with eltrombopag and romiplostim in immune thrombocytopenia. Am J Hematol. 2018;93(12):1501–1508. doi: 10.1002/ajh.25275. [DOI] [PubMed] [Google Scholar]
- 21.Al-samkari H, Kuter DJ. Optimal use of thrombopoietin receptor agonists in immune thrombocytopenia. Ther Adv Hematol. 2019;10:2040620719841735. doi: 10.1177/2040620719841735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waghe P, Sarkar SN, Sarath TS, Kandasamy K, Choudhury S, Gupta P, et al. Subchronic arsenic exposure through drinking water alters lipid profile and electrolyte status in rats. Biol Trace Elem Res. 2017;176(2):350–354. doi: 10.1007/s12011-016-0851-8. [DOI] [PubMed] [Google Scholar]
- 23.Son DJ, Hwang SY, Kim M, Park UK, Kim BS. Anti-diabetic and hepato-renal protective effects of ziyuglycoside ii methyl ester in type 2 diabetic mice. Nutrients. 2015;7(7):5469–5483. doi: 10.3390/nu7075232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghosh S, Sinha JK, Putcha UK, Raghunath M. Severe but not moderate vitamin B12 deficiency impairs lipid profile, induces adiposity, and leads to adverse gestational outcome in female C57BL/6 mice. Front Nutr. 2016;3:1. doi: 10.3389/fnut.2016.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mirmalek SA, Boushehrinejad AG, Yavari H, Kardeh B, Parsa Y, Salimi-tabatabaee SA, et al. Antioxidant and anti-inflammatory effects of coenzyme q10 on l-arginine-induced acute pancreatitis in rat. Oxid Med Cell Longev. 2016;2016:5818479. doi: 10.1155/2016/5818479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall MN, Niedzwiecki M, Liu X, Harper KN, Alam S, Slavkovich V, et al. Chronic arsenic exposure and blood glutathione and glutathione disulfide concentrations in Bangladeshi adults. Environ Health Perspect. 2013;121(9):1068–1074. doi: 10.1289/ehp.1205727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otuechere CA, Adewuyi A, Bankole O. Green synthesized hydroxamic acid administered in high dose disrupts the antioxidant balance in the hepatic and splenic tissues of albino rats. Clin Phytosci. 2020;6:10. doi: 10.1186/s40816-020-00157-0. [DOI] [Google Scholar]
- 28.Shakya B, Siddique YH. Exploring the neurotoxicity and changes in life cycle parameters of Drosophila melanogaster exposed to arecoline. J Basic Appl Zool. 2018;79:47. doi: 10.1186/s41936-018-0057-z. [DOI] [Google Scholar]
- 29.Schneider L, Giordano S, Zelickson BR, Johnson S, Benavides G, Ouyang X, et al. Differentiation of SH-SY5Y cells to a neuronal phenotype changes cellular bioenergetics and the response to oxidative stress. Free Radic Biol Med. 2011;51(11):2007–17. doi: 10.1016/j.freeradbiomed.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turgut S, Enlib Y, Kaptanoglub B, Turguta S, Gença O. Changes in the levels of MDA and GSH in mice serum, liver and spleen after aluminum administration. East J Med. 2006;2006(11):7–12. [Google Scholar]
- 31.Sugihara T, Koda M, Okamoto T, Miyoshi K, Matono T, Oyama K. Falsely elevated serum vitamin B12 levels were associated with the severity and prognosis of chronic viral liver disease. Yonago Acta Med. 2017;60(1):31–39. [PMC free article] [PubMed] [Google Scholar]
- 32.Miranda TS, Heluy SL, Cruz DF, Doyle H, Feres M, Figueiredo LC, et al. The ratios of pro-inflammatory to anti-inflammatory cytokines in the serum of chronic periodontitis patients with and without type 2 diabetes and /or smoking habit. Clin Oral Investig. 2019;23(2):641–650. doi: 10.1007/s00784-018-2471-5. [DOI] [PubMed] [Google Scholar]
- 33.Vila Y, Cavalcanti N, Carolina M, Brelaz A, Kelle J, Lemoine DA, et al. Role of TNF-alpha, IFN-gamma and IL-10 in the development of pulmonary tuberculosis. Pulm Med. 2012;2012:745483. doi: 10.1155/2012/745483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehta NN, Teague HL, Swindell WR, Baumer Y, Ward NL, Xing X, et al. IFN- γ and TNF- α synergism may provide a link between psoriasis and inflammatory atherogenesis. Sci Rep. 2017;7(1):13831. doi: 10.1038/s41598-017-14365-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sikka G, Miller KL, Steppan J, Pandey D, Jung SM, Fraser CD, 3rd, et al. Interleukin 10 knockout frail mice develop cardiac and vascular dysfuction with increased age. Exp Gerontol. 2013;48(2):128–135. doi: 10.1016/j.exger.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su D, Lu Z, Shen M, Li X, Sun L. Roles of pro- and anti-inflammatory cytokines in the pathogenesis of SLE. J Biomed Biotechnol. 2012;2012:347141. doi: 10.1155/2012/347141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mikkelsen K, Apostolopoulos V. Vitamin B12, folic acid, and the immune system. In: Mahmoudi M, Rezaei N, editors. Nutrition and immunity. Cham: Springer; 2019. [Google Scholar]
- 38.Tamura J, Kubota K, Murakami H, Sawamura M, Matsushima T, Tamura T, et al. Immunomodulation by vitamin B12: augmentation of CD8+ T lymphocytes and natural killer (NK) cell activity in vitamin B12-deficient patients by methyl-B12 treatment. Clin Exp Immunol. 1999;116(1):28–32. doi: 10.1046/j.1365-2249.1999.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vellema P, Rutten VP, Hoek A, Moll L, Wentink GH. The effect of cobalt supplementation on the immune response in vitamin B12 deficient Texel lambs. Vet Immunol Immunopathol. 1996;55(1–3):151–161. doi: 10.1016/S0165-2427(96)05560-2. [DOI] [PubMed] [Google Scholar]
- 40.Tousson E, El-atrsh A, Mansour M, Assem A. Histopathological and immunohistochemical studies on the effects of Ethephon on liver and kidney in male rats Histopathological and immunohistochemical studies on the effects of Ethephon on liver and kidney in male rats. J Med Life Sci. 2019;1(4):104–109. doi: 10.21608/jmals.2019.179684. [DOI] [Google Scholar]
- 41.Ford TC, Downey LA, Simpson T, Mcphee G, Oliver C, Stough C. The effect of a high-dose vitamin B multivitamin supplement on the relationship between brain metabolism and blood biomarkers of oxidative stress : a randomized control trial. Nutrients. 2018;10(12):1860. doi: 10.3390/nu10121860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mcmahon GM, Hwang S, Tanner RM, Jacques PF, Selhub J, Muntner P, et al. The association between vitamin B12, albuminuria and reduced kidney function : an observational cohort study. BMC Nephrol. 2015;16:7. doi: 10.1186/1471-2369-16-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bini M, Rajesh B, Babu TD. Acute and subacute toxicity evaluation of calcium carbide and ethylene glycol in Wistar albino rats. J Basic Clin Physiol Pharmacol. 2019 doi: 10.1515/jbcpp-2019-0126. [DOI] [PubMed] [Google Scholar]
- 44.Hajihashemi S, Hamidizad Z, Rahbari A, Ghanbari F, Motealeghi ZA. Effects of cobalamin (Vitamin B 12) on gentamicin induced nephrotoxicity in Rat. Drug Res (Stuttg) 2017;67(12):710–718. doi: 10.1055/s-0043-117418. [DOI] [PubMed] [Google Scholar]
- 45.Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data that support the findings of this study are available on request from the corresponding author.