Abstract
Objective
As a progressive chronic condition, osteoarthritis (OA) causes substantial pain and impairment. Secrete proinflammatory cytokines are essential mediators involved in the pathophysiology of OA. In this regard, the clinical effectiveness of autologous conditioned serum (ASC) has been shown through its injection into OA tissues. This study aimed to assess the effectiveness and concentration level of ACS components produced by Nano-carbon glass beads.
Intravenous whole blood was obtained from each New Zealand male rabbit by 10-ml syringes, comprising 33 medical-grade Nano carbon-coated glass beads. Serum retrieving was performed after 6–8 h incubation (37 C, 5% Co2), and then centrifuged. The ACS was then injected into OA rabbits to assess its function.
Results
Glass beads-prepared ACS coated with Nano-carbon, induced a huge amount of cytokines and growth factors production. The concentration level of anti-inflammatory cytokines and proinflammatory cytokines was improved throughout Nano-carbon coated glass beads stimulation. ACS also shortened the recovery time and improved the function and mobility of OA rabbits.
We showed that ACS improved the function and mobility of OA rabbits, as well as shortened the recovery time. It is suggested that further studies evaluate this effectiveness.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13104-022-06166-1.
Keywords: Osteoarthritis, Autologous conditioned serum, Cytokines, Growth factors
Introduction
Osteoarthritis (OA) is a common degradative articular cartilage disease, leading to joint dilution, hypertrophic bone modifications, pain, discomfort, and disability [1], thereby putting a significant strain on healthcare systems [2, 3]. According to recent estimates, nearly 67 million Americans over the age of 18 will be diagnosed with OA by 2030, with medical expenses of more than $100 billion [4]. Joint pain is the most frequent manifestation of OA, and the most often affected joints are the hips, hands, back, and knee. The risk of developing OA increases with age, obesity, and past trauma [5, 6]. There are very few pharmacological OA treatment choices, including non-steroidal anti-inflammatory drugs (NSAIDs), analgesics, and the intra-articular injection of hyaluronan or steroids [7, 8]. Unfortunately, no definite therapies for OA are available. The key objectives of modern OA treatment are pain relief and joint function improvements [9].
The pathogenesis and etiology behind this debilitating disease are not very well understood. The interactions between catabolic factors, including interleukine-1 (IL-1), tumor necrosis factor (TNF) alpha, proteinases, and anabolic factors, including insulin-like growth factor (IGF) I and II, affect the complex balance between the development and decomposition of cartilage matrix [6]. In this respect, local cytokine production, which causes hyaline cartilage and its matrix degradation, is one of the principal molecular pathologies [10]. Epidemiological trials demonstrate a direct connection between the development and the existence of inflammatory synovium and tibiofemoral cartilage injury [11, 12]. Thus, secreted proinflammatory cytokines are a serious mediator for increased catabolism of joint tissues implicated in OA. The major proinflammatory cytokines involved in OA pathogenesis are IL-1β, IL-6, and TNF [9]. In this regard, the IL-1 receptor antagonist (IL-1Ra), can theoretically regulate intranet activities of IL-1 and thereby regulate the disease process as a naturally occurring regulator [13, 14].
Endogenous IL-1Ra-enriched autologous conditioned serum (ACS) was designed as a novel injectable substance for OA therapy in the mid-1990s. Meijer et al. [15] reported a rapid elicitation of several anti-inflammatory cytokines, such as IL-1ra, IL-4, and IL-10, as a result of exposing blood to glass beads. Following monocyte activation, the level of IL-13 is also increased, leading to improved IL-1Ra and IL-1β production hindering [16]. Exposing blood to glass beads also facilitates the extraction of many growth factors, including transforming growth factor-beta (TGF-β), Platelet-derived growth factor (PDGF), and IGF-1, in the liquid phase of blood [17]. The current study aimed to evaluate the macroscopic and microscopic effect of glass beads-produced ACS coated with Nano-carbon substances in OA rabbits. Moreover, the concentration level of cytokines and growth factors produced by polished glass beads and Nano-carbon-coated glass beads was also compared.
Main text
Materials and methods
Animals
The New Zealand Male rabbits (2.2 ± 0.2 kg) were purchased from Pasture Institute, Tehran, Iran, and held at 22 ± 1 °C in the Stem Cell Research Center, Tabriz University of Medical Sciences. In a metabolic cage under a light/dark 12/12 h cycle, every animal had free access to food and water. The experiments were conducted in compliance with national standards and endorsed by the local ethics committee. Collagenase was used to develop OA in the knee of rabbits. The OA animals were divided into two ACS-treated and normal saline-treated groups.
ACS preparation
From each rabbit, 10 ml of whole blood was taken (without anti-coagulants) by syringes containing 33 medical-grade Nano carbon-coated glass beads with a diameter of 2.5 mm and a surface area of 21 mm2. In an aseptic condition, whole blood was incubated at 37 °C, and 5% CO2 for 6–8 h. Then, the serum was recovered and centrifuged at 1000 g for 10 min. The prepared ACS was stored at −20 °C until use. The control serum was immediately centrifuged without any incubation, and preserved at −20 °C until use.
ACS safety tests on serum
External, certified clinical laboratories evaluated the existence of microbial pollutants (bacteria, fungi, and mycoplasma) and serologic criteria in serum generated by syringes.
ACS ELISA tests
The ACS cytokines and growth factor levels were evaluated by ELISA in a pre-pilot experiment. The blood samples of 10 different rabbits were used, and ELISA tests were performed according to the manufacturer’s instruction for the analysis of basic fibroblast growth factor (b-FGF), epidermal growth factor (EGF), PDGF-AB, IGF-1, TGF-β, IL-1Ra, IL-1β, IL-4, IL-6, IL-8, IL-10, and IL-13 (MyBioSource, San Diego, California).
Macroscopic evaluation of ACS effects
Rabbits were put in a 2 square meter area to assess the improvement of rabbit lameness in the knee. Two physiotherapists who were oblivious of animal health assessed the flexion, gait, extension, and rotation of animals for 28 days for 30 min. The Knee Position Comparison Scale was used for the comparison of a healthily extended and flexible knee. The damaged knee had abnormal, non-weight bearing extension and flexibility. When the rabbit’s knee extension and flexibility returned to the normal day, it was taken into account as the knee recovery time. The degeneration status of cartilage was macroscopically assessed. For the assessment of gross morphological changes in the knee joint, rabbits were anesthetized, and the knee joint was removed.
Microscopic and histological evaluation of ACS effects
Hematoxylin and eosin (H&E) staining were used for the microscopic analysis of rabbit knee cartilage destruction. For this purpose, 10% of neutral buffered formalin was used for the fixation of separate bone samples. The samples were then decalcified using 20% ethylenediaminetetraacetic acid (EDTA) and soaked in paraffin. Then, 5 μm sections were prepared by microtone and stained by H&E. Samples were assessed by a pathologist without any information about the intervention group and controls. At the end of the study to avoid further harassment to animals we began to euthanized them. To do this, the animals were injected with the Pentobarbital overdose ≥ 150 mg/kg intravenously.
Statistical analysis
SPSS Version 15.0 (SPSS Inc., Chicago, IL, USA) was applied to perform data analysis. The mean level of studied parameters between ACS-treated (polished glass beads and coated glass beads) and baseline were compared by paired t-test. P < 0.05 was considered statistically significant.
Results
Nano-carbon glass beads improved the cytokines and growth factors production in ACS
The results of the ELISA test revealed that the concentration of cytokines, either anti-inflammatory (IL-1Ra, IL-4, IL-10, and IL-13) or proinflammatory (IL-1β, IL-6, and IL-8) were improved in ACS produced coated glass beads as compared to polished glass beads (Fig. 1 and Additional file 1). The concentration level of anti-inflammatory cytokines (IL-1Ra, IL-4, IL-10, and IL-13) stimulated by the polished glass were 8028 ± 3269, 7.11 ± 4.12, 24.99 ± 8.05, and 92.92 ± 26.36 while the concentration level of these cytokines stimulated by Nano-carbon-coated glass beads were 12953 ± 4090, 12.3 ± 5.47, 32.14, 32.14 ± 9.948, and 95.47 ± 23.33, respectively. Although polished glass beads provoked proinflammatory cytokines (IL-1β, IL-6, and IL-8), the concentration levels of proinflammatory cytokines induced by Nano-carbon-coated glass beads were relatively higher with the number of 61.19 ± 43.06, 59.76 ± 19.14, and 34.16 ± 10.80, respectively. Additionally, the concentration of growth factors including b-FGF, EGF, PDGF-AB, IGF-1, and TGF-β were significantly increased in ACS exposed to Nano-carbon coated glass beads, as compared to polished glass beads alone. The concentration of growth factors for Nano-carbon coated glass beads were 2.577 ± 1.085, 31.44 ± 7.007, 40.71 ± 10.20, 164.5 ± 47.09, and 177.1 ± 66.54 while the concentration for polished glass beads was 1.673 ± 0.73, 24.57 ± 6.24, 33.74 ± 12.49, 129.8 ± 47.41, and 164.3 ± 89.84, respectively (Fig. 2 and Additional file 1).
Fig. 1.
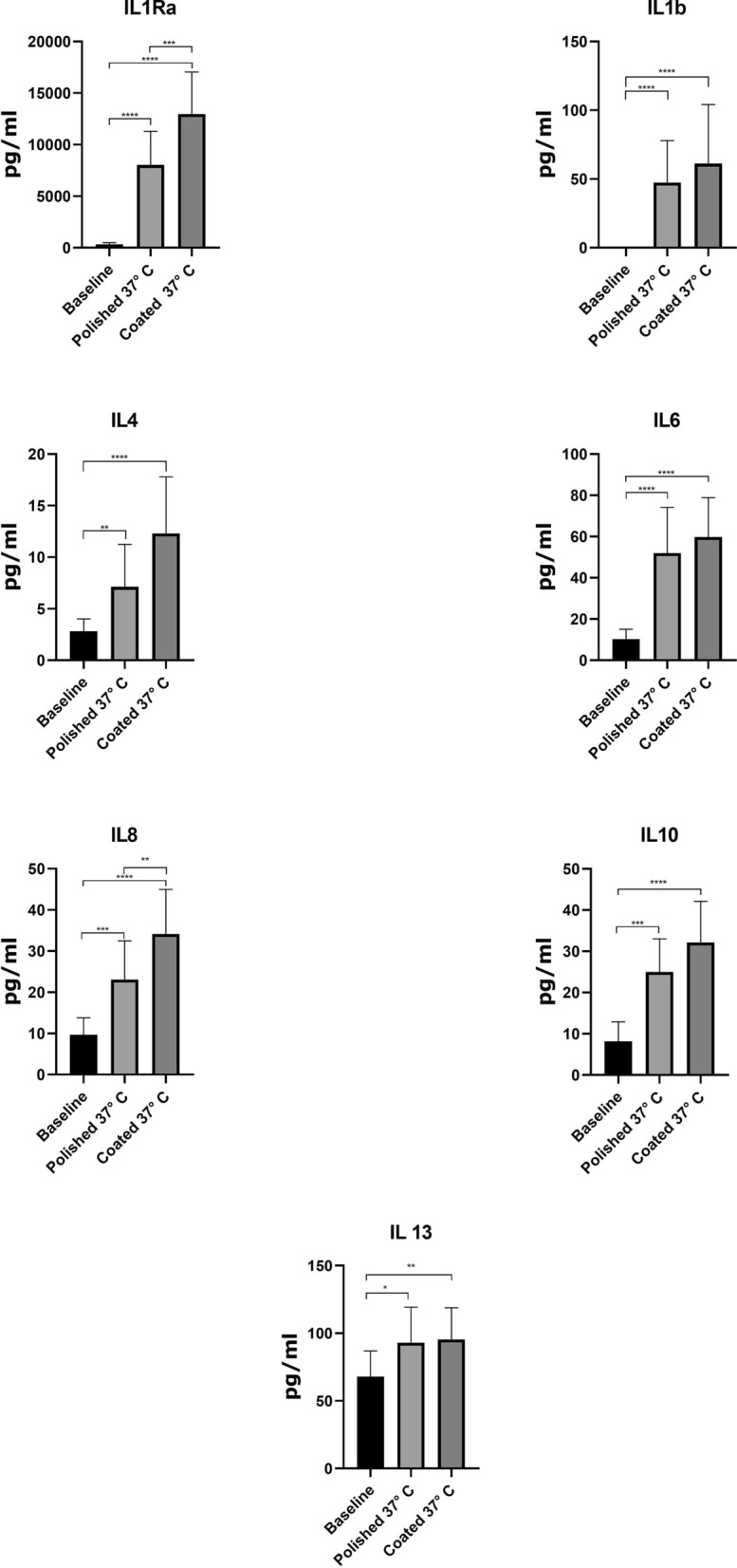
Cytokines level in Nano-carbon-stimulated or polished glass beads ACS. Data are presented as mean ± standard division (SD). P < 0.05 was considered statistically significant
Fig. 2.
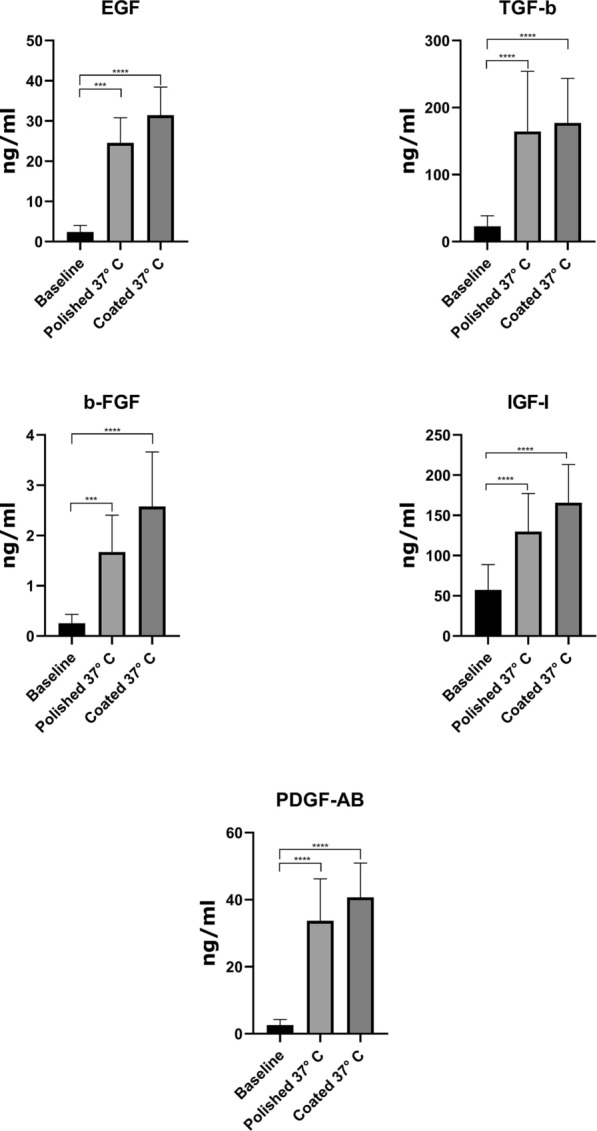
Growth factors concentration level in Nano-carbon-stimulated or polished glass beads ACS. Data are presented as mean ± standard division (SD). P < 0.05 was considered statistically significant
ACS improved the macroscopic and lameness condition of the cartilage
In the ACS-treated group, on the 14th ± 2 day, and the normal saline-treated group on the 20th ± 4 day, non-weight bearing of the injured knee was relieved and the knee flexibility and extension returned to normal, which demonstrated a considerable difference between the two groups. Macroscopic evaluation of the knee joint revealed that in the ACS group, the severity of knee joint destruction was less than in the control group. Less surface irregularity was found with cartilage in the ACS treatment group. Also, more cell growth and greater regeneration were observed in this group, in comparison to the normal saline treatment group (Fig. 3 A–C).
Fig. 3.
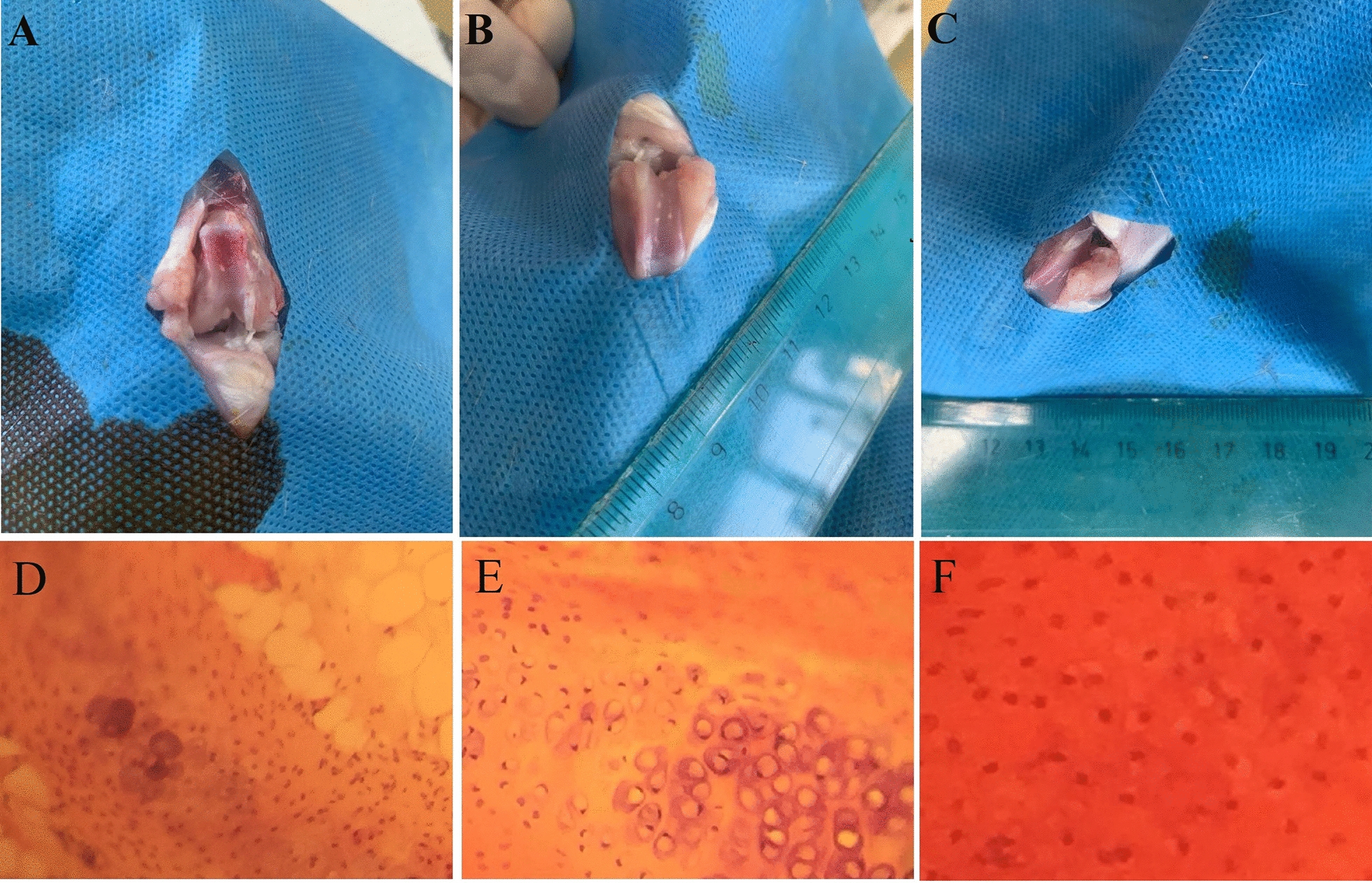
Gross morphological and histological sample stained with hematoxylin and eosin. A Day 1 experiment, B Normal saline group at day 28, C Autologous conditioned serum group at day 28, D Day1 experiment, E Normal saline group at day 28, and F autologous conditioned serum group at day 28
ACS improved the microscopic and histological condition of cartilage degeneration
Outcomes evaluation of overall cartilage degeneration score revealed a lower score for the ACS-treated group compared to the normal saline-treated group. Additionally, the ACS-treated group showed normal cellularity in transitional and radial zones, as well as better chondrocyte cloning in the former zone, compared to the normal saline-treated group (Fig. 3 D–F).
Discussion
A variety of functional and biological disabilities are associated with osteoarthritis. Synoviocyte-excreted cytokines and intraarticular environment growth factors, as well as activated immune cells, partly mediate OA associated cartilage deterioration [18, 19]. This leads to the generation of both destructive and pro-inflammatory intracellular factors which encourage further degradation of hyaline cartilage [20]. Pro-inflammatory cytokine IL-1β has a central function in OA progression through triggering inflammatory and catabolic cascade processes. Deterioration of the articular cartilage matrix is controlled by IL-1β and TNF. Along with IL-1β and TNF, other factors such as LIF, IL-21, IL-18, IL-17, IL-15, IL-8, and chemokines have also been involved in OA pathogenesis [9, 21]. IL-1ra is a competing IL-1 antagonist with a Type I and II IL-1 relation [22].
In the early 1980s, it was suggested that IL1 inhibitors can be used therapeutically in certain diseases [23, 24]. The strategies for prohibiting IL-1 biological function include using soluble forms of IL-1 receptors, IL-1Ra, and cytokines IL-4, IL-10, and IL-13 [9]. Injecting ACS into affected tissues has also demonstrated therapeutic efficacy for the prevention of osteoarthritis, disk prolapse, lumbar stenosis, and muscle injuries in animal models and human clinical trial studies [17]. This treatment in many European countries is currently available for patients and is still more commonly applied for equine OA [25]. Baltzer et al. [26] have shown that ACS is effective and preventive against painful knee OA. In parallel to previous studies, our study also demonstrated that Nano-carbon glass beads-stimulated ACS was capable to improve flexibility and extension of OA rabbits, as well as total cartilage degeneration reduction and shortening the recovery time.
The secretion of ACS repeatedly increases the volume of growth factors (TGF-β, IGF-1, bFGF, and PDGF-AB) and anti-inflammatory cytokines (IL-10, IL-1Ra, and IL-1β) [17]. Another study in 2010 revealed that increased amounts of anti-inflammatory cytokines (IL-1Ra and IL-10) and pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) were achieved after ACS injection; However, serum conditioning seems to exert no net effect on cartilage metabolism [21]. It has been revealed that blood-derived ACS is rich in IL-1Ra, IL-4, IL-10, and IL-13 [15]. Additionally, ACS can also improve and hinder IL-1Ra and IL-1β production, respectively [15, 16]. Our results demonstrated that blood exposure to Nano-carbon-coated glass beads provokes a vigorous, prompt improvement in the synthesis of many anti-inflammatory (IL-1Ra, IL-4, IL-10, and IL-13), and proinflammatory (IL-1β, IL-6, and IL-8) cytokines, and growth factors (EGF, bFGF, IGF-1, PDGF-ab, and TGF-β).
Adequate levels of IL-1Ra are necessary for intra-articular availability. Since IL-1β is known to be functional at low levels, relatively huge amounts of IL-1Ra are needed to inhibit the effect of IL-1β [27]. In vivo studies have demonstrated the increased level of IL-1Ra in an equine synovial fluid almost 35 days after the last ACS injections [25]. It was determined that the IL-1/IL1Ra ratio of the joint was 1:170. For IL-1 inhibition, the minimum required IL-1/IL-1Ra ratio is 1:10 [19]. Thus, ACS injection can strongly inhibit the bioactivities of IL-1 [22]. Moreover, our findings revealed a higher concentration level of IL-1Ra in Nano-carbon glass beads-prepared ACS, compared to polished glass beads. Besides, the concentration levels of all cytokines and growth factors were considerably higher in ACS stimulated by coated glass beads, compared to glass beads alone.
Conclusion
Prepared Nano-carbon-coated ACS comprises a huge amount of growth factors and cytokines, indicating the higher stimulatory effects on cells. We also showed that ACS improved the function and mobility of OA rabbits, as well as shortened the recovery time. It is suggested that future studies be directed on conducting randomized controlled trials capable to confirm the results of pilot clinical studies and investigating ACS mechanisms of action.
Limitation
Although the ACS manufacturing process has been found to consistently raise IL-1Ra and other variables, the methods by which the effects are mediated are not entirely known. The observed clinical impact may be explained by a large number of synergistic, active therapeutic molecules, but its long-term durability is more difficult to explain.
Supplementary Information
Additional file 1: Table S1. Concentration of cytokine and growth factors in Autologous conditioned serum.
Acknowledgements
The authors gratefully acknowledge the financial support of this project from the Stem Cell Research Center at Tabriz University of Medical Sciences, Tabriz, Iran (Grant No. 66350).
Abbreviations
- ACS
Autologous conditioned serum
- b-FGF
Basic fibroblast growth factor
- ELISA
Enzyme-linked immunosorbent assay
- EGF
Epidermal growth factor
- EDTA
Ethylenediaminetetraacetic acid
- H&E
Hematoxylin and eosin
- IL1-Ra
IL-1 receptor antagonist
- IGF
Insulin-like growth factor
- IL
Interleukin
- LIF
Leukemia inhibitory factor
- NSAIDs
Non-steroidal anti-inflammatory drugs
- OA
Osteoarthritis
- PDGF
Platelet-derived growth factor
- TGF-β
Transforming growth factor-beta
- TNF
Tumor necrosis factor
Author contributions
AP wrote the manuscript, contributed in study design, performed laboratory tests, responsible for injection of ACS and taking care of animals, MJ wrote some parts of the manuscript, contributed in ACS Preparation, performed laboratory tests, and taking care of animals, MAD and LR Investigation of microscopic and macroscopic recovery status of animals, BP contributed in data analysis, AM edited the manuscript and submitted the paper, MY designed and approved the protocols in the study, whole correspondence during the paper submission, handling the revisions and re-submission of revised manuscripts up to the acceptance of the manuscripts. All authors read and approved the final manuscript.
Funding
The current study was supported by the Stem Cell Research Center at Tabriz University of Medical Sciences, Tabriz, Iran (Grant Number: 66350).
Availability of data and materials
Raw data were generated at Tabriz University of Medical Sciences. Derived data supporting the findings of this study are available from the corresponding author on request.
Declarations
Ethics approval and consent to participate
Ethics approval for this animal study was granted by the Tabriz University of Medical Sciences Ethics Committee (Ethics No. IR.TBZMED.VCR.REC.1399.409).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sinusas K. Osteoarthritis: diagnosis and treatment. Am Fam Physician. 2012;85(1):49–56. [PubMed] [Google Scholar]
- 2.Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis. 2001;60(2):91–97. doi: 10.1136/ard.60.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta S, Hawker G, Laporte A, Croxford R, Coyte P. The economic burden of disabling hip and knee osteoarthritis (OA) from the perspective of individuals living with this condition. Rheumatology. 2005;44(12):1531–1537. doi: 10.1093/rheumatology/kei049. [DOI] [PubMed] [Google Scholar]
- 4.Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54(1):226–229. doi: 10.1002/art.21562. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26(3):355–369. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michael JW-P, Schlüter-Brust KU, Eysel P. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Dtsch Arztebl Int. 2010;107(9):152. doi: 10.3238/arztebl.2010.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernández-Díaz S, Varas-Lorenzo C, García Rodríguez LA. Non-steroidal antiinflammatory drugs and the risk of acute myocardial infarction. Basic Clin Pharmacol Toxicol. 2006;98(3):266–274. doi: 10.1111/j.1742-7843.2006.pto_302.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhang W, Moskowitz R, Nuki G, Abramson S, Altman R, Arden N, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part I: critical appraisal of existing treatment guidelines and systematic review of current research evidence. Osteoarthritis Cartilage. 2007;15(9):981–1000. doi: 10.1016/j.joca.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 9.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier J-P, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1):33. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 10.Fukui N, Purple CR, Sandell LJ. Cell biology of osteoarthritis: the chondrocyte’s response to injury. Curr Rheumatol Rep. 2001;3(6):496–505. doi: 10.1007/s11926-001-0064-8. [DOI] [PubMed] [Google Scholar]
- 11.Ayral X, Pickering E, Woodworth T, Mackillop N, Dougados M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis–results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage. 2005;13(5):361–367. doi: 10.1016/j.joca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Požgan U, Caglič D, Rozman B, Nagase H, Turk V, Turk B. Expression and activity profiling of selected cysteine cathepsins and matrix metalloproteinases in synovial fluids from patients with rheumatoid arthritis and osteoarthritis. Biol Chem. 2010;391(5):571–579. doi: 10.1515/bc.2010.035. [DOI] [PubMed] [Google Scholar]
- 13.Arend WP, Malyak M, Guthridge CJ, Gabay C. Interleukin-1 receptor antagonist: role in biology. Annu Rev Immunol. 1998;16(1):27–55. doi: 10.1146/annurev.immunol.16.1.27. [DOI] [PubMed] [Google Scholar]
- 14.Dinarello CA. The role of the interleukin-1–receptor antagonist in blocking inflammation mediated by interleukin-1. N Engl J Med. 2000;343(10):732–734. doi: 10.1056/NEJM200009073431011. [DOI] [PubMed] [Google Scholar]
- 15.Meijer H, Reinecke J, Becker C, Tholen G, Wehling P. The production of anti-inflammatory cytokines in whole blood by physico-chemical induction. Inflamm Res. 2003;52(10):404–407. doi: 10.1007/s00011-003-1197-1. [DOI] [PubMed] [Google Scholar]
- 16.de Waal MR, Figdor CG, Huijbens R, Mohan-Peterson S, Bennett B, Culpepper J, et al. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes. Comparison with IL-4 and modulation by IFN-gamma or IL-10. J Immunol. 1993;151(11):6370–81. [PubMed] [Google Scholar]
- 17.Wehling P, Moser C, Frisbie D, McIlwraith CW, Kawcak CE, Krauspe R, et al. Autologous conditioned serum in the treatment of orthopedic diseases. BioDrugs. 2007;21(5):323–332. doi: 10.2165/00063030-200721050-00004. [DOI] [PubMed] [Google Scholar]
- 18.Smith MD, Triantafillou S, Parker A, Youssef PP, Coleman M. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J Rheumatol. 1997;24(2):365–371. [PubMed] [Google Scholar]
- 19.Goldring SR, Goldring MB. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res. 2004;427:S27–S36. doi: 10.1097/01.blo.0000144854.66565.8f. [DOI] [PubMed] [Google Scholar]
- 20.Arrich J, Piribauer F, Mad P, Schmid D, Klaushofer K, Müllner M. Intra-articular hyaluronic acid for the treatment of osteoarthritis of the knee: systematic review and meta-analysis. CMAJ. 2005;172(8):1039–1043. doi: 10.1503/cmaj.1041203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rutgers M, Saris DB, Dhert WJ, Creemers LB. Cytokine profile of autologous conditioned serum for treatment of osteoarthritis, in vitro effects on cartilage metabolism and intra-articular levels after injection. Arthritis Res Ther. 2010;12(3):1–11. doi: 10.1186/ar3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Granowitz EV, Clark B, Mancilla J, Dinarello C. Interleukin-1 receptor antagonist competitively inhibits the binding of interleukin-1 to the type II interleukin-1 receptor. J Biol Chem. 1991;266(22):14147–14150. doi: 10.1016/S0021-9258(18)98655-2. [DOI] [PubMed] [Google Scholar]
- 23.Dinarello CA. Interleukin-1. Rev Infect Dis. 1984;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- 24.Malemud CJ. Anticytokine therapy for osteoarthritis. Drugs Aging. 2010;27(2):95–115. doi: 10.2165/11319950-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 25.Frisbie DD, Kawcak CE, Werpy NM, Park RD, McIlwraith CW. Clinical, biochemical, and histologic effects of intra-articular administration of autologous conditioned serum in horses with experimentally induced osteoarthritis. Am J Vet Res. 2007;68(3):290–296. doi: 10.2460/ajvr.68.3.290. [DOI] [PubMed] [Google Scholar]
- 26.Baltzer A, Moser C, Jansen S, Krauspe R. Autologous conditioned serum (Orthokine) is an effective treatment for knee osteoarthritis. Osteoarthritis Cartilage. 2009;17(2):152–160. doi: 10.1016/j.joca.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 27.Arend WP, Welgus H, Thompson RC, Eisenberg S. Biological properties of recombinant human monocyte-derived interleukin 1 receptor antagonist. J Clin Investig. 1990;85(5):1694–1697. doi: 10.1172/JCI114622. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Concentration of cytokine and growth factors in Autologous conditioned serum.
Data Availability Statement
Raw data were generated at Tabriz University of Medical Sciences. Derived data supporting the findings of this study are available from the corresponding author on request.


