Abstract
FetA, formerly designated FrpB, an iron-regulated, 76-kDa neisserial outer membrane protein, shows sequence homology to the TonB-dependent family of receptors that transport iron into gram-negative bacteria. Although FetA is commonly expressed by most neisserial strains and is a potential vaccine candidate for both Neisseria gonorrhoeae and Neisseria meningitidis, its function in cell physiology was previously undefined. We now report that FetA functions as an enterobactin receptor. N. gonorrhoeae FA1090 utilized ferric enterobactin as the sole iron source when supplied with ferric enterobactin at approximately 10 μM, but growth stimulation was abolished when an omega (Ω) cassette was inserted within fetA or when tonB was insertionally interrupted. FA1090 FetA specifically bound 59Fe-enterobactin, with a Kd of approximately 5 μM. Monoclonal antibodies raised against the Escherichia coli enterobactin receptor, FepA, recognized FetA in Western blots, and amino acid sequence comparisons revealed that residues previously implicated in ferric enterobactin binding by FepA were partially conserved in FetA. An open reading frame downstream of fetA, designated fetB, predicted a protein with sequence similarity to the family of periplasmic binding proteins necessary for transporting siderophores through the periplasmic space of gram-negative bacteria. An Ω insertion within fetB abolished ferric enterobactin utilization without causing a loss of ferric enterobactin binding. These data show that FetA is a functional homolog of FepA that binds ferric enterobactin and may be part of a system responsible for transporting the siderophore into the cell.
With the possible exception of lactobacilli, all known bacteria have an absolute requirement for iron (37). Although iron is one of the most abundant elements in the Earth’s crust, it is found mainly in the insoluble ferric (Fe3+) state. Consequently, bacteria have evolved efficient ways to solubilize and obtain iron. One common mechanism is the production and secretion of low-molecular-weight iron scavengers called siderophores. Under iron-limiting conditions, many bacteria secrete siderophores that bind iron and subsequently enter the cell via a receptor-mediated event.
Pathogens have an especially difficult time obtaining iron because host organisms sequester free iron within chelators such as transferrin, lactoferrin, and hemoglobin-haptoglobin. Some pathogens use siderophores to strip these carrier proteins of iron. Neisseria gonorrhoeae does not produce siderophores (37) but uses various other mechanisms to scavenge iron, through the use of TonB-dependent transferrin, lactoferrin, and hemoglobin receptors (6, 11, 12). Recently, Cornelissen et al. (13) showed that iron acquisition is critical for gonococcal experimental infection of human male volunteers. Although gonococci do not produce their own siderophores, they are able to scavenge siderophores made by other bacteria, including the Escherichia coli hydroxamate siderophore aerobactin (3, 39). Neisserial species also transport the E. coli phenolate siderophore ferric enterobactin (32), although utilization of ferric enterobactin as an iron source has never been demonstrated. Bacterial utilization of siderophores from other organisms sharing the same ecological niche is well known, (e.g., the use of the Ustilago sphaerogena siderophore, ferrichrome, by E. coli and the use of enterobactin by various nonproducing species) (34, 36), but there are no reports of specific siderophore receptors in any neisserial species.
FrpB, a 76-kDa iron-regulated outer membrane protein common among neisserial species, has homology to the TonB-dependent class of outer membrane proteins of gram-negative bacteria (3, 29). It is closely related to CopB, a protein with a possible role in transferrin and lactoferrin utilization by Moraxella catarrhalis (71% similarity) (1). FrpB has generated interest as a potential vaccine candidate for both N. gonorrhoeae and N. meningitidis (29), but its function is unknown. Beucher and Sparling (3) reported a 60% reduction in 55Fe uptake from heme in a FrpB mutant compared to the wild type, but careful analysis suggested that the effect was nonspecific. Furthermore, frpB did not affect growth on heme as the sole iron source (reference 3 and unpublished data).
The reasons to investigate FrpB as a ferric enterobactin receptor were twofold. First, neisserial species are able to transport enterobactin, and a neisserial protein of approximately 70 kDa cross-reacts with monoclonal antibodies against FepA in immunoblot analyses (32). In fact, the epitope recognized by one of the cross-reactive monoclonal antibodies mapped to a surface loop of FepA that was implicated in ligand binding (25). Second, sequences located directly downstream of frpB have a high degree of similarity to components of siderophore transport machinery in other species (2, 9, 10). We now present evidence that FrpB functions as a ferric enterobactin receptor. We suggest that it would be appropriate to change the gene name frpB (iron-repressed protein B) to the functionally descriptive name fetA (ferric enterobactin transport).
MATERIALS AND METHODS
Strains and growth conditions.
The bacterial strains and plasmids used in this study are described in Table 1. Gonococcal strains were routinely cultured on gonococcal base agar (Difco Laboratories) containing Kellogg’s supplement I (21) and 10 μM ferric nitrate and grown for 14 to 16 h at 37°C in an atmosphere of 5% CO2. The gonococci were iron stressed by growth in a chelexed defined medium (CDM) (38). They were inoculated to an optical density of 15 to 20 Klett units by using a Klett-Summerson colorimeter with a green filter and incubated at 37°C in an atmosphere of 5% CO2 for 2 h to allow the bacteria to acclimate to the medium. The cells were then diluted 1:4 in fresh medium and incubated for the appropriate time. Iron-sufficient Neisseria cells were grown similarly, but with 100 μM ferric nitrate added to the medium. E. coli strains were routinely grown on Luria-Bertani agar at 37°C overnight and rendered iron deficient by growth to mid-log phase in morpholine propanesulfonic acid (MOPS) minimal medium (27). Bacterial growth was measured by determining the optical density either with a Klett-Summerson colorimeter with a green filter or spectrophotometrically at 600 nm.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype | Source or reference |
|---|---|---|
| N. gonorrhoeae | ||
| FA1090 | Wild type | 26 |
| FA6959 | fetA::Ω | This study |
| FA6990 | tonB::cat | This study |
| FA6991 | fetA::Ω tonB::cat | This study |
| FA7015 | fetB::Ω | This study |
| AR89 | Wild-type clinical isolate | Clinical isolate, collection of P. Klebba |
| E. coli | ||
| DH5αMCR | F−mcrA mcrB mrr j80 dlacZΔM15 Δ(argF-lac)U169 recA1 endA1 hsdR hsdM supE44 l−thi-1 gyrA96 relA1 F−thi entA pro trp rpsL | Bethesda Research Laboratories |
| BN1071 | 22 | |
| Plasmids | ||
| pUNCH170 | Partial gonococcal tonB clone | 5 |
| pUNCH320 | 5.3-kb clone containing fetA fetB | 3 |
| pUNCH666 | Contains fetA::Ω | This study |
| pUNCH1154 | Promoterless fetA clone | This study |
| pUNCH1162 | Partial fetB clone | This study |
| pUNCH1165 | Contains fetB::Ω | This study |
| pUNCH1166 | Contains fetB::Ω with gonococcal DNA uptake sequence | This study |
| pUNCH1171 | Contains partial tonB::cat | This study |
| pUNCH1173 | fetA in pUC18 | This study |
| pHP45Ω | Source of the Ω fragment | 30 |
| pCR II | TA cloning vector | Invitrogen |
| pNC40 | Source of the cat cassette | 33 |
| pUP1 | Source of the gonococcal uptake sequence | 15 |
Transformations.
Gonococcal transformations were performed with piliated isolates by the plate-spot method as previously described (18). E. coli transformations were as previously described (20).
PCR amplification of chromosomal DNA.
Template DNA was prepared by picking a single colony from 16-h plates and resuspending it in 100 μl of water. The sample was boiled for 10 min, and 10 μl was used as the template in a 100-μl PCR mixture. The amplification conditions for all reactions were as follows: denaturing for 1 min at 94°C, annealing for 1 min at 56°C, and extension for 3 min at 72°C for 30 cycles in a RoboCycler Gradient 40 temperature cycler (Stratagene). The primers used are as indicated individually.
Construction of gonococcal mutants.
Gonococcal fetA, formerly designated frpB, was previously cloned, and an insertion was constructed in strain FA19 (3). FA19, however, expresses fetA at a low level, and so we constructed a similar fetA mutation in an isolate of FA1090 that expresses high levels of FetA. This was done by PCR cloning FA1090 fetA with the forward primer ATCCTGCCAAACCTTAACGG and the reverse primer ATGCCGTCTGAAAGGCTTTC. This yielded a 2.3-kb product that contained the entire fetA coding sequence but not the putative promoter. This product was cloned into the TA cloning vector pCR II (Invitrogen Corp., San Diego, Calif.) to yield pUNCH1154, which was then digested with NdeI, a unique restriction site on the plasmid in the coding sequence of fetA, and Klenow treated to leave blunt ends. The SmaI omega (Ω) fragment was then isolated from pHP45Ω (30) and ligated into the NdeI site of pUNCH1154 to yield the plasmid pUNCH666, in which fetA was insertionally interrupted by the Ω cassette. pUNCH666 was then transformed into N. gonorrhoeae FA1090, and spectinomycin-resistant colonies were selected. Homologous recombination yielded FetA− strain FA6959. The Ω insertion was confirmed by PCR, and the inability of the mutant to express FetA was verified by Western blot analysis (see Fig. 5). The PCR confirmation was achieved by comparing the PCR product size from wild-type and mutant DNA, using fetA-specific primers that flanked the 2-kb Ω insertion. The forward primer was ATCCTGCCAAACCTTAACGG, and the reverse primer was CAAACTCTTCACGCACAGTG. An approximately 850-bp product was amplified from the wild-type DNA and a 2.8-kb product was amplified from the fetA::Ω DNA, as expected.
FIG. 5.
FetA expression in gonococcal strains. Whole-cell lysates were prepared from iron-stressed cultures of FA1090 (wild type) (lane 1), FA6959 (fetA::Ω) (lane 2), FA6990 (tonB::cat) (lane 3), FA6991 (fetA::Ω tonB::cat) (lane 4), and FA7015 (fetB::Ω) (lane 5). The Western blot was probed with FetA polyclonal antiserum.
Cloning and mutagenesis of N. gonorrhoeae FA1090 tonB was previously described (5). The original TonB− strain was tonB::Ω. In this study, we constructed a tonB::cat insertion mutant by using the previously described partial tonB clone (pUNCH170). The 1-kb chloramphenicol acetyltransferase cassette was isolated from pNC40 (33) by digestion with BglII. The 1-kb fragment was treated with Klenow to give blunt ends and was ligated into the Klenow-treated Bsu36I site of pUNCH170, creating pUNCH1171. pUNCH1171 was then transformed into N. gonorrhoeae, and chloramphenicol-resistant colonies were selected. The presence of a tonB::cat mutant was then confirmed by lack of growth on transferrin and hemoglobin as sole iron sources, as previously described (5), and by PCR. PCR confirmation was achieved by comparing the PCR product size from wild-type and mutant DNA. tonB-specific primers were designed that flank the unique Bsu36I site in which the 1-kb cat cassette was inserted. The forward primer was GGATGCGGATATTCAGCAAC, and the reverse primer was ATCAAACATCCAAGCTGCCG. An approximately 600-bp product was produced for the wild-type strain and a 1.6-kb fragment was amplified from the tonB::cat DNA, as expected.
A strain with an insertion in the open reading frame directly downstream of fetA (fetB) was constructed as follows. fetB was sequenced from pUNCH320 (3), a 5.3-kb clone that contained fetA, fetB, and two additional open reading frames. A 937-bp fragment (lacking the promoter region) of fetB was PCR amplified from strain FA1090 by using the forward primer ATTGACCCTCTGCACCGTC and the reverse primer AAGCATCCGCAACCTGTTTG, and the product was confirmed by DNA sequencing. This product was cloned into the TA cloning vector pCR II (Invitrogen Corp., San Diego, Calif.) to yield pUNCH1162. pUNCH1162 was then digested with HincII, a unique restriction site on the plasmid in the coding sequence of fetB. The SmaI omega fragment was then isolated from pHP45Ω and ligated into the HincII site of pUNCH1162 to yield plasmid pUNCH1165, in which the fetB fragment was insertionally interrupted by the Ω cassette. Because pUNCH1165 contained no gonococcal DNA uptake sequence, the fetB::Ω EcoRI fragment was excised and inserted into the EcoRI site of the vector pUP1, which contains the gonococcal uptake sequence (15). This final construct was named pUNCH1166. The latter plasmid was transformed into gonococcal strain FA1090, and transformants were selected for spectinomycin resistance. The transformant was confirmed by PCR analysis with the same primers as for cloning. An approximately 937-bp product was amplified from the wild-type strain, and an approximately 2.9-kb product was amplified from the putative fetB mutant. The strain was further confirmed by Western blot analysis (see Fig. 2).
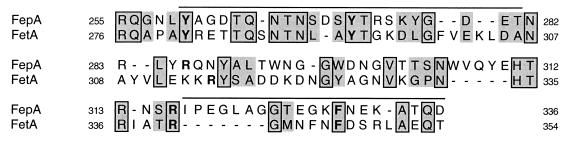
FIG. 2.
FetA conservation of the FepA binding site. This lineup shows sequence conservation between FepA and FetA in the central region of the FepA structure bounded by residues 255 to 336. Residues within this region are implicated in ferric enterobactin binding (8a, 28). Shading indicates similar amino acids, and outlining indicates amino acid identities. Amino acids indicated in bold lettering are important in ferric enterobactin binding of FepA; the corresponding residues in FetA are also in bold. The lines above FepA residues 269 to 281 and residues 317 to 336 indicate extracellular loops L3 and L4, respectively, as determined by the recently solved crystal structure of FepA (8).
Production of FetB-specific polyclonal antisera.
Two predicted antigenic peptides were identified from the deduced amino acid sequence of fetB and were synthesized by fluoroenylmethoxycarbonal (Fmoc) chemistry by the Microchemical Core Facility, Department of Microbiology and Immunology, University of North Carolina, Chapel Hill, N.C. The synthetic peptide sequences were as follows: SPQNSDPAPQAKGGC and CGGEYLKEKNDP (GGC and CGG functioned for sulfhydryl coupling and are not part of the FetB protein sequence). After purification by reverse-phase high-performance liquid chromatography, the sequences were confirmed by fast atom bombardment mass spectrophotometry. The peptides were conjugated to keyhole limpet hemocyanin. Elite rabbits (Covance Research Products Inc., Denver, Pa.) were immunized a total of four times, 3 weeks apart, with 1 mg of each peptide conjugate for the first immunization and 0.5 mg for the remaining immunizations. Freund’s complete adjuvant was used for the first immunization, and Freund’s incomplete adjuvant was used for the remainder. Antiserum was used at a 1:1,000 dilution.
Western blot analysis.
Iron-stressed whole-cell lysates were prepared from bacteria grown in CDM to late log phase. Cultures were pelleted by centrifugation, and bacteria were boiled in sodium dodecyl sulfate sample buffer for 10 min. Proteins from whole-cell lysates were separated on 7.5% polyacrylamide gels (23) and electrophoretically transferred to nitrocellulose (35). FetA-specific polyclonal antibody (7) was used at a dilution of 1:1,000 for detection of FetA, and FepA-specific monoclonal antibodies (MAbs) 2 and 35 were used as described previously (25, 32).
Ferric enterobactin-dependent growth assays.
Bacteria were grown in CDM as described above. To scavenge all traces of free iron for this assay, 2.5 μM apo-lactoferrin was added to each culture after the initial 2-h growth period and dilution. Because strain FA1090 and its derivatives are unable to utilize lactoferrin as an iron source due to a natural deletion in the lactoferrin receptor (4, 24), no iron was available for growth. If a utilizable iron source is then added to this medium, the bacteria regain the ability to grow. Therefore, bacterial growth was measured in duplicate cultures of each strain, containing either 10 μM ferric enterobactin or no enterobactin. Ferric enterobactin was prepared as previously described (25). The preparations were used within 16 h of purification for growth assays. Each preparation was assayed spectrophotometrically for purity to ensure the absence of significant breakdown products of enterobactin.
Ferric enterobactin binding assays.
For binding assays, ferric enterobactin was used immediately following preparation and spectrophotometric characterization (25). Neisserial cultures were grown for 2 h at 37°C with CO2 before being subjected to fourfold dilution into fresh medium and further growth for an additional 4 h to late log phase. Measurements of ferric siderophore binding to bacteria require a metabolically inactive state in the cells, which we normally achieve by extended (1-h) preincubation at 0°C. However, the relative fragility N. gonorrhoeae at such low temperatures necessitated modifications to this procedure. The cultures of N. gonorrhoeae were chilled on ice for 10 min to drop the temperature below 10°C. [59Fe]enterobactin was added to 10-ml aliquots of culture over a range of concentrations and incubated with the cells on ice. After 6 and 11 min, 5-ml aliquots were removed, the cells were collected on fiberglass filters (pore size, 1.0 μm; LIDA), and the filters were counted in a Beckman gamma counter. The procedure achieved the desired metabolically inactive state, as demonstrated by the lack of [59Fe]enterobactin transport (absence of a differential in the cell-associated ferric siderophore between the 6- and 11-min measurements), while minimizing any detrimental effects of the cold incubation (data not shown).
Because significant nonspecific adsorption of ferric enterobactin to the gonococcal cell surface occurred (see Results), FetA-specific binding was determined by subtracting the amount adsorbed to a fetA strain from the amount adsorbed to an otherwise isogenic fetA+ strain. Furthermore, FetA is negatively regulated by iron, and effective iron deprivation of the strains was essential to the detection of ferric enterobactin binding. In several experiments, the capacity of ferric enterobactin binding was considerably lower than usual, which correlated with decreased levels of FetA in the cell envelope, as measured by Western blotting. The results of such experiments were excluded from the calculation of the thermodynamic parameters of the binding interaction. Binding data were analyzed and plotted with Grafit 4 software (Erithacus Software Ltd., Middlesex, United Kingdom) by using the bound-versus-total equation.
Nucleotide sequence accession number. The DNA and deduced amino acid sequences of fetB have been deposited in GenBank under accession no. AF115385.
RESULTS
FetA cross-reactivity with FepA MAbs.
A previous study revealed that a protein of approximately 70 kD in three neisserial species, N. gonorrhoeae, N. meningitidis, and N. lactamica, cross-reacted with one or more MAbs against E. coli FepA (32). To determine whether the cross-reactive protein was FetA, we performed Western blots with anti-FepA MAbs and whole-cell lysates of wild-type (FA1090) and fetA (FA6959) strains. As previously observed, anti-FepA MAbs 2 and 35 reacted weakly yet specifically with a 70-kDa protein in the wild-type gonococcal strain (32). No cross-reactivity occurred in the gonococcal FetA mutant (Fig. 1). These MAbs identified two FepA epitopes that are conserved in FetA. The first, recognized by MAb 2, exists within residues 100 to 178 of FepA. The second, recognized by MAb 35, lies in the central region of FepA that interacts with ferric enterobactin, between residues 290 and 339 (25, 28).
FIG. 1.
FetA cross-reactivity with FepA MAbs. Western blots were probed with FepA MAb 2 (A) or FepA MAb 35 (B). Whole-cell lysates were prepared from iron-stressed bacteria. Lanes: 1, E. coli BN1071; 2, FA1090 (wild type); 3, FA6959 (fetA::Ω).
Sequence similarity to the putative binding site of FepA.
Initial amino acid sequence alignments between FetA and FepA suggested that the proteins were not closely related or, at least, were no more closely related than other TonB-dependent proteins. However, in light of the MAb cross-reactivity data, we looked more closely at the amino acid sequences in the putative ligand binding domain. While little was previously known about the function of FetA, the structure and function of FepA are well understood. Immunological (25) and genetic (28) evidence suggested that residues in the central, surface-exposed domain of FepA are important for the binding of ferric enterobactin. Amino acids R286, R316, Y260, Y272, and F329 (8a, 28), which were implicated in the ferric enterobactin-FepA interaction, were conserved in the alignment with FetA (Fig. 2). However, in some cases these residues were not spaced identically to those in the FepA binding site.
Characterization of the open reading frame downstream of fetA.
Initial DNA sequencing revealed a region located 152 bp downstream of fetA with no intervening coding sequence and no rho-independent transcriptional terminator, which had similarity to the sequence encoding members of the family of periplasmic binding proteins responsible for transporting ferric siderophores through the periplasmic space (2). Further sequence analysis demonstrated a 972-bp open reading frame that we designated fetB. FetB had a high degree of similarity to periplasmic siderophore binding proteins throughout its sequence. The most closely related protein was the periplasmic enterobactin binding protein of Campylobacter spp., CeuE (31), which was 46% similar and 35% identical. FetB was 48% similar and 25% identical to the E. coli ferric enterobactin periplasmic binding protein, FepB (16).
Because the Ω cassette used to create the FetA mutant contains transcriptional stops, the possibility existed that the Ω insertion in fetA had polar effects on downstream genes. Therefore, Western blot analysis was performed with anti-FetB peptide sera and whole-cell lysates from strains FA1090 (wild type), FA7015 (fetB::Ω), and FA6959 (fetA::Ω) (Fig. 3). As expected, the FetB-specific antisera recognized a protein of approximately 35 kDa from the wild-type strain but did not react with the FetB mutant or the fetA::Ω strain, confirming that the Ω insertion in fetA did block the expression of fetB. This suggested that fetA and fetB were cotranscribed on a single operon.
FIG. 3.
FetB expression. A Western blot was probed with FetB-peptide polyclonal antiserum. Whole-cell lysates were prepared from iron-stressed bacteria. Lanes: 1, FA1090 (wild type); 2, FA7015 (fetB::Ω); 3, FA6959 (fetA::Ω).
FetA-dependent enterobactin growth stimulation.
By utilizing an iron-deficient growth medium, we examined whether the various strains (FA1090, FA6959, FA6990 [tonB::cat], and FA7015) used ferric enterobactin as the sole iron source. A marked increase in the growth of the wild-type strain was observed when ferric enterobactin was added to the medium (Fig. 4), but no detectable growth stimulation in the fetA, tonB, or fetB mutants was observed. This indicated that FetA and/or products of genes downstream of fetA were necessary for ferric enterobactin utilization and that both TonB and FetB (and/or genes transcriptionally linked downstream of fetB) also were critical for enterobactin utilization in the gonococcus (Fig. 4). Western blot analysis confirmed that FetA expression levels were not altered in the TonB or FetB mutants (Fig. 5).
FIG. 4.
Growth stimulation by ferric enterobactin. ○, FA1090 with no added ferric enterobactin; solid symbols, cultures containing 10 μM enterobactin. ●, FA1090 (wild type); ■, FA6959 (fetA::Ω); ⧫, FA6990 (tonB::cat); ▴, FA7015 (fetB::Ω). The graph is representative of a minimum of three experiments.
FetA-specific enterobactin binding.
We tested the wild type and each of the relevant mutant strains for adsorption of [59Fe]enterobactin. The measurements were complicated by the tendency of the Neisseria to nonspecifically adsorb the siderophore. The nonsaturable binding by the fetA mutant suggests that the fetA-independent binding is due to a molecule on the cell surface that is present at an extremely high concentration, such as lipooligosaccharide, and is not due to specific binding of an additional receptor. Figure 6A shows total ferric enterobactin binding by wild-type FA1090 and by the fetA mutant FA6959. Similar results were observed with another gonococcal clinical isolate, AR89, and its fetA mutant (data not shown). Comparison between wild-type and FetA− strains allowed us to quantitate FetA-specific ferric enterobactin binding (Fig. 6B). In every case, the fetA-corrected values showed concentration-dependent saturation-type binding. The rectangular hyperbolic curves from the binding equilibria yielded dissociation constants of approximately 5 μM. This measure of the affinity of ferric enterobactin adsorption to FetA was about 250-fold lower than the strength of ferric enterobactin binding to E. coli FepA (Kd = 20 nM [Fig. 6B]) (34). In most cases, the binding equilibria saturated at a level of about 35 pmol/109 cells, almost identical to the capacity of E. coli cells expressing chromosomal FepA (34). A receptor affinity in the micromolar range is not unique to FetA, since the E. coli maltoporin, LamB, has a similar affinity for its ligand, maltose (17).
FIG. 6.
Ferric enterobactin binding by gonococci. The amount of [59Fe]enterobactin bound (in picomoles per 109 cells) was determined as a function of ferric siderophore concentration. (A) Ferric enterobactin (FeEnt) binding to wild-type (FA1090) (●) and fetA mutant (FA6959) (○) strains of N. gonorrhoeae. (B) FetA-specific binding (see the text) of ferric enterobactin by FA1090 (wild type) (●), FA6990 (tonB) (■), and FA7015 (fetB) (▴). Binding to wild-type E. coli K-12 strain BN1071, which expresses chromosomally encoded FepA, is also shown (⧫). The Kd values for binding to FA1090, FA6990, FA7015, and BN1071 were 5.7, 4.17, 6.3, and 0.018 μM, respectively; the mean standard error on these measurements was 30.4%. The capacities of the four strains were 31, 34, 22, and 32 pmol/109 cells, respectively; the mean standard error on these measurements was 11.7%. For FA1090 and FA6990, data were collected from six or more experiments and the mean values are plotted. For FA7015, the data represent the mean of three separate experiments.
The thermodynamic parameters were essentially identical for the tonB mutant (FA6990); as expected, TonB did not affect siderophore binding to FetA. The fetB mutant (FA7015) adsorbed ferric enterobactin with the same Kd and a somewhat reduced capacity (22 pmol/109 cells). The discrepancy in capacity between FA1090 and FA7015 is quite small and could be explained by a slight difference in iron starvation and FetA expression levels in strain FA7015 that was not detected by Western blot analysis. The ability of the FetB− strain to bind ferric enterobactin showed that the specificity of the interaction of ferric enterobactin with the gonococci did not originate from polar effects on the downstream fetB gene or another gene product transcriptionally linked to fetA. If fetB is a periplasmic transporter for ferric enterobactin, as suggested by its predicted protein sequence, then normal binding affinity but reduced transport (see above) was the expected phenotype for the fetB mutant.
We also tested for general effects of fetA on iron acquisition, by determining whether the mutation impaired transferrin binding. To do so, we compared transferrin adsorption to wild-type (FA1090) and fetA::Ω (FA6959) strains of N. gonorrhoeae by the liquid-phase transferrin binding assay (14). We observed no differences in transferrin binding between these strains (data not shown).
DISCUSSION
This study provides evidence that the gonococcal 76-kDa outer membrane protein, FetA, is a receptor capable of binding, and most probably important for transporting, the E. coli siderophore ferric enterobactin. Although it was previously shown that Neisseria spp. transport iron from ferric enterobactin (32), this is the first demonstration that any neisserial species can utilize ferric enterobactin as an iron source for growth and that FetA may act as a classic siderophore receptor, binding its ligand and transporting it through the outer membrane in a TonB-dependent manner.
The ability to utilize ferric enterobactin in a receptor-mediated fashion in vitro implies that ferric enterobactin utilization could play a role in vivo. Approximately 40% of women with cervical gonococcal infection are also infected anorectally (19). The cocolonization of those tissues by E. coli will undoubtedly result in a high concentration of ferric enterobactin in the microenvironment of the rectal mucosa, available to other bacteria in the same niche. Thus, the gonococcus represents another example of the several bacterial species that do not synthesize enterobactin but produce a FepA homolog and utilize ferric enterobactin as an iron source (34).
The low affinity of the ferric enterobactin-FetA interaction, which at first glance appears to render the transport system much less efficient than comparable uptake pathways of the enteric bacteria, may function indirectly as a mechanism that saves energy. Our biochemical characterizations indicate that ferric enterobactin binding and transport occur only when the siderophore accumulates to high concentrations in the bacterial milieu. In other words, this energy-dependent active transport system will only function in particular environments, where sufficient ferric enterobactin concentrations exist to fully support growth as a sole iron source. An alternative explanation for the low affinity of the binding is that FetA may act primarily as a receptor in vivo for another, currently unknown phenolate siderophore or a breakdown product of ferric enterobactin and only secondarily as a ferric enterobactin receptor.
It was surprising that although FetA is dissimilar to FepA at the amino acid level over the majority of the protein, it contains important similarities within the putative binding region of FepA. Additionally, it cross-reacted with at least two different FepA MAbs. This result demonstrates two conserved epitopes between FetA and FepA, providing further evidence for their functional relatedness. One of these antibodies (MAb 35) recognizes an epitope near the ligand binding site of FepA and blocks ferric enterobactin binding (25). Hence, structural conservation exists in FetA and FepA at the primary level, as seen from both sequence identities and MAb recognition. Differences in the spacing of putative residues critical for ferric enterobactin binding between FepA and FetA may account for the lower affinity of the FetA-ferric enterobactin interaction. The gonococcus evidently has evolved means of scavenging iron from transferrin and lactoferrin by using specific receptors and for scavenging siderophores from other bacteria sharing the same ecological niche. The significant sequence divergence of FetA from FepA suggests either that a genetic exchange event during which neisserial species gained the siderophore receptor happened early in the evolution of the genus or that the phenolate siderophore receptor evolved by a convergent pathway.
The siderophore utilization function of FetA is supported by the presence of downstream open reading frames homologous to other siderophore transport machinery. A polar insertion into the first of these open reading frames (fetB) had no effect on the binding of ferric enterobactin to FetA but abolished gonococcal utilization of ferric enterobactin as an iron source. Therefore, FetA was capable of binding but was not sufficient alone for utilization of ferric enterobactin. While FetA specifically bound ferric enterobactin and a polar mutation in fetA abolished ferric enterobactin utilization, it is conceivable that FetA may not be necessary for utilization. To test the absolute requirement for FetA in ferric enterobactin utilization, a nonpolar mutation would have to be tested.
We also cannot conclusively rule out the existence of an additional ferric enterobactin receptor on the cell. FetA-independent binding of ferric enterobactin was nonsaturable and thus apparently nonspecific, but it is possible that a low-level specific binding was masked by this background. However, as shown in Fig. 6A, the FetA-independent (nonspecific) adsorption was seen only at relatively high concentrations (greater than 1 μM ferric enterobactin). If another receptor system of equivalent or higher affinity with respect to FetA (i.e., Kd < 5 μM) existed, we would have observed binding to it, as evidenced by saturable adsorption to the FetA-deficient strain at concentrations of <5 μM. Since we saw no saturation of the FetA-deficient strain, any other receptor system must have even lower affinity. It cannot be ruled out that FetA may have functions other than as a ferric enterobactin receptor.
We identified three additional open reading frames downstream of fetB that may also play a role in ferric enterobactin utilization (data not shown). Two of these open reading frames are homologous to siderophore cytoplasmic membrane permeases, and one is homologous to the class of ATPases located in the inner membrane, involved in siderophore transport (9, 10). The transcriptional regulation of these genes and the functional involvement of their gene products in siderophore utilization are current foci of investigation.
ACKNOWLEDGMENTS
This work was supported by NIH grants R37 AI26837 and U19 AI31496 to P.F.S., NIH grant T32 AI07001 to S.D.B.C., and NIH grant 1R01-GM53837 to P.E.K.
We thank P. Thulasiraman, G. Biswas, C. Thomas, C. Elkins, and C. Cornelissen for helpful discussions; M. Montague and A. Rountree for technical assistance; and M. Beucher for the initial observation of a 5′ fragment of fetB.
REFERENCES
- 1.Aebi C, Stone B, Beucher M, Cope L D, Maciver I, Thomas S E, McCracken G H, Jr, Sparling P F, Hansen E J. Expression of the CopB outer membrane protein by Moraxella catarrhalis is regulated by iron and affects iron acquisition from transferrin and lactoferrin. Infect Immun. 1996;64:2024–2030. doi: 10.1128/iai.64.6.2024-2030.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beucher M. Ph.D. thesis. Chapel Hill: University of North Carolina; 1995. [Google Scholar]
- 3.Beucher M, Sparling P F. Cloning, sequencing, and characterization of the gene encoding FrpB, a major iron-regulated, outer membrane protein of Neisseria gonorrhoeae. J Bacteriol. 1995;177:2041–2049. doi: 10.1128/jb.177.8.2041-2049.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biswas G D, Anderson J E, Chen C J, Cornelissen C N, Sparling P F. Identification and functional characterization of the Neisseria gonorrhoeae lbpB gene product. Infect Immun. 1999;67:455–459. doi: 10.1128/iai.67.1.455-459.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biswas G D, Anderson J E, Sparling P F. Cloning and functional characterization of Neisseria gonorrhoeae tonB, exbB, and exbD genes. Mol Microbiol. 1997;24:169–179. doi: 10.1046/j.1365-2958.1997.3421692.x. [DOI] [PubMed] [Google Scholar]
- 6.Biswas G D, Sparling P F. Characterization of lbpA, the structural gene for a lactoferrin receptor in Neisseria gonorrhoeae. Infect Immun. 1995;63:2958–2967. doi: 10.1128/iai.63.8.2958-2967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Black J R, Dyer D W, Thompson M K, Sparling P F. Human immune response to iron-repressible outer membrane proteins of Neisseria meningitidis. Infect Immun. 1986;54:710–713. doi: 10.1128/iai.54.3.710-713.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchanan S K, Smith B S, Venkatramani L, Xia D, Esser L, Palnitkar M, Chakraborty R, van der Helm D, Deisenhofer J. Crystal structure of the outer membrane active transporter FepA from Escherichia coli. Nat Struct Biol. 1999;6:56–63. doi: 10.1038/4931. [DOI] [PubMed] [Google Scholar]
- 8a.Cao, Z. Unpublished data.
- 9.Carson S D B, Sparling P F. Abstracts of the 10th International Pathogenic Neisseria Conference. 1996. Gonococcal FrpB: a possible role in siderophore uptake, poster 209; p. 566. [Google Scholar]
- 10.Carson S B D, Newton S M C, Klebba P E, Sparling P F. Abstracts of the 98th General Meeting of the American Society for Microbiology 1998. Washington, D.C: American Society for Microbiology; 1998. Ferric enterobactin binding by gonococcal FrpB, abstr. B-242; p. 96. [Google Scholar]
- 11.Chen C J, Sparling P F, Lewis L A, Dyer D W, Elkins C. Identification and purification of a hemoglobin-binding outer membrane protein from Neisseria gonorrhoeae. Infect Immun. 1996;64:5008–5014. doi: 10.1128/iai.64.12.5008-5014.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornelissen C N, Biswas G D, Tsai J, Adams J, Parachuri D K, Thompson S A, Sparling P F. Gonococcal transferrin-binding protein 1 is required for transferrin utilization and is homologous to TonB-dependent outer membrane receptors. J Bacteriol. 1992;174:5788–5797. doi: 10.1128/jb.174.18.5788-5797.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornelissen C N, Kelley M, Hobbes M M, Anderson J E, Cannon J G, Cohen M S, Sparling P F. The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers. Mol Microbiol. 1998;27:611–616. doi: 10.1046/j.1365-2958.1998.00710.x. [DOI] [PubMed] [Google Scholar]
- 14.Cornelissen C N, Sparling P F. Binding and surface exposure characteristics of the gonococcal transferrin receptor are dependent on both transferrin-binding proteins. J Bacteriol. 1996;178:1437–1444. doi: 10.1128/jb.178.5.1437-1444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elkins C, Thomas C E, Seifert H S, Sparling P F. Species-specific uptake of DNA by gonococci is mediated by a 10-base-pair sequence. J Bacteriol. 1991;173:3911–3913. doi: 10.1128/jb.173.12.3911-3913.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elkins M F, Earhart C F. Nucleotide sequence and regulation of the Escherichia coli gene for ferrienterobactin transport protein FepB. J Bacteriol. 1989;171:5443–5451. doi: 10.1128/jb.171.10.5443-5451.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferenci T, Boos W. The role of Escherichia coli λ receptor in the transport of maltose and maltodextrins. J Supramol Struct. 1980;13:101–116. doi: 10.1002/jss.400130110. [DOI] [PubMed] [Google Scholar]
- 18.Gunn J S, Stein D C. Use of a non-selective transformation technique to construct a multiply restriction/modification-deficient mutant of Neisseria gonorrhoeae. Mol Gen Genet. 1996;251:509–517. doi: 10.1007/BF02173639. [DOI] [PubMed] [Google Scholar]
- 19.Handsfield H H. Gonorrhea and uncomplicated gonococcal infection. In: Holmes K K, Mardh P A, Sparling P F, Wiesner P J, editors. Sexually transmitted diseases. New York, N.Y: McGraw-Hill Book Co.; 1984. p. 209. [Google Scholar]
- 20.Inoue H, Nojima H, Okayama H. High efficiency transformation of Escherichia coli with plasmids. Gene. 1990;96:23–28. doi: 10.1016/0378-1119(90)90336-p. [DOI] [PubMed] [Google Scholar]
- 21.Kellogg D S, Jr, Peacock W L, Jr, Deacon W E, Brown L, Pirkle C I. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J Bacteriol. 1963;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klebba P E, McIntosh M A, Neilands J B. Kinetics of biosynthesis of iron-regulated membrane proteins in Escherichia coli. J Bacteriol. 1982;149:880–888. doi: 10.1128/jb.149.3.880-888.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Lewis L A, Rohde K, Gipson M, Behrens B, Gray E, Toth S I, Roe B A, Dyer D W. Identification and molecular analysis of lbpBA, which encodes the two-component meningococcal lactoferrin receptor. Infect Immun. 1998;66:3017–3023. doi: 10.1128/iai.66.6.3017-3023.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy C K, Kalve V I, Klebba P E. Surface topology of the Escherichia coli K-12 ferric enterobactin receptor. J Bacteriol. 1990;172:2736–2746. doi: 10.1128/jb.172.5.2736-2746.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nachamkin I, Cannon J G, Mittler R S. Monoclonal antibodies against Neisseria gonorrhoeae: production of antibodies directed against a strain-specific cell surface antigen. Infect Immun. 1981;33:555–564. doi: 10.1128/iai.32.2.641-648.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neidhardt F C, Bloch P L, Smith D F. Culture media for enterobacteria. J Bacteriol. 1974;119:736. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newton S M C, Allen J S, Cao Z, Qi Z, Jiang X, Sprencel C, Igo J D, Foster S B, Payne M A, Klebba P E. Double mutagenesis of a positive charge cluster in the ligand-binding site of the ferric enterobactin receptor, FepA. Proc Natl Acad Sci USA. 1997;94:4560–4565. doi: 10.1073/pnas.94.9.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pettersson A, Maas A, Van Wassenaar D, Van der Ley P, Tommassen J. Molecular characterization of FetA, the 70-kilodalton iron-regulated outer membrane protein of Neisseria meningitidis. Infect Immun. 1995;63:4181–4184. doi: 10.1128/iai.63.10.4181-4184.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prentki P, Krisch H M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984;29:303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- 31.Richardson P T, Park S F. Enterochelin acquisition in Campylobacter coli: characterization of a binding-protein-dependent transport system. Microbiology. 1995;141:3181–3191. doi: 10.1099/13500872-141-12-3181. [DOI] [PubMed] [Google Scholar]
- 32.Rutz J M, Abdullah T, Singh S P, Kalve V I, Klebba P E. Evolution of the ferric enterobactin receptor in gram-negative bacteria. J Bacteriol. 1991;173:5964–5974. doi: 10.1128/jb.173.19.5964-5974.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas C E, Carbonetti N H, Sparling P F. Pseudo-transposition of a Tn5 derivative in Neisseria gonorrhoeae. FEMS Microbiol Lett. 1996;145:371–376. doi: 10.1111/j.1574-6968.1996.tb08603.x. [DOI] [PubMed] [Google Scholar]
- 34.Thulasiraman P, Newton S M C, Xu J, Raymond K N, Mai C, Hall A M, Montague M A, Klebba P E. Selectivity of ferric enterobactin binding and cooperativity of transport in gram-negative bacteria. J Bacteriol. 1998;180:6689–6696. doi: 10.1128/jb.180.24.6689-6696.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Towbin J, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrilamide gels to nitrocellulose sheets: procedures and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wayne R, Neilands J B. Evidence for common binding sites for ferrichrome compounds and bacteriophage phi 80 in the cell envelope of Escherichia coli. J Bacteriol. 1975;121:497–503. doi: 10.1128/jb.121.2.497-503.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weinberg E D. The Lactobacillus anomaly: total iron abstinence. Perspect Biol Med. 1997;40:578–583. doi: 10.1353/pbm.1997.0072. [DOI] [PubMed] [Google Scholar]
- 38.West S E H, Sparling P F. Response of Neisseria gonorrhoeae to iron limitation: alterations in expression of membrane proteins without apparent siderophore production. Infect Immun. 1985;47:388–394. doi: 10.1128/iai.47.2.388-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.West S E H, Sparling P F. Aerobactin utilization by Neisseria gonorrhoeae and cloning of a genomic DNA fragment that complements Escherichia coli fhuB mutations. J Bacteriol. 1987;169:3414–3421. doi: 10.1128/jb.169.8.3414-3421.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]