Abstract
Preeclampsia is a multifactorial condition associated with significant morbidity and mortality. Fluid therapy in these patients is challenging since volume expansion may precipitate pulmonary edema, and fluid restriction may worsen renal function. Furthermore, cardiac impairment may introduce an additional component to the hemodynamic management. This article reviews the repercussions of preeclampsia on renal and cardiovascular systems and the development of pulmonary edema, as well as to discuss fluid management, focusing on the mitigation of adverse outcomes and monitoring alternatives. The literature review was carried out using PubMed, Embase, and Google Scholar databases from May 2019 to March 2020. Papers addressing the subjects of interest were included regardless of the publication language. There is a current trend towards restricting the administration of fluids in women with non-complicated preeclampsia. However, patients with preeclampsia may experience hemorrhagic shock, requiring volume resuscitation. In this case, hemodynamic monitoring is recommended to guide fluid therapy while avoiding complications.
Keywords: Review article, Preeclampsia, Acute kidney injury, Cardiovascular, Pulmonary edema, Fluid management
Introduction
Preeclampsia is a multifactorial condition characterized by new-onset hypertension and proteinuria, or hypertension associated with significant organic dysfunction after 20-weeks of pregnancy.1 Worldwide, it complicates 2% to 8% of pregnancies and is responsible for up to 14% of maternal deaths.2, 3 Preeclampsia is also a significant cause of morbidity; patients presenting with severe disease are at a higher risk of developing severe complications, including Acute Kidney Injury (AKI), cardiovascular diseases, and Pulmonary Edema (PE).4
The current knowledge in physiopathology of preeclampsia relies on abnormal placentation and imperfect invasion of the uterine spiral arteries by cytotrophoblast cells, causing inadequate blood flow and relative placental ischemia.5, 6 In consequence, a state of oxidative stress is established, leading to defective fetoplacental angiogenesis and endothelial dysfunction.7
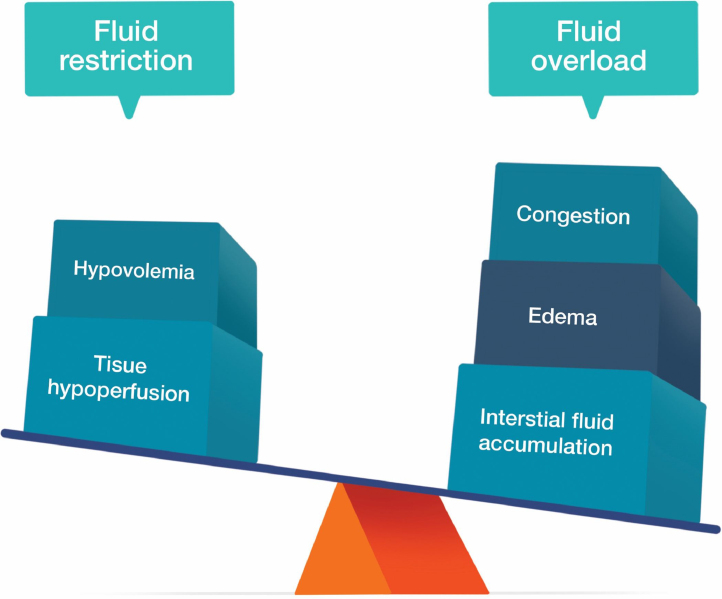
The endothelial dysfunction in preeclampsia is associated with an increase in both peripheral vascular resistance and vascular permeability, which ultimately results in a state of relative intravascular hypovolemia.8 Fluid therapy in this scenario may be challenging, especially during cesarean delivery. Although volume expansion may precipitate fluid overload and PE, fluid restriction may worsen tissue hypoperfusion and increase the risk of AKI (Fig. 1).9, 10, 11 Furthermore, cardiac impairment may play an essential role in preeclampsia, introducing an additional component to the hemodynamic management.12, 13
Figure 1.
Considerations while choosing the fluid management strategy in women with preeclampsia.
This article reviews the repercussions of preeclampsia on renal and cardiovascular systems, and the development of pulmonary edema, as well as to discuss fluid management, focusing on the mitigation of adverse outcomes and monitoring alternatives.
Methodology
The literature review was carried out by searching the PubMed, Embase, and Google Scholar databases from May 2019 to March 2020. Eligible studies were identified by using different combinations of the following search terms: “preeclampsia”, “pathogenesis”, “acute kidney failure”, “cardiovascular”, “pulmonary edema”, and “fluid management”.
Articles were initially selected after reviewing the title and the abstract. Papers addressing the subjects of interest were selected for a full review and included regardless of the publication language.
Preeclampsia-related renal repercussions
Preeclampsia is the leading cause of pregnancy-related AKI,14, 15 a condition associated with high rates of maternal mortality and fetal loss.16 In the United States, between 1998 and 2009, up to 17% of deaths during hospitalized deliveries occurred among women with AKI.11 Additionally, women who develop AKI during pregnancy, regardless of etiology, are at higher risk of poor outcomes, including obstetrical hemorrhage, placental abruption, and intensive care unit admission.17, 18 Approximately 2% of women with severe preeclampsia and 15% of women with HELLP (hemolysis, elevated liver enzymes, low platelet count) syndrome, a variant of severe preeclampsia, will develop AKI.15, 18, 19, 20, 21
The diagnosis of pregnancy-related AKI is challenging. During pregnancy, the physiological increase in Glomerular Filtration Rate (GFR) decreases the concentration of serum creatinine.22, 23 Thus, serum creatinine that is within the normal range for the general population may actually mask a significant impairment in renal function.24 Pregnant women may experience 30% to 40% reduction in GFR before a significant increase in serum creatinine occurs.15
An additional issue is the absence of consensus regarding the diagnostic criteria for pregnancy-related AKI. Several classifications have been developed for application in the general population, including the RIFLE (Risk, Injury, Failure, Loss, and End-Stage Kidney Disease – ESKD)25 and the AKIN (Acute Kidney Injury Network)26, 27 criteria. They are commonly used in the obstetric population, even without validation. The American College of Obstetricians and Gynecologists (ACOG) has its definition for AKI as a pregnancy-related hypertensive disorder (Table 1).1
Table 1.
RIFLE, AKIN, and ACOG definitions for AKI.
| RIFLE25 | |
| Risk | 1.5-fold increase in serum creatinine OR 25% decrease in GFR OR < 0.5 mL.kg-1.h-1 for > 6 h |
| Injury | 2-fold increase in serum creatinine OR 50% decrease in GFR OR UO < 0.5 mL.kg-1.h-1 for > 12 h |
| Failure | 3-fold increase in serum creatinine OR 75% decrease in GFR OR UO < 0.3 mL.kg-1.h-1 for > 24 h OR no UO for 12 h |
| Loss of kidney function | Complete loss of kidney function (> 4 weeks) |
| ESKD | Complete loss of kidney function (> 3 months) |
| AKIN26 | |
| Absolute increase in serum creatinine 0.3 mg.dL-1 or more OR 1.5-fold increase in baseline serum creatinine OR UO < 0.5 mL.kg-1.h-1 for > 6 h | |
| ACOG1 | |
| Baseline serum creatinine > 1.1 mg.dL-1 OR 2-fold increase in baseline serum creatinine in the absence of renal disease | |
ESKD, End Stage Kidney Disease; GFR, Glomerular Filtration Rate; UO, Urinary Output.
AKI management during pregnancy is focused on providing supportive measures, dialysis, and correction of the underlying etiology.28 Avoidance of nephrotoxic drugs and correction of complications, such as hypertension, hyperkalemia, and metabolic acidosis, should be promptly initiated along with judicious fluid management, aiming to provide adequate uteroplacental perfusion and fetal well-being while avoiding volume overload and PE.29
In the presence of uremic symptoms (encephalopathy, pericarditis, or neuropathy) or complications that are refractory to pharmacological interventions, renal replacement therapy is indicated. Dialysis prescription during pregnancy should minimize hemodynamic fluctuations; in this scenario, longer and more frequent sessions are preferred.24, 29 As many as 30% to 50% of those who develop AKI associated with HELLP syndrome will require dialysis temporarily.30
The identification of the underlying etiology of pregnancy-related AKI is essential; however, since several causes may share similar clinical and laboratory findings, the exact etiology may remain indefinite.24 Regarding preeclampsia, diagnosis relies on the presence of Blood Pressure (BP) > 140/90 mmHg with proteinuria of ≥ 300 mg/day after 20 weeks of gestation in a previously normotensive woman or the evidence of organ damage.31
Specific treatment depends on the illness severity, fetal well-being, and gestational age. Before 24 weeks, pregnancy is discontinued because no fetal survival benefit is observed.32 Between 24 and 32 weeks, expectant management is recommended32, 33; after 32-weeks, delivery is the treatment of choice.34 Development of severe preeclampsia, HELLP syndrome, or fetal compromise are indications for prompt delivery regardless of gestational age.33
In the past, preeclampsia-related AKI was considered completely reversed after delivery. Although the risk of ESKD after pregnancy is low, recent studies indicate that AKI during pregnancy increases the risk of long-term renal dysfunction.18, 22, 35, 36, 37 A study that followed women with HELLP syndrome and AKI for up to one year after delivery reported that 21% of the patients needed dialysis.35 Patients with preexisting hypertension or renal disease have a higher likelihood of requiring long-term dialysis.38
Preeclampsia-related cardiovascular repercussions
Preeclampsia and cardiovascular diseases share several predisposing conditions, such as obesity, smoking, sedentary lifestyle, diabetes, chronic kidney disease, chronic hypertension, and abnormal serum lipid profile.39 For a long time, the overlap of risk factors for both illnesses was thought to be spurious. However, recent studies hypothesize that disorders of the cardiovascular system may have a direct effect on preeclampsia pathogenesis and that preeclampsia is an independent risk factor for cardiovascular disease.12, 13, 40, 41, 42
Preeclamptic women experience a derangement in renin-angiotensin-aldosterone system regulation when compared to their healthy counterparts. While refractoriness to angiotensin II is found in uncomplicated pregnancies,43 an increase in vascular sensitivity to angiotensin II occurs in preeclampsia.44, 45 This imbalance, which is clinically expressed as high BP and peripheral arterial resistance, is present long before the diagnosis of preeclampsia.39 Likewise, when compared to healthy women, those who develop preeclampsia may have a lower cardiac output and cardiac index, impaired myocardial relaxation, and increased prevalence of abnormal heart geometry even before conception.46, 47, 48
Besides the compelling evidence that preeclampsia has a cardiovascular etiology, the American Heart Association now recognizes that this condition is a risk factor for long-term postpartum cardiovascular diseases.49 A cohort study that included over one million maternities demonstrated that preeclamptic women have a higher risk of major adverse cardiovascular events and that this risk remains significantly higher long after the delivery.50 Another study found that recurrent preeclampsia is associated with increased rates of hypertension, ischemic heart disease, heart failure, and cerebrovascular accident.51 There is also evidence for increased cardiovascular risk in the offspring of the preeclamptic mother.52
The cardiovascular management in uncomplicated preeclampsia is focused on BP control. Although BP thresholds and goals vary in international guidelines, there is a consensus that BP should be treated when it is severe (systolic BP ≥ 160 mmHg or diastolic BP ≥ 110 mmHg).42 In complicated preeclampsia, delivery after stabilization of the patient is the only specific treatment.31 During labor, the use of invasive hemodynamic monitoring might be considered to guide fluid therapy, especially if severe cardiac disease, PE, persistent oliguria, and severe hypertension are present.10, 53
In the postpartum period, women should be followed during the first 6 to 8 weeks; patients with a persistent need for antihypertensive medication require a referral to a cardiologist.54 It is known that cardiovascular diseases have a slow progression, from asymptomatic to symptomatic stage. In this setting, the diagnosis of preeclampsia, which typically occurs in young women, poses an opportunity for early identification of high-risk patients when they are still in the asymptomatic stage. At this point, lifestyle and therapeutic interventions are more effective in controlling other cardiovascular risk factors.42
Pulmonary edema in preeclampsia
PE is the most common cardiopulmonary complication of preeclampsia, occurring in 3% to 5% of preeclamptic pregnancies, mainly in the peripartum or postpartum stage.42, 55, 56 Despite the low rate of incidence, PE is a life-threatening event, being a frequent cause of admission to the intensive care unit and the leading cause of death among preeclamptic women.57, 58
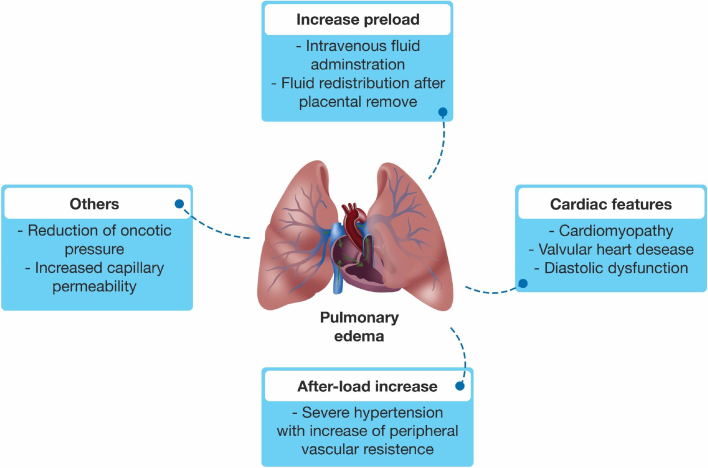
The etiology of PE in preeclampsia is multifactorial (Fig. 2). The decrease in oncotic pressure secondary to preeclampsia-related hypoproteinemia, the disruption in pulmonary endothelium leading to increased capillary permeability, and the increase in afterload due to severe hypertension all appear to play a role.10, 59 As previously discussed, preeclampsia may be associated with cardiac function impairment, which may contribute to PE.60 PE is also related to preeclampsia severity, with higher rates among women who develop AKI, HELLP syndrome, and eclampsia.18, 61, 62
Figure 2.
Multifactorial etiology of pulmonary edema in preeclampsia.
Nearly 70% of the PE events occur in the postpartum period.61 Following the placental removal, a fluid shift from the extravascular space restores the intravascular volume, increasing the preload.63, 64 Furthermore, the inadvertent administration of fluid, frequently used to increase plasma volume or treat oliguria, may also exacerbate the preload and trigger PE.10 It is recognizable that iatrogenic fluid administration is a major preventable cause of PE.65
The management of preeclampsia complicated by PE poses a significant challenge to the medical team. The superimposed issues of the physiological changes of pregnancy, the presence of the fetus, and the knowledge gaps regarding the physiopathology of preeclampsia all contribute to the high rates of morbidity and mortality associated with this condition.10
The initial goal while treating PE during preeclampsia relies on the reduction of BP with intravenous antihypertensive agents (Table 2).42 Rapid reduction of blood pressure may be accomplished with the use of intravenous labetatol or hydralazine.42, 66 Some authors also recommend the use of nitroglycerine in hypertensive crises, despite the risk of aggravate the depletion of intravascular volume.42, 67, 68 Furosemide should be used with caution because it may worsen the placental perfusion.69
Table 2.
Intravenous antihypertensive medications used in hypertensive pulmonary edema during preeclampsia.
| Drug | Starting dose | Repeating doses and intervals | Maximum total dose | Comments |
|---|---|---|---|---|
| Labetalol42, 66 | 20 mg | 40 mg after 10 minutes | 220 mg | Avoid in asthma, chronic obstructive airways disease, and heart failure; associated with neonatal bradycardia and hypoglycemia |
| Hydralazine42, 66 | 5 mg | 5–10 mg after 20 minutes | 20 mg | Risk of sudden hypotension and maternal tachycardia; may need preloading or simultaneous fluid infusion |
| Nitroglycerine10, 42, 67, 68 | 5 μg.min−1 | Gradually increase every 3–5 minutes | 100 μg.min−1 | Considered the drug of choice by some authors; may aggravate the depletion of intravascular volume |
| Furosemide10 | 20–40 mg | 40–60 mg after 30 minutes | 120 mg | May worsen placental perfusion |
In the case of left ventricular systolic dysfunction, inotropic support should be considered.10 There is a gap in the literature regarding the choice of a specific inotrope to manage acute heart failure during pregnancy, especially due to safety concerns.70 Therefore, the selection of an inotrope should be based upon the clinical scenario.
Regarding ventilatory support, noninvasive modalities are initially preferred due to the risks associated with tracheal intubation in hypertensive pregnant women, such as intracerebral hemorrhage.10, 71, 72
After stabilization of the patient, consideration needs to be given to the delivery of the fetus if PE occurs in the antenatal period. Assessment of fetal well-being and multidisciplinary planning for safe birth is necessary.10
Fluid management and hemodynamic monitoring
Despite the lack of paramount evidence favoring a specific fluid management protocol, current guidelines advocate a restrictive approach, with additional fluid administration only recommended in selected scenarios (Table 3).73, 74, 75
Table 3.
Intravenous fluid indications in preeclampsia.
| Maintenance73, 80 | |
| 60 to 80 mL.h-1 OR UO + stool + insensible losses | Consider the volume used for drug administration |
| Monitoring based on clinical observation | |
| Replacement73, 74, 88, 89 | |
| Titrate to systolic BP > 90 mmHg OR Shock index < 0.9 | Consider arterial line insertion if pressure control is difficult or there is severe bleeding |
| Consider noninvasive hemodynamic monitoring | |
| Preload before neuraxial anesthesia73, 74, 76, 77 | |
| 300 mL fluid challenge | Not routinely indicated |
| Consider if high dose of anesthetic is administered | |
| Consider if hydralazine is the antenatal antihypertensive | |
| Oliguria73, 74, 77 | |
| 300 mL fluid challenge | Not routinely indicated |
| Maintain UO ≥ 100 mL/4 h | If persistent oliguria, consider repeat the fluid challenge (in case of negative fluid balance) |
| Consider noninvasive hemodynamic monitoring | |
BP, Blood Pressure; UO, Urinary Output.
Usually, preeclamptic women admitted for delivery may need fluid therapy for maintenance of water and electrolyte balance, or replacement of lost intravascular volume.73 A routine fluid bolus should not be administered before neuraxial anesthesia, or to treat oliguria.73, 74, 76, 77 The available data do not suggest an absolute maternal or fetal benefit of colloids over crystalloids in preeclamptic women.75, 78, 79
Maintenance fluid therapy is required in uncomplicated patients who are expected to fast for several hours during labor. The infusion rate of crystalloids may be fixed 60 to 80 mL.h-1, or calculated to match the Urinary Output (UO) combined with stool and insensible losses (lungs and skin).73, 80 It is also important to consider the volume used as a vehicle for drug administration when calculating the total amount of fluid. Given the low risk of complications following slow administration of intravenous fluids, hemodynamic monitoring may be based only upon clinical observation.73
Patients with severe preeclampsia may experience hemorrhagic shock due to several causes, such as placental abruption, operative blood loss, and rupture of subcapsular liver hematoma.81, 82, 83 These women require immediate resuscitative measures, including intravenous volume replacement and blood typing.84 The primary goal in this setting is to maintain a systolic BP above 90 mmHg.73
The shock index may also be considered as a predictor of adverse maternal outcomes. A threshold ≥ 0.9 indicates rigorous monitoring, ≥ 1.4 indicates an urgent need for intervention, and ≥ 1.7 indicates a high chance of adverse outcomes, including severe end-organ dysfunction and death.85 UO is not a good predictor of fluid responsiveness in preeclamptic women and should not be routinely used as a therapeutic guide.86
The possibility of fluid overload and PE should be considered in the case of over-transfusion, especially when the fluid balance is above 2,000 mL.58, 73 However, inadequate resuscitation may increase the likelihood of AKI.9 To balance the risks of both complications, hemodynamic monitoring is recommended to guide replacement therapy.87
Arterial line insertion is valuable in the presence of severe bleeding or difficult BP control. Other invasive methods, such as central venous pressure and pulmonary artery catheterization, are not routinely encouraged.53, 74 Ultimately, particular attention has been given to the use of noninvasive methods. Transthoracic echocardiography is recommended as a diagnostic and monitoring tool for hemodynamic complications, such as PE, severe arterial hypertension, and chest pain.88 Lung ultrasound is another modality with growing importance. It can indicate both PE and increased left ventricular end-diastolic pressures.89
Conclusion and future directions
Preeclampsia is an important complication in pregnancy, resulting in significant rates of morbidity and mortality worldwide. Given its intricate and not still completely understood renal and cardiovascular repercussions, the management of fluid administration in preeclamptic women can be challenging. Fluid restriction may precipitate or accentuate ischemic kidney lesions, while fluid overload may increase the hydrostatic pressure in the pulmonary capillaries, leading to PE.
Despite the lack of paramount evidence, there is a current trend towards restricting the administration of fluids in women with non-complicated preeclampsia. However, patients with severe preeclampsia may experience hemorrhagic shock, requiring volume resuscitation. In this case, hemodynamic monitoring is recommended to guide fluid therapy while avoiding complications. Noninvasive methods, such as transthoracic echocardiography and lung ultrasound, are preferred.
Future studies should focus on the influence of fluid management on patient outcomes. A randomized controlled trial could help to define the appropriate volume, as well as to describe which outcomes are significantly impacted by different volume management strategies. Additionally, basic sciences research could help to clarify the physiopathology of preeclampsia.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.ACOG Practice Bulletin No. 202 Gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1–e25. doi: 10.1097/AOG.0000000000003018. [DOI] [PubMed] [Google Scholar]
- 2.Abalos E., Cuesta C., Grosso A.L., et al. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2013;170:1–7. doi: 10.1016/j.ejogrb.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Vigil-De Gracia P. Maternal deaths due to eclampsia and HELLP syndrome. Int J Gynaecol Obstet. 2009;104:90–94. doi: 10.1016/j.ijgo.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Hutcheon J.A., Lisonkova S., Joseph K.S. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25:391–403. doi: 10.1016/j.bpobgyn.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Roberts JM, Redman CW. Pre-eclampsia: more than pregnancy-induced hypertension. Lancet. 1993;341:1447–1451. doi: 10.1016/0140-6736(93)90889-o. [DOI] [PubMed] [Google Scholar]
- 6.Meekins J.W., Pijnenborg R., Hanssens M., et al. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1994;101:669–674. doi: 10.1111/j.1471-0528.1994.tb13182.x. [DOI] [PubMed] [Google Scholar]
- 7.Uzan J., Carbonnel M., Piconne O., et al. Pre-eclampsia: pathophysiology, diagnosis, and management. Vasc Health Risk Manag. 2011;7:467–474. doi: 10.2147/VHRM.S20181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mabie W.C., Ratts T.E., Sibai B.M. The central hemodynamics of severe preeclampsia. Am J Obstet Gynecol. 1989;161:1443–1448. doi: 10.1016/0002-9378(89)90901-0. [DOI] [PubMed] [Google Scholar]
- 9.Mehrabadi A., Liu S., Bartholomew S., et al. Hypertensive disorders of pregnancy and the recent increase in obstetric acute renal failure in Canada: population based retrospective cohort study. BMJ. 2014;349:g4731. doi: 10.1136/bmj.g4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dennis AT, Solnordal CB. Acute pulmonary oedema in pregnant women. Anaesthesia. 2012;67:646–659. doi: 10.1111/j.1365-2044.2012.07055.x. [DOI] [PubMed] [Google Scholar]
- 11.Callaghan W.M., Creanga A.A., Kuklina E.V. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. 2012;120:1029–1036. doi: 10.1097/aog.0b013e31826d60c5. [DOI] [PubMed] [Google Scholar]
- 12.Thilaganathan B. Pre-eclampsia and the cardiovascular-placental axis. Ultrasound Obstet Gynecol. 2018;51:714–717. doi: 10.1002/uog.19081. [DOI] [PubMed] [Google Scholar]
- 13.Perry H., Khalil A., Thilaganathan B. Preeclampsia and the cardiovascular system: an update. Trends Cardiovasc Med. 2018;28:505–513. doi: 10.1016/j.tcm.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Prakash J., Niwas S.S., Parekh A., et al. Acute kidney injury in late pregnancy in developing countries. Ren Fail. 2010;32:309–313. doi: 10.3109/08860221003606265. [DOI] [PubMed] [Google Scholar]
- 15.Fakhouri F., Vercel C., Fremeaux-Bacchi V. Obstetric nephrology: AKI and thrombotic microangiopathies in pregnancy. Clin J Am Soc Nephrol. 2012;7:2100–2106. doi: 10.2215/CJN.13121211. [DOI] [PubMed] [Google Scholar]
- 16.Bentata Y., Housni B., Mimouni A., et al. Acute kidney injury related to pregnancy in developing countries: etiology and risk factors in an intensive care unit. J Nephrol. 2012;25:764–775. doi: 10.5301/jn.5000058. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y., Ma X., Zheng J., et al. Pregnancy outcomes in patients with acute kidney injury during pregnancy: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2017;17:235. doi: 10.1186/s12884-017-1402-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gul A., Aslan H., Cebeci A., et al. Maternal and fetal outcomes in HELLP syndrome complicated with acute renal failure. Ren Fail. 2004;26:557–562. doi: 10.1081/jdi-200031750. [DOI] [PubMed] [Google Scholar]
- 19.Nzerue C.M., Hewan-Lowe K., Nwawka C. Acute renal failure in pregnancy: a review of clinical outcomes at an inner-city hospital from 1986-1996. J Natl Med Assoc. 1998;90:486–490. [PMC free article] [PubMed] [Google Scholar]
- 20.Kuklina E.V., Ayala C., Callaghan W.M. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113:1299–1306. doi: 10.1097/AOG.0b013e3181a45b25. [DOI] [PubMed] [Google Scholar]
- 21.Sibai B.M., Ramadan M.K., Usta I., et al. Maternal morbidity and mortality in 442 pregnancies with hemolysis, elevated liver enzymes, and low platelets (HELLP syndrome) Am J Obstet Gynecol. 1993;169:1000–1006. doi: 10.1016/0002-9378(93)90043-i. [DOI] [PubMed] [Google Scholar]
- 22.Szczepanski J., Griffin A., Novotny S., et al. Acute kidney injury in pregnancies complicated with preeclampsia or HELLP syndrome. Front Med (Lausanne) 2020;7:22. doi: 10.3389/fmed.2020.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheung KL, Lafayette RA. Renal physiology of pregnancy. Adv Chronic Kidney Dis. 2013;20:209–214. doi: 10.1053/j.ackd.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rao S., Jim B. Acute kidney injury in pregnancy: the changing landscape for the 21st century. Kidney Int Rep. 2018;3:247–257. doi: 10.1016/j.ekir.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellomo R., Ronco C., Kellum J.A., et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta R.L., Kellum J.A., Shah S.V., et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopes J.A., Jorge S. The RIFLE and AKIN classifications for acute kidney injury: a critical and comprehensive review. Clin Kidney J. 2013;6:8–14. doi: 10.1093/ckj/sfs160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balofsky A., Fedarau M. Renal failure in pregnancy. Crit Care Clin. 2016;32:73–83. doi: 10.1016/j.ccc.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Prakash J., Ganiger V.C. Acute kidney injury in pregnancy-specific disorders. Indian J Nephrol. 2017;27:258–270. doi: 10.4103/0971-4065.202406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ganesan C., Maynard S.E. Acute kidney injury in pregnancy: the thrombotic microangiopathies. J Nephrol. 2011;24:554–563. doi: 10.5301/JN.2011.6250. [DOI] [PubMed] [Google Scholar]
- 31.American College of O., Gynecologists, Task Force on Hypertension in P. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 32.Magee L.A., Yong P.J., Espinosa V., et al. Expectant management of severe preeclampsia remote from term: a structured systematic review. Hypertens Pregnancy. 2009;28:312–347. doi: 10.1080/10641950802601252. [DOI] [PubMed] [Google Scholar]
- 33.Krane N.K., Hamrahian M. Pregnancy: kidney diseases and hypertension. Am J Kidney Dis. 2007;49:336–345. doi: 10.1053/j.ajkd.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 34.Gaugler-Senden I.P., Huijssoon A.G., Visser W., et al. Maternal and perinatal outcome of preeclampsia with an onset before 24-weeks’ gestation. Audit in a tertiary referral center. Eur J Obstet Gynecol Reprod Biol. 2006;128:216–221. doi: 10.1016/j.ejogrb.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 35.Ye W., Shu H., Yu Y., et al. Acute kidney injury in patients with HELLP syndrome. Int Urol Nephrol. 2019;51:1199–1206. doi: 10.1007/s11255-019-02111-7. [DOI] [PubMed] [Google Scholar]
- 36.Sibai BM, Ramadan MK. Acute renal failure in pregnancies complicated by hemolysis, elevated liver enzymes, and low platelets. Am J Obstet Gynecol. 1993;168:1682–1687. doi: 10.1016/0002-9378(93)90678-c. discussion 7-90. [DOI] [PubMed] [Google Scholar]
- 37.Vikse B.E., Irgens L.M., Leivestad T., et al. Preeclampsia and the risk of end-stage renal disease. N Engl J Med. 2008;359:800–809. doi: 10.1056/NEJMoa0706790. [DOI] [PubMed] [Google Scholar]
- 38.Gammill H.S., Jeyabalan A. Acute renal failure in pregnancy. Crit Care Med. 2005;33:S372–84. doi: 10.1097/01.ccm.0000183155.46886.c6. [DOI] [PubMed] [Google Scholar]
- 39.Thilaganathan B., Kalafat E. Cardiovascular system in preeclampsia and beyond. Hypertension. 2019;73:522–531. doi: 10.1161/HYPERTENSIONAHA.118.11191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Behrens I., Basit S., Melbye M., et al. Risk of post-pregnancy hypertension in women with a history of hypertensive disorders of pregnancy: nationwide cohort study. BMJ. 2017;358:j3078. doi: 10.1136/bmj.j3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalafat E., Thilaganathan B. Cardiovascular origins of preeclampsia. Curr Opin Obstet Gynecol. 2017;29:383–389. doi: 10.1097/GCO.0000000000000419. [DOI] [PubMed] [Google Scholar]
- 42.Melchiorre K., Sharma R., Thilaganathan B. Cardiovascular implications in preeclampsia: an overview. Circulation. 2014;130:703–714. doi: 10.1161/CIRCULATIONAHA.113.003664. [DOI] [PubMed] [Google Scholar]
- 43.Sanghavi M, Rutherford JD. Cardiovascular physiology of pregnancy. Circulation. 2014;130:1003–1008. doi: 10.1161/CIRCULATIONAHA.114.009029. [DOI] [PubMed] [Google Scholar]
- 44.Gant N.F., Worley R.J., Everett R.B., et al. Control of vascular responsiveness during human pregnancy. Kidney Int. 1980;18:253–258. doi: 10.1038/ki.1980.133. [DOI] [PubMed] [Google Scholar]
- 45.Gant N.F., Daley G.L., Chand S., et al. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest. 1973;52:2682–2689. doi: 10.1172/JCI107462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foo F.L., Mahendru A.A., Masini G., et al. Association Between prepregnancy cardiovascular function and subsequent preeclampsia or fetal growth restriction. Hypertension. 2018;72:442–450. doi: 10.1161/HYPERTENSIONAHA.118.11092. [DOI] [PubMed] [Google Scholar]
- 47.Castleman J.S., Ganapathy R., Taki F., et al. Echocardiographic structure and function in hypertensive disorders of pregnancy: a systematic review. Circ Cardiovasc Imaging. 2016;9 doi: 10.1161/CIRCIMAGING.116.004888. [DOI] [PubMed] [Google Scholar]
- 48.Guy G.P., Ling H.Z., Garcia P., et al. Maternal cardiac function at 35-37 weeks’ gestation: prediction of pre-eclampsia and gestational hypertension. Ultrasound Obstet Gynecol. 2017;49:61–66. doi: 10.1002/uog.17300. [DOI] [PubMed] [Google Scholar]
- 49.Mosca L., Benjamin E.J., Berra K., et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women–2011 update: a guideline from the american heart association. Circulation. 2011;123:1243–1262. doi: 10.1161/CIR.0b013e31820faaf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin Y.S., Tang C.H., Yang C.Y., et al. Effect of pre-eclampsia-eclampsia on major cardiovascular events among peripartum women in Taiwan. Am J Cardiol. 2011;107:325–330. doi: 10.1016/j.amjcard.2010.08.073. [DOI] [PubMed] [Google Scholar]
- 51.Brouwers L., van der Meiden-van Roest A.J., Savelkoul C., et al. Recurrence of pre-eclampsia and the risk of future hypertension and cardiovascular disease: a systematic review and meta-analysis. BJOG. 2018;125:1642–1654. doi: 10.1111/1471-0528.15394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alsnes I.V., Vatten L.J., Fraser A., et al. Hypertension in pregnancy and offspring cardiovascular risk in young adulthood: prospective and sibling studies in the HUNT study (Nord-Trondelag Health Study) in Norway. Hypertension. 2017;69:591–598. doi: 10.1161/HYPERTENSIONAHA.116.08414. [DOI] [PubMed] [Google Scholar]
- 53.Li Y.H., Novikova N. Pulmonary artery flow catheters for directing management in pre-eclampsia. Cochrane Database Syst Rev. 2012 doi: 10.1002/14651858.CD008882.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hypertension in Pregnancy: The Management of Hypertensive Disorders During Pregnancy. National Institute for Health and Clinical Excellence: Guidance; London: 2010. [Google Scholar]
- 55.Amorim M.M., Katz L., Valenca M., et al. Severe maternal morbidity in an obstetric ICU in Recife, Northeast of Brasil. Rev Assoc Med Bras (1992) 2008;54:261–266. doi: 10.1590/s0104-42302008000300021. [DOI] [PubMed] [Google Scholar]
- 56.Sibai B.M., Mabie B.C., Harvey C.J., et al. Pulmonary edema in severe preeclampsia-eclampsia: analysis of thirty-seven consecutive cases. Am J Obstet Gynecol. 1987;156:1174–1179. doi: 10.1016/0002-9378(87)90135-9. [DOI] [PubMed] [Google Scholar]
- 57.Lowe S.A., Brown M.A., Dekker G.A., et al. Guidelines for the management of hypertensive disorders of pregnancy 2008. Aust N Z J Obstet Gynaecol. 2009;49:242–246. doi: 10.1111/j.1479-828X.2009.01003.x. [DOI] [PubMed] [Google Scholar]
- 58.Pordeus A.C.B., Katz L., Soares M.C., et al. Acute pulmonary edema in an obstetric intensive care unit: a case series study. Medicine (Baltimore) 2018;97 doi: 10.1097/MD.0000000000011508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bauer ST, Cleary KL. Cardiopulmonary complications of pre-eclampsia. Semin Perinatol. 2009;33:158–165. doi: 10.1053/j.semperi.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 60.Vaught A.J., Kovell L.C., Szymanski L.M., et al. Acute cardiac effects of severe pre-eclampsia. J Am Coll Cardiol. 2018;72:1–11. doi: 10.1016/j.jacc.2018.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Norwitz E.R., Hsu C.D., Repke J.T. Acute complications of preeclampsia. Clin Obstet Gynecol. 2002;45:308–329. doi: 10.1097/00003081-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 62.Gandhi S., Sun D., Park A.L., et al. The pulmonary edema preeclampsia evaluation (PEPE) study. J Obstet Gynaecol Can. 2014;36:1065–1070. doi: 10.1016/S1701-2163(15)30383-2. [DOI] [PubMed] [Google Scholar]
- 63.Brichant J.F., Brichant G., Dewandre P.Y., et al. Circulatory and respiratory problems in preeclampsia. Ann Fr Anesth Reanim. 2010;29:e91–5. doi: 10.1016/j.annfar.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 64.Poole JH, Spreen DT. Acute pulmonary edema in pregnancy. J Perinat Neonatal Nurs. 2005;19:316–331. doi: 10.1097/00005237-200510000-00007. [DOI] [PubMed] [Google Scholar]
- 65.Thornton C.E., von Dadelszen P., Makris A., et al. Acute pulmonary oedema as a complication of hypertension during pregnancy. Hypertens Pregnancy. 2011;30:169–179. doi: 10.3109/10641950902972140. [DOI] [PubMed] [Google Scholar]
- 66.Wilkerson RG, Ogunbodede AC. Hypertensive Disorders of Pregnancy. Emerg Med Clin North Am. 2019;37:301–316. doi: 10.1016/j.emc.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 67.Coppage KH, Sibai BM. Treatment of hypertensive complications in pregnancy. Curr Pharm Des. 2005;11:749–757. doi: 10.2174/1381612053381864. [DOI] [PubMed] [Google Scholar]
- 68.European Society of G, Association for European Paediatric C, German Society for Gender M, et al. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC) Eur Heart J. 2011;32:3147–3197. doi: 10.1093/eurheartj/ehr218. [DOI] [PubMed] [Google Scholar]
- 69.Dolley P., Lebon A., Beucher G., et al. Acute pulmonary edema and pregnancy: a descriptive study of 15 cases and review of the literature. J Gynecol Obstet Biol Reprod (Paris) 2012;41:638–644. doi: 10.1016/j.jgyn.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 70.van Hagen I.M., Cornette J., Johnson M.R., et al. Managing cardiac emergencies in pregnancy. Heart. 2017;103:159–173. doi: 10.1136/heartjnl-2015-308285. [DOI] [PubMed] [Google Scholar]
- 71.Elliott MW. Non-invasive ventilation: established and expanding roles. Clin Med (Lond) 2011;11:150–153. doi: 10.7861/clinmedicine.11-2-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Perbet S., Constantin J.M., Bolandard F., et al. Non-invasive ventilation for pulmonary edema associated with tocolytic agents during labour for a twin pregnancy. Can J Anaesth. 2008;55:769–773. doi: 10.1007/BF03016350. [DOI] [PubMed] [Google Scholar]
- 73.Anthony J, Schoeman LK. Fluid management in pre-eclampsia. Obstet Med. 2013;6:100–104. doi: 10.1177/1753495X13486896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Magee L.A., Pels A., Helewa M., et al. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can. 2014;36:416–441. doi: 10.1016/s1701-2163(15)30588-0. [DOI] [PubMed] [Google Scholar]
- 75.Pretorius T., van Rensburg G., Dyer R.A., et al. The influence of fluid management on outcomes in preeclampsia: a systematic review and meta-analysis. Int J Obstet Anesth. 2018;34:85–95. doi: 10.1016/j.ijoa.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 76.Hofmeyr G., Cyna A., Middleton P. Prophylactic intravenous preloading for regional analgesia in labour. Cochrane Database Syst Rev. 2004 doi: 10.1002/14651858.CD000175.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.NICE Guideline [NG133]. Hypertension in pregnancy: diagnosis and management. https://www.nice.org.uk/guidance/ng133. Published: June 2019. Accessed March 12, 2020.
- 78.Duley L., Williams J., Henderson-Smart D.J. Plasma volume expansion for treatment of women with pre-eclampsia. Cochrane Database Syst Rev. 2000 doi: 10.1002/14651858.CD001805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ganzevoort W., Rep A., Bonsel G.J., et al. A randomised controlled trial comparing two temporising management strategies, one with and one without plasma volume expansion, for severe and early onset pre-eclampsia. BJOG. 2005;112:1358–1368. doi: 10.1111/j.1471-0528.2005.00687.x. [DOI] [PubMed] [Google Scholar]
- 80.Mol B.W.J., Roberts C.T., Thangaratinam S., et al. Pre-eclampsia. Lancet. 2016;387:999–1011. doi: 10.1016/S0140-6736(15)00070-7. [DOI] [PubMed] [Google Scholar]
- 81.von Schmidt auf Altenstadt JF, Hukkelhoven CW, van Roosmalen J, et al. Pre-eclampsia increases the risk of postpartum haemorrhage: a nationwide cohort study in the Netherlands. PLoS One. 2013;8 doi: 10.1371/journal.pone.0081959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Singh Y., Kochar S., Biswas M., et al. Hepatic rupture complicating HELLP syndrome in pregnancy. Med J Armed Forces India. 2009;65:89–90. doi: 10.1016/S0377-1237(09)80072-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ananth C.V., Lavery J.A., Vintzileos A.M., et al. Severe placental abruption: clinical definition and associations with maternal complications. Am J Obstet Gynecol. 2016;214:272.e1–272.e9. doi: 10.1016/j.ajog.2015.09.069. [DOI] [PubMed] [Google Scholar]
- 84.Ruth D., Kennedy B.B. Acute volume resuscitation following obstetric hemorrhage. J Perinat Neonatal Nurs. 2011;25:253–260. doi: 10.1097/JPN.0b013e31822539e3. [DOI] [PubMed] [Google Scholar]
- 85.El Ayadi A.M., Nathan H.L., Seed P.T., et al. Vital sign prediction of adverse maternal outcomes in women with hypovolemic shock: the role of shock index. PLoS One. 2016;11 doi: 10.1371/journal.pone.0148729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brun C., Zieleskiewicz L., Textoris J., et al. Prediction of fluid responsiveness in severe preeclamptic patients with oliguria. Intensive Care Med. 2013;39:593–600. doi: 10.1007/s00134-012-2770-2. [DOI] [PubMed] [Google Scholar]
- 87.Lambert G., Brichant J.F., Hartstein G., et al. Preeclampsia: an update. Acta Anaesthesiol Belg. 2014;65:137–149. [PubMed] [Google Scholar]
- 88.Dennis A.T. Transthoracic echocardiography in women with preeclampsia. Curr Opin Anaesthesiol. 2015;28:254–260. doi: 10.1097/ACO.0000000000000182. [DOI] [PubMed] [Google Scholar]
- 89.Zieleskiewicz L., Contargyris C., Brun C., et al. Lung ultrasound predicts interstitial syndrome and hemodynamic profile in parturients with severe preeclampsia. Anesthesiology. 2014;120:906–914. doi: 10.1097/ALN.0000000000000102. [DOI] [PubMed] [Google Scholar]